Abstract
An accumulation in cells of unfolded proteins is believed to be the common signal triggering the induction of heat shock proteins (hsps). Accordingly, in Saccharomyces cerevisiae, inhibition of protein breakdown at 30°C with the proteasome inhibitor MG132 caused a coordinate induction of many heat shock proteins within 1 to 2 h. Concomitantly, MG132, at concentrations that had little or no effect on growth rate, caused a dramatic increase in the cells’ resistance to very high temperature. The magnitude of this effect depended on the extent and duration of the inhibition of proteolysis. A similar induction of hsps and thermotolerance was seen with another proteasome inhibitor, clasto-lactacystin β-lactone, but not with an inhibitor of vacuolar proteases. Surprisingly, when the reversible inhibitor MG132 was removed, thermotolerance decreased rapidly, while synthesis of hsps continued to increase. In addition, exposure to MG132 and 37°C together had synergistic effects in promoting thermotolerance but did not increase hsp expression beyond that seen with either stimulus alone. Although thermotolerance did not correlate with hsp content, another thermoprotectant trehalose accumulated upon exposure of cells to MG132, and the cellular content of this disaccharide, unlike that of hsps, quickly decreased upon removal of MG132. Also, MG132 and 37°C had additive effects in causing trehalose accumulation. Thus, the resistance to heat induced by proteasome inhibitors is not just due to induction of hsps but also requires a short-lived metabolite, probably trehalose, which accumulates when proteolysis is reduced.
Exposure of cells or organisms to elevated temperatures triggers the synthesis of heat shock proteins (hsps), which help protect cells against high temperatures and a variety of other potentially toxic agents (39, 51). Many of these hsps function as molecular chaperones that help prevent the accumulation of unfolded or aggregated polypeptides (21). In growing cells, the hsps catalyze the proper folding of nascent polypeptide chains, and upon heat shock, these chaperones prevent protein aggregation and promote the refolding of damaged polypeptides (15). Another important function of certain hsps is to promote the rapid degradation of such abnormal proteins (28, 32, 47). In eukaryotes, ubiquitin and certain ubiquitin-conjugating enzymes are hsps that function in the rapid breakdown of denatured proteins (39). In addition, certain molecular chaperones have been shown to serve as cofactors in the selective degradation of abnormal polypeptides (28, 32).
The induction of heat shock response can lead to increased tolerance of cells to otherwise lethal, high temperatures. For example, when yeast cells growing at 25°C are shifted to an intermediate temperature, e.g., 37°C, to cause induction of hsps, the fraction of cells able to survive a subsequent exposure to 50°C increases markedly. This increase in thermotolerance is generally believed to require the induction of hsps (39), although this requirement has been questioned (3, 20, 48). The induction of the heat shock response can also protect cells against a variety of other toxic insults, such as ethanol and hydrogen peroxide (42, 49). In fact, in experimental animals, the exposure to 42°C to induce hsps has been shown to protect heart and brain against subsequent anoxic injury (36). Consequently, there has been appreciable medical interest in the possibility of inducing this response in patients. Because elevating body temperatures is an inconvenient and potentially dangerous procedure, the identification of pharmacological agents that could elicit this protective response would be highly desirable.
Hsps are also induced by a variety of other insults to the cell, such as ethanol, heavy metals, and oxidants (42). One common feature of these various conditions is that they damage or denature cell proteins. Other treatments that prevent the proper folding of newly synthesized proteins (e.g., incorporation of amino acid analogs) or introduction of unfolded proteins into bacterial or vertebrate cells also causes the induction of hsps (1, 22, 40). Thus, it is widely believed that the common feature of the various conditions that elicit this response is the accumulation of abnormal polypeptides in cells. Similarly, it is now well established that the accumulation of unfolded proteins in the endoplasmic reticulum (ER) signals the induction of many ER-specific molecular chaperones (8, 35).
The cells’ capacity to degrade rapidly such unfolded proteins is therefore likely to be one important determinant influencing the expression of hsps. The major pathway for the selective degradation of abnormal proteins in the cytosol and nucleus is the ubiquitin-proteasome pathway (7, 18). A failure of function of this degradative system should lead to the induction of hsps. In fact, increased thermotolerance was observed in a yeast mutant in which genes encoding the ubiquitin-conjugating enzymes UBC4 and UBC5 were deleted (45).
