Abstract
Pregnant women are vulnerable to folate deficiency as its requirement is substantially greater than folate requirements for non-pregnant women. Folic acid is a synthetic form of folate and has been used in the fortified foods and nutritional supplements. Since the 1990s, maternal folic acid supplementation has been adopted by the governments and health organizations around the world as the policy to prevent the birth defects, especially neural tube defects. Under the promotion of folic acid supplementation, however, the global prevalence of congenital heart disease continues to be increased. In the recent years, our research group has evaluated that the heterogeneity concerning the association between folic acid supplementation and congenital heart disease is high. Based on experiments with animal models such as zebrafish and mice, we have demonstrated that excessive folic acid supplementation led to cardiovascular development disorders and even early embryo death. In this review article, we first summarize the discovery of folic acid and the achievement of folic acid supplementation in the prevention of congenital diseases. We then discuss the transport and metabolism of folic acid particularly in the form of unmetabolized folic acid. Finally, we comment on the association of folic acid supplementation with congenital heart disease. Better understanding the dual character of folic acid supplementation on congenital heart disease may provide new insights into the potential role of folic acid and offer a fresh perspective on the prevention of congenital heart disease.
Keywords: Congenital heart disease, Folic acid, Metabolism, Neural tube defects, 6S-5-methyltetrahydrofolate-calcium
Graphical abstract
Highlights
-
•
Maternal folic acid (FA) supplementation has been adopted by the governments and health organizations in the world as the policy to prevent the birth defects, especially neural tube defects.
-
•
Under the promotion of FA supplementation, the global prevalence of congenital heart disease continues to be increased.
-
•
Evidence has demonstrated that excessive FA supplementation led to cardiovascular development disorders and even early embryo death based upon the experiments with animal models such as zebrafish and mice.
-
•
This review is focused on discussion of the transport and metabolism of FA particularly in the form of unmetabolized FA and the association of FA supplementation with congenital heart disease.
Abbreviations
- Angpt2:
angiopoietin 2
- Arnt:
aryl hydrocarbon receptor nuclear translocator
- AV:
atrioventricular
- CHD:
congenital heart disease
- DHFR:
dihydrofolate reductase
- Epas1:
endothelial PAS domain protein 1
- FA:
folic acid
- FR:
folate receptor
- L-5-MTHF-Ca:
6S-5-methyltetrahydrofolate-calcium
- 5-MTHF:
5-methyltetrahydrofolate
- MAIT:
Mucosal-associated invariant T
- MR1:
complex-related molecule 1
- MTHFD1:
formyltetrahydrofolate synthetase 1
- MTHFR:
5,10-methylenetetrahydrofolate reductase
- NK:
Natural killer
- NTD:
neural tube defects
- UMFA:
unmetabolized folic acid
- RFC:
reduced folate carrier
- PCFT:
proton-coupled folate transporter
- PE:
pre-eclampsia
- Prmt6:
protein arginine N-methyltransferase 6
- Sphk2:
sphingosine kinase 2
- Spns2:
spinster homolog 2
- S1P:
sphingosine-1-phosphate
- 6- FP:
6-formylpterin
- Hcy:
homocysteine
1. Introduction
The significance of sufficient folate consumption during pregnancy, lactation, and infancy for the well-being of both mothers and infants is unquestionable (Lamers, 2011). Folate and folic acid (FA) are different forms of vitamin B9, with the latter being a synthetic form of folate incorporated into fortified foods and nutritional supplements. Pregnant women are particularly susceptible to folate deficiency due to their significantly higher requirement compared to non-pregnant women. Currently, the prevention of birth defects, especially neural tube defects (NTD), is the main clinical use of FA. Since the 1990s, governments and health organizations worldwide have adopted the policies designed to increase the intake of FA for women prior to and during pregnancy to reduce the risk of birth defects (Jouanne et al., 2021; van Gool et al., 2018). Under the promotion of FA fortification of food and maternal FA supplementation policies, however, the global prevalence of congenital heart disease (CHD) persists in its upward trajectory (Liu et al., 2019). Although clinical observation has demonstrated that low maternal folate levels may increase the risk of CHD, the relationship between FA and CHD is controversial (Obeid et al., 2019; Cheng et al., 2022; Ruan et al., 2024; Qu et al., 2024). CHD, characterized by cardiovascular function and structure abnormalities, is the most common type of congenital malformation and is gaining recognition as a significant global public health issue. Therefore, understanding whether maternal periconceptional FA or higher doses of FA reduce CHD is of utmost importance.
In recent years, our research group has focused on investigation of the correlation between FA supplementation and CHD. First, we conducted a meta-analysis to evaluate the data of previous epidemiological studies and found that high heterogeneity in these studies concerning the association between maternal FA supplementation and the risk of CHD (Cheng et al., 2022). We then conducted the animal model experiments utilizing zebrafish and mice, wherein we observed that excessive FA supplementation resulted in the manifestation of cardiovascular developmental disorders and premature embryo mortality (Lian et al., 2022). It is worth noting that many researchers have not considered the effects of FA and natural dietary folates on the differences in metabolism. At higher intakes, FA fails to undergo conversion into its active form and instead accumulates in plasma as unmetabolized folic acid (UMFA). In this review, we first summarize the discovery of FA and the achievement of FA supplementation in the prevention of congenital diseases. Subsequently, we put the focus shifts towards elucidating the mechanisms underlying FA transport and metabolism, with particular emphasis on its impact on cardiovascular development. Lastly, we comment on the association of FA supplementation with CHD. By gaining a deeper comprehension of dual character of FA supplementation on CHD, novel perspectives on the preventive role of FA may emerge, thereby contributing to better understanding of CHD prevention.
2. Folic acid
Natural folates and FA possess the same vitamin activity, while their names are easily confused. Dr. Lucy Wills discovered a factor in yeast that corrected macrocytic anemia of pregnancy in 1931, and this unknown substance was later named “Wills factor”, and “Wills factor” was shown to be found in a different fraction of liver extract from which was curative for pernicious anemia (Wills, 1931; Watson and Castle, 1946). Subsequently, it was determined that yeast or yeast extract, known as “Marmite,” could prevent nutritional cytopenias in monkeys, where the factor was named vitamin M (Day et al., 1938; Langston et al., 1938). In 1941, this factor received its name “folic acid” when it was isolated from spinach (Mitchell et al., 1941). In 1945, FA was synthesized by Lederle laboratories that is composed of pterin, p-aminobenzoyl, and L-glutamic acid (Angier et al., 1945). Soon after its synthesis, it was recognized that the structure of naturally occurring folates typically varied from this compound (Table 1) (Pfiffner and Calkins, 1946). Due to the convenience and stability of newly synthesized FA, many scholars use this material for research and the name FA eventually came into wide usage. FA is now used to denote the fully oxidized synthetic compounds used in supplements and for food fortification. Whereas folate is a substance (or class of substances) that is documented occurrence in certain leafy vegetables and liver, as well as various other biological materials, for example, spinach (Fig. 1).
Table 1.
The discovery and application of folic acid.
| Date | Description | References |
|---|---|---|
| 1931 | Wills&Mehta found that yeast extract prevents dietary anemia in rats and named it ‘Wills’ factor’ | (Wills, 1931), (Watson and Castle, 1946) |
| 1938 | Yeast and liver extracts are effective in reducing monkey vegetative cells. This factor is named vitamin M | (Day et al., 1938), (Langston et al., 1938) |
| 1941 | ‘FA’ receives its name and shown to be a growth factor for Streptococcus lactis R (S. faecalis) | Mitchell et al. (1941) |
| 1945 | Synthesis of FA and called pteroylglutamic acid | Angier et al. (1945) |
| 1946 | Naturally occurring folate in liver is a heptaglutamate | Pfiffner and Calkins (1946) |
| 1964 | folate deficiency and NTD occurrence was hypothesized | Hibbard (1964) |
| 1991 | The effectiveness of FA supplementation to prevent the recurrence of NTD was determined | MRC Vitamin Study Research Group (1991) |
| 1992 | Prevention by FA of a first occurrence of NTD was proven | Czeizel and Dudás (1992) |
| 1998 | In the United States and Canada, mandatory fortification of enriched cereal grain products with FA was implemented | Crider et al. (2011a) |
| 1999 | Reduction of the risk in NTD by 0.4 mg FA was proven by China-US Collaborative Project | Berry et al. (1999) |
| 2009 | The Chinese government provides free FA for rural planned pregnant women | Ren (2015) |
| 2010 | Regulations for mandatory fortification of wheat flour with FA have been in place in 53 countries | Crider et al. (2011a) |
| 2025 | 94 countries have mandates that require wheat flour, maize flour, and/or rice to be fortified with micronutrients | Food fortification initiative (2025) |
Fig. 1.
Differences between folic acid (A) and natural dietary folate (B) chemical structure.
Numerous aspects of folate metabolism such as the biochemical reactions involving folates in single carbon unit transfer in amino acid conversions including homocysteine (Hcy) conversion to methionine and in purine and pyrimidine synthesis have been elucidated (Ducker and Rabinowitz, 2017). Folate plays a crucial role in facilitating rapid cell division and growth, making it essential for embryonic and fetal development (Marangoni et al., 2016; Cawley et al., 2016). Humans depend entirely on dietary intake for folate, but many staple crops like potatoes, rice, cassava, and corn are low in folates (Blancquaert et al., 2014). There is a potential gap between theoretical recommendations for folate in literature and benefits of application in clinical practice. The majority of current understandings regarding the effectiveness of folate therapy are based on studies using synthetic FA. The unfavorable outcomes or lack of effectiveness observed in these trials regarding folate may not be solely attributed to the incorrect theoretical efficacy, but rather to limitations in the form of FA.
3. Folic acid and birth defects prevention
Neural tube defects (NTD) are severe structural birth defects affecting offspring mortality and morbidity that result from a failure of embryonic neural tube closure. Failure to complete low spinal closure can lead to spina bifida, and incomplete cranial closure causes anencephaly. To prevent the occurrence of this malignant disorder, enhancement of FA supplementation has been undertaken. Evidence has demonstrated that FA supplementation plays an important role in preventing NTD. The Medical Research Council (MRC) trial and Czeizel & Dudas have successively demonstrated that FA supplementation prevents the recurrence and first occurrence of NTD (MRC Vitamin Study Research Group, 1991; Czeizel and Dudás, 1992). Reduction of the risk in NTD by 0.4 mg FA was subsequently proven by China-US Collaborative Project involving more than 200,000 Chinese women (Berry et al., 1999). Subsequently, two main approaches have been implemented by public health agencies around the world: (1) fortification, whereby FA intake is artificially increased by adding FA to cereals and (2) information provision and providing FA pill supplementation to perinatal women. Since 1998, the United States and Canada have implemented mandatory fortification of enriched cereal grain products with FA (Ray, 2004). Afterward, over 90 countries currently have mandatory wheat and/or maize flour fortification legislation, including FA (Food fortification initiative, 2025). In China, FA supplements have been promoted, and the government provides all women who have a rural household registration and who plan to become pregnant with FA supplements, free of charge, through a nation-wide program started in 2009 (Ren, 2015).
The incidence of spina bifida and anencephaly in the United States and Canada has experienced a significant decrease following the fortification of enriched grain products with FA. This fortification initiative, aimed at reducing neural tube defects (NTD), is widely regarded as one of the most successful public health endeavors of the past 50–75 years (Crider et al., 2011a). NTD is reduced by 46 % in Canada and 19 % in the United States after FA fortification (De Wals et al., 2007; Honein et al., 2001). Currently, after an immediate initial decline, the prevalence of NTD reported in the United States after the implementation of mandatory FA fortification has been relatively flat for more than a decade after implementation (Williams et al., 2015). China has one of the highest reported birth prevalences of NTD in the world (Li et al., 2006). Since 2009, local maternal health care workers are responsible for the distribution of the supplements free of charge. Nowadays, the vast majority of pregnant women report having taken FA supplements (Cui et al., 2021). The incidence of certain major birth defects has exhibited a declining trend over the years (Fig. 2). For example, the incidence of perinatal neural tube defects decreased from 27.4 per 10,000 in 1987 to about 1.5 per 10,000 in 2018, a decrease of 94.5 % (National Health Commission of the People's Republic of China, 2019; Xu et al., 2020). Because the beneficial effects were so pronounced, health authorities in many countries recommended FA supplementation for pregnant women, a decline in severe fetal CHD in populations worldwide has been seen, but a rise in total CHD.
