Abstract
Amyloidosis, which is caused by misfolded proteins that form amyloid fibrils, is predominantly diagnosed in older adults. Although previously considered a rare disease, increased awareness and noninvasive diagnostic methods have resulted in a rise in diagnoses. As a multisystem disease that affects multiple organ systems (cardiac, gastrointestinal, renal, and neurological), there is significant overlap with both geriatric conditions and common conditions in heart failure. Frailty is recognized as a distinct biological syndrome of declines across multiple physiological systems, which prevents maintenance of homeostasis and limits the ability to respond to stressors. Frailty was initially characterized as physical frailty alone; however, it is increasingly recognized that it is multidimensional with components including nutrition, cognitive, psychological, and social. Frailty in cardiovascular disease has become an important risk factor, indicator for disease severity, and can help guide decisions around intervention. In certain patients, frailty may be reversible. Given the lack of consensus definitions, tools, and implementation of frailty in both clinical and research settings in the field of amyloidosis, we convened a group of experts from cardiology, geriatric cardiology, geriatrics, hematology, and allied health to form this state-of-the-art review. There are many points of intersectionality between amyloidosis, aging, and frailty which herald a need for multidisciplinary care. This review document aims to provide guidance in how to understand and address frailty in older patients with a specific focus on cardiac amyloidosis.
Key words: aging, amyloidosis, frailty, geriatric syndromes, inflammation, multimorbidity, multisystem dysregulation, older adults
Central Illustration
Amyloidosis, a cause of heart failure (HF), is a systemic condition affecting a range of organs including the heart, peripheral nerves, gastrointestinal (GI) system, and kidneys. Amyloidosis occurs when misfolded proteins form amyloid fibrils that deposit in organs. Disease severity is correlated with the degree of fibril deposition, type and toxicity of the fibrils, and resultant organ dysfunction. There are 2 main types of amyloidosis that account for 95% of cardiac cases transthyretin amyloidosis (ATTR) or light chain amyloidosis (AL) due to plasma cell dyscrasia. Transthyretin is divided into hereditary (ATTRv), or wild type (ATTRwt). As ATTR and AL disproportionately affect older populations (age 75 or older) and are the leading cause of cardiac disease, these will form the focus of this review paper.
Amyloidosis is of heightened awareness within the medical community and is now understood to be more common than previously believed. This is largely due to noninvasive techniques that facilitate the diagnosis of amyloidosis in the appropriate clinical setting, and the development of several effective disease modifying therapies, with more in the pipeline. Historically, the treatment approach for amyloidosis was focused on managing symptoms and manifestations of the disease rather than addressing the production of amyloidogenic precursor protein. Therefore, many patients were diagnosed late, if at all. Amyloidosis is associated with progressive loss of organ and often physical function. As patients with amyloidosis are living longer, both AL and ATTR, it is time to focus on the long-term management of comorbidities, and frailty is a major consideration. Frailty is common and may serve as a target for intervention, especially as we now have therapies that address amyloid production. Overall, frailty is relevant since amyloidosis disproportionately affects older adults, since frailty is highly common in cardiac amyloidosis, and because of the potential bidirectional relationship between frailty and this HF syndrome.
Frailty is increasingly recognized as an independent marker of prognosis, particularly among patients with cardiovascular disease. It is also an independent predictor of survival.1 It is now considered one of the “giant” challenges facing the aging population worldwide.2 The assessment of frailty is a powerful tool for risk stratifying patients, guiding appropriate selection of therapies and following progression of disease in domains that often matter most to patients. Recently, the International Society of Heart and Lung Transplant (ISHLT) have released a consensus document on frailty in HF, and though this has proved an important step forward,3 there is currently no universal definition of frailty for clinical practice.4,5 No consensus document on frailty in cardiac amyloidosis exists, despite it being a burgeoning area for clinical trials. Therefore, this document aims to provide multidisciplinary information in this important area.
Cardiac amyloidosis in the older patient
Transthyretin cardiac amyloidosis (ATTR-CM) affects an estimated 50,000 to 150,000 people in the United States.6 The most common variant in North America is p.Val142Ile (classical nomenclature Val122Ile), this is closely associated with the aging process. It is estimated that 1 million years of life are lost due to this variant in the United States.7 The penetrance at 65 years of age is approximately 10%; this increases with age and has recently been reported to be as high as 39% by the eighth decade.8
The average age at diagnosis for cardiac amyloidosis across studies is 74 to 90 years—the median age for ATTR-CM is estimated to be in the 8th and 9th decade of life, while the median age at the time of diagnosis for AL amyloidosis is 65 years.9 Moreover, ATTR-CM affects a significant proportion of other common age-associated conditions—it is estimated that ATTR-CM is present in up to 16% with degenerative aortic stenosis10 and in 6% to 17% of patients with HF with preserved ejection fraction.11 In fact, the prevalence of amyloid deposition in the heart is likely even higher—autopsy studies indicate that up to 25% of hearts from decedents aged over 80 years without a known HF diagnosis had transthyretin (TTR) amyloid deposits in their hearts.12
Light chain amyloidosis cardiomyopathy results from clonal plasma cell expansion which produces amyloidogenic immunoglobulin light chains that aggregate to form insoluble fibrils that deposit in tissues including the heart (>75% of cases) and cause organ dysfunction. In the United States, light chain amyloidosis cardiomyopathy has an estimated annual incidence of 1 in 75,000 to 100,000 and a prevalence of 1 in 25,000.13
The epidemiology of ATTR-CM reflects cellular aging and the consequent declines in intracellular quality control systems. More specifically, ATTR-CM is a consequence of impaired proteostasis—a well-known hallmark of aging that occurs with advancing age and has been implicated in age-associated disease.14 Broadly speaking, proteostasis is a critical element of the cell's regulation of protein and involves a complex machinery of molecular chaperones (such as heat shock proteins). Proteostasis ensures proper folding and translocation of proteins, stress-responsive pathways (such as the unfolded protein response), and proteolytic pathways (such as the ubiquitin-proteasome and autophagosome-lysosome systems). These components collectively dictate whether misfolded proteins will be identified, and subsequently refolded or eliminated. When impaired, misfolded and/or aggregated proteins can accumulate and cause damage to cells—this is particularly important for postmitotic cells such as cardiomyocytes and nerves which are vulnerable to proteotoxicity.15 In the case of ATTR-CM, there are impairments in endoplasmic reticulum–associated degradation of misfolded TTR,16 and age-associated defects in hepatic chaperone and unfolded protein response capacity that increase cardiac TTR deposition17 and ultimately cause disease.
AL is a systemic syndrome secondary to the deposition as amyloid fibril aggregates of conformationally unstable immunoglobulin free light chains in target organs.18 AL is generally driven by underlying plasma cell disorders and incidence increases with age, peaking in the 6th to 7th decade of life.19 Any organ can be involved by AL amyloidosis with cardiac involvement being present in 80% of patients, and renal involvement in 60%.20 Multiorgan involvement is common, and deposition of amyloid fibrils leads to disruption of normal histological architecture and rapidly progressive organ failure. Due to the insidious nature of early symptoms and insufficient awareness around this disorder, diagnosis is often delayed, resulting in patients being critically ill with advanced organ failure and debilitated diagnosis.21 It follows that frailty is common among patients with AL, regardless of age, and bears significant repercussions on treatment decision-making and outcome.22
Older adults with cardiac amyloidosis contend with health deficits in multiple frailty and/or geriatric domains that go well beyond HF.23,24 It has been suggested that geriatric conditions serve as a window to underlying accelerated aging processes and reflect pathological aging.25,26 This is important because pathological age is potentially modifiable and could be a target for therapy, whereas chronologic age is fixed.25,26 In short, much can be learned about amyloidosis and frailty by understanding the natural and disrupted processes of aging.
Geriatric conditions such as malnutrition, frailty, cognitive impairment, mood disorders, loneliness, and social isolation have important implications on overall amyloid disease management. They impact risk and subsequent diagnostic and therapeutic decision-making. They also impact prognosis, eg, those with significant cognitive impairment in the form of dementia have limited life expectancy and may not benefit from usual therapies.27 Geriatric conditions also impact self-care, which is a critical aspect of managing patients. Since many geriatric conditions co-occur with one another, conversations related to risk and medical decision-making, prognosis, self-care, and counseling can be complex.28 Taken together, characterizing and incorporating frailty into routine management of patients with cardiac amyloidosis is important to maximize outcomes in this highly vulnerable population.
Key points
-
•
Amyloidosis is a heterogenous group of diseases caused by misfolded proteins that form amyloid fibrils which deposit variably in different organ systems.
-
•
Cardiac amyloidosis is a treatable cause of HF.
-
•
Ninety-five percent of cardiac amyloidosis cases occur due to transthyretin (hereditary or ATTRwt) or AL.
-
•
Wild-type transthyretin cardiomyopathies (ATTR-CM) typically present and are diagnosed, over the age of 70 years.
-
•
Older adults with amyloidosis can be expected to have health deficits across multiple components.
Clinical manifestations of cardiac amyloidosis in older adults
Cardiac amyloidosis involvement
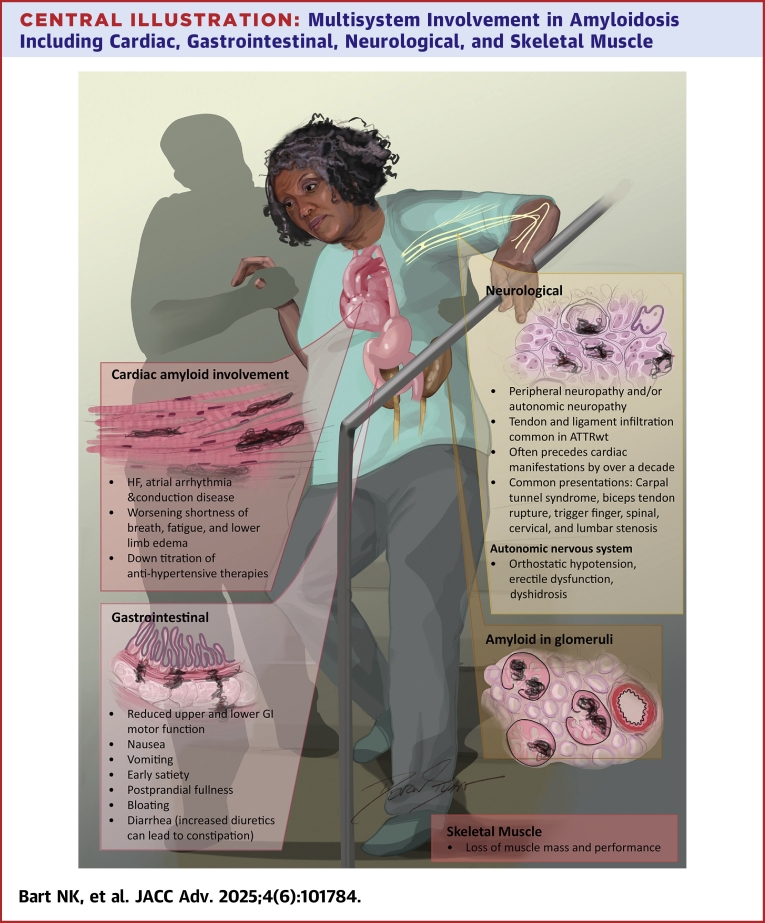
Cardiac infiltration is the most common and clinically significant manifestation of ATTRwt, resulting in HF, atrial arrhythmia, and conduction system disease (Central Illustration). Patients often present with worsening shortness of breath, fatigue, and lower limb edema. In addition, patients may demonstrate a down-titration of antihypertensive therapies. “Red flags” for ATTR-CM, such as aortic stenosis, cardiac disease plus orthopedic and neurologic manifestations such as carpel tunnel syndrome and lumbar canal stenosis, have considerable overlap with common disorders in a geriatric population.
Central Illustration.
Multisystem Involvement in Amyloidosis Including Cardiac, Gastrointestinal, Neurological, and Skeletal Muscle
ATTRwt = wild-type transthyretin; GI = gastrointestinal; HF = heart failure.
There is a strong association with HF and frailty, especially in older populations. In many of the therapeutic clinical trials for ATTRwt, 6-minute walk test distance is a key secondary endpoint and has been found to be an important prognostic indicator longitudinally. Many amyloidosis centers incorporate 6-minute walk test into routine clinical assessment. Clinical assessment includes history, physical examination, 12-lead electrocardiogram, and echocardiogram. Differentiating between the 2 main amyloidosis subtypes is essential. This involves simultaneous bone scintigraphy, blood, and urine testing to exclude a paraprotein, which may point to a diagnosis of AL amyloidosis. Where a noninvasive diagnosis is not possible (which can occur in 30% of cases), patients require a tissue diagnosis. This may be “on target” (such an endomyocardial biopsy), or a surrogate site biopsy (such as a fat pad biopsy or salivary gland biopsy). Where a case of AL is suspected, a tissue diagnosis is typically required for a definitive diagnosis. In patients aged 70 years or older, case-based multidisciplinary team (MDT) discussion should be undertaken to determine whether a patient should undergo invasive testing.
