Abstract
Metabolic acidosis is a common disorder in patients with chronic kidney disease (CKD). As eGFR decreases, a reciprocal decrease in bicarbonate (HCO3−) and an increase in chloride (Cl−) concentration is observed. The aim of this study is to determine whether the Cl−/HCO3− ratio can be used to predict metabolic acidosis with pH decline. A total of 115 patients (age 63 ± 17 years), with CKD stage G4 or G5 were enrolled in this cross-sectional study. The arterial (A) and venous (V) blood samples were taken during AV fistula creation and evaluated in a point of care testing analyzer. The ratio of arterial Cl− and HCO3− concentration were calculated. According to mean arterial pH (pH-A) the group was divided into group with pH-A ≤ 7.33 and with pH-A > 7.33. The group with pH-A ≤ 7.33 showed significantly lower HCO3−, and higher Cl−, Cl−/HCO3−-ratio than group with pH-A > 7.33. Cl−/HCO3−-A ratio negatively correlated with pH (r=-0.77,p < 0.01). The discriminative power of Cl−/HCO3−-A ratio for predicting pH-A ≤ 7.33 was 0.917 ([CI] = 0.87–0.97,p < 0.01) which provided 87% sensitivity and 84% specificity. The best cut-off was 6.22(mmol/l)/(mmol/l). In conclusion, a Cl−/HCO3− ratio higher than 6.22(mmol/l)/(mmol/l) may be used as predictor of advanced metabolic acidosis in CKD stages G4 and G5.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-05633-6.
Keywords: Metabolic acidosis; Bicarbonate, chloride, anion gap; CKD
Subject terms: Kidney diseases, Renal replacement therapy
Introduction
The kidneys play a key role in acid-base balance, maintaining pH in the normal range (isohydria) by three mechanisms: bicarbonate reabsorption, ammogenesis, and titratable acidity. Endogenous acids accumulate in renal failure patients, and bicarbonate is utilized as a buffer. A bicarbonate level of less than 22 mmol/l was commonly used to diagnose metabolic acidosis (MA)1.
Acidosis in chronic kidney disease (CKD) can be associated with both normal and high anion gaps. The anion gap (AG) is the difference between serum concentrations of cations and anions. It is commonly determined using the formula AG = Na+-(Cl−+HCO3−). However, it may be affected by other particles such as potassium (cation), phosphate (anion), albumin (anion), calcium (cation), and magnesium (cation). In renal failure, anions such as sulfone compounds (indoxyl, p-cresol) accumulate, potentially increasing AG.
Anions of the utmost significance in serum are chlorides (Cl−) and bicarbonates (HCO3−). The more advanced the CKD, the greater the AG. As glomerular filtration rate (GFR) falls, HCO3− concentrations decline in order to correct MA and chloride concentrations rise in order to correct the AG. In earlier our study we found the strong negative relationship between Cl− and HCO3− concentration2. The main objective of this study is to examine whether the Cl−/HCO3− ratio can predict advanced metabolic acidosis with pH declines. The second goal is to determine if the Cl−/HCO3− ratio is related to the estimated glomerular filtration rate (eGFR) in CKD patients stages G4 and G5.
To our knowledge it is the first study characterizing the Cl−/HCO3− ratio in CKD patients.
In the study, we used a method of collecting arterial blood for POCT directly from the artery during the creation of an arteriovenous fistula.
Results
Basic demographic, clinical and laboratory parameters of patients included in the study
The group of 115 CKD patients was divided into two groups based on their mean arterial pH (pH-A): pH-A ≤ 7.33 and pH-A > 7.33 and reported in Table 1. There were no differences in regard demographic, cause of CKD and comorbidity.
Table 1.
Basic demographic and clinical parameters of patients included in the study, divided into group with pH-A ≤ 7.33 and group with pH-A > 7.33. Student’s t-test for independent variables, mean ± sd, *chi quadrat test. Abbreviations: PWV, pulse wave velocity; CCI, Charlson comorbidity index; CKD, chronic kidney disease; DM, diabetes mellitus; HA, hypertension; GN, glomerulonephritis; ADPKD, autosomal dominant polycystic kidney disease; IN, interstitial nephritis. ** comorbidities were listed in groups in the material and methods section.
| Demographic and clinical parameters | ||||
|---|---|---|---|---|
| pH-A ≤ 7.33 N = 52 | pH-A > 7.33 N = 63 | p | All study patients N = 115 | |
| Age (y.) | 63.63 ± 17.82 | 62.26 ± 15.67 | > 0.05 | 62.88 ± 16.61 |
| BMI (kg/m2) | 26.81 ± 4.64 | 27.01 ± 5.59 | > 0.05 | 26.92 ± 5.17 |
| CCI (point) | 6.19 ± 2.96 | 6.68 ± 3.13 | > 0.05 | 6.46 ± 3.05 |
| PWV (m/s) | 11.16 ± 2.08 | 9.18 ± 2.34 | 0.07 | 10.12 ± 2.38 |
| Causes of CKD | ||||
| pH-A ≤ 7.33 N = 52 | pH-A > 7.33 N = 63 | p* | No (%) | |
| DM and HA (%) | 26 (23) | 29 (25) | > 0.05 | 55 (47) |
| Chronic GN (%) | 17 (15) | 14 (12) | > 0.05 | 31 (27) |
| ADPKD (%) | 4 (3) | 8 (7) | > 0.05 | 12 (10) |
| IN (%) | 3 (3) | 2 (2) | > 0.05 | 5 (4) |
| others (%) | 2 (2) | 10 (9) | > 0.05 | 12 (10) |
| Comorbidities | ||||
| Heart diseases (%) | 26 (23) | 48 (42) | > 0.05 | 74 (64) |
| Peripheral vascular disease (%) | 14 (12) | 13 (11) | > 0.05 | 27 (23) |
| Cerebrovascular accident (%) | 10 (9) | 24 (21) | > 0.05 | 34 (39) |
| Chronic obturative disease (%) | 2 (2) | 9 (8) | > 0.05 | 10 (9) |
| Connective tissue disease (%) | 3 (3) | 9 (8) | > 0.05 | 12 (10) |
| Gastrointerstitial complications (%) | 7 (6) | 9 (8) | > 0.05 | 16 (14) |
| Diabetes mellitus (%) | 19 (17) | 20 (17) | > 0.05 | 39 (34) |
| Neoplasmatic disease (%) | 7 (6) | 8 (7) | > 0.05 | 15 (13) |
In group with pH-A ≤ 7.33 was significantly lower pH-A, pH-V, HCO3−-A, HCO3−-V, pCO2-A, pCO2-V, BE-A, BE-V, ΔHCO3− and significantly higher deltaAG/deltaHCO3−index and both arterial (A) and venous (V), K+, Cl− Cl−/HCO3− ratios compared to group with pH-A > 7.33. No such differences were observed in the case of AG (Table 2). The percentage of patients without metabolic acidosis (non-MA) was significantly higher in subgroup with pH-A > 7.33 (Table 3). In the subgroup with lower pH (pH-A ≤ 7.33), significantly more advanced renal failure was observed with lower eGFR, hemoglobin level and higher phosphate and PTH level (Table 2).
Table 2.