Fig. 2.
The incidence of congenital heart defects and neural tube defects in China, 1996–2019.
After providing free folic acid supplements to women planning pregnancy in rural areas of China (dashed gray line), the incidence of NTD has been significantly decreased, while the incidence of CHD increased.
4. Folic acid supplementation and congenital heart disease
Congenital heart disease (CHD) is the most common type of congenital malformation. Generally, FA supplementation is considered advantageous in the prevention of CHD. In 1959, Warkany et al. reported that the use of FA antagonists increased the risk of CHD in infants (Warkany et al., 1959). On the other hand, the presence of maternal MTHFR C677T polymorphism contributes to the risk of fetal CHD (Wang et al., 2013). However, with the advancement of the FA supplement policy in China, the overall prevalence of birth defects has not been effectively controlled as anticipated, and there has been a puzzling increase in the incidence of CHD (Fig. 2), rising from 40.95/10,000 in 2011 to 126.62/10,000 in 2019 (Xu et al., 2020; Zhao et al., 2020). Case-control studies (Bean et al., 2011; Li et al., 2013a; Qu et al., 2020; Kolmaga et al., 2024) and cohort studies (Mao et al., 2017; Wang et al., 2022) suggest maternal FA supplementation in the periconceptional period reduces the risk of CHD, but there are also irrelevant reports (Ruan et al., 2024; Qu et al., 2024; Øyen et al., 2019; Bedard et al., 2013). We do not question the effectiveness of FA interventions to reduce the risk of severe CHD. However, there is a concern that FA supplementation may not decrease, but rather increase, the overall incidence of CHD (Bedard et al., 2013; Lamichhane et al., 2016; Egbe et al., 2015). Some scholars suggested that the reason for the lack of significant effect is that FA should be supplemented more regularly prior to pregnancy (Qu et al., 2020; Kolmaga et al., 2024; Chen et al., 2022). Nevertheless, the magnitude of the observed discrepancies with the NTD trend remains unexplained. It is important to confirm the preventive effect of FA on CHD, as the incidence of CHD is considerably higher than that of NTD and can have a significant impact on public health.
The effects of FA on cardiac development in animal models are summarized in Table 2. FA supplementation prevents early exposures in mice, including alcohol, lithium from interfering with Wnt/β-linked protein signaling, which allows normal gene activation and cardiogenesis (Han et al., 2009; Serrano et al., 2010; Linask and Huhta, 2010; Yue et al., 2017), and was also found to be effective in preventing CHD caused by vitamin A-related metabolic imbalances (Amati et al., 2010; Cipollone et al., 2009). Early arsenic exposure may also induce alterations in NKX2.5, GATA4, and TBX5 gene expression, and FA is also able to mitigate this damage (Lin et al., 2018). In addition to Wnt signaling changes in lipid metabolism and changes in DNA methylation may also be a consequence of early embryonic risk exposure, and FA supplementation may likewise ameliorate these changes (Han et al., 2016; Jiang et al., 2019). However, FA has been found to harm cardiac development at high doses, and although it was shown to reduce the cardiac defects induced by extractable organic matter in PM2.5 in zebrafish, concentrations increased to 0.2 μM were shown to increase the incidence of defects (Yue et al., 2017). 10-fold of recommended daily intake of FA in mice results in embryonic loss and embryonic delay and affects cardiac development (increased septal defects and reduced ventricular wall thickness) (Pickell et al., 2011; Mikael et al., 2013), and in zebrafish excess FA also leads to atrial and ventricular stretching of embryo (Han et al., 2021). Recent experiments conducted by our team have provided evidence that FA but not L-5-Methyltetrahydrofolate calcium (L-5-MTHF-Ca) inhibited the angiogenesis in zebrafish and resulted in abnormal cardiovascular development leading to embryonic death owing to the downregulation of eif1axb (Lian et al., 2022). Show that studies related to folate-associated health outcomes cannot be based upon the premise that FA possesses an identical biological function as L-5-MTHF. The existence of a secure threshold of exposure to FA in humans, below which its detrimental effects cease, remains uncertain. The dihydrofolate reductase activity in liver of humans is low and variable (Bailey and Ayling, 2009).
Table 2.
Effects of folic acid supplementation on cardiac development in offspring of various animal models.
| Animal | Teratogen | Folic aicd dose | Outcome | Literature |
|---|---|---|---|---|
| Mice | Retinoic acid competitive antagonist | 4 mg/kg(i.p) | + | Amati et al. (2010) |
| Mice | Retinoic acid competitive antagonist | 4 mg/kg(i.p) | + | Cipollone et al. (2009) |
| Mice | Alcohol | 10.5 mg/kg (high dose) or 6.2 mg/kg (medium dose) FA supplementation diet | + | Serrano et al. (2010) |
| Mice | Li | 10.5 mg/kg FA supplemented diet | + | Han et al. (2009) |
| Mice | Li | 10.5 mg/kg FA supplemented diet | + | Han et al. (2016) |
| Mice | HCY | 10.5 mg/kg FA supplemented diet | + | Serrano et al. (2010) |
| Mice | HCY | 10.5 mg/kg FA supplemented diet | + | Han et al. (2016) |
| Mice | - | 40 mg/kg FA supplementation diet | - | Pickell et al. (2011) |
| Mice | - | 20 mg/kg FA supplementation diet | - | Mikael et al. (2013) |
| Rat | Arsenic | 0.53 mg/kg,5.3 mg/kg,10.6 mg/kg of FA supplementation diet | + | Lin et al. (2018) |
| Rat | Arsenic | 0.53 mg/kg,5.3 mg/kg,10.6 mg/kg of FA supplementation diet | + | Na et al. (2020) |
| Quail | Alcohol | 3.2 μg each egg | + | Ford et al. (2021) |
| Quail | Alcohol | 10 μg/mL added to the medium | + | Serrano et al. (2010) |
| Chick | Li | 10 μg/mL added to the medium | + | Han et al. (2009) |
| Chick | HCY | 10 μg/mL added to the medium | + | Han et al. (2009) |
| Zebrafish | PM2.5 organic extract | 0.02、0.05、0.2 μM | +/− | Yue et al. (2017) |
| Zebrafish | Selenite | 0.01、0.1、1 mM | + | Ma et al. (2012) |
| Zebrafish | - | 1、2、3、4、5 mM | - | Han et al. (2021) |
| Zebrafish | - | 0.25 mM, 0.5 mM, 1 mM, 2.5 mM, 5 mM | - | Lian et al. (2022) |
FA: folic acid; Bolded indicates the presence of negative effects and their doses; +No. means reducing CHD risk, while -No. means increasing CHD risk.
5. Folic acid transport and metabolism
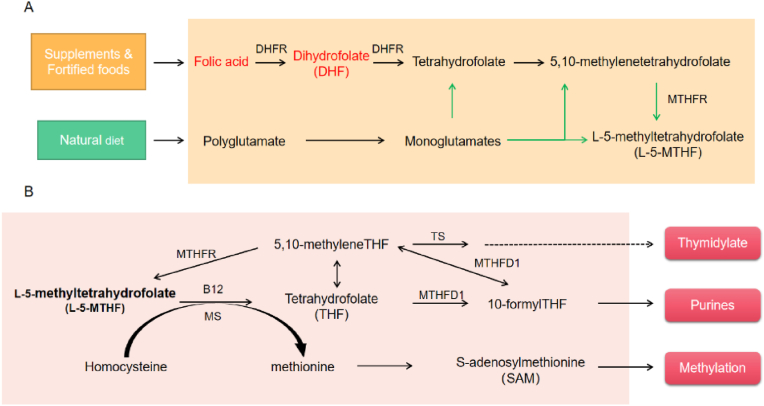
FA does not occur in nature and is rarely found in unfortified foods, which is the most common form of folate in fortified foods, dietary supplements, and drugs because it is more stable. FA and monoglutamate-folate are absorbed in the proximal small intestine through the proton-coupled folate transporter (PCFT), a saturable transporter that exhibits comparable efficiency in transporting oxidized and reduced folate (Pietrzik et al., 2010). Following absorption, the assimilated FA undergoes biotransformation within the intestinal mucosa and is subsequently translocated through the mesenteric vein to the hepatic portal vein, facilitating its transportation to the liver (Rogers et al., 1997; Ohrvik and Witthoft, 2011). After ingestion, this water-soluble vitamin is converted by dihydrofolate reductase (DHFR) to the dihydrofolate and then to the tetrahydrofolate, and it is then metabolized via serine hydroxymethyltransferase and 5,10-methylenetetrahydrofolate reductase (MTHFR) to L-5-methyltetrahydrofolate(L-5-MTHF), and finally come into the peripheral blood circulation (Fig. 3A) (Scaglione and Panzavolta, 2014). Initially, there was a prevalent belief that physiological doses of FA underwent reduction and methylation within the intestinal epithelium, resulting in the exportation of L-5-MTHF to the hepatic portal vein, in the same way as dietary folates (Tani and Iwai, 1983; Perry and Chanarin, 1970). However, recent findings have refuted this hypothesis, indicating that the typical oral dosage of FA is not entirely converted into L-5-MTHF within the human intestine. Instead, a portion of it enters the hepatic portal vein in its original form (Patanwala et al., 2014). High FA intake can overwhelm enterocyte and/or liver metabolism, leading to the presence of UMFA in the circulation.
Fig. 3.
Intestinal absorption and metabolism of natural folate and folic acid.
(A) Folate is available in the form of FA from supplementation and fortification or tetrahydrofolate derivatives (for example, L-5-MTHF) from natural sources. FA must be reduced to tetrahydrofolate in a two-step reaction by DHFA. Folates in natural diet, mostly polyglutamates, are hydrolyzed to monoglutamates by gamma-glutamyl hydrolase in the gut. Both food folates and FA are ultimately metabolized to L-5-MTHF by MTHFR. (B) After metabolism, it finally enters circulation in the form of L-5-MTHF. In cells, L-5-MTHF is converted to tetrahydrofolate (THF) via the action of the methionine synthase (MS) which requires vitamin B12 and, via S-adenosylmethionine (SAM). Upon accepting a one-carbon group (from formate, or from amino acids like serine, glycine, and histidine) THF is converted to other forms of folate, such as 10-formyl-THF and 5,10-methylene-THF, implicated in the biosynthesis of nucleotides, such as thymidine monophosphate (TMP) synthesized via thymidylate synthase (TS).
Defective or impaired folate transport or metabolism can hinder the expected preventive effect of FA; these include MTHFR, DHFR, formyltetrahydrofolate synthetase 1 (MTHFD1), and folate transport proteins (Fig. 3B). The major product of the MTHFR gene is a catalytically active 77 KDa protein that catalyzes the conversion of 5,10-methylenetetrahydrofolate into L-5-methyltetrahydrofolate, the major circulation form of folate. Two common genetic polymorphisms associated with reduced MTHFR activity have been identified, one of which is the MTHFR C677T mutation at exon 4 resulted in a partial reduction of thermostability, and the other is the MTHFRA1298C mutation, which leads to reduced enzyme activity in vitro (Petrone et al., 2021; Jacques et al., 1996). A double-blind trial showed that serum folate and erythrocyte folate levels were lower in the MTHFR677TT genotype than in the CC and CT genotype at the same FA dose (Crider et al., 2011b). Excessive intake of FA (10-fold of recommended daily) has been shown to result in decreased MTHFR protein levels as well as production of a less active form of MTHFR in liver (Christensen et al., 2015a). Several meta-analyses have shown that MTHFR polymorphisms are associated with a significantly increased risk of fetal CHD (Xuan et al., 2014; Wang et al., 2013; Yu et al., 2017; Li et al., 2015; Zhang et al., 2018). Indeed, maternal, but not embryonic, MTHFR deficiency increases the risk of CHD in mice (Li et al., 2005). This suggests that getting adequate L-5-MTHF from the mother may be more critical.
