Abstract
The apicomplexan parasites Cryptosporidium spp. are the causative agents of a severe diarrhoeal disease called cryptosporidiosis. Cryptosporidium species are capable of infecting a wide range of vertebrate hosts, including humans and livestock. In cattle, cryptosporidiosis is now one of the most important causes of neonatal scour globally, either as a sole agent or co-infecting with other pathogens. Cryptosporidiosis is considered globally endemic, with a prevalence of Cryptosporidium in stool samples from 13% to 93% in European cattle. This disease has a significant economic burden, with costs associated with veterinary diagnosis and medication, animal rearing, and supplemental nutrition. It is also associated with a reduced long-term growth rate in calves, causing huge economic losses in the livestock industry. Moreover, cattle act as a zoonotic reservoir for Cryptosporidium parvum, a species that is capable of infecting humans as well. As such, monitoring the prevalence of Cryptosporidium spp. in cattle is important due to the public health risk and financial burden the clinical disease causes. Publications reporting on the prevalence of Cryptosporidium spp. in cattle were collected from PubMed and Google Scholar. Information regarding the age of the animals, the species of Cryptosporidium in positive samples, the genotype of C. parvum found in samples, and the diarrhoeic status of the cattle was collected where available. A total of 248 publications were collected for this meta-analysis from six continents and 63 countries to provide an estimation for global bovine Cryptosporidium prevalence. The global prevalence of Cryptosporidium infection ranged between 27.0% and 37.5% in calves and pre-weaned cattle, respectively, with C. parvum being the most frequently identified species, particularly the IIa subfamily. Diarrhoea was reported in 7080 pre-weaned cattle samples, of which 38.1% tested positive for Cryptosporidium. Regarding symptoms, we found that in countries reporting over 50% of diarrhoeic positive cattle, C. parvum was the most common species. Continued monitoring and reporting of Cryptosporidium spp. in cattle are crucial for both public health and economic reasons. Consequently, efforts should focus on underreported regions and the development of control measures to reduce prevalence and limit zoonotic transmission.
Keywords: Cryptosporidium, Cryptosporidiosis, Cattle, Meta-analysis, Epidemiology
Graphical abstract
Highlights
-
•
We analysed 248 publications from 65 countries to estimate global bovine Cryptosporidium spp. prevalence.
-
•
The global prevalence of Cryptosporidium infection ranged between 27.0% and 37.5% in calves and pre-weaned cattle.
-
•
C. parvum was the most common species, with the IIa subfamily found globally, while IId subfamily found in China.
-
•
Diarrhoea was reported in 7080 pre-weaned cattle, of which 38.1% tested positive for Cryptosporidium.
1. Introduction
Since its identification in mice in 1907 (Tyzzer, 1907) there are now more than 49 recognised species of the apicomplexan parasite genus Cryptosporidium (Ryan et al., 2021a; Tůmová et al., 2023), the causative agents of the diarrhoeal disease cryptosporidiosis. Of these species, four are common in cattle: Cryptosporidium parvum, Cryptosporidium bovis, Cryptosporidium andersoni, and Cryptosporidium ryanae (Fayer et al., 2005, 2008; Xiao, 2010). There is an age-related distribution of the common species, with C. parvum being the predominant species in pre-weaned dairy calves, C. bovis and C. ryanae in post-weaned dairy calves, and C. andersoni in yearling calves and adults (Santín et al., 2004; Díaz et al., 2021). It is likely that the same distribution exists in beef cattle (Geurden et al., 2007; Feltus et al., 2008).
While four species are commonly found in cattle, only C. parvum and C. andersoni have been associated with clinical disease and thus can financially burden cattle farms. Cryptosporidium parvum infection frequently leads to diarrhoeal disease in pre-weaned calves (Harp and Goff, 1998; Thomson et al., 2017), and C. andersoni has been associated with a reduction in milk production and weight loss in adult cattle (Ralston et al., 2003, 2010). Genotyping of C. parvum at the 60 kDa glycoprotein (gp60) has resulted in the identification of over 120 different genotypes (Ryan et al., 2021a). In cattle, three subfamilies have been identified: IIa, IId, and IIl. The IIa subfamily, which includes the highly transmissible IIaA15G2R1 genotype (Feng et al., 2013), is most frequently found in Europe, while the IId subfamily is more prevalent in Asia, especially in China (Wang et al., 2017).
Cryptosporidium parvum is a zoonotic pathogen which occurs in cattle and was the second most frequent diagnosis in diseased cattle between 2015 and 2023 in Great Britain (https://www.gov.uk/government/publications/cattle-gb-disease-surveillance-and-emerging-threats-reports). Rises in the global number of Concentrated Animal Feeding Operations (CAFOs), particularly in industrialised countries have been associated with increased transmission of several pathogens including C. parvum, posing a further risk for increased transmission to humans (Guo et al., 2021).
As the global demand for livestock products, including cattle, is expected to increase in developing countries with a growing population (Thornton, 2010), monitoring the global distribution of Cryptosporidium spp. in cattle is important due to the public health risk of transmission to humans and for the health of farmed animals. Two previous meta-analyses have given estimations of global Cryptosporidium infections in cattle. Hatam-Nahavandi et al. (2019) presented the prevalence of Cryptosporidium spp. in terrestrial ungulates, including cattle, and Chen et al. (2023) presented the prevalence of C. parvum genotypes in dairy calves. Here, we expand on previous publications by first conducting a systematic review and meta-analysis of the global prevalence of Cryptosporidium spp. including C. parvum genotypes in cattle of all age groups. We analysed the health status of the animals and discuss the link between C. parvum and the prevalence of diarrhoea during infection. By not restricting the characteristics of sampled cattle, we present data for a larger sample size of 124,150 cattle to provide an updated estimation of global species and C. parvum genotype distribution.
2. Materials and methods
2.1. Search strategy
To identify publications reporting on Cryptosporidium prevalence in cattle, PubMed and manual searching via Google Scholar were used. Only papers published between January 2003 and October 2023 were included in the meta-analysis. In Google Scholar, searches were made using combinations of keywords “Cattle”, “Calf”, “Calves”, “Cryptosporidium”, and “cryptosporidiosis”. In PubMed, publications were identified using MeSH terms with the formula (((((cattle[MeSH Terms])) OR (calf)) OR (calves)) AND (Cryptosporidium[MeSH Terms])) OR (cryptosporidiosis[MeSH Terms])).