POCT and laboratory parameters of patient’s divided in two subgroups according pH-A. Student’s t-test for independent variables, *chi quadrat test. Abbreviations: SD, standard deviations; BMI, body mass index; pO2, oxygen partial pressure; pCO2, carbon dioxide partial pressure; HCO3−, bicarbonate; BE, base excess; K+, potassium; Na+, sodium; Ca2+, ionized calcium; Cl−, chloride; AG, uncorrected anion gap; Pi, phosphate; PTH, parathormone; eGFR, estimated glomerular filtration rate. *The index deltaAG/deltaHCO3− includes AG corrected to potassium and albumin.
| POCT and laboratory parameters | ||||
|---|---|---|---|---|
| pH-A ≤ 7.33 N = 52 | pH-A > 7.33 N = 63 | p | All study patients N = 115 | |
| pH-A | 7.28 ± 0.04 | 7.38 ± 0.03 | < 0.01 | 7.33 ± 0.06 |
| pCO2-A (mmHg) | 32.91 ± 4.9 | 35.72 ± 4.72 | < 0.01 | 34.45 ± 4.98 |
| pO2-A (mmHg) | 88.34 ± 17.05 | 83.42 ± 15.5 | > 0.05 | 85.65 ± 16.33 |
| HCO3−-A (mmol/l) | 15.53 ± 2.76 | 20.95 ± 3.08 | < 0.01 | 18.50 ± 3.99 |
| BE-A (mmol/l) | (−10.22 ± 2.81) | (−3.66 ± 2.91) | < 0.01 | (−6.63 ± 4.34 |
| K+-A (mmol/l) | 4.60 ± 0.71 | 4.26 ± 0.81 | < 0.05 | 4.41 ± 0.78 |
| Na+-A (mmol/l) | 141.29 ± 2.44 | 139.97 ± 3.57 | < 0.05 | 140.57 ± 3.17 |
| Cl−-A (mmol/l) | 115.98 ± 4.03 | 109.29 ± 4.77 | < 0.01 | 112.31 ± 5.55 |
| AG-A (mmol/l) | 9.86 ± 2.35 | 9.72 ± 2.33 | > 0.05 | 9.78 ± 2.33 |
| Cl−/HCO3−-A (mmol/l)/(mmol/l) | 7.76 ± 1.7 | 5.36 ± 0.99 | < 0.01 | 6.44 ± 1.81 |
| deltaAG/deltaHCO3− * | 1.52 ± 1.36 | −0.4 ± 5.3 | < 0.05 | 0.47 ± 4.13 |
| pH-V | 7.24 ± 0.07 | 7.31 ± 0.06 | < 0.01 | 7.28 ± 0.07 |
| pCO2-V (mmHg) | 35.25 ± 4.99 | 38.57 ± 4.6 | < 0.01 | 37.07 ± 5.04 |
| pO2-V (mmHg) | 63.50 ± 15.27 | 58.12 ± 11 | < 0.05 | 60.53 ± 13.3 |
| HCO3−-V (mmol/l) | 15.09 ± 3.01 | 19.71 ± 3.54 | < 0.01 | 17.62 ± 4.02 |
| BE-V (mmol/l) | −11.44 ± 3.58 | −6.03 ± 4.01 | < 0.01 | −8.48 ± 4.67 |
| K+-V (mmol/l) | 4.67 ± 0.71 | 4.31 ± 0.75 | < 0.05 | 4.47 ± 0.75 |
| Na+-V (mmol/l) | 141.54 ± 2.6 | 140.67 ± 3.31 | > 0.05 | 141.06 ± 3.03 |
| Cl−-V (mmol/l) | 115.85 ± 3.93 | 109.46 ± 4.7 | < 0.01 | 112.35 ± 5.4 |
| AG-V (mmol/l) | 10.81 ± 2.67 | 11.47 ± 2.3 | > 0.05 | 11.17 ± 2.49 |
| Cl−/HCO3−-V (mmol/l)/(mmol/l) | 8.05 ± 2.02 | 5.77 ± 1.26 | < 0.01 | 6.80 ± 2.0 |
| ΔHCO3− (mmol/l) | 0.89 ± 1.7 | 1.95 ± 1.79 | < 0.01 | 1.47 ± 1.82 |
| Total protein (g/dl) | 5.75 ± 0.78 | 6.12 ± 1.01 | < 0.05 | 5.96 ± 0.93 |
| Pi (mg/dl) | 5.90 ± 1.29 | 5.13 ± 1.15 | < 0.01 | 5.48 ± 1.27 |
| PTH (pg/ml) | 424.28 ± 262.27 | 294.3 ± 185.8 | < 0.01 | 350.01 ± 229.76 |
| Hemoglobin (g/dl) | 9.63 ± 1.33 | 10.33 ± 1.5 | < 0.01 | 10.02 ± 1.46 |
| Creatinine (mg/dl) | 5.58 ± 1.68 | 5.23 ± 1.95 | > 0.05 | 5.39 ± 1.83 |
| eGFR (ml/min/1.73 m2) | 10.45 ± 3.06 | 12.69 ± 4.8 | < 0.01 | 11.68 ± 4.24 |
| Urea (mg/dl) | 152.66 ± 31.62 | 141.74 ± 43.08 | > 0.05 | 146.68 ± 38.55 |
Table 3.
Type of acid-base disorder. Abbreviations: non-MA, non-metabolic acidosis; AGMA, metabolic acidosis with normal anion Gab; HAGMA, high anion Gab metabolic acidosis.
| Type of acid-base disorder | ||||
|---|---|---|---|---|
| pH-A ≤ 7.33 N = 52 | pH-A > 7.33 N = 63 | p* | All study patients N = 115 | |
| non-MA-A (%) | 0 (0) | 24 (21) | < 0.01 | 24 (21) |
| AGMA-A (%) | 26 (23) | 23 (20) | > 0.05 | 49 (43) |
| HAGMA-A (%) | 26 (23) | 16 (14) | 0.06 | 42 (37) |
| non-MA-V (%) | 0 (0) | 16 (14) | < 0.01 | 16 (14) |
| AGMA-V (%) | 18 (16) | 10 (9) | 0.07 | 28 (24) |
| HAGMA-V (%) | 34 (30) | 37 (32) | > 0.05 | 71 (62) |
Predictors of advanced metabolic acidosis with pH-A ≤ 7.33
Among several point of care testing (POCT) parameters, there were three that most strongly indicated advanced acidosis with pH-A ≤ 7.33, namely BE-A, Cl−/HCO3−-A, and HCO3−−A with area under curve (AUC) more then 0.9 (Table 4). The discriminative power of Cl−/HCO3−-A ratio for predicting pH-A ≤ 7.33 was 0.917 (95% confidence interval [CI] = 0.87–0.97; p < 0.01) which provided 87% sensitivity and 84% specificity. The best cut-off point was 6.22 (mmol/l)/(mmol/l) (Fig. 1). Cl− and HCO3− concentrations alone were found to be poorer predictors for decompensated acidosis (pH-A ≤ 7.33), with AUCs of 0.857 and 0.915, respectively. The arterial anion gap (AG-A) value was not related to the arterial pH (pH-A) value (AUC = 0.589, 95%CI = 0.483–0.696, p > 0.05) (Table 4).
Table 4.