MTHFD1 is the gene for the methylenetetrahydrofolate dehydrogenase, cyclohydrolase and formyltetrahydrofolate synthetase 1 (MTHFD1) trifunctional enzyme, which were the pivotal enzymes in the folate one-carbon metabolic cycle (Fox and Stover, 2008). A common variant of MTHFD1(1958G→A) may reduce enzyme stability and increase the risk of congenital heart disease (Yi et al., 2025; Christensen et al., 2015b). 10 mg/kg FA supplemented diet (5-fold of recommended daily) in female Mthfd1S+/− mice also found a similar pseudo-MTHFR deficiency, resulting in lower MTHFR protein and higher MTHFR phosphorylation, and interaction between high FA intake and Mthfd1S genotype increased the incidence of developmental defects (Christensen et al., 2016). The incidence of defects was similarly increased by a low intake of FA in this model (Christensen et al., 2018). The metabolic block in purine synthesis that is caused by synthetase deficiency is not mitigated by increasing the available FA. On the contrary, the high dose of FA exacerbates the effects of synthetase deficiency.
In order to be utilized, FA must undergo conversion into biologically useable forms of folate by DHFR which plays a crucial role in FA metabolism. Suppression of DHFR activity has been observed to lead to the occurrence of cardiac malformations (Sun et al., 2007, 2011; Gong et al., 2021). In contrast to mice, humans are less adapted to tolerate high levels of FA (Bailey and Ayling, 2009). It has been known for some time that oral FA above 200 μg results in the appearance of UMFA in serum (Chen et al., 2023). Low enzyme activity of DHFR may compromise both mucosal and hepatic biotransformation of FA in humans. The human DHFR gene has a 19-bp deletion polymorphism in the intron 1 sequence, which is very common (Hayashi et al., 2010; Philip et al., 2015), and the proportion of del/del genotypes has been reported to be significantly higher in East Asian populations than in European-American populations (Wu et al., 2022), implying that the potential role of UMFA is higher for East Asian populations than for Caucasians. Additionally, it is worth noting that UMFA exposures to the fetus can occur through the placenta due to the circulation of maternal blood.
Placental and fetal development involves complex molecular events like proliferation, migration, and invasion of placental trophoblasts. To meet the high demand for folate during the first trimester to provide for cell division and growth of fetus the placental folate levels are very high (Mohanraj et al., 2019). It is essential to understand potential interactions with folate delivery pathways, since it is the mother who provides fetal folate requirements, through the placenta. Transplacental folate delivery involves the coordinated activity of three specific folate transport mechanisms: the folate receptor isoforms α (FRα) and β (FRβ), the reduced folate carrier (RFC), and PCFT. There are four human FR cDNA isoforms (α, β, γ, and δ); FRα is crucial and is highly expressed in the placenta, where it exerts essential functions (Strandgaard et al., 2017). PCFT is a proton/folate cotransport protein that exhibits a low affinity for folate in the placental environment due to its pH dependency (Zhao et al., 2011). Finally, RFC is a bidirectional transporter protein that is expressed on both apical and basolateral membranes, which further facilitates folate transport across the placenta (Solanky et al., 2010). Functioning as an anion exchanger, the RFC exhibits a high affinity for L-5-MTHF and a low affinity for FA, operating optimally at a physiologic pH (Matherly et al., 2007). FRα, essential for maternal-to-fetal folate transport during embryonic development, is demonstrated by the early embryonic lethality observed in FRα knockout mice (Piedrahita et al., 1999). The deficiency of FRα has been found to have a detrimental effect on the migration of neural crest cells. This impaired folate transportation results in apoptosis-mediated cell death and also causes alterations in gene expression in construal development, ultimately contributing to the development of CHD (Tang et al., 2004; Zhu et al., 2007). It is worth noting that FA and 5-MTHF have varying affinities for different folate transport carriers, with FR family members exhibiting a 6- to 10-fold higher binding affinity for FA compared to 5-methyltetrahydrofolate (KD = 10−10-10−11 M) (Maruvada et al., 2020). In addition, the presence of FA in endothelial cells appears to competitively inhibit 5-MTHF uptake (Smith et al., 2017). Maternal circulating UMFA may be captured by FRα on the maternally facing chorionic surface, a process favored by a high affinity of FRα for the UMFA. Following the administered of intraperitoneal biotin-conjugate FA, a rapid transport and accumulation of FA in the placenta and subsequent distribution to the fetal cardiac tissue were observed (Bobrowski-Khoury et al., 2022). Thus, S-adenosylmethionine (SAM) was significantly elevated in the plasma of mice on a high-FA diet, while showing a significantly reduced in embryonic liver as well as in the placenta (Luan et al., 2021).
6. Anti-angiogenesis and congenital heart disease
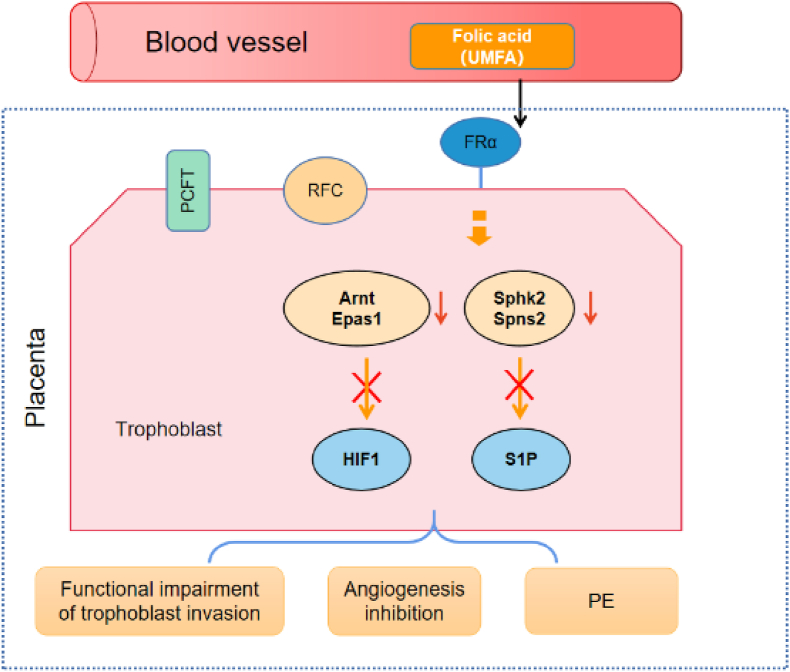
The investigation into the impact of FA on placental angiogenesis may help us in understanding the effects on CHD from another point of view. It is known that placental folate levels in patients with pre-eclampsia (PE) are significantly lower than those in normal patients (Mohanraj et al., 2019). Of note is that 4 mg/d of FA not only did not prevent PE but potentially heightened the risk (Wen et al., 2018). Inadequate trophoblast invasion, followed by abnormalities in the development of placental vasculature and resultant placental under perfusion has been implicated in the pathology of PE (Young et al., 2010). FA has been shown to have a pro-angiogenic effect (Sasaki et al., 2003), whereas in vitro it inhibits angiogenesis in contrast (Lin et al., 2012; Hou et al., 2013). A recent study showed that excessive FA intake during pregnancy induces alterations in placental metabolism and gene expression, particularly in angiogenesis (Fig. 4) (Luan et al., 2021). FA over-supplementation decreases sphingosine kinase 2 (Sphk2) and spinster homolog 2 (Spns2) while upregulating protein arginine N-methyltransferase 6 (Prmt6) and angiopoietin 2 (Angpt2) gene expression. Furthermore, the expression levels of aryl hydrocarbon receptor nuclear translocator (Arnt) and endothelial PAS domain protein 1(Epas1) decrease significantly or tendency to decrease in high FA diet. HIF-1, composed of the Arnt bHLH-PAS proteins and HIF1α,is critical for normal placental development. Placentas lacking Arnt (Arnt−/−) displayed impaired vascularization and compromised maintenance of spongiotrophoblast cells, resulting in underdeveloped endocardial cushions. These cushions are responsible for lining the atrioventricular (AV) canal and eventually developing into the leaflets of the AV valves and septum (Adelman et al., 2000). Spns2 and Sphk2 are involved in sphingosine-1-phosphate (S1P) signaling that promotes proliferation, migration, and angiogenesis, while the S1P synthetic pathway could be involved in extra villous trophoblast migration and spiral artery remodeling during pregnancy (Patanapirunhakit et al., 2021). Certain mechanisms, such as the release of estrogen, counteract the anti-angiogenic effects of FA in the placenta (Lee et al., 2018). Perturbations in estrogen levels and the dysregulation of enzymes involved in estrogen biosynthesis have been observed in cases of PE (Berkane et al., 2017). The high-dose FA or UMFA induces an imbalance between angiogenic and antiangiogenic factors, potentially resulting in an elevated risk of PE. Placental insufficiency may have an effect on heart development (Matthiesen et al., 2016). Further studies suggest that PE may significantly increase the risk of atrial septal defect (ASD) (Boyd et al., 2017; Zhang et al., 2022). Given the common developmental window and common molecular pathways of the heart and placenta (Courtney et al., 2018), it is necessary to confirm the association of placental effects due to FA with cardiac development.
Fig. 4.
High-dose folic acid and its un-metabolized form alter the placental angiogenic gene expression.
In mice, excess FA down-regulates the Arnt, Epas1, Sphk2, and Spns2 gene expression, and subsequently inhibits the HIF1 and S1P signaling pathways. UMFA in circulation is transported into the trophoblast cell primarily by FRα but no PCFT and RFC. Arnt, Epas1, Sphk2 and Spns2 expession are dramatically down-regulated in placental cells of mice on high FA diet. Arnt is a known heterodimerization partner of HIF-1α to form an active HIF-1 complex. Epas1, also known as HIF-2α,is closely related to HIF-1α in structure and is likewise activated during hypoxia. Nuclear Sphk2 generates S1P that regulates histone acetylation. Spns2 has been identied as an S1P transporter. HIF-1 and S1P have been implicated in angiogenesis.
Our research group has carried out the experiments to explore the potential effects of FA on early embryonic development. We found that FA but not L-5-MTHF suppressed early expression of the eif1axb gene in zebrafish embryos. Subsequently, the knockdown of the eif1axb gene resulted in abnormal cardiovascular development, particularly vascular development (Lian et al., 2022). A significant reduction in the incidence of overall PE, severe PE, and early-onset PE has been reported in women with prior PE taking oral 5-MTHF 15 mg daily (Saccone et al., 2016). Recent studies indicate that L-5-MTHF, but not FA, can prevents pregnancy-induced hypertension and intrauterine growth restriction in mice and restore BH4 levels and NOS activity in endothelial cells from women with pregnancy-induced hypertension (Dickinson et al., 2024). Therefore, it is hypothesized that the ineffectiveness of FA in preventing CHD may be attributed to the presence of various forms of folate (Fig. 5). In contrast to FA that does not occur in foods in significant amounts, L-5-MTHF is the predominant natural form of folates. It is also the essential form in which folates occur and are stored in the human body. The concentration of folate (primarily 5-MTHF) in the bloodstream is estimated to be around 5–30 nM (Crider et al., 2019), whereas the intracellular folate pool in placental tissue is considerably higher, surpassing the magnitude of the former by several orders (Bobrowski-Khoury et al., 2022; Stark et al., 2021). In addition, unlike synthetic FA, studies have demonstrated that L-5-MTHF does not exhibit embryotoxic or teratogenic effects on rat embryos even at a high dose of 1000 mg/kg (European Food Safety Authority (EFSA), 2004). Therefore, naturally occurring L-5-MTHF has important advantages over synthetic FA. Using L-5-MTHF instead of FA overcomes metabolic defects caused by MTHFR polymorphism, and subsequently reduces the potential for pseudo-MTHFR deficiency.
