Abstract
Introduction
Kaposi sarcoma is a vascular tumour associated with human herpesvirus-8 (HHV-8) infection. Due to immunosuppressive treatment, solid organ transplant recipients infected with HHV-8 have increased risk of Kaposi sarcoma. The risk of being infected with HHV-8 is associated with higher age, male sex, non-White ethnicity, being a man who has sex with men (MSM) and use of recreational drugs. Post-transplant Kaposi sarcoma is rare in HHV-8 low-prevalence areas. We report the first case of a liver transplant recipient developing Kaposi sarcoma following donor-transmitted HHV-8 infection in Denmark.
Case presentation
A 51-year-old male underwent uncomplicated liver transplantation at Copenhagen University Hospital – Rigshospitalet, and received standard immunosuppressive treatment with tacrolimus, mycophenolate mofetil and prednisolone. Five months post-transplantation the patient was admitted with abdominal pain, diarrhoea, and dehydration. Ultrasound imaging and PET/CT scan revealed multiple liver tumours. Biopsy from liver tumour diagnosed visceral post-transplantation Kaposi sarcoma. Kaposi sarcoma was successfully treated with reduction of immunosuppressive treatment and conversion from tacrolimus to everolimus, resulting in viral clearance and complete metabolic and structural tumour response. A review of the donor post-transplantation revealed multiple risk factors for being infected with HHV-8, with subsequent analysis confirming the donor was positive for HHV-8.
Discussion
Even in low-prevalence areas, there are benefits of targeted screening for HHV-8 in high-risk organ donors and recipients described in the literature and highlighted in the current case. Post-transplantation Kaposi sarcoma due to HHV-8 may be managed with reduction in immunosuppressive treatment and conversion from calcineurin inhibitors to mTOR inhibitors.
Keywords: Human herpesvirus-8, liver transplantation, Kaposi sarcoma, immunosuppressive treatment, case report
Highlights
-
•
First case of post-transplantation HHV-8 induced Kaposi sarcoma in Denmark.
-
•
Complete response following reduction in immunosuppressive treatment.
-
•
Potential HHV-8 screening of organ donors with risk of HHV-8.
Introduction
Solid organ transplantation is carried out as a standard of care lifesaving procedure for a wide range of end-stage organ diseases [1]. Solid organ transplant recipients require immunosuppressive treatment to reduce the risk of acute cellular rejection (ACR) of the transplanted, which increases the risk of both infectious and malignant diseases [2]. One rare post-transplant malignant disease is Kaposi sarcoma. Kaposi sarcoma is a vascular tumour arising from endothelial cells that most commonly presents as skin lesions but may also present as mucosal or visceral tumours. Kaposi sarcoma lesions can range from purple patches and papules to aggressive ulcerated plaques and nodules with dissemination to visceral organs [3]. Kaposi sarcoma manifests as 4 different subtypes [4]: Classic, endemic, epidemic, and iatrogenic. Classic Kaposi sarcoma primarily affects middle-aged to elderly men from the Mediterranean and Middle Eastern areas [4]. Endemic Kaposi sarcoma is most prevalent in sub-Saharan Africa [4]. Epidemic Kaposi sarcoma is associated with human immunodeficiency virus (HIV) infection or acquired immunodeficiency syndrome (AIDS) [4]. Iatrogenic Kaposi sarcoma emerges after immunosuppressive treatment primarily observed after solid organ or haematopoietic stem cell transplantation, but it is also observed after immunosuppressive treatment for autoimmune diseases [4], [5]. General risk factors identified for development of Kaposi sarcoma in transplant recipients are higher age, non-White ethnicity, male sex, and specific vascular endothelial growth factor (VEGF) genotypes [6], [7]. Also, men who have sex with men (MSM) and people who use recreational drugs are at higher risk [9]. Kaposi sarcoma is directly associated with infection with Human Herpesvirus-8 (HHV-8) [10].
