Abstract
Background
Diabetes mellitus-induced erectile dysfunction (DMED) is a common complication of diabetes mellitus. Earthworm protein (EWP) is an active protein extracted from the Chinese herbal medicine earthworm, which is used in clinical practice for treating DMED.
Objective
To investigate the mechanism of action of EWP in improving DMED in rats using network pharmacology and in vivo experimental validation.
Materials and methods
Network pharmacology predicts key targets. After identifying the DMED targets of EWP, a protein-protein interaction network was constructed using the STRING platform. The target genes were then enriched using Gene Ontology and the Kyoto Encyclopedia of Genes and Genomes. A “drug-component-disease-target-pathway” network map with Cytoscape 3.9.1 software was constructed. The nuclear factor-kappa B (NF-κB) signaling pathway was selected for further in vivo experimental validation in rats.
Results
EWP was mainly involved in the inflammatory response and NF-κB signaling pathway to regulate DMED. In vivo experiments showed that EWP was able to reduce Interleukin-1β, Interleukin-6 and Tumour Necrosis Factor-α levels, significantly inhibit NF-κB, nuclear factor-κB inhibitor protein α and mRNA expression, increase serum testosterone (T), and improve the erectile function of DMED rats.
Conclusion
EWP improves erectile function in DMED rats. This mechanism may be related to the inhibition of the NF-κB signaling pathway, reduction of the inflammatory response in testicular tissue, promotion of testicular and penile tissue repair, and increase in serum T levels.
Keywords: Earthworm protein, Diabetes, Erectile dysfunction, Inflammatory response, NF-κB signaling pathway
Graphical abstract

Highlights of the findings and novelties
-
•
In this study, network pharmacology was used to explore the mechanism EWP (earthworm protein) for the treatment of DMED, and a rat diabetes-induced ED model was constructed and validated.
-
•
The earthworm proteins were proposed to regulate NF- κ B signaling pathway, which affects the inflammatory response of testicular tissue to improve diabetes erectile dysfunction for the first time.
List of abbreviations
- APO
apomorphine
- CASP3
cysteinyl aspartate specific proteinases 3
- DM
diabetes mellitus
- DMED
diabetes mellitus-induced erectile dysfunction
- ECL
enhanced chemiluminescence
- ED
erectile dysfunction
- EGFR
epidermal growth factor receptor
- EWP
earthworm protein
- Elisa
enzyme-linked immunosorbent assay
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GO
gene ontology
- HE
hematoxylin-eosin staining
- ICPmax
maximum intracavernosal pressure
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IκB-α
inhibiting kappa B kinase alpha
- lKK
lκB kinase complex
- KEGG
kyoto Encyclopedia of Genes and Genomes
- MAP
mean Arterial Pressure
- PDE5
type 5 phosphodiesterase inhibitors
- PPI
protein-protein interaction
- STZ
streptozotocin
- T
testosterone
- TCM
traditional chinese medicine
- TNF-α
tumor necrosis factor-α
1. Introduction
Diabetes mellitus-induced erectile dysfunction (DMED) is a common complication of diabetes mellitus (DM). According to the survey, the incidence of erectile dysfunction (ED) in men in China is 0.1%–18 % in the healthy population,1 while that in patients with DM it is as high as 35 %–90 %.2 Moreover, the onset is 10 years earlier in patients with DM than in the healthy population, and the symptoms are more serious.3,4 In DMED rats, due to the persistent high glucose environment in the body, proteins, nucleic acids, and lipids undergo a non-enzymatic glycosylation reaction with reducing sugars (the Melad reaction). This subsequently produces glycosylation end products, which in turn activate inflammatory responses and oxidative stress, damaging the testes and penile structures of the rats and affecting erectile function.5 Inflammatory response and oxidative stress are the main mechanisms leading to endothelial dysfunction in the vasculature. Inflammation in the vasculature promotes the expression of vascular endothelial cell adhesion factors, which leads to endothelial cell dysfunction in the penile vasculature.6 Currently, the main clinical treatments for DMED include type 5 phosphodiesterase inhibitors (PDE5), negative pressure erection devices, intracavernous injections, penile prosthesis implants, intraurethral suppositories, and gene therapy. However, all treatments have certain limitations and adverse effects, leading to limited application.7 Therefore, there is an urgent need to find a new, comprehensive, and effective treatment for DMED.
The pathogenesis of DMED is a complex pathophysiological process involving multiple factors such as vascular endothelial dysfunction, peripheral nervous system disorder, and endocrine system disorder. Endothelial dysfunction and a decrease in the diastolic capacity of the vascular smooth muscle of the penile corpus cavernosum have been identified as the central causes of DMED pathophysiology. In recent years, serum testosterone (T) has been reported as an important determinant of penile erectile function, which can regulate penile erection by influencing aspects such as cavernous nerve function and structure and smooth muscle contractile activity.8,9
According to Traditional Chinese Medicine (TCM), obstruction of collaterals by blood stasis is the main pathogenesis of DMED.10 The TCM earthworm is a key drug for resolving blood stasis and promoting the circulation of blood vessels: it has the effects of clearing heat and stopping spasms, calming the liver and quenching wind, removing paralysis, diuretics, and calming asthma. The TCM earthworm has also been widely used for treating complications of diabetic vascular disease, including DMED.11,12 Earthworm protein (EWP) is an active protein extracted from TCM earthworms through modern separation techniques. EWP contains collagenase, plasmin, lumbrokinase, nucleic acid, trace elements, and other components, which have a variety of pharmacological effects such as anti-inflammatory, antioxidant, anti-fibrotic, anti-clotting, and vascular protection.13,14 Studies have shown that blood-activating and blood-stasis-removing Chinese medicines reduce the inflammatory response of vascular endothelial cells, inhibit oxidative stress, and improve vascular endothelial dysfunction.15 Our research team found in the early stage that EWP can improve penile erection function in DMED rats, although the specific mechanism remains unclear.16
In recent years, network pharmacology has become a research approach based on systems biology theory to elucidate the active ingredients and underlying mechanisms of TCM formulations, which are characterized by multi-component, multi-target, and multi-pathway synergy in the treatment and prevention of diseases. This is also consistent with TCM theory's system perspective. Our research aims to investigate the mechanism of action of EWP for treating DMED in rats through a combination of network pharmacology and in vivo experiments to provide an experimental basis for the clinical prevention and treatment of DMED.