2.2. Publication inclusion and exclusion criteria
Publications were initially excluded based on abstract and title screening for relevance. Publications identified as potentially relevant were read in full and were included in the meta-analysis if the inclusion criteria were met. Publications were excluded based on several factors: incorrect host species; less than 20 samples in the overall study (n < 20); no clear age separation; publication was a review article; the full text was not available; was not in English or Spanish; the prevalence of Cryptosporidium was not stated; or prevalence results in the publication were already published in a different publication.
2.3. Data extraction
Data were extracted from publications meeting the inclusion criteria by two independent researchers. The following data were collected: publication year, first author, location of study (country), host species, clinical signs (diarrhoeic or non-diarrhoeic), age(s) of sampled cattle, detection method used, keeping status, total number of samples collected, total number of samples tested positive for Cryptosporidium, Cryptosporidium species identified, and C. parvum gp60 genotypes identified. Cattle were classified as pre-weaned (≤ 24 days) (Pardue et al., 1962), calf (24 days to 12 months) or adult (≥ 12 months) (Nafziger et al., 2021).
2.4. Quality assessment
Publications were quality assessed into high-, medium-, and low-quality studies following previous publications (Chen et al., 2023). The criteria were largely unchanged from Chen et al. (2023), with a study scoring one point for containing the following: (i) a clear research goal; (ii) a clear research period; (iii) a study size over 200 cows; (iv) a clear detection method; and (v) diarrhoea reported in cows.
2.5. Meta-analysis
The meta-analysis was conducted with the aim of enhancing knowledge of global Cryptosporidium prevalence and the distribution of species and C. parvum gp60 genotypes, given the burden of cattle losses to cryptosporidiosis and considering that cattle play a role in zoonotic transmission. All data were analysed in RStudio (version 4.3.1). The R packages meta (version 6.5-0) and metafor (version 4.4-0) were used to calculate the pooled prevalence of Cryptosporidium infection in cattle along with the 95% confidence interval (CI). Due to high heterogeneity (I2 > 50%, P < 0.1), a random effects model was used for meta-analysis. Forest plots were generated to display results by continent and by method used for Cryptosporidium detection. If multiple methods were used for Cryptosporidium detection in the same samples, the molecular method was included in analysis. The extent of publication bias was also tested using a funnel plot and Egger’s test. Sources of heterogeneity were assessed through meta-regression using the R package metafor (version 4.4-0). This meta-analysis and literature review were conducted in accordance with the PRISMA guidelines (Moher et al., 2009) (Supplementary Table S1) and following meta-analysis textbook (Harrer et al., 2022). Figures containing maps of identified Cryptosporidium spp. were generated using R packages dplyr (version 1.1.4), ggplot2 (version 3.5.1), rnaturalearth (version 1.0.1), sf (version 1.0.19), and scatterpie (version 0.2.4).
3. Results
Database searching revealed 1629 potentially relevant publications, of which 1262 were removed during initial screening of the publication title and abstract. The remaining 367 publications were further assessed, with 119 publications excluded due to not meeting the inclusion criteria. After all publications were assessed, 248 publications met the inclusion criteria and were included in the meta-analysis. These 248 publications resulted in 248 studies for use in the meta-analysis (Supplementary Fig. S1, Supplementary Table S2). This includes a total of 29 studies from Africa, 104 studies from Asia, 61 studies from Europe, 22 studies from North America, 19 studies from Oceania, and 13 studies from South America (Fig. 1 and Supplementary Table S2).
Fig. 1.
Global prevalence of Cryptosporidium spp. in calves. Estimation of Cryptosporidium spp. prevalence in calves and the number of studies per country.
The quality assessment identified 67.7% (168/248) of publications as of medium quality, followed by 30.2% (75/248) of studies as of high quality. Only 2.02% (5/248) of papers were of low quality and still included in the subsequent analysis.
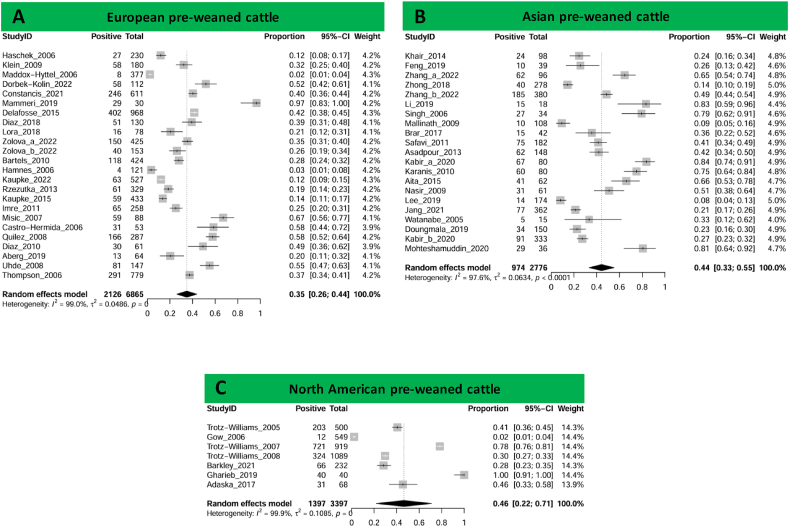
The overall pooled prevalence of Cryptosporidium worldwide was 26.5% (95% CI: 24.0–29.0%), comprising samples from 124,150 cattle (Supplementary Fig. S2). This prevalence ranges from 23.5% (95% CI: 16.0–31.0%) in South America to 33.5% (95% CI: 28.0–39.0%) in Europe, with North America and Asia presenting similar prevalence 25.5% (95% CI: 17.0–34.0%) and 24.5% (95% CI: 21.0–28.0%), respectively (Supplementary Fig. S3). Focusing only on pre-weaned cattle, the global prevalence was 37.5% (95% CI: 32.0–43.0%), ranging from 19.0% (95% CI: 10.0–28.0%) to 46.5% (95% CI: 22.0–71.0%) in Africa and North America, respectively (Fig. 2). Instead, in calves, the prevalence ranges from 16.5% (95% CI: 9.0–24.0%) to 38.5% (95% CI: 31.0–46.0%) in South America and Europe, respectively, with a global estimate of 27.0% (95% CI: 24.0–30.0%) (Supplementary Figs. S3 and S4).
Fig. 2.
Forest plots of estimated Cryptosporidium spp. prevalence in pre-weaned cattle from studies conducted in Europe (A), Asia (B), and North America (C).
