Abstract
Background and Aims
The pathophysiology of inflammatory bowel disease (IBD) including ulcerative colitis (UC) and Crohn’s disease (CD) remains unclear. While IBD is heterogeneous, most molecular-targeted drugs (MTDs) are effective for both UC and CD. The immunological pathoetiology can be considered to overlap regardless of clinical manifestations. Classifying IBD based on its immune profile could contribute to understanding its pathophysiology and predict the efficacy of therapy in individual cases. Machine learning has the advantage of being able to analyze complex data and could provide insights into the subcategorization of IBD using its immune profile.
Methods
The study used 20 cytokines and chemokines in serum samples from 69 patients with active UC (n = 51) or CD (n = 18) who were MTD-naïve before starting induction therapy. Multidimensional immune profiles considering the balance of items were used for machine learning to classify samples. The clinical outcome was the steroid-free clinical remission rate at 6 months in the patients treated with an MTD (n = 59).
Results
Levels of 13 cytokines and chemokines were analyzed. The balance of these 13 cytokines and chemokines was categorized into 5 groups. Cytokines and chemokines appeared to be more balanced in CD than in UC. Machine learning classified 69 patients with IBD into 5 clusters regardless of diagnosis. Among the 59 patients who started an MTD, the steroid-free clinical remission rate at 6 months was 68.4%, 52.6%, 50.0%, 37.5%, and 28.6% in each cluster. A significant association trend was observed between clustering and clinical outcome (P = .043).
Conclusion
This proof-of-concept study indicates that machine learning using the serum immune profile can classify active IBD regardless of the clinical diagnosis.
Keywords: Inflammatory Bowel Disease, Ulcerative Colitis, Crohn’s Disease, Immune Profile, Machine Learning
Graphical abstract
Introduction
Inflammatory bowel disease (IBD) is a chronic disorder that includes 2 major clinical phenotypes, namely, ulcerative colitis (UC) and Crohn’s disease (CD). UC causes continuous circumferential inflammation in the colonic mucosa. The inflammation in UC extends from the rectum to the proximal colon, whereas CD causes skip lesions that may occur at any site in the gastrointestinal tract. The transmural lesions in CD can develop into deep ulcers, leading to fistulas and abscesses, and may lead to strictures. These diseases are diagnosed according to their clinical manifestations. The incidence and prevalence of IBD are increasing dramatically worldwide.1 Appropriate therapeutic intervention before accumulation of damage in the gastrointestinal tract contributes to a better prognosis.2 While the pathoetiology of IBD remains unclear and no radical treatment has been established, various molecular-targeted drugs (MTDs) are now used in patients with the disease. Broadening the treatment options has improved the prognosis in these patients.3 Despite the marked differences in clinical manifestations between UC and CD, most MTDs, including anti–tumor necrosis factor (TNF)-α antibody, anti-α4β7 integrin antibody, anti-interleukin (IL)-12/23p40 antibody, anti-IL-23p19 antibody, and Janus kinase inhibitor, have clinical efficacy in both of these diseases. This clinical finding is compatible with the notion that the pathophysiology of UC and CD overlap to some extent. Genome-wide association studies have demonstrated that over 200 single nucleotide polymorphisms are associated with an increased risk of IBD.4 Many of these single-nucleotide polymorphisms are related to genes that are involved in inflammatory pathways, and a significant number of these genes are common to both UC and CD.4 Meanwhile, in daily practice, individual patients with IBD are heterogeneous in clinical course and response to treatment even with the same clinical diagnosis. We hypothesized that IBD, including UC and CD, could be classified based on its immunological pathophysiology independent of the clinical diagnosis. We also speculated that the classification of IBD by its immune profile would reflect the immunological characteristics of the patient and have the potential to predict the response to molecular-targeted therapy before starting treatment, which could contribute to the optimization of the treatment strategy for individual patients.
Collecting and analyzing serum samples is feasible and practical in the clinical setting. The serum biomarkers classifying IBD and predicting treatment response have much clinical significance. Previous studies have investigated various serum cytokines and chemokines associated with IBD,5,6 and these can be candidates for biomarkers. However, no practical classifiers have been established to date in the clinical setting. We assumed that there were methodological restrictions in other studies that focused on individual cytokines and chemokines and subjected their serum concentrations to statistical analysis. The absolute amounts of serum cytokines and chemokines could be affected by various factors, such as disease activity and interindividual variability in the production of proteins. Furthermore, analysis of each cytokine or chemokine one by one may not reflect the overall picture of the immune status of a specific patient. We speculated that if the balance of cytokines and chemokines is similar (eg, some cytokines and chemokines are relatively more dominant than others) among patients, these patients could be considered to have a similar immune profile, suggesting a common underlying pathophysiology for this inflammatory condition. However, complex data that contain multidimensional information are too challenging to analyze and categorize using conventional statistical methods. In this regard, a machine learning approach could provide novel insights. We have previously reported that machine learning can identify predictors and developed tools that can predict the efficacy of molecular-targeted therapy in patients with UC using meta-clinical data even with a limited sample size7, 8, 9 and proven the concept of using machine learning to develop innovative approaches in the IBD field.