Neurological and orthopedic involvement
Neurological involvement is seen in many forms of ATTRv, this can present as either peripheral neuropathy and/or autonomic neuropathy. Tendon and ligament infiltration (eg, carpal tunnel syndrome, biceps tendon rupture, trigger finger, and spinal/cervical and/or lumbar canal stenosis) is a common manifestation of ATTRwt and can present over a decade before the onset of cardiac manifestations (Central Illustration). Collectively, these manifestations may contribute significantly to mobility and, hence, impact frailty.
Autonomic nervous system
Autonomic neuropathy is more frequently seen in ATTRv and AL, manifesting as orthostatic hypotension, erectile dysfunction, or dyshidrosis (Central Illustration). Orthostatic hypotension is a significant risk factor for falls, which can contribute to overall frailty. Autonomic dysfunction that affects the GI tract may also lead to malnutrition and reduced muscle mass.
Gastrointestinal system
GI issues can be due to direct involvement of the autonomic nervous system in specific variants of ATTRv; patients complain of a large spectrum of disabling symptoms that are mostly attributable to reduced upper and lower GI motor function (eg, nausea, vomiting, early satiety, postprandial fullness, bloating, and diarrhea; Central Illustration). Like the neurological manifestations of ATTR, however, there is also a significant indirect effect of ATTRwt. As ATTR-CM advances and HF symptoms worsen, patients frequently develop edema of the GI tract, resulting in malabsorption and cardiac cachexia, contributing to frailty. Increasing diuretic requirements may lead to constipation. The modified body mass index (mBMI) is a useful metric for GI manifestations. mBMI is the product of body mass index and serum albumin concentration, which is intended to adjust nutritional status, fluid retention and can be tracked longitudinally.29
Skeletal muscle
Loss of muscle mass is frequently identified in patients with ATTR (Central Illustration). Whether there is a direct effect of amyloid on the skeletal muscle is under investigation.30 Again, HF has a significant impact on skeletal muscle mass and function. Promoting factors for loss of muscle mass and performance, including high levels of inflammatory cytokines, low levels of anabolic hormones, micronutrient deficiencies, and physical inactivity, are prevalent in both frailty and HF.31
Key points
-
•
Amyloidosis may have cardiac, neurological, autonomic, renal, GI, psychological, or other sequelae.
-
•
Amyloidosis that affects the autonomic nervous system may result in postural instability and falls.
The between frailty and cardiac amyloidosis
Frailty is defined as a distinct biologic syndrome of decline across multiple physiological systems that may occur either independently of, or potentiated by, advanced HF. Frailty results in decreased reserves and increased vulnerability to stressors, and may be reversible with a combination of cardiovascular and/or noncardiovascular therapies.3,32 Systems affected by frailty include the intersection of physical, cognitive, nutritional, and psychosocial domains as detailed further below.
Two main methods of conceptualizing frailty exist. The first considers physical frailty using the Fried's Frailty Phenotype. The second takes a more multidimensional approach using the “Frailty Index” by Rockwood et al. The latter counts the cumulative impact of aging-related deficits (including functional impairments, symptoms, signs, and comorbidities). Newer methods, including the Clinical Frailty Score, are emerging.33 We summarize these in Table 1 below.
Table 1.
Commonly Used Assessment Instruments to Evaluate or Screen for Frailty
| Instruments | Type of Assessment | Time Required | Components Covered | How Scored | Data in Amyloidosis | Potential Limitations/Comments |
|---|---|---|---|---|---|---|
| Clinical Frailty Scale | Clinician judgment | Seconds | Physical Other: comorbidities, ADLs |
Graded with 9 pictures. varying from 1 (very fit) to 8 (very severe frailty) or 9 (terminally ill). Frail if score ≥5 | ATTR34 | Subjective |
| Essential Frailty Toolkit35 | Performance based, laboratory parameters | 10 min | Physical, cognitive, anemia, and hypoalbuminemia |
1 point for each criterion except chair stands which is 2 points if unable to perform. 0 criteria met = nonfrail; 1-2 criteria met = prefrail; 3+ criteria met = frail |
Nil | Mainly used in TAVR |
| FRAIL Scale36 | Self-reported | <1 min | Physical Other: comorbidities |
0 criteria = nonfrail; 1-2 criteria met = prefail; 3+ criteria | Nil | Validation data lacking |
| Electronic Frailty Index (eFI)37,38 | Population screening | Need relevant data from EHR | Physical Cognitive Psychological Other: comorbidities |
Calculated by dividing the number of deficits by the total number considered Reported as a ratio ranging from 0-1 with >0.25 indicative of frailty |
ATTR-CA39 | Validation data lacking |
| Comprehensive geriatric assessment frailty index | Clinician judgment | Usually >1 h | Detailed assessments, including medical history, physical examination, cognitive assessment, and social factors. | Frailty index (FI) = (sum of deficit scores)/(total number of deficits assessed) | Nil | Validation data lacking |
| Fried frailty phenotype40 | Clinical assessment | ∼20-30 min Requires dynamometer |
Physical | 5 items with 0 criteria = nonfrail; 1-2 criteria = prefrail; 3+ criteria = frail | ATTRwt-CA41 | Difficult to perform in routine clinical setting |
| Gait speed42 | Clinical assessment | ∼5 min | Physical | Measured in meters (4-10 m) divided by time in meters/second <0.8-1.0 m/s is slow |
ATTRv-PN43,44 | Measures single domain only |
| SPPB45,46 | Clinical assessment | ∼5 min | Physical (gait speed, balance, and chair stands) | Scores range from 0-12 in 3 domains | Nil | Widely used in other diseases |
| SHARE-FI47 | Clinical assessment or population screening | ∼3-5 min | Physical (appetite, gait speed, grip strength, exhaustion, physical activity) |
0 criteria = nonfrail; 1-2 criteria = prefrail; 3+ criteria = frail | Nil | Mainly used in primary care |
| TFI48 | Clinical assessment or population screening | Self-reported | Physical, psychological and cognitive (also social domains) | A score of ≥5 indicates frailty | Nil | Not widely used |
| G8 Score49 | Clinical screening or population screening | <3 min | Nutritional, weight loss, cognitive, polypharmacy, age, self-reported heath status | Score <14, a full geriatric evaluation is indicated | Nil | Mainly used in cancer |
ADL = activities of daily living; ATTR = transthyretin amyloidosis; ATTR-CA = transthyretin cardiac amyloidosis; ATTRv-PN = hereditary transthyretin amyloidosis with polyneuropathy; ATTRwt-CA = wild-type transthyretin cardiac amyloidosis; EHR = electronic health record; SHARE-FI = Survey of Health, Ageing and Retirement in Europe - Frailty Instrument; TFI = Tilburg frailty indicator; TAVR = transcatheter aortic valve replacement.
When considering frailty, most clinicians solely think about physical frailty. In reality, frailty is multidimensional, with additional nutritional, cognitive, psychological, and social components compounding vulnerability to stressors. Many of these components are commonly referred to as geriatric conditions, and geriatric-specific conditions (such as falls and polypharmacy) may also contribute to the overall clinical picture of the patient. Frailty and geriatric conditions are highly prevalent in HF and can occur independent of age (although are admittedly more common in older patients).50
Frailty frequently interacts with amyloidosis, creating a complex multifaceted milieu for affected individuals. The pathophysiology of frailty involves multiple systems but overall is characterized by a decline in homeostatic reserve and resilience.33 The key mechanisms include chronic inflammation, hormonal dysregulation, sarcopenia (loss of muscle mass, quality, and function), and oxidative stress.51,52 Chronic low-grade inflammation, or “inflammaging,” is a hallmark of aging and is implicated in the pathogenesis of both conditions.25,53 In frailty, persistent inflammation leads to muscle wasting, weakness, and impaired physiological functions, with elevated levels of proinflammatory cytokines such as interleukin 6 and tumor necrosis factor α contributing to muscle catabolism and functional decline.54 The chronic inflammatory state often seen in frailty may exacerbate amyloid fibril formation and deposition, accelerating disease progression,55 while amyloid deposits can further drive inflammation, creating a concatenation of events that worsens both conditions.56,57 Ongoing research into shared mechanisms and potential therapeutic targets is essential for developing effective therapies and interventions.
Physical frailty
Physical frailty, as defined by Fried et al,40 includes 5 components: unintentional weight loss, weakness or poor handgrip strength, self-reported exhaustion, slow walking speed, and low physical activity.40 The relationship between physical frailty and amyloidosis is synergistic. Both ATTRwt and ATTRv amyloidosis can lead to cardiac and systemic manifestations (including fatigue, exercise intolerance, and muscle weakness) that exacerbate frailty.6,58,59 Moreover, the systemic nature of amyloidosis can lead to organ dysfunction and metabolic disturbances, further promoting weight loss and decreased physical activity. The latter are both critical aspects of the frailty phenotype.
It is also important to note that the presence of physical frailty in individuals with amyloidosis may complicate disease management and prognosis. Frail patients can have diminished resilience to the physiological stress imposed by amyloidosis, leading to a more rapid progression of symptoms and a higher risk of adverse outcomes. Taken together, this bidirectional relationship suggests interventions aimed at mitigating physical frailty may improve overall health and quality of life in patients with amyloidosis.33
Malnutrition
Malnutrition is characterized by the simultaneous presence of undernutrition and physical frailty, where inadequate dietary intake, poor nutrient absorption, and/or altered metabolism can lead to weight loss, diminished muscle mass, strength, and function, exacerbating the frailty phenotype.60 The link between nutritional status and amyloidosis is particularly significant. For those with GI involvement, malabsorption (eg, early satiety and chronic diarrhea) contributes to nutritional deficiencies and weight loss.52,61 Taken together, these nutritional challenges may worsen physical frailty, leading to a causal nexus where malnutrition and frailty exacerbate each other and complicate management.
Cognitive impairment
Cognitive impairment in the context of frailty (excluding dementia) represents a state of increased vulnerability to stressors due to diminished reserves and resistance in both cognitive and physical function.27,62,63 This condition is particularly relevant for older adults with cardiovascular disease, where the interplay between reduced cognitive function and physical frailty can exacerbate overall health decline.27 Importantly, cognitive frailty has been associated with a higher risk of adverse outcomes, including disability, hospitalization, and mortality.27,64 There exist specific concerns regarding ATTR amyloidosis and cognitive function. In ATTRv patients who undergo liver transplant, formation of intracranial ATTRv has been observed, with a mean time to central nervous system complications being 21 years.65 Emerging evidence also suggests amyloid deposits may cause neuroinflammation, which further impairs cognitive function.66 Large-scale studies are required to elucidate whether there is a clear association between amyloid deposits and cognitive impairment. Importantly, there is a difference between the type of amyloidosis which is implicated in Alzheimer disease (amyloid beta deposition in the brain), and ATTR. Both are age related disorders of protein misfolding, but the relationship is poorly understood. Regardless, in patients with chronic HF (including those with ATTR-CM), there exists an established relationship between symptom onset, impaired cognition,67,68 and poorer clinical outcomes.69 Patients with cognitive dysfunction may also have barriers adhering to lifestyle and medical regimes requirements, although the opposite may also where there is increased caregiver involvement, closer monitoring, and support.70
Mood disorders
Mood disorders encompass cognitive decline, depressive symptoms, and a decrease in mental resilience, significantly impacting an individual's overall well-being and ability to cope with stressors.33,71 Depression and/or anxiety are prevalent in ATTR-CM and have been reported in almost 50% of patients, being more common in females than males.72 Of note, this is between 3- and 4-fold higher than that reported in the general population. Depression is also more common in those with advanced HF.72,73
Social isolation
Social isolation is characterized by a decline in relationships, support, and engagement in activities, which increases vulnerability and reduces an individual's ability to cope with stressors.28,74 Bunt et al74 emphasize that social isolation includes loneliness, lack of social networks, and limited social participation. The junction between social status and amyloidosis is particularly important because the chronic and debilitating nature of the disease can intensify social isolation and decrease participation. Patients with amyloidosis may also experience increased dependency or encounter significant physical limitations, the effect of which is social withdrawal, reduced engagement in community activities, and feelings of loneliness.75,76 Addressing social isolation in patients with amyloidosis necessitates a comprehensive approach that includes building social support and undertaking community engagement initiatives.77,78
Key points
-
•
Frailty is decreased physiological reserve and increased vulnerability to stressors.
-
•
Physical frailty includes unintentional weight loss, muscle weakness, exhaustion, slow walking speed, and low physical activity.
-
•
Frailty is multidimensional and goes beyond the physical domain.
-
•
Nutritional, cognitive impairment, mind/body disorders, and social isolation are well-known geriatric conditions and intersect with aspect of frailty.