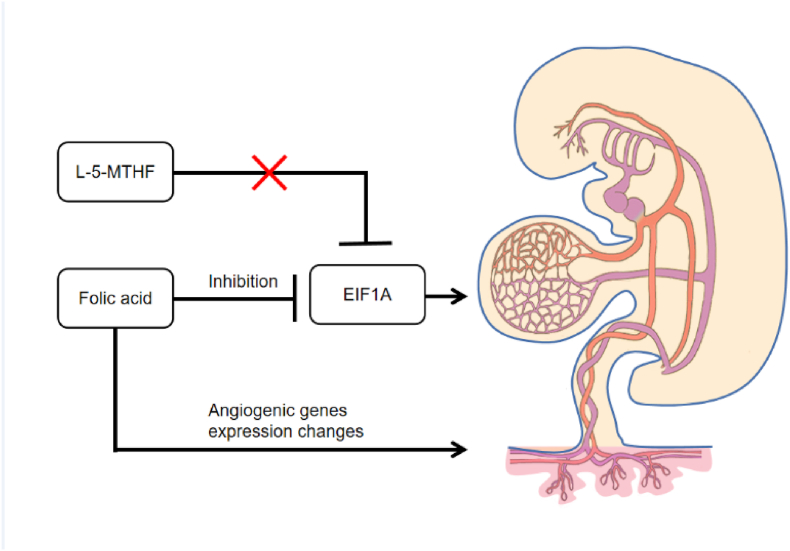
Fig. 5.
A supposed mechanism of folic acid causing congenital heart disease.
FA but not L-5-MTHF exposure in zebrafish embryos inhibits eif1axb gene expression and induces vascular developmental disorders. A zebrafish of eif1axb knockdown also showed suppressed angiogenesis and vascular developmental malformations. The zebrafish gene eif1axb is orthologues of human EIF1A. Since the gene functions are typically conserved between orthologs, We hypothesized that FA might affect the gene expression in humans. Human embryonic heart begins beating around 21 days postfertilization, it is subject to the pulsatile pressures and flows generated by its own pumping action. Yolk sac and/or early placental vasculature, which account for a substantial portion of the total vascular impedance to flow sensed by the embryonic heart, poor vascularity in these organs can increase cardiac workload. FA may cause changes in the vasculature of the yolk sac and/or early placenta as a cause of congenital heart disease.
7. Immune homeostasis in pregnancy: a changing balance of immunity caused by phote-degradation product of FA
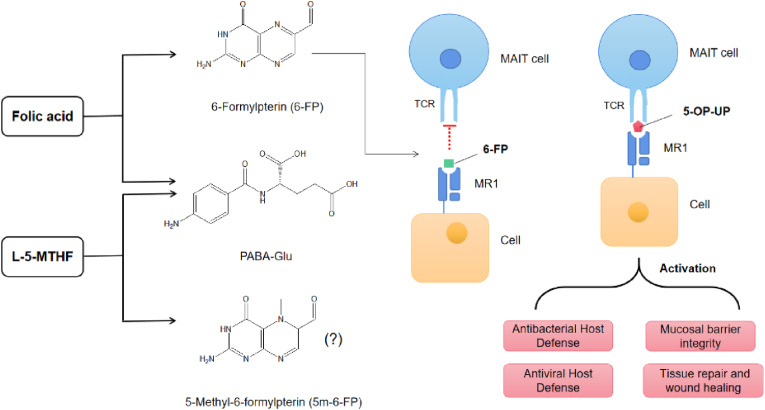
Mucosal-associated invariant T (MAIT) cells are non-classical T cells with both innate and adaptive immune properties intertwined with barrier immunity ever since they were coined (Toubal et al., 2019; Porcelli et al., 1993; Treiner et al., 2003). MAIT cells have the ability to recognize the major histocompatibility complex-related molecule 1 (MR1), presenting microbial vitamin B metabolites from certain bacteria and yeast (Kjer-Nielsen et al., 2012). One of the main breakdown products of folic acid (FA), known as 6-formylpterin (6-FP), has been identified as a ligand for MR1 (Patel et al., 2013). Interestingly, 6-FP efficiently stabilizes MR1, up-regulates its cell surface expression but does not stimulate MAIT cells. As a phytometabolite of FA and various pterin derivatives, 6-FP can be found in human serum and urine, notably in the urine of cancer patients (Halpern et al., 1977). Increased levels of 6-carboxypterin and pterin, the downstream oxidative metabolic products of 6-FP, under the effect of FA supplementation (Juzeniene et al., 2016; Burton et al., 2016). Of note, UMFA/6-FP acts as a competitive inhibitor of MAIT activation by diminishing the activation of cells induced by riboflavin derivatives (Patel et al., 2013). In comparison to FA, L-5-MTHF exhibits greater photostability, moreover, if photo-degradation of L-5-MTHF should occur, it does not release 6-FP and does not affect MAIT cell activation to any significant extent (Fig. 6) (Steindal et al., 2006; Naidoo et al., 2021; Tang et al., 2022).
Fig. 6.
Oxidative degradation products of folic acid and L-5-MTHF and their relevance to MR1 immunobiology.
FA photodegrades rapidly forming 6-formylpterin (6-FP) and p-aminobenzoylglutamate (PABA-Glu) as major degradation products. 6-FP as a MR1-ligand binding to MR1 that drives MR1 to fully mature, associate with β2-microglobulin, and traffic to the cell surface but does not activate MAIT cells. Photodegradation products of L-5-MTHF does not affect MAIT cell activation to any significant extent. MR1-restricted T cells have varied biological functions, which are blocked by 6-formylpterin (6-FP).
The immune system faces a delicate balancing act during pregnancy, and the disruption caused by environmental exposure to chemicals that perturb the balance of inflammatory status is possibly harmful. MAIT cells were highly enriched in intervillous space following term pregnancies and MR1 was expressed in placental villous and decidual macrophages (Solders et al., 2017). Notably, MAIT cells in the intervillous space have a higher expression of intracellular IFN-γ and Granzyme B when stimulated with Escherichia coli compared to the periphery, blocking of MR1 decreased the production of IFN-γ. Early-onset preeclamptic patients often present with a significantly lower expression by MAIT cells, yet there remains limited understanding regarding their presence and functional engagement in pregnancy (Solders et al., 2017; Raffetseder et al., 2021). Mammalian uterine tissues undergo tissue remodeling during pregnancy. Natural killer (NK) cells are members of the emerging family of innate lymphoid cells that play important roles in innate immunity and tissue remodeling. Previous studies have shown that lack of uterine NK cells or impaired IFN-γ signaling fail to initiate normal pregnancy-induced modification of decidual arteries and display hypocellularity or necrosis of decidua (Ashkar et al., 2000; Yagel, 2009; Wei and Yang, 2023). The activation of human decidual NK cells secretes trophoblast migration-promoting factors and angiogenic factors, thereby facilitating the remodeling of uterine spiral arteries and regulating trophoblast invasion (Hanna et al., 2006; Wang et al., 2021). Evidence from studies shows that high FA intake and UMFA can reduce the number and effectiveness of NK cells in serum, increasing the potential for immune dysfunction (Troen et al., 2006; Sawaengsri et al., 2016; Paniz et al., 2017; Alnabbat et al., 2022). Based on the aforementioned observations, a hypothesis has been formulated. The development of the placenta and its vascular system were in a state of immune homeostasis, maintaining a delicate balance between interactions of maternal immune cells and trophoblast cells. However, this equilibrium could potentially be disrupted by high level of UMFA which could be defined as immunosuppressant. In that context, abnormal placental hemodynamics and other dysfunctions emerge as a conceivable etiology for the association between high FA and CHD.
8. Summary
In regions with increasing utilization of FA supplements or with established policies of FA supplementation, the anticipated control over the occurrence of CHD has not been achieved. Particularly noteworthy is the upward trend observed in the prevalence of mild lesions of CHD (Liu et al., 2019). The observed decrease in the incidence of severe CHD, coupled with a simultaneous rise in milder cases, particularly septal defects, leading to an overall increase in CHD prevalence, may suggests the intricate and multifaceted interactions of FA in cardiovascular development. Similarly, the efficacy of treatments aimed at reducing Hcy concentrations in mitigating cardiovascular disease risk has produced inconsistent findings (Bazzano et al., 2006; Li et al., 2016). Hcy and its metabolites are implicated in the induction of oxidative stress, the promotion of inflammation, and the impairment of endothelial function (Currò et al., 2009; Welch and Loscalzo, 1998). Epidemiological studies have consistently demonstrated a correlation between elevated Hcy levels and the incidence of coronary heart disease and stroke (Homocysteine Studies Collaboration, 2002; Unadkat et al., 2024). Initial meta-analyses failed to substantiate the purported cardiovascular benefits of FA supplementation (Bazzano et al., 2006). However, the expanded cohort of individuals without prior cardiovascular disease revealed a modest reduction in overall cardiovascular disease risk of 4 % (Li et al., 2016). It is noteworthy that no significant differences in the effects of the intervention were observed based on the dosage of FA supplementation, particularly in subgroup analyses related to stroke, where high doses did not confer any additional benefit. Is there a devil in the details? Early studies in rodents results that overestimated the extent to which FA undergoes biotransformation in the human mucosa and liver. This led to a widespread belief that FA, akin to dietary folates, underwent near-complete conversion to L-5-MTHF in human gut. However, subsequent to the year 2005, numerous studies have redefined the model of FA biotransformation when applied specifically to humans (Bailey and Ayling, 2009; Patanwala et al., 2014; Wright et al., 2005). As scientific evidence and data accumulate, the understanding of the cardiovascular benefits of FA supplementation continues to deepen. Earlier studies suggested that FA could lower PE risk, but recent trials indicate high doses might not protect and could even increase risk (Wen et al., 2008, 2018; Hernández-Díaz et al., 2002). Recent studies support that high-dose FA supplementation may elevate the risk of hypertension during pregnancy (Li et al., 2013b, 2020; Timmermans et al., 2011). These findings align with emerging evidence from a case-control study investigating the relationship between maternal serum folate levels during pregnancy and the risk of CHD in offspring. Utilizing conditional logistic regression analysis, this study identified a U-shaped association between maternal folate levels and the risk of CHD (Qu et al., 2024). The conflicting findings in various studies may be attributed, in part, to variations in the form of folate used, as FA has both beneficial as well as harmful effects. These biological effects may arise from the independent actions of UMFA unrelated to one-carbon cycle metabolism, including anti-angiogenic and immunosuppressive effects (Fig. 7).
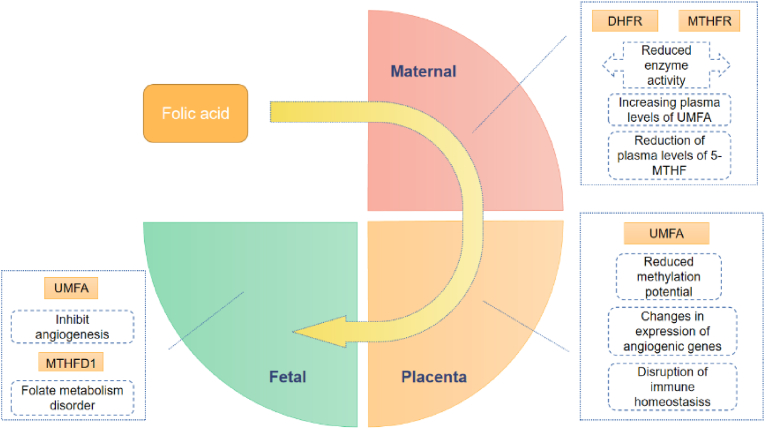
Fig. 7.
Various factors affecting the absorption and transport of folic acid may reduce the effect of preventing CHD.
Restriction of DHFR and MTHFR enzyme activities in the mother will reduce the availability of FA. FA is converted to biologically useable forms of folate (dihydrofolate or tetrahydrofolate) by DHFR and, the presence and persistence of UMFA in the blood are thought to be a result of low and variable DHFR activity in humans. UMFA targets FRα which is highly expressed in placental tissue. UMFA may have adverse effects in the placenta and embryo to increase the risk of congenital heart defects. In the placenta UMFA may have immunosuppressive and angiogenesis inhibitory effects which disturb the balance of pro- and antiangiogenic factors in the direction of angiogenesis. In early fetal embryos, UMFA may represses the expression of EIF1A and influences vascular development. All of the above abnormalities could affect the effect of preventing CHD.