Discriminative power of various acid-base indicators from POCT in the diagnosis of pH-A ≤ 7.33. Abbreviations: AUC, area under curve, CI, confidence interval.
| Discriminative power of different acid-base indices for diagnosis pH < 7.33 | ||||||
|---|---|---|---|---|---|---|
| AUC | 95% CI | p | Proposed Cut-Off Point | Sensitivity (%) | Specificity (%) | |
| pCO2-A (mmol/l) | 0.652 | 0.505–0.798 | < 0.05 | 27.1 | 35.0 | 93.7 |
| HCO3−A (mmol/l) | 0.911 | 0.854–0.968 | < 0.01 | 17.5 | 95.0 | 73.7 |
| BE-A (mmol/l) | 0.956 | 0.922–0.990 | < 0.01 | −8.6 | 100.0 | 83.2 |
| Cl−-A (mmol/l) | 0.903 | 0.839–0.967 | < 0.01 | 115 | 95.0 | 73.7 |
| Cl−/HCO3−A (mmol/l)/(mmol/l) | 0.922 | 0.870–0.974 | < 0.01 | 6.22 | 90.0 | 82.1 |
| eGFR (ml/min/1.73 m2) | 0.584 | 0.452–0.716 | 0.209 | 11 | 80.0 | 45.3 |
| Creatinine (mg/dl) | 0.626 | 0.492–0.759 | 0.066 | 4.6 | 82.4 | 44.6 |
| ΔHCO3− (mmol/l) | 0.563 | 0.426–0.701 | 0.367 | 0.8 | 55.0 | 57.9 |
| Cl−+HCO3−A (mmol/l) | 0.593 | 0.49–0.697 | 0.08 | 128.5 | 90.4 | 27.0 |
Fig. 1.
Discriminative power of Cl-/HCO3--A for diagnosis pH-A≤7.33. The proposed cut-off point was 6.22 (mmol/l)/(mmol/l).
Pearson’s correlation of POCT parameters
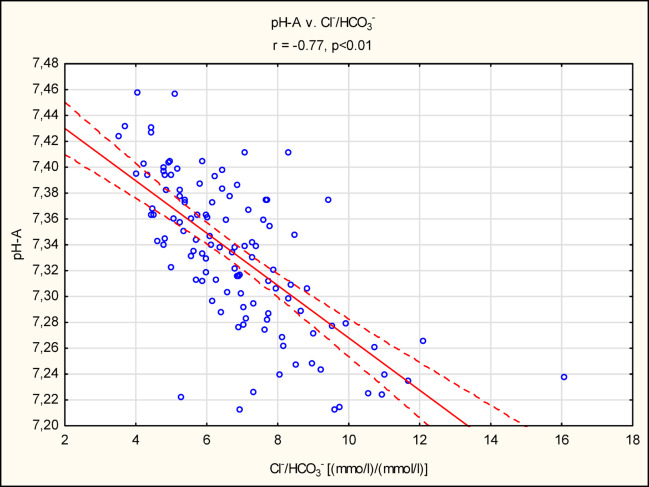
pH-A correlated significantly with HCO3−-A (r = 0.78, p < 0.01) and with Cl− (r=−0.71, p < 0.01). A strong negative correlation was found between the Cl−/HCO3−A ratio and pH-A (r=−0.77, p < 0.01) (Fig. 2). However, no correlation was found between AG-A and pH-A (r = 0.14, p > 0.05). There were positive correlation between AG-A and serum creatinine (r = 0.4, p < 0.05), urea (r = 0.43, p < 0.05) and negative correlation with eGFR (r=−0.36, p < 0.05) (Supplementary Tables 1 and Supplementary Fig. 1).
Fig. 2.
Pearson’s correlation between pH-A and Cl-/HCO3--A ratio. Abbreviation; A-arterial sample.
Predictors of high anion Gab metabolic acidosis (HAGMA)
Parameters of kidney function including serum creatinine, eGFR, serum urea and were analyzed as indicator of HAGMA with AG-A ≥ 10 mmol/l and HCO3−≤22 mmol/l. The area under the curve (AUC) estimate with the proposed best cut-off point was presented in Supplementary Table 2. The discriminative power of eGFR for predicting HAGMA was 0.7 (95% confidence interval [CI] = 0.6–0.8; p < 0.01) which provided 67% sensitivity and 72% specificity. The best cut-off point was 10.67 ml/min./1.73 m2 (Supplementary Fig. 2).
The characteristics of three study subgroups with no metabolic acidosis (non-AG), normal anion gap (AGMA) and high anion gap (HAGMA)
Significant intergroup differences were discovered using the ANOVA test (Supplementary Table 3). In both subgroups with metabolic acidosis, i.e., AGMA and HAGMA, significantly higher concentrations of Cl− and lower pHs, pCO2 and HCO3− were observed compared to the non-MA group. The anion gap (AG) was the highest in the HAGMA group and the lowest in the AGMA group. The HAGMA group had the lowest estimated glomerular filtration rate (eGFR) and more severe renal insufficiency. Patients with HAGMA have higher Cl−/HCO3−-A ratio and started dialysis much faster than patients with AGMA (Suplementary Table 2). Additionally, survival analysis was performed to determine the probability of initiating HD treatment in the three groups (Supplementary Fig. 3) and separately in the AGMA and HAGMA groups (Supplementary Fig. 4). The likehood of starting HD treatment within 5 months was significantly higher in HAGMA than in AGMA group, with 73%, 55% and 85% for non-MA, AGMA and HAGMA, respectively.
Fig. 3.
Kaplan-Maier survival analysis. The probability of starting HD treatment in group with Cl-/HCO3-≥ 6.22 and Cl-/HCO3-<6.22.
Fig. 4.
Kaplan-Maier survival analysis. The probability of starting HD treatment in group with pH≤7.33 and pH>7.33.
Clinical, demographic laboratory parameters describing subgroups in relation to Cl−/HCO3−-A ratio
We finally analyzed the POCT and laboratory parameters, dividing the study group into two subgroups with Cl−/HCO3−-A < 6.22 and Cl−/HCO3−-A ≥ 6.22. There were no significant differences in clinical and demographic parameters (Table 5). In the group with higher Cl−/HCO3−-A more advanced metabolic acidosis was observed with lower pH, pCO2, HCO3− and base excess (BE) in both arterial and venous samples and higher K+ and Cl− concentration (Table 6). All individuals in the Cl−/HCO3−-A ≥ 6.22 ratio group had metabolic acidosis. This group was characterized by lower eGFR, lower hemoglobin and higher phosphate and PTH.
Table 5.
The demographic and clinical laboratory characteristics of the study population divided in two groups regarding Cl−/HCO3−-A ratio. Student’s t-test for independent variables, *chi quadrat test. Abbreviations: BMI; body mass index, CCI; Charlson comorbidity index, PWV; pulse wave velocity, DM; diabetes mellitus, HA, arterial hypertension, GN; glomerulonephritis, ADPKD; autosomal dominant polycystic kidney disease, IN; interstitial nephritis, BMI, body mass index.