In this study, we developed a novel classification of active IBD based on serum cytokine and chemokine profiles in patients with UC and CD using a machine learning approach. We observed a significant trend of an association between IBD classification and the response to molecular-targeted therapy.
Methods
Study Design and Subjects
Residues of serum samples obtained in everyday clinical practice when patients with UC and CD started systemic steroids or an MTD for active diseases at Kyorin University Hospital are stored at −80 °C (Institutional Ethics Committee of Kyorin University School of Medicine Approval Numbers 1378). Serum samples collected from MTD-naïve patients between June 2020 and July 2023 were enrolled consecutively in this study. UC and CD were diagnosed based on the Inflammatory Bowel Disease Guidelines of the Japanese Society of Gastroenterology.10 Clinical activity scores, including the Lichtiger index and Harvey–Bradshaw index, are assessed for patients with IBD in daily practice. Clinical data, including age at the time of sample collection, sex, diagnosis, type, duration, and activity of diseases, and therapeutic drugs administered, were obtained from the Kyorin University Hospital medical records system (Institutional Ethics Committee of Kyorin University School of Medicine Approval Numbers 1845).
Clinical Assessment
Clinical remission was defined as a Lichtiger index11 of ≤3 for UC and a Harvey–Bradshaw index12 of ≤3 for CD. Steroid-free clinical remission (SFCR) at 6 months after starting molecular-targeted therapy was defined as clinical remission without (1) terminating the MTD (ie, switching to other medication), (2) undergoing gastrointestinal surgery because of insufficient control of UC or CD disease activity, and (3) steroid induction before 6 months.
Measurement and Profiling of Serum Cytokines and Chemokines
Serum cytokine and chemokine levels were measured using the Milliplex Human Cytokine/Chemokine/Growth Factor Panel A (Merck, Boston, MA), Milliplex Multi-Species transforming growth factor (TGF)-β1 Panel Single-Plex (Merck), and Human IL-23 Quantikine enzyme-linked immunosorbent assay Kit (R&D systems, MN) according to the manufacturers’ protocols. Referencing previous reports on the cytokines and chemokines involved in the pathophysiology of IBD5,6 and considering the targets of MTDs for IBD and commercial availability of enzyme-linked immunosorbent assay kits, we measured the levels of 20 cytokines and chemokines, including IL-1β, IL-2, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17A, IL-17F, IL-22, IL-23, interferon-gamma, TNF-α, TGF-β, CCL2, CCL4, and CXCL10. The standardized value for the measured concentration of each cytokine and chemokine was used for the analysis. If the concentration of an item was not determined owing to a low level for more than 40% of subjects, this item was not included in the analysis.
Classifying Subjects with IBD
The standardized value of each cytokine and chemokine was calculated by subtracting the mean from the measured value and dividing by the standard deviation. These standardized values were used for the analysis in this study. The standardized values for the cytokines and chemokines were described using radar charts. The polygons in the charts were categorized into 5 groups based not on the area but on the shape of the polygon so that each group contained almost the same number of patients to visualize the balance of cytokines and chemokines as the 5-stage rating from “balanced” to “unbalanced”, focusing on the maximum value among the cytokines and chemokines: cyan (∼0.7), blue (0.7–1.5), green (1.5–2.0), red (2.0–3.4), and purple (3.4∼). We generated a dendrogram to classify patients into clusters based on overall multidimensional information using Ward’s method; each cluster contained only one sample of the data at the beginning, and the process was repeatedly applied until we obtained one cluster, in which 2 clusters showing the minimum distance (the Euclidean distance between centroids) were merged. Principal component analysis was used to assess the clustering of the data.
Statistical Analysis
Continuous variables are presented as the mean ± standard error and categorical variables as the median (interquartile range [IQR]). Kruskal–Wallis and Dunn’s tests were used to compare nonparametric data among groups. Fisher’s exact test was used to analyze contingency tables. The chi-square test for trend was used to examine the association between response to treatment and the IBD classification. The statistical analysis was performed using GraphPad Prism Version 9.5.1 (GraphPad Software Inc, San Diego, CA). A P value < .05 was considered statistically significant.
