Abstract
The Rel/NF-κB family of transcription factors is sequestered in the cytoplasm of most mammalian cells by inhibitor proteins belonging to the IκB family. Degradation of IκB by a phosphorylation-dependent ubiquitin-proteasome (inducible) pathway is believed to allow nuclear transport of active Rel/NF-κB dimers. Rel/NF-κB (a p50–c-Rel dimer) is constitutively nuclear in murine B cells, such as WEHI231 cells. In these cells, p50, c-Rel, and IκBα are synthesized at high levels but only IκBα is rapidly degraded. We have examined the mechanism of IκBα degradation and its relation to constitutive p50–c-Rel activation. We demonstrate that all IκBα is found complexed with c-Rel protein in the cytoplasm. Additionally, rapid IκBα proteolysis is independent of but coexistent with the inducible pathway and can be inhibited by calcium chelators and some calpain inhibitors. Conditions that prevent degradation of IκBα also inhibit nuclear p50–c-Rel activity. Furthermore, the half-life of nuclear c-Rel is much shorter than that of the cytoplasmic form, underscoring the necessity for its continuous nuclear transport to maintain constitutive p50–c-Rel activity. We observed that IκBβ, another NF-κB inhibitor, is also complexed with c-Rel but slowly degraded by a proteasome-dependent process in WEHI231 cells. In addition, IκBβ is basally phosphorylated and cytoplasmic. We thus suggest that calcium-dependent IκBα proteolysis maintains nuclear transport of a p50–c-Rel heterodimer which in turn activates the synthesis of IκBα, p50, and c-Rel to sustain this dynamic process in WEHI231 B cells.
Proteolysis is one mechanism by which cells irreversibly control protein functions. The functions of many regulatory proteins, such as oncoproteins, tumor suppressors, cell cycle control proteins, and transcription factors, are controlled by modulated proteolysis (14, 41). In the case of Rel/NF-κB, a family of transcription factors important for regulation of many cellular functions (5, 58), the proteolytic control is imposed not on the factors themselves but on the associated inhibitor protein, IκB. Thus, an important area of Rel/NF-κB studies focuses on the molecular mechanisms of IκB degradation pathways.
IκB comprises a family of related proteins that includes IκBα, IκBβ, IκBγ/p105, IκBδ/p100, and IκBɛ (4). IκB members form trimeric complexes with dimers of Rel/NF-κB family members, p50 (NFKB1), p52 (NFKB2), RelA (p65), c-Rel, and RelB (4, 5, 58). Different IκB members preferentially associate with specific Rel/NF-κB dimers and sequester them in the cytoplasm (37). Upon stimulation with extracellular signals, such as cytokines, growth factors, chemical stresses, UV or ionizing radiation, bacterial lipopolysaccharide (LPS), or tetradecanoyl phorbol acetate, many IκB members undergo phosphorylation-dependent degradation to release active Rel/NF-κB dimers (5, 58). Signal-inducible degradation of IκBα, IκBβ, and IκBɛ requires site-specific phosphorylation of serines 32 and 36, 19 and 23, and 157 and 161, respectively (9, 10, 16, 32, 60). These serines are conserved among family members; therefore, the same or similar kinases may be responsible for phosphorylation (4). Phosphorylation serves as a signal for subsequent attachment of multiple 76-amino-acid ubiquitin polypeptides (1, 12, 43). Ubiquitination targets IκBα to degradation by the 26S proteasome (12). Consequently, signal-inducible IκB degradation and Rel/NF-κB activation pathways can be efficiently blocked by various cell-permeable proteasome inhibitors (5, 58). Extracellular signal and cell type dictate which of coexisting Rel/NF-κB/IκB complexes become targeted for IκB degradation and transient or long-term NF-κB activation (54, 58, 60). The activated Rel/NF-κB dimers migrate into the nucleus, bind to decameric κB DNA binding sites, and regulate transcription of a wide variety of genes. These include Rel/NF-κB/IκB members (37) and those involved in immune, inflammatory, and acute-phase responses (28). Rel/NF-κB proteins may also regulate oxidative stress responses (46), proliferation (17, 27, 49, 50), and apoptosis (7, 56, 59). Thus, IκB degradation is one essential event in signaling pathways leading to Rel/NF-κB activation and subsequent target gene activation. To date, degradation by the 26S proteasome is the only known process for IκB degradation in cells (4, 5, 58).
In mouse splenic B cells and B-cell lines, Rel/NF-κB activity is constitutively nuclear and is believed to regulate immunoglobulin kappa light chain (Igκ) gene transcription (45, 48). The major constitutive dimers in these cells are a p50 homodimer and a p50–c-Rel heterodimer (31, 36). c-Rel contains a C-terminal transactivation domain which p50 lacks (6, 26); therefore, p50–c-Rel is considered to be the major transcriptional activator. In these B cells, the expression of p50/p105, c-Rel, and IκBα is augmented, compared to pre-B cells (36), presumably by autoregulation through the κB sites in their genes (13, 22, 53). Other IκB members are also expressed in B cells, but the level of IκBγ is lower than that in pre-B cells (25, 30). IκBγ preferentially blocks the DNA binding of homodimeric p50 protein (30). Coincidentally, the DNA binding of p50 homodimer is increased in B cells. Among the IκB members, IκBα is selectively and rapidly degraded in B cells despite its high synthetic rate (34). IκBα can efficiently inhibit the DNA binding of p50–c-Rel present in B cells (34). In the present study, we examined this rapid IκBα proteolysis and its relationship to constitutive p50–c-Rel activity in WEHI231 murine B cells. Specifically, we examined the role of IκBα S32/36 phosphorylation and ubiquitin-proteasome degradation. In addition, we analyzed degradation, basal phosphorylation, and nuclear localization of IκBβ in relation to constitutive p50–c-Rel activation. Our results suggest that a novel calcium-dependent but proteasome-independent IκBα proteolysis maintains constitutive p50–c-Rel activity in WEHI231 murine B cells.
MATERIALS AND METHODS
Cell culture.
WEHI231 cells were maintained in RPMI 1640 medium (Cellgro; Mediatech) supplemented with 10% fetal bovine serum (HyClone Laboratory, Inc.), 5 × 10−5 M β-mercaptoethanol, 1,250 U of penicillin G (Sigma), and 0.5 mg of streptomycin sulfate (Sigma) per ml in a 5% CO2 humidified incubator (Forma). 70Z/3-CD14 cells were maintained as described above in the presence of 1 mg of G418 (Gibco-BRL) per ml in the medium. The cells were passaged twice weekly before reaching a cell density of 2 × 106 ml.
Chemicals.
Calpain inhibitor I (ALLnL), calpain inhibitor II (ALLM), tosylphenylalanine chloromethyl ketone (TPCK), pyrrolidine dithiocarbamate (PDTC), NH4Cl, dimethyl sulfoxide (DMSO), bacterial LPS, and cycloheximide were purchased from Sigma. Calpeptin (ZLnL) was from Calbiochem, and BAPTA-AM was from NovaBiochem. E64-d and ZLLF were generous gifts from K. Hanada (Taisho Pharmaceutical, Japan) and F. Mercurio (Signal Pharmaceutical), respectively. Lactacystin was generously provided by E. J. Corey (Harvard University). The stock solutions were prepared in DMSO at 50 mg/ml or 100 mM (ALLnL, ALLM, calpeptin, and E64-d), 50 mM (tosylleucine chloromethyl ketone [TLCK] and TPCK), 100 mM (PDTC), 4 mg/ml (ZLLF), 30 mM (BAPTA-AM), and 25 mM (lactacystin and 25% DMSO). NH4Cl and cycloheximide were prepared in H2O at 1 M and 10 mg/ml, respectively. LPS was prepared in the growth medium at 1 mg/ml. In every experiment presented, the amount of DMSO was corrected in each sample such that the effect of DMSO was controlled. All stocks were stored in aliquots either at −70 or −20°C.
Cell preparation and Western blotting.
All incubations were performed in 1 ml of growth medium in 1.5-ml Eppendorf tubes secured on Labquaker (Barnstead/Thermolyne) which was placed in a 37°C incubator. The samples were continuously mixed by slow rotation for the period described for each experiment. Cells were then pelleted at 13,000 × g for 10 s in an Eppendorf centrifuge, rinsed twice with phosphate-buffered saline (PBS), resuspended in small amounts of PBS, and lysed by addition of 2× Laemmli buffer. The cell samples were immediately boiled for 10 min to inactivate proteases and phosphatases, electrophoresed in sodium dodecyl sulfate (SDS)–10 or 12.5% polyacrylamide gels, electroblotted (Bio-Rad) onto an Immobilon-P nylon membrane (GIBCO), and then incubated with appropriate IgG fractions in PBS containing 5% nonfat dry milk (Carnation), 0.2% Tween 20 (Sigma), and 0.02% sodium azide (Sigma). IgGs against IκBα (C21), IκBβ (C20), c-Rel (C), RelA (A), and Sp-1 (1C6) were from Santa Cruz Biotechnology. The antibody against lamin B was from MatriTect. Following an overnight incubation, the blots were washed twice with a wash buffer (PBS–0.2% Tween 20) for 30 min each time at room temperature and then further incubated for 2 h with a secondary horseradish peroxidase (HRP)-conjugated donkey anti-rabbit antibody (Amersham), HRP-conjugated protein A (Amersham), or HRP-conjugated donkey anti-mouse antibody (Oncogene Science). The blots were washed twice as above and developed by using the enhanced chemiluminescence (ECL) procedure as specified by the manufacturer (Amersham).
Samples used for coimmunoprecipitation experiments were resuspended in hypotonic buffer A (2) supplemented with various protease and phosphatase inhibitors as described previously (35), the nuclei were removed by centrifugation at 13,000 × g for 10 s, and 4 volumes of co-IP (coimmunoprecipitation) buffer (10 mM Tris-Cl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 0.4% Nonidet P-40) was added to the supernatants. The protein A-Sepharose and appropriate antibodies for each protein were then added. For nuclear and cytoplasmic partitioning experiments, cells were lysed in hypotonic buffer A in the presence of above-specified protease and phosphatase inhibitors followed by fivefold dilution with the nuclear preparation buffer as described previously (11), and the fractions were separated by centrifugation through a 50% sucrose cushion in the nuclear preparation buffer. The upper supernatant fractions and the pellets formed at the bottom of the sucrose layer represented the cytoplasmic and nuclear fractions, respectively.
In vivo labeling.