A major goal of the present study was to test whether pharmacological agents that block proteasome function, by causing an accumulation of abnormal proteins, might increase the expression of hsps and thermotolerance. The magnitude and rapidity of such a response should depend on the extent of inhibition of protein breakdown and the frequency of production of abnormal polypeptides in normal cells. Recently, several selective inhibitors of the proteasome that can enter mammalian cells and inhibit the ubiquitin-proteasome pathway (e.g., the reversible peptide aldehydes such as MG132 or the irreversible modifiers lactacystin and clasto-lactacystin β-lactone) have been identified (11, 14, 25, 41). Certain of these inhibitors also selectively block the degradation of short-lived and abnormal proteins in intact Saccharomyces cerevisiae cells (31). In yeast cells, unlike in mammalian cells, these proteasome inhibitors do not affect the breakdown of bulk of cell proteins, which are long-lived and degraded in the vacuole (31). In related studies of MDCK cells, we have recently found that exposure to proteasome inhibitors can cause an induction of hsps (4). The present study of yeast not only establishes the generality of this effect but also systematically investigated the mechanism of this response and its physiological consequences. We demonstrate here that proteasome inhibitors at concentrations that do not appear harmful, through their inhibition of protein degradation, cause an induction of hsps in yeast and concomitantly cause an increase in the cells’ resistance to high temperature. However, this protection against high temperature could not be explained simply by the buildup of hsps. We present evidence that proteasome inhibitors also cause the accumulation of another thermoprotectant molecule, the disaccharide trehalose, whose content correlates with the cells’ resistance to high temperature.
MATERIALS AND METHODS
Measurement of the synthesis of hsps.
The S. cerevisiae ise1 strain used in this study, which is permeable to several proteasome inhibitors (31), is JN 284 (MATα his7 leu2 ura3 ise1; kindly provided by J. C. Wang, Harvard University). This strain was grown exponentially in methionine-free glucose minimal (SD) medium at 30°C. At different times after exposure to the proteasome inhibitors, the cells were incubated with 200 μCi of [35S]methionine (Tran35S-label; 1,000 Ci/mmol; ICN) for 5 or 30 min. Preparation of cell extracts and immunoprecipitation were carried out as described previously (32).
Measurement of protein breakdown in vivo.
The ise1 cells grown exponentially in methionine-free SD medium were incubated with proteasome inhibitors or phenylmethylsulfonyl fluoride (PMSF) for 90 min prior to labeling. These cells were then labeled for 5 min with 100 μCi of [35S]methionine. After two washes, cells were resuspended in fresh SD medium containing methionine (0.5 mg/ml) and cycloheximide (0.5 mg/ml) to prevent reincorporation of radioactive amino acids released from proteins. At different time intervals, aliquots of cells were taken and mixed with 100% trichloroacetic acid to give a final concentration of 10%. After incubation at 4°C for 1 h, the samples were centrifuged, and the radioactivity in the trichloroacetic acid-insoluble material (precipitates) was measured. The rate of protein degradation is expressed as the percentage of incorporated radioactivity that is converted into acid-soluble fragments from the cells during the chase period (means ± standard deviations [SD]).
Assay of cell resistance to heat.
Thermotolerance assays were carried out as described elsewhere (44), with some modifications. Prior to heat treatment, ise1 cells growing exponentially at 30°C in SD medium were incubated with proteasome inhibitors for 2 h (except in Fig. 3C) and then shifted to 52°C for the indicated time. Cells were then diluted 200-fold and plated onto YPD medium to determine the number of viable colonies.
FIG. 3.
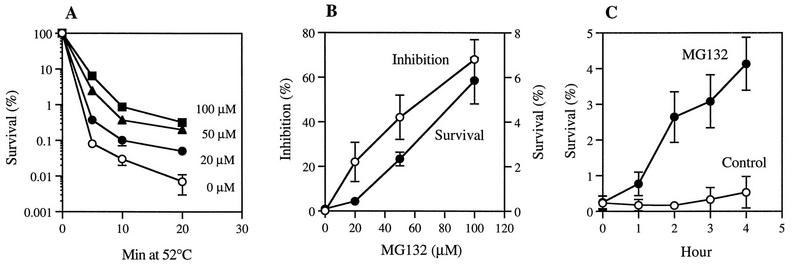
MG132 increases thermotolerance in yeast cells. (A) ise1 cells growing at 30°C were incubated with different concentrations of MG132 or with 0.1% DMSO (control) for 2 h and then exposed to 52°C for the indicated times. The cells were diluted 200-fold and plated onto YPD medium, and the fraction of viable cells was measured as the number of colonies formed. (B) The ability to block the breakdown of short-lived proteins by MG132 is proportional to its ability to increase thermotolerance. Degradation of cell proteins after 5-min pulse-labeling with [35S]methionine was measured in the presence or absence of MG132 as described previously (32). (C) Time course for the increase in thermotolerance by MG132. The ise1 cells were incubated at 30°C with or without 50 μM MG132 for the indicated times. After exposure to 52°C, cell survival was measured. Data are mean values ± SD from four independent experiments.