Results
Patient Demographics
Data for 69 patients who were MTD-naïve at baseline and started remission induction with systemic steroids or MTDs were analyzed (Table 1). These patients with active IBD were consecutively enrolled to minimize the selection bias of subjects. The therapeutic option for each patient was determined by a physician who saw the patient. Fifty-one of the 69 patients were diagnosed with UC and 18 with CD. Among the 51 patients with UC, 36 had pancolitis and 15 had left-sided colitis. At baseline, the median Lichtiger index was 9 (IQR 7–10). All patients with UC had clinically active disease at baseline. Colonoscopy was performed for 49 patients at baseline, and their median Mayo endoscopic subscore13 was 2 (IQR 2–3). The medication used for remission induction therapy in the patients with UC was adalimumab, golimumab, infliximab, ustekinumab, vedolizumab, filgotinib, carotegrast methyl, and prednisolone in 2, 2, 9, 10, 16, 3, 1, and 8 patients, respectively. One of the 18 patients with CD was diagnosed with the ileal type, 14 were diagnosed with the ileocolonic type, and 3 were diagnosed with the colonic type. The median Harvey–Bradshaw index at baseline was 4 (IQR 3–5). Six patients with CD had a Harvey–Bradshaw index of ≤3 on the day of starting medication, but their clinical course and other examinations, including endoscopy and biomarkers, indicated overt active disease, so they started advanced induction treatment. The remission induction therapy for CD was adalimumab, infliximab, ustekinumab, vedolizumab, and prednisolone in 1, 7, 6, 3, and 1 patients, respectively.
Table 1.
Patient Demographic and Clinical Data
| Demographics | Summary |
|---|---|
| All patients with IBD, n | 69 |
| Sex, female/male | 27/42 |
| Age at induction therapy, y | 41 (23.5, 51.8) |
| Patients with UC, n | 51 |
| Sex, female/male | 19/32 |
| Age at induction therapy, y | 43 (27, 54) |
| Disease duration, y | 3 (1, 6) |
| Disease type, pancolitis/left-sided/proctitis | 36/15 |
| Lichtiger index at baseline | 9 (7, 10) |
| Medications used for induction therapy | |
| Adalimumab | 2 |
| Golimumab | 2 |
| Infliximab | 9 |
| Ustekinumab | 10 |
| Vedolizumab | 16 |
| Filgotinib | 3 |
| Carotegrast methyl | 1 |
| Prednisolone | 8 |
| Patients with CD, n | 18 |
| Sex, female/male | 8/10 |
| Age at induction therapy, y | 26 (21.3, 44) |
| Disease duration, y | 2.5 (0.3, 7.0) |
| Disease type, ileitis/ileocolitis/colitis | 1/14/3 |
| Harvey–Bradshaw index at baseline | 4 (3, 5) |
| Medications for induction therapy | |
| Adalimumab | 1 |
| Infliximab | 7 |
| Ustekinumab | 6 |
| Vedolizumab | 3 |
| Prednisolone | 1 |
The data are presented as the number or median (IQR).
Profiling of Serum Cytokines and Chemokines
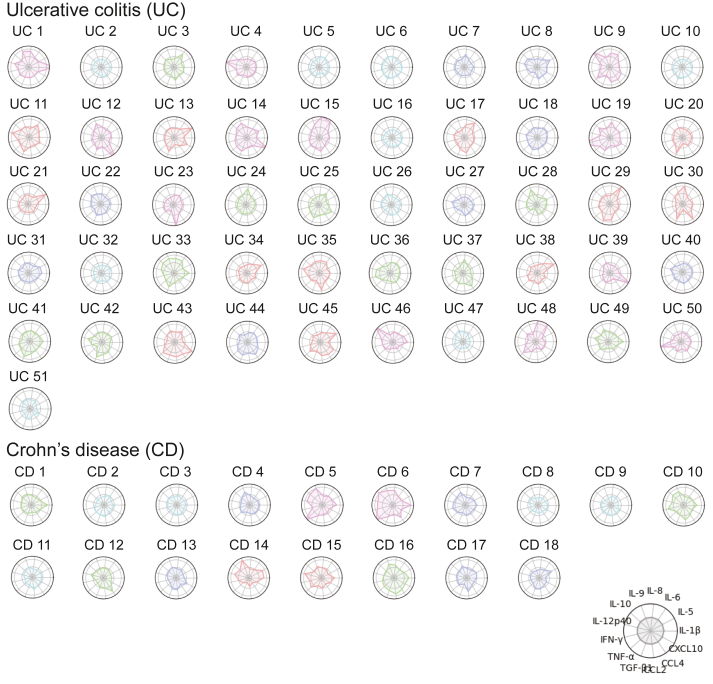
Cytokine and chemokine concentrations were measured in serum samples from the 69 patients who were MTD-naïve at baseline (ie, before starting induction therapy with prednisolone or MTDs). Among the 20 items, 7 were excluded from further analyses because they were not detected in more than 40% of subjects. Eventually, 13 cytokines and chemokines (IL-1β, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12p40, interferon-gamma, TNF-α, TGF-β, CCL2, CCL4, and CXCL10) were used for the analyses. The standardized values for these 13 items were described in a radar chart for each patient (Figure 1). A distinctive feature in this study was that the categorization was based on shape rather than area so that we could focus on the balance of the cytokine and chemokine profile. The shape of the polygon in the chart was categorized as follows: group A, a balanced shape similar to a regular 13-gon without slanting to a specific item or items (colored with cyan in Figure 1); group B, a shape close to that of group A (blue); group C, intermediate (green); group D, a shape close to that of group E (red); and group E, an imbalanced (distorted) shape with predominant specific item or items (purple) (Figure 1). Groups A–E included 14 patients (UC, n = 9; CD, n = 5), 13 patients (UC, n = 8; CD, n = 5), 14 patients (UC, n = 10; CD, n = 4), 14 patients (UC, n = 12; CD, n = 2), and 14 patients (UC, n = 12; CD, n = 2), respectively. The shape was more balanced in patients with CD than in those with UC.