| Demographic and clinical parameters | |||
|---|---|---|---|
| Cl−/HCO3−-A ≥ 6.22 No = 55 | Cl−/HCO3−-A < 6.22 No = 60 | p | |
| Age (y.) | 62.63 ± 17.12 | 63.11 ± 16.27 | > 0.05 |
| Age < 60y. (%) | 23 (41) | 18 (30) | > 0.05* |
| Age ≥ 60y. (%) | 32 (49) | 42 (70) | > 0.05* |
| BMI (kg/m2) | 26.22 ± 4.32 | 27.57 ± 5.81 | > 0.05 |
| CCI (point) | 6.09 ± 3.06 | 6.8 ± 3.03 | > 0.05 |
| PWV (m/s) | 10.53 ± 2.21 | 9.66 ± 2.62 | > 0.05 |
| Causes of CKD | |||
| Cl−/HCO3−-A ≥ 6.22 | Cl−/HCO3−-A < 6.22 | p* | |
| DM and HA (%) | 23 (20) | 33 (29) | > 0.05 |
| Chronic GN (%) | 17 (15) | 14 (12) | > 0.05 |
| ADPKD (%) | 5 (4) | 7 (6) | > 0.05 |
| IN (%) | 3 (3) | 2 (2) | > 0.05 |
| others (%) | 7 (6) | 4 (3) | > 0.05 |
| Comorbidities | |||
| Heart diseases (%) | 29 (52) | 45 (75) | > 0.05 |
| Peripheral vascular disease (%) | 12 (21) | 15 (25) | > 0.05 |
| Cerebrovascular acciden (%) | 10 (18) | 24 (40) | 0.06 |
| Chronic obturative disease (%) | 3 (5) | 7 (11) | > 0.05 |
| Connective tissue disease (%) | 5 (9) | 7 (11) | > 0.05 |
| Gastrointerstitial complications (%) | 5 (9) | 7 (11) | > 0.05 |
| Diabetes mellitus (%) | 18 (32) | 21 (35) | > 0.05 |
| Neoplasmatic disease (%) | 8 (14) | 7 (11) | > 0.05 |
Table 6.
Laboratory characteristics of the study population divided in two groups regarding Cl−/HCO3− -A index. Student’s t-test for independent variables, *chi quadrat test. Abbreviations: non-MA, non-metabolic acidosis; AGMA, metabolic acidosis with normal anion Gab; HAGMA, high anion Gab metabolic acidosis; SD, standard deviations; BMI, body mass index; pO2, oxygen partial pressure; pCO2, carbon dioxide partial pressure; HCO3−, bicarbonate; BE, base excess; K+, potassium; Na+, sodium; Ca2+, ionized calcium; Cl−, chloride; AG, uncorrected anion gap; pi, phosphate; PTH, parathormone; eGFR, estimated glomerular filtration rate.
| POCT and laboratory parameters | |||
|---|---|---|---|
| Cl-/HCO3–A ≥ 6.22 No = 55 | Cl-/HCO3–A < 6.22 No = 60 | p | |
| pH-A | 7.29 0.05 | 7.37 ± 0.04 | < 0.01 |
| pCO2-A (mmHg) | 31.47 ± 4.57 | 37.17 ± 3.61 | < 0.01 |
| pO2-A (mmHg) | 89.75 ± 19.20 | 81.88 ± 12.15 | < 0.01 |
| HCO3−-A (mmol/l) | 15.22 ± 2.36 | 21.50 ± 2.55 | < 0.01 |
| BE-A (mmol/l) | −11.33 ± 2.82 | −3.74 ± 2.99 | < 0.01 |
| K+-A (mmol/l) | 4.43 ± 0.78 | 4.40 ± 0.79 | > 0.05 |
| Na+-A (mmol/l) | 141 ± 2.62 | 139.83 ± 3.46 | < 0.01 |
| Cl−-A (mmol/l) | 116.35 ± 3.66 | 108.62 ± 4.27 | < 0.01 |
| AG-A (mmol/l) | 9.89 ± 2.25 | 9.68 ± 2.41 | > 0.05 |
| Cl−/HCO3−-A (mmol/l)/(mmol/l) | 7.87 ± 1.54 | 5.14 ± 0.71 | < 0.01 |
| pH-V | 7.24 ± 0.07 | 7.31 ± 0.06 | < 0.01 |
| pCO2-V (mmHg) | 34.35 ± 4.53 | 39.56 ± 4.14 | < 0.01 |
| pO2-V (mmHg) | 62.96 ± 14.80 | 58.34 ± 11.46 | > 0.05 |
| HCO3−-V (mmol/l) | 14.78 ± 2.63 | 20.23 ± 3.24 | < 0.01 |
| BE-V (mmol/l) | −12.63 ± 3.48 | −5.94 ± 4.08 | < 0.01 |
| K+-V (mmol/l) | 4.54 ± 0.76 | 4.41 ± 0.74 | > 0.05 |
| Na+-V (mmol/l) | 141.63 ± 2.77 | 140.53 ± 3.18 | > 0.05 |
| Cl−-V (mmol/l) | 116.05 ± 3.62 | 108.95 ± 4.43 | < 0.01 |
| AG-V (mmol/l) | 10.95 ± 2.75 | 11.38 ± 2.22 | > 0.05 |
| Cl−/HCO3−-V (mmol/l)/(mmol/l) | 8.16 ± 1.86 | 5.55 ± 1.11 | < 0.01 |
| ΔHCO3− (mmol/l) | 1.03 ± 1.76 | 1.88 ± 1.78 | < 0.01 |
| Total protein (g/dl) | 5.75 ± 0.81 | 6.15 ± 0.99 | < 0.01 |
| Pi (mg/dl) | 5.82 ± 1.23 | 5.17 ± 1.23 | < 0.01 |
| PTH (pg/ml) | 436 ± 257 | 276 ± 174 | < 0.01 |
| Hemoglobin (g/dl) | 9.77 ± 1.45 | 10.23 ± 1.45 | > 0.05 |
| Creatinine (mg/dl) | 5.66 ± 1.75 | 5.15 ± 1.92 | > 0.05 |
| eGFR (ml/min/1.73 m2) | 10.21 ± 3.07 | 12.9 ± 4.75 | < 0.01 |
| Urea (mg/dl) | 150.89 ± 39.80 | 143.37 ± 44.09 | > 0.05 |
| Type of acid-base disorder | |||
| Cl−/HCO3−-A ≥ 6.22 No = 55 | Cl−/HCO3−-A < 6.22 No = 60 | p* | |
| non-MA-A (%) | 0 | 24 (21) | < 0.01 |
| AGMA-A (%) | 29 (25) | 20 (17) | 0.08 |
| HAGMA-A (%) | 26 (23) | 16 (14) | > 0.05 |
| non-MA-V (%) | 0 | 16 (14) | < 0.01 |
| AGMA-V (%) | 19 (17) | 9 (8) | 0.06 |
| HAGMA-V (%) | 36 (31) | 35 (30) | > 0.05 |
Clinical follow-up after two years
Three patients were lost to follow-up. Among 111 patients, 21 underwent kidney transplantation, 55 suffered at least one cardiovascular event, and 35 died. There were no statistical differences in the number of the above-mentioned events relative to the baseline Cl−/HCO3−-A ratio (Table 7). In 93 patients out of 115 (80%), the date of hemodialysis initiation could be determined. Patients with a Cl−/HCO3−-A ratio higher than 6.22(mmol/l)/(mmol/l) started hemodialysis significantly earlier, with a median number of days of 49 vs. 94 days (p < 0.05). Similar follow-up analysis was performed in group with pH-A ≤ 7.33 and pH-A > 7.33 (Table 7). Although the two analyses (Cl−/HCO3−-A ratio and pH-A) were similar, subtle changes in the Cl−/HCO3−ratio were discovered, demonstrating that the Cl−/HCO3−ratio is associated with pH but not identical. Kaplan-Meier analysis showed these small differences in the number of days to start hemodialysis (HD) (Figs. 3 and 4). The live table reviled that, the likehood of starting HD treatment in 5 months was 84% and 69% for pH ≤ 7.33 and pH > 7.33, respectively. In comparison, the probability of starting HD treatment in 5 months was 80% and 61% for Cl−/HCO3−≥6.22 and Cl−/HCO3−<6.22, respectively.