HHV-8 is a gamma-2 herpesvirus that infects endothelial cells and B cells. The virus is associated with different severe diseases including Kaposi sarcoma, multicentric Castleman’s disease, primary effusion lymphoma, and Kaposi sarcoma-associated herpesvirus inflammatory cytokine syndrome [6]. The prevalence of HHV-8 varies across the world. In sub-Saharan Africa, which is considered an endemic area, the prevalence of HHV-8 is > 50 % [10]. Countries in the Mediterranean area and Middle Eastern countries have an intermediate prevalence of HHV-8 around 10–20 % [10], and low-prevalence areas are Northern European and North American countries with < 5 % being infected with HHV-8 [10]. HHV-8 DNA is found in 95 % of all Kaposi sarcoma tissue [5], but HHV-8 infection alone is not sufficient for development of Kaposi sarcoma. A reduction of host immune competency is required for the progression of Kaposi sarcoma [11].
In this case report we describe the first case of transplantation-transmitted HHV-8 inducing Kaposi sarcoma in a liver transplant recipient in Denmark, which is an HHV-8 low-prevalence area.
Case
This case report covers a 51-year-old male who underwent technically uncomplicated orthotopic liver transplantation at Copenhagen University Hospital – Rigshospitalet, a national centre of liver transplantation, due to metabolic dysfunction-associated steatohepatitis (MASH) cirrhosis with duct-to-duct bile anastomosis. The patient was born in Turkey and emigrated to Denmark 27 years before his transplantation. His medical history included insulin-treated type 2 diabetes mellitus and asthma. Post-transplantation the patient received standard immunosuppressive treatment with tacrolimus, mycophenolate mofetil (MMF), and prednisolone. Both the donor and recipient were positive for cytomegalovirus (CMV) IgG, leading to valganciclovir as antiviral prophylaxis for the first three months post-transplantation according to local protocol [12]. In addition, trimethoprim-sulfamethoxazole was administered as Pneumocystis jiroveci prophylaxis for six months. There were no postoperative complications, and the patient was discharged after 13 days with normalized liver and kidney biochemistry and serum tacrolimus trough concentration of 9 µg/L. Initially, the patient was followed with weekly outpatient clinic visit with normal biochemistry.
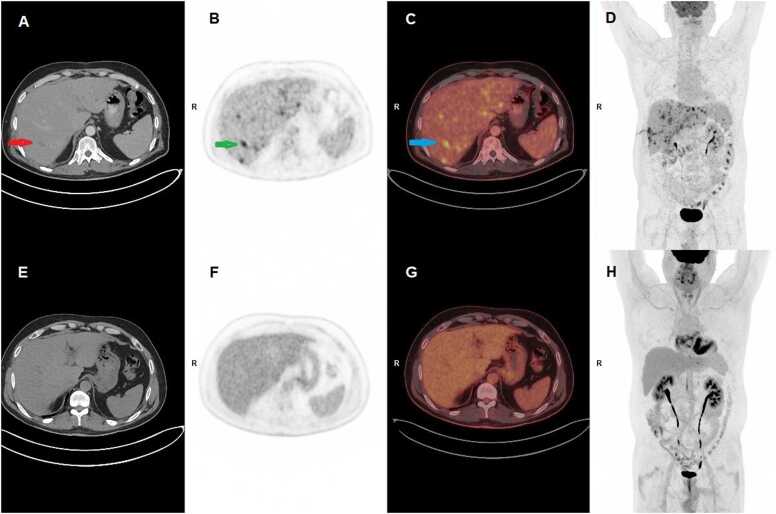
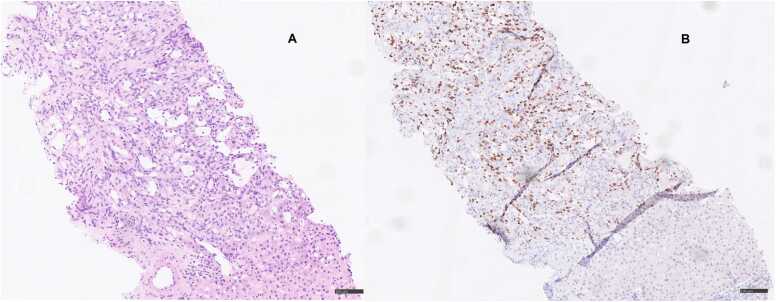
Five months post-transplantation, the patient was admitted with abdominal pain, diarrhoea, and dehydration. On examination, his abdomen was soft and without distention, but with slight tenderness during palpation of the lower abdomen, primarily in the lower right quadrant. There was no rebound or percussion tenderness. Ultrasound imaging of the abdomen showed multiple tumours and a subsequent 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) revealed pathological metabolic activity in the liver (Fig. 1). Liver biopsy demonstrated morphologically and immunohistochemically confirmed Kaposi sarcoma (based on ERG1, CD31, CD34, FVIII, and nuclear HHV-8 stainings) (day 0) (Fig. 2). MMF was discontinued, tacrolimus was switched to everolimus, and the dose of prednisolone was reduced. Quantitative polymerase chain reaction (PCR) in serum was positive for HHV-8 with a cycle threshold of 31. The cycle threshold value is a surrogate marker for viral material present in a sample, and higher number of cycles required indicates lower viral load.