Figure 1.
Radar charts describing the level and balance of cytokines and chemokines in the serum of patients with active IBD. The standardized values for 13 cytokines and chemokines (IL-1β, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12p40, IFN-γ, TNF-α, TGF-β, CCL2, CCL4, and CXCL10) in 51 patients with UC and 18 with CD are described. The subjects were categorized into 5 groups based on the balance of 13 polygons by focusing on the maximum value among the cytokines and chemokines. Cyan, blue, green, red, and purple were used to describe a balanced pattern through to an imbalanced pattern. IFN-γ, interferon-gamma.
Classifying IBD by Machine Learning Using the Serum Cytokine/Chemokine Profile
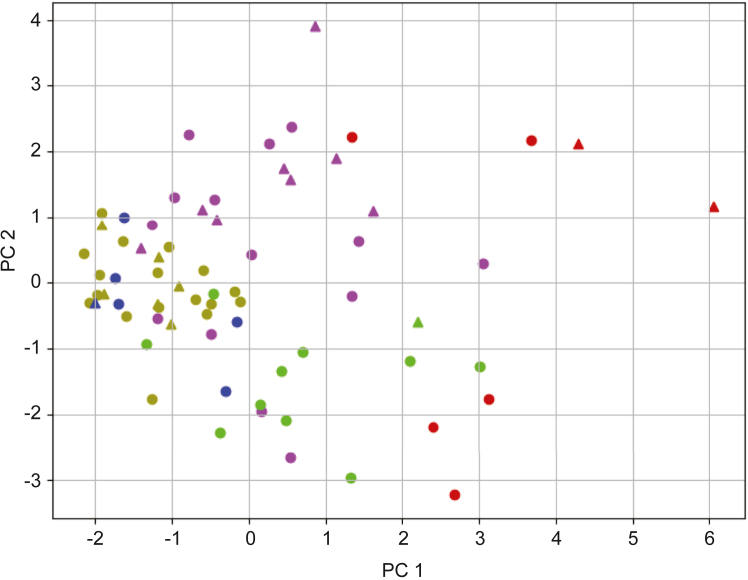
Using the 13-dimensional cytokine and chemokine profile, the dendrogram generated by Ward’s method demonstrated that the 69 patients with active IBD could be classified into 5 clusters (Figure 2). Each cluster included patients with UC and CD. There was no significant difference in the ratio of patients with UC to those with CD among the clusters. This finding supported the notion that IBD could be subcategorized based on the immune profile independent of its clinical manifestations and the diagnosis. Each of the 5 clusters appeared to cluster on the 2-dimensional principal component analysis plot (Figure 3). The sum of the contribution ratio of the first and second axes was 37%. Samples forming a dense cluster near the center (ie, x-axis = 0 and y-axis = 0) belonged to the ochre-colored cluster in Figures 2 and 3. Based on this principal component analysis plot and the clustering dendrogram by machine learning (Figure 2), the clusters were defined as follows: cluster I, ochre; cluster II, purple; cluster III, indigo; cluster IV, yellow-green; and cluster V, red. Cluster I was considered the core and clusters II–V were separated from cluster I in that order (ie, cluster II was closest to cluster I, while cluster V was far from cluster I). Clusters I–V included 23 patients (UC, n = 17; CD, n = 6), 22 patients (UC, n = 4; CD, n = 8), 6 patients (UC, n = 5; CD, n = 1), 11 patients (UC, n = 10; CD, n = 1), and 7 patients (UC, n = 5; CD, n = 2), respectively.
Figure 2.
Serum cytokine/chemokine profiles for patients with IBD were classified into 5 groups using machine learning. The dendrogram demonstrates that the serum cytokine/chemokine profiles consisting of 13 items in 69 patients with IBD could be classified into 5 groups. Samples in the same group have the same color.
Figure 3.
Principal component analysis plot of the serum cytokine/chemokine profiles of patients with IBD. The serum immune profiles including 13 cytokines and chemokines for the 69 patients with IBD are described in a 2-dimensional principal components analysis plot. The colors for each sample are the same as in Figure 2.