2. Materials and methods
2.1. Network pharmacology
2.1.1. Databases and software
PubMed (https://pubmed.ncbi.nlm.nih.gov/); China National Knowledge Internet (https://www.cnki.net/); WanfangDatabase (http://www.wanfangdata.com.cn/); SwissADEM (http://www.swissadme.ch/); PubChem (https://pubchem.ncbi.nlm.nih.gov/); SwissTargetPrediction (http://www.swisstargetprediction.ch/); Human Genetic Data Bank (https://www.genecards.org/); Metascape platform (https://metascape.org/); STRING Database (https://string-db. org/); Cytoscape 3.9.1 Platform (http://cytoscape.org/).
2.1.2. Collection of EWP- and DMED-related targets
The active ingredients of EWP were obtained using the PubMed, CNKI, and Wanfang databases,17,18 and the simplified molecular linear input specification (SMILES) structure of EWP was queried using the PubChem platform. The active ingredients of EWP that met Lipinski's rule (gastrointestinal absorption showed high, while drug-like properties showed Yes ≥2) were screened using the SwissADEM platform. The obtained active ingredients were subjected to target prediction in the SwissTargetPrediction database and screened according to probability ≥0. The GeneCard database was searched for disease targets related to DMED. The EWP and DMED targets were then entered into the Venny 2.1 platform, and Venny diagrams were drawn to obtain common EWP-DMED targets.
2.1.3. Construction of the PPI network and GO function and KEGG pathway enrichment analyses
The active components of EWP and DMED intersecting targets were uploaded to the STRING database with a confidence level set at ≥ 0.4 to construct a protein-protein interaction (PPI) network of target proteins. The intersecting targets were then uploaded to the Metascape and Microbiotope platforms for gene ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis, and network maps were drawn.
2.1.4. Construction of an active ingredient-target-disease network
The active components of EWP and the corresponding targets, pathways, and corresponding disease targets were created into network files, and the above elements were categorized and saved into type files, before being uploaded to the Cytoscape 3.9.1 platform to draw network diagrams.
2.2. Experimental verification
2.2.1. Drug preparation and reagents
EWP was purchased from Baoji F.S. Biological Development Co., Ltd. Baoji, China; sildenafil tablets were purchased from QILU Pharmaceutical Co., Ltd. Shandong, China; apomorphine (APO) injection was purchased from APExBO, Houston, USA; streptozotocin (STZ) and the BCA Protein Concentration Assay Kit were purchased from Beijing Solarbio Science & Technology Co., Ltd. Beijing, China; the Interleukin-1β (IL-1β) kit was purchased from Shanghai Enzyme Link Biotechnology Co., Ltd. Beijing, China; the Interleukin-6 (IL-6), Tumour Necrosis Factor-α (TNF-α) kits were purchased from Jiangsu Sufia Biotechnology Co., Ltd. Jiangsu, China; the T kit was purchased from Sinobestbio, Beijing, China; the Nuclear factor-κB (NF-κB) antibody was purchased from Immunoway, California, USA; the Nuclear factor-κB inhibitor protein alpha (IκB-α) antibody was purchased from Abcam, Cambridge, UK; and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was purchased from Genetex, Southern California, USA.
2.2.2. Laboratory animals
Sixty specific pathogen free (SPF) grade Sprague-Dawley (SD) rats, aged 6–8 weeks, with a body weight of (200 ± 20) g, were selected for the experiment. The experimental animals were raised in the Animal Experiment Center of Gansu University of Chinese Medicine, where the room temperature was maintained at 22–25 °C, relative humidity at 45–55 %, and 12 h of light (08:00–20:00). The mating test showed that all SD rats had normal sexual function. This experiment was conducted in strict accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health. This study was approved by the Animal Experiment Center Committee of Gansu University of Traditional Chinese Medicine (Lanzhou, China) (No. 2020–149).
2.2.3. Establishment of the DMED model and its treatment
Sixty SPF grade SD rats, aged 6–8 weeks, were randomly divided into two groups (normal group: 10 and molding group: 50) after 1 week of acclimatization feeding. Molding group rats were intraperitoneally injected with STZ (concentration 0.1 mmol/L, dose 50 mg/kg) dissolved in citrate buffer solution with pH 4.0–4.5 to induce type I DM rats.19 After 3 days of injection, blood was randomly collected from the tail vein of rats by puncture, and blood glucose was measured. If blood glucose was >16.7 mmol/L, the rat was successfully molded with type I DM; otherwise, the experiment was rejected. Thereafter, blood glucose was measured randomly once a week, while those with blood glucose <16.7 mmol/L were excluded from the trial. After continuous feeding of DM rats with normal feed for 8 weeks, according to the method of Heaton,20 rats were weighed and placed in transparent glass boxes. The room was kept quiet, the light was dark, and the environment was adapted for 10 min. APO (100 μg/kg) was injected into the neck of the rats, and the penile erection of the rats was observed and recorded within 30 min. Those without penile erection were considered as DMED rats. The 30 rats in which the DMED model was successfully constructed were randomly divided into the following five groups: model group, sildenafil group, low-dose EWP group, medium-dose EWP group, and high-dose EWP group (n = 6). Six of the original ten rats in the normal group were randomly selected for inclusion in the final experiment. According to the conversion method of dosage given to humans and experimental animals, the sildenafil group was administered 0.005 g/kg (preparation of physiological saline) of sildenafil by gavage, whereas the low-dose EWP group, medium-dose EWP group, and high-dose EWP group were individually administered 0.045 g/kg, 0.090 g/kg, and 0.180 g/kg of EWP (preparation of physiological saline) by gavage, and the normal and model groups were given the same amount of normal saline every day for 4 weeks.