Table 7.
Two years follow-up data in subgroups divided according to Cl−/HCO3− ratio and pH. * U Mann-Whitney test. ** Chi quadrat test. Abbreviations: POCT; point of care testing. IQR; inter quartile range. HD; hemodialysis.
| Follow-up data after 2 years | |||
|---|---|---|---|
| Cl−/HCO3−≥6.22 N = 44 | Cl−/HCO3−<6.22 N = 49 | p | |
| Time between POCT assessment and HD start, days(IQR) | 47(13–124) | 94(29–236) | 0.04* |
| Cl−/HCO3−≥6.22 N = 53 | Cl−/HCO3−<6.22 N = 59 | p | |
| Number of patients who didn’t start HD (%) | 3 (2.7) | 4 (3.6) | > 0.05** |
| Number of patients who started HD (%) | 49 (44.9) | 55 (49.5) | > 0.05** |
| Number of patients who received a kidney transplant (%) | 9 (8) | 12 (11) | > 0.05** |
| Number of patients who experienced a cardiovascular event***(%) | 24 (21.6) | 31 (27.9) | > 0.05** |
| Number of patients who died (%) | 17 (15.3) | 18 (16.2) | > 0.05** |
| pH-A ≤ 7.33 N = 44 | pH-A > 7.33 N = 49 | p | |
| Time between POCT assessment and HD start, days(IQR) | 42 (18–116) | 96 (29–236) | 0.02* |
| pH-A ≤ 7.33 N = 50 | pH-A > 7.33 N = 62 | p | |
| Number of patients who didn’t start HD (%) | 1 (0.9) | 6 (5.3) | > 0.05** |
| Number of patients who started HD (%) | 48 (43.2) | 56 (50) | > 0.05** |
| Number of patients who received a kidney transplant (%) | 9 (8) | 12 (10.7) | > 0.05** |
| Number of patients who experienced a cardiovascular event***(%) | 24 (21.4) | 31 (27.6) | > 0.05** |
| Number of patients who died (%) | 17 (15.1) | 18 (16) | > 0.05** |
Discussion
One of the significant complications observed in patients with chronic kidney disease (CKD) is metabolic acidosis (MA). The main objective of our study was to determine whether the Cl−/HCO3− ratio can be used as a predictor of advanced metabolic acidosis (MA) in patients with CKD in stages G4 and G5. The results indicated that this index strongly correlates with arterial blood pH, suggesting its usefulness in assessing the depth of acidosis. Further analysis, including follow-up, revealed that the likelihood of starting dialysis was comparable when estimated in pH-A ≤ 7.33 v. pH-A > 7.33 and Cl-/HCO3-ratio ≥ 6.22 vs. Cl-/HCO3-ratio < 6.22, however not identical. We discovered that the Cl−/HCO3−-A ratio had a higher predictive power than the concentration of the commonly used bicarbonate. The idea of using the product of two anions in a single indicator to describe acid-base disturbance arises from the fact that their levels are negatively correlated2 and both anions influence the calculated anion gap value. In the situation of worsening kidney function, the level of HCO3− decreases3 and the concentration of Cl− tends to increase in groups of patients with lower eGFR4,5. As indicated by the results of numerous studies, neither one nor the other are ideal parameters for assessing the acid-base disorders including the depth of acidosis and the anion gap, and thus criteria for potentially initiating treatment6.
As chronic kidney disease progresses, the percentage of patients with metabolic acidosis increases, and in stages G4 and G5 of CKD, it affects 79% of patients2 if the criterion of bicarbonate below 22 mmol/l was accepted (till 2024) according to the 2012 Guideline (KDIGO 2012 CKD)7and this level was the commonly recognized indication for bicarbonate supplementation8. As some studies show, serum bicarbonate levels correlate with the progression of CKD3 and even with mortality9although others contradict this6. A 2021 meta-analysis based on 15 studies with a 3-month observation period in CKD patients (eGFR < 60 ml/min per 1.73 m² and/or proteinuria) (2445 participants with a median observation period of 12 months) comparing the effect of oral sodium bicarbonate versus placebo or no treatment showed that sodium bicarbonate delayed the decline in kidney function (standardized mean difference [SMD]: 0.26; 95% confidence interval [CI]: 0.13–0.40; I² = 50%), but the evidence is of low certainty and this study does not confirm any significant modifying effect of oral sodium bicarbonate on the risk of kidney failure (HR: 0.81; 95% CI: 0.54–1.22)10. Due to these controversies, the working group in the new KDIGO 2024 CKD Guideline lowered the bicarbonate level threshold to 18 mmol/l solely to prevent the development of severe acidosis11. It should be noted that overly aggressive correction of acidosis can be harmful. In one study, the administration of bicarbonates at a concentration above 24 mmol/l increased the risk of congestive heart failure, although no association was observed with mortality or cardiovascular events12. Moreover, in a reanalysis of the CRIC study (The Chronic Renal Insufficiency Cohort) on chronic kidney disease, a sustained serum bicarbonate level above 26 mmol/l was associated with an increased risk of heart failure and mortality12. For this reason, current recommendations suggest bringing serum bicarbonate levels to normal11.
Generally, in patients with MA in the course of advanced CKD, two subgroups of patients with metabolic acidosis can be distinguished: those with normal anion gap (AGMA) and those with high anion gap (HAGMA). The anion gap is the difference between cations and anions and is usually calculated using the simple formula AG = (Na+) - (HCO3− + Cl−), with bicarbonate and chloride being the most important anions in serum. Acidosis with a normal anion gap is usually diagnosed in the early stages of chronic kidney disease, while acidosis with a high anion gap is observed in the later stages of chronic kidney disease due to the accumulation of non-chloride anions, including phosphate, sulfate, and a wide range of organic acids13. There is a lot of data indicating that AG increases with the decline in GFR and, as a result, accelerates CKD progression6. This is confirmed by our analyses in which we found a negative correlation between eGFR and AG and more advanced kidney failure in HAGMA group (see Supplementary file with Supplementary Tables 1, 2 and 3 and Supplementary Fig. 1). This group started dialysis earlier than AGMA patients (see Supplementary Figs. 3 and 4). Furthermore, Lee et al. in a group of 440 patients with advanced CKD [with an estimated glomerular filtration rate (eGFR) of 15–60 mL/min/1.73 m²] demonstrated that the size of the anion gap also correlates with all-cause mortality5. Similar results were shown by Abramowitz et al. in an observational study of a population of 11,957 adults in the National Health and Nutrition Examination Survey6 and by Asahina et al. who used cohort data from 1,168 Japanese patients with chronic kidney disease14. Current clinical knowledge about how high anion gap acidosis affects kidney outcomes, especially in the later stages of CKD, is limited. Some preliminary studies have shown that uremic acids responsible for AG, such as indoxyl sulfate, p-cresyl sulfate, and trimethylamine N-oxide, cause renal interstitial fibrosis15,16. Many studies have confirmed the association between high AG and hypertension, and insulin resistance, which can directly translate into cardiovascular events17.