-
•
Key mechanisms underlying frailty include chronic inflammation, hormonal dysregulation, sarcopenia (loss of muscle mass), and oxidative stress.
-
•
There are many points of intersection between aging, frailty and amyloidosis.
Operationalizing an approach to measure frailty in cardiac amyloidosis
To date, a widely accepted, universal, commonly employed measure to characterize the frailty phenotype has not yet been embraced in clinical practice, either in general or in amyloidosis specifically. The absence of an established, easy to perform, clinically useful measure of frailty is 1 reason for lack of systematic, routine quantification of frailty over the course of a patient's disease trajectory. Additionally, in health care systems in which performance of procedures is linked to reimbursement, the lack of a Current Procedural Terminology code for frailty assessment is a potential hindrance to widespread adoption, ironically despite those same health care systems acknowledging the impact of multiple comorbidities, frailty, and disability on the complexity of administering care and providing additional compensation for administering care in this complex patient population. In amyloidosis, more so than in advanced HF, data on the measurement of frailty are in their infancy and thus there are little empiric data to guide recommendations. Irrespective of these issues, consensus has emerged that frailty should be assessed in patients with advanced HF,3 and the same holds true to patients with cardiac amyloidosis across the spectrum of causes. Frailty provides independent prognostic information beyond traditional cardiovascular assessments and risk scores in patients with cardiac amyloidosis who are treated with disease modifying therapies34 and for those considering additional interventions (eg, ablations for atrial fibrillation or transcatheter aortic valve replacement for aortic stenosis)79 and can help to guide the complex decision-making for such patients thereby facilitating patient centered care.80 There are many instruments and approaches to frailty can be seen in Table 2 which shows tools for both physical frailty and accumulation deficit frailty. Table 2 demonstrates ways to screen for or identify frailty or related conditions such as sarcopenia, disability, cognitive dysfunction, and affective disorders or those who need more comprehensive geriatric assessments. Choosing the most appropriate frailty assessment tool is highly dependent on its intended use. While multidimensional frailty assessments are more comprehensive and were more accurate in prediction of mortality and disability,35 it is important to not let the attainment of the “perfect” instrument hinder the routine assessment of frailty. While it is premature to recommend 1 approach to the ascertainment of frailty at this time, patients with systemic amyloidosis should have a frailty assessment, which minimally should evaluate physical frailty but ideally would include cognitive, affective, and nutritional components.
Table 2.
Operationalizing Frailty Assessments for Research and Clinical Practice in Cardiac Amyloidosisa
| Component | Preferred Tool | Time | Completed by | Optional Approaches/Research needed | Research Needed |
|---|---|---|---|---|---|
| Physical frailty: Modified fried frailty | Robust frailty biomarkers differentiating HF from amyloidosis as a cause of frailty |
||||
| Loss of muscle mass/unintentional weight loss | SHARE-FI Scale: What has your appetite been like? Or have you been eating more, the same or less than usual? | <5 min | Medical/nursing | DXA or BIA scan or preferably MRI/ CT scan of psoas muscle or pectoralis muscle. |
Potential use of noninvasive muscle ultrasound, and which muscle to measure?81 |
| Weakness | 5-repeat chair stands (eg, rising up and down from a chair 5 times without using arms/hands) | <1 min | Nursing | Handgrip strength measured with a dynamometer |
Role of strength training earlier in life to prevent weakness |
| Slowness | Gait speed over 5 m | <5 min | Nursing | Gait speed over 4-10 m | |
| Physical exhaustion | SHARE-FI Scale: “In the last week, did you feel on at least 3 d, that everything you did was an effort?” | <5 min | Medical/nursing | Validated fatigue questionnaire (eg, FACIT-Fatigue, PROMIS Fatigue) | |
| Low physical activity | SHARE-FI Scale: “How often do you engage in activities that require a low or moderate level of energy such as gardening, cleaning the car, or doing a walk? | <5 min | Medical/nursing | Validated physical activity questionnaire which captures low to moderate intensity activities (eg, CHAMPS, PASE) or Duke Activity Status index, accelerometer, or daily tracking device |
Role of strength training earlier in life to prevent weakness |
| Cognitive function | |||||
| Montreal Cognitive Assessment | 10 min | Medical/nursing | Mini-Cog, MMSE ,and formal neurocognitive testing | Link between amyloidosis and cognition | |
| Depressive symptoms/anxiety | |||||
| Patient Health Questionnaire-2 or -9 Hospital Anxiety and Depression Scale |
<1 min (−2) or 2-5 min (−9) | Medical/nursing | Generalized Anxiety Disorder Instrument (GAD-7) | ||
BIA = bioelectrical impedance analysis; CHAMPS = Community Healthy Activities Model Program for Seniors; CT = computed tomography; DXA = dual-energy X-ray absorptiometry; FACIT = functional assessment of chronic illness therapy; MMSE = Mini-Mental State Examination; MRI = magnetic resonance imaging; PASE = Physical Activity Scale for the Elderly; PROMIS = Patient-Reported Outcomes Measurement Information System.
Adopted from International Society of Heart and Lung Transplant consensus document.3
In studies of patients with cardiac amyloidosis to date, data are from either single-center experiences or in the context of randomized clinical trials of novel therapeutics. Fine et al34 utilized the Clinical Frailty Scale to demonstrate that this “eyeball assessment” of worsening frailty was associated with advancing age, lower body mass index, lower hemoglobin and albumin, and higher cardiac biomarkers and was in multivariable analysis associated with greater mortality even after adjusting for ATTR-CM disease stage, New York Heart Association class and treatment with tafamidis.34 In the context of clinical trials, submaximal functional assessment using six-minute hall walk and gait speed (predominately in trials of silencer-based therapy for ATTRv polyneuropathy patients) has been employed.43,44,82, 83, 84, 85 These measures have generally shown efficacy of disease modifying therapies (eg, tafamidis, acoramidis, patisiran, vutrisiran, and eplontersen). The six-minute hall walk assesses physical capacity, exercise tolerance, is strongly linked to HF can be used as a functional measure. In addition to this, the short physical performance battery has been incorporated as key secondary or exploratory endpoints into several ongoing late phase clinical trials of silencer, degrader, and gene editing trials, which will provide invaluable evidence regarding the impact of these therapies on patient centered outcomes relevant to frailty.
Recommendations for operationalizing an approach to frailty in amyloidosis are outlined in Table 2. The outlined approach is similar to the ISHLT consensus recommendations regarding the measurement of frailty in advanced HF.3 First, it is recommended that all patients with symptomatic amyloidosis have a frailty evaluation and especially those with advanced disease or planning to undergo a procedure. Secondly, a performance-based measurement of physical function is suggested along with additional assessments of cognitive function and mood/affect (eg, depression and anxiety). Suggestions for assessing frailty domains including loss of muscle mass, weakness, slowness, physical exhaustion, and low physical activity in clinical practice as well as measured to screen for cognitive dysfunction and mood/affect are outlined. The goal is to facilitate the performance of these critical evaluations in clinical practice while fostering additional research to further refine or modify these initial recommendations. As outlined in the ISHLT document,3 the ideal test for frailty in amyloidosis would be reproducible, valid, practical, and sensitive to change. To screen large data sets or the electronic medical record to quantify frailty, especially retrospectively, the ISHLT consensus document recommended using the Frailty Index. Thirdly, cognitive testing and screening for depression/anxiety should be implemented at baseline. Where symptoms of weight loss, weakness, slowness, physical exhaustion, or low physical activity occur, we provide specific recommendations in Table 2.
Key points
-
•
Frailty tools can measure different and/or multiple components. Choosing the right tool depends on its intended use.
-
•
Multidimensional frailty assessments improve prediction of mortality and disability.
-
•
All symptomatic patients with amyloidosis should undergo frailty evaluation.
-
•
Longitudinal assessment with the same frailty tool is clinically useful.
Recommendations on care of the older patient with cardiac amyloidosis
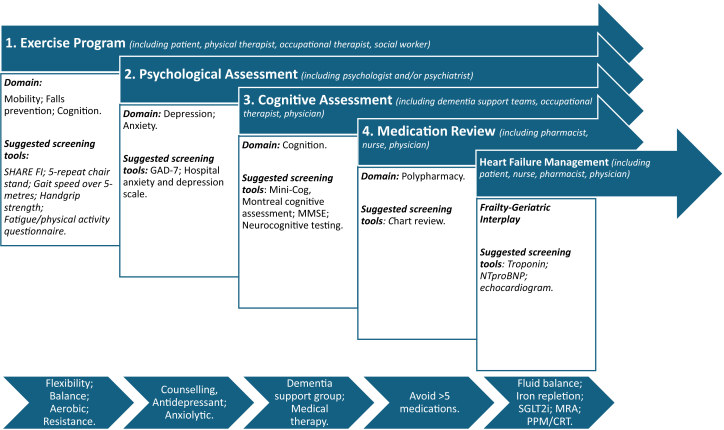
The primary goal of management should be to prevent progression of frailty and to identify and address reversible causes (Figure 2).33 The importance of an individualized patient care approach cannot be overemphasized. Employing a multidisciplinary team approach to the management of frail patients with ATTR-CM is recommended, and in addition to the HF cardiologist, should include physical and occupational therapy, pharmacy, speech/language therapy, nutrition, genetic counseling, geriatric medicine, psychologists, social workers, and palliative care where appropriate. They require closer follow-up with the HF specialists, more frequent monitoring, and individualized self-care support.86 Importantly, many of these recommendations are in line with the recommendations for the management of HF in older adults. However, as amyloidosis is multisystem, it is important to consider amyloidosis involvement beyond the HF diagnosis alone.
Figure 2.
Flowchart of Key Recommendations
GAD-7 = Generalized Anxiety Disorder Instrument; MMSE = Mini-Mental State Examination; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Mobility
There are several aspects of mobility that need to be considered as it pertains to frail patients with ATTR-CM. Mobility cannot be enhanced without the necessary strength and balance required, both of which are affected by the multisystem manifestations of ATTR amyloidosis including cardiovascular, neurologic, autonomic, and musculoskeletal. As such, exercise prescription needs to be tailored to the individual older patient with consideration of frequency, intensity, time, type (flexibility, balance, aerobic, and resistance), volume (per week), and progression.87 The REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial enrolled patients with acute HF over 60 years of age, the goal being to improve balance, mobility, strength, and endurance with a tailored progressive rehabilitation intervention that included multiple physical-function domains.88 In contrast to other studies in patients with chronic HF, at baseline, 97% of patients were either frail or prefrail. The intervention resulted in greater improvement in physical function than usual care (which included traditional cardiac or pulmonary rehabilitation), as assessed by the Short Physical Performance Battery at 3 months and clinical benefits in frailty status, quality of life, and depression. Although exercise training is known to improve physical performance in frail patients, it needs to be continued long term to maintain the benefits. Home-based exercises help to address the regression which occurs after focused interventions and engaging the help of physical therapy, occupational therapy, and social workers may be beneficial.89
Falls
Patients with HF are more susceptible to falls, in part due to a reduction in cerebral perfusion, which accelerates the development of frailty8 and disability. In addition, falls may be exacerbated by deficits in balance and mobility, medications, and visual impairment. The American College of Sports Medicine/American Heart Association guidelines state that to reduce risk of injury from falls, community-dwelling older adults with substantial risk of falls (eg, with frequent falls or mobility problems) should perform exercises that maintain or improve balance.90 Lower limb muscle strength, gait, reaction time, and balance play a major role in falls, and exercise programs which challenge balance are more effective in preventing falls than those in which balance is not addressed.91 Resistance training is also a fundamental aspect of preventing falls and should be incorporated into exercise programs. Resistance training promotes increases in skeletal muscle mass and quality, muscular strength and endurance, as well as improves coordination and vascular function.92 Improvements in upper and lower body strength are consistently described in studies of resistance training programs in patients with HF, the extent of improvement being dependent on the intensity of training. In addition to exercise training, occupational therapy mobility tools that reduce risk of falls at home should be addressed.93 Attention should be paid to timing of medications and to limit nighttime dosing, particularly of those that promote micturition, to minimize the risk of nighttime falls. An assessment of bone health is important to consider in conjunction with prevention of or treatment for osteoporosis to ameliorate the complications of falls.