Extraction and assay of trehalose.
Trehalose was extracted from yeast cells and assayed as described previously (29), with some modifications. Exponentially growing yeast cells were collected by centrifugation and then washed twice in cold water to remove free glucose. Cells were resuspended in 10 to 20 volumes of ice-cold water and incubated at 95°C for 20 min, and then the supernatant was collected by centrifugation. The amount of trehalose was measured by treatment of this supernatant with trehalase (20 mU/sample; Sigma Chemical Co.), which hydrolyzes trehalose to glucose. After 6 to 8 h of incubation at 37°C, the amount of glucose generated was assayed with a glucose assay kit (Sigma) containing hexokinase and glucose-6-phosphate dehydrogenase. The preexistent glucose in each sample (usually less than 5% of the amount generated by trehalase) was assayed in a parallel tube without trehalase and was subtracted from total glucose. The total amount of proteins in each sample was also measured by the Bradford method (Pierce) for the calibration. The cellular content of trehalose was expressed as the nanomoles of trehalose per microgram of cell protein.
RESULTS
Induction of hsps by the inhibitor of proteasome.
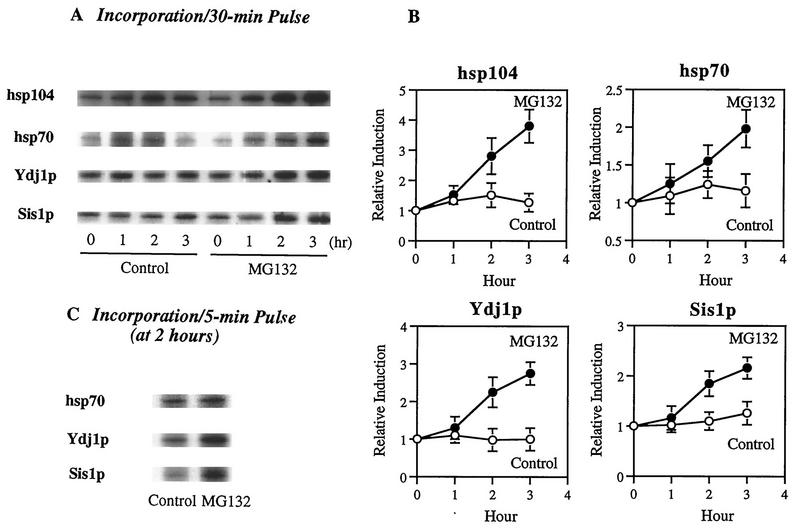
To test whether the inhibition of proteasome function influences the synthesis of various hsps, yeast cells growing at 30°C were exposed to [35S]methionine for a 30-min pulse in the presence or absence (dimethyl sulfoxide [DMSO] control) of a potent inhibitor of proteasomes, MG132 (Cbz-LLLal). We then measured the rates of incorporation of 35S into four different hsps (hsp104, hsp70, Ydj1p, and Sis1p) after isolation of each by immunoprecipitation. Because these inhibitors fail to penetrate into wild-type cells (31), we used an ise1 permeability mutant strain (38). Upon incubation with 50 μM MG132, [35S]methionine incorporation into all of these hsps increased within 1 h and continued to increase linearly for 3 h (Fig. 1). Synthesis of hsp104 showed the largest relative increase (three- to fourfold) after exposure to MG132. The DnaJ homologs Ydj1p and Sis1p also showed a 2- to 3-fold increase in synthetic rates, while incorporation into hsp70 seemed to rise only 1.5- to 2-fold, perhaps because the antibody used cannot distinguish the heat-inducible species from the several constitutive species of hsp70 (Fig. 1). These findings clearly indicate steadily increasing rates of labeling of multiple hsps during a 30-min pulse of [35S]methionine with longer exposure to MG132. To ensure that these findings represent increased rates of synthesis and are not complicated by changes in degradation of the labeled hsps in the presence of MG132, cells were exposed to MG132 for 2 h and then to the 5-min pulse of [35S]methionine. The data shown in Fig. 1C also indicated two- to threefold more rapid labeling of hsps under these conditions. Control studies showed that MG132 did not stimulate the synthesis of these hsps in wild-type yeast, where this agent does not penetrate and thus cannot affect protein breakdown (data not shown). Therefore, these data must reflect increased rates of synthesis and cannot be explained by the inhibitor’s preventing degradation of hsps (especially since hsps are rather stable proteins). Also, these findings are in accord with observation in mammalian cells, where this inhibitor causes hsp accumulation through enhanced gene expression (4, 52).