2.2.4. General condition, blood glucose, body mass testing in rats, and erectile function assessment
The rats were observed daily to evaluate their mental status, diet, water intake, hair shine, activity, urination, and defecation, and blood glucose and body mass levels were recorded weekly.
After the rats were anesthetized with 3 % sodium pentobarbital (40 mg/kg), the carotid vessels and the penile cavernous nerve were exposed, the prepuce of the penis was cut, the PE-50 tube was inserted into the carotid vessels and the penile corpus cavernosum, the cavernous nerve was stimulated with electrodes (electrical stimulation parameters: voltage 2.5V, frequency 20 z Hz, pulse 1.2 ms, duration 60 s) and the values of maximum intracavernosal pressure (ICPmax) and mean arterial pressure (MAP) were recorded under electrical stimulation. The ratio of ICPmax to MAP was used as a criterion for the evaluation of erectile function in rats.
2.2.5. Sample collection
The blood was taken from the rats using a disposable venous blood collection needle, before executing the rats, the testes and the penile corpus cavernosum were stripped, the penile corpus cavernosum tissue was divided into three sections, one section of the penile corpus cavernosum and one side of the testis were fixed with 4 % paraformaldehyde, and the rest of the tissue was stored in a freezer at −80 °C for subsequent testing.
2.2.6. Histopathology
Testicular tissues fixed in 4 % formaldehyde solution were dehydrated and transparent using xylene and ethanol, embedded in paraffin, made into 5-μm-thick testicular tissue sections, and subjected to hematoxylin-eosin (HE) staining. Pathological changes in the testicular tissue structure of rats were observed under a microscope. Following the same method as above, penile tissue fixed in 4 % formaldehyde solution was made into 5-μm-thick penile tissue sections, Masson's staining was performed, and the pathological changes in the penile tissue structure of rats were observed under a microscope.
2.2.7. Enzyme-linked immunosorbent assay (ELISA)
Testicular tissues from each group of rats were collected and tested for IL-1β, IL-6, TNF-α and serum T levels according to the operating instructions of the ELISA kit.
2.2.8. Western blot
The total protein of each group of rat testis tissue was extracted, and the BCA kit was used to determine the protein concentration of the samples. After denaturation, the samples were subjected to electrophoresis, transmigration, and blocking steps in a 30 μg loading volume. After 2 h of blocking, PVDF membranes were incubated with primary antibodies, including NF-κB (1:3000), IκB-α (1:2000), and GAPDH (1:2000), at 4 °C overnight. The next day, the membrane was incubated with a secondary antibody (1:2000) at a constant temperature for 1 h. The bands were exposed using enhanced chemiluminescence (ECL), and their gray values were calculated using the analysis software Image-J. The relative expression of the target protein was calculated using GAPDH as an internal reference.
2.2.9. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
The testicular tissue stored at −80 °C in the refrigerator was weighed out to 50 mg, which was then cut and placed in a centrifuge tube for grinding. Total RNA was extracted by adding 1 mL of TRIzol Total RNA Extraction Reagent. Subsequently, 1 μg of total RNA was taken from each group for reverse transcription into synthetic cDNA and amplified using cDNA as a template. The reaction conditions were pre-denaturation at 95 °C for 5 min, denaturation at 95 °C for 10 s, annealing at 55 °C for 10 s, and full extension at 72 °C for 20 s, with a total of 40 cycles performed. NF-κB and IκB-α mRNA expression was analyzed using the 2−ΔΔCT method using GAPDH as the internal reference gene.
The primer sequences are shown in Supplementary Table 1.
2.2.10. Statistical analyses
SPSS 21.0 software was used for data analysis, the continuous data are expressed as the mean ± standard deviation (), and one-way ANOVA was used for comparison between multiple groups. Two-by-two comparisons were made between groups; the LSD-t test was used if the variance was uniform, and Tamhane's T2 was used if the variance was not uniform. P-values <0.05 were taken to indicate statistical significance.
A schematic illustration of this study is shown in Fig. 1.
Fig. 1.
Analysis process of this study.