We identified studies on pre-weaned cattle comprising 38 countries, with one study in 20 countries, while the rest had between 2 and 6 studies. The country with the lowest prevalence was Denmark (2.1%); however, this was only based on one study. Similarly, the country with the highest prevalence was the United Arab Emirates (80.6%); however, this was again based on one study. Japan, with three studies, has a prevalence of 75.0%, giving it the highest prevalence among countries with more than two studies. Contrariwise, Argentina, with six studies, has a prevalence of 24.5%, the lowest prevalence of countries with more than three studies. In calves, 56 countries with at least one study were identified, with 31 countries with one study, and 22 countries with two to 10 studies, India and Iran with 12 studies each and China with 40 (Fig. 1). Prevalence of infection in calves ranged between 0.6% in South Africa and 76.8% in Myanmar.
Cryptosporidium spp. can be detected using several methods, including conventional microscopy (CM), immunochromatographic test (ICT), immunofluorescence antibody test (IFA), PCR and ELISA, all with different detection capacities. To assess whether the method of detection used in each study might impact the estimated pooled prevalence, we first grouped methods as microscopic detection (CM), molecular detection (PCR) or immunological detection (IFA, ICT and ELISA). Then, we grouped the studies by method of detection and calculated the pooled prevalence in each type of method used. A total of 52 studies used immunological detection methods, while 88 and 108 studies used molecular and microscopy methods, respectively. Immunological detection methods resulted in a higher pooled prevalence (32.5%; 95% CI: 26.0–39.0%) than the other detection methods, while the pooled prevalence of the most used methods (microscopic and molecular detection methods) ranged between 25.0% (95% CI: 22.0–28.0%) and 24.0% (95% CI: 20.0–28.0%) (Table 1, Supplementary Fig. S5).
Table 1.
Summary of bovine Cryptosporidium prevalence and statistical analysis by continent, and detection method.
| Variable | Overall prevalence (95% CI) (%) | Age prevalence (95% CI) (%) |
||
|---|---|---|---|---|
| Pre-weaned | Calf | Adult | ||
| Continent | ||||
| Africa | 21.0 (18.0–24.0) | 19.0 (10.0–28.0) | 23.0 (18.0–28.0) | 19.5 (15.0–23.0) |
| Asia | 24.5 (21.0–28.0) | 44.0 (32.0–55.0) | 25.0 (21.0–29.0) | 15.0 (9.0–21.0) |
| Europe | 33.5 (28.0–39.0) | 35.0 (26.0–44.0) | 38.5 (31.0–46.0) | 23.5 (12.0–35.0) |
| North America | 25.5 (17.0–34.0) | 46.5 (22.0–71.0) | 26.5 (17.0–36.0) | 10.5 (5.0–16.0) |
| South America | 23.5 (16.0–31.0) | 33.0 (22.0–44.0) | 16.5 (9.0–24.0) | 3.0 (0.0–6.0) |
| Oceania | 27.0 (15.0–39.0) | 37.0 (19.0–55.0) | 28.0 (8.0–48.0) | 10.0 (0.0–20.0) |
| Method | ||||
| Molecular | 24.0 (20.0–28.0) | 33.5 (24.0–43.0) | 27.0 (22.0–32.0) | 12.5 (8.0–17.0) |
| Microscopy | 25.0 (22.0–28.0) | 39.0 (32.0–46.0) | 23.5 (20.0–27.0) | 15.5 (10.0–21.0) |
| Immunological | 32.5 (26.0–39.0) | 40.5 (26.0–55.0) | 36.5 (28.0–45.0) | 21.0 (9.0–33.0) |
Abbreviation: CI, confidence interval.
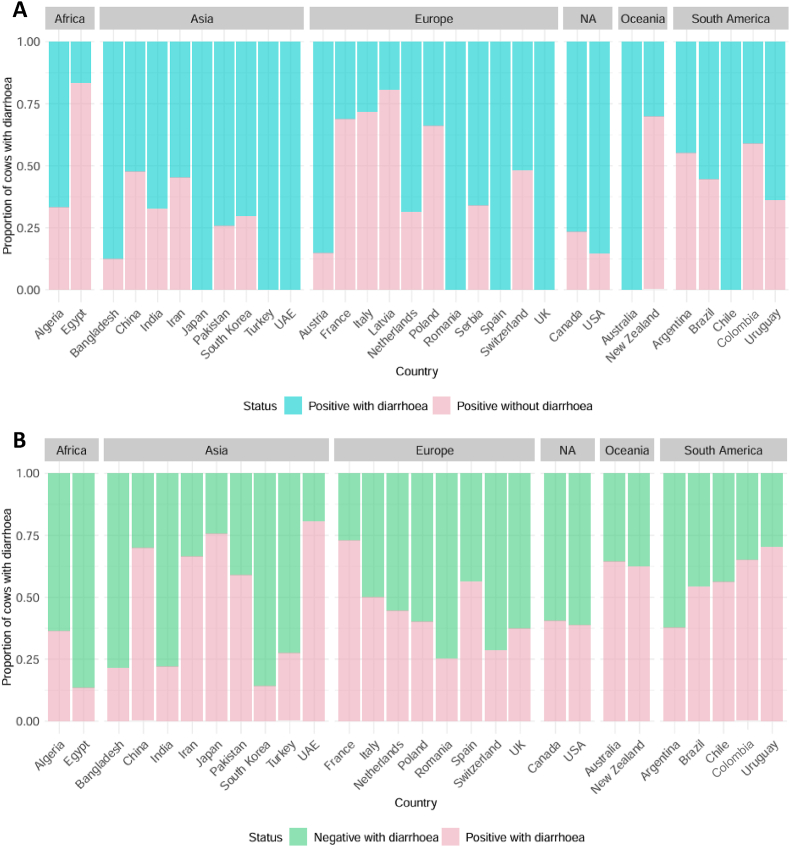
Cryptosporidium usually leads to diarrhoea in most animals infected; however, in some instances, infected animals do not develop symptoms. To estimate the proportion of these cases around the globe, we analysed a total of 60 studies across 32 countries reporting on the prevalence of diarrhoea in the pre-weaned cattle (Fig. 3A). Of these studies, 17 countries only had data from one study reporting on diarrhoea prevalence, 13 countries had 2–4 studies, with Argentina and China having 5 studies each.
Fig. 3.
Global estimates of infected pre-weaned cattle with or without diarrhoea (A) and diarrhoeic pre-weaned cattle that are either positive or negative for Cryptosporidium spp. infection (B). Abbreviation: NA, North America.