FIG. 1.
MG132 increases the synthesis of hsps in yeast cells. ise1 cells were incubated during growth at 30°C with either 50 μM MG132 (dissolved in DMSO) or 0.1% DMSO (control). (A) The rates of synthesis of several hsps (hsp104, hsp70, Ydj1p, and Sis1p) were then measured by pulse-labeling cells with 200 μCi of [35S]methionine for 30 min at different times. Shown are the times at the end of the 30-min pulse (1, 2, or 3 h after MG132 was added). After cell lysis, immunoprecipitation with antibodies against these hsps was performed with equal amounts of radioactive proteins in control or MG132-treated cells. (B) Relative induction rates of these four hsps based on data in panel A. Data shown are the mean values ± SD from three independent experiments. (C) To show that these effects of MG132 were due to enhanced synthesis of hsps, the ise1 cell were incubated with either 50 μM MG132 or 0.1% DMSO (control) for 2 h and then labeled with 200 μCi of [35S]methionine for 5 min. The synthesis of hsp70, Ydj1p, and Sis1p was measured as for panel A.
Proteasome inhibitor induces hsps without reducing cell growth.
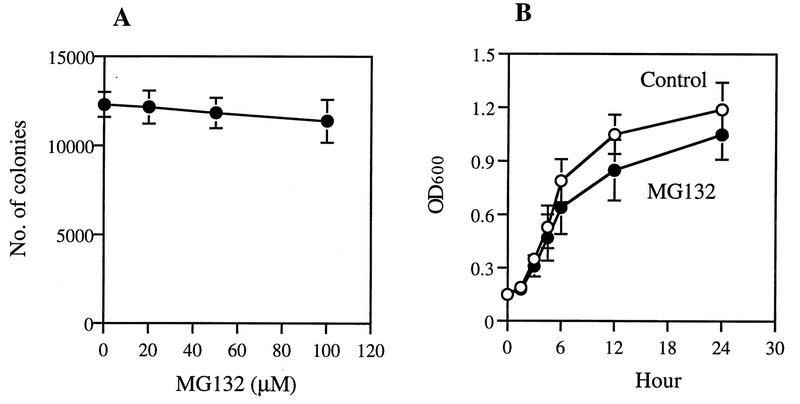
Most treatments that cause induction of hsps (e.g., incorporation amino acid analogs, puromycin, or heavy metals) are themselves harmful to cells and can rapidly reduce growth rate or viability. Surprisingly, MG132, at concentrations that caused induction of hsps, had no or very little effect on the growth of yeast at 30°C (Fig. 2). Incubation of growing cells with increasing concentrations of MG132 (20 to 100 μM) for 2 h did not significantly reduce the number of colonies on YPD plates (Fig. 2A), even though this agent caused a marked inhibition of intracellular proteolysis (Fig. 3B). Also, in liquid cultures, exposure to 50 μM MG132 did not reduce cell growth for 3 h, and by 24 h, the optical density of the treated culture was only 15 to 30% lower than that in the control (Fig. 2B). These experiments all used the ise1 strain, whose growth rate was about 50% lower than those of typical wild-type strains, e.g., W303 (data not shown), presumably because of the defect in its cell membrane (19).
FIG. 2.
MG132 does not affect the overall growth of yeast cells. (A) After incubation with different concentrations of MG132 for 2 h, ise1 cells were diluted 200-fold and then inoculated onto YPD plates to determine the numbers of surviving colonies. (B) For measurement of cell growth in the presence of 50 μM MG132 (up to 24 h) or with 0.1% DMSO (control), we took aliquots at the indicated times and measured their optical densities at 600 nm (OD600). Data shown here are the mean values ± SD from three independent experiments.