3. Result
3.1. Network pharmacology analysis
3.1.1. Chemical composition and drug targets
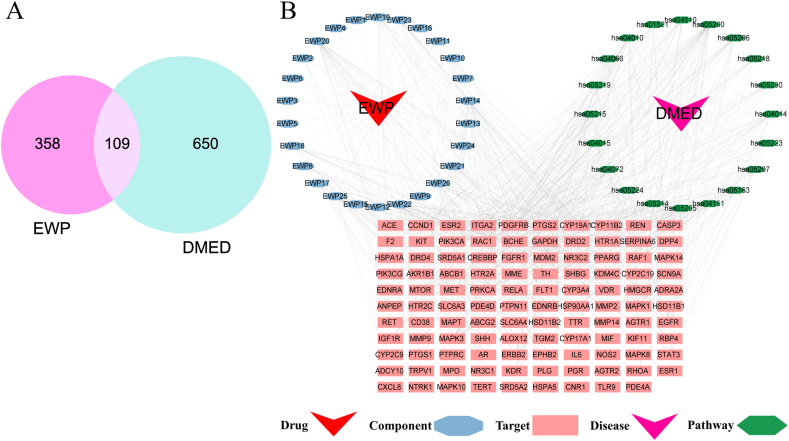
Twenty-six EWP active components were identified after a manual search of the literature and the SwissADEM platform (Supplementary Table 2). These components were predicted and screened using SwissTargetPrediction to obtain 467 pertinent targets. The GeneCards database was searched for “Diabetic erectile dysfunction” and 759 disease targets were obtained. The compound drug targets and disease targets obtained were imported into R software using Venny 2.1.0. Consequently, 109 target genes were obtained, and Venn diagrams were drawn, as shown in Fig. 2A.
Fig. 2.
Network pharmacology analysis. (A) Venn diagram of the target of EWP for DMED treatment; (B) Drug-component-disease-target-pathway network diagram The target genes are shown as purple rectangle. The EWPs are shown as red hexagon and active ingredient of EWPs are shown as green hexagon. DMED is shown as yellow rectangle. Top 20 most significant pathways were shown as blue diamond.
3.1.2. Construction of drug component disease target pathway network diagrams
The constructed network file and attribute type file were uploaded to the Cytoscape 3.9.1 platform, and the drug-component-disease-target-pathway networks were mapped based on their nodes and edges (Fig. 2B). The network was also topologically analyzed, involving 157 nodes and 640 edges, of which the active components with high degree values included N-Cbz-l-glutamic acid 5-ethyl ester, dl-methionine, hypoxanthine, Lauric acid, and l-glutamic acid, which may be the main active components of EWP for the treatment of DMED.
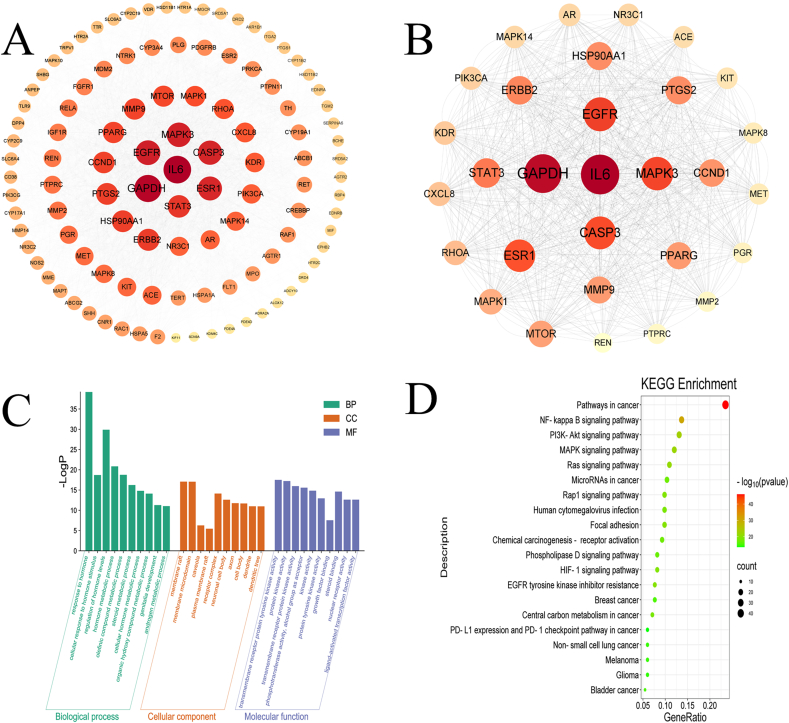
3.1.3. PPI networks
The PPI network was constructed by importing the intersection targets of EWP and DMED into the STRING database by setting the species category as “Homo sapiens” to obtain the PPI network map. The network comprised 109 nodes and 1357 edges (Fig. 3A). The PPI network was imported into Cytoscape 3.9.1 for topological analysis, and the top 30 were selected as key nodes based on the degree values (Fig. 3B–Supplementary Table 3). The main targets include IL-6, cysteinyl aspartate specific proteinase 3 (CASP3), GAPDH, epidermal growth factor receptor (EGFR), and mitogen-activated protein kinase 3 (MAPK3), among which IL-6, CASP3, EGFR, and MAPK3 are key targets in the inflammatory response. The larger the node and the darker the color, the more critical the node is in the network.
Fig. 3.
PPI networks construction and GO enrichment analysis and KEGG pathway analysis. (A) PPI network diagram; (B) Visualization of key targets; (C) GO enrichment analysis; (D) KEGG enrichment analysis.
3.1.4. GO enrichment and KEGG pathway analyses
A total of 1661 GO items with p-values <0.05 were obtained using GO analysis, including 1461 biological process entries, 85 cell component entries, and 115 molecular function entries. In biological processes, the target genes were involved in hormone metabolism, steroid metabolism, and genital development. In the cell components, the target genes are mainly concerned with the membrane microdomain, caveola, and receptor complexes. In molecular functions, the target genes may play roles in transmembrane receptor protein tyrosine kinase activity and protein kinase activity. The top ten entries with the most significant p-value of each component were visualized and were shown in Fig. 3C. A total of 183 KEGG pathways were analyzed. The target genes were mainly involved in the cancer, NF-κB, PI3K/Akt, and MAPK signaling pathways. The top 20 pathways with significant p-values were converted into a bubble chart (Fig. 3D).