Numerous polymorphisms in the FA metabolism-related genes have been identified, and certain polymorphisms in the genes encoding MTHFR or DHFR have the potential to diminish enzymatic activity, resulting in low concentration of L-5-MTHF and the appearance of UMFA (Menezo et al., 2022). Since the developing embryo are entirely dependent on maternal folate, folate receptors are crucial for transplacental maternal-to-fetal folate transport. UMFA circulating in the bloodstream seem target FRα, which is highly expressed in placental tissue (Mohanraj et al., 2019). Vasculogenesis and angiogenesis are essential for placental development, which are critical for the successful progression of gestation. Aberrant vascular development within the placenta results in its under perfusion, thereby contributing to the onset of pregnancy-related disorders such as gestational hypertension and PE. In zebrafish, The knockdown of eif1axb during the initial stages of embryonic development by FA results in impaired cardiovascular development (Lian et al., 2022). Moreover, UMFA could function as an immunosuppressant, have the potential to impact vascular development. High-dose FA supplementation may be associated with CHD as explained by the aforementioned mechanism. Based upon the current information, it is reasonable to conclude that excessive FA intake is not without risks. Replicating previous clinical studies is impractical in assessing the cardiovascular development of the unborn fetus due to ethical considerations. L-5-MTHF is already available as an alternative source of folate supplementation and does increase erythrocyte folate levels to reduce the risk of NTD. Further investigation such as clinical study of L-5-MTHF supplementation has been taken into our consideration.
Declaration of competing interest
The authors declare that they have no known competing interests.
Acknowledgement
This study is supported by Fundamental Research Grant of Henan Province, China (JBKY2023002).
Data availability
Data will be made available on request.
References
- Adelman D.M., Gertsenstein M., Nagy A., Simon M.C., Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Gene Dev. 2000;14(24):3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnabbat K.I., Fardous A.M., Shahab A., James A.A., Bahry M.R., Heydari A.R. High dietary folic acid intake is associated with genomic instability in peripheral lymphocytes of healthy adults. Nutrients. 2022;14(19):3944. doi: 10.3390/nu14193944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati F., Diano L., Campagnolo L., Vecchione L., Cipollone D., Bueno S., Prosperini G., Desideri A., Siracusa G., Chillemi G., Marino B., Novelli G. Hif1α down-regulation is associated with transposition of great arteries in mice treated with a retinoic acid antagonist. BMC Genom. 2010;11:497. doi: 10.1186/1471-2164-11-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angier R.B., Boothe J.H., Hutchings B.L., Mowat J.H., Semb J., Stokstad E.L., Subbarow Y., Waller C.W., Cosulich D.B., Fahrenbach M.J., Hultquist M.E., Kuh E., Northey E.H., Seeger D.R., Sickels J.P., Smith J.M., Jr. Synthesis of a compound identical with the l. Casei factor isolated from liver. Science (New York, N.Y.) 1945;102(2644):227–228. doi: 10.1126/science.102.2644.227. [DOI] [PubMed] [Google Scholar]
- Ashkar A.A., Di Santo J.P., Croy B.A. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J. Exp. Med. 2000;192(2):259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S.W., Ayling J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(36):15424–15429. doi: 10.1073/pnas.0902072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzano L.A., Reynolds K., Holder K.N., He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296(22):2720–2726. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- Bean L.J., Allen E.G., Tinker S.W., Hollis N.D., Locke A.E., Druschel C., Hobbs C.A., O'Leary L., Romitti P.A., Royle M.H., Torfs C.P., Dooley K.J., Freeman S.B., Sherman S.L. Lack of maternal folic acid supplementation is associated with heart defects in Down syndrome: a report from the National Down Syndrome Project. Birth defects research. Part A. Clinical. molecular teratology. 2011;91(10):885–893. doi: 10.1002/bdra.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard T., Lowry R.B., Sibbald B., Harder J.R., Trevenen C., Horobec V., Dyck J.D. Folic acid fortification and the birth prevalence of congenital heart defect cases in Alberta, Canada. Birth Defects Res. Part A Clin. Mol. Teratol. 2013;97(8):564–570. doi: 10.1002/bdra.23162. [DOI] [PubMed] [Google Scholar]
- Berkane N., Liere P., Oudinet J.P., Hertig A., Lefèvre G., Pluchino N., Schumacher M., Chabbert-Buffet N. From pregnancy to preeclampsia: a key role for estrogens. Endocr. Rev. 2017;38(2):123–144. doi: 10.1210/er.2016-1065. [DOI] [PubMed] [Google Scholar]
- Berry R.J., Li Z., Erickson J.D., Li S., Moore C.A., Wang H., Mulinare J., Zhao P., Wong L.Y., Gindler J., Hong S.X., Correa A. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for neural tube defect prevention. N. Engl. J. Med. 1999;341(20):1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- Blancquaert D., De Steur H., Gellynck X., Van Der Straeten D. Present and future of folate biofortification of crop plants. J. Exp. Bot. 2014;65(4):895–906. doi: 10.1093/jxb/ert483. [DOI] [PubMed] [Google Scholar]
- Bobrowski-Khoury N., Sequeira J.M., Arning E., Bottiglieri T., Quadros E.V. Absorption and tissue distribution of folate forms in rats: indications for specific folate form supplementation during pregnancy. Nutrients. 2022;14(12):2397. doi: 10.3390/nu14122397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd H.A., Basit S., Behrens I., Leirgul E., Bundgaard H., Wohlfahrt J., Melbye M., Øyen N. Association between fetal congenital heart defects and maternal risk of hypertensive disorders of pregnancy in the same pregnancy and across pregnancies. Circulation. 2017;136(1):39–48. doi: 10.1161/CIRCULATIONAHA.116.024600. [DOI] [PubMed] [Google Scholar]
- Burton C., Shi H., Ma Y. Daily variation and effect of dietary folate on urinary pteridines. Metabolomics. 2016;12:1–10. [Google Scholar]
- Cawley S., Mullaney L., McKeating A., Farren M., McCartney D., Turner M.J. A review of European guidelines on periconceptional folic acid supplementation. Eur. J. Clin. Nutr. 2016;70(2):143–154. doi: 10.1038/ejcn.2015.131. [DOI] [PubMed] [Google Scholar]
- Chen H., Zhang Y., Wang D., Chen X., Li M., Huang X., Jiang Y., Dou Y., Wang Y., Ma X., Sheng W., Jia B., Yan W., Huang G., SPCC (Shanghai Preconception Cohort) Group Periconception red blood cell folate and offspring congenital heart disease : nested case-control and mendelian randomization studies. Annals. internal med. 2022;175(9):1212–1220. doi: 10.7326/M22-0741. [DOI] [PubMed] [Google Scholar]
- Chen P., Tang L., Song Y., Wang B., Qin X., Zhang N., Wei Y., Xu X., Zhou Z., He Q., Liu L., Siddiqi S.M., Huang X., Cheng X., Tang G., Duan Y., Zhou H., Jiang J., Li S. Association of folic acid dosage with circulating unmetabolized folic acid in Chinese adults with H-type hypertension: a multicenter, double-blind, randomized controlled trial. Front. Nutr. 2023;10 doi: 10.3389/fnut.2023.1191610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Gu R., Lian Z., Gu H.F. Evaluation of the association between maternal folic acid supplementation and the risk of congenital heart disease: a systematic review and meta-analysis. Nutr. J. 2022;21(1):20. doi: 10.1186/s12937-022-00772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K.E., Mikael L.G., Leung K.Y., Lévesque N., Deng L., Wu Q., Malysheva O.V., Best A., Caudill M.A., Greene N.D., Rozen R. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 2015;101(3):646–658. doi: 10.3945/ajcn.114.086603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K.E., Deng L., Bahous R.H., Jerome-Majewska L.A., Rozen R. MTHFD1 formyltetrahydrofolate synthetase deficiency, a model for the MTHFD1 R653Q variant, leads to congenital heart defects in mice. Birth Defects Res. Part A Clin. Mol. Teratol. 2015;103(12):1031–1038. doi: 10.1002/bdra.23451. [DOI] [PubMed] [Google Scholar]
- Christensen K.E., Hou W., Bahous R.H., Deng L., Malysheva O.V., Arning E., Bottiglieri T., Caudill M.A., Jerome-Majewska L.A., Rozen R. Moderate folic acid supplementation and MTHFD1-synthetase deficiency in mice, a model for the R653Q variant, result in embryonic defects and abnormal placental development. Am. J. Clin. Nutr. 2016;104(5):1459–1469. doi: 10.3945/ajcn.116.139519. [DOI] [PubMed] [Google Scholar]
- Christensen K.E., Bahous R.H., Hou W., Deng L., Malysheva O.V., Arning E., Bottiglieri T., Caudill M.A., Jerome-Majewska L.A., Rozen R. Low dietary folate interacts with MTHFD1 synthetase deficiency in mice, a model for the R653Q variant, to increase incidence of developmental delays and defects. J. Nutr. 2018;148(4):501–509. doi: 10.1093/jn/nxy013. [DOI] [PubMed] [Google Scholar]
- Cipollone D., Carsetti R., Tagliani A., Rosado M.M., Borgiani P., Novelli G., D'Amati G., Fumagalli L., Marino B., Businaro R. Folic acid and methionine in the prevention of teratogen-induced congenital defects in mice. Cardiovasc. Pathol. official j. Soc. Cardiovasc. Pathol. 2009;18(2):100–109. doi: 10.1016/j.carpath.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Courtney J.A., Cnota J.F., Jones H.N. The role of abnormal placentation in congenital heart disease; cause, correlate, or consequence? Front. Physiol. 2018;9:1045. doi: 10.3389/fphys.2018.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider K.S., Bailey L.B., Berry R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011;3(3):370–384. doi: 10.3390/nu3030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider K.S., Zhu J.H., Hao L., Yang Q.H., Yang T.P., Gindler J., Maneval D.R., Quinlivan E.P., Li Z., Bailey L.B., Berry R.J. MTHFR 677C->T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am. J. Clin. Nutr. 2011;93(6):1365–1372. doi: 10.3945/ajcn.110.004671. [DOI] [PubMed] [Google Scholar]
- Crider K.S., Devine O., Qi Y.P., Yeung L.F., Sekkarie A., Zaganjor I., Wong E., Rose C.E., Berry R.J. Systematic review and Bayesian meta-analysis of the dose-response relationship between folic acid intake and changes in blood folate concentrations. Nutrients. 2019;11(1):71. doi: 10.3390/nu11010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M., Lu X.L., Lyu Y.Y., Wang F., Xie X.L., Cheng X.Y., Zhang T. Knowledge and intake of folic acid to prevent neural tube defects among pregnant women in urban China: a cross-sectional study. BMC Pregnancy Childbirth. 2021;21(1):432. doi: 10.1186/s12884-021-03893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currò M., Condello S., Caccamo D., Ferlazzo N., Parisi G., Ientile R. Homocysteine-induced toxicity increases TG2 expression in Neuro2a cells. Amino Acids. 2009;36(4):725–730. doi: 10.1007/s00726-008-0122-x. [DOI] [PubMed] [Google Scholar]
- Czeizel A.E., Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992;327(26):1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- Day P.L., Langston W., Darby W.J. Failure of nicotinic acid to prevent nutritional cytopenia in the monkey. PSEBM (Proc. Soc. Exp. Biol. Med.) 1938;38:860–863. [Google Scholar]