Appropriate number of cells were rinsed with Met− Cys− RPMI 1640 medium (CellGro), resuspended at 107 cells/ml, and pulse-labeled with [35S]Met-Cys mixture (Amersham) as described previously (34). Cells were then rinsed twice with growth medium without the label and chased for indicated periods. Cells were then pelleted, rinsed twice with PBS, and frozen at −70°C until all samples were terminated. Samples used for immunoprecipitation experiments were resuspended in IP buffer (20 mM Tris-Cl [pH 8.0], 250 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS, protease and phosphatase inhibitors) supplemented with 0.5% SDS, boiled for 10 min, diluted fivefold with IP buffer, and immunoprecipitated as described above. Following washes, the immunoprecipitates were boiled in the presence of 1 mg of bovine serum albumin and 0.5% SDS in IP buffer, diluted fivefold, and reprecipitated for the second time to reduce backgrounds. The immunoprecipitates were rinsed four times with IP buffer, resuspended in 2× Laemmli buffer, boiled for 10 min, and electrophoresed in SDS–10 or 12.5% polyacrylamide gels. The gels were processed as described previously (34). Nuclear and cytoplasmic fractions were prepared as described above, using the 50% sucrose cushion from cell pellets prepared at each time point of the pulse-chase experiments. The gels were exposed to either X-ray film for generation of figures or a PhosphorImager for quantification using the ImageQuant program.
Retrovirus construction and infection.
The murine IκBα cDNA was cloned into the pLHL-CA retroviral vector as described previously (57). An oligonucleotide (5′-TATACGCGTTATGGCTAGCTACCCATACGACGTCCCAGATTACGCGGACTTAGGATCCGTTAACAAGCTTAGATCTTC-3′) containing the hemagglutinin (HA) tag (underlined) (19) and three amino acids at both N and C termini was cloned into the MluI and BglII sites within the multiple cloning sites of the retroviral vector. The resulting vector is pLHL-CAHA. The murine IκBα cDNA clone was amplified by PCR, and the product was digested with BamHI and HindIII and cloned into the BamHI and HindIII sites downstream of the HA tag. This cloning procedure generated murine IκBα with an N-terminal HA tag in frame with a total of a 15-amino-acid extension. The S32/36A mutant was generated by site-directed mutagenesis using an oligonucleotide (5′-GTGGACGATCGCCACGACGCAGGTCTAGACGCCATGAAGGACGAGGAGTAC-3′) which introduced a unique XbaI site along with change of serines 32 and 36 to alanines. The mutant clones were identified by the presence of a unique XbaI site and confirmed by sequencing. The mutant cDNA was then digested with ApaI and HindIII and cloned into ApaI and HindIII sites of the pLHL-CAHA-mIκBα, replacing the N-terminal wild-type (WT) sequence.
Retrovirus was generated by transient cotransfection of 293 human embryonic kidney cells (grown in Dulbecco’s modified Eagle’s medium with 10% bovine serum in 0.1% gelatin-coated culture dishes in 10% CO2 incubators) with a retroviral construct and a helper virus, pCLeco (38), followed by coincubation of either 70Z/3-CD14 or WEHI231 cells for 24 h with Polybrene (4 μg/ml) in RPMI 1640 with the above-specified supplements. The infected cells were separated from adherent 293 cells and then selected with hygromycin (1 mg/ml; Boehringer Mannheim). For cloning, cells were diluted immediately following infection, and individual cells were picked under a microscope and grown from a single cell into a mass culture in the presence of hygromycin. The WEHI231 and 70Z/3-CD14 cells stably expressing WT and S32/36A mutant IκBα were maintained in the growth medium supplemented with 1 mg of hygromycin per ml.
EMSA.
Cell pellets were prepared as described above except that the concentration of cells used for initial incubations was 5 × 106 to 107 /ml. The cell pellets were stored frozen at −70°C until nuclear extract preparation. Nuclear extracts were prepared as described previously (2), and the conditions for the electrophoretic mobility shift assay (EMSA) were as published previously (36). The nature of the inducible and constitutive NF-κB complexes in 70Z/3 and WEHI231 cells, respectively, has been previously published (36). The oligonucleotide used was a double-stranded 27-mer containing the Igκ intronic κB site (5′-CTCAACAGAGGGGACTTTCCGAGAGGCCAT-3′). Following electrophoresis in a 4% native acrylamide gel, the gels were dried, exposed to X-ray films, and developed as described above.
RESULTS
Total IκBα is rapidly degraded in WEHI231 murine B cells.
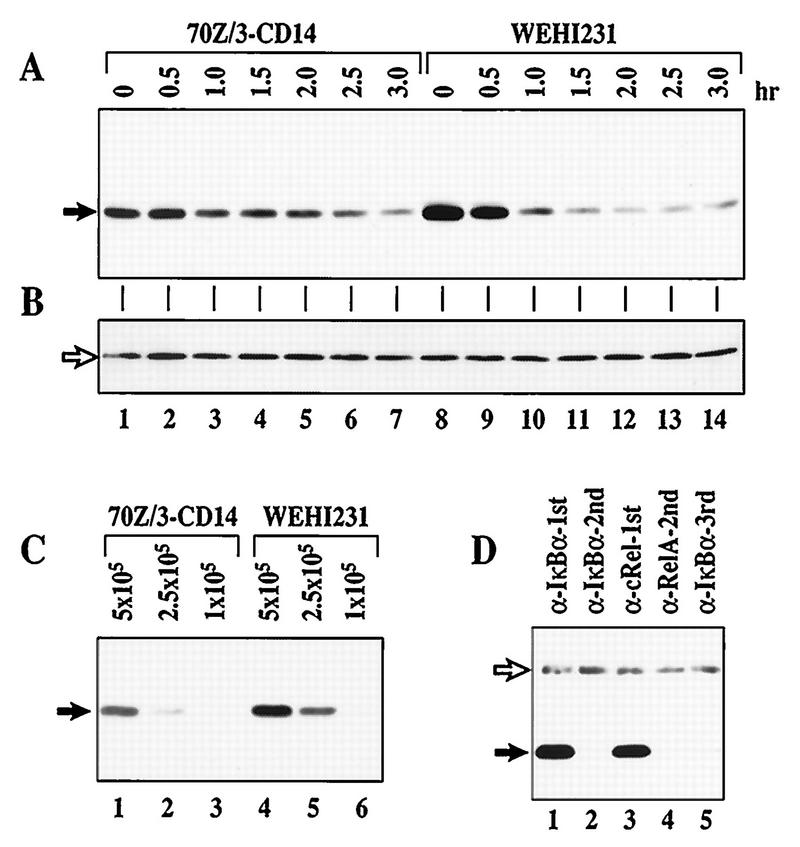
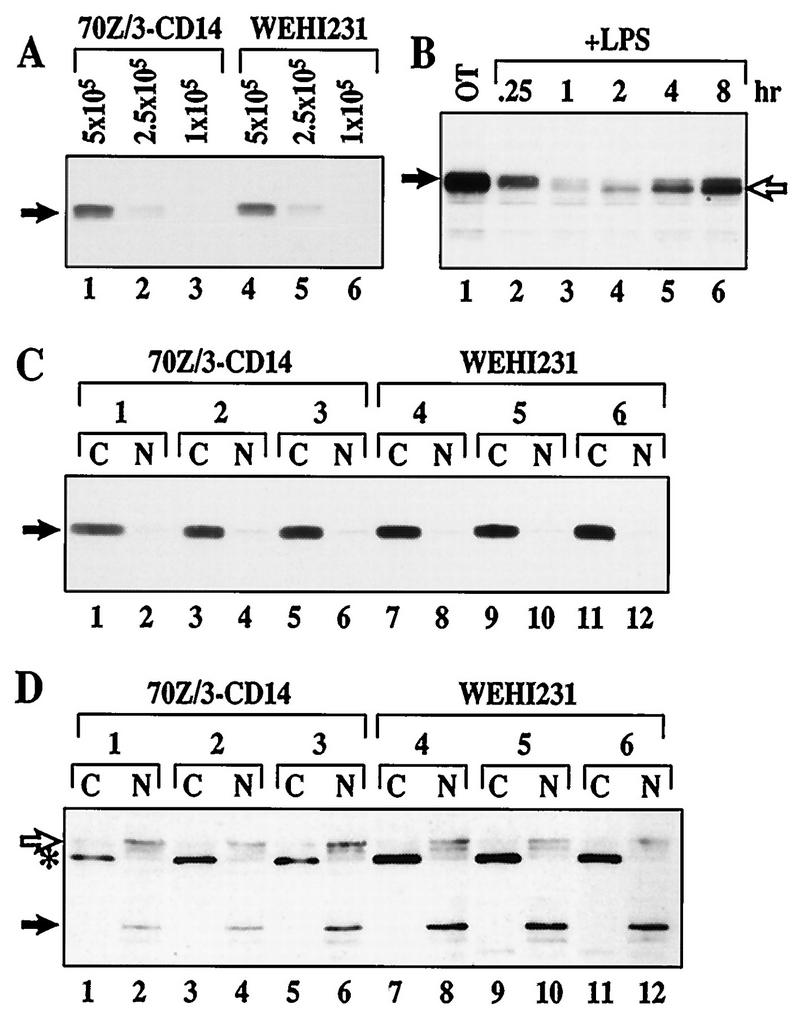
The p50–c-Rel dimer is constitutively nuclear in WEHI231 murine B cells (36). We previously demonstrated that newly synthesized IκBα is rapidly degraded in these cells (34). In contrast, rapid proteolysis of newly synthesized IκBα is not seen in 70Z/3 murine pre-B cells. Coincidentally, these pre-B cells lack constitutive NF-κB activity. To further address the question of rapid IκBα degradation in B cells, we examined the degradation of the total IκBα population. We used anti-IκBα to probe immunoblots of total cellular proteins from WEHI231 cells treated for various lengths of time with the protein synthesis inhibitor cycloheximide (Fig. 1A). A 70Z/3 cell line expressing human CD14, 70Z/3-CD14 (23), was similarly examined. The results shown in Fig. 1A demonstrate that total IκBα turns over more rapidly in WEHI231 (lanes 8 to 14) than 70Z/3-CD14 (lanes 1 to 7) cells. Equivalent loading between samples is shown by the presence of nonspecific protein (Fig. 1B). Although degradation is augmented, the net steady-state level of IκBα protein is ∼2-fold higher in WEHI231 cells (Fig. 1C; compare lanes 1 and 5) due to an even greater augmentation of synthesis (34). To determine whether this rapid proteolysis is due to the presence of excess free IκBα protein, which has a half-life of about 30 min in Cos cells (51), we performed coimmunoprecipitation using antisera against NF-κB subunit proteins. We reasoned that if free IκBα was produced in a large quantity to account for the overall half-life of about 40 min, then we should be able to detect some IκBα protein which was not bound to NF-κB subunit proteins at steady state. As shown in Fig. 1D, all detectable IκBα coimmunoprecipitated with c-Rel (lanes 1 and 3) and no free IκBα was seen (lane 5). These results demonstrate that most, if not all, IκBα is bound to c-Rel and suggest that excess free IκBα is unlikely to account for faster IκBα turnover in B cells (WEHI231) than in pre-B cells (70Z/3-CD14).
FIG. 1.