3.2. Animal experimental validation
3.2.1. General condition, blood glucose levels, and body mass testing in rats
Before the construction of the animal experimental model, the rats had shiny hair, flexible activities, good mental status, and normal urination and defecation. After the construction of the experimental animal model, DM rats showed excessive drinking, urination and feeding, slow weight gain, loss of shiny hair, reduced activity and depression, and some rats showed cataracts.
3.2.2. Effect of EWP on blood glucose levels and body mass in rats
The blood glucose and body mass of the rats in each group were not significantly different before the animal model was constructed (p > 0.05). After 3 days of animal modeling, the blood glucose levels of rats in the model, sildenafil, and EWP groups were significantly higher than those in the normal group (p < 0.01), while there was no significant difference between the blood glucose of rats in the sildenafil group and EWP groups compared to those in the model group (p > 0.05) (Fig. 4A).
Fig. 4.
Effects of EWP on blood glucose and body weight in DMED rats. (A) Blood glucose changes in DMED rats during EWP treatment; (B) Body weight changes in DMED rats during EWP treatment **p < 0.01; sildenafil group and EWP groups compared with normal group.
The body weight of rats in the normal group tended to increase over time, and there was no significant change in the remaining groups. After 2 weeks of animal modeling, the body mass of the rats in the model, sildenafil, and EWP groups decreased significantly compared to those in the normal group (p < 0.01). There was no significant change in body weight between any of the intervention groups compared to the model group (p > 0.05) (Fig. 4B).
3.2.3. Effect of EWP on the ICP max, MAP, and ICP max/MAP in rats
In addition, to analyze the effect of EWP on erectile function in DMED rats, we assessed the ICPmax/MAP based on the initial state of MAP at 5 V.
Compared to the normal group, the ICPmax and ICPmax/MAP ratio were significantly lower in the model group (p < 0.01), while there was no significant change in MAP (p > 0.05); compared to the model group, the ICPmax and ICP max/MAP ratio were significantly higher in the sildenafil group and all EWP groups (p < 0.01), but there was no significant change in MAP (p > 0.05). Compared to the normal group, the ICPmax and ICPmax/MAP ratio were significantly lower in the model group (p < 0.01), while there was no significant change for MAP (p > 0.05); compared to the model group, the ICPmax and ICP max/MAP ratio were significantly higher in the sildenafil group and all EWP groups (p < 0.01), but there was no significant change in MAP (p > 0.05) (Fig. 5A–C).
Fig. 5.
Effects of EWP on erectile function in DMED rats. (A) EWP increased ICPmax levels in DMED rats; (B) No significant changed in DMED rats after EWP treatment; (C) EWP increased ICPmax/MAP levels in DMED rats **p < 0.01; normal group compared with model group; ##p < 0.01; sildenafil group and EWP groups compared with model group.
3.2.4. Effect of EWP on the testicular tissue of rats
Testicular tissues were harvested and stained with HE. In the normal group, the seminiferous tubules were structurally intact and tightly arranged, and the spermatocytes and mesenchymal cells at all levels were neatly arranged; in the model group, the volume of seminiferous tubules and mesenchymal cells was reduced, the nuclei were solidified and deformed, and the seminiferous epithelial cells appeared to be necrotic and detached to different degrees. Seminiferous tubules and interstitial cells with essentially intact structures were found in the sildenafil and high-dose EWP groups. Compared to the model group, a significant improvement was found with the injury of seminiferous tubules and interstitial cell atrophy and deformation in the low- and medium-dose EWP groups (Fig. 6).
Fig. 6.
HE staining of testicular tissue of rats in each group; scale bar: 40 μm, image at × 200; scale bar: 20 μm, image at × 400; the blue arrows indicate spermatogonia detachment, the green arrows indicate spermatogonia shrinkage, the red arrows indicate Sertoli cell shrinkage, and the black arrows indicate interstitial cell deformation.
3.2.5. Effect of EWP on the penile tissue of rats
Penile tissues were harvested and stained by Masson's staining. Breakage of myofibrils and collagen fibers and a large amount of collagen deposition in corpus cavernosum tissues were found in the model group; collagen and muscle fibers were largely intact structurally in the sildenafil and high-dose EWP groups, but the number of muscle fibers was still low. A significant reduction in the number of muscle fibers and breakage of collagen fibers was found in the low- and medium-dose EWP groups (Fig. 7).
Fig. 7.
Masson staining of penile tissue of rats in each group; scale bar: 40 μm, image at × 200; scale bar: 20 μm, image at × 400; the blue arrows indicate collagen fiber rupture, the red arrows indicate muscle fiber rupture, and the yellow arrows indicate collagen fibers.
3.2.6. Effect of EWP on the IL-1β, IL-6, TNF-α, and serum T levels in rat testicular tissue
Compared to the normal group, the IL-1β, IL-6, and TNF-α levels in the testicular tissues were significantly increased in the model group (p < 0.01); compared to the model group, the IL-1β, IL-6, and TNF-α levels in testicular tissues were significantly decreased in each treatment group (p < 0.01) (Fig. 8A–C). Compared to the normal group, the serum T level of rats in the model group was significantly lower (p < 0.01); compared to the model group, the serum T level of rats in each treatment group was significantly higher (p < 0.01) (Fig. 8D).
Fig. 8.