Depression
It is recommended that all patients with ATTR-CM should be screened for psychopathological difficulties.94 Assessment should include an evaluation of patient safety and social supports available but must also attempt to rule out other conditions which may mimic depression or anxiety. Consideration should be given to referral for counseling or psychiatric evaluation and antidepressant or anxiolytic therapy, where indicated, with attention being paid to adverse effects which can be potentiated in the older patient.93
Cognition
Cognitive impairment is common in older patients with HF and exacerbates frailty. Attention should be paid to factors which influence cognition including age, education, sociocultural, and linguistic background, and assessment should involve evaluation for underlying causes.93 Accurate diagnosis requires a multidisciplinary approach and should involve cognitive screening, appropriate laboratory evaluation, structural imaging, functional neuroimaging, and neuropsychological testing.95 Home care support, social work, referral to day programs, Alzheimer's society, and medical therapy, where indicated, should be considered.93 Exercise has a beneficial effect on cognitive function and should be a part of treatment.96 Wherever possible the amyloidosis MDT should aim to enhance treatment adherence in collaboration with specialist dementia support teams, utilizing medication compliance aids, providing tailored self-care advice and mitigating adverse self-care behaviors, and encouraging involvement of family and caregivers.86
Polypharmacy
Polypharmacy (the use of ≥5 daily medications) has been shown to impact mobility, is an independent risk factor for falls leading to hip fractures in older adults and is associated with adverse outcomes in HF.97 In addition, polypharmacy leads to increased risk of adverse drug reactions, elevated treatment burden, reduced quality of life, and difficulties with medication adherence. Many agents which are typically used for the treatment of HF are not well tolerated in the ATTR-CM population.
Medications in an older patient with ATTR-CM should be carefully reviewed, and unnecessary medications should be ceased. Importantly, in these complex patients' limiting medications to <5 to avoid polypharmacy is often not possible due to the number of disease-specific medications. Therapy should be tailored to the individual patient and their clinical scenario.
The American Geriatrics Society Beers Criteria is a useful aid to addressing harmful or inappropriate medication use in older patients and specifically addresses many agents used in the HF setting.98 Education is one component in addressing polypharmacy, as is the development of better systems and approaches for clinicians. The primary objective should be to simplify the medication regimen by removing nonessential medications, promoting once-daily regimens, and frequently reviewing drug-drug interactions. Again, this should be targeted at the individual patient. For example, sodium-glucose cotransporter-2 inhibitors are listed on the Beers criteria for older patients, but the benefits may outweigh the risks in older patients with amyloidosis. Special attention should be paid to dose (adjusted for renal or hepatic impairment), duration, timing, and indication for each medication, including over-the-counter medications or supplements. Only medications that treat symptoms or the underlying disease process should be maintained and if not prescribed, should be added. Furthermore, the use of medication boxes or organizers should be considered particularly for those with cognitive impairment or limited social support.
HF management
Individualization of therapeutic strategies to treat HF is an important concept in patients with cardiac amyloidosis (Figure 1). The mainstay of symptomatic management remains the control of volume intake and administration of diuretics, in large part to target dyspnea and edema, which promotes enhanced exercise tolerance. Furthermore, by relieving intestinal congestion we can promote enhanced appetite, address early satiety and weight loss which can lead to cachexia. Patients with ATTR-CM require higher filling pressures to maintain cardiac output and are prone to organ hypoperfusion with aggressive diuresis which often manifests as acute kidney injury, necessitating judicious use of loop diuretics.99 Sodium-glucose cotransporter-2 inhibitors appear to be well-tolerated in ATTR-CM, enhancing loop diuretic response and volume status, and their use is associated with a reduced risk of HF hospitalizations, cardiovascular mortality,8 and all-cause mortality.100 Fatigue is a universal complaint in this patient population, which may be exacerbated by HF therapies. Beta-blockers can be considered in the right patients with cardiac amyloidosis but need to be considered in the context of other comorbidities and risk of hemodynamic intolerance and bradycardia;70,101,102 however there is a subset who may derive benefit from continued, low-dose beta-blockade.103 Mineralocorticoid receptor antagonists are synergistic with loop diuretics, promote potassium sparing, and may be associated with improved outcomes, while angiotensin-converting enzyme (inhibitor)/angiotensin II receptor blocker/angiotensin receptor-neprilysin inhibitor should be used with caution due to their propensity to induce or worsen hypotension, orthostasis, and exacerbate falls.101,103 Neurogenic orthostatic hypotension may occur, particularly in ATTRv and may benefit from treatment with midodrine, droxidopa, and compression hosiery, in addition to referral to neurology for assessment.104 Note that although drugs like midodrine are relatively contraindicated in HF, they are recommended in cases of ATTR-CM where patients remain hypotensive despite cessation of antihypertensive agents. Newly prescribed agents should be initiated at a low dose and titrated slowly. Attention should be paid to correcting iron deficiency, and consideration given to invasive hemodynamic monitoring, permanent pacemaker implantation, or cardiac resynchronization therapy, where appropriate.99
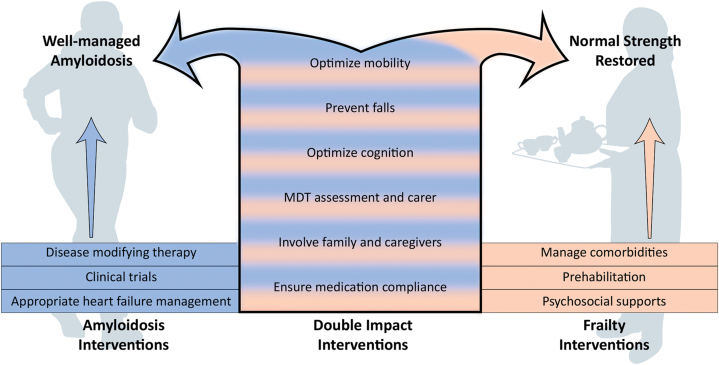
Figure 1.
Representation of Specific Interventions for Frailty and Amyloidosis That Have “Double Impact” by Resulting in Improvements in Both
Goals of care
Goals of care discussions are particularly relevant for those with advanced stages of disease, though should be considered for all patients.28 At various stages in the disease process, these may include self-care, caregiver support, social support, support of patient-caregiver relationship, palliative care, and advanced directives (to include place of death, resuscitation wishes, and deactivation of implantable cardioverter defibrillators and/or pacemakers). Promoting self-care is paramount for the older frail patient and supporting this is imperative. Social support is a fundamental aspect of promoting self-care behaviors, particularly medication adherence, monitoring fluid intake, reporting weight gain to the health care team, and physical activity.105 Understanding the patient's expectations and perspectives on their disease is paramount and consideration should be given to whether the patient places more importance on quality of life and symptom control over life prolongation alone, with evidence to suggest the majority of patients with HF attach more weight to quality over longevity.93,106 Palliative care specialists can provide assistance in addressing goals of care for the patient and their family/caregivers in addition to providing education and support. Management should include frequent assessment of symptoms of both HF and comorbidities (such as pain, anxiety, depression), nonessential medications, access to psychological and spiritual support for both patient and caregivers, and advanced care planning.86 Unfortunately, access to palliative care specialists is not universal, so HF cardiologists should familiarize themselves with local options. Communication and coordination between involved teams is paramount to ensure optimal patient care and support and goals of care should be reviewed at frequent intervals.
Key points
-
•
A multidisciplinary care team approach is recommended.
-
•
The overall clinical context should be considered on a case-by-case approach including mobility, falls depression, cognition, polypharmacy, HF management, and goals of care.
Frailty in clinical decision-making
Frailty can help reset actuarial, population-derived life expectancy estimates.107,108 In the general population, an 80-year-old who is frail may have a life expectancy of <2 years, while a nonfrail 80-year-old may have 10 years or more of additional life expectancy. When frailty and multimorbidity occur in tandem, survival is further decreased.109 Knowing a patient's frailty can help with treatment decisions, particularly when time to benefit requires years. Tools such as ePrognosis can be useful in determining a patient's overall prognosis, taking into account their living environment, cognition, and functional status.110 Although the scores used are not specific to frailty, these tools are useful in understanding overall remaining life expectancy.
A key principle in caring for older adults is recognizing that frailty is not a disease state or a reason to withhold a given intervention. Rather aging does not occur uniformly and this variability in biologic age can be captured in part by frailty.111 When an individual is identified as frail (using any valid assessment), this represents an opportunity to pause and consider risks and benefits in the context of biologic age, physiologic reserve, and overall clinical trajectory.112 Additionally, frailty is at least partly reversible,113 which has led to a series of investigations on interventions that may reverse frailty or prevent acceleration of frailty, particularly prior to a stressful intervention.114
Frailty can help guide decisions for both initiating treatment and stopping a given therapy. It may also allow the option for a time limited trial if a given individual's frailty is driven by a dominant disease (eg, amyloidosis). Once a patient screens positive for frailty, the Comprehensive Geriatric Assessment can be conducted to help clinicians identify driving and potentially reversible contributors to frailty such as nutrition or physical function.115 If a decision is made to start a therapy even in the context of frailty, it is important to monitor changes in frailty, ideally using the same assessment tool at follow-up, to see if treatment is worsening frailty. In a transcatheter aortic valve replacement cohort, those who were frail at baseline had worse functional outcomes in the year following the procedure.26,116
Identifying frailty allows clinicians to personalize care plans, minimize risk, and the opportunity to practice “precision gerontology.” Including a geriatrician in the multidisciplinary heart team can ensure that a complete picture of the patient is considered beyond their amyloidosis.117 In addition to frailty, many older adults have competing morbidities, cognitive and functional changes, polypharmacy, as well as complex social and financial environments that need to be considered.118 Geriatricians have expertise in managing this overall complexity and can provide context and perspective for incorporating geriatric syndromes, into the care plan.119 Including geriatricians may help prevent adverse outcomes such as falls and delirium, and that interventions align with goals of care; however this has not been studied specifically in amyloidosis care.
Key points
-
•
Aging does not occur uniformly and this variability in biologic age can be captured in part by frailty.
-
•
Frailty can help guide decisions for both initiating treatment and stopping a given therapy.
-
•
A geriatrician as part of the MDT heart team can ensure that a complete picture of the patient is considered.
Intersection of nutrition and frailty in cardiac amyloidosis
Malnutrition in cardiac amyloidosis is often multifactorial.52 Patients with HF commonly have symptoms such as nausea, poor appetite, and early satiety that directly affect food intake. Edema and decreased perfusion of the gut wall can reduce the absorption of key nutrients. These issues are exacerbated by GI amyloid deposits, if present. Loop diuretics to manage volume retention increase urinary excretion of water-soluble micronutrients. Chronic undernutrition is central to the model of physical frailty through contributing to loss of muscle mass through calorie and protein intake deficits in concert with other aging-related mechanisms such as cellular senescence,120 which plays a role in cardiac amyloidosis. In addition, depression, anxiety, fatigue, poor mobility, limited support, complex dietary requirements, and cognitive impairment frequently pose challenges to shopping and meal preparation.120 Social determinants of health compound these difficulties; 1 recent analysis estimated that over 40% of U.S. pa tients with HF have food insecurity, defined as limited or uncertain access to adequate food.120
Patients with cardiac amyloidosis frequently have malnutrition. A recent focused review noted 25% to 65% prevalence in patients with AL (most with cardiac involvement), varying by the severity of illness and assessment methodology used.121 Several indices of malnutrition are closely associated with quality of life and clinical outcomes. The mBMI is inversely related to the risk of death in amyloidosis. Due to its simplicity and prognostic capacity, mBMI is commonly assessed in clinical trials and in practice. The more complex Patient-Generated Subjective Global Assessment (PG-SGA) combines subjective information on food intake, physical function, and symptoms with weight loss, estimated metabolic demand, and physical examination findings of malnutrition such as muscle and fat pad wasting. The PG-SGA score independently predicts survival in AL, and additio nally triages for the intensity of recommended nutritional support.120 Bioelectrical impedance vector analysis is an inexpensive and rapid technique used to assess body composition and volume status using low-grade electrical current.52,122 The phase angle is derived from resistance and reactance measurements and is associated both with sarcopenia and malnutrition. In a study of 127 treatment-naive patients with AL, phase angle predicted mortality independent of age, sex, volume status, hematologic response to treatment, and Mayo clinic staging.122 Less information is available regarding the prevalence and importance of malnutrition in TTR amyloidosis but given overlap in HF and GI manifestations the implications are likely similar.
Key points
-
•
Nutritional deficits are an important contributor to frailty in amyloidosis.
-
•
Autonomic gut dysfunction and edema are prognostically important in ATTR-CM.
-
•
The mBMI adjusts for nutritional status and fluid retention and is prognostic of mortality.
-
•
The PG-SGA is prognostic of mortality in AL.
Frailty in AL
Systemic, rapidly progressive maladies with vital multiorgan involvement and a high catabolic state play a critical role in increasing frailty.123,124 Consistently, patients with hematologic malignancies bear a high risk of being frail due to the direct effect of malignant clones on healthy organs and systems, the consequences of chemotherapy and polypharmacy and the frequent presence of malnutrition and unintentional weight loss.125 This is particularly true for patients with AL given the major decline in vital organ reserve often associated with this illness.
Determining whether a patient with a hematological cancer is fit/robust, prefrail or frail bears important implications regarding therapy, prognosis, and supportive care. An accurate assessment of frailty is therefore critical to ensure the right therapy for the right patient. There is a fine balance of chosen treatment regimen and its toxicities due to multiorgan involvement with AL.