IκBα is associated with c-Rel and undergoes rapid proteolysis in WEHI231 cells. (A) Total IκBα degrades faster in WEHI231 cells than in 70Z/3-CD14 cells. The same number of 70Z/3-CD14 and WEHI231 cells (1.4 × 106) were incubated with cycloheximide (20 μg/ml) and terminated at the time points shown. Total cell pellets were dissolved in 2× Laemmli sample buffer and immediately boiled to preserve potentially modified IκBα forms. The samples were electrophoresed in SDS–12.5% polyacrylamide gels, transferred to a nylon membrane, and probed with IgG against IκBα protein. The protein bands (arrow) were visualized by ECL reaction. (B) Loading control for blot in panel A. The blot in panel A was reprobed with IgG against RelA and developed as described above; a nonspecific band (arrow) is shown. (C) Relative steady-state levels of IκBα in WEHI231 and 70Z/3-CD14 cells. Serial dilutions of 70Z/3-CD14 and WEHI231 cells (shown above each lane in cell number) were loaded. Positions of IκBα are shown on the left (arrow). (D) IκBα is complexed exclusively with c-Rel in WEHI231 cells. WEHI231 cells (106) were lysed in a hypotonic buffer in the presence of various protease inhibitors and phosphatase inhibitors as described in Materials and Methods, and the cytoplasmic fraction was split into two equal fractions. One fraction was immunoprecipitated with antibody against IκBα (lane 1), and the unprecipitated supernatant was reimmunoprecipitated to examine the efficiency of the first precipitation (lane 2). The other half of the original fraction was first immunoprecipitated with anti-c-Rel (lane 3). The unprecipitated proteins were then immunoprecipitated with anti-RelA (lane 4), and the same procedure was repeated for final IκBα precipitation (lane 5). The immunoprecipitates were electrophoresed in SDS–10% polyacrylamide gels, blotted, and probed with anti-IκBα antibody. The IκBα band (filled arrow) was visualized by ECL reaction using HRP-conjugated protein A to reduce reactivity with the rabbit Igμ heavy chains used for immunoprecipitation (open arrow).
Rapid IκBα degradation is insensitive to proteasome inhibitors.
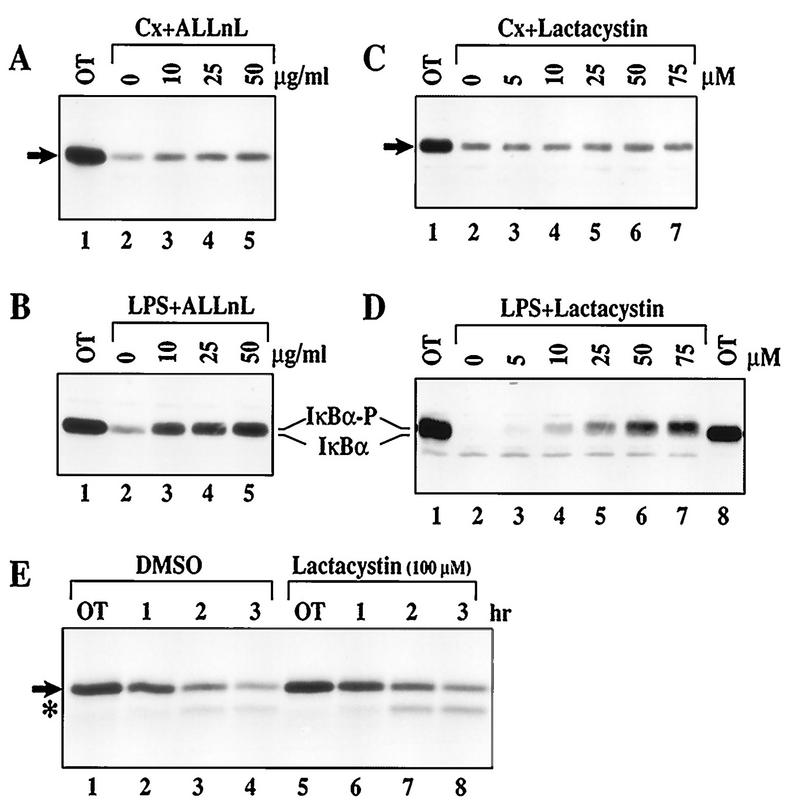
Since 26S proteasome is the only known in vivo IκBα protease (4, 5, 58), the requirement of proteasome activity for rapid IκBα proteolysis in WEHI231 cells was next examined. We treated WEHI231 cells with cycloheximide and the proteasome inhibitor ALLnL (58). ALLnL poorly inhibited IκBα degradation in these cells (Fig. 2A). As a positive control, we showed that ALLnL blocks IκBα degradation induced by LPS in pre-B cells (Fig. 2B). We then examined the effects of a highly specific proteasome inhibitor, lactacystin (18). High doses of lactacystin (up to 75 μM) show no detectable inhibitory activity in WEHI231 cells (Fig. 2C) but block the signal-inducible IκBα degradation in pre-B cells (Fig. 2D). Pulse-chase experiments also demonstrated that lactacystin (even at 100 μM) is ineffective at blocking IκBα degradation in these B cells (Fig. 2E and quantification by PhosphorImager not shown). These results provide evidence for proteasome-independent IκBα proteolysis in WEHI231 cells.
FIG. 2.
Proteasome inhibitors fail to block rapid IκBα proteolysis in WEHI231 cells. (A) ALLnL only slightly blocks basal IκBα turnover. WEHI231 cells were preincubated with various concentrations of ALLnL as shown for 30 min and then treated with cycloheximide (Cx) for an additional 2 h. Cell samples were processed, and IκBα (arrow) was visualized. OT, samples terminated prior to addition of inhibitors. (B) ALLnL efficiently blocks LPS-induced IκBα degradation in 70Z/3-CD14 cells. 70Z/3-CD14 cells were pretreated with various doses of ALLnL for 30 min and then treated with LPS (1 μg/ml; lanes 2 to 5) for 15 min. Cells were processed, and IκBα was visualized. A slight mobility shift of IκBα associated with hyperphosphorylation in lanes 3 to 5 is shown by IκBα-P. (C) Lactacystin fails to block rapid IκBα degradation in WEHI231 cells. WEHI231 cells were pretreated with various doses of lactacystin for 30 min then treated with cycloheximide for 1.5 h followed by Western blot analysis of IκBα. (D) Lactacystin is capable of inhibiting LPS-inducible IκBα degradation in 70Z/3-CD14 cells. 70Z/3-CD14 cells were preincubated with various doses of lactacystin for 30 min and then stimulated with LPS (1 μg/ml) for 15 min, and the level of IκBα was determined. Lane 8 is loaded with half as many untreated WEHI231 cells as a migration control. Hyperphosphorylated IκBα (IκBα-P) is in lanes 4 to 7. (E) Pulse-chase of IκBα in untreated and lactacystin-treated WEHI231 cells. WEHI231 cells (8 × 106) were pulse-labeled with [35S]Met-Cys for 3.5 h, rinsed with excess growth medium, and incubated with either 0.1% DMSO or 100 μM lactacystin (final DMSO concentration, 0.1%) for various periods. OT was taken immediately after addition of DMSO or lactacystin. Cell samples were processed as described in Materials and Methods, and the resulting dry gel was exposed to X-ray films to visualize IκBα. Scanning with a PhosophorImager and quantification by ImageQuant show a slight overloading in lanes 5 and 6, but there was no difference of degradation between these two treatment groups. In addition, a proteolytic intermediate (asterisk) was observed in both the control and treated cells. A similar proteolytic intermediate was not seen during LPS-induced IκBα degradation (not shown).
S32/36 phosphorylation is not an absolute requirement for rapid IκBα proteolysis.
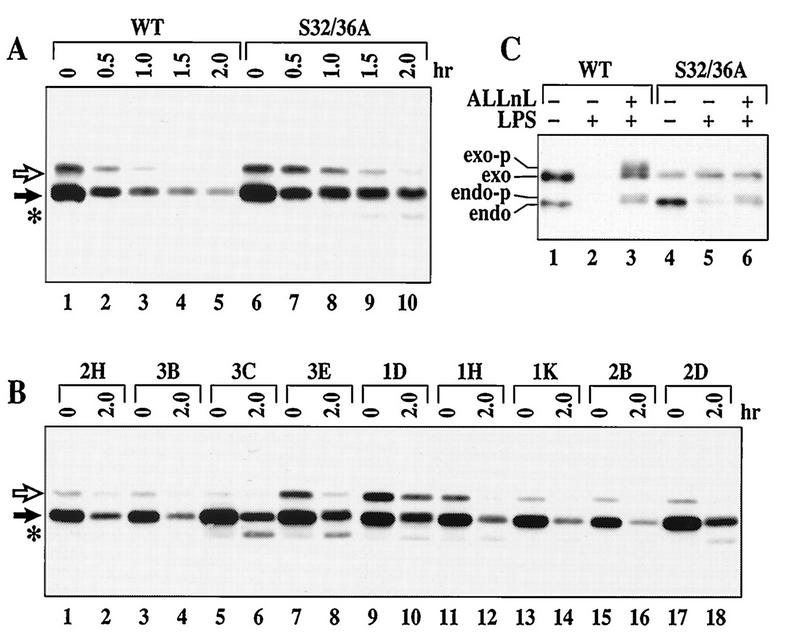
Prior phosphorylation at S32/36 is an essential requirement for most signal-inducible IκBα degradation pathways (9, 10). This phosphorylation induces a characteristic mobility shift of the IκBα protein during polyacrylamide gel electrophoresis (Fig. 2B and D, IκBα-P). However, this mobility shift of IκBα was not observed in WEHI231 cells treated with proteasome inhibitors (Fig. 2A and C). To determine if S32/36 phosphorylation is an essential requirement for rapid IκBα proteolysis in WEHI231 cells, we examined the degradation of an HA epitope-tagged S32/36A IκBα mutant. This mutant contains alanines at positions 32 and 36. As a positive control, WT IκBα was also expressed in these cells. Both the WT and endogenous IκBα proteins are rapidly degraded in control cells (Fig. 3A, lanes 1 to 5). Therefore, the N-terminal HA epitope does not interfere with degradation. The S32/36A mutant and the endogenous IκBα protein are also rapidly degraded in a pool of cells stably expressing this mutant protein (Fig. 3A, lanes 6 to 10). Rapid proteolysis of the S32/36A mutant also occurs in nine independent stable clones that express various levels of the S32/36A mutant (Fig. 3B). These results are in sharp contrast to the absolute requirement of the S32/36 phosphorylation sites for LPS-inducible IκBα phosphorylation (Fig. 3C, lanes 3 and 6) and degradation (lanes 2 and 5) in pre-B cells. A mutant of IκBα with lysine-to-arginine changes at ubiquitination sites, positions 21 and 22 (K21/22R), also degrades efficiently in WEHI231 cells (not shown). Thus, these results demonstrate that rapid IκBα proteolysis can occur in the absence of S32/36 phosphorylation and K21/22 ubiquitination in WEHI231 cells.
FIG. 3.