- De Wals P., Tairou F., Van Allen M.I., Uh S.H., Lowry R.B., Sibbald B., Evans J.A., Van den Hof M.C., Zimmer P., Crowley M., Fernandez B., Lee N.S., Niyonsenga T. Reduction in neural-tube defects after folic acid fortification in Canada. N. Engl. J. Med. 2007;357(2):135–142. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- Dickinson Y., Boehni R., Obeid R., Knapp J.P., Moser R., Lewandowski A.J., Douglas G., Leeson P., Channon K.M., Chuaiphichai S. Novel role of 5-Methyl-(6S)-Tetrahydrofolate in mediating endothelial cell tetrahydrobiopterin in pregnancy and implications for gestational hypertension. Hypertension. 2024;81(9):1910–1923. doi: 10.1161/HYPERTENSIONAHA.124.22838. (Dallas, Tex. : 1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducker G.S., Rabinowitz J.D. One-carbon metabolism in health and disease. Cell Metab. 2017;25(1):27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbe A., Uppu S., Lee S., Stroustrup A., Ho D., Srivastava S. Temporal variation of birth prevalence of congenital heart disease in the United States. Congenit. Heart Dis. 2015;10(1):43–50. doi: 10.1111/chd.12176. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA) Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Calcium L‐Methylfolate. EFSA J. 2004;2(11):135. doi: 10.2903/j.efsa.2004.135. [DOI] [Google Scholar]
- Food fortification initiative . The Food Fortification Initiative Website. 2025. Global progress.https://www.ffinetwork.org/globalprogress Retrieved April 26, 2025, from. [Google Scholar]
- Ford S.M., Pedersen C.J., Ford M.R., Kim J.W., Karunamuni G.H., McPheeters M.T., et al. Folic acid prevents functional and structural heart defects induced by prenatal ethanol exposure. Am. J. Physiol. Heart Circ. Physiol. 2021;320(4):H1313–H1320. doi: 10.1152/ajpheart.00817.2020. [DOI] [Google Scholar]
- Fox J.T., Stover P.J. Folate-mediated one-carbon metabolism. Vitam. Horm. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- Gong K., Xie T., Yang Y., Luo Y., Deng Y., Chen K., Tan Z., Guo H., Xie L. Establishment of a dihydrofolate reductase gene knock-in zebrafish strain to aid preliminary analysis of congenital heart disease mechanisms. Frontiers cardiovasc. med. 2021;8 doi: 10.3389/fcvm.2021.763851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern R., Halpern B.C., Stea B., Dunlap A., Conklin K., Clark B., Ashe H., Sperling L., Halpern J.A., Hardy D., Smith R.A. Pterin-6-aldehyde, a cancer cell catabolite: identification and application in diagnosis and treatment of human cancer. Proceedings of the National Academy of Sciences of the United States of America. 1977;74(2):587–591. doi: 10.1073/pnas.74.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Serrano M.C., Lastra-Vicente R., Brinez P., Acharya G., Huhta J.C., Chen R., Linask K.K. Folate rescues lithium-, homocysteine- and Wnt3A-induced vertebrate cardiac anomalies. Dis. Model. Mech. 2009;2(9–10):467–478. doi: 10.1242/dmm.001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Evsikov A.V., Zhang L., Lastra-Vicente R., Linask K.K. Embryonic exposures of lithium and homocysteine and folate protection affect lipid metabolism during mouse cardiogenesis and placentation. Reprod. Toxicol. 2016;61:82–96. doi: 10.1016/j.reprotox.2016.03.039. [DOI] [PubMed] [Google Scholar]
- Han X., Wang B., Jin D., Liu K., Wang H., Chen L., Zu Y. Precise dose of folic acid supplementation is essential for embryonic heart development in zebrafish. Biology. 2021;11(1):28. doi: 10.3390/biology11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J., Goldman-Wohl D., Hamani Y., Avraham I., Greenfield C., Natanson-Yaron S., Prus D., Cohen-Daniel L., Arnon T.I., Manaster I., Gazit R., Yutkin V., Benharroch D., Porgador A., Keshet E., Yagel S., Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006;12(9):1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Horino M., Morishita M., Tazoe Y., Tsuboi S., Matsuyama T., Kosuge K., Yamada H., Tsuji D., Inoue K., Itoh K. Dihydrofolate reductase gene intronic 19-bp deletion polymorphisms in a Japanese population. Drug Metabol. Pharmacokinet. 2010;25(5):516–518. doi: 10.2133/dmpk.dmpk-10-sc-036. [DOI] [PubMed] [Google Scholar]
- Hernández-Díaz S., Werler M.M., Louik C., Mitchell A.A. Risk of gestational hypertension in relation to folic acid supplementation during pregnancy. Am. J. Epidemiol. 2002;156(9):806–812. doi: 10.1093/aje/kwf129. [DOI] [PubMed] [Google Scholar]
- Hibbard B.M. The role of folic acid in pregnancy; with particular reference to anaemia, abruption and abortion. J. Obstet. Gynaecol. Br. Commonwealth. 1964;71:529–542. doi: 10.1111/j.1471-0528.1964.tb04317.x. [DOI] [PubMed] [Google Scholar]
- Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- Honein M.A., Paulozzi L.J., Mathews T.J., Erickson J.D., Wong L.Y. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981–2986. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- Hou T.C., Lin J.J., Wen H.C., Chen L.C., Hsu S.P., Lee W.S. Folic acid inhibits endothelial cell migration through inhibiting the RhoA activity mediated by activating the folic acid receptor/cSrc/p190RhoGAP-signaling pathway. Biochem. Pharmacol. 2013;85(3):376–384. doi: 10.1016/j.bcp.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Jacques P.F., Bostom A.G., Williams R.R., Ellison R.C., Eckfeldt J.H., Rosenberg I.H., Selhub J., Rozen R. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93(1):7–9. doi: 10.1161/01.cir.93.1.7. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Li J., Ren F., Ji C., Aniagu S., Chen T. PM2.5-induced extensive DNA methylation changes in the heart of zebrafish embryos and the protective effect of folic acid. Environ. Pollut. 2019;255(Pt 3) doi: 10.1016/j.envpol.2019.113331. (Barking, Essex : 1987) [DOI] [PubMed] [Google Scholar]
- Jouanne M., Oddoux S., Noël A., Voisin-Chiret A.S. Nutrient requirements during pregnancy and lactation. Nutrients. 2021;13(2):692. doi: 10.3390/nu13020692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juzeniene A., Grigalavicius M., Ma L.W., Juraleviciute M. Folic acid and its photoproducts, 6-formylpterin and pterin-6-carboxylic acid, as generators of reactive oxygen species in skin cells during UVA exposure. J. Photochem. Photobiol. B Biol. 2016;155:116–121. doi: 10.1016/j.jphotobiol.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Kjer-Nielsen L., Patel O., Corbett A.J., Le Nours J., Meehan B., Liu L., Bhati M., Chen Z., Kostenko L., Reantragoon R., Williamson N.A., Purcell A.W., Dudek N.L., McConville M.J., O'Hair R.A., Khairallah G.N., Godfrey D.I., Fairlie D.P., Rossjohn J., McCluskey J. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- Kolmaga A., Trafalska E., Gaszyńska E., Murlewska J., Witkowski S., Sylwestrzak O., Sokołowski Ł., Respondek-Liberska M., Strzelecka I. Folic acid and selected risk factors for fetal heart defects-preliminary study results. Nutrients. 2024;16(17):3024. doi: 10.3390/nu16173024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers Y. Folate recommendations for pregnancy, lactation, and infancy. Ann. Nutr. Metabol. 2011;59(1):32–37. doi: 10.1159/000332073. [DOI] [PubMed] [Google Scholar]
- Lamichhane D.K., Leem J.H., Park M., Kim J.A., Kim H.C., Kim J.H., Hong Y.C. Increased prevalence of some birth defects in Korea, 2009-2010. BMC Pregnancy Childbirth. 2016;16:61. doi: 10.1186/s12884-016-0841-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston W.C., Darby W.J., Shukers C.F., Day P.L. Nutritional cytopenia (vitamin m deficiency) in the monkey. J. Exp. Med. 1938;68(6):923–940. doi: 10.1084/jem.68.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.S., Lu Y.C., Kuo C.T., Chen C.T., Tang P.H. Effects of female sex hormones on folic acid-induced anti-angiogenesis. Acta Physiol. 2018;222(4) doi: 10.1111/apha.13001. [DOI] [PubMed] [Google Scholar]
- Li D., Pickell L., Liu Y., Wu Q., Cohn J.S., Rozen R. Maternal methylenetetrahydrofolate reductase deficiency and low dietary folate lead to adverse reproductive outcomes and congenital heart defects in mice. Am. J. Clin. Nutr. 2005;82(1):188–195. doi: 10.1093/ajcn.82.1.188. [DOI] [PubMed] [Google Scholar]
- Li Z., Ren A., Zhang L., Ye R., Li S., Zheng J., Hong S., Wang T., Li Z. Extremely high prevalence of neural tube defects in a 4-county area in Shanxi Province, China. Birth Defects Res. Part A Clin. Mol. Teratol. 2006;76(4):237–240. doi: 10.1002/bdra.20248. [DOI] [PubMed] [Google Scholar]
- Li X., Li S., Mu D., Liu Z., Li Y., Lin Y., Chen X., You F., Li N., Deng K., Deng Y., Wang Y., Zhu J. The association between periconceptional folic acid supplementation and congenital heart defects: a case-control study in China. Prev. Med. 2013;56(6):385–389. doi: 10.1016/j.ypmed.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Li Z., Ye R., Zhang L., Li H., Liu J., Ren A. Folic acid supplementation during early pregnancy and the risk of gestational hypertension and preeclampsia. Hypertension. 2013;61(4):873–879. doi: 10.1161/HYPERTENSIONAHA.111.00230. (Dallas, Tex. : 1979) [DOI] [PubMed] [Google Scholar]
- Li Z., Jun Y., Zhong-Bao R., Jie L., Jian-Ming L. Association between MTHFR C677T polymorphism and congenital heart disease. A family-based meta-analysis. Herz. 2015;40(Suppl. 2):160–167. doi: 10.1007/s00059-014-4144-8. [DOI] [PubMed] [Google Scholar]
- Li Y., Huang T., Zheng Y., Muka T., Troup J., Hu F.B. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2016;5(8) doi: 10.1161/JAHA.116.003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Xu S., Chen X., Zhang X., Li X., Lin L., Gao D., Wu M., Yang S., Cao X., Tan T., Hu W., Guo J., Huang L., Chen R., Zhou X., Cui W., Xiong T., Gao Q., Wu Y., et al. Folic acid supplement use and increased risk of gestational hypertension. Hypertension. 2020;76(1):150–156. doi: 10.1161/HYPERTENSIONAHA.119.14621. (Dallas, Tex. : 1979) [DOI] [PubMed] [Google Scholar]
- Lian Z., Wu Z., Gu R., Wang Y., Wu C., Cheng Z., He M., Wang Y., Cheng Y., Gu H.F. Evaluation of cardiovascular toxicity of folic acid and 6S-5-Methyltetrahydrofolate-Calcium in early embryonic development. Cells. 2022;11(24):3946. doi: 10.3390/cells11243946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.Y., Lee W.R., Su Y.F., Hsu S.P., Lin H.C., Ho P.Y., Hou T.C., Chou Y.P., Kuo C.T., Lee W.S. Folic acid inhibits endothelial cell proliferation through activating the cSrc/ERK 2/NF-κB/p53 pathway mediated by folic acid receptor. Angiogenesis. 2012;15(4):671–683. doi: 10.1007/s10456-012-9289-6. [DOI] [PubMed] [Google Scholar]
- Lin Y., Zhuang L., Yi H., Xu L., Huang H., He D., Zhao X., Ma H., Wu L. Embryonic protective role of folate in arsenic-induced cardiac malformations in rats. Int. J. Clin. Exp. Pathol. 2018;11(4):1946–1955. [PMC free article] [PubMed] [Google Scholar]
- Linask K.K., Huhta J. Folate protection from congenital heart defects linked with canonical Wnt signaling and epigenetics. Curr. Opin. Pediatr. 2010;22(5):561–566. doi: 10.1097/MOP.0b013e32833e2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen S., Zühlke L., Black G.C., Choy M.K., Li N., Keavney B.D. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019;48(2):455–463. doi: 10.1093/ije/dyz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y., Leclerc D., Cosín-Tomás M., Malysheva O.V., Wasek B., Bottiglieri T., Caudill M.A., Rozen R. Moderate folic acid supplementation in pregnant mice results in altered methyl metabolism and in sex-specific placental transcription changes. Mol. Nutr. Food Res. 2021;65(14) doi: 10.1002/mnfr.202100197. [DOI] [PubMed] [Google Scholar]
- Ma Y., Wu M., Li D., Li X.Q., Li P., Zhao J., Luo M.N., Guo C.L., Gao X.B., Lu C.L., Ma X. Embryonic developmental toxicity of selenite in zebrafish (Danio rerio) and prevention with folic acid. Food Chem. Toxicol. : an Int J. Pub. British Ind. Bio.Res. Association. 2012;50(8):2854–2863. doi: 10.1016/j.fct.2012.04.037. [DOI] [PubMed] [Google Scholar]