Novel Classification of IBD was Associated with the Clinical Response to MTDs
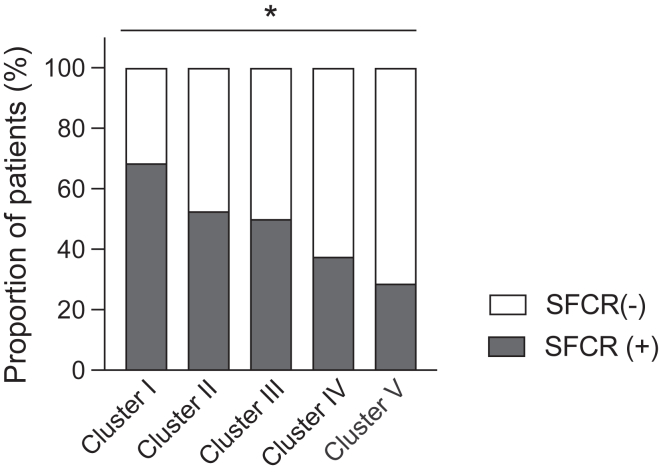
Among the 69 patients, 1 patient started carotegrast methyl and 9 started prednisolone as their induction therapy. Given that these medicines cannot be used for maintenance therapy, these 10 patients were excluded from the assessment of drug efficacy at 6 months. Overall, 42 patients with UC and 17 with CD were analyzed further: 19 patients (UC, n = 13; CD, n = 6) belonged to cluster I, 19 patients (UC, n = 12; CD, n = 7) to cluster II, 6 patients (UC, n = 5; CD, n = 1) to cluster III, 8 patients (UC, n = 7; CD, n = 1) to cluster IV, and 7 patients (UC, n = 5; CD, n = 2) to cluster V. There was a significant difference in the distribution of cytokine and chemokine balance patterns (groups A–E) among clusters I–V (P < .0001), ie, cluster I consisted of samples with balanced patterns, and clusters II–V, particularly clusters IV and V, included more imbalanced samples (Table 2). The balance of items appeared to be an important factor in clustering by machine learning. There was no significant difference in the ratio of patients with UC to those with CD or in disease activity of UC or CD among the clusters. Furthermore, there was no significant difference in the MTDs selected among the clusters (Table 2). The SFCR rate at 6 months after starting MTDs was 52.5% (31 of 59) in the overall cohort, 68.4% (13 of 19) in cluster I, 52.6% (10 of 19) in cluster II, 50.0% (3 of 6) in cluster III, 37.5% (3 of 8) in cluster IV, and 28.6% (2 of 7) in cluster V (Figure 4). There was a significant association trend between clustering and the SFCR rate at 6 months (P = .043). Clusters I–III tended to demonstrate better SFCR rates regardless of modes of action of MTDs compared to clusters IV and V (anti-TNF-α antibodies, 73.3% [11 of 15] vs 33.3% [2 of 6]; ustekinumab, 57.1% [8 of 14] vs 33.3% [1 of 3]; vedolizumab, 42.9% [6 of 14] vs 20.0% [1 of 5]; filgotinib, 100% [1 of 1] vs 50.0% [1 of 2]) (Figure A1).
Table 2.
Clinical Characteristics in 5 Clusters Based on the Immune Profile
| Demographics | Summary |
|---|---|
| Cluster I | |
| Patients, n | 19 |
| Cytokine/chemokine balance subgroup | (A) 10, (B) 6, (C) 3, (D) 0, (E) 0 |
| UC/CD | 13/6 |
| Disease activity at baseline | LI: 8 (6, 10.5), HB: 5 (2.25, 5) |
| Molecular-targeted medications | TNF, n = 9; IL-12/23, n = 5; integrin, n = 4; JAKi, n = 1 |
| Cluster II | |
| Patients, n | 19 |
| Cytokine/chemokine balance subgroup | (A) 2, (B) 4, (C) 6, (D) 5, (E) 2 |
| UC/CD | 12/7 |
| Disease activity at baseline | LI: 8.5 (7, 9.75), HB: 4 (2, 4) |
| Molecular-targeted medications | TNF, n = 5; IL-12/23, n = 6; integrin, n = 8; JAKi, n = 0 |
| Cluster III | |
| Patients, n | 6 |
| Cytokine/chemokine balance subgroup | (A) 0, (B) 1, (C) 0, (D) 5, (E) 0 |
| UC/CD | 5/1 |
| Disease activity at baseline | LI: 10 (8.5, 10), HB: 2 |
| Molecular-targeted medications | TNF, n = 1; IL-12/23, n = 3; integrin, n = 2; JAKi, n = 0 |
| Cluster IV | |
| Patients, n | 8 |
| Cytokine/chemokine balance subgroup | (A) 0, (B) 1, (C) 2, (D) 2, (E) 3 |
| UC/CD | 7/1 |
| Disease activity at baseline | LI: 10 (7, 12), HB: 5 |
| Molecular-targeted medications | TNF, n = 3; IL-12/23, n = 1; integrin, n = 2; JAKi, n = 2 |
| Cluster V | |
| Patients, n | 7 |
| Cytokine/chemokine balance subgroup | (A) 0, (B) 0, (C) 0, (D) 0, (E) 7 |
| UC/CD | 5/2 |
| Disease activity at baseline | LI: 9 (6.5, 11), HB: 4 (3, 5) |
| Molecular-targeted medications | TNF, n = 3; IL-12/23, n = 1; integrin, n = 3; JAKi, n = 0 |
The data are presented as the number or median (IQR).