These findings indicate that the increases in hsp production are not due to nonspecific toxic effects of the inhibitor; otherwise, growth would have been reduced. This continued growth at normal rates in the presence of the proteasome inhibitor was an unexpected finding, since progression through multiple stages of the cell cycle requires degradation of cyclins and other regulatory proteins by the ubiquitin-proteasome pathway (7). Presumably, the residual activity of the proteasome under these conditions, which allowed protein degradation to proceed at 20% of the normal rate (Fig. 3B), is sufficient for the selective degradation of these important proteins.
Inhibition of proteasome function induces thermotolerance.
To test if exposure to proteasome inhibitors also increases the cells’ resistance to high temperatures, we incubated exponentially growing ise1 cells at 30°C with different concentrations of MG132 for 2 h and then exposed them to 52°C for 5 to 20 min. To measure the number of cells still viable and able to form colonies, the cells were then diluted 200-fold and plated in medium lacking the inhibitor. After dilution of the treated cells, rates of protein breakdown should have returned to control level, since MG132 is a reversible inhibitor, and its effects on proteolysis in vivo are rapidly reversed upon removal of this inhibitor (31). After a 5-min exposure to 52°C, less than 0.1% of control cells could form colonies. However, the cells incubated with MG132 showed 5- to 100-fold-greater survival, depending on the concentration used. Similarly, after 20 min at 52°C, when less than 0.01% of control cells survived, the MG132-treated cells showed 10- to 50-fold-higher survival rates (Fig. 3A). The ise1 strain was two to three times more sensitive to killing at 52°C than typical wild-type strains (e.g., W303), presumably due to its defect in the biosynthesis of principal membrane sterol (19). Nevertheless, MG132 caused a 50- to 100-fold increase in thermotolerance, such that this mutant strain became much more resistant to high temperature than wild-type cells. These results are also consistent with the finding for mammalian cells, where this same inhibitor also increased thermotolerance dramatically (4).
If this response is signalled by the accumulation of nondegraded proteins, it should depend on the degree of inhibition of protein breakdown. Upon incubation with increasing concentrations of MG132, the extent of inhibition of intracellular proteolysis increased, as did the resistance of cells to high temperature (Fig. 3A). In fact, the number of surviving cells increased almost in parallel with the extent of the inhibition of intracellular protein breakdown (Fig. 3B). A significant (about 10-fold) increase in thermotolerance was also seen when overall proteolysis was reduced by only 20 to 30%, which corresponds to about a 30 to 40% reduction in the proteasome-mediated breakdown of short-lived proteins (since MG132 does not affect the vacuolar degradation of long-lived proteins [31]).
If the development of thermotolerance results from the accumulation of abnormal or short-lived proteins, this effect should also depend on the duration of the inhibition of protein breakdown. To determine how the length of the exposure to MG132 actually influences this response and to learn how rapidly thermotolerance rises after the inhibitor is added, we incubated ise1 cells with 50 μM MG132 for different periods, shifted them to 52°C for 5 min, and measured cell survival. Within an hour after MG132 addition, cell survival began to rise and increased linearly with the duration of incubation for up to 4 h, which was the longest time studied (Fig. 3C). These findings support the conclusion that the rise in thermotolerance was triggered by the accumulation of short-lived protein(s), which would otherwise be rapidly degraded. The buildup of such a short-lived regulatory component(s) would require continued protein synthesis, and blocking synthesis by addition of cycloheximide (100 μg/ml) together with MG132 for 2 h prevented the rise in thermotolerance (Table 1). Alternatively, this requirement for protein synthesis may also indicate that new protective proteins have to be synthesized for the thermotolerant state.
TABLE 1.
Effect of cycloheximide treatment on MG132-induced thermotolerance in ise1 cellsa
| Treatment | Survival (%) |
|---|---|
| None (0.1% DMSO) | 0.08 ± 0.01 |
| MG132 (50 μM) | 2.5 ± 0.3 |
| Cycloheximide (100 μg/ml) | 0.1 ± 0.02 |
| MG132 + cycloheximide | 0.2 ± 0.02 |
Exponentially growing ise1 cells at 30°C were treated with MG132 (50 μM), cycloheximide (100 μg/ml), or MG132 plus cycloheximide for 2 h and then shifted to 52°C for 5 min to measure the cell survival as described in Materials and Methods. Data presented are mean values ± SD from three independent experiments.