The majority of countries reported ≤ 50% of positive cattle exhibiting diarrhoea; Egypt reported only 13.3% of animals testing positive and having diarrhoea (Fig. 3A). Interestingly, France and Poland are two examples with larger available datasets where less than 50% of all positive cattle exhibited diarrhoea. African countries reported 23.1% of infected animals presenting diarrhoea, while Asian and Oceanian countries presented 39.3% and 64.2% of infected symptomatic animals (Fig. 3A). Because diarrhoea can be caused by other infections, studies also analysed the presence of Cryptosporidium in symptomatic animals (Fig. 3B). In cattle with diarrhoea, 38.1% tested positive across all countries, but a large variation between countries was observed, with Egypt and reporting the lowest percentage of 13.3%, while UAE had the highest percentage of 85.2%.
A total of 59.8% (16,201/27,083) of animals infected by Cryptosporidium spp. were assessed to determine the exact species causing the infection. The number of samples with detailed Cryptosporidium species ranged from 85.7% (3281/3827), 80.8% (953/1180), 65.6% (5941/9052), 56.3% (5092/9052), 28.0% (614/2192), and 15.3% (320/2089) of samples in North America, Oceania, Europe, Asia, Africa, and South America, respectively. By country, this percentage varied, with several countries' studies determining all or more than 75% of samples, while other countries’ studies determined none or less than 25% of samples. From all studies, 98.3% (15933/16201) were mono-infections, with 1.7% (268/16201) reported incidents of mixed infections - the majority of which were identified as C. parvum + C. bovis (32.5%; 87/268) or C. bovis + C. rynae (29.5%; 79/268). Mono-infection of C. parvum was identified in 67.8% (10,983/16,210) of samples, C. andersoni in 15.3% (2488/16,210) of samples, C. bovis in 9.6% (1558/16,210) of samples, and C. ryanae in 3.7% (594/16,210) of samples. Within the identified Cryptosporidium spp. in Asia, 86.2% (629/730) of pre-weaned cattle were mono-infected with C. parvum compared to 30.6% (217/708) of adults. However, 58.8% (416/708) of adult cow infections were due to C. andersoni, the highest in all three age groups in Asia. Most mono-infections in calves were with C. parvum (54.8%; 2002/3654). Asian calves had the highest proportion of C. bovis infection (23.3%; 850/3654) compared to Asian pre-weaned (10.3%; 75/730) and adult cattle (7.1%; 50/708). Other Cryptosporidium species in Asia were mostly identified in calves (1.0%; 35/3654), with the highest reports of C. canis (0.2%; 9/3654). Determined mixed infections were highest in calves (3.2%; 117/3654) reported only in China and Malaysia (Fig. 4). Most reported mixed infections were C. bovis and C. rynae (35.9%; 42/117), followed by C. parvum and C. bovis (23.9%; 28/117) in Asian calves. The trend of species most predominantly found within mono-infections across age groups is similar to other continents, although South American calves were mainly infected with C. andersoni. North America had the greatest proportion of other Cryptosporidium infections (11.8%; 227/1927) in calf and adult cattle with Cryptosporidium (bovine b) (54.9%; 79/144), and C. muris (84.3%; 70/83) in each age group, respectively. In Africa, co-infections reports were highest in calves and followed the same trend seen in Asia, C. bovis and C. rynae (48.9%; 21/43), with C. parvum and C. bovis (23.3%; 10/43) joined by C. parvum and C. rynae (23.3%; 10/43). Globally, C. parvum was the major species identified, while mixed infections made up a small proportion of cryptosporidiosis on each continent, with all cattle ages considered (Supplementary Fig. S6).
Fig. 4.
Distribution and frequency of Cryptosporidium spp. in Asia by pre-weaned (A), calf (B), and adult (C) cattle.
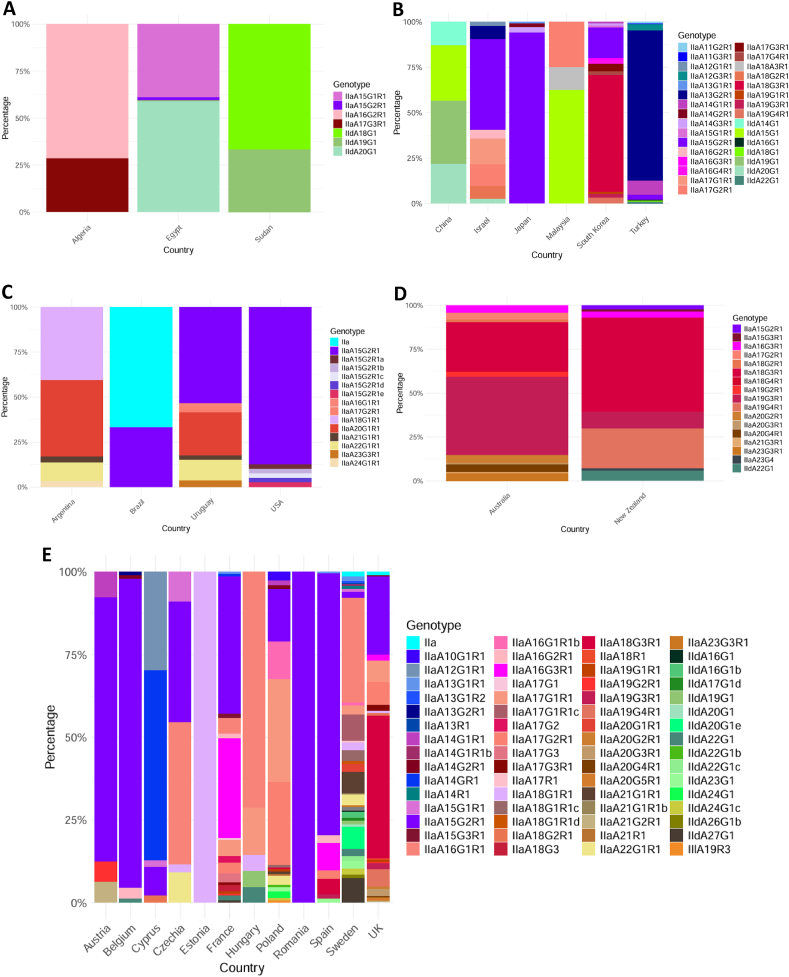
For all studies reporting C. parvum cases, 30.5% (3405/11160) were sub-typed, with three sub-type families identified: IIa (2336/3405; 68.6%), IId (1068/3405; 31.4%), and IIl (1/3405; 0.03%). The IIa family was found in all six continents with data, while the IId family was identified in all except the Americas, and the IIl family was only found in Europe (Fig. 5).
Fig. 5.