Since AG is traditionally calculated from two anions (bicarbonate and chloride) and sodium as the main cation, the main idea of the work was to find a simple index connecting the element of acidosis advancement (HCO3−) and the element strongly associated with AG (Cl−), which was captured in a simple numerical ratio Cl−/HCO3−. This indicator was a good predictor of advanced acidosis with a cutoff point of 6.22 for pH < 7.33. The ultimate goal of our study was to build a profile of patients in terms of the Cl−/HCO3− ratio. The analysis presented in Table 6 showed that the group of patients with a higher Cl−/HCO3−A ratio [≥ 6.22(mmol/l)(mmol/l)] was characterized by lower eGFR and HCO3− levels, as well as higher Cl−, phosphate, and PTH values, suggesting that these patients had more advanced kidney disease and metabolic disorders. In this subgroup, shorter time to initiate renal replacement treatment provides a supplementary proof (Table 7; Fig. 3). The question remains whether this ratio can play a role in making therapeutic decisions. Considering that it includes two important factors (Cl− and HCO3−) in its formula, which are the main components of AG and the latter has documented prognostic significance, this indicator may have a significant impact on the choice of therapy in the future.
The 2012 KDIGO recommendations advised that metabolic acidosis should be diagnosed when serum bicarbonate levels are below 22 mmol/l and, importantly, it then requires pharmacological correction7. However, as we mentioned above, the significance of bicarbonate supplementation has diminished recently following the publication of the randomized BiCARB study comparing the use of oral sodium bicarbonate to placebo18. In a study of individuals with chronic kidney disease G3-G4 aged over 60 years and sodium bicarbonate levels < 22 mmol/l, oral sodium bicarbonate did not improve physical function or kidney function, but did increase the risk of adverse effects. This was confirmed by other observational studies, which have shown that a serum bicarbonate level ≥ 27 mmol/l is associated with an increased risk of heart failure, raising concerns12. Additionally, a meta-analysis of 15 studies (2445 participants, with a median follow-up period of 12 months) in individuals with chronic kidney disease (eGFR < 60 ml/min per 1.73 m² and/or proteinuria) comparing the effect of oral sodium bicarbonate versus placebo or no treatment did not confirm any significant modifying effect of oral sodium bicarbonate on the risk of kidney failure (HR: 0.81; 95% CI: 0.54–1.22). Therefore, in the new KDIGO recommendations, the recommended bicarbonate concentration has been lowered to 18 mmol/l as the threshold for starting supplementation.
Another strategy for controlling metabolic acidosis in patients with CKD is dietary changes that limit the intake of acid-rich foods and/or increase the intake of alkaline-rich foods, thereby reducing total endogenous acid production. Goraya et al.19. shown that a diet rich in fruits and vegetables that produce alkalinity allows for equally good control of HCO3− levels and maintenance of eGFR as oral sodium bicarbonate administration, and additionally better improves cardiovascular disease risk indicators, probably due to reduced arterial blood pressure and higher vitamin K levels19.
Veverimer is a new hydrochloric acid-binding drug that removes acid from the gastrointestinal tract, resulting in an increase in bicarbonate concentration in the serum. This drug is being developed to treat metabolic acidosis, and its aim was to slow the progression of chronic kidney disease. Veverimer is free of sodium counterions, such as sodium or potassium. The initial series of clinical studies on veverimer showed encouraging results in cases of metabolic acidosis in chronic kidney disease20,21. In a multicenter, randomized controlled trial, treatment with veverimer improved kidney outcomes, identified as the occurrence of renal replacement therapy or a decrease in the estimated glomerular filtration rate (eGFR) of at least 50% over 52 weeks22. However, in the recent randomized VALOR-CKD study23,24, veverimer did not slow the progression of CKD. The authors comment that the small difference in bicarbonate levels between the study arms may have been the main reason for the negative study outcome.
These controversial experiences with interventions targeting bicarbonate in the treatment of MA in CKD patients, combined with a lack of obvious benefits, have encouraged interest in the anion gap and its associated uremic toxins.
The anion gap is a reflection of the accumulation of non-volatile anions, such as sulfonic acid derivatives25. These uremic toxins are formed in the large intestine as a consequence of dysbiosis. The increase in serum urea concentration promotes the influx of urea and uric acid into the intestinal lumen through the enterohepatic cycle, significantly increasing the number of bacterial species that produce urease, uricase, and enzymes that generate indole (the precursor of indoxyl sulfate [IS]) and p-cresol (two well-known uremic toxins), while simultaneously reducing the number of species that produce butyrate26. In our study, the size of the anion gap correlate negatively with eGFR (r=−0.36, p < 0.05) and with the sum Cl− and HCO3− (Cl− + HCO3−) (r=−0.42, p < 0.05) (Supplementary Tables 1 and Supplementary Fig. 1), however there were no correlation between AG and Cl−/HCO3− ratio. This suggests that in less advanced CKD, a normal anion gap metabolic acidosis (AGMA) is common; acidosis is mainly corrected by bicarbonate buffer, which lowers bicarbonate concentrations; however, the anion gap is maintained at a normal level due accompanied compensatory increase in chloride27. In more advanced CKD the anion gap cannot be compensated by an increase in bicarbonate resorption and only accumulation of chloride may limit, but not normalize, the size of AG. In other words, in patients with high anion gap metabolic acidosis (HAGMA), there is practically no possibility of bicarbonate reabsorption and also the ability of the kidneys to retain Cl− is also exhausted. It should be noted that when analyzing the predictors of conversion from AGMA to HAGMA (Supplementary Table 2), we found that creatinine levels above 5 mg/dl, eGFR < 10.7 ml/min/1.73 m2, and urea levels of 177 mg/dl may indicate high anion gap.
Studies show that intervention at the point of anion gap can lead to a reduction in toxin levels and thus improve prognosis. The AST-120 resin effectively improves intestinal function, reduces endotoxemia, and lowers the levels of uremic toxins28,29. Since there is no evidence that correcting metabolic acidosis under the control of bicarbonate concentration bring clear benefits, one should consider whether the parameters used to calculate the anion gap would be simpler for determining the threshold for intervention in the treatment of acid-base disorders.
Shifts between bicarbonate and chloride compartments within the kidney constitute a crucial element shaping acid-base and ionic balance30. Bicarbonate is mainly (in 85–90% of filtered bicarbonate) reabsorbed in the proximal tubules. The loop of Henle reabsorbs about 10%, and the remaining 5–10% is reabsorbed in the collecting ducts31. Various types of carbonic anhydrase (CA), namely membrane-bound and cytoplasmic CA, are involved in this process. In the proximal tubule, filtered bicarbonate reacts with hydrogen ions to form carbonic acid (H2CO3), which is broken down into CO2 and H2O by the membrane-bound enzyme carbonic anhydrase IV (CA IV). CO2 diffuses into the tubular cells, where it reacts with H2O and, in the presence of cytoplasmic carbonic anhydrase II (CA II), recombines with HCO3− and H+. Next, HCO3− is transported to the blood in exchange for chlorides. The Cl−/HCO3− exchanger conducts the final reversible electroneutral exchange process, which maintains the anion gap within normal limits32. These physiological processes indirectly explain the interaction between Cl− and HCO3− in CKD. We believe that this physiological dependence may be of significant importance in shaping acid-base disorders in patients with CKD, although in uremic conditions, changes in ion channels may also involve a complex mechanism of interaction, antagonism, and reinforcement, which constitutes a significant limitation of the study.