Fig. 1.
Whole-body FDG PET/CT, December 2020 (A-D) and November 2023 (E-H). A, CT of upper abdomen with multiple hypodense foci in the liver parenchyma with a red arrow pointing at the largest focus. B, PET of the same part of upper abdomen as seen in A with several foci with pathological high FDG-uptake corresponding to foci in B. The green arrow points at the same finding as the green arrow in A. C, Fused PET/CT of A and B with a blue arrow pointing at the same finding as shown in A and B. D, Whole body FDG-PET/CT with multiple pathological foci with high FDG uptake in the liver parenchyma. The long finding in the right lateral side of the patient is due to a liver biopsy prior to the PET/CT. Physiological high FDG uptake in the included part of the brain and excretion to the urinary tract, the bladder, and intestines. E, CT image of the same part of the liver as seen in A. No pathological findings in the liver. F, PET corresponding to B with normal FDG uptake in the liver parenchyma with no pathological foci. G, Fused PET/CT of E and F. H, Whole-body PET with normal FDG distribution in the body. Physiological FDG uptake in the heart. FDG, 18F-fluorodeoxyglucose; PET, positron-emission tomography; CT, computed tomography.
Fig. 2.
Liver biopsy, November 2020. A, Liver biopsy with a vascular tumour with spindle cells and irregular vascular spaces with atypical endothelial lining, morphologically consistent with Kaposi sarcoma. B, Immunohistochemical staining for HHV-8 showing positivity in tumour cells, immunohistochemically consistent with Kaposi sarcoma.
The patient developed a small superficial intrahepatic abscess as a complication to liver biopsy, which was treated successfully with drainage and antibiotics (day 25).
Control FDG-PET/CT after diagnosis demonstrated significant progression with multiple bilateral metabolic active tumours (day 28). Following this, everolimus was reduced further resulting in serum trough steady-state concentrations ranging from 3.0 to 3.8 µg/L as compared to 4.7–7.5 µg/L in the initial phase after conversion to everolimus. At the same time, PCR demonstrated increased cycle threshold values of 36.4, indicating reduced HHV-8 virus load.
At day 56, control FDG-PET/CT demonstrated unchanged structural changes, but there was no longer metabolic activity in the liver tumours. Following continuous low dose immunosuppressive treatment at day 90, the serum HHV-8 PCR analysis was negative, and FDG-PET/CT remained unchanged with structural lesions with complete metabolic remission.
At day 113, liver enzymes increased, and control liver biopsy demonstrated mild ACR with Banff 2–3, and low dose tacrolimus was added to the treatment resulting in normalized liver parameters. Since then, all HHV-8 serum PCR analyses have been negative, and at day 293, FDG-PET/CT demonstrated significant reduction in structural changes. At day 470, complete remission was observed on FDG-PET/CT. Three years after liver transplantation, the patient was well with no signs of Kaposi sarcoma recurrence on FDG-PET/CT, normal liver parameters and negative HHV-8 analyses (Fig. 1).
A post hoc review of donor risk factors revealed that the donor originated from a country with intermediate prevalence of HHV-8, belonged to the category of MSM and was using pre-exposure HIV prophylaxis. Post-transplantation tests showed HHV-8 positivity in tissue from the donor. Lungs from the same donor were used for another recipient who also tested positive for HHV-8 without development of Kaposi sarcoma. Other organs from this donor were not used for transplantation.
Discussion
The current case report describes the first case of Kaposi sarcoma after transplantation-transmitted HHV-8 in a liver transplant recipient in Denmark, which is an HHV-8 low-prevalence area, and the organ donor had multiple risk factors for having HHV-8 [7], [8]. Currently, adult organ transplant recipients and donors at the Copenhagen University Hospital – Rigshospitalet are routinely screened for human parvovirus B19, BK virus (kidney transplant recipients), CMV, Epstein-Barr virus, hepatitis B and C viruses, herpes simplex virus, and HIV [12], while recipients are additionally screened for latent tuberculosis [13]. Solid organ transplant recipients and donors are not screened for HHV-8, and this case therefore raises the question of additional HHV-8 screening in organ transplantation.