The International Myeloma Working Group frailty index and the Revised Myeloma Comorbidity Index for frailty successfully predicted overall survival and risk of toxicities in patients with multiple myeloma, a plasma cell cancer similar to AL.126,127 There is no frailty index validated specifically for patients with AL, and the Fried scoring system has been generally used in other hematologic conditions.128 Recently, the Spanish group proposed a frailty score based on combination of age, Eastern Cooperative Oncology Group performance status, and N-terminal pro–B-type natriuretic peptide level exceeding 8,500 pg/mL for patients with AL. These parameters predicted early mortality and survival in a cohort of 611 patients with AL, although it is unclear that they are sufficient to comprehensively capture frailty and assess risk of excessive toxicity.129
Differently from other hematological malignancies, the staging systems in AL reflect severity of cardiac involvement, rather than clonal plasma cell burden itself, serendipitously providing a frailty metric.130, 131, 132 Furthermore, the criteria for autologous stem cell transplantation eligibility in patients with AL include metrics that reflect organ reserve, thus providing a useful tool to help in the assessment of frailty.133, 134, 135, 136 From this perspective, frail patients, including but not limited to patients with advanced amyloid cardiomyopathy (stage IIIA and IIIB), are not considered eligible for full high dose melphalan followed by autologous stem cell transplantation given the increased mortality risk.137,138 Age per se should not be a disqualifying criterion for intensive treatment, as older patients who are not frail can withstand therapy with acceptable toxicities, including autologous stem cell transplant, resulting in significant benefit as measured by quantity and quality of life.139,140 Daratumumab, cyclophosphamide, bortezomib, and dexamethasone (DaraCyBorD) is Food and Drug Administration–approved for the treatment of patients with newly diagnosed amyloidosis stage I to IIIA based on the results of the randomized, phase 3 clinical trial ANDROMEDA (the Efficacy and Safety of Daratumumab in Combination with Cyclophosphamide, Bortezomib and Dexamethasone (CyBorD) Compared 1 with CyBorD Alone in Newly Diagnosed Systemic AL Amyloidosis) comparing this quadruplet to CyBorD.141 Approximately half of the patients (45%-50%) enrolled in this study were above age 65 years, including patients older than 85 years. Advanced age did not negatively impact response to DaraCyBorD, and there was a trend toward increased benefit of quadruple therapy in patients with more advanced staging. While patients were not clearly stratified based on frailty, and stage IIIB patients were excluded from this study, these data suggest that DaraCyBorD can be safely administered in patients who may be frail with AL, although formal testing would be required to confirm this. Retrospective studies suggest that this regimen is effective with no overt signal of added toxicities in patients with stage IIIB AL with an estimated 1-year survival of 67.5%.142 With the caveat of the retrospective nature of this study and consequent selection bias and small number of patients examined, these data compare favorably with the median overall survival of 10.3 months observed in a prospective multicenter study of daratumumab single therapy in patients with stage IIIB AL.143
Considering that rapid and deep normalization of free light chains is necessary to maximize the chances of organ response, and therefore survival and improved quality of life, administration of highly active treatment is of critical importance. The decision to reduce the intensity of treatment upfront due to the critical illness of a newly diagnosed patient with AL can therefore be detrimental and should be weighed against the likelihood of long-term survival of patients and suitability for solid organ transplantation. The availability of highly effective and well tolerated salvage therapies for patients with AL has led to improved outcomes after cardiac transplant, shifting the field view regarding the suitability of these patients to receive a solid organ.144 In particular, we recommend considering administration of DaraCyBorD in newly diagnosed young and previously fit patients with advanced AL amyloid cardiomyopathy who are potentially suitable candidates for heart transplant. In this population of patients, achievement of a deep and rapid hematologic remission is considered a necessary condition for solid organ transplant listing in many high-volume centers.145 A multidisciplinary approach to the care of frail patients, with optimized management of organ failure and drug-related toxicities is critical to ensure the safety of patients and maximize the benefits of care. In older and frail patients with advanced AL in the context of pre-existing comorbidities negatively affecting quality of life and/or life expectancy, the option of focusing on palliation of symptoms should be discussed as even the most active treatment is unlikely to result in meaningful and lasting benefit as measured by alleviation of symptoms, improvement in quality of life, or prolongation of survival.
Finally, it is important to bear in mind that frailty is a dynamic measure and hematological control of AL often leads to a significant improvement in frailty score even in older patients with resultant improved quality of life and increased options for subsequent therapies.
Key points
-
•
Concomitant hematological malignancies bear a high risk of frailty.
-
•
There is currently no frailty index for AL.
-
•
The International Myeloma Working Group frailty index and the Revised Myeloma Comorbidity Index may be helpful in AL.
-
•
Frailty assessment at baseline is recommended.
-
•
Frailty assessment should be considered in the context of stem cell transplant assessment.
-
•
Hematological control of AL often leads to frailty improvement.
Role of specialist nursing and allied health
The World Health Federation Consensus document on amyloidosis explained the essential role of allied health in an amyloidosis MDT, which is concurrent with the literature surrounding geriatric care. In a survey of specialized amyloidosis centers, patients and patient advocates, multidisciplinary care was recognized as one of the most important aspects of amyloidosis care.146 Allied health plays a vital role as part of the MDT and has several functions in nursing, physical therapy, genetic counseling, nutritional support, and psychology.
Nursing
The complex nature of both amyloidosis and frailty requires an individualized person-centered approach to target the physical, psychological, and social aspects of health. Specialist nurses are uniquely positioned at the juncture between clinical expertise and patient advocacy to provide this holistic approach, in collaboration with the MDT. Patient and family education in amyloidosis is crucial for effective self-management. Nurse-led self-management programs improve individuals' understanding of their condition and help them develop the skills necessary to make informed health decisions.147 By improving patients understanding of their diagnosis, disease progression, treatment options, and the importance of adherence to prescribed regimens, nurse-led education programs support patients to make lifestyle choices that promote health and decrease their risk of frailty and, in certain cases, may reverse frailty.148
Support groups and patient with amyloidosis and family associations are recommended to address psychosocial needs.149 Nurses may facilitate or lead these support groups, allowing patients and families to share experiences and coping strategies, or connect patients with community resources, social services, and financial assistance programs. For a multisystemic disease like amyloidosis, specialist nurses serve as patient advocates, equipping patients with the health literacy skills to navigate the complex health care system and enhancing their access to correct information and quality care. Consequently, patients receive appropriate and timely care within the health care system. The multifaceted role of the specialist nurse within the amyloidosis MDT enhances patient outcomes through personalized care, education, and support, ultimately improving the quality of life for these patients.
Clinical care coordinator
A clinical care coordinator, who is usually a member of nursing staff, plays an important role in the continuity of care from the point of diagnosis through to streamlining and supporting ongoing care. Diagnosis of a patient with amyloidosis is complex and involves multimodality imaging and blood work. For older patients, navigating the health care system with a chronic disease can be difficult and the care coordinator can reduce some of this burden on the patient. By ensuring the workup is complete and available for the MDT team, this also reduces time within the clinic visit.
The care coordinator facilitates the development of a trusting therapeutic relationship and patient management across the MDT, ensuring continuity and quality of care. Continuity of care and a relationship of trust with the MDT are recognized as key factors in the care of those with amyloidosis.150
Physical therapy
Patients with amyloidosis, particularly those with AL with cardiac involvement, demonstrate significantly impaired functional mobility with impaired exercise tolerance and capacity a key prognostic indicator.151,152 Evidence supports the need for physical therapy assessment and intervention in their care.151 A neurologic based physical therapist may be most beneficial for patients with amyloidosis. The role of the physical therapist is to assess the history, ascertain therapeutic goals, conduct a physical examination including general mobility and posture, strength and range of motion, coordination, and conduct standardized tests of balance and gait. Interval reassessments are crucial to optimize symptom management and achieve the goals of care.
Nutritional support
Malnutrition is a well-established pathophysiologic mechanism underlying the development of frailty (See section on Nutrition).33 The involvement of a dietitian in the MDT is associated with improved nutritional outcomes and decreased hospitalizations.153 Dietitians offer a broad range of services to help individuals manage their nutritional requirements.
Occupational therapy
Occupational therapy is proven to complement the medical and other psychosocial treatments in ATTRv.154 Occupational therapists aim to improve functional capacity, enhance accessibility of living environment, increase resilience, and address individual concerns and needs. The use of self-management techniques, energy conservation techniques, and work simplification improves personal autonomy and empowers patients to overcome disease-related barriers, such as impaired performance in daily living activities or frailty.155
Proactive intervention and reversing frailty
Frailty is a dynamic process and a potential therapeutic target either mechanistically or by addressing individual domains.156 In patients with advanced HF, frailty is an independent predictor of mortality.156, 157, 158 Moreover, frailty detected in the days or weeks prior to implantation of a durable mechanical circulatory support device (MCS) or within the 6 months prior to heart transplantation (HTx) is associated with an increased operative mortality from these major surgical interventions.159, 160, 161, 162 However, for patients who survive the perioperative period, frailty is largely reversible in the majority of patients following durable MCS or HTx.162, 163, 164 These observations have prompted the investigation of strategies to prevent or reverse frailty in advanced patients with HF who are being considered for durable MCS or HTx, a concept known as prehabilitation.
A systematic review and meta-analysis of studies aimed at preventing or reversing frailty in patients with HF identified 7 studies (3 retrospective and 4 prospective) that evaluated the impact of a structured cardiac rehabilitation on frailty status.165 Collectively, these studies found that frailty was at least partially reversible in patients with HF. Additionally, 2 studies reported that interruption of rehabilitation programs resulted in deterioration of the frailty status.165 In a more recent retrospective review of patients with advanced heart or lung failure who were referred for transplant assessment, it was found that for both groups of patients, frailty scores were significantly lower (ie, they were less frail) in patients who were participating in a structured rehabilitation program compared with patients who were not.166 Interestingly, patients participating in a home-based rehabilitation program had similar frailty scores to patients participating in a hospital-based rehabilitation program.
Frailty interventions have not been studied in an amyloidosis cohort. In a French cohort of patients with ATTR, Broussier et al41 reported that frailty affected multiple components including autonomy, balance, muscle strength, nutrition, cognitive function, and mood. Potential therapeutic interventions targeting frailty include structured exercise programs, nutritional support, and psychosocial support.167 Ideally, a multimodal approach should be adopted.
Structured exercise programs include resistance exercise, flexibility training, balance training, and aerobic training including walking (Figure 2). The starting intensity of exercise is individualized based on the initial patient assessment with graded intensity based on patient progress.168,169 In a recent systematic review of structured exercise programs in patients with a diagnosis of cognitive frailty, Li, et al,169 reported identified 10 studies which collectively demonstrated significant improvements in physical frailty, cognitive function, and depression with the exercise intervention. The frequency of exercise in most studies has been 3 to 4 times per week and the duration of each exercise session 30 to 60 minutes.169
A potential limitation to the use of structured exercise programs is the capacity of hospital-based exercise/physiotherapy services to accommodate the number of frail patients who might benefit from such a program. One potential solution is the use of a home-based exercise program, either as a follow-on or as an alternative to a hospital-based program. As mentioned above, there is observational evidence that (and lung) patients with HF undertaking home-based as opposed to hospital-based rehabilitation have similar frailty scores.166 A possible enhancement of a home-based program is the use of mobile health technology to provide a structured exercise program adapted to the individual's baseline performance, as well as to monitor their progress over time. There are reports of successful utilization of mobile health applications to reduce falls risk in older community-dwelling people170 and to improve frailty in patients awaiting lung transplantation.171 However, it is yet to be seen whether this is applicable in an elderly complex amyloidosis population.
Few nutritional support interventions have been performed in patients with cardiac amyloidosis. In a single-center Italian randomized trial, 144 patients with AL were randomized to dietitian-guided nutritional counseling over 12 months including weight maintenance and nutritional supplements compared with usual care.172 Patients' mental health score on the 36-item Short Form General Health Survey increased; however, mid-arm circumference and the 36-item Short Form General Health Survey physical health score did not improve. The benefits of protein/calorie supplementation for reversing frailty need further evidence. A taskforce established by the International Conference for Frailty and Sarcopenia Research167 recommended that protein/caloric supplementation could be considered for persons with frailty when unintended weight loss or undernutrition has been diagnosed; however, the taskforce noted that there was only low-level evidence to support this recommendation. A wide range of other nutritional supplements including amino acids, vitamins C, D, and E, omega free fatty acids, creatine, inorganic nitrate, probiotics, minerals, collagen peptides, and polyphenols have been investigated for the treatment of frailty and sarcopenia;167,173 however, the benefit of these supplements if any remains to be established.