Distribution of Cryptosporidium parvum genotypes in different regions: Africa (A), Asia (B), North and South America (C), Oceania (D), and Europe (E).
The IIa family was the most prevalent one in all continents with data except for Africa and Asia, where the IId family was most frequently identified, with the majority (886/1068; 83.0%) of IId cases originating from China. For the 65 genotypes identified in the IIa family, 18 were identified once, 22 were identified 2–10 times, 20 were identified 11–99 times, and five were identified ≥ 100 times. The eight most common genotypes were IIaA15G2R1 (758/2336; 32.4%), IIaA18G3R1 (292/2336; 12.5%), IIaA13G2R1 (176/2336; 7.5%), IIaA16G1R1 (150/2336; 6.4%), IIaA19G3R1 (109/2336; 4.7%), IIaA17G1R1 (87/2336; 3.7%), IIaA17G2R1 (86/2336; 3.7%), and IIaA16G3R1 (77/2336; 3.3%). For the 18 identified IId genotypes, one was identified once, ten were identified 2–10 times, three were identified 11–99 times, and four were identified ≥ 100 times. The four most common genotypes were IIdA19G1 (312/1068; 29.2%), IIdA20G1 (282/1068; 26.4%), IIdA15G1 (275/1068; 25.7%), and IIdA14G1 (115/1068; 10.8%).
Publication bias was assessed using funnel plots and Egger’s test (Supplementary Fig. S7). The funnel plot for all data showed obvious asymmetry with many datapoints falling outside of the funnel, which are both indicators of publication bias. This is consistent with the results of the Egger’s test, indicating strong publication bias with P < 0.0001. There is also evidence of publication bias with P < 0.01 for all continents with enough studies to assess except for South America. By methodology, the results of the Egger’s test suggest publication bias (P = 0.002) for immunological methods but not for conventional microscopy or molecular techniques (Table 2).
Table 2.
Assessment of heterogeneity and publication bias in publications reporting prevalence of Cryptosporidium in pre-weaned cattle.
| Variable | Pooled prevalence |
Heterogeneity |
Publication bias |
|||||
|---|---|---|---|---|---|---|---|---|
| Pooled (%) | OR (95% CI) | I2 (%) | Q | df | Egger bias | t-value | Egger P-value | |
| Global | 37.51 | 32.14–42.89 | 99.3 | 10053.26 | 75 | 9.41 | 4.48 | <0.0001 |
| Africa | 19.03 | 10.15–27.91 | 79.9 | 14.93 | 3 | Too small (3 studies) | ||
| Asia | 44.3 | 33.29–55.32 | 97.6 | 818.91 | 20 | 9.21 | 3.66 | 0.0017 |
| Europe | 34.91 | 25.98–43.83 | 99.0 | 2208.8 | 23 | 11.21 | 5.51 | <0.0001 |
| North America | 46.44 | 21.95–70.93 | 99.9 | 4878.06 | 6 | Too small (7 studies) | ||
| Oceania | 36.92 | 18.78–55.06 | 98.7 | 297.12 | 4 | Too small (5 studies) | ||
| South America | 33.01 | 22.09–43.93 | 99.1 | 1561.23 | 14 | 7.75 | 1.19 | 0.250 |
| Microscopy | 39.07 | 32.36–45.79 | 99.0 | 3360.43 | 33 | −1.35 | −0.33 | 0.742 |
| Immunological | 40.47 | 26.14–54.79 | 99.3 | 2133.8 | 16 | 12.92 | 4.89 | 0.002 |
| Molecular | 33.44 | 23.71–43.17 | 98.7 | 1879.19 | 24 | 7.17 | 2.03 | 0.054 |
Abbreviations: CI, confidence interval; df, degrees of freedom.
The meta-analysis revealed substantial heterogeneity across the included studies (Table 2). Potential sources of heterogeneity were explored using meta-regression and backward elimination of predictors (Supplementary Table S3). The best model included age and diarrhoea status of the animals and accounted for 11.60% of heterogeneity.
4. Discussion
This meta-analysis and systematic review identified a global pooled prevalence of 26.5% from 248 studies encompassing 124,150 cattle samples across 63 countries. Interestingly, when splitting publications by detection method, PCR and CM yielded very similar pooled prevalence ∼24.5%, despite PCR being a higher sensitivity method (Ahmed and Karanis, 2018). CM is less sensitive, but cheaper and thus may still be capable of providing accurate estimates of Cryptosporidium prevalence in lower-income countries, which are the most underrepresented in this meta-analysis.
Cattle serve as a known reservoir of zoonotic cryptosporidiosis (Chako et al., 2010; Ryan et al., 2021b), with the majority of human cryptosporidiosis cases caused by either C. hominis or C. parvum. Additionally, the implications of cryptosporidiosis in cattle contribute to large financial losses, with the USDA in 2015 reporting losses of $3.87 billion due to cattle deaths from non-predator causes, of which 15.4% were due to digestive problems, including cryptosporidiosis (USDA, 2015; Roblin et al., 2023). This, coupled with increasing global demand for livestock products to support the increasing population (Alexandratos and Bruinsma, 2012), makes monitoring the global distribution of Cryptosporidium in cattle vital. Concentrated Animal Feeding Operations (CAFOs) have been associated with a rise in zoonotic pathogens including Cryptosporidium, and the findings of this meta-analysis show that a lower prevalence of Cryptosporidium is found in Africa and Asia, where cattle rearing is typically less intensive (Fig. 1). Higher rates were found in Japan, Europe, and North America, which are countries with higher intensity rearing (Guo et al., 2021), with outbreaks reportedly rising in recent years in China due to increasing cattle CAFOs (Guo et al., 2022).
Cryptosporidium parvum is a known causative of diarrhoea in neonatal calves (Harp and Goff, 1998; Thomson et al., 2017), and the majority of included studies sampled this age group. A total of 14 of 31 countries with data reported over 50% of samples being diarrhoeic, and C. parvum was the most frequent species identified in these countries (Fig. 3, Fig. 4). In the UK, it has been reported that costs associated with severe cryptosporidiosis symptoms in calves can amount to approximately £200 per calf when totalling veterinary costs and loss of market value due to weight loss (Wells and Thomson, 2014), thus outbreaks of Cryptosporidium can lead to a significant financial burden for farms. Globally, there were also cases of cattle having diarrhoea that was not linked to a positive Cryptosporidium sample, indicating the potential presence other infectious agents frequently found in cattle as well as Cryptosporidium (Fig. 3) (Blanchard, 2012).