HB, Harvey–Bradshaw index; Integrin, anti-α4β7 integrin antibody (including vedolizumab); IL-12/23, anti-IL-12/23p40 antibody (including ustekinumab); JAKi, Janus kinase inhibitor (including filgotinib); LI, Lichtiger index.
Figure 4.
The SFCR rate at 6 months after starting molecular-targeted therapy. The percentage of patients who achieved SFCR at 6 months differed among the 5 clusters (clusters I–V) determined by machine learning. There was a significant association trend between clustering and the SFCR rate. ∗P < .05.
Discussion
In this study, we examined multiple cytokines and chemokines in serum and developed a novel approach for determining the immune profile of IBD based on not only the amount (individual measured levels) but also the balance (overall patterns) of cytokines and chemokines. We demonstrated that machine learning could subcategorize the immune profile of patients with IBD beyond their clinical diagnosis as UC or CD. Furthermore, subgrouping by machine learning using comprehensive immune profiling was associated with the efficacy of molecular-targeted therapy. This proof-of-concept study indicates that machine learning using an immune profile consisting of multiple items can classify active IBD regardless of its clinical manifestations and that categorization of IBD based on immunological patterns can contribute to the prediction of the response to molecular-targeted therapy.
While an increasing number of MTDs have become available for IBD in clinical practice, predicting therapeutic efficacy remains a challenge. Clinical studies have demonstrated that the long-term efficacy of an individual MTD is eventually 50% at best, regardless of its mode of action.14,15 This finding suggests that an MTD could be appropriate for the pathophysiology in almost half of the patients who start treatment, but alternative MTDs could be better for other patients. Furthermore, it can be speculated that some patients can respond to various MTDs while others are refractory to any MTD. Although there has been considerable progress in medical therapy and outcomes have improved, surgery still plays an important role in the management of IBD.3 Therefore, focusing too closely on medical treatment should be avoided. An ability to predict the efficacy of molecular-targeted therapy before starting medication, ie, when patients have active inflammation, could allow prompt clinical decision-making, including selection of medication and assessment of the indication for surgery, and contribute to better clinical outcomes as well as less socioeconomic burden. Given that the therapeutic targets of MTDs are molecules related to inflammatory pathways, categorizing IBD based on immunological characteristics seems a promising strategy for the prediction of the efficacy of these agents.
Machine learning has the advantages of being able to analyze complex data and to discover rules and classifiers that are not discernible by the human eye. In this study, our machine learning approach using 13-dimensional information of the serum cytokine and chemokine profile classified active IBD into 5 clusters (clusters I–V). Among our 59 patients with active IBD who started an MTD as induction and maintenance therapy, the SFCR rate at 6 months was 52.5%. However, the SFCR rate ranged widely from 68.4% to 28.6% in clusters I–V, and there was a significant association trend between our IBD clustering by machine learning and the SFCR rate. Nineteen of our 59 patients (32.2%) were classified into cluster I, which had the best SFCR rate (68.4%) overall and tended to have a more balanced cytokine and chemokine profile. In contrast, patients in clusters IV and V showed more imbalanced profiles and lower SFCR rates. Also, patients in clusters IV and V demonstrated lower SFCR rates regardless of modes of action of MTDs compared to those in clusters I–III. These results suggest that a clinical study of patients with a cluster I–type immune profile (possibly almost a third of patients with IBD, who may respond to medication better than the patients overall) could overestimate the efficacy of an MTD. Furthermore, patients with IBD who have a cluster IV–type or cluster V-type immune profile could be refractory to medical treatment.