S32/36 phosphorylation of IκBα is not required for rapid IκBα proteolysis in WEHI231 cells. (A) Both WT and S32/36A mutant IκBα degrade in WEHI231 cells. Pooled WT (lanes 1 to 5)- or S32/36A (lanes 6 to 10)-expressing WEHI231 cells were treated with cycloheximide and terminated at different time points. Exogenous IκBα proteins are shown by an open arrow, while the endogenous protein is shown by a filled arrow. An asterisk shows a possible proteolytic intermediate. (B) Degradation of the S32/36A mutant in stable clones. Nine independent clones of WEHI231 cells expressing the S32/36A mutant IκBα protein were analyzed for the ability to degrade the mutant IκBα protein as described above. The open arrow points to the S32/36A mutant, while the filled arrow points to the endogenous IκBα protein. An asterisk shows a possible proteolytic intermediate. (C) The endogenous and exogenous WT but not the exogenous S32/36A mutant IκBα undergo LPS-induced hyperphosphorylation and degradation in 70Z/3-CD14 cells. 70Z/3-CD14 cells expressing either WT (lanes 1 to 3) or S32/36A (lanes 4 to 6) were treated with LPS (lanes 2, 3, 5, and 6) without (lanes 2 and 5) or with ALLnL (lanes 3 and 6) (50 μg/ml). Cells were processed and blotted with anti-IκBα antibody as for Fig. 1A. The positions of exogenous (exo), endogenous (endo), phosphorylated exogenous (exo-p) and phosphorylated endogenous (endo-p) IκBα proteins are shown.
Constitutive p50–c-Rel activity is insensitive to proteasome inhibitors.
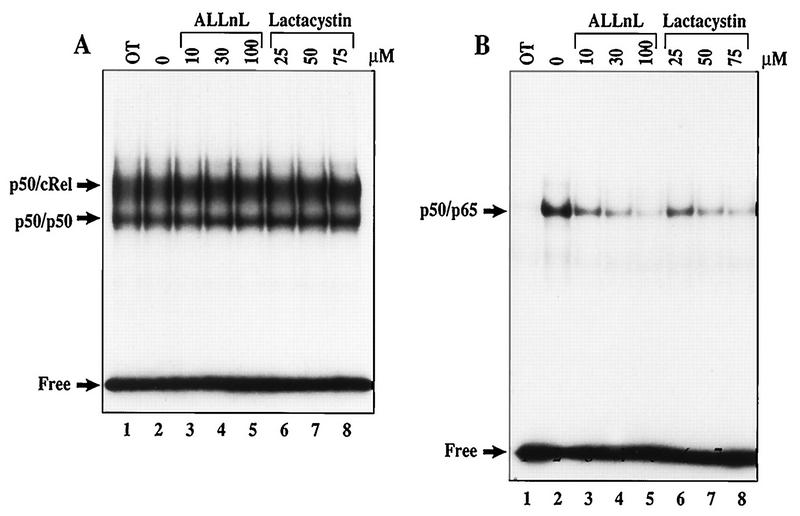
Even though rapid IκBα proteolysis cannot be blocked by proteasome inhibitors in WEHI231 cells, constitutive p50–c-Rel activity may require a proteasome-dependent process. For example, proteasome-dependent degradation of other IκB members, such as IκBβ or IκBɛ, may be required (32, 39, 60). To directly examine this possibility, WEHI231 cells were treated with doses of ALLnL and lactacystin for times up to 3.5 h. Although these inhibitors have been shown to efficiently block signal-induced NF-κB activation (58), EMSAs demonstrate that neither proteasome inhibitor is able to inhibit constitutive p50–c-Rel activity (Fig. 4A). The control experiments shown in Fig. 4B demonstrate that both proteasome inhibitors efficiently block LPS-inducible NF-κB activation in a dose-dependent manner. Thus, like rapid IκBα proteolysis, constitutive p50–c-Rel activation is a proteasome-independent process in WEHI231 B cells.
FIG. 4.
Proteasome inhibitors fail to block constitutive p50–c-Rel activity in WEHI231 cells. (A) ALLnL and lactacystin fail to block constitutive p50–c-Rel activity in WEHI231 cells. WEHI231 cells were treated with various doses of ALLnL (lanes 3 to 5) and lactacystin (lanes 6 to 8) or with DMSO (0.2%) alone (lane 2) for 3 h. Nuclear extracts were analyzed by EMSA using the Igκ κB site (see Materials and Methods). Lane 1 contains untreated WEHI231 cells. The positions of p50–c-Rel, p50 homodimer, and free probe are shown on the left. (B) ALLnL and lactacystin efficiently block LPS-inducible NF-κB activation in 70Z/3-CD14 cells. 70Z/3-CD14 cells were pretreated with inhibitors and concentrations as indicated for 30 min, followed by stimulation with LPS (1 μg/ml) for 15 min. Lane 1 contains untreated cells. The nuclear extracts were analyzed as described above. The positions of the inducible p50-p65 (RelA) complex and the free probe are shown on left.
IκBα is a target of at least two proteolytic processes in WEHI231 cells.
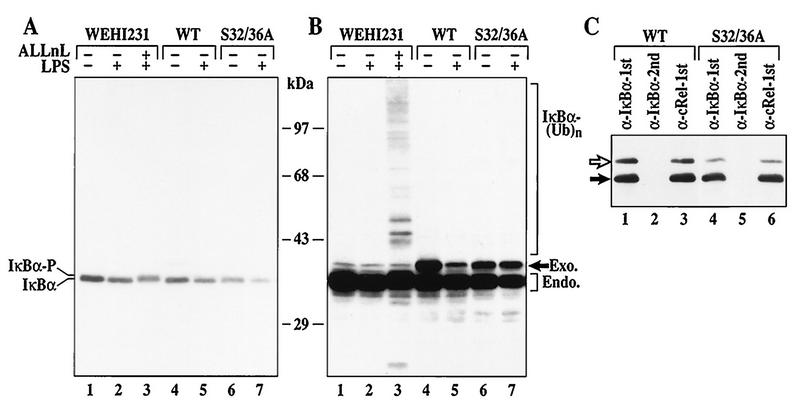
The foregoing data demonstrate that rapid IκBα proteolysis and constitutive p50–c-Rel activity in WEHI231 cells are mechanistically distinct from known signal-inducible pathways. This may be due to the lack of the signal-inducible pathway or insufficient uptake of proteasome inhibitors. To directly address these possibilities, WEHI231 cells were stimulated with LPS in the presence and absence of proteasome inhibitors. Stimulation of WEHI231 cells with LPS induces modest IκBα degradation in 30 min (Fig. 5A; compare lanes 1, 4, and 6 to lanes 2, 5, and 7). Even though the rate of degradation induced by LPS is lower in this cell type than in pre-B cells (Fig. 2B and D), ALLnL can efficiently block this degradation. Additionally, ALLnL induces accumulation of the slower-migrating S32/36 phosphorylated IκBα protein (lane 3). Longer exposure shows the accumulation of a high-molecular-weight IκBα ladder, consistent with formation of multiubiquitinated IκBα proteins (Fig. 5B, lane 3). More pronounced effects of LPS-inducible degradation and proteasome inhibitors can be seen when WEHI231 cells are treated with cycloheximide to eliminate high-level IκBα synthesis (not shown). Finally, the S32/36A mutant is resistant to degradation by LPS stimulation (Fig. 5B, lanes 6 and 7) and remains associated with c-Rel (Fig. 5C, lanes 3 and 6). These results demonstrate that the LPS-inducible IκBα degradation processes in B (WEHI231) and pre-B (70Z/3-CD14) cells are indistinguishable and dependent on the S32/36 phosphorylation-dependent ubiquitin-proteasome pathway. Furthermore, the uptake of proteasome inhibitors is sufficient to block LPS-stimulated IκBα degradation in these cells. Thus, the results demonstrate that IκBα is targeted to at least two proteolytic systems, signal-inducible proteasome-dependent and constitutive proteasome-independent systems, in WEHI231 B cells.
FIG. 5.
Inducible IκBα degradation requires the S32/36 phosphorylation-dependent ubiquitin-proteasome pathway in WEHI231 cells. (A) IκBα degradation induced by LPS in control, WT, or S32/36 WEHI231 cells. WEHI231 cells were treated with LPS (10 μg/ml) for 30 min with (lane 3) and without (other lanes) 30-min pretreatment with ALLnL (50 μg/ml). Similar numbers of cells stably expressing either WT or S32/36A mutant IκBα were also stimulated with LPS as described above. Hyperphosphorylated IκBα (IκBα-P) is shown in lane 3. (B) Longer exposure of the blot in panel A. A high-molecular-weight ladder and a smaller antiserum-reactive band are seen in lane 3. WT IκBα is degraded (compare lanes 4 and 5, band labeled Exo.), while S32/36A is not (lanes 6 and 7). (C) Both exogenous and endogenous IκBα are complexed with c-Rel in WEHI231 cells. WEHI231 cells expressing WT (lanes 1 to 3) or S32/36A (lanes 4 to 6) were processed for coimmunoprecipitation as described in legend to Fig. 1D. The positions of exogenous (open arrow) and endogenous (filled arrow) proteins are shown on the left.
Calpain inhibitors and calcium chelators selectively block rapid IκBα proteolysis in WEHI231 cells.
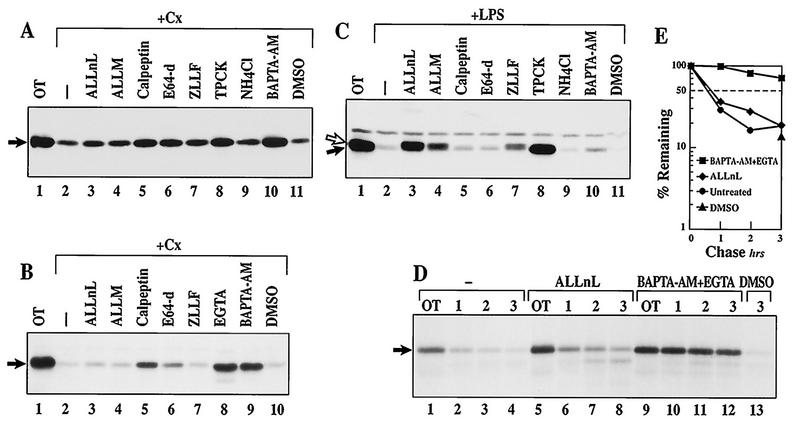
To further distinguish between constitutive and inducible IκBα degradation processes in WEHI231 cells, effects of various protease inhibitors were next examined. Our previous study (Fig. 2A) demonstrated that ALLnL, but not lactacystin, slightly inhibits IκBα turnover in unstimulated WEHI231 B cells. Since ALLnL can inhibit the activity of calpain, a calcium-dependent cysteine protease, other calpain inhibitors were also examined. The results shown in Fig. 6A (1.5-h treatment) and B (3.5-h treatment) demonstrate that ALLM can also slightly block basal IκBα turnover (lane 4). ALLnL and ALLM possess distinct potencies toward 26S proteasome activity (Ki of 0.67 and 28 μM for ALLnL and ALLM, respectively [42]), but they block the activity of calpain equivalently (Ki of 190 and 120 nM for ALLnL and ALLM, respectively [21]). Consequently, their similar effects on IκBα degradation suggest that this process may be calpain mediated. Other calpain inhibitors, such as calpeptin (50% inhibitory dose of 52 nM against calpain I) and a cysteine protease inhibitor, E64-d, are also effective at partially inhibiting IκBα proteolysis (Fig. 6A and B, lanes 5 and 6, respectively). Higher doses of ALLnL, ALLM, calpeptin, and E64-d are toxic to the cells and do not further inhibit IκBα turnover (not shown). Since calpains require calcium for their activity, we also examined the effects of BAPTA-AM, an intracellular calcium chelator, and EGTA, an extracellular calcium chelator (55). BAPTA-AM and EGTA show marked inhibitory activities both in the Western blot assay (Fig. 6A and B) and the pulse-chase assay (Fig. 6D and E). Equivalent doses of calpeptin, E64-d, EGTA, and BAPTA-AM do not affect LPS-stimulated IκBα degradation in 70A/3-CD14 cells (Fig. 6C, lanes 5, 6, and 10; results for EGTA not shown). Also, calpeptin, E64-d, and EGTA are incapable of blocking IκBα degradation induced by LPS and cycloheximide in WEHI231 cells (not shown). In contrast, the proteasome inhibitors ALLnL, ALLM, and ZLLF block LPS-induced degradation of IκBα, resulting in the accumulation of hyperphosphorylated forms in pre-B (Fig. 6C, lanes 3, 4, and 7) and WEHI231 cells (Fig. 5). A lysosomal inhibitor, NH4Cl, is ineffective but TPCK is effective for inhibiting both processes (Fig. 6A and C, lanes 9 and 8, respectively) as reported previously (34, 35). NH4Cl does not block calpain activity, but TPCK does (8). These results demonstrate that calcium chelators and some calpain inhibitors can selectively block high constitutive IκBα turnover.