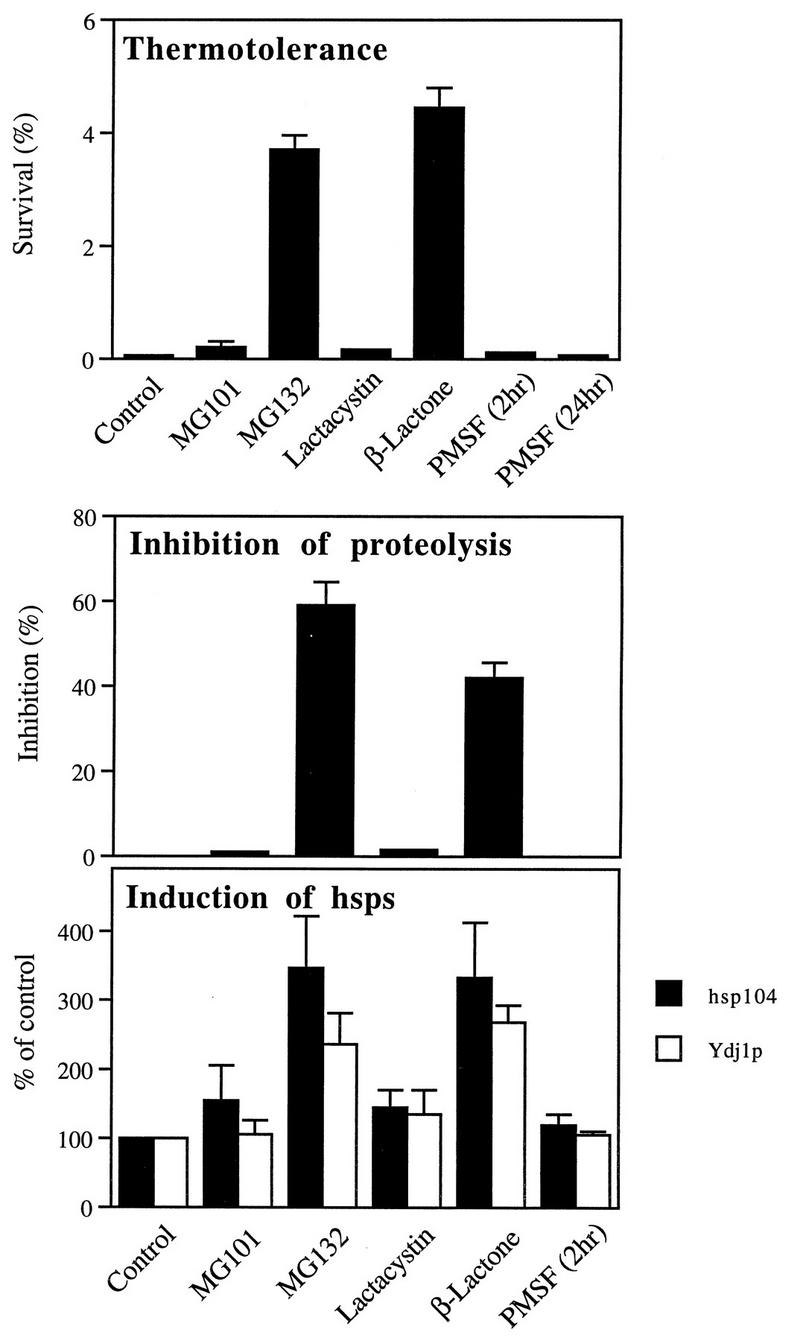
To confirm that the increase in thermotolerance induced by MG132 is really due to the inhibition of protein breakdown by the proteasome, we examined whether other proteasome inhibitors or inhibitors of other cell proteases also could increase thermotolerance in ise1 cells. NAc-LLnLal (calpain inhibitor-1, MG101) is also a hydrophobic peptide aldehyde but is a much weaker inhibitor of proteasomes than MG132 (41), and in intact yeast, this agent does not block protein degradation (31). Accordingly, incubation of cells with this inhibitor (50 μM) did not induce thermotolerance (Fig. 4). The irreversible inhibitor lactacystin covalently modifies the active-site threonine residues on the proteasome’s β subunits and thus blocks multiple peptidase activities (14). However, lactacystin is quite impermeable to yeast, even to ise1 cells, and therefore is ineffective in reducing proteolysis in intact cells (31). As expected, this agent did not enhance thermotolerance (Fig. 4). The active derivative of lactacystin that actually reacts with the proteasome is the spontaneous hydrolysis product, clasto-lactacystin β-lactone (11), which enters yeast cells readily (31). Like MG132, the β-lactone is highly effective in reducing the degradation of short-lived and abnormal polypeptides by the ubiquitin-proteasome pathway (Fig. 4). Incubation of cells for 2 h with the β-lactone (20 μM), which reduced proteolysis by about 40%, increased cell survival 100-fold, similarly to MG132 (Fig. 4).