Recommendation
All patients with ATTR and AL should be screened for frailty including sarcopenia. As recommended by the International Conference for Frailty and Sarcopenia Research, all patients with frailty should receive social support as part of a comprehensive care plan. The care plan should include a structured physical exercise program with a resistance-based training component. Protein/caloric supplementation is recommended for patients with sarcopenia or unintended weight loss.
Key points
-
•
Frailty is dynamic and modifiable geriatric syndrome.
-
•
Therapeutic interventions include structured exercise programs, nutritional and psychosocial support.
-
•
Structured exercise programs include resistance exercise, flexibility, balance, and aerobic training.
-
•
Nutrition plays an important role in frailty reversal.
Conclusions
Cardiac amyloidosis is common in older adults, and often exists within the context of the known geriatric syndromes. Frailty is a dynamic syndrome that has many overlapping features with the end-organ systems affected by amyloidosis. Importantly, frailty is highly prevalent within the population of patients with amyloidosis, especially those that suffer from ATTM-CM (with or without HF symptoms). Amyloid is now recognized as a treatable condition, with disease modifying therapy available. Frailty itself is also reversible if identified and treated appropriately. Understanding the interplay between frailty, aging, and amyloidosis, therefore, can help our understanding of the pathophysiology of disease, as well as early implementation of frailty assessments and interventions.
Funding support and author disclosures
Dr Bart has received research funding from Pfizer; speaking fees from Pfizer; and advisory board fees from Bridge Bio, Novo Nordisk, and Bristol Myers Squibb. Dr Cuddy has received grant support from NIH 1K23HL166686-01 and AHA 23CDA857664; site support for Helios-B (Alnylam), Cardio-TTRansform (Ionis, Astra Zeneca), and DepleTTR (Alexion); and personal fees from Pfizer, Bridgebio, Ionis, AstraZeneca, Alexion, and Novo-Nordisk. Dr Griffin has received grant support from Pfizer; and personal fees from Pfizer and BridgeBio. Dr Maurer has received grant support from NIH R01HL139671 and AG081582 and grants from Alnylam, BridgeBio, Intellia, and Ionis; and personal fees from Alnylam, Novo-Nordisk, Roche, Prothena Astra Zeneca, Akcea, and Intellia. Dr Nanne has received current research support from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation, the Patient-Centered Outcomes Research Institute (PCORI), and the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342). Dr Sanchorawala has received research support (to institution) from Celgene, Millennium-Takeda, Janssen, Prothena, Sorrento, Karyopharm, Oncopeptide, and Caelum–Alexion; has served as a consultant for Pfizer, Janssen, Attralus, GateBio, Abbvie, and BridgeBio; has served on the Scientific advisory board for Proclara, Caelum, Abbview, Janssen, Regeneron, Protego, Pharmatrace, Telix, Prothena, AstraZeneca, and Nexcella. Dr Damluji has received research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30-AG021334; has mentored patient-oriented research career development award from the National Heart, Lung, and Blood Institute K23-HL153771, the NIH National Institute of Aging R01-AG078153, and the Patient-Centered Outcomes Research Institute (PCORI). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Peng Y., Zhong G.C., Zhou X., Guan L., Zhou L. Frailty and risks of all-cause and cause-specific death in community-dwelling adults: a systematic review and meta-analysis. BMC Geriatr. 2022;22(1):725. doi: 10.1186/s12877-022-03404-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morley J.E. The new geriatric giants. Clin Geriatr Med. 2017;33(3):xi–xii. doi: 10.1016/j.cger.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Denfeld Q.E., Jha S.R., Fung E., et al. Assessing and managing frailty in advanced heart failure: an International Society for Heart and Lung Transplantation consensus statement. J Heart Lung Transplant. 2024;43(1):1–27. doi: 10.1016/j.healun.2023.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Bart N.K., Powell A., Macdonald P.S. The role of frailty in advanced HF and cardiac transplantation. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1082371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damluji A.A., Cohen M.G. The influence of frailty on cardiovascular disease: the time for a “Frailty Academic Research Consortium” is now! Circ Cardiovasc Interv. 2022;15(1) doi: 10.1161/CIRCINTERVENTIONS.121.011669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruberg F.L., Maurer M.S. Cardiac amyloidosis due to transthyretin protein: a review. Jama. 2024;331(9):778–791. doi: 10.1001/jama.2024.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvaraj S., Claggett B., Shah S.H., et al. Cardiovascular burden of the V142I transthyretin variant. JAMA. 2024;331(21):1824–1833. doi: 10.1001/jama.2024.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wardhere A., Bampatsias D., Cohn E., et al. Unlocking diversity in cardiovascular clinical research: lessons from the screening for cardiac amyloidosis with nuclear imaging in a minority populations study. J Card Fail. 2024;30:1507–1511. doi: 10.1016/j.cardfail.2024.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aimo A., Merlo M., Porcari A., et al. Redefining the epidemiology of cardiac amyloidosis. A systematic review and meta-analysis of screening studies. Eur J Heart Fail. 2022;24(12):2342–2351. doi: 10.1002/ejhf.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castano A., Haq M., Narotsky D.L., et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1(8):880–889. doi: 10.1001/jamacardio.2016.2839. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed S.F., Mirzoyev S.A., Edwards W.D., et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2(2):113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanskanen M., Peuralinna T., Polvikoski T., et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40(3):232–239. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 13.Kittleson M.M., Ruberg F.L., Ambardekar A.V., et al. 2023 ACC Expert Consensus Decision Pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis. J Am Coll Cardiol. 2023;81(11):1076–1126. doi: 10.1016/j.jacc.2022.11.022. [DOI] [PubMed] [Google Scholar]
- 14.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal P., Maurer M.S., Roh J. Aging in heart failure. JACC Heart Fail. 2024;12(5):795–809. doi: 10.1016/j.jchf.2024.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekijima Y., Wiseman R.L., Matteson J., et al. The biological and chemical basis for tissue-selective amyloid disease. Cell. 2005;121(1):73–85. doi: 10.1016/j.cell.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Buxbaum J.N., Tagoe C., Gallo G., Walker J.R., Kurian S., Salomon D.R. Why are some amyloidoses systemic? Does hepatic “chaperoning at a distance” prevent cardiac deposition in a transgenic model of human senile systemic (transthyretin) amyloidosis? FASEB J. 2012;26(6):2283–2293. doi: 10.1096/fj.11-189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merlini G., Dispenzieri A., Sanchorawala V., et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. doi: 10.1038/s41572-018-0034-3. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi G., Kumar S. Systemic amyloidosis due to clonal plasma cell diseases. Hematol Oncol Clin North Am. 2020;34(6):1009–1026. doi: 10.1016/j.hoc.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Sanchorawala V. Systemic light chain amyloidosis. N Engl J Med. 2024;390(24):2295–2307. doi: 10.1056/NEJMra2304088. [DOI] [PubMed] [Google Scholar]
- 21.Oubari S., Naser E., Papathanasiou M., et al. Impact of time to diagnosis on Mayo stages, treatment outcome, and survival in patients with AL amyloidosis and cardiac involvement. Eur J Haematol. 2021;107(4):449–457. doi: 10.1111/ejh.13681. [DOI] [PubMed] [Google Scholar]
- 22.Gavriatopoulou M., Fotiou D., Ntanasis-Stathopoulos I., Kastritis E., Terpos E., Dimopoulos M.A. How I treat elderly patients with plasma cell dyscrasias. Aging (Albany NY) 2018;10(12):4248–4268. doi: 10.18632/aging.101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubenstein L.Z., Siu A.L., Wieland D. Comprehensive geriatric assessment: toward understanding its efficacy. Aging (Milano) 1989;1(2):87–98. doi: 10.1007/BF03323881. [DOI] [PubMed] [Google Scholar]
- 24.Molnar F., Frank C.C. Optimizing geriatric care with the GERIATRIC 5Ms. Can Fam Physician. 2019;65(1):39. [PMC free article] [PubMed] [Google Scholar]
- 25.Damluji A.A., Nanna M.G., Rymer J., et al. Chronological vs biological age in interventional cardiology: a comprehensive approach to care for older adults: JACC Family Series. JACC Cardiovasc Interv. 2024;17(8):961–978. doi: 10.1016/j.jcin.2024.01.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damluji A.A., Bernacki G., Afilalo J., et al. TAVR in older adults: moving toward a comprehensive geriatric assessment and away from chronological age: JACC Family Series. JACC Adv. 2024;3(4) doi: 10.1016/j.jacadv.2024.100877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ijaz N., Jamil Y., Brown C.H., et al. Role of cognitive frailty in older adults with cardiovascular disease. J Am Heart Assoc. 2024;13(4) doi: 10.1161/JAHA.123.033594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalra K., Moumneh M.B., Nanna M.G., Damluji A.A. Beyond MACE: a multidimensional approach to outcomes in clinical trials for older adults with stable ischemic heart disease. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1276370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wixner J., Mundayat R., Karayal O.N., Anan I., Karling P., Suhr O.B. THAOS: gastrointestinal manifestations of transthyretin amyloidosis - common complications of a rare disease. Orphanet J Rare Dis. 2014;9:61. doi: 10.1186/1750-1172-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J., Zhou X., Wang Y., et al. Skeletal muscle involvement in systemic amyloidosis is often overlooked. Neuropathol Appl Neurobiol. 2024;50(4) doi: 10.1111/nan.12996. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki T., Palus S., Springer J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. 2018;5(6):1099–1107. doi: 10.1002/ehf2.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO . World Health Organization; 2015. World Report on Ageing and Health. [Google Scholar]
- 33.Ijaz N., Buta B., Xue Q.-L., et al. Interventions for frailty among older adults with cardiovascular disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79(5):482–503. doi: 10.1016/j.jacc.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fine N.M., McMillan J.M. Prevalence and prognostic significance of frailty among patients with transthyretin amyloidosis cardiomyopathy. Circ Heart Fail. 2021;14(6) doi: 10.1161/CIRCHEARTFAILURE.120.008105. [DOI] [PubMed] [Google Scholar]
- 35.Afilalo J., Lauck S., Kim D.H., et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. 2017;70(6):689–700. doi: 10.1016/j.jacc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Morley J.E., Malmstrom T.K., Miller D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitnitski A.B., Mogilner A.J., MacKnight C., Rockwood K. The accumulation of deficits with age and possible invariants of aging. ScientificWorldJournal. 2002;2:1816–1822. doi: 10.1100/tsw.2002.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rockwood K., Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 39.Fumagalli C., Ponti L., Smorti M., et al. Determinants of health status in older patients with transthyretin cardiac amyloidosis: a prospective cohort study. Aging Clin Exp Res. 2024;36(1):89. doi: 10.1007/s40520-024-02750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 41.Broussier A., David J.P., Kharoubi M., et al. Frailty in wild-type transthyretin cardiac amyloidosis: the tip of the iceberg. J Clin Med. 2021;10(15):3415. doi: 10.3390/jcm10153415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Studenski S., Perera S., Patel K., et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams D., Tournev I.L., Taylor M.S., et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid. 2023;30(1):1–9. doi: 10.1080/13506129.2022.2091985. [DOI] [PubMed] [Google Scholar]
- 44.Obici L., Ajroud-Driss S., Lin K.P., et al. Impact of vutrisiran on quality of life and physical function in patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy. Neurol Ther. 2023;12(5):1759–1775. doi: 10.1007/s40120-023-00522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guralnik J.M., Simonsick E.M., Ferrucci L., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 46.Guralnik J.M., Ferrucci L., Simonsick E.M., Salive M.E., Wallace R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero-Ortuno R., Walsh C.D., Lawlor B.A., Kenny R.A. A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE) BMC Geriatr. 2010;10:57. doi: 10.1186/1471-2318-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gobbens R.J., van Assen M.A., Luijkx K.G., Wijnen-Sponselee M.T., Schols J.M. The tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc. 2010;11(5):344–355. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Bellera C.A., Rainfray M., Mathoulin-Pelissier S., et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 50.Boxer R.S., Shah K.B., Kenny A.M. Frailty and prognosis in advanced heart failure. Curr Opin Support Palliat Care. 2014;8(1):25–29. doi: 10.1097/SPC.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 51.Pothier K., Gana W., Bailly N., Fougère B. Associations between frailty and inflammation, physical, and psycho-social health in older adults: a systematic review. Front Psychol. 2022;13 doi: 10.3389/fpsyg.2022.805501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Damluji A.A., Alfaraidhy M., AlHajri N., et al. Sarcopenia and cardiovascular diseases. Circulation. 2023;147(20):1534–1553. doi: 10.1161/CIRCULATIONAHA.123.064071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 54.Soysal P., Stubbs B., Lucato P., et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Furman D., Campisi J., Verdin E., et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sack G.H., Jr. Serum amyloid A - a review. Mol Med. 2018;24(1):46. doi: 10.1186/s10020-018-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simons J.P., Al-Shawi R., Ellmerich S., et al. Pathogenetic mechanisms of amyloid A amyloidosis. Proc Natl Acad Sci U S A. 2013;110(40):16115–16120. doi: 10.1073/pnas.1306621110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruberg F.L., Grogan M., Hanna M., Kelly J.W., Maurer M.S. Transthyretin amyloid cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(22):2872–2891. doi: 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruberg F.L., Berk J.L. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286–1300. doi: 10.1161/CIRCULATIONAHA.111.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bales C.W., Ritchie C.S. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309–323. doi: 10.1146/annurev.nutr.22.010402.102715. [DOI] [PubMed] [Google Scholar]