The distribution of Cryptosporidium species was the most underreported in the African and South American continents. Cryptosporidium parvum, C. bovis, C. andersoni, and C. ryanae are the most common species infecting cattle (Feng et al., 2018; Díaz et al., 2021), and this is shown in the meta-analysis, with C. parvum being the most frequently identified species, likely due to the majority of included studies involving neonatal calves (Fig. 4). Cryptosporidium andersoni was the most frequently identified species in the Czech Republic, Ethiopia, and Mongolia, and C. rynae was the most frequently identified species in Nepal, Sri Lanka, and Vietnam. Cryptosporidium bovis was majorly identified in Colombia and Nigeria, while Cryptosporidium deer-like in Kenya. All five of the most frequent species reported in humans (C. hominis, C. parvum, C. meleagridis, C. canis, and C. felis) were identified (Ryan et al., 2021b), furthering the evidence of cattle acting as a zoonotic reservoir. Not only is the global C. parvum distribution capable of compromising the health of calves, but the identification of species capable of infecting humans also poses a public health risk. Global estimates suggest that the yearly oocyst load of livestock manure is 3.23 × 1023, with cattle being large contributors (Vermeulen et al., 2017). Contamination of food and water sources with oocysts shed by cattle has been associated with food-borne (Budu-Amoako et al., 2011; Ryan et al., 2018; Zahedi and Ryan, 2020) and water-borne (Mac Kenzie et al., 1994; Zahedi and Ryan, 2020; Gururajan et al., 2021) outbreaks.
The C. parvum IIa subfamily was the most widely distributed one, making up the majority of infections in Europe, North America, Oceania, and South America, while the IId subfamily was the source of most infections in Africa and Asia (Fig. 5). North America was significantly under-reported, with 1.5% (40/2712) of C. parvum cases genotyped, while the five other continents had genotyped between 32.4% and 90.5% of cases. There was also a large variation in the number of different genotypes identified in each country, particularly noticeable in Sweden, where 34 different genotypes were found. The highly transmissible IIaA15G2R1 (Xiao, 2010; Feng et al., 2013) was the dominant IIa subfamily genotype, particularly in North and South America. This genotype is also dominant in human C. parvum infections in countries where it is a common bovine genotype (Xiao, 2010; Zahedi and Ryan, 2020). The IIaA18G3R1 genotype was also frequently identified in Asia and Oceania and has been the cause of human infections in Ireland (O’Leary et al., 2020). The dominant IId subfamily genotype, IIdA19G1, was identified primarily in cattle from China, with only several cases in Africa and Europe. This genotype has been linked to human infections in China (Yu et al., 2019) and Scotland (Deshpande et al., 2015).
Our meta-analysis aims to provide a recent estimate of global Cryptosporidium prevalence in cattle using studies published in the last 20 years. This search strategy gave a large sample size of 124,150 cattle across six continents and 63 countries. However, despite this large dataset, our study has several limitations. First, only data from publicly accessible journals and published in English or Spanish are included. Additionally, there are still many countries with no published data, and for some included countries, there were few publications available, meaning many regions were underrepresented. This became evident particularly when quantifying Cryptosporidium spp. and C. parvum genotype distribution, where the number of species or genotypes identified from positive samples varied greatly between continents and countries. Statistical analysis also revealed high levels of publication bias and heterogeneity, which can affect the results (Table 2) (Cochrane Handbook for Systematic Reviews of Interventions, https://training.cochrane.org/handbook/current). This was explored using meta-regression, and age and diarrhoea status were identified as predictors; however, there are still further factors that could be explored such as the farming status, rearing intensity, or sex of sampled cattle, but these data are not frequently provided in detail in publications. The sampled cattle from the various continents are likely sources of heterogeneity due to the sampling bias created by the selection of symptomatic cattle, and through different continents having differing prevalence and reporting rates of Cryptosporidium. Lastly, the majority of studies were of medium quality, where the majority of studies did not report diarrhoea within the sampled cows or included sample sizes below 200 animals. Given the limitations from the lack of predictor data, it is evident that further work is needed to ensure equal and accurate reporting of global Cryptosporidium prevalence, especially regarding the public health risk posed by cryptosporidiosis and zoonotic species of Cryptosporidium.
5. Conclusions
The findings of this meta-analysis suggest widespread bovine Cryptosporidium infection, with approximately one in five sampled cattle testing positive. The data also showed significant variance by continent and country, although the underrepresentation of certain regions is a likely contributor to this. Regions with higher numbers of CAFOs also had higher infection rates, but additional research is needed to establish causality. Cryptosporidium parvum was the most identified Cryptosporidium species, with the IIa subfamily found globally and the IId subfamily most frequently found in China. The prevalence of diarrhoea in sampled cattle also varied; however, in countries with higher rates of cattle exhibiting diarrhoea, C. parvum was the dominant species. Effective control measures should continue to be developed and implemented to prevent infection in cattle, which can limit the risk of transfer to humans. Additionally, further efforts should be undertaken to report on the prevalence and transmission in countries with limited or no data to ensure that control measures are implemented effectively where needed. To that end, adopting a One Health approach, which underscores the interconnectedness of human, animal, and environmental health, is crucial for developing comprehensive strategies to control and prevent Cryptosporidium infections, thereby safeguarding public health and reducing economic losses in the livestock industry.
CRediT authorship contribution statement
Rachel Buchanan: Investigation, Formal analysis, Software, Writing - original draft. Przemyslaw Wieckowski: Investigation, Formal analysis, Software, Data curation, Writing - review & editing. Eleni Matechou: Methodology, Software. Frank Katzer: Conceptualization, Writing - review & editing. Anastasios Tsaousis: Conceptualization, Resources, Writing - review & editing. Marta Farré: Supervision, Conceptualization, Project administration, Investigation, Formal analysis, Software, Writing – original draft, Writing - review & editing.
Ethical approval
Not applicable.
Data availability
The data supporting the conclusions of this article are included within the article and its supplementary files.
Funding
Rachel Buchanan is supported by SoCoBio DTP studentship. Frank Katzer is supported by the Moredun Foundation and the Scottish Government through the Rural and Environment Science and Analytical Services (RESAS) Strategic Research Programme 2022–2027. Marta Farré is supported by the Royal Society Research Grant (RGS\R1\211047) and by the Biotechnology and Biological Sciences Research Council Grant (BB/X511158/1).