Protein and Gene Expression of Key Targets and Pathways and Changes in Testosterone Levels after EWP Treatment. (A) EWP decreased the IL-1β levels in the testicular tissue of DMED rats; (B) EWP decreased the IL-6 levels in the testicular tissue of DMED rats; (C) EWP decreased the TNF-α levels in the testicular tissue of DMED rats; (D) EWP increased the serum T levels in DMED rats; (E) EWP decreased the expression of NF-κB protein in the testicular tissue of DMED rats; (F) EWP decreased the expression of IκB-α protein in the testicular tissue of DMED rats; (G) Western blotting assay for NF-κB and IκB-α proteins; (H) EWP decreased the expression of NF-κB mRNA in the testicular tissue of DMED rats; (I) EWP decreased the expression of IκB-α mRNA in the testicular tissue of DMED rats **p < 0.01; normal group compared with model group; ##p < 0.01; sildenafil group and EWP groups compared with model group.
3.2.7. Effect of EWP on NF-κB and IκB-α levels in rat testicular tissue
Compared to the normal group, the expression of NF-κB and IκB-α protein in testis tissues of rats in the model group was significantly higher (p < 0.01); compared to the model group, the expression of NF-κB and IκB-α protein in the testis tissues of rats in each treatment group was significantly lower (p < 0.01) (Fig. 8E–G).
3.2.8. Effect of EWP on mRNA expression of NF-κB and IκB-α in testicular tissue
Compared to the normal group, the mRNA expression of NF-κB and IκB-α in the testis of rats in the model group was significantly higher (p < 0.01); compared to the model group, the mRNA expression of NF-κB and IκB-α in the testis of rats in each treatment group was significantly lower (p < 0.01) (Fig. 8H and I).
4. Discussion
ED is defined as hypoperfusion of the corpus cavernosum of the penis, such that an erection sufficient for satisfactory sexual intercourse cannot be achieved and maintained.21 The pathogenesis of DMED is closely related to endothelial dysfunction and smooth muscle lesions of the penis, sensory nerve dysfunction, sex hormone level disorders, and psychological factors caused by circulating hyperglycemia toxicity in patients with DM, among which the decrease in T level is an important pathogenic mechanism.22 Numerous glycosylation end products can be produced during the persistent hyperglycemic state in patients with DM, which bind to specific receptors on the cell membrane and release a large amount of reactive oxygen species (ROS), which in turn initiates the NLRP3 inflammatory vesicles and contributes to the expression of inflammatory factors, such as IL-1β, IL-6, and TNF-α. The expression of inflammatory factors can stimulate the activation of NF-κB located at the junction of the pathway downstream of TLR4, which can contribute to the release of numerous inflammatory factors, which in turn generate an inflammatory response in testicular tissues, damage testicular interstitial cells, and ultimately lead to a decrease in testosterone secretion, affecting the erectile function of the penis.23 Recent studies have found that testosterone deficiency can lead to penile tissue atrophy, alterations in dorsal nerve structure, endothelial cell morphology, decreased trabecular smooth muscle content, and increased extracellular matrix deposition, which can lead to impairments in penile erectile function.24,25 Our preliminary studies found that the T levels in the serum of DMED rats were significantly lower than those of the normal group, suggesting that T levels might affect the erectile function of the rat penis. EWP can improve the erectile function of DMED rats by increasing the serum T level, inhibiting oxidative stress and inflammation in the cavernous tissue of the penis, and protecting the vascular endothelial cells of the cavernous body of the penis; however, the exact mechanism of action of its increase in the serum T level remains unclear.16 In the present study, our findings showed that EWP could improve erectile function in DMED rats by modulating the NF-κB signaling pathway and affecting downstream inflammatory factors such as IL-6, IL-1, and TNF-α, which increased serum T levels.
A total of 26 potential active components and 109 targets of EWP for treating DMED were screened using network pharmacological methods. Among these, active components such as N-Cbz-l-glutamic acid 5-ethyl ester, dl-Methionine, Hypoxanthine, Lauric acid, and l-glutamic acid have more targets, which are the main active components for improving DMED. Analysis of the PPI network results revealed that EWP may play a role for treating DMED by regulating targets such as IL-6, CASP3, GAPDH, EGFR, and MAPK3. IL-6 plays a key role in the inflammatory immune response, CASP3 is the executive protein of apoptosis, and EGFR plays a crucial role in the growth, multiplication, and repair of tumor cells. GO analysis revealed that hormone metabolism, steroid metabolism, and genital development were the main biological processes. KEGG enrichment analysis revealed that the active components of EWP mainly act on the NF-κB, PI3K/Akt, HIF-1, Ras, and MAPK signaling pathways, among which the NF-κB signaling pathway was enriched to a higher extent and the number of enriched genes was higher. Additionally, EWP is the upstream regulator of these key targets and plays a role in regulating inflammatory responses. Therefore, the mechanism of EWP treatment of DMED may be related to the NF-κB signaling pathway-mediated regulation of the inflammatory response.