- 61.Gertz M.A., Dispenzieri A., Sher T. Pathophysiology and treatment of cardiac amyloidosis. Nat Rev Cardiol. 2015;12(2):91–102. doi: 10.1038/nrcardio.2014.165. [DOI] [PubMed] [Google Scholar]
- 62.Dartigues J.F., Amieva H. Cognitive frailty: rational and definition from an (I.a.N.a./i.a.g.g.) international consensus group. J Nutr Health Aging. 2014;18(1):95. doi: 10.1007/s12603-013-0437-5. [DOI] [PubMed] [Google Scholar]
- 63.Halil M., Cemal Kizilarslanoglu M., Emin Kuyumcu M., Yesil Y., Cruz Jentoft A.J. Cognitive aspects of frailty: mechanisms behind the link between frailty and cognitive impairment. J Nutr Health Aging. 2015;19(3):276–283. doi: 10.1007/s12603-014-0535-z. [DOI] [PubMed] [Google Scholar]
- 64.Shimada H., Makizako H., Tsutsumimoto K., Doi T., Lee S., Suzuki T. Cognitive Frailty and incidence of dementia in older persons. J Prev Alzheimers Dis. 2018;5(1):42–48. doi: 10.14283/jpad.2017.29. [DOI] [PubMed] [Google Scholar]
- 65.Wange N., Anan I., Ericzon B.-G., et al. Atrial fibrillation and central nervous complications in liver transplanted hereditary transthyretin amyloidosis patients. Transplantation. 2018;102(2):e59–e66. doi: 10.1097/TP.0000000000001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merlini G., Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349(6):583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 67.Cannon J.A., Moffitt P., Perez-Moreno A.C., et al. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail. 2017;23(6):464–475. doi: 10.1016/j.cardfail.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 68.Ovsenik A., Podbregar M., Fabjan A. Cerebral blood flow impairment and cognitive decline in heart failure. Brain and Behavior. 2021;11(6) doi: 10.1002/brb3.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agarwal K.S., Kazim R., Xu J., Borson S., Taffet G.E. Unrecognized cognitive impairment and its effect on heart failure readmissions of elderly adults. J Am Geriatr Soc. 2016;64(11):2296–2301. doi: 10.1111/jgs.14471. [DOI] [PubMed] [Google Scholar]
- 70.Dobner S., Bernhard B., Asatryan B., et al. SGLT2 inhibitor therapy for transthyretin amyloid cardiomyopathy: early tolerance and clinical response to dapagliflozin. ESC Heart Fail. 2023;10(1):397–404. doi: 10.1002/ehf2.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gobbens R.J., Luijkx K.G., Wijnen-Sponselee M.T., Schols J.M. Towards an integral conceptual model of frailty. J Nutr Health Aging. 2010;14(3):175–181. doi: 10.1007/s12603-010-0045-6. [DOI] [PubMed] [Google Scholar]
- 72.Smorti M., Ponti L., Soffio F., et al. Prevalence of anxiety and depression symptoms in a sample of outpatients with ATTR cardiac amyloidosis. Front Psychol. 2023;13 doi: 10.3389/fpsyg.2022.1066224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sbolli M., Fiuzat M., Cani D., O'Connor C.M. Depression and heart failure: the lonely comorbidity. Eur J Heart Fail. 2020;22(11):2007–2017. doi: 10.1002/ejhf.1865. [DOI] [PubMed] [Google Scholar]
- 74.Bunt S., Steverink N., Olthof J., van der Schans C.P., Hobbelen J.S.M. Social frailty in older adults: a scoping review. Eur J Ageing. 2017;14(3):323–334. doi: 10.1007/s10433-017-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donovan N.J., Okereke O.I., Vannini P., et al. Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry. 2016;73(12):1230–1237. doi: 10.1001/jamapsychiatry.2016.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lovley A., Raymond K., Guthrie S.D., Pollock M., Sanchorawala V., White M.K. Patient-reported burden of hereditary transthyretin amyloidosis on functioning and well-being. J Patient Rep Outcomes. 2021;5(1):3. doi: 10.1186/s41687-020-00273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kojima G., Liljas A.E.M., Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23–30. doi: 10.2147/RMHP.S168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berglund H., Hasson H., Wilhelmson K., Dunér A., Dahlin-Ivanoff S. The impact of socioeconomic conditions, social networks, and health on frail older people's life satisfaction: a cross-sectional study. Health Psychol Res. 2016;4(1):5578. doi: 10.4081/hpr.2016.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Green P., Woglom A.E., Genereux P., et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5(9):974–981. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Afilalo J., Alexander K.P., Mack M.J., et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63(8):747–762. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu H., Wang L., Zhang W., Lu J., Yang M. Diagnostic test accuracy of ultrasound for sarcopenia diagnosis: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(1):57–70. doi: 10.1002/jcsm.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gillmore J.D., Judge D.P., Cappelli F., et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390(2):132–142. doi: 10.1056/NEJMoa2305434. [DOI] [PubMed] [Google Scholar]
- 83.Maurer M.S., Kale P., Fontana M., et al. Patisiran treatment in patients with transthyretin cardiac amyloidosis. N Engl J Med. 2023;389(17):1553–1565. doi: 10.1056/NEJMoa2300757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maurer M.S., Schwartz J.H., Gundapaneni B., et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 85.Coelho T., Marques W., Jr., Dasgupta N.R., et al. Eplontersen for hereditary transthyretin amyloidosis with polyneuropathy. JAMA. 2023;330(15):1448–1458. doi: 10.1001/jama.2023.18688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 87.Zaleski A.L., Taylor B.A., Panza G.A., et al. Coming of age: considerations in the prescription of exercise for older adults. Methodist Debakey Cardiovasc J. 2016;12(2):98–104. doi: 10.14797/mdcj-12-2-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kitzman D.W., Whellan D.J., Duncan P., et al. Physical rehabilitation for older patients hospitalized for heart failure. N Eng J Med. 2021;385(3):203–216. doi: 10.1056/NEJMoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bibas L., Levi M., Bendayan M., Mullie L., Forman D.E., Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57(2):134–143. doi: 10.1016/j.pcad.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 90.Nelson M.E., Rejeski W.J., Blair S.N., et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 91.Tiedemann A., Sherrington C., Close J.C., Lord S.R. Exercise and Sports Science Australia position statement on exercise and falls prevention in older people. J Sci Med Sport. 2011;14(6):489–495. doi: 10.1016/j.jsams.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 92.Laddu D.R., Ozemek C., Sabbahi A., Severin R., Phillips S.A., Arena R. Prioritizing movement to address the frailty phenotype in heart failure. Prog Cardiovasc Dis. 2021;67:26–32. doi: 10.1016/j.pcad.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Irabor B., McMillan J.M., Fine N.M. Assessment and management of older patients with transthyretin amyloidosis cardiomyopathy: geriatric cardiology, frailty assessment and beyond. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.863179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):e876–e894. doi: 10.1161/CIR.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 95.Rahman M.M., Mim S.A., Islam M.R., et al. Exploring the recent trends in management of dementia and frailty: focus on diagnosis and treatment. Curr Med Chem. 2022;29(32):5289–5314. doi: 10.2174/0929867329666220408102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu L., Gu H., Cai X., et al. The effects of exercise for cognitive function in older adults: a systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health. 2023;20(2):1088. doi: 10.3390/ijerph20021088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dagli R.J., Sharma A. Polypharmacy: a global risk factor for elderly people. J Int Oral Health. 2014;6(6):i–ii. [PMC free article] [PubMed] [Google Scholar]
- 98.By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052–2081. doi: 10.1111/jgs.18372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griffin J.M., Rosenthal J.L., Grodin J.L., Maurer M.S., Grogan M., Cheng R.K. ATTR amyloidosis: current and emerging management strategies. JACC CardioOncology. 2021;3(4):488–505. doi: 10.1016/j.jaccao.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lang F.M., Teruya S., Weinsaft A., et al. Sodium-glucose cotransporter 2 inhibitors for transthyretin amyloid cardiomyopathy: analyses of short-term efficacy and safety. Eur J Heart Fail. 2024;26(4):938–947. doi: 10.1002/ejhf.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng R.K., Vasbinder A., Levy W.C., et al. Lack of association between neurohormonal blockade and survival in transthyretin cardiac amyloidosis. J Am Heart Assoc. 2021;10(24) doi: 10.1161/JAHA.121.022859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Porcari A., Cappelli F., Nitsche C., et al. SGLT2 inhibitor therapy in patients with transthyretin amyloid cardiomyopathy. J Am Coll Cardiol. 2024;83(24):2411–2422. doi: 10.1016/j.jacc.2024.03.429. [DOI] [PubMed] [Google Scholar]
- 103.Ioannou A., Massa P., Patel R.K., et al. Conventional heart failure therapy in cardiac ATTR amyloidosis. Eur Heart J. 2023;44(31):2893–2907. doi: 10.1093/eurheartj/ehad347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Palma J.-A., Gonzalez-Duarte A., Kaufmann H. Orthostatic hypotension in hereditary transthyretin amyloidosis: epidemiology, diagnosis and management. Clin Auton Res. 2019;29:33–44. doi: 10.1007/s10286-019-00623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uchmanowicz I., Nessler J., Gobbens R., et al. Coexisting frailty with heart failure. Front Physiol. 2019;10:791. doi: 10.3389/fphys.2019.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kraai I.H., Vermeulen K.M., Luttik M.L.A., Hoekstra T., Jaarsma T., Hillege H.L. Preferences of heart failure patients in daily clinical practice: quality of life or longevity? Eur J Heart Fail. 2013;15(10):1113–1121. doi: 10.1093/eurjhf/hft071. [DOI] [PubMed] [Google Scholar]
- 107.Herr M., Arvieu J.J., Ankri J., Robine J.M. What is the duration of life expectancy in the state of frailty? Estimates in the SIPAF study. Eur J Ageing. 2018;15(2):165–173. doi: 10.1007/s10433-017-0438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Orkaby A.R., Nussbaum L., Ho Y.L., et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257–1264. doi: 10.1093/gerona/gly232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schoenborn N.L., Blackford A.L., Joshu C.E., Boyd C.M., Varadhan R. Life expectancy estimates based on comorbidities and frailty to inform preventive care. J Am Geriatr Soc. 2022;70(1):99–109. doi: 10.1111/jgs.17468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.ePrognosis - University of California San Francisco. http://eprognosis.ucsf.edu/
- 111.Ferrucci L., Kuchel G.A. Heterogeneity of aging: individual risk factors, mechanisms, patient priorities, and outcomes. J Am Geriatr Soc. 2021;69(3):610–612. doi: 10.1111/jgs.17011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abellan van Kan G., Rolland Y., Bergman H., Morley J.E., Kritchevsky S.B., Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12(1):29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 113.He D., Wang Z., Li J., et al. Changes in frailty and incident cardiovascular disease in three prospective cohorts. Eur Heart J. 2024;45:1058–1068. doi: 10.1093/eurheartj/ehad885. [DOI] [PubMed] [Google Scholar]
- 114.Norris C.M., Close J.C.T. Prehabilitation for the frailty syndrome: improving outcomes for our most vulnerable patients. Anesth Analg. 2020;130(6):1524–1533. doi: 10.1213/ANE.0000000000004785. [DOI] [PubMed] [Google Scholar]