- Mao B., Qiu J., Zhao N., Shao Y., Dai W., He X., Cui H., Lin X., Lv L., Tang Z., Xu S., Huang H., Zhou M., Xu X., Qiu W., Liu Q., Zhang Y. Maternal folic acid supplementation and dietary folate intake and congenital heart defects. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni F., Cetin I., Verduci E., Canzone G., Giovannini M., Scollo P., Corsello G., Poli A. Maternal diet and nutrient requirements in pregnancy and breastfeeding. An Italian consensus document. Nutrients. 2016;8(10):629. doi: 10.3390/nu8100629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruvada P., Stover P.J., Mason J.B., Bailey R.L., Davis C.D., Field M.S., Finnell R.H., Garza C., Green R., Gueant J.L., Jacques P.F., Klurfeld D.M., Lamers Y., MacFarlane A.J., Miller J.W., Molloy A.M., O'Connor D.L., Pfeiffer C.M., Potischman N.A., Rodricks J.V., et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am. J. Clin. Nutr. 2020;112(5):1390–1403. doi: 10.1093/ajcn/nqaa259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matherly L.H., Hou Z., Deng Y. Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev. 2007;26(1):111–128. doi: 10.1007/s10555-007-9046-2. [DOI] [PubMed] [Google Scholar]
- Matthiesen N.B., Henriksen T.B., Agergaard P., Gaynor J.W., Bach C.C., Hjortdal V.E., Østergaard J.R. Congenital heart defects and indices of placental and fetal growth in a nationwide study of 924 422 liveborn infants. Circulation. 2016;134(20):1546–1556. doi: 10.1161/CIRCULATIONAHA.116.021793. [DOI] [PubMed] [Google Scholar]
- Menezo Y., Elder K., Clement A., Clement P. Folic acid, folinic acid, 5 Methyl TetraHydroFolate supplementation for mutations that affect epigenesis through the folate and one-carbon cycles. Biomolecules. 2022;12(2):197. doi: 10.3390/biom12020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikael L.G., Deng L., Paul L., Selhub J., Rozen R. Moderately high intake of folic acid has a negative impact on mouse embryonic development. Birth Defects Res. Part A Clin. Mol. Teratol. 2013;97(1):47–52. doi: 10.1002/bdra.23092. [DOI] [PubMed] [Google Scholar]
- Mitchell H.K., Snell E.E., Williams R.J. The concentration of ‘folic acid’. J. Am. Chem. Soc. 1941;63:2284. [Google Scholar]
- Mohanraj P.S., Rahat B., Mahajan A., Bagga R., Kaur J. Temporal expression of genes involved in folate metabolism and transport during placental development, preeclampsia and neural tube defects. Mol. Biol. Rep. 2019;46(3):3193–3201. doi: 10.1007/s11033-019-04776-w. [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group Prevention of neural tube defects: results of the medical research Council vitamin study. Lancet. 1991;338(8760):131–137. [PubMed] [Google Scholar]
- Na L., Q B., Xiumei Z., Lingzi Z., Deqin H., Xuanxuan Z., Huanhuan G., Yuan L., Xiujuan C. Research into the intervention effect of folic acid on arsenic-induced heart abnormalities in fetal rats during the periconception period. BMC Cardiovasc. Disord. 2020;20(1):139. doi: 10.1186/s12872-020-01418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo K., Woods K., Pellefigues C., Cait A., O'Sullivan D., Gell K., Marshall A.J., Anderson R.J., Li Y., Schmidt A., Prasit K., Mayer J.U., Gestin A., Hermans I.F., Painter G., Jacobsen E.A., Gasser O. MR1-dependent immune surveillance of the skin contributes to pathogenesis and is a photobiological target of UV light therapy in a mouse model of atopic dermatitis. Allergy. 2021;76(10):3155–3170. doi: 10.1111/all.14994. [DOI] [PubMed] [Google Scholar]
- National Health Commission of the People's Republic of China China maternal and child health development report. 2019. http://www.nhc.gov.cn/fys/jdt/201905/bbd8e2134a7e47958c5c9ef032e1dfa2.shtml Retrieved April 226, 2025, from.
- Obeid R., Holzgreve W., Pietrzik K. Folate supplementation for prevention of congenital heart defects and low birth weight: an update. Cardiovasc. Diagn. Ther. 2019;9(Suppl. 2):S424–S433. doi: 10.21037/cdt.2019.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrvik V.E., Witthoft C.M. Human folate bioavailability. Nutrients. 2011;3(4):475–490. doi: 10.3390/nu3040475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øyen N., Olsen S.F., Basit S., Leirgul E., Strøm M., Carstensen L., Granström C., Tell G.S., Magnus P., Vollset S.E., Wohlfahrt J., Melbye M. Association between maternal folic acid supplementation and congenital heart defects in offspring in birth cohorts from Denmark and Norway. J. Am. Heart Assoc. 2019;8(6) doi: 10.1161/JAHA.118.011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz C., Bertinato J.F., Lucena M.R., De Carli E., Amorim P.M.D.S., Gomes G.W., Palchetti C.Z., Figueiredo M.S., Pfeiffer C.M., Fazili Z., Green R., Guerra-Shinohara E.M. A daily dose of 5 mg folic acid for 90 Days is associated with increased serum unmetabolized folic acid and reduced natural killer cell cytotoxicity in healthy Brazilian adults. J. Nutr. 2017;147(9):1677–1685. doi: 10.3945/jn.117.247445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanapirunhakit P., Karlsson H., Mulder M., Ljunggren S., Graham D., Freeman D. Sphingolipids in HDL - potential markers for adaptation to pregnancy? Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2021;1866(8) doi: 10.1016/j.bbalip.2021.158955. [DOI] [PubMed] [Google Scholar]
- Patanwala I., King M.J., Barrett D.A., Rose J., Jackson R., Hudson M., Philo M., Dainty J.R., Wright A.J., Finglas P.M., Jones D.E. Folic acid handling by the human gut: implications for food fortification and supplementation. Am. J. Clin. Nutr. 2014;100(2):593–599. doi: 10.3945/ajcn.113.080507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel O., Kjer-Nielsen L., Le Nours J., Eckle S.B., Birkinshaw R., Beddoe T., Corbett A.J., Liu L., Miles J.J., Meehan B., Reantragoon R., Sandoval-Romero M.L., Sullivan L.C., Brooks A.G., Chen Z., Fairlie D.P., McCluskey J., Rossjohn J. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat. Commun. 2013;4:2142. doi: 10.1038/ncomms3142. [DOI] [PubMed] [Google Scholar]
- Perry J., Chanarin I. Intestinal absorption of reduced folate compounds in man. Br. J. Haematol. 1970;18(3):329–339. doi: 10.1111/j.1365-2141.1970.tb01447.x. [DOI] [PubMed] [Google Scholar]
- Petrone I., Bernardo P.S., Dos Santos E.C., Abdelhay E. MTHFR C677T and A1298C polymorphisms in breast cancer, gliomas and gastric cancer: a review. Genes. 2021;12(4):587. doi: 10.3390/genes12040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfiffner J.J., Calkins D.G. On the peptide nature of vitamin Bc conjugate from yeast. J. Am. Chem. Soc. 1946;68:1392. doi: 10.1021/ja01211a515. [DOI] [PubMed] [Google Scholar]
- Philip D., Buch A., Moorthy D., Scott T.M., Parnell L.D., Lai C.Q., Ordovás J.M., Selhub J., Rosenberg I.H., Tucker K.L., Troen A.M. Dihydrofolate reductase 19-bp deletion polymorphism modifies the association of folate status with memory in a cross-sectional multi-ethnic study of adults. Am. J. Clin. Nutr. 2015;102(5):1279–1288. doi: 10.3945/ajcn.115.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickell L., Brown K., Li D., Wang X.L., Deng L., Wu Q., Selhub J., Luo L., Jerome-Majewska L., Rozen R. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res. Part A Clin. Mol. Teratol. 2011;91(1):8–19. doi: 10.1002/bdra.20754. [DOI] [PubMed] [Google Scholar]
- Piedrahita J.A., Oetama B., Bennett G.D., van Waes J., Kamen B.A., Richardson J., Lacey S.W., Anderson R.G., Finnell R.H. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat. Genet. 1999;23(2):228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- Pietrzik K., Bailey L., Shane B. Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2010;49(8):535–548. doi: 10.2165/11532990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Yockey C.E., Brenner M.B., Balk S.P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J. Exp. Med. 1993;178(1):1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Lin S., Zhuang J., Bloom M.S., Smith M., Nie Z., Mai J., Ou Y., Wu Y., Gao X., Tan H., Liu X. First-trimester maternal folic acid supplementation reduced risks of severe and most congenital heart diseases in offspring: a large case-control study. J. Am. Heart Assoc. 2020;9(13) doi: 10.1161/JAHA.119.015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Liu X., Lin S., Bloom M.S., Wang X., Li X., Wang H., Han F., Liu J.E., Pan W., Zhang W., Zou X., Zhuang J., Li J., Chen J. Maternal serum folate during pregnancy and congenital heart disease in offspring. JAMA Netw. Open. 2024;7(10) doi: 10.1001/jamanetworkopen.2024.38747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffetseder J., Lindau R., van der Veen S., Berg G., Larsson M., Ernerudh J. MAIT cells balance the requirements for immune tolerance and anti-microbial defense during pregnancy. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.718168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J.G. Folic acid food fortification in Canada. Nutr. Rev. 2004;62:S35–S39. doi: 10.1111/j.1753-4887.2004.tb00072.x. 6 Pt 2. [DOI] [PubMed] [Google Scholar]
- Ren A.G. Prevention of neural tube defects with folic acid: the Chinese experience. World J. Clin. Pediatr. 2015;4(3):41–44. doi: 10.5409/wjcp.v4.i3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L.M., Pfeiffer C.M., Bailey L.B., Gregory J.F. A dual-label stable-isotopic protocol is suitable for determination of folate bioavailability in humans: evaluation of urinary excretion and plasma folate kinetics of intravenous and oral doses of [13C5] and [2H2]folic acid. J. Nutr. 1997;127(12):2321–2327. doi: 10.1093/jn/127.12.2321. [DOI] [PubMed] [Google Scholar]
- Ruan X., Shang W., Lu J., Li Z., Yang J., Cheng J., Wu Y., Sun K., Sun J. Maternal multivitamin supplementation mitigates the risk of fetal congenital heart disease associated with high indoor total volatile organic compounds exposure in east China: a case-control study. Environ. Health : Glob. Access Sci. Source. 2024;23(1):110. doi: 10.1186/s12940-024-01150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone G., Sarno L., Roman A., Donadono V., Maruotti G.M., Martinelli P. 5-Methyl-tetrahydrofolate in prevention of recurrent preeclampsia. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies. Int. Soc. Perinatal Obstetricians. 2016;29(6):916–920. doi: 10.3109/14767058.2015.1023189. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Duan J., Murohara T., Ikeda H., Shintani S., Shimada T., Akita T., Egami K., Imaizumi T. Rescue of hypercholesterolemia-related impairment of angiogenesis by oral folate supplementation. J. Am. Coll. Cardiol. 2003;42(2):364–372. doi: 10.1016/s0735-1097(03)00629-6. [DOI] [PubMed] [Google Scholar]
- Sawaengsri H., Wang J., Reginaldo C., Steluti J., Wu D., Meydani S.N., Selhub J., Paul L. High folic acid intake reduces natural killer cell cytotoxicity in aged mice. J. Nutr. Biochem. 2016;30:102–107. doi: 10.1016/j.jnutbio.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Scaglione F., Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica; the fate of foreign compounds in biological systems. 2014;44(5):480–488. doi: 10.3109/00498254.2013.845705. [DOI] [PubMed] [Google Scholar]
- Serrano M., Han M., Brinez P., Linask K.K. Fetal alcohol syndrome: cardiac birth defects in mice and prevention with folate. Am. J. Obstet. Gynecol. 2010;203(1) doi: 10.1016/j.ajog.2010.03.017. 75.e7–75.e15. [DOI] [PubMed] [Google Scholar]