When evaluating the findings of this study, it is critical to take into account the other study limitations. The main is cross-sectional design, therefore we were unable to observe changes in POCT values over time, but we did conduct a clinical 2-year follow-up and found that patients with high Cl−/HCO3− ratio started hemodialysis treatment earlier. This statement has limits because the decision to initiate renal replacement treatment was made by a leading nephrologist and was based on the patient’s overall status rather than just acid-base abnormalities. The study group was adequately large, but consisted of a narrow range of individuals, mainly those with CKD stages G4 and G5, which may have impacted the relationship between the measures. We also did not measure any urine indicators, including urine ammonia, titratable acid excretion, or urine anion gap, to correlate with AG in serum, and we did not correlate our results with the patients’ diets. Participants in our study did not receive any additional dietary recommendations beyond those provided by the attending physician during follow-up visits at the clinic. Moreover, arterial blood was used for POCT, whereas venous blood is used in most acid-base studies and some studies indicate that these differences may be significant33.
Conclusion
A Cl−/HCO3−A ratio higher than 6.22 (mmol/l)/(mmol/l) may indicate advanced metabolic acidosis, and dialysis treatment may be initiated earlier in these individuals than in those with a lower ratio. Further studies are necessary to determine whether this indicator can be comprehensively used for individualized therapy selection.
Methods
From January 2021 to June 2022, 188 patients with CKD in stages G4 and G5 who had been admitted to our department for arteriovenous fistula surgery were screened for participation in the study. The inclusion criteria were: (1) age over 18 years old; (2) patients with CKD stages G4 and G5 who qualified for fistula creation; and (3) written consent. To increase cohort homogeneity, individuals undergoing haemodialysis were excluded due to high bicarbonate concentrations in dialysis fluid (usually 32 mmol/l), which can quickly correct metabolic acidosis during haemodialysis. 72 patients were excluded due to already ongoing hemodialysis treatment. The rest of 115 patients (72 male, 43 female) (mean age 63 ± 17 years), with CKD stages G4 or G5 were enrolled in this cross-sectional study. 14 of 115 patients were given oral bicarbonate, although they were instructed to discontinue medication at least one week before creating fistula.
Collecting blood samples and measuring POCT parameters
During the arteriovenous fistula (AVF) creation operation, arterial (A) and venous (V) blood samples were collected and analysed using a point-of-care testing analyser (POCT). After dissecting the radial artery (RA) and clamping the distal and proximal sections, a longitudinal incision was made. Then the clamp was released, blood was taken into a heprinized syringe through a plastic needle put into the proximal section of the RA. The delay between RA closure and sample collection was no more than 30 s. A venous blood sample was aspirated after the vein was cut off.
The assisting nurse subsequently took the samples to the POCT in an adjacent room and immediately completed the test. The overall time from sample collection to measurement was no more than three minutes. The POCT parameters were examined using an analyser (RADIOMETER ABL90 SERIES, RADIOMETER MEDICAL APS, Denmark). This is a totally automatic equipment that obtains diagnostic results while the patient is present or close by. It uses a cartridge containing test reagents to accomplish an 8-hour calibration.
The following parameters were evaluated: HCO3−, Bicarbonate; pCO2, Carbon dioxide partial pressure; BE, Base Excess; Na+, Sodium; K+, Potassium; Cl−, Chloride; Ca2+, Ionized calcium; urea, and creatinine. The estimated glomerular filtration rate (eGFR) was determined using the MDRD formula because our device did not automatically report it34. The ratio of arterial (A) and venous (V) Cl− and HCO3− concentration were calculated (Cl−/HCO3−-A and Cl−/HCO3−-V ratio). In addition, the difference in concentrations (AV gradient) (Δ = A - V) of HCO3− was calculated (ΔHCO3−).
In the study, AG was analysed as uncorrected anion gap (AG) and calculated using the equation AG = [Na+] - ([Cl−] + [HCO3−]). The anion gap, including potassium, was calculated as [AG (K+)] = ([Na+] + [K+] - ([Cl−] + [HCO3−]). The anion gap was corrected to albumin and counted using the equation: corr.AG = AG (K+) + 2.5×[4,5-albumin (g/dl)]. Finally, the deltaAG/deltaHCO3− index was determined using the following equation: deltaAG/deltaHCO3−=[corr.AG(mmol/l)−10]/[22-HCO3−(mmol/l)].
Types of metabolic acidosis
According to the type of acidosis study patients were divided into 3 subgroups: (1) patients without metabolic acidosis (non-MA) with HCO3−>22 mmol/l, (2) MA with normal anion gap (AGMA) (AG ≤ 10 mmol/l, HCO3− ≤22 mmol/l) and (3) high anion gab MA (HAGMA) AG > 10 mmol/l, HCO3− ≤22 mmol/l.
Demographic characteristics of the study group, causes of chronic kidney disease, and comorbidities
The primary causes of chronic kidney disease (CKD), as well as demographic and comorbidity data, were gathered from medical records and direct interviews. Charlson Comorbid Index (CCI) was calculated using the rule described in the previous study4,5. In brief, this score incorporates the patient’s age and the following disorders set in groups: (1) Heart diseases; Myocardial infarction (MI) with history of definite or probable MI (EKG changes and/or enzyme changes); Chronic heart failure with exertional or paroxysmal nocturnal dyspnea that responded to digitalis, diuretics, or afterload reducing agents; (2) Peripheral obstructive arterial disease (PAOD) with intermittent claudication or past bypass for chronic arterial insufficiency, history of gangrene or acute arterial insufficiency, or untreated thoracic or abdominal aneurysm (≥ 6 cm); (3) Cerebrovascular accident (CVA) or transient ischemic attacks (TIA) with minor or no residual deficit; Dementia with chronic cognitive deficit; Hemiplegia (4) chronic obturative pulmonary disease (COPD); (5) Connective tissue disease; (6) Gastrointestinal diseases: Peptic ulcer disease with any history of treatment for ulcer disease or history of ulcer bleeding; Liver disease; (7) Diabetes mellitus; (8) Neoplasmatic disease: Solid tumor; Leukemia; Lymphoma. Depending on the severity of the comorbidity, 1–6 points were assigned, and the sum was calculated.
In addition, ambulatory measurements of hemodynamic parameters with Mobil-O-Graph monitor (Industrielle Entwicklung Medizintechnik und Vertriebsgesellschaft GmbH -IEM, Stolberg, Germany), which records oscillometric arm blood pressure: systolic and diastolic blood pressure (SBP and DBP) and pulse pressure (pPP). It calculates the pulse wave velocity (PWV) as a measure of arterial stiffness.
Two-year clinical follow-up
A two-year clinical follow-up was conducted in June 2024 to assess the clinical status of each participant by reviewing their medical records and telephone interview. The time at which dialysis was initiated, cardiovascular events, kidney transplantation and deaths were all recorded.
Statistical analysis
Statistical analysis was carried out using standard software (Statistica Version 13.3, StatSoft, Tulsa, OK, USA). Continuous variables between groups were expressed as mean and standard deviation (± SD) and compared using the independent t-test, an analysis of variance (ANOVA), or Mann-Whitney U test, depending on normality of the variables (checked using the Browna and Forsytha test). Categorical variables were compared using the χ2with absolute (n) and percentage (%) values. The association between POCT parameters was investigated using Pearson’s correlation analysis. A p-value less than 0.05 was considered significant.