NF-κB is a transcription factor that plays an important role in the inflammatory response, cell proliferation, apoptosis, tissue remodeling, immune response, and cellular stress response.26 The NF-κB and IκB proteins in the cytoplasm bind tightly to form a trimer, which inhibits the activation of NF-κB and thus prevents it from regulating the expression of many inflammatory factors (e.g., IL-1β, IL-6, TNF-α) in the nucleus in a normal physiological state.27 In contrast, the IκB kinase complex (lKK) is activated when the cell is stimulated, which in turn leads to the ubiquitination of IκB-α, resulting in trimer dissociation and nuclear translocation of NF-κB, facilitating the regulation of the transcription of many inflammatory factors. These processes ultimately lead to the massive release of numerous inflammatory factors that damage tissues. The existing literature has also shown that abnormal NF-κB expression is closely associated with DMED-induced testicular damage.28
ICPmax is the preferred physiological index to assess erectile function in rodents, whereas MAP can minimize the experimental errors caused by individual differences.29,30 In this study, the ICPmax measurements confirmed that low, medium, and high doses of EWP could increase ICPmax and ICPmax/MAP, indicating that EWP could effectively improve DMED in rats. The results of HE of testicular tissue and Masson's staining of penile tissue in this study showed that the morphology and structure of testicular and penile tissues were normal in the normal group of rats, but severely damaged in the model group of rats, while the damage in rats administered low, medium, and high doses of EWP was improved. In addition, the release of inflammatory factors such as IL-1β, IL-6, and TNF-α was increased, and the expression of NF-κB and IκB-α proteins and mRNA were upregulated in the model group. In parallel, serum T levels were reduced in the model rats, which in turn affected their erectile function. After intervention with different doses of EWP, the testis and penile tissue structure of DMED rats was restored, the release of inflammatory factors (IL-1β, IL-6, and TNF-α) were reduced, and the expression of NF-κB and IκB-α proteins and mRNA were down-regulated; in addition, the serum T level of DMED rats was significantly increased, and the erectile function of rats was improved.
EWP may exert a therapeutic effect on type II DM by increasing insulin sensitivity of receptor cells.31 However, the subjects of this study were STZ-induced type I DM rats; therefore, the low, medium, and high doses of EWP in this study had no significant effect on blood glucose.
This study has several limitations. First, the study data were obtained from an online database; therefore, there were some components and targets that might not have been included. Second, NF-κB inhibitors were excluded from the study as positive drug controls. Third, only the NF-κB signaling pathway was validated, and the mechanism of action of the remaining pathways needs to be further investigated.
5. Conclusion
This study combined network pharmacology with in vivo experiments to investigate the potential mechanism of EWP for treating DMED. The results showed that EWP could reduce the inflammatory response of testicular tissue, promote the repair of testicular and penile tissues, and increase the serum T level by regulating the NF-κB signaling pathway, thereby improving the erectile function of DMED rats. These findings provide a theoretical basis for further investigation into the mechanism of EWP against DMED and its clinical application.
Authors’ contributions
L.M.L. and X.P.X. conceived and designed the project. A.P.Z, Y.R., X.W.J. and J.S.Y. implemented the methods and conducted the analysis. Y.F.Z, X.P.W, and Y.Z.G. drafted the manuscript. L.M.L. and X.P.X. revised the manuscript. All authors read and approved the final manuscript.
Availability of data and materials
Any data and R script in this study can be obtained from the corresponding author on reasonable request. Publicly available datasets were analyzed.
Funding
This study was financially supported by Gansu Provincial Key Laboratory of Chinese Medicine for Prevention and Treatment of Chronic Diseases Open Fund (Grant Number: GSMBKY2015-13); Natural Science Foundation of Gansu Province (Grant Number: 23JRRA1198); Guidance Plan for Science and Technology Development Projects in Lanzhou City (Grant Number: 2023-ZD-102); Guangdong Basic and Applied Basic Research Foundation Provincial-Enterprise Joint Fund (Grant No.2023A1515220184).
Taxonomy (classification by EVISE)
The experimental approach, the methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2024.06.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ryan J.G., Gajraj J. Erectile dysfunction and its association with metabolic syndrome and endothelial function among patients with type 2 diabetes mellitus. J Diabet Complicat. 2012;26(2):141–147. doi: 10.1016/j.jdiacomp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y., Yang C., Meng F., et al. Nerve growth factor improves the outcome of type 2 diabetes-induced hypotestosteronemia and erectile dysfunction. Reprod Sci. 2019;26(3):386–393. doi: 10.1177/1933719118773421. [DOI] [PubMed] [Google Scholar]
- 3.Irwin G.M. Erectile dysfunction. Prim Care. 2019;46(2):249–255. doi: 10.1016/j.pop.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Ding F., Shan C., Li H., et al. Simvastatin alleviated diabetes mellitus-induced erectile dysfunction in rats by en-hancing AMPK pathway-induced autophagy. Andrology. 2020;8(3):780–792. doi: 10.1111/andr.12758. [DOI] [PubMed] [Google Scholar]
- 5.Peng T.M., Zhao C., Liu H.S., et al. Role of advanced glycation end products in diabetic erectile dysfunction and its treatment. J Clin Urol. 2021;36(10):834–838. [Google Scholar]