While this proof-of-concept study demonstrated that machine learning regarding the immune profile allows the classification of IBD and the classification can be associated with response to treatment, one of the important future directions is the subcategorization of IBD for prediction of the response to each individual MTD. Further considerations on study design and analysis methodology and algorithms are needed to address the crucial clinical challenge of distinguishing responders and nonresponders before starting molecular-targeted therapy. Considering the differences in mode of action between the different agents (eg anti-TNF-α, anti-IL-12/23p40 antibody, and anti-IL-23p19), a prediction model for each mode of action would be needed. We have previously reported that machine learning using clinical data at baseline could be used to develop a clinical efficacy prediction model for the anti-α4β7 integrin antibody and anti-IL-12/23p40 antibody in patients with UC7,8 but that a prediction model for the anti-α4β7 integrin antibody could not predict the efficacy of the anti-IL-12/23p40 antibody.8 Obraztsov et al. developed a prediction model for anti-TNF-α antibody in UC using the Fisher linear discriminant analysis model with seven cytokines.16 These findings, together with the present study, support the notion that machine learning using the immune profile with or without clinical data can contribute to developing an accurate prediction model for each mode of action. If prediction models for various MTDs are available, we can predict the response to multiple medications at baseline and determine the best option for individual patients. As another future direction, understanding the mechanism of the loss of response to an MTD can contribute to better clinical practice. Some patients who initially respond to an MTD lose their response during treatment. While antidrug antibodies are considered to cause this phenomenon, there is also a possibility that the inflammatory pathways involved in disease activity change as a result of immunological modifications or alterations in the underlying pathophysiology.17,18 An immune profile classification system could be used in patients with IBD who have a loss of response to evaluate the changes in immunological conditions that cause active inflammation. Assessment of the immune profile at the time of loss of response, particularly when compared with that at baseline, could provide a clue to appropriate interventions (eg, dose escalation or a switch to a specific MTD) as the next step.
This study had some limitations. It was performed as a single-center study, so our ability to predict the efficacy of each MTD was limited by the small sample size for each medication. Also, although the diagnosis of UC and CD was made based on the guidelines,10 the possibility that the diagnosis might be biased cannot be strictly excluded. In addition, while this study employed the commercially available kits for measuring cytokines and chemokines with a limited amount of serum considering the potential clinical application, a different measurement methodology could be useful to detect more cytokines and chemokines. However, the proof-of-concept provided by this study paves the way for a large multicenter study of machine learning using immune profiles and clinical metadata. The validation of our findings with external cohorts is crucial to obtaining robust evidence. This clustering can be applied to new patients for further studies by processing their measured data as conducted in the present study. Furthermore, we analyzed serum samples from MTD-naïve patients with IBD to exclude the possibility of immunological modifications by previous MTDs. The study design was appropriate for understanding the immunological pathophysiology of active IBD and is critical in a proof-of-concept study designed to determine if machine learning can classify immune profiles in patients with IBD. However, it was not possible to evaluate possible immunological shifts during MTD therapy. A further study that analyzes serum samples before and after MTD induction therapy could provide some insights.
Conclusion
This study proposed a novel classification of IBD beyond clinical diagnosis by applying machine learning to serum cytokine and chemokine profiles. It also demonstrated that this classification is associated with treatment responses to MTDs.
Acknowledgments
The authors thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Authors’ Contributions
Jun Miyoshi: Study conceptualization, data collection, data analysis, experiments, design, writing – original draft. Satoshi Tamura: Study conceptualization, sample collection, data collection, data analysis, experiments, design, writing – original draft. Tadakazu Hisamatsu: Study conceptualization, design, writing – original draft, supervision. Noriaki Oguri: Writing – original draft, sample collection, data collection, data analysis, experiments, design. Daisuke Saito: Sample collection, data collection, data analysis, experiments. Yuu Nishinarita: Sample collection, data collection, data analysis, experiments. Haruka Wada: Sample collection, data collection, data analysis, experiments. Nobuki Nemoto: Sample collection, data collection, data analysis, experiments. Minoru Matsuura: Writing – original draft, supervision.
Footnotes
Conflicts of Interest: These authors disclose the following: Jun Miyoshi has received grant support from AbbVie GK and consulting and lecture fees from EA Pharma Co, Ltd, AbbVie GK, Janssen Pharmaceutical K.K., Jansen Asia Pacific Pte. Ltd, Pfizer Inc, Mitsubishi Tanabe Pharma Corporation, JIMRO Co Miyarisan Co Ltd, and Takeda Pharmaceutical Co Ltd. Minoru Matsuura has received consulting and lecture fees from Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co Ltd, AbbVie GK, Mitsubishi Tanabe Pharma Corporation, Kyorin Pharmaceutical Co Ltd, Mochida Pharmaceutical Co Ltd, JIMRO Co Nippon Kayaku Co, Ltd, Mylan EPD G.K., and Aspen Japan Co Ltd. Tadakazu Hisamatsu has undertaken joint research with Kissei Pharmaceutical Co, Ltd, and EA Pharma Co Ltd; received grant support from Mitsubishi Tanabe Pharma Corporation, EA Pharma Co, Ltd, AbbVie GK, JIMRO Co, Ltd, Zeria Pharmaceutical Co Ltd, Kyorin Pharmaceutical Co Ltd, Nippon Kayaku Co, Ltd, Takeda Pharmaceutical Co Ltd, Pfizer Inc, Boston Scientific Corporation, and Mochida Pharmaceutical Co Ltd; and received consulting and lecture fees from EA Pharma Co, Ltd, AbbVie GK, Janssen Pharmaceutical K.K., Pfizer Inc, Mitsubishi Tanabe Pharma Corporation, Kyorin Pharmaceutical Co Ltd, JIMRO Co Mochida Pharmaceutical Co Ltd, Bristol Myers Squibb Co Eli Lilly and Company, Gilead Sciences, Inc, and Takeda Pharmaceutical Co Ltd. The remaining authors disclose no conflicts.