Retrospective registry studies indicate an incidence rate of Kaposi sarcoma in the United States of around 8.8–12.4 per 100.000 person years between 1987 and 2014, with an estimated cumulative incidence 8 years after solid organ transplantation of 0.06 % [8]. The primary risk factor for development of Kaposi sarcoma in solid organ transplantation is infection with HHV-8 either due to transmission of HHV-8 through organ transplantation [10], post-transplantation de novo HHV-8 infection, or reactivation of latent infection [5]. In solid organ transplant recipients, HHV-8 is demonstrated in Kaposi sarcoma tumour tissue [14], and an increase in circulating HHV-8 DNA is observed when host immune competency decreases following immunosuppressive treatment [14]. However, a study screening 20 HHV-8 seropositive kidney transplant recipients and 7 seropositive donors between 2000 and 2006 in Italy did not find any cases of positive HHV-8 PCR at scheduled six months intervals for 18 months after transplantation [15]. This study was limited by being conducted in an HHV-8 low-prevalence area, lack of information of immunosuppressive regimen, and incomplete follow-up.
Some studies have suggested systematic screening for HHV-8, especially in individuals from high-prevalence areas [14]. These studies also suggest that HHV-8 positive individuals should not be excluded from organ transplantation, but screening may identify those who need close monitoring for Kaposi sarcoma and alternative post-transplantation immunosuppressive treatment [14]. The optimal pre-transplantation screening of donors is hampered by the fact that fast and reliable, validated HHV-8 serological assays are not generally available [14]. Based on this, several authors recommend post-transplant recipient monitoring of HHV-8 to modify immune suppression as soon as a potential increase in viral DNA is observed [14].
The recipient who received a lung from the same donor was infected with HHV-8 with detectable HHV-8 DNA in plasma but did not develop Kaposi sarcoma. This is in line with a report of six HHV-8 positive donors with organs transplanted to 22 recipients, of whom 14 demonstrated HHV-8 infection, while only 6 recipients developed Kaposi sarcoma [9].
In this case, immunosuppression was changed from a calcineurin inhibitor to an mTOR inhibitor combined with discontinuation of MMF and steroid reduction following the diagnosis of Kaposi sarcoma. The rationale for using mTOR inhibitors is that the angiogenic and to some extent carcinogenic effects of HHV-8 are mediated through the Akt- and MAP kinase pathway that stimulates mTOR [14], [15]. Several studies covering optimal treatment of iatrogenic Kaposi sarcoma recommend reduction in immunosuppression and conversion from calcineurin inhibitors to mTOR inhibitors [11]. This results in a high rate of tumour response while maintaining graft function. Before introduction of mTOR inhibitors, reduction of immunosuppression was associated with acute cellular graft rejection [11], but recent studies report that conversion from calcineurin inhibitors to mTOR inhibitors in Kaposi sarcoma results in significant antitumour effect with lower risk of ACR than compared to reduction of immunosuppressive treatment alone [11]. In the current case, a mild ACR was observed, and low dose tacrolimus was supplemented to everolimus. In two recent case series, including kidney, liver, and lung transplant recipients with Kaposi sarcoma following reduction and conversion of immunosuppression led to antitumour response in 9/13 and 8/8, respectively González-Cruz et al., 2021, [16]. However, randomised trials have not been performed to investigate optimal management, and changes in immunosuppression may result in rejection as seen in this case.
In aggressive disseminated cases of Kaposi sarcoma, chemotherapy can be required when reduction in immunosuppression is not effective [11], while for Kaposi sarcoma limited to the skin, a double case report of 2 kidney recipients supported the use of topical imiquimod 5 % [20].