- Smith D., Hornstra J., Rocha M., Jansen G., Assaraf Y., Lasry I., Blom H., Smulders Y.M. Folic acid impairs the uptake of 5-methyltetrahydrofolate in human umbilical vascular endothelial cells. J. Cardiovasc. Pharmacol. 2017;70(4):271–275. doi: 10.1097/FJC.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanky N., Requena Jimenez A., D'Souza S.W., Sibley C.P., Glazier J.D. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta. 2010;31(2):134–143. doi: 10.1016/j.placenta.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Solders M., Gorchs L., Erkers T., Lundell A.C., Nava S., Gidlöf S., Tiblad E., Magalhaes I., Kaipe H. MAIT cells accumulate in placental intervillous space and display a highly cytotoxic phenotype upon bacterial stimulation. Sci. Rep. 2017;7(1):6123. doi: 10.1038/s41598-017-06430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M., Raz S., Assaraf Y.G. Folylpoly-ɣ-glutamate synthetase association to the cytoskeleton: implications to folate metabolon compartmentalization. J. Proteonomics. 2021;239 doi: 10.1016/j.jprot.2021.104169. [DOI] [PubMed] [Google Scholar]
- Steindal A.H., Juzeniene A., Johnsson A., Moan J. Photodegradation of 5-methyltetrahydrofolate: biophysical aspects. Photochem. Photobiol. 2006;82(6):1651–1655. doi: 10.1562/2006-06-09-RA-915. [DOI] [PubMed] [Google Scholar]
- Strandgaard T., Foder S., Heuck A., Ernst E., Nielsen M.S., Lykke-Hartmann K. Maternally contributed folate receptor 1 is expressed in ovarian follicles and contributes to preimplantation development. Front. Cell Dev. Biol. 2017;5:89. doi: 10.3389/fcell.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.N., Gui Y.H., Wang Y.X., Qian L.X., Jiang Q., Liu D., Song H.Y. Effect of dihydrofolate reductase gene knock-down on the expression of heart and neural crest derivatives expressed transcript 2 in zebrafish cardiac development. Chin. Med. J. (Taipei) 2007;120(13):1166–1171. [PubMed] [Google Scholar]
- Sun S., Gui Y., Jiang Q., Song H. Dihydrofolate reductase is required for the development of heart and outflow tract in zebrafish. Acta Biochim. Biophys. Sin. 2011;43(12):957–969. doi: 10.1093/abbs/gmr098. [DOI] [PubMed] [Google Scholar]
- Tang L.S., Wlodarczyk B.J., Santillano D.R., Miranda R.C., Finnell R.H. Developmental consequences of abnormal folate transport during murine heart morphogenesis. Birth Defects Res. Part A Clin. Mol. Teratol. 2004;70(7):449–458. doi: 10.1002/bdra.20043. [DOI] [PubMed] [Google Scholar]
- Tang J.S., Cait A., White R.M., Arabshahi H.J., O'Sullivan D., Gasser O. MR1-dependence of unmetabolized folic acid side-effects. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.946713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani M., Iwai K. High-performance liquid chromatographic separation of physiological folate monoglutamate compounds. Investigation of absorption and conversion of pteroylglutamic acid in the small intestine of the rat in situ. J. Chromatogr. 1983;267(1):175–181. doi: 10.1016/s0021-9673(01)90830-1. [DOI] [PubMed] [Google Scholar]
- Timmermans S., Jaddoe V.W., Silva L.M., Hofman A., Raat H., Steegers-Theunissen R.P., Steegers E.A. Folic acid is positively associated with uteroplacental vascular resistance: the Generation R study. Nutr. Metabol. Cardiovasc. Dis. : Nutr. Metabol. Cardiovasc. Dis. 2011;21(1):54–61. doi: 10.1016/j.numecd.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Toubal A., Nel I., Lotersztajn S., Lehuen A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 2019;19(10):643–657. doi: 10.1038/s41577-019-0191-y. [DOI] [PubMed] [Google Scholar]
- Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F., Affaticati P., Gilfillan S., Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- Troen A.M., Mitchell B., Sorensen B., Wener M.H., Johnston A., Wood B., Selhub J., McTiernan A., Yasui Y., Oral E., Potter J.D., Ulrich C.M. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J. Nutr. 2006;136(1):189–194. doi: 10.1093/jn/136.1.189. [DOI] [PubMed] [Google Scholar]
- Unadkat S.V., Padhi B.K., Bhongir A.V., Gandhi A.P., Shamim M.A., Dahiya N., Satapathy P., Rustagi S., Khatib M.N., Gaidhane A., Zahiruddin Q.S., Sah R., Serhan H.A. Association between homocysteine and coronary artery disease-trend over time and across the regions: a systematic review and meta-analysis. Egyptian heart J. (EHJ) : official bulletin Egyptian Soci. Cardiol. 2024;76(1):29. doi: 10.1186/s43044-024-00460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool J.D., Hirche H., Lax H., De Schaepdrijver L. Folic acid and primary prevention of neural tube defects: a review. Reprod. Toxicol. 2018;80:73–84. doi: 10.1016/j.reprotox.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Wang W., Wang Y., Gong F., Zhu W., Fu S. MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 case-control and TDT studies. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Qualls A.E., Marques-Fernandez L., Colucci F. Biology and pathology of the uterine microenvironment and its natural killer cells. Cell. Mol. Immunol. 2021;18(9):2101–2113. doi: 10.1038/s41423-021-00739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Jin L., Zhang J., Meng W., Ren A., Jin L. Maternal periconceptional folic acid supplementation and risk for fetal congenital heart defects. J. Pediatr. 2022;240:72–78. doi: 10.1016/j.jpeds.2021.09.004. [DOI] [PubMed] [Google Scholar]
- Warkany J., Beaudry P.H., Hornstein S. Attempted abortion with aminopterin (4-amino-pteroylglutamic acid); malformations of the child. AMA J.diseases.child. 1959;97(3):274–281. doi: 10.1001/archpedi.1959.02070010276003. [DOI] [PubMed] [Google Scholar]
- Watson J., Castle W.B. Nutritional macrocytic anemia, especially in pregnancy; response to a substance in liver other than that effective in pernicious anemia. Am. J. Med. Sci. 1946;211:513–530. [PubMed] [Google Scholar]
- Wei X., Yang X. The central role of natural killer cells in preeclampsia. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1009867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch G.N., Loscalzo J. Homocysteine and atherothrombosis. N. Engl. J. Med. 1998;338(15):1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- Wen S.W., Chen X.K., Rodger M., White R.R., Yang Q., Smith G.N., Sigal R.J., Perkins S.L., Walker M.C. Folic acid supplementation in early second trimester and the risk of preeclampsia. Am. J. Obstet. Gynecol. 2008;198(1):45.e1–45.e457. doi: 10.1016/j.ajog.2007.06.067. [DOI] [PubMed] [Google Scholar]
- Wen S.W., White R.R., Rybak N., Gaudet L.M., Robson S., Hague W., Simms-Stewart D., Carroli G., Smith G., Fraser W.D., Wells G., Davidge S.T., Kingdom J., Coyle D., Fergusson D., Corsi D.J., Champagne J., Sabri E., Ramsay T., Mol B.W.J., et al. Effect of high dose folic acid supplementation in pregnancy on pre-eclampsia (FACT): double blind, phase III, randomised controlled, international, multicentre trial. Br. Med. J. 2018;362 doi: 10.1136/bmj.k3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J., Mai C.T., Mulinare J., Isenburg J., Flood T.J., Ethen M., Frohnert B., Kirby R.S., Centers for Disease Control and Prevention Updated estimates of neural tube defects prevented by mandatory folic Acid fortification - United States, 1995-2011. MMWR. Morbidity and mortality weekly report. 2015;64(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Wills L. Treatment of ‘pernicious anaemia of pregnancy’ and ‘tropical anaemia’ with special reference to yeast extract as curative agent. Br. Med. J. 1931;1:1059–1064. [PubMed] [Google Scholar]
- Wright A.J., Finglas P.M., Dainty J.R., Wolfe C.A., Hart D.J., Wright D.M., Gregory J.F. Differential kinetic behavior and distribution for pteroylglutamic acid and reduced folates: a revised hypothesis of the primary site of PteGlu metabolism in humans. J. Nutr. 2005;135(3):619–623. doi: 10.1093/jn/135.3.619. [DOI] [PubMed] [Google Scholar]
- Wu Y., Smith A.D., Bastani N.E., Refsum H., Kwok T. The dihydrofolate reductase 19-bp deletion modifies the beneficial effect of B-vitamin therapy in mild cognitive impairment: pooled study of two randomized placebo-controlled trials. Hum. Mol. Genet. 2022;31(7):1151–1158. doi: 10.1093/hmg/ddab246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Deng C., Li W., Wang K., Tao J., Gao Y., et al. National perinatal prevalence of selected major birth defects—China, 2010− 2018. China CDC Weekly. 2020;2(37):711–717. [Google Scholar]
- Xuan C., Li H., Zhao J.X., Wang H.W., Wang Y., Ning C.P., Liu Z., Zhang B.B., He G.W., Lun L.M. Association between MTHFR polymorphisms and congenital heart disease: a meta-analysis based on 9,329 cases and 15,076 controls. Sci. Rep. 2014;4:7311. doi: 10.1038/srep07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagel S. The developmental role of natural killer cells at the fetal-maternal interface. Am. J. Obstet. Gynecol. 2009;201(4):344–350. doi: 10.1016/j.ajog.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Yi K., He S.E., Guo T., Wang Z.Q., Zhang X., Xu J.G., Zhang H.Y., Liu W.G., You T. Association of MTHFD1 G1958A (rs2236225) gene polymorphism with the risk of congenital heart disease: a systematic review and meta-analysis. BMC Med. Genom. 2025;18(1):20. doi: 10.1186/s12920-024-02052-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.C., Levine R.J., Karumanchi S.A. Pathogenesis of preeclampsia. Annual rev. pathology. 2010;5:173–192. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- Yu D., Zhuang Z., Wen Z., Zang X., Mo X. MTHFR A1298C polymorphisms reduce the risk of congenital heart defects: a meta-analysis from 16 case-control studies. Ital. J. Pediatr. 2017;43(1):108. doi: 10.1186/s13052-017-0425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C., Ji C., Zhang H., Zhang L.W., Tong J., Jiang Y., Chen T. Protective effects of folic acid on PM2.5-induced cardiac developmental toxicity in zebrafish embryos by targeting AhR and Wnt/β-catenin signal pathways. Environ. Toxicol. 2017;32(10):2316–2322. doi: 10.1002/tox.22448. [DOI] [PubMed] [Google Scholar]
- Zhang R., Huo C., Wang X., Dang B., Mu Y., Wang Y. Two common MTHFR gene polymorphisms (C677T and A1298C) and fetal congenital heart disease risk: an updated meta-analysis with trial sequential analysis. Cell. Physiol. Biochem. : Int.l J. Experimental Cellular Physiology, Biochem. pharmacology. 2018;45(6):2483–2496. doi: 10.1159/000488267. [DOI] [PubMed] [Google Scholar]
- Zhang S., Qiu X., Wang T., Chen L., Li J., Diao J., Li Y., Qin J., Chen L., Jiang Y. Hypertensive disorders in pregnancy are associated with congenital heart defects in offspring: a systematic review and meta-analysis. Frontiers in cardiovascular medicine. 2022;9 doi: 10.3389/fcvm.2022.842878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Diop-Bove N., Visentin M., Goldman I.D. Mechanisms of membrane transport of folates into cells and across epithelia. Annu. Rev. Nutr. 2011;31:177–201. doi: 10.1146/annurev-nutr-072610-145133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Chen L., Yang T., Wang T., Zhang S., Chen L., Ye Z., Luo L., Qin J. Birth prevalence of congenital heart disease in China, 1980-2019: a systematic review and meta-analysis of 617 studies. Eur. J. Epidemiol. 2020;35(7):631–642. doi: 10.1007/s10654-020-00653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Cabrera R.M., Wlodarczyk B.J., Bozinov D., Wang D., Schwartz R.J., Finnell R.H. Differentially expressed genes in embryonic cardiac tissues of mice lacking Folr1 gene activity. BMC Dev. Biol. 2007;7:128. doi: 10.1186/1471-213X-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.