Funding: This work was supported by a Takeda Japan Medical Office Funded Research Grant, the Kyorin Medical Society Research Grant, and Grants-in-Aid for Scientific Research (KAKENHI: 22K07970) as well as grants from the Japan Sciences Research Grant for Research on Intractable Diseases (Japanese Inflammatory Bowel Disease Research Group) affiliated with the Japan Ministry of Health, Labour and Welfare.
Ethical Statement: The study was approved by the Institutional Ethics Committee of Kyorin University School of Medicine (approval numbers 1378 and 1845) and conducted in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived in view of the use of stored samples and recorded data.
Data Transparency Statement: The data underpinning this research will be shared by the corresponding author upon reasonable request.
Reporting Guidelines: Helsinki Declaration.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2025.100667.
Contributor Information
Jun Miyoshi, Email: jmiyoshi@ks.kyorin-u.ac.jp.
Satoshi Tamura, Email: tamura@info.gifu-u.ac.jp.
Tadakazu Hisamatsu, Email: thisamatsu@ks.kyorin-u.ac.jp.
Supplementary Materials
References
- 1.Ng S.C., Shi H.Y., Hamidi N., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2.Noor N.M., Sousa P., Paul S., et al. Early diagnosis, early stratification, and early intervention to deliver precision medicine in IBD. Inflamm Bowel Dis. 2022;28:1254–1264. doi: 10.1093/ibd/izab228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai L., Ma C., Dulai P.S., et al. Contemporary risk of surgery in patients with ulcerative colitis and crohn's disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol. 2021;19:2031–2045.e11. doi: 10.1016/j.cgh.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Hadad J., Schreiner P., Vavricka S.R., et al. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28:27–35. doi: 10.1007/s40291-023-00678-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen P., Zhou G., Lin J., et al. Serum biomarkers for inflammatory bowel disease. Front Med (Lausanne) 2020;7:123. doi: 10.3389/fmed.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurumi H., Yokoyama Y., Hirano T., et al. Cytokine profile in predicting the effectiveness of advanced therapy for ulcerative colitis: a narrative review. Biomedicines. 2024;12:952. doi: 10.3390/biomedicines12050952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyoshi J., Maeda T., Matsuoka K., et al. Machine learning using clinical data at baseline predicts the efficacy of vedolizumab at week 22 in patients with ulcerative colitis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-96019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morikubo H., Tojima R., Maeda T., et al. Machine learning using clinical data at baseline predicts the medium-term efficacy of ustekinumab in patients with ulcerative colitis. Sci Rep. 2024;14:4386. doi: 10.1038/s41598-024-55126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinton P. Prediction of vedolizumab treatment outcomes by machine learning. J Biopharm Stat. 2022;32:802–804. doi: 10.1080/10543406.2022.2065501. [DOI] [PubMed] [Google Scholar]
- 10.Nakase H., Uchino M., Shinzaki S., et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56:489–526. doi: 10.1007/s00535-021-01784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtiger S., Present D.H., Kornbluth A., et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–1845. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 12.Harvey R.F., Bradshaw J.M. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder K.W., Tremaine W.J., Ilstrup D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 14.Haider M., Lashner B. Dual targeted therapy for the management of inflammatory bowel disease. J Clin Gastroenterol. 2021;55:661–666. doi: 10.1097/MCG.0000000000001583. [DOI] [PubMed] [Google Scholar]
- 15.Wetwittayakhlang P., Lakatos P.L. Current evidence for combined targeted therapy for the treatment of inflammatory bowel disease. J Can Assoc Gastroenterol. 2024;7:22–29. doi: 10.1093/jcag/gwad032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obraztsov I.V., Shirokikh K.E., Obraztsova O.I., et al. Multiple cytokine profiling: a new model to predict response to tumor necrosis factor antagonists in ulcerative colitis patients. Inflamm Bowel Dis. 2019;25:524–531. doi: 10.1093/ibd/izy358. [DOI] [PubMed] [Google Scholar]
- 17.Fine S., Papamichael K., Cheifetz A.S. Etiology and management of lack or loss of response to anti-tumor necrosis factor therapy in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2019;15:656–665. [PMC free article] [PubMed] [Google Scholar]
- 18.Khoury T., Ilan Y. Introducing patterns of variability for overcoming compensatory adaptation of the immune system to immunomodulatory agents: a novel method for improving clinical response to anti-TNF therapies. Front Immunol. 2019;10:2726. doi: 10.3389/fimmu.2019.02726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.