FIG. 4.
The ability of different protease inhibitors to induce thermotolerance and hsps correlates with their ability to inhibit degradation of short-lived proteins. ise1 cells were incubated with MG101 (50 μM), MG132 (50 μM), lactacystin (20 μM), or β-lactone (20 μM) for 2 h or PMSF (1 mM) for 2 to 24 h and exposed to 52°C for 5 min, and then cell survival was measured. In parallel, cells were pulse-labeled for 5 min with [35S]methionine to measure the degradation of short-lived proteins as described previously (32) and labeled for 30 min to measure the content of hsp104 and Ydj1p as described for Fig. 1. Data presented are the mean values + SD from three independent experiments.
The inhibitors that increased cell survival at 52°C were also the only ones that enhanced the expression of hsps. Like MG132, the β-lactone increased two- to threefold the expression of hsps, such as hsp104 and Ydj1p, while the inhibitors that do not affect proteasome function in intact yeast and did not increase thermotolerance (e.g., MG101 and lactacystin) also did not induce hsps (Fig. 4). Thus, the ability of these agents to increase hsps and thermotolerance appears to be directly related to their ability to inhibit proteasome function. We also tested whether the effect of the β-lactone on thermotolerance, like that of MG132, also depends on the duration of the inhibition. Upon incubation for 1 to 2 h with the β-lactone, thermotolerance increased progressively, and at 2 h, cell survival at 52°C was 20- to 100-fold higher than that in control cells. With longer exposure, however, thermotolerance fell, and after 4 h, the cell survival was similar to that of control (data not shown). Presumably thermotolerance did not continue to increase with the β-lactone, because it caused irreversible inhibition of proteasome function which eventually blocked viability.
The bulk of cell proteins are long-lived components that are degraded in yeast by the vacuolar system and not by the ubiquitin-proteasome pathway (31) (which degrades such proteins in mammalian cells [41]). PMSF is a serine protease inhibitor which inhibits multiple vacuolar proteases but not the proteasomes (12, 26). In growing yeast, this agent blocks vacuolar protein breakdown and autophagic body formation (50) but does not affect the breakdown of short-lived proteins (31). When cells were treated with 1 mM PMSF for up to 24 h, no significant effect on thermotolerance was seen (Fig. 4). Thus, this increase in thermotolerance appears to be a specific consequence of the reduction in degradation by the proteasomes of abnormal or short-lived, normal proteins. The finding that inhibition of proteasome function leads to an increased resistance to high temperature suggested that certain mutant strains with defects in the 20S proteasome might also show greater thermotolerance than wild-type cells. We therefore examined thermotolerance of several mutant strains (kindly provided by M. Hochstrasser), in which the active-site threonine residues were mutated to alanines. A strain lacking the chymotryptic activity exhibited severe defect in growth even at 30°C, and therefore any effects seen upon heat shock and subsequently plating at 30°C could not be interpreted. However, the strain lacking the tryptic site grew normally at 30°C and showed two- to threefold greater survival at 52°C for 5 min than did isogenic wild type (data not shown). While the results might support the present findings with proteasome inhibitors, further experiments with these mutant strains were not pursued since the use of selective inhibitors offered many experimental advantages (e.g., the effects could be reversed or the degree of inhibition could be altered).
Dissociation of thermotolerance from hsp production.