FIG. 6.
Calpain inhibitors and calcium chelators block rapid IκBα proteolysis in WEHI231 cells. (A) Calpain inhibitors and calcium chelators block basal IκBα turnover in WEHI231 cells. WEHI231 cells were incubated with cycloheximide (Cx; 20 μg/ml) and the inhibitors for 1.5 h prior to Western blot analysis using IκBα antibody. The inhibitors used were ALLnL (50 μg/ml), ALLnM (50 μg/ml), calpeptin (10 μg/ml), E64-d (25 μg/ml), ZLLF (2 μg/ml), TPCK (12.5 μg/ml), NH4Cl (30 mM), BAPTA-AM (30μM), and DMSO (0.2%). Lanes 3 to 11 had same final DMSO concentrations. (B) Longer treatment of WEHI231 cells with inhibitors. WEHI231 cells were treated with various inhibitors plus cycloheximide for 3.5 h and analyzed as described above. The concentrations of inhibitors were the same as specified above. EGTA (lane 8) was at 2.5 mM (final concentration). (C) Calpain inhibitors and calcium chelators do not block LPS-inducible IκBα degradation in 70Z/3-CD14 cells. 70Z/3-CD14 cells were pretreated with inhibitors at concentrations as in panel A for 30 min and then treated with LPS (1 μg/ml) for 15 min. IκBα was visualized as described above, and the positions of IκBα (filled arrow) and hyperphosphorylated IκBα (open arrow) are shown on the left. Note the presence of IκBα-P in lanes 3, 4, and 7. A band seen above the IκBα protein is a nonspecific band. (D) Pulse-chase experiment of IκBα in WEHI231 cells treated with various inhibitors. WEHI231 cells were pulse-labeled for 2 h, rinsed with growth medium and incubated with 50 μg of ALLnL per ml (lanes 5 to 8) and 30 μM BAPTA-AM plus 1.25 mM EGTA (lanes 9 to 12) or untreated for 15 min. Samples were terminated immediately after addition of inhibitors (lanes 1, 5, and 9). At various time points thereafter, equivalent numbers of cells were terminated for each condition. The DMSO control (lane 13) was treated for a total of 3 h 15 min (equivalent to 3-h time points in other conditions). IκBα was immunoprecipitated and visualized as for Fig. 2E. (E) Quantification of IκBα bands in panel D. The dried gel was exposed to a PhosphorImager cassette, scanned with a PhosphorImager, and quantified by ImageQuant, and the values were plotted by using the OT values as 100% against time in hours.
Calcium chelators also block constitutive p50–c-Rel activity.
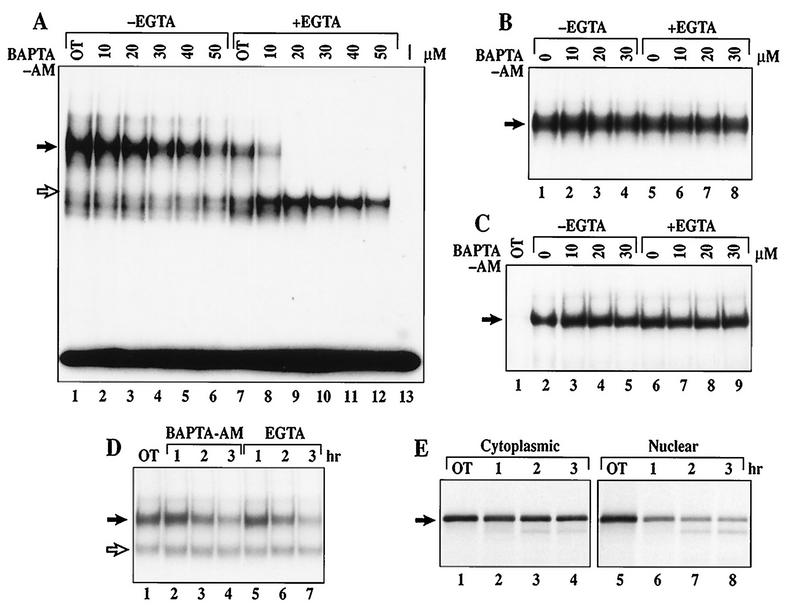
We previously showed that TPCK blocks both rapid IκBα degradation and constitutive p50–c-Rel activity (34). If rapid IκBα proteolysis is involved in constitutive p50–c-Rel activation, inhibitors of rapid but not signal-inducible IκBα degradation should also block constitutive p50–c-Rel activity. Thus, we examined the effects of doses of calpeptin, E64-d, and BAPTA-AM with or without EGTA on the level of constitutive p50–c-Rel activity. BAPTA-AM is able to selectively reduce the level of nuclear p50–c-Rel DNA binding in a dose-dependent manner (Fig. 7A, lanes 2 to 6). This inhibitory effect of BAPTA-AM can be augmented by simultaneous addition of EGTA (lanes 8 to 12). EGTA can also inhibit this process alone (compare lanes 1 and 7). This inhibitory effect is not due to direct inhibition of the DNA binding activity, because BAPTA-AM with or without EGTA does not inhibit p50–c-Rel DNA binding activity when directly added to nuclear extracts isolated from untreated WEHI231 cells (Fig. 7B). This inhibitory effect is also not a result of NF-κB nuclear transport blockage, because BAPTA-AM with or without EGTA did not block LPS-induced NF-κB nuclear transport (Fig. 7C). The effects of BAPTA-AM and EGTA are not only dose dependent but also time dependent, because the level of constitutive p50–c-Rel activity is progressively reduced over the 3-h period examined (Fig. 7D, lanes 2 to 4 and 5 to 7, respectively). Similarly, calpeptin and E64-d can also selectively reduce the level of p50–c-Rel activity, although not as efficiently as BAPTA-AM and EGTA (not shown). These results demonstrate that inhibitors capable of blocking rapid IκBα proteolysis can also selectively block constitutive p50–c-Rel activity in murine B cells. Furthermore, there is a correlation between the degree of inhibition of IκBα degradation and p50–c-Rel activity in WEHI231 cells.
FIG. 7.
Calcium is essential for the maintenance of constitutive p50–c-Rel activity in WEHI231 cells. (A) EMSA of WEHI231 cells treated with BAPTA-AM with or without EGTA. WEHI231 cells were treated with various doses of BAPTA-AM without (lanes 2 to 6) or with (lanes 8 to 12) EGTA (2.5 mM). Lane 1, DMSO alone (0.2%); lane 7, DMSO plus EGTA; lane 13, without a nuclear extract. Position of the p50–c-Rel heterodimer is shown by the filled arrow, whereas a p50 homodimer is shown by an open arrow. (B) BAPTA-AM and EGTA do not directly block p50–c-Rel DNA binding activity. A nuclear extract prepared from untreated WEHI231 cells was incubated with doses of BAPTA-AM (lanes 2 to 4), EGTA (lane 5), or BAPTA-AM plus EGTA (lanes 6 to 8) for 40 min and analyzed by EMSA. An area of the gel with p50–c-Rel complex is shown (arrow). (C) BAPTA-AM and EGTA do not block LPS-induced p50–RelA binding activity in 70Z/3-CD14 cells. 70Z/3-CD14 cells were treated with doses of BAPTA-AM without (lanes 3 to 5) or with (lanes 7 to 9) EGTA or with EGTA alone (lane 6) and treated with LPS (1 μg/ml) for 15 min, and nuclear extracts were analyzed by EMSA. Lane 1, unstimulated cells; lane 2, DMSO- and LPS-treated cells. An area of the gel with p50-RelA complex is shown (arrow). (D) Time course of inhibition of p50–c-Rel binding in WEHI231 cells by BAPTA-AM and EGTA. WEHI231 cells were treated with either BAPTA-AM (30 μM; lanes 2 to 4) or EGTA (2.5 mM; lanes 5 to 7) for the indicated periods of time. Nuclear extracts were analyzed by EMSA as described above. Lane 1, untreated cells. The filled arrow points to p50–c-Rel, while the open arrow points to p50 homodimer. (E) Pulse-chase of cytoplasmic and nuclear c-Rel protein in WEHI231 cells. WEHI231 cells were pulse-labeled with [35S]Met-Cys for 3.5 h, washed with growth medium, and incubated in growth medium, and equal cell numbers were terminated at time points shown. The cells were then fractionated into cytoplasmic and nuclear pools, and each pool was immunoprecipitated with anti-c-Rel antibody. Cytoplasmic fractions used for immunoprecipitation were one-fourth the level of the nuclear fractions for each time point. The exposure time for nuclear and the cytoplasmic fractions was the same (3 days). Quantification by PhosphorImager demonstrated that the half-life of cytoplasmic c-Rel was >3 h, while that for nuclear c-Rel was 57 min.
Nuclear c-Rel is short-lived.