- 115.Brunetti E., Lucà F., Presta R., et al. A comprehensive geriatric workup and frailty assessment in older patients with severe aortic stenosis. J Clin Med. 2024;13(14):4169. doi: 10.3390/jcm13144169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim D.H., Afilalo J., Shi S.M., et al. Evaluation of changes in functional status in the year after aortic valve replacement. JAMA Intern Med. 2019;179(3):383–391. doi: 10.1001/jamainternmed.2018.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Batchelor W.B., Anwaruddin S., Wang D.D., et al. The multidisciplinary heart team in cardiovascular medicine: current role and future challenges. JACC Adv. 2023;2(1) doi: 10.1016/j.jacadv.2022.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goyal P., Kwak M.J., Al Malouf C., et al. Geriatric cardiology: coming of age. JACC Adv. 2022;1(3) doi: 10.1016/j.jacadv.2022.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nanna M.G., Sutton N.R., Kochar A., et al. A geriatric approach to percutaneous coronary interventions in older adults, Part II: a JACC: Advances Expert Panel. JACC Adv. 2023;2(5) doi: 10.1016/j.jacadv.2023.100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prayman T.S., Thirusha L., Zoe F., et al. A prospective study of nutritional status in immunoglobulin light chain amyloidosis. Haematologica. 2012;98(1):136–140. doi: 10.3324/haematol.2012.070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grigoletti S.S., Zuchinali P., Lemieux-Blanchard É., et al. Focused review on nutritional status of patients with immunoglobulin light chain amyloidosis. Curr Probl Cancer. 2022;46(3) doi: 10.1016/j.currproblcancer.2021.100833. [DOI] [PubMed] [Google Scholar]
- 122.Caccialanza R., Cereda E., Klersy C., et al. Bioelectrical impedance vector analysis-derived phase angle predicts survival in patients with systemic immunoglobulin light-chain amyloidosis. Amyloid. 2020;27(3):168–173. doi: 10.1080/13506129.2020.1737004. [DOI] [PubMed] [Google Scholar]
- 123.Ahmed N., Mandel R., Fain M.J. Frailty: an emerging geriatric syndrome. Am J Med. 2007;120(9):748–753. doi: 10.1016/j.amjmed.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 124.Fried L.P., Ferrucci L., Darer J., Williamson J.D., Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 125.Abel G.A., Klepin H.D. Frailty and the management of hematologic malignancies. Blood. 2018;131(5):515–524. doi: 10.1182/blood-2017-09-746420. [DOI] [PubMed] [Google Scholar]
- 126.Palumbo A., Bringhen S., Mateos M.V., et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Engelhardt M., Domm A.S., Dold S.M., et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102(5):910–921. doi: 10.3324/haematol.2016.162693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McCurdy S.R., Gier S.H., Babushok D.V., et al. Fried frailty phenotype predicts mortality for newly diagnosed older patients with acute myeloid leukemia or high risk myelodysplastic syndrome. Blood. 2019;134(Supplement_1):2209. [Google Scholar]
- 129.Rios-Tamayo R., Lecumberri R., Cibeira M.T., et al. A simple frailty score predicts survival and early mortality in systemic AL amyloidosis. Cancers (Basel) 2024;16(9):1689. doi: 10.3390/cancers16091689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dispenzieri A., Gertz M.A., Kyle R.A., et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18):3751–3757. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 131.Wechalekar A.D., Schonland S.O., Kastritis E., et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121(17):3420–3427. doi: 10.1182/blood-2012-12-473066. [DOI] [PubMed] [Google Scholar]
- 132.Kumar S., Dispenzieri A., Lacy M.Q., et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gustine J.N., Staron A., Szalat R.E., et al. Predictors of hematologic response and survival with stem cell transplantation in AL amyloidosis: a 25-year longitudinal study. Am J Hematol. 2022;97(9):1189–1199. doi: 10.1002/ajh.26641. [DOI] [PubMed] [Google Scholar]
- 134.Sidiqi M.H., Aljama M.A., Buadi F.K., et al. Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol. 2018;36(13):1323–1329. doi: 10.1200/JCO.2017.76.9554. [DOI] [PubMed] [Google Scholar]
- 135.Venner C.P., Gillmore J.D., Sachchithanantham S., et al. Stringent patient selection improves outcomes in systemic light-chain amyloidosis after autologous stem cell transplantation in the upfront and relapsed setting. Haematologica. 2014;99(12):e260–e263. doi: 10.3324/haematol.2014.108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bianchi G., Zhang Y., Comenzo R.L. AL amyloidosis: current chemotherapy and immune therapy treatment strategies: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2021;3(4):467–487. doi: 10.1016/j.jaccao.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jaccard A., Moreau P., Leblond V., et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357(11):1083–1093. doi: 10.1056/NEJMoa070484. [DOI] [PubMed] [Google Scholar]
- 138.D'Souza A., Dispenzieri A., Wirk B., et al. Improved outcomes after autologous Hematopoietic cell transplantation for light chain amyloidosis: a Center for International Blood and Marrow Transplant Research Study. J Clin Oncol. 2015;33(32):3741–3749. doi: 10.1200/JCO.2015.62.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Acevedo J., Doros G., Szalat R., Sanchorawala V. Clinical characteristics, treatment regimens, and survival in elderly patients with AL amyloidosis. Clin Lymphoma Myeloma Leuk. 2021;21(6):425–426. doi: 10.1016/j.clml.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 140.Seldin D.C., Anderson J.J., Skinner M., et al. Successful treatment of AL amyloidosis with high-dose melphalan and autologous stem cell transplantation in patients over age 65. Blood. 2006;108(12):3945–3947. doi: 10.1182/blood-2006-06-029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kastritis E., Palladini G., Minnema M.C., et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46–58. doi: 10.1056/NEJMoa2028631. [DOI] [PubMed] [Google Scholar]
- 142.Chakraborty R., Rosenbaum C., Kaur G., et al. First report of outcomes in patients with stage IIIb AL amyloidosis treated with Dara-VCD front-line therapy. Br J Haematol. 2023;201(5):913–916. doi: 10.1111/bjh.18733. [DOI] [PubMed] [Google Scholar]
- 143.Kastritis E., Minnema M.C., Dimopoulos M.A., et al. Efficacy and safety of daratumumab monotherapy in newly diagnosed patients with stage 3B light-chain amyloidosis: a phase 2 study by the European myeloma network. Blood. 2023;142(Supplement 1):539. [Google Scholar]
- 144.Griffin J.M., Chiu L., Axsom K.M., et al. United network for organ sharing outcomes after heart transplantation for al compared to ATTR cardiac amyloidosis. Clin Transplant. 2020;34(10) doi: 10.1111/ctr.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bagratuni T., Wu P., Gonzalez de Castro D., et al. XBP1s levels are implicated in the biology and outcome of myeloma mediating different clinical outcomes to thalidomide-based treatments. Blood. 2010;116(2):250–253. doi: 10.1182/blood-2010-01-263236. [DOI] [PubMed] [Google Scholar]
- 146.Nativi-Nicolau J., Sarswat N., Fajardo J., et al. Best practices in specialized amyloidosis centers in the United States: a survey of cardiologists, nurses, patients, and patient advocates. Clin Med Insights Cardiol. 2021;15 doi: 10.1177/11795468211015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ski C.F., Cartledge S., Foldager D., et al. Integrated care in cardiovascular disease: a statement of the association of cardiovascular nursing and allied professions of the European Society of Cardiology. Eur J Cardiovasc Nurs. 2023;22(5):e39–e46. doi: 10.1093/eurjcn/zvad009. [DOI] [PubMed] [Google Scholar]
- 148.Kasa A.S., Drury P., Traynor V., Lee S.-C., Chang H.-C. The effectiveness of nurse-led interventions to manage frailty in community-dwelling older people: a systematic review. Systematic Reviews. 2023;12(1):182. doi: 10.1186/s13643-023-02335-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Magliano L., Obici L., Sforzini C., et al. Psychosocial burden and professional and social support in patients with hereditary transthyretin amyloidosis (ATTRv) and their relatives in Italy. Orphanet J Rare Dis. 2021;16(1):163. doi: 10.1186/s13023-021-01812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ihne-Schubert S.M., Radovic T., Fries S., et al. Needs of amyloidosis patients and their care providers: design & first results of the AMY-NEEDS research and care program. Orphanet J Rare Dis. 2024;19(1):58. doi: 10.1186/s13023-024-03052-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Redder E., Zhao Q., Bumma N., et al. Functional impairments of amyloidosis patients: physical therapy assessment. Hemato. 2022;3(3):414–421. [Google Scholar]
- 152.Dasgupta N.R. Care of patients with transthyretin amyloidosis: the roles of nutrition, supplements, exercise, and mental health. Am J Cardiol. 2022;185(Suppl 1):S35–S42. doi: 10.1016/j.amjcard.2022.10.053. [DOI] [PubMed] [Google Scholar]
- 153.Kuehneman T., Gregory M., de Waal D., et al. Academy of nutrition and dietetics evidence-based practice guideline for the management of heart failure in adults. J Acad Nutr Diet. 2018;118(12):2331–2345. doi: 10.1016/j.jand.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 154.Gayà-Barroso A., González-Moreno J., Rodríguez A., et al. Occupational practice in patients with hereditary transthyretin amyloidosis, a qualitative study. Orphanet J Rare Dis. 2023;18(1):352. doi: 10.1186/s13023-023-02964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gayà-Barroso A., González-Moreno J., Rodríguez A., et al. Establishing occupational therapy needs: a semi-structured interview with hereditary transthyretin amyloidosis patients. Int J Environ Res Public Health. 2022;19(18):11721. doi: 10.3390/ijerph191811721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jha S.R., Hannu M.K., Chang S., et al. The prevalence and prognostic significance of frailty in patients with advanced heart failure referred for heart transplantation. Transplantation. 2016;100(2):429–436. doi: 10.1097/TP.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 157.Aili S.R., De Silva R., Wilhelm K., et al. Validation of cognitive impairment in combination with physical frailty as a predictor of mortality in patients with advanced heart failure referred for heart transplantation. Transplantation. 2022;106(1):200–209. doi: 10.1097/TP.0000000000003669. [DOI] [PubMed] [Google Scholar]
- 158.Jha S.R., Hannu M.K., Gore K., et al. Cognitive impairment improves the predictive validity of physical frailty for mortality in patients with advanced heart failure referred for heart transplantation. J Heart Lung Transplant. 2016;35(9):1092–1100. doi: 10.1016/j.healun.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 159.Macdonald P.S., Gorrie N., Brennan X., et al. The impact of frailty on mortality after heart transplantation. J Heart Lung Transplant. 2021;40(2):87–94. doi: 10.1016/j.healun.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 160.Muthiah K., Wilhelm K., Robson D., et al. Impact of frailty on mortality and morbidity in bridge to transplant recipients of contemporary durable mechanical circulatory support. J Heart Lung Transplant. 2022;41(6):829–839. doi: 10.1016/j.healun.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 161.Dunlay S.M., Park S.J., Joyce L.D., et al. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant. 2014;33(4):359–365. doi: 10.1016/j.healun.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chung C.J., Wu C., Jones M., et al. Reduced handgrip strength as a marker of frailty predicts clinical outcomes in patients with heart failure undergoing ventricular assist device placement. J Card Fail. 2014;20(5):310–315. doi: 10.1016/j.cardfail.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maurer M.S., Horn E., Reyentovich A., et al. Can a left ventricular assist device in individuals with advanced systolic heart failure improve or reverse frailty? J Am Geriatr Soc. 2017;65(11):2383–2390. doi: 10.1111/jgs.15124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jha S.R., Hannu M.K., Newton P.J., et al. Reversibility of frailty after bridge-to-transplant ventricular assist device implantation or heart transplantation. Transplant Direct. 2017;3(7) doi: 10.1097/TXD.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aili S.R., Lo P., Villanueva J.E., Joshi Y., Emmanuel S., Macdonald P.S. Prevention and reversal of frailty in heart failure - a systematic review. Circ J. 2021;86(1):14–22. doi: 10.1253/circj.CJ-21-0819. [DOI] [PubMed] [Google Scholar]
- 166.Dinesh V., Pierce R., Hespe L., et al. The relationship between rehabilitation and frailty in advanced heart or lung disease. Transplant Direct. 2024;10(4) doi: 10.1097/TXD.0000000000001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dent E., Morley J.E., Cruz-Jentoft A.J., et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23(9):771–787. doi: 10.1007/s12603-019-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Angulo J., El Assar M., Álvarez-Bustos A., Rodríguez-Mañas L. Physical activity and exercise: strategies to manage frailty. Redox Biol. 2020;35 doi: 10.1016/j.redox.2020.101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li X., Zhang Y., Tian Y., Cheng Q., Gao Y., Gao M. Exercise interventions for older people with cognitive frailty—a scoping review. BMC Geriatrics. 2022;22(1):721. doi: 10.1186/s12877-022-03370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Delbaere K., Valenzuela T., Lord S.R., et al. E-health StandingTall balance exercise for fall prevention in older people: results of a two year randomised controlled trial. BMJ. 2021;373 doi: 10.1136/bmj.n740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Singer J.P., Soong A., Bruun A., et al. A mobile health technology enabled home-based intervention to treat frailty in adult lung transplant candidates: a pilot study. Clin Transplant. 2018;32(6) doi: 10.1111/ctr.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Caccialanza R., Palladini G., Cereda E., et al. Nutritional counseling improves quality of life and preserves body weight in systemic immunoglobulin light-chain (AL) amyloidosis. Nutrition. 2015;31(10):1228–1234. doi: 10.1016/j.nut.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 173.Liu S., Zhang L., Li S. Advances in nutritional supplementation for sarcopenia management. Front Nutr. 2023;10 doi: 10.3389/fnut.2023.1189522. [DOI] [PMC free article] [PubMed] [Google Scholar]