The prognosis of Kaposi sarcoma in organ transplant recipients depends on the extent of disease involvement [4]. Post-transplant Kaposi sarcoma limited to the skin has a good prognosis with low mortality [5]. When the disease spreads to one or more visceral organs, mortality ranges from 25 % to 80 %, depending on disease severity and treatment response [4], [8]. Data on mortality in cases with only a single visceral organ involved are limited, but the prognosis may be better than for patients with multiple visceral organs affected, particularly if early intervention, such as change of immunosuppression, is possible [3], [4], González-Cruz et al., 2021. Therefore, a risk evaluation of the donor organ and recipient need is warranted, even in HHV-8 low-prevalence areas. Introduction of universal HHV-8 screening of solid organ donors and recipients may not be cost-efficient in HHV-8 low-prevalence areas, but we propose targeted serological screening of those with multiple risk factors for HHV-8 infection.
This is the first case of transmission of HHV-8 through liver transplantation leading to Kaposi sarcoma in Denmark. HHV-8 screening in high-risk patients is recommended and may potentially impact clinical decisions, but universal screening in an HHV-8 low-prevalence area is questionable. If Kaposi sarcoma develops after transplantation, reduction of immunosuppression and conversion from calcineurin inhibitor to mTOR inhibitor can be effective, but further trials are needed to guide changes in immunosuppression while maintaining organ function.
CRediT authorship contribution statement
Clemmesen Julius Leander Ross: Writing – original draft, Data curation, Conceptualization. Willemoe Gro Linno: Validation, Writing – review & editing, Data curation, Investigation, Resources. Nielsen Susanne Dam: Writing – original draft, Validation, Supervision, Resources, Project administration, Investigation, Data curation, Conceptualization. Loft Annika: Writing – review & editing, Validation, Resources, Investigation, Data curation. Suarez-Zdunek Moises Alberto: Writing – original draft, Data curation, Conceptualization. Schultz Nicolai Aagaard: Writing – review & editing, Validation, Investigation, Data curation. Arentoft Nicoline: Writing – original draft, Data curation, Conceptualization. Pedersen Christian Ross: Writing – review & editing, Validation, Resources, Investigation, Data curation, Conceptualization. Perch Michael: Writing – review & editing, Validation, Investigation, Data curation. Hillingsø Jens Georg: Writing – review & editing, Validation, Investigation, Data curation. Kirkby Nikolai: Writing – review & editing, Validation, Resources, Investigation, Data curation.
Ethical approval
Under Danish law, case reports are not subjects to approval from ethical committees. The patient has provided oral and written informed consent to publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. All transplantations in Denmark are performed after obtaining autonomous consent free from coercion from the donor or their next of kin. Organs are not sourced from executed prisoners or prisoners of conscience.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: S.D.N. has received unrestricted research grants from Novo Nordic Foundation, Independent Research Fund Denmark, Rigshospitalet Research Fund, Svend Andersen Fonden and honoraria from Gilead and has served on advisory boards for Gilead, GSK and Takeda. Other authors declare no competing interests.
Acknowledgements
We extend our thanks to the patient for allowing his case to be presented.
References
- 1.Lucey M.R., Furuya K.N., Foley D.P. Liver transplantation. N Engl J Med. 2023;389(20):1888–1900. doi: 10.1056/NEJMra2200923. [DOI] [PubMed] [Google Scholar]
- Ruiz R., Kirk A.D. Transplantation of the liver. Elsevier; 2015. Long-term toxicity of immunosuppressive therapy; pp. 1354–1363. [DOI] [Google Scholar]