IκBα is complexed with c-Rel in the cytoplasm (Fig. 1D) and undergoes rapid degradation. Since inhibition of IκBα degradation reduces nuclear p50–c-Rel DNA binding activity in a time-dependent manner (Fig. 7D), this process is associated with the maintenance of constitutive p50–c-Rel activity. Thus, continuous nuclear transport of cytoplasmic p50–c-Rel dimers may be required to maintain nuclear p50–c-Rel activity. If this model is correct, then the nuclear p50–c-Rel complex must have a relatively short half-life to account for the progressive loss of the nuclear DNA binding activity. To examine this possibility, the nuclear half-life of c-Rel protein was measured by pulse-chase experiments. The half-life of the cytoplasmic c-Rel was also measured as an internal control. The cytoplasmic c-Rel has a half-life of more than 3 h (Fig. 7E, lanes 1 to 4). In contrast, the half-life of nuclear c-Rel is only 57 min (lanes 5 to 8; data quantified with a PhosphorImager not shown). This short nuclear half-life correlates with the progressive loss of the p50–c-Rel DNA binding activity seen in Fig. 7D. Consequently, reduced DNA binding activity is likely due to reduced protein levels. Thus, these results suggest that a continuous nuclear transport of c-Rel complex is required to maintain nuclear p50–c-Rel DNA binding activity. They further suggest that p50–c-Rel nuclear transport is maintained by rapid proteolysis of associated IκBα in the cytoplasm.
Basal IκBβ degradation is slow and proteasome dependent in WEHI231 cells.
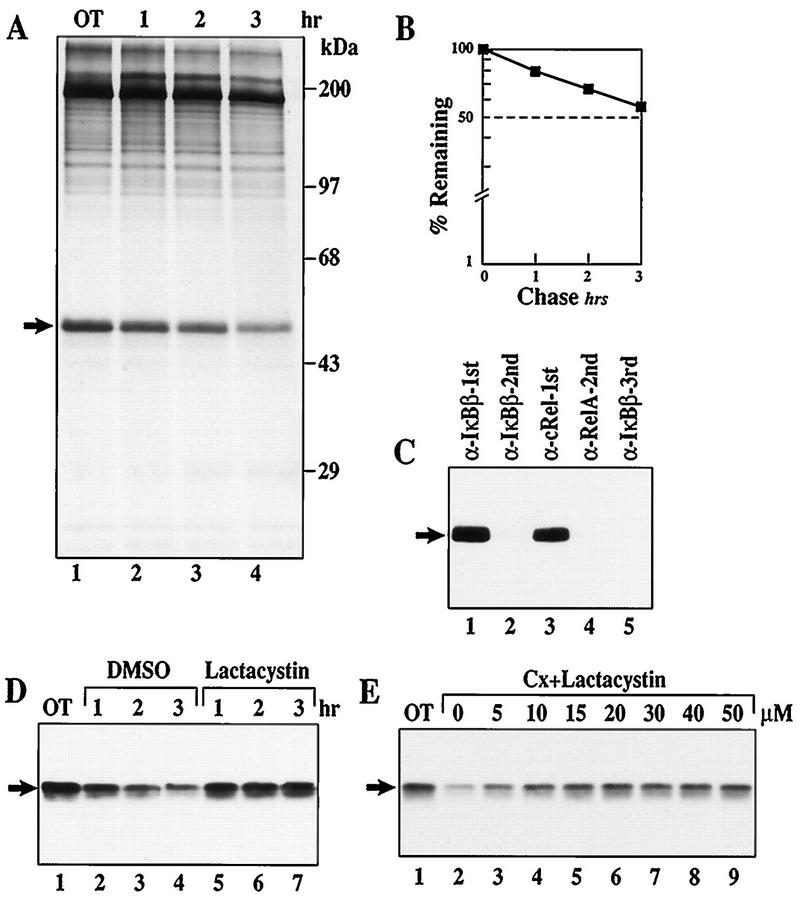
The results thus far are consistent with the hypothesis that rapid IκBα proteolysis in the cytoplasm maintains nuclear p50–c-Rel activity in WEHI231 B cells. If basal IκBβ degradation is rapid in unstimulated B cells, it may also significantly contribute to constitutive p50–c-Rel activity. Accordingly, IκBβ degradation has been suggested to regulate prolonged and constitutive NF-κB activities (32, 54). To directly examine this possibility, the level of IκBβ degradation and its proteasome dependence were examined in unstimulated WEHI231 cells. The pulse-chase experiment shown in Fig. 8A and quantification shown in 8B demonstrate that the half-life of IκBβ was >3 h, much longer than that of IκBα (∼40 min [Fig. 6E]). The different degradation levels of IκBα (rapid) and IκBβ (slow) are not due to associated Rel/NF-κB proteins, because most IκBβ is also found complexed with c-Rel (Fig. 8C). To determine if the difference of degradation is due to the different protease systems, the effect of the proteasome-specific inhibitor lactacystin was examined. Figure 8D shows a Western blot analysis demonstrating that lactacystin can efficiently block basal IκBβ degradation over a 3-h period (compare lanes 2 to 4 and 5 to 7). The dose response shown in Fig. 8E demonstrates that relatively low doses (15 to 20 μM) of lactacystin are sufficient to completely block basal IκBβ degradation (lanes 5 and 6). These results demonstrate that both IκBβ and IκBα are associated with c-Rel but basal IκBβ degradation is proteasome dependent in WEHI231 cells whereas IκBα degradation is not.
FIG. 8.
IκBβ is complexed with c-Rel and degraded slowly by the proteasome-dependent pathway in WEHI231 cells. (A) Pulse-chase of IκBβ in WEHI231 cells. WEHI231 cells were pulse-labeled with [35S]Met-Cys for 3.5 h and chased with growth medium for the indicated periods. The labeled IκBβ protein was immunoprecipitated with anti-IκBβ antibody in the presence of various protease inhibitors and phosphatase inhibitors as described in Materials and Methods. The position of IκBβ is shown by the arrow, and the molecular weight markers are shown on the right. (B) Quantification of IκBβ by PhosphorImager. The gel in panel A was exposed to a PhosphorImager, and IκBβ bands were quantified. The value at the start of the chase (OT) was used as 100%, and the fractions remaining were plotted against time. The half-life was slightly greater than 3 h. (C) IκBβ is associated with c-Rel in WEHI231 cells. Coimmunoprecipitation and Western blotting were performed as for Fig. 1D. The blots were first incubated with HRP-protein A in the presence of sodium azide to saturate the Igμ reactivity. Sodium azide inactivated the HRP activity of HRP-protein A bound to the Igμ heavy chain. The blot was then washed extensively, incubated with anti-IκBβ antibody, rinsed, incubated with HRP-protein A without sodium azide, and developed by ECL. No Igμ chain is visible in the blot shown. The arrow points to the IκBβ band. (D) Time course of lactacystin-mediated inhibition of basal IκBβ degradation in WEHI231 cells. WEHI231 cells were treated with cycloheximide (20 μg/ml) and lactacystin (25 μM) for the indicated periods of time, and the IκBβ was detected by Western blotting and ECL reaction using HRP-conjugated goat anti-rabbit antibody. (E) Dose response of lactacystin-mediated inhibition of basal IκBβ degradation in WEHI231 cells. WEHI231 cells were treated with cycloheximide (Cx; 20 μg/ml) and lactacystin at doses shown for 3 h, and IκBβ was detected as described above.
IκBβ is basally phosphorylated and cytoplasmic in WEHI231 cells.
Prolonged activation of NF-κB has been suggested to involve production of hypophosphorylated IκBβ which shields NF-κB from IκBα proteins and allows nuclear transport of NF-κB (52). To examine if such hypophosphorylated nuclear IκBβ is constitutively expressed in WEHI231 cells, we compared the forms (slower-migrating phosphorylated versus faster-migrating hypophosphorylated) and subcellular localization (nuclear versus cytoplasmic) of IκBβ in unstimulated WEHI231 B cells. 70Z/3-CD14 pre-B cells were also analyzed as a control. There is no difference in level and form of IκBβ in these two cell types (Fig. 9A), suggesting that hypophosphorylated IκBβ is not present at augmented level in WEHI231 cells. The absence of mobility difference between IκBβ in these cell types is not technical, because the faster-migrating hypophosphorylated IκBβ protein can be detected when pre-B cells are stimulated with LPS for prolonged periods (Fig. 9B). Furthermore, IκBβ is mostly cytoplasmic in unstimulated WEHI231 cells (Fig. 9C, lanes 7, 9, and 11) as in 70Z/3-CD14 cells (lanes 1, 3, and 5), further arguing against the presence of significant level of constitutively nuclear hypophosphorylated IκBβ protein. In contrast, two nuclear proteins, Sp-1 and lamin B, are seen only in the nuclear fractions, demonstrating that the cytoplasmic/nuclear fractionation is complete in these experiments. These results together with the results shown in Fig. 8 suggest that IκBβ is not a regulator of constitutive p50–c-Rel activity in WEHI231 immature B cells.
FIG. 9.
Level, migration pattern, and subcellular localization of IκBβ are the same in WEHI231 cells as in 70Z/3-CD14 cells. (A) Steady-state level of IκBβ in WEHI231 and 70Z/3-CD14 cells. The Western blot shown in Fig. 1C was also probed with anti-IκBβ antibody to examine the relative level of IκBβ protein (arrow). Samples were as in Fig. 1C. (B) Hypophosphorylated IκBβ produced following prolonged stimulation of 70Z/3-CD14 with LPS. 70Z/3-CD14 cells were treated with LPS (1 μg/ml) for up to 8 h. Equal fractions of cells were terminated at each time point and analyzed by Western blotting using anti-IκBβ antibody. The filled arrow shows basally phosphorylated IκBβ, while the open arrow points to newly synthesized hypophosphorylated IκBβ (lanes 4 to 6). (C) IκBβ is cytoplasmic in both WEHI231 and 70Z/3-CD14 cells. Three sets of 70Z/3-CD14 and WEHI231 cells were fractionated into cytoplasmic (C) and nuclear (N) fractions independently as described in Materials and Methods. The resulting fractions were analyzed by Western blotting using anti-IκBβ antibody. (D) Sp-1 and lamin B are nuclear. The blot in panel C was reprobed with antibodies against nuclear proteins Sp-1 (open arrow) and lamin B (closed arrow). The bands were visualized with HRP-conjugated anti-mouse antibody followed by ECL reaction. An asterisk points to an unknown protein which is exclusively localized in the cytoplasmic fraction.