- 6.Byun K., Yoo Y., Son M., et al. Advanced glycation end-products produced systemically and by macrophages: a common contributor to inflammation and degenerative diseases. Pharmacol Ther. 2017;177:44–55. doi: 10.1016/j.pharmthera.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Mobley D.F., Khera M., Baum N. Recent advances in the treatment of erectile dysfunction. Postgrad Med. 2017;93(1105):679–685. doi: 10.1136/postgradmedj-2016-134073. [DOI] [PubMed] [Google Scholar]
- 8.Ma J.X., Wang B., Li H.S., et al. Uncovering the mechanisms of leech and centipede granules in the treatment of diabetes mellitus-induced erectile dysfunc-tion utilising network pharmacology. J Ethnopharmacol. 2021;265 doi: 10.1016/j.jep.2020.113358. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka T., Hotta Y., Yamamoto Y., et al. Effect of late androgen replacement therapy on erectile function through structural changes in castrated rats. Sex Med. 2021;9(4) doi: 10.1016/j.esxm.2021.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J.X., Wang B., Li H.S., et al. Uncovering the mechanisms of leech and centipede granules in the treatment of diabetes mellitus-induced erectile dysfunc-tion utilising network pharmacology. J Ethnopharmacol. 2021;265 doi: 10.1016/j.jep.2020.113358. [DOI] [PubMed] [Google Scholar]
- 11.Yang A.M., Meng F.C., Dai H.H., et al. Experience in treating diabetic erectile dysfunction from collateral diseases. Beijing J Tradit Chin Med. 2021;40(8):856–858. [Google Scholar]
- 12.Li X.T., Zhang J., Wang S. Professor Zhang Jun's treatment of diabetic erectile dysfunc-tion: 2 medical cases. Med Diet Health. 2021;19(8):170–171. [Google Scholar]
- 13.Han Y.B., Feng T.T., Tian M., et al. Effect of effective components of dilong (Pheretima) on secretion of lInflammatory factors in human mesanaial cell under hiah glucose. J Liaoning Univ Tradit Chin Med. 2021;23(2):25–28. [Google Scholar]
- 14.Wang X.M., Fan S.C., Chen Y., et al. Earthworm protease in anti-thrombosis and anti-fibrosis. Biochim Biophys Acta Gen Subj. 2019;1863(2):379–383. doi: 10.1016/j.bbagen.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Seo M., Lee J.H., Baek M., et al. A novel role for earthworm peptide Lumbricusin as a regulator of neuroinflammation. Biochem Biophys Res Commun. 2017;490(3):1004–1010. doi: 10.1016/j.bbrc.2017.06.154. [DOI] [PubMed] [Google Scholar]
- 16.Zhang A.P., Wu B.B., Liu L.M., et al. Mech-anism of earthworm protein in improving erectile dysfunction in diabetic rats. Chin J Androl. 2022;36(4):29–35. [Google Scholar]
- 17.Garczyńska M., Kostecka J., Pączka G., et al. Chemical composition of earthworm (Dendrobaena veneta Rosa) biomass is suitable as an alterna-tive protein source. Int J Environ Res Publ Health. 2023;20(4):3108. doi: 10.3390/ijerph20043108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q., Bi Q.R., Tan N.H. Research progress on proteins and peptides from earthworm. Chin Tradit Herb Drugs. 2019;50(1):252–261. [Google Scholar]
- 19.Liu K., Sun T., Luan Y., et al. Berberine ameliorates erectile dysfunction in rats with strepto-zotocin-induced diabetes mellitus through the attenuation of apoptosis by inhibiting the SPHK1/S1P/S1PR2 and MAPK pathways. Andrology. 2022;10(2):404–418. doi: 10.1111/andr.13119. [DOI] [PubMed] [Google Scholar]
- 20.Wen Y., Liu G., Zhang Y., et al. MicroRNA-205 is associated with diabetes melli-tus-induced erectile dysfunction via down-regulating the androgen receptor. J Cell Mol Med. 2019;23(5):3257–3270. doi: 10.1111/jcmm.14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang A.P., Liu L.M., Ji X.W., et al. Research progress on the action mechanism of protein from earthworm in the treatment of erectile. J Gansu Univ Chin Med. 2023;40(1):101–105. [Google Scholar]
- 22.Onyeji I.C., Clavijo R.I. Testosterone replacement therapy and erectile dysfunction. Int J Impot Res. 2022;34(7):698–703. doi: 10.1038/s41443-021-00512-w. [DOI] [PubMed] [Google Scholar]
- 23.Yu H., Lin L., Zhang Z., et al. Targeting NF-kappaB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Targeted Ther. 2020;5(1):209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kataoka T., Hotta Y., Yamamoto Y., et al. Effect of late androgen replacement therapy on erectile function through structural changes in castrated rats. Sex Med. 2021;9(4) doi: 10.1016/j.esxm.2021.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin J., Guo Y.M., Chen P., et al. Testosterone regulates the expression and functional activity of sphingosine-1-phosphate receptors in the rat corpus cavernosum. J Cell Mol Med. 2018;22(3):1507–1516. doi: 10.1111/jcmm.13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zappavigna S., Cossu A.M., Grimaldi A., et al. Anti-inflammatory drugs as anticancer agents. Int J Mol Sci. 2020;21(7):2605. doi: 10.3390/ijms21072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z.M. Effects of vitexin on streptozotocin-induced testiculardysfunction and its un-deryling mechanisms in diabetic mice. J Ningxia Med Univ. 2019;45(4):376–381. [Google Scholar]
- 28.Chen T.Q., Deng Y.F., Wang Y.Y., et al. Butyrolactone I attenuates inflammation in murine NASH by inhibiting the NF-kappaB signaling pathway. Biochem Biophys Res Commun. 2022;626:167–174. doi: 10.1016/j.bbrc.2022.07.050. [DOI] [PubMed] [Google Scholar]
- 29.Ye M.Y., Zhao F., Ma K., et al. Comparison of the use of intravenous infusion needle and self-made PE-50 tube needle in the measarement of intracavernosal pressure in rat penis. Acta Lab Anim Sci Sin. 2019;27(6):753–759. [Google Scholar]
- 30.Cellek S., Bivalacqua T.J., Burnett A.L., et al. Common pitfalls in some of the experimental studies in erectile function and dysfunction:a consensus article. J Sex Med. 2012;9(11):2770–2784. doi: 10.1111/j.1743-6109.2012.02916.x. [DOI] [PubMed] [Google Scholar]
- 31.Cooper E.L., Hrzenjak T.M., Grdisa M. Alternative sources of fibrinolytic, anticoagulative, antimicrobial and anticancer molecules. Int J Immunopathol Pharmacol. 2004;17(3):237–244. doi: 10.1177/039463200401700303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any data and R script in this study can be obtained from the corresponding author on reasonable request. Publicly available datasets were analyzed.