To evaluate the discriminant value of Cl−/HCO3−-A ratio, pCO2-A, HCO3−-A, BE-A, Cl−-A, eGFR, creatinine and urea concentration receiver operating characteristic curves were constructed for patients with pH ≤ 7.33. We investigated area under curve (AUC), cut-offs, sensitivity and specificity. Kaplan-Maier analysis and live table was used to assessed of probability of initiating hemodialysis treatment.
Ethics approval was granted by the Ethics Board of Wroclaw Medical University No KB-609/2019.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization, T.G.; methodology, T.G. and M.K.; formal analysis, T.G. and M.G.; investigation, T.G., J.K., M.G., J.S., M.S., M.Ż., D.B., K.K-K., M.Ś.; writing—original draft preparation, T.G., M.G., M.K.; writing—review and editing, T.G., M.K.,; data curation, M.G., J.K.; supervision, M.K.; project administration, T.G; funding acquisition, M.B., T.G.; analysis of results T.G. and M.K. All authors reviewed the manuscript.
Funding
The study is supported by the Wroclaw Medical University statutory funds (SUBK.C160.24.056). It was investigator-initiated research. The funding body had no role in the study design, data collection, analyses, and interpretation, or in writing the manuscript.
Data availability
The data presented in this study are available on request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee at Wroclaw Medical University No KB-609/2019.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maciej Gołębiowski, Email: maciej.golebiowski@student.umw.edu.pl.
Tomasz Gołębiowski, Email: tomasz.golebiowski@umw.edu.pl.
References
- 1.Chap. 3: Management of progression and complications of CKD. Kidney International Supplements 3, 73–90, (2013). 10.1038/kisup.2012.66 [DOI] [PMC free article] [PubMed]
- 2.Gołębiowski, T. et al. Point-of-Care testing to differentiate various Acid–Base disorders in chronic kidney disease. Diagnostics13, 3367 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inker, L. A. et al. Relationship of estimated GFR and albuminuria to concurrent laboratory abnormalities: an individual participant data meta-analysis in a global consortium. Am. J. Kidney Dis.73, 206–217 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WAKABAYASHI, Y., SAKAMOTO, H., MISHINA, T. & MARUMO, F. Hyperchloremia in patients with chronic renal failure. Tohoku J. Exp. Med.149, 145–150 (1986). [DOI] [PubMed] [Google Scholar]
- 5.Lee, S. W. et al. Serum anion gap predicts all-cause mortality in patients with advanced chronic kidney disease: a retrospective analysis of a randomized controlled study. PLoS One. 11, e0156381 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramowitz, M. K., Hostetter, T. H. & Melamed, M. L. The serum anion gap is altered in early kidney disease and associates with mortality. Kidney Int.82, 701–709 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin, A. & Stevens, P. E. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int.85, 49–61 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Di Iorio, B. R. et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI study. J. Nephrol.32, 989–1001 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navaneethan, S. D. et al. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin. J. Am. Soc. Nephrol.6, 2395–2402 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hultin, S. et al. A systematic review and meta-analysis on effects of bicarbonate therapy on kidney outcomes. Kidney Int. Rep.6, 695–705 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens, P. E. et al. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int.105, S117–S314. 10.1016/j.kint.2023.10.018 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Dobre, M. et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the chronic renal insufficiency cohort (CRIC) study. Am. J. Kidney Dis.62, 670–678 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, H. J. et al. Metabolic acidosis is associated with pulse wave velocity in chronic kidney disease: results from the KNOW-CKD study. Sci. Rep.9, 16139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asahina, Y. et al. Association of time-updated anion gap with risk of kidney failure in advanced CKD: a cohort study. Am. J. Kidney Dis.79, 374–382 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Vanholder, R., Schepers, E., Pletinck, A., Nagler, E. V. & Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J. Am. Soc. Nephrol.25, 1897–1907 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta, N. et al. Targeted Inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. Arterioscler. Thromb. Vasc. Biol.40, 1239–1255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramowitz, M. K., Hostetter, T. H. & Melamed, M. L. Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney Int.81, 1033–1042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical cost-effectiveness of oral sodium bicarbonate therapy for older patients with chronic kidney disease and low-grade acidosis (BiCARB): a pragmatic randomised, double-blind, placebo-controlled trial. BMC Med.18, 1–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goraya, N., Munoz-Maldonado, Y., Simoni, J. & Wesson, D. E. Fruit and vegetable treatment of chronic kidney disease-related metabolic acidosis reduces cardiovascular risk better than sodium bicarbonate. Am. J. Nephrol.49, 438–448 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Bushinsky, D. A. et al. Randomized, controlled trial of TRC101 to increase serum bicarbonate in patients with CKD. Clin. J. Am. Soc. Nephrol.13, 26–35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adrogué, H. J. & Madias, N. E. Veverimer: an emerging potential treatment option for managing the metabolic acidosis of CKD. Am. J. Kidney Dis.76, 861–867 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Wesson, D. E. et al. Veverimer versus placebo in patients with metabolic acidosis associated with chronic kidney disease: a multicentre, randomised, double-blind, controlled, phase 3 trial. Lancet393, 1417–1427 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Mathur, V. S. et al. Design and population of the VALOR-CKD study: a multicenter, randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of veverimer in slowing progression of chronic kidney disease in patients with metabolic acidosis. Nephrol. Dialysis Transplantation. 38, 1448–1458 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Tangri, N. et al. VALOR-CKD: A multicenter, randomized, Double-Blind Placebo-Controlled trial evaluating veverimer in slowing progression of chronic kidney disease in patients with metabolic acidosis. J. Am. Soc. Nephrol.10, 1681 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raphael, K. L. Metabolic acidosis in CKD: core curriculum 2019. Am. J. Kidney Dis.74, 263–275 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Wong, J. et al. Expansion of urease-and uricase-containing, indole-and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol.39, 230–237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golebiowski, T. et al. Point-of-Care testing to differentiate various Acid-Base disorders in chronic kidney disease. Diagnostics (Basel). 1310.3390/diagnostics13213367 (2023). [DOI] [PMC free article] [PubMed]
- 28.Yoshifuji, A. et al. Oral adsorbent AST-120 ameliorates gut environment and protects against the progression of renal impairment in CKD rats. Clin. Exp. Nephrol.22, 1069–1078 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaziri, N. D. et al. Oral activated charcoal adsorbent (AST-120) ameliorates chronic kidney disease-induced intestinal epithelial barrier disruption. Am. J. Nephrol.37, 518–525 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widmer, B., Gerhardt, R. E., Harrington, J. T. & Cohen, J. J. Serum electrolyte and acid base composition: the influence of graded degrees of chronic renal failure. Arch. Intern. Med.139, 1099–1102 (1979). [PubMed] [Google Scholar]
- 31.Berend, K. Review of the diagnostic evaluation of normal anion gap metabolic acidosis. Kidney Dis.3, 149–159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonar, P. T. & Casey, J. R. Plasma membrane Cl-/HCO3-exchangers: structure, mechanism and physiology. Channels2, 337–345 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Gołębiowski, T. et al. Exhausted capacity of bicarbonate buffer in renal failure diagnosed using point of care analyzer. Diagnostics11, 226 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levey, A. S. et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med.145, 247–254. 10.7326/0003-4819-145-4-200608150-00004 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.