DISCUSSION
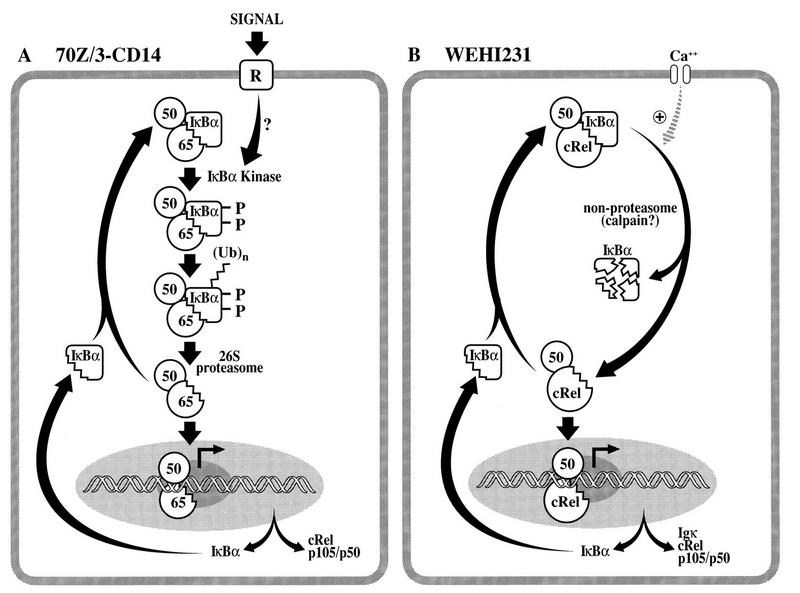
In this study, we present several lines of evidence for a novel IκBα degradation process that coexists with the well-characterized S32/36 phosphorylation-K21/22 ubiquitination-proteasome pathway (Fig. 10A). We suggest a model (Fig. 10B) in which continuous nuclear transport of p50–c-Rel dimer is induced by a high-level basal degradation of associated IκBα protein. This continuous nuclear transport counterbalances the short half-life of nuclear c-Rel complex in WEHI231 cells. The constitutive p50–c-Rel presumably activates transcription of genes encoding IκBα, c-Rel, and p50 to replace the degraded pool for the maintenance of this dynamic cycle. We further suggest that rapid IκBα proteolysis requires free calcium, likely imported from outside the cell.
FIG. 10.
Signal-inducible and constitutive Rel/NF-κB activation pathways in murine B cells. (A) A signal-inducible NF-κB activation pathway in 70Z/3-CD14 pre-B cells. The NF-κB activation pathway induced by extracellular stimuli involves activation of a specific IκBα kinase resulting in site-specific phosphorylation at serine residues 32 and 36. This phosphorylation event signals the multiubiquitination event primarily at lysine residues 21 and 22 by a ubiquitin-conjugating enzyme system, E1-E2-E3 (14). Finally, the multiubiquitinated IκBα protein while still complexed with NF-κB is then selectively and extensively degraded by the 26S proteasome complex. The liberated NF-κB migrates into the nucleus and regulates target genes, including that of IκBα. If an activating signal is terminated or downregulated, the newly synthesized IκBα will terminate the NF-κB activity, resulting in transient NF-κB activation. Extracellular signals can also induce degradation of IκBβ, resulting in prolonged NF-κB activation. (B) Constitutive p50/c-Rel activation pathway in WEHI231 cells. This pathway is a novel Rel/NF-κB activation pathway which is distinct from the conventional signal-inducible pathway shown in panel A. This constitutive pathway does not require the S32/36 phosphorylation or the ubiquitin-proteasome pathway. It likely depends on high-level constitutive IκBα degradation. This degradation requires free calcium, likely maintained by continuous influx through a calcium channel. Continuous IκBα degradation allows continuous nuclear transport of p50–c-Rel complex, which has a relatively short half-life of only 1 h in the nucleus. p50–c-Rel then activates the transcription of genes encoding IκBα, c-Rel, and p50/p105 to replenish the degrading pools.
IκBα is a target of two distinct proteases, constitutive proteasome-independent protease and the signal-inducible 26S proteasome.
Rel/NF-κB activation induced by a wide variety of extracellular signals requires proteolysis of the associated IκBα by the phosphorylation-dependent ubiquitin-proteasome pathway (reviewed in references 4, 5, and 58). This conclusion is supported by evidence showing that (i) various proteasome inhibitors prevent IκBα degradation, (ii) the stabilized IκBα is hyperphosphorylated at S32/36 and multiubiquitinated, (iii) S32/36A phosphorylation site mutant is resistant to signal-inducible ubiquitination and degradation, (iv) K21/22R mutant allows signal-inducible S32/36 phosphorylation but retards ubiquitination and degradation, (v) the modified forms of IκBα are still bound to NF-κB, (vi) proteasome inhibitors also block Rel/NF-κB appearance in the nucleus, and finally (vii) an S32/36A mutation or deletion of the N-terminal phosphorylation and ubiquitination sites produces a dominant-negative mutant of IκBα that is capable of preventing Rel/NF-κB activation induced by many extracellular signals. The only reported exception thus far is NF-κB activation following exposure to hypoxia-reoxygenation, which induces IκBα phosphorylation at tyrosine 42 and dissociation from NF-κB without degradation (24).
In the present study, we used many of these criteria to test whether rapid IκBα proteolysis in unstimulated WEHI231 cells is a result of constitutive activation of a signal-inducible degradation pathway. Our results demonstrate that high-level constitutive IκBα proteolysis is not mediated by a phosphorylation-ubiquitin-proteasome pathway. Since this process involves degradation of IκBα protein without a shift of mobility of IκBα in polyacrylamide gel electrophoresis, it is also not a tyrosine phosphorylation-mediated event (24). Additionally, it is not due to overproduction of free IκBα protein, which degrades rapidly through a phosphorylation-ubiquitination-independent but proteasome-dependent pathway in HeLa cells (29). Rapid degradation of free IκBα also requires basal phosphorylation in the C-terminal PEST sequence (47). It is not known whether this basal PEST phosphorylation is required for high turnover in WEHI231 cells. Although constitutive IκBα degradation cannot be inhibited by different proteasome inhibitors, the proteasome-dependent pathway can be induced by LPS (with or without cycloheximide) stimulation of WEHI231 cells. Thus, constitutive IκBα proteolysis in WEHI231 cells represents a novel IκBα degradation pathway which is present together with the signal-inducible pathway. Identification of amino acid sequence requirements, such as the C-terminal PEST sequence and the basal phosphorylation sites, for constitutive IκBα degradation in WEHI231 cells will help to further define this novel IκBα degradation pathway.
Protease(s) responsible for rapid IκBα proteolysis in WEHI231 cells.
What is the protease(s) responsible for constitutive IκBα degradation in WEHI231 cells? This degradation process cannot be prevented by lysosomal or proteasome inhibitors. This degradation pathway is, however, sensitive to various inhibitors of calpains. Calpains are calcium-dependent cysteine proteases that play important physiological roles, including those for platelet functions (15). Calpains are also associated with a number of pathological conditions, such as ischemia, cataract, muscular dystrophy, and arthritis (15, 44). There are two major forms of calpains, calpain I (μ-calpain) and calpain II (m-calpain). Either or both can be found in the cytoplasm of most mammalian cells (15). Calpain activity can be blocked by high levels of cell-permeable inhibitors, such as ALLnL, ALLM, and calpeptin, as well as cysteine protease inhibitors, such as leupeptin (ALLR) and E64-d (15, 33). Additionally, removal of free calcium can also block in vivo calpain activity. We have shown that high basal IκBα degradation is blocked by calpain inhibitors. The rank order of inhibitor potency against IκBα proteolysis in WEHI231 cells is BAPTA-AM and EGTA > calpeptin and E64-d > ALLnL and ALLM. These inhibitors may directly modulate the activity of the IκBα protease, or they may inhibit an upstream event involved in activation of the protease. These results nevertheless suggest a potential role for calpain in high constitutive IκBα degradation in WEHI231 cells.
Rapid IκBα proteolysis maintains continuous nuclear transport of p50–c-Rel to counterbalance short-lived nuclear p50–c-Rel complex.
We hypothesized that high IκBα proteolysis in unstimulated B cells is responsible for the maintenance of constitutive p50–c-Rel activity (34). This hypothesis predicts that conditions which prevent high basal IκBα degradation should also block constitutive p50–c-Rel activity. Additionally, conditions that fail to block the former would not inhibit the latter. Consistent with this hypothesis, proteasome inhibitors or lysosomal inhibitors fail to block IκBα turnover and also p50–c-Rel activity. In contrast, calcium chelators and some calpain inhibitors inhibit IκBα degradation and constitutive p50–c-Rel activity. Additionally, there is a correlation between the effectiveness of these inhibitors for blocking IκBα degradation and inhibiting constitutive p50–c-Rel activity. These results strongly suggest a causal relationship between high basal IκBα degradation and constitutive p50–c-Rel activity in WEHI231 cells. However, it is also possible that rapid IκBα degradation is a mechanistically unrelated paralleling event and that its inhibition increases the relative level of IκBα resulting in artificial blockage of constitutive p50–c-Rel activity.
Inhibition of IκBα degradation reduces nuclear p50–c-Rel activity in a time-dependent manner. Maximum loss of p50–c-Rel activity in the nucleus may require 3 to 4 h, depending on the inhibitors used. This time lag may be due to a slow inactivation of the existing nuclear p50–c-Rel complex. Correspondingly, the progressive loss of p50–c-Rel DNA binding activity correlates with the half-life of nuclear c-Rel protein. The short half-life of nuclear c-Rel is not due to inhibition by nuclear IκBα protein, since none is detected in WEHI231 nuclei. Therefore, this inactivation mechanism appears distinct from the autoregulatory mechanism where excess free IκBα enters the nucleus and rapidly shuts off an otherwise long-lasting NF-κB activity (3). It is of interest to determine if the loss of nuclear c-Rel is due to degradation in the nucleus or to cytoplasmic export. Regardless of the exact mechanism, these results demonstrate that continuous nuclear import of the c-Rel complex is necessary to maintain constitutive p50–c-Rel activity in WEHI231 cells.
IκBβ does not regulate constitutive p50–c-Rel activity.
IκBβ degradation has been suggested to regulate prolonged NF-κB activity induced by extracellular stimuli or constitutive activity induced by infection with the human T-cell leukemia virus type 1 (20, 32, 54). These studies raise the possibility that IκBβ degradation also contributes to constitutive p50–c-Rel activity in WEHI231 cells. However, IκBβ degradation is relatively slow (half-life of >3 h) and proteasome dependent. Since proteasome inhibitors capable of blocking IκBβ degradation do not affect constitutive p50–c-Rel activity, IκBβ degradation is not a regulatory contributor for constitutive p50–c-Rel activation.
A study by Suyang et al. (52) suggests that hypophosphorylated IκBβ can induce prolonged NF-κB activity. Hypophosphorylated IκBβ is induced by prolonged stimulation with certain agents, such as LPS or interleukin-1. Prolonged stimulation induces IκBβ degradation by proteasome-mediated pathway followed by new synthesis of hypophosphorylated IκBβ. In the continual presence of stimuli, newly synthesized IκBβ remains hypophosphorylated and is believed to associate with NF-κB at a higher affinity than IκBα and allow NF-κB nuclear translocation. Thus, this form of IκBβ is found in the nucleus in association with NF-κB and the target DNA binding site (52). If hypophosphorylated IκBβ is constitutively produced in unstimulated WEHI231 cells, it may contribute to constitutive p50–c-Rel activity. While this report was under review, a report by the above-cited group demonstrated that WEHI231 cells contained this hypophosphorylated IκBβ protein in the nucleus (40). However, our results demonstrate that the majority of detectable IκBβ is not hypophosphorylated (Fig. 9A), is cytoplasmic (Fig. 9C), and is associated with c-Rel (Fig. 8C). Although it was also suggested that IκBβ degradation is not inhibited by proteasome inhibitors by the same group (40), we were able to block its degradation by various proteasome inhibitors. We were also able to detect IκBα mobility shift in Western blots due to S32/36 phosphorylation and multiubiquitination which were not detected by the above-cited investigators in WEHI231 cells. These discrepancies may stem from the differences of procedures used or cell line variation. Our results, however, demonstrate that IκBβ is unlikely to play a regulatory role for constitutive p50–c-Rel activity in WEHI231 cells. Furthermore, they suggest an intriguing proteolytic process that induces selective degradation of IκBα protein without affecting IκBβ proteins, even though both are associated with c-Rel in the cytoplasm of WEHI231 cells. Thus, future investigations aim to define the protease responsible for high basal IκBα proteolysis as well as the regulatory mechanism(s) for its activity in WEHI231 cells and in other B cells.