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Given their role as Co-Editor, Frank Katzer had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Professor Aneta Kostadinova (Editor-in-Chief).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2025.100264.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Supplementary Figure S1. Flow diagram of the publication inclusion process. Multimedia component 1
Supplementary Table S3. Meta-regression analysis. Multimedia component 10
Supplementary Figure S2. Forest plots of Cryptosporidium prevalence across age groups globally. Multimedia component 2
Supplementary Figure S3. Forest plots of Cryptosporidium prevalence, separated by age and continent. Multimedia component 3
Supplementary Figure S4. Map of Cryptosporidium global prevalence within calves and study count. Multimedia component 4
Supplementary Figure S5. Forest plot of Cryptosporidium prevalence, separated by age, depending on the method of detection used: immunological detection, microscopic detection, and molecular detection. Multimedia component 5
Supplementary Figure S6. Proportion of animals infected by Cryptosporidium with the exact species identified across continents separated by age. Multimedia component 6
Supplementary Figure S7. Funnel plots to assess publication bias in the included studies. Multimedia component 7
Supplementary Table S1. PRISMA checklist. Multimedia component 8
Supplementary Table S2. List of publications and data gathered for the meta-analysis. Multimedia component 9
References
- Ahmed S.A., Karanis P. Comparison of current methods used to detect Cryptosporidium oocysts in stools. Int. J. Hyg Environ. Health. 2018;221:743–763. doi: 10.1016/j.ijheh.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Alexandratos N., Bruinsma J. Food and Agriculture Organization of the United Nations; Rome: 2012. World agriculture towards 2030/2050: the 2012 revision. ESA Working paper No. 12-03.https://www.fao.org/4/ap106e/ap106e.pdf [Google Scholar]
- Blanchard P.C. Diagnostics of dairy and beef cattle diarrhea. Vet. Clin. North Am. Food Anim. Pract. 2012;28:443–464. doi: 10.1016/j.cvfa.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budu-Amoako E., Greenwood S.J., Dixon B.R., Barkema H.W., McClure J.T. Foodborne illness associated with Cryptosporidium and Giardia from livestock. J. Food Protect. 2011;74:1944–1955. doi: 10.4315/0362-028X.JFP-11-107. [DOI] [PubMed] [Google Scholar]
- Chako C.Z., Tyler J.W., Schultz L.G., Chiguma L., Beerntsen B.T. Cryptosporidiosis in people: It’s not just about the cows. J. Vet. Intern. Med. 2010;24:37–43. doi: 10.1111/j.1939-1676.2009.0431.x. [DOI] [PubMed] [Google Scholar]
- Chen Y., Huang J., Qin H., Wang L., Li J., Zhang L. Cryptosporidium parvum and gp60 genotype prevalence in dairy calves worldwide: A systematic review and meta-analysis. Acta Trop. 2023;240 doi: 10.1016/j.actatropica.2023.106843. [DOI] [PubMed] [Google Scholar]
- Deshpande A.P., Jones B.L., Connelly L., Pollock K.G., Brownlie S., Alexander C.L. Molecular characterization of Cryptosporidium parvum isolates from human cryptosporidiosis cases in Scotland. Parasitology. 2015;142:318–325. doi: 10.1017/S0031182014001346. [DOI] [PubMed] [Google Scholar]
- Díaz P., Navarro E., Remesar S., García-Dios D., Martínez-Calabuig N., Prieto A., et al. The age-related Cryptosporidium species distribution in asymptomatic cattle from north-western Spain. Animals. 2021;11:256. doi: 10.3390/ani11020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R., Santín M., Trout J.M. Cryptosporidium ryanae n. sp. (Apicomplexa: Cryptosporidiidae) in cattle (Bos taurus) Vet. Parasitol. 2008;156:191–198. doi: 10.1016/j.vetpar.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Fayer R., Santín M., Xiao L. Cryptosporidium bovis n. sp. (Apicomplexa: Cryptosporidiidae) in cattle (Bos taurus) J. Parasitol. 2005;91:624–629. doi: 10.1645/GE-3435. [DOI] [PubMed] [Google Scholar]
- Feltus D.C., Giddings C.W., Khaitsa M.L., McEvoy J.M. High prevalence of Cryptosporidium bovis and the deer-like genotype in calves compared to mature cows in beef cow-calf operations. Vet. Parasitol. 2008;151:191–195. doi: 10.1016/j.vetpar.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Feng Y., Ryan U.M., Xiao L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018;34:997–1011. doi: 10.1016/j.pt.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Feng Y., Torres E., Li N., Wang L., Bowman D., Xiao L. Population genetic characterisation of dominant Cryptosporidium parvum subtype IIaA15G2R1. Int. J. Parasitol. 2013;43:1141–1147. doi: 10.1016/j.ijpara.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Geurden T., Berkvens D., Martens C., Casaert S., Vercruysse J., Claerebout E. Molecular epidemiology with subtype analysis of Cryptosporidium in calves in Belgium. Parasitology. 2007;134:1981–1987. doi: 10.1017/S0031182007003460. [DOI] [PubMed] [Google Scholar]
- Guo Y., Ryan U., Feng Y., Xiao L. Association of common zoonotic pathogens with concentrated animal feeding operations. Front. Microbiol. 2021;12:810142. doi: 10.3389/fmicb.2021.810142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Ryan U., Feng Y., Xiao L. Emergence of zoonotic Cryptosporidium parvum in China. Trends Parasitol. 2022;38:335–343. doi: 10.1016/j.pt.2021.12.002. [DOI] [PubMed] [Google Scholar]
- Gururajan A., Rajkumari N., Devi U., Borah P. Cryptosporidium and waterborne outbreaks - a mini review. Tropenmed. Parasitol. 2021;11:11–15. doi: 10.4103/tp.TP_68_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harp J.A., Goff J.P. Strategies for the control of Cryptosporidium parvum infection in calves. J. Dairy Sci. 1998;81:289–294. doi: 10.3168/jds.S0022-0302(98)75578-X. [DOI] [PubMed] [Google Scholar]
- Harrer M., Cuijpers P., Furukawa T.A., Ebert D.D. CRC Press; Boca Raton, FL, USA: 2022. Doing Meta-Analysis with R: A Hands-On Guide.https://www.routledge.com/Doing-Meta-Analysis-with-R-A-Hands-On-Guide/Harrer-Cuijpers-Furukawa-Ebert/p/book/9780367610074 [Google Scholar]