- 2.Etemad S.A., Dewan A.K. Kaposi sarcoma updates. Dermatol Clin. 2019;37(4):505–517. doi: 10.1016/J.DET.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Cesarman E., Damania B., Krown S.E., Martin J., Bower M., Whitby D. Kaposi sarcoma. Nat Rev Dis Prim. 2019;5(1):9. doi: 10.1038/s41572-019-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saowapa S., Polpichai N., Siladech P., Wannaphut C., Tanariyakul M., Wattanachayakul P., et al. Evaluating Kaposi Sarcoma in kidney transplant patients: a systematic review and meta-analysis. Cureus. 2024;16(1) doi: 10.7759/cureus.52527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cesaro S., Tridello G., van der Werf S., Bader P., Sociè G., Ljungman P., et al. Incidence and outcome of Kaposi sarcoma after hematopoietic stem cell transplantation: a retrospective analysis and a review of the literature, on behalf of infectious diseases working party of EBMT. Bone Marrow Transpl. 2020;55(1):110–116. doi: 10.1038/s41409-019-0644-8. [DOI] [PubMed] [Google Scholar]
- 6.Zanetti E., Barozzi P., Brown E.E., Bosco R., Vallerini D., Riva G., et al. Common vascular endothelial growth factor variants and risk for posttransplant Kaposi sarcoma. Transplantation. 2010;90(3):337–338. doi: 10.1097/TP.0b013e3181e4e4d9. [DOI] [PubMed] [Google Scholar]
- 7.Cahoon E.K., Linet M.S., Clarke C.A., Pawlish K.S., Engels E.A., Pfeiffer R.M. Risk of Kaposi sarcoma after solid organ transplantation in the United States. Int J Cancer. 2018;143(11):2741–2748. doi: 10.1002/ijc.31735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dollard S.C., Annambhotla P., Wong P., Meneses K., Amin M.M., La Hoz R.M., et al. Donor-derived human herpesvirus 8 and development of Kaposi sarcoma among 6 recipients of organs from donors with high-risk sexual and substance use behavior. Am J Transpl. 2021;21(2):681–688. doi: 10.1111/ajt.16181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elboukhari K., Achehboune K., Gallouj S., Elloudi S., BayBay H., Mernissi F.Z. Iatrogenic kaposi sarcoma: case report with review of the literature. MOJ Gerontol Geriatr. 2020;5(1):1–3. doi: 10.15406/mojgg.2020.05.00221. [DOI] [Google Scholar]
- 9.Delyon J., Rabate C., Euvrard S., Harwood C.A., Proby C., Güleç A.T., et al. Management of Kaposi sarcoma after solid organ transplantation: a European retrospective study. J Am Acad Dermatol. 2019;81(2):448–455. doi: 10.1016/j.jaad.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Ekenberg C., da Cunha-Bang C., Lodding I.P., Sørensen S.S., Sengeløv H., Perch M., et al. Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22(2) doi: 10.1111/tid.13252. [DOI] [PubMed] [Google Scholar]
- 11.Harboe Z.B., Hald A., Ekenberg C., Ete Wareham N., Fogt Lundbo L., Holler J.G., et al. Implementation of a vaccination clinic for adult solid organ transplant candidates: a single-center experience. Vaccine. 2023;41(45):6637–6644. doi: 10.1016/j.vaccine.2023.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Mikulska M., Balletto E., Mularoni A. Human herpesvirus 8 and Kaposi sarcoma: how should we screen and manage the transplant recipient? Curr Opin Infect Dis. 2021;34(6):646–653. doi: 10.1097/QCO.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 13.Bergallo M., Costa C., Margio S., Sidoti F., Re D., Segoloni G.P., et al. Human herpes virus 8 infection in kidney transplant patients from an area of northwestern Italy (Piemonte region. Nephrol Dial Transpl. 2007;22(6):1757–1761. doi: 10.1093/ndt/gfm056. [DOI] [PubMed] [Google Scholar]
- 14.Kim H., Jang J.H., Song Y.E., Seo T. Kaposi’s sarcoma-associated herpesvirus viral protein kinase phosphorylates extracellular signal-regulated kinase and activates MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 2020;521(4):1083–1088. doi: 10.1016/j.bbrc.2019.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Liu X., Cohen J.I. The role of PI3K/Akt in human herpesvirus infection: from the bench to the bedside. Virology. 2015;479–480:568–577. doi: 10.1016/j.virol.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Cruz C., Ferrándiz-Pulido C., Ferrer Fàbregas B., García-Patos Briones V. Vol. 157. 2021. Posttransplant Kaposi sarcoma: analysis of a series of 13 patients; pp. 339–343. (Med Clin (Barc)). [DOI] [PubMed] [Google Scholar]
- 16.Bohelay G., Arzouk N., Lévy P., Rabaté C., Le Cleach L., Barete S., et al. Outcome of second kidney transplantation in patients with previous post-transplantation Kaposi’s sarcoma: a French retrospective study. Clin Transpl. 2017;31(11) doi: 10.1111/ctr.13091. [DOI] [PubMed] [Google Scholar]
- 17.Prinz Vavricka B.M., Hofbauer G.F.L., Dummer R., French L.E., Kempf W. Topical treatment of cutaneous Kaposi sarcoma with imiquimod 5% in renal-transplant recipients: a clinicopathological observation. Clin Exp Dermatol. 2012;37(6):620–625. doi: 10.1111/j.1365-2230.2011.04278.x. [DOI] [PubMed] [Google Scholar]