ACKNOWLEDGMENTS
We thank members of the Verma laboratory, M. Maki, and Z. J. Chen for many discussions. We appreciate R. Wisdom, E. Oltz, E. T. Alarid, and J. Stevenson for critical reading of the manuscript. We also acknowledge P. Chaio for murine IκBα cDNA, J. Stevenson for generating pLHL-CAHA and pLHL-CAHA-mIκBα clones, E. J. Corey for providing lactacystin, M. Maki for E64-d (through Taisho Pharmaceutical), F. Mercurio for ZLLF, R. Naviaux for pCLeco vector, C. Akazawa for CA promoter, and R. J. Ulevitch for 70Z/3-CD14 cells.
This work was funded by a Howard Hughes Medical Institute grant through the UW Medical School and a Shaw Scientist Award from Milwaukee Foundation to S.M. S.D.S. is a trainee under NIH predoctoral training grant T32GM07215.
REFERENCES
- 1.Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. Stimulation-dependent IκBα phosphorylation marks the NF-κB inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1995;92:10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N C, Faller D V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arenzana-Seisdedos F, Thompson J, Rodriguez M S, Bachelerie F, Thomas D, Hay R T. Inducible nuclear expression of newly synthesized IκBα negatively regulates DNA-binding and transcriptional activities of NF-κB. Mol Cell Biol. 1995;15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeuerle P A, Baltimore D. NF-κB—ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin A S J. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 6.Ballard D W, Walker W H, Doerre S, Sista P, Molitor J A, Dixon E P, Peffer N J, Hannink M, Greene W C. The v-rel oncogene encodes a κB enhancer binding protein that inhibits NF-κB function. Cell. 1990;63:803–814. doi: 10.1016/0092-8674(90)90146-6. [DOI] [PubMed] [Google Scholar]
- 7.Beg A A, Baltimore D. An essential role of NF-κB in preventing TNFα-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 8.Bond J S, Butler P E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- 9.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκBα proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 11.Bunce C M, Thick J A, Lord J M, Mills D, Brown G. A rapid procedure for isolating hemopoietic cell nuclei. Anal Biochem. 1988;175:67–73. doi: 10.1016/0003-2697(88)90362-4. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 13.Chiao P J, Miyamoto S, Verma I M. Autoregulation of IκBα activity. Proc Natl Acad Sci USA. 1994;91:28–32. doi: 10.1073/pnas.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 15.Croall D E, Dermartino G N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- 16.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi T S, Takahashi T, Taguchi O, Azuma T, Obata Y. NF-κB RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J Exp Med. 1997;185:953–961. doi: 10.1084/jem.185.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 19.Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson I A, Lerner R A, Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Good L, Sun S C. Persistent activation of NF-κB/Rel by human T-cell leukemia virus type 1 tax involves degradation of IκBβ. J Virol. 1996;70:2730–2735. doi: 10.1128/jvi.70.5.2730-2735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griscavage J M, Wilk S, Ignarro L J. Serine and cysteine proteinase inhibitors prevent nitric oxide production by activated macrophages by interfering with transcription of the inducible NO synthase gene. Biochem Biophys Res Commun. 1995;215:721–729. doi: 10.1006/bbrc.1995.2523. [DOI] [PubMed] [Google Scholar]
- 22.Grumont R J, Richardson I B, Gaff C, Gerondakis S. rel/NF-κB nuclear complexes that bind κB sites in the murine c-rel promoter are required for constitutive c-rel transcription in B-cells. Cell Growth Differ. 1993;4:731–743. [PubMed] [Google Scholar]
- 23.Han J, Lee J D, Tobias P S, Ulevitch R J. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009–25014. [PubMed] [Google Scholar]
- 24.Imbert V, Rupec R A, Livolsi A, Pahl H L, Traenckner E B M, Muellerdieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle P A, Peyron J F. Tyosine phosphorylation of IκBα activates NF-κB without proteolytic degradation of IκBα. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 25.Inoue J, Kerr L D, Kakizuka A, Verma I M. IκBγ, a 70 kd protein identical to the C-terminal half of p110 NF-κB: a new member of the IκB family. Cell. 1992;68:1109–1120. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- 26.Inoue J, Kerr L D, Ransone L J, Bengal E, Hunter T, Verma I M. c-rel activates but v-rel suppresses transcription from κB sites. Proc Natl Acad Sci USA. 1991;88:3715–3719. doi: 10.1073/pnas.88.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontgen F, Grumont R J, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 28.Kopp E B, Ghosh S. NF-κB and rel proteins in innate immunity. Adv Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- 29.Krappmann D, Wulczyn F G, Scheidereit C. Different mechanisms control signal-induced degradation and basal turnover of the NF-κB inhibitor IκBα in vivo. EMBO J. 1996;15:6716–6726. [PMC free article] [PubMed] [Google Scholar]
- 30.Liou H C, Nolan G P, Ghosh S, Fujita T, Baltimore D. The NF-κB p50 precursor, p105, contains an internal IκB-like inhibitor that preferentially inhibits p50. EMBO J. 1992;11:3003–3009. doi: 10.1002/j.1460-2075.1992.tb05370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liou H C, Sha W C, Scott M L, Baltimore D. Sequential induction of NF-κB/Rel family proteins during B-cell terminal differentiation. Mol Cell Biol. 1994;14:5349–5359. doi: 10.1128/mcb.14.8.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKinsey T A, Brockman J A, Scherer D C, Al-Murrani S W, Green P L, Ballard D W. Inactivation of IκBβ by the tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-κB. Mol Cell Biol. 1996;16:2083–90. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehdi S. Cell-penetrating inhibitors of calpain. Trends Biochem Sci. 1991;16:150–153. doi: 10.1016/0968-0004(91)90058-4. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto S, Chiao P J, Verma I M. Enhanced IκBα degradation is responsible for constitutive NF-κB activity in mature murine B-cell lines. Mol Cell Biol. 1994;14:3276–3282. doi: 10.1128/mcb.14.5.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto S, Maki M, Schmitt M J, Hatanaka M, Verma I M. Tumor necrosis factor α-induced phosphorylation of IκBα is a signal for its degradation but not dissociation from NF-κB. Proc Natl Acad Sci USA. 1994;91:12740–12744. doi: 10.1073/pnas.91.26.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto S, Schmitt M J, Verma I M. Qualitative changes in the subunit composition of κB-binding complexes during murine B-cell differentiation. Proc Natl Acad Sci USA. 1994;91:5056–5060. doi: 10.1073/pnas.91.11.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto S, Verma I M. Rel/NF-κB/IκB story. Adv Cancer Res. 1995;66:255–292. [PubMed] [Google Scholar]
- 38.Naviaux R K, Costanzi E, Haas M, Verma I M. The pCL vector system-rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palombella V J, Rando O J, Goldberg A L, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 40.Phillips R J, Ghosh S. Regulation of IκBβ in WEHI231 mature B cells. Mol Cell Biol. 1997;17:4390–4396. doi: 10.1128/mcb.17.8.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rechsteiner M, Rogers S W. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 42.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez M S, Wright J, Thompson J, Thomas D, Baleux F, Virelizier J L, Hay R T, Arenzanaseisdedos F. Identification of lysine residues required for signal-induced ubiquitination and degradtion of IκBα in vivo. Oncogene. 1996;12:2425–2435. [PubMed] [Google Scholar]
- 44.Saido T C, Sorimachi H, Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994;8:814–822. [PubMed] [Google Scholar]
- 45.Scherer D C, Brockman J A, Bendall H H, Zhang G M, Ballard D W, Oltz E M. Corepression of RelA and c-rel inhibits immunoglobulin κ gene transcription and rearrangement in precursor B lymphocytes. Immunity. 1996;5:563–574. doi: 10.1016/s1074-7613(00)80271-x. [DOI] [PubMed] [Google Scholar]
- 46.Schreck R, Baeuerle P A. Assessing oxygen radicals as mediators in activation of inducible eukaryotic transcription factor NF-κB. Methods Enzymol. 1994;234:151–163. doi: 10.1016/0076-6879(94)34085-4. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz E M, VanAntwerp D, Verma I M. Constitutive phosphorylation of IκBα by casein kinase II occurs preferentially at serine 293: requirement for degradation of free IκBα. Mol Cell Biol. 1996;16:3554–3559. doi: 10.1128/mcb.16.7.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 49.Sha W C, Liou H C, Tuomanen E I, Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 50.Snapper C M, Rosas F R, Zelazowski P, Moorman M A, Kehry M R, Bravo R, Weih F. B cells lacking RelB are defective in proliferative responses, but undergo normal B cell maturation to Ig secretion and Ig class switching. J Exp Med. 1996;184:1537–1541. doi: 10.1084/jem.184.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun S C, Ganchi P A, Ballard D W, Greene W C. NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 52.Suyang H, Phillips R, Douglas I, Ghosh S. Role of unphosphorylated, newly synthesized IκBβ in persistent activation of NF-κB. Mol Cell Biol. 1996;16:5444–5449. doi: 10.1128/mcb.16.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ten R M, Paya C V, Israel N, Le Bail O, Mattei M G, Virelizier J L, Kourilsky P, Israel A. The characterization of the promoter of the gene encoding the p50 subunit of NF-κB indicates that it participates in its own regulation. EMBO J. 1992;11:195–203. doi: 10.1002/j.1460-2075.1992.tb05042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. IκBβ regulates the persistent response in a biphasic activation of NF-κB. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 55.Tsien R Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981;290:527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- 56.VanAntwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNFα-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 57.VanAntwerp D J, Verma I M. Signal-induced degradation of IκBα: association with NF-κB and the PEST sequence in IκBα are not required. Mol Cell Biol. 1996;16:6037–6045. doi: 10.1128/mcb.16.11.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 59.Wang C Y, Mayo M W, Baldwin A S. TNF- and cancer therapy-induced apoptosis—potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 60.Whiteside S T, Epinat J-C, Rice N R, Israel A. IκBɛ, a novel member of the IκB family, controls RelA and cRel NF-κB activity. EMBO J. 1997;16:1413–1426. doi: 10.1093/emboj/16.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]