- Hatam-Nahavandi K., Ahmadpour E., Carmena D., Spotin A., Bangoura B., Xiao L. Cryptosporidium infections in terrestrial ungulates with focus on livestock: A systematic review and meta-analysis. Parasites Vectors. 2019;12:453. doi: 10.1186/s13071-019-3704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Kenzie W.R., Hoxie N.J., Proctor M.E., Gradus M.S., Blair K.A., Peterson D.E., et al. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafziger S.R., Tenley S.C., Summers A.F., Abedal-Majed M.A., Hart M., Bergman J.W., et al. Attainment and maintenance of pubertal cyclicity may predict reproductive longevity in beef heifers. Biol. Reprod. 2021;104:1360–1372. doi: 10.1093/biolre/ioab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary J.K., Blake L., Corcoran G.D., Sleator R.D., Lucey B. Increased diversity and novel subtypes among clinical Cryptosporidium parvum and Cryptosporidium hominis isolates in Southern Ireland. Exp. Parasitol. 2020;218 doi: 10.1016/j.exppara.2020.107967. [DOI] [PubMed] [Google Scholar]
- Pardue F.E., Jacobson D.R., Graden A.P., Seath D.M. Performance of dairy calves weaned at 24 days of age and fed vegetable vs. animal source protein in the dry starter. J. Dairy Sci. 1962;45:986–989. doi: 10.3168/jds.S0022-0302(62)89539-3. [DOI] [Google Scholar]
- Ralston B., Thompson R.C.A., Pethick D., McAllister T.A., Olson M.E. Cryptosporidium andersoni in Western Australian feedlot cattle. Aust. Vet. J. 2010;88:458–460. doi: 10.1111/j.1751-0813.2010.00631.x. [DOI] [PubMed] [Google Scholar]
- Ralston B.J., Cockwill C.L., Guselle N.J., Van Herk F.H., McAllister T.A., Olson M.E. Prevalence of Giardia and Cryptosporidium andersoni and their effects on performance in feedlot beef cattle. Can. J. Anim. Sci. 2003;83:153–159. doi: 10.4141/A01-001. [DOI] [Google Scholar]
- Roblin M., Canniere E., Barbier A., Daandels Y., Dellevoet-Groenewegen M., Pinto P., et al. Study of the economic impact of cryptosporidiosis in calves after implementing good practices to manage the disease on dairy farms in Belgium, France, and The Netherlands. Curr. Res. Parasitol. Vector Borne Dis. 2023;4 doi: 10.1016/j.crpvbd.2023.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U., Hijjawi N., Xiao L. Foodborne cryptosporidiosis. Int. J. Parasitol. 2018;48:1–12. doi: 10.1016/j.ijpara.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Ryan U., Zahedi A., Feng Y., Xiao L. An update on zoonotic Cryptosporidium species and genotypes in humans. Animals. 2021;11:3307. doi: 10.3390/ani11113307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U.M., Feng Y., Fayer R., Xiao L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia - a 50 year perspective (1971-2021) Int. J. Parasitol. 2021;51:1099–1119. doi: 10.1016/j.ijpara.2021.08.007. [DOI] [PubMed] [Google Scholar]
- Santín M., Trout J.M., Xiao L., Zhou L., Greiner E., Fayer R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 2004;122:103–117. doi: 10.1016/j.vetpar.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Thomson S., Hamilton C.A., Hope J.C., Katzer F., Mabbott N.A., Morrison L.J., Innes E.A. Bovine cryptosporidiosis: Impact, host-parasite interaction and control strategies. Vet. Res. 2017;48:42. doi: 10.1186/s13567-017-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:2853–2867. doi: 10.1098/rstb.2010.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tůmová L., Ježková J., Prediger J., Holubová N., Sak B., Konečný R., et al. Cryptosporidium mortiferum n. sp. (Apicomplexa: Cryptosporidiidae), the species causing lethal cryptosporidiosis in Eurasian red squirrels (Sciurus vulgaris) Parasites Vectors. 2023;16:235. doi: 10.1186/s13071-023-05844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzzer E.E. A sporozoan found in the peptic glands of the common mouse. Proc. Soc. Exp. Biol. Med. 1907;5:12–13. doi: 10.3181/00379727-5-5. [DOI] [Google Scholar]
- USDA . United States Department of Agriculture; Fort Collins, USA: 2015. Death loss in U.S. cattle and calves due to predator and nonpredator causes, 2015.https://www.aphis.usda.gov/sites/default/files/cattle_calves_deathloss_2015.pdf [Google Scholar]
- Vermeulen L.C., Benders J., Medema G., Hofstra N. Global Cryptosporidium loads from livestock manure. Environ. Sci. Technol. 2017;51:8663–8671. doi: 10.1021/acs.est.7b00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Zhao G., Gong Y., Zhang L. Advances and perspectives on the epidemiology of bovine Cryptosporidium in China in the past 30 years. Front. Microbiol. 2017;8:1823. doi: 10.3389/fmicb.2017.01823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells B., Thomson S. Cryptosporidiosis in cattle. The Moredun Foundation. News Sheet. 2014;6(1) February 2014. [Google Scholar]
- Xiao L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Yu F., Li D., Chang Y., Wu Y., Guo Z., Jia L., et al. Molecular characterization of three intestinal protozoans in hospitalized children with different disease backgrounds in Zhengzhou, central China. Parasites Vectors. 2019;12:543. doi: 10.1186/s13071-019-3800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A., Ryan U. Cryptosporidium - an update with an emphasis on foodborne and waterborne transmission. Res. Vet. Sci. 2020;132:500–512. doi: 10.1016/j.rvsc.2020.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Flow diagram of the publication inclusion process. Multimedia component 1
Supplementary Table S3. Meta-regression analysis. Multimedia component 10
Supplementary Figure S2. Forest plots of Cryptosporidium prevalence across age groups globally. Multimedia component 2
Supplementary Figure S3. Forest plots of Cryptosporidium prevalence, separated by age and continent. Multimedia component 3
Supplementary Figure S4. Map of Cryptosporidium global prevalence within calves and study count. Multimedia component 4
Supplementary Figure S5. Forest plot of Cryptosporidium prevalence, separated by age, depending on the method of detection used: immunological detection, microscopic detection, and molecular detection. Multimedia component 5
Supplementary Figure S6. Proportion of animals infected by Cryptosporidium with the exact species identified across continents separated by age. Multimedia component 6
Supplementary Figure S7. Funnel plots to assess publication bias in the included studies. Multimedia component 7
Supplementary Table S1. PRISMA checklist. Multimedia component 8
Supplementary Table S2. List of publications and data gathered for the meta-analysis. Multimedia component 9
Data Availability Statement
The data supporting the conclusions of this article are included within the article and its supplementary files.