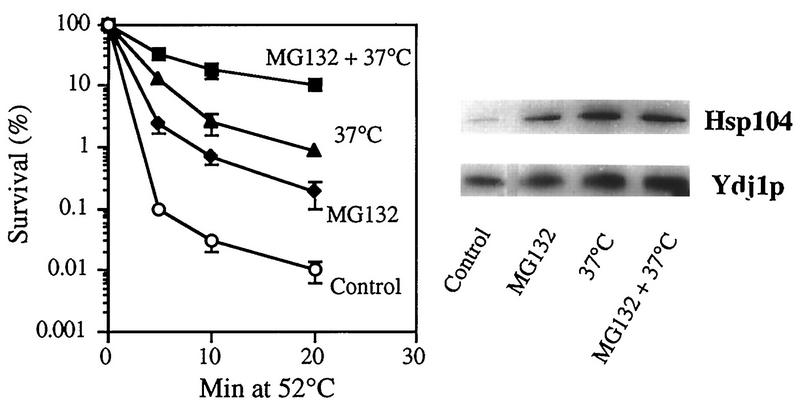
When yeast (or other) cells are preincubated at a high but not lethal temperature (e.g., 37°C), hsps are induced, and a larger fraction of cells survive a subsequent exposure to 50°C than when they are switched directly from 28 to 50°C (39). If the induction of hsps and induction of thermotolerance at 37°C are in fact signalled by the generation of abnormal proteins, these effects should be greater in the presence of proteasome inhibitors, which prevent the rapid breakdown of such proteins. To test if the protective effects of preincubation at 37°C and proteasome inhibitors are additive or synergistic, we incubated exponentially growing ise1 cells at 30°C with MG132 for 2 h and then exposed them to 37°C for another 30 min prior to the shift to 52°C for 5 to 20 min. As expected, incubation with either MG132 or 37°C increased cell survival 10- to 50-fold (Fig. 5). However, the cells pretreated with MG132 and then incubated at 37°C showed an additional 3- to 10-fold-greater survival than that induced by incubation at 37°C alone (Fig. 5). As a result of the combined treatment, 25 to 30% of cells survived exposure to 52°C for 10 min, while only 0.1% of control cells did. Even after 20 min at 52°C, 8 to 10% of cells preincubated with MG132 and 37°C survived, while less than 0.01% of control cells and 1% of those preincubated only at 37°C were viable (Fig. 5). Thus, these two stimuli are clearly synergistic in enhancing thermotolerance.
FIG. 5.
Effects of combined exposure to MG132 and 37°C on thermotolerance and the level of hsps. Exponentially growing ise1 cells were preincubated for 2 h with 50 μM MG132 or with 0.1% DMSO (control). The cultures were then divided in half, and one half was incubated at 37°C for an additional 30 min. The cells were then exposed to 52°C for 5 to 20 min, and survival was measured. In parallel, equal volumes of these cells were collected before the exposure to 52°C, and their hsp104 and Ydj1p contents were determined by Western blotting. For quantitation, the blots were incubated with 125I-protein A for 2 h and subsequently subjected to autoradiography. Data presented are the mean values ± SD from four independent experiments.
Additional experiments were carried out to test whether this marked increase in cell survival, when 37°C and MG132 were combined, resulted from a similarly large increase in the synthesis of hsps. Since all hsps appeared to be induced in similarly by MG132 (Fig. 1), we focused on hsp104 and Ydj1p, which are particularly important for thermotolerance in yeast (5, 43) and showed large relative increases upon inhibitor treatment (Fig. 1). Surprisingly, the total amount of these two hsps (assayed by Western blotting) in yeast treated with both MG132 and 37°C was not significantly greater than that in cells exposed to 37°C alone or incubated only with MG132 at 30°C, despite their 3- to 10-fold greater survival at 52°C (Fig. 5). In other words, these experiments failed to show additive effects on hsp contents, and the dramatic increase in thermotolerance with the combined treatment is not due to an increased content of these hsps.
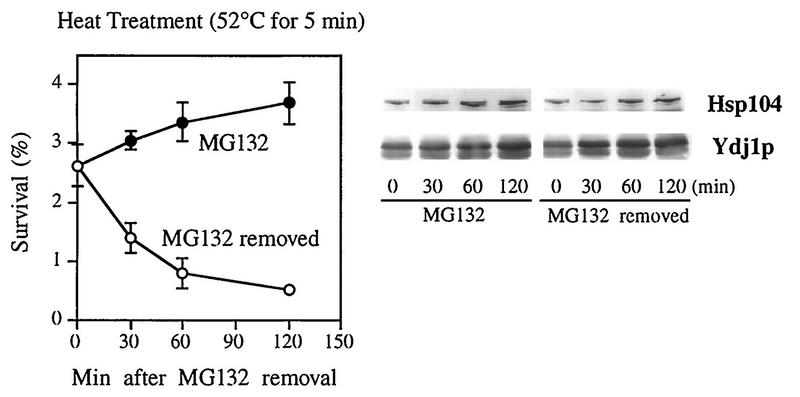
These findings suggest that the increase in cell resistance to high temperature is not simply due to the enhanced production of hsps. To further examine the relationship between the expression of hsps and the induction of thermotolerance, we studied the changes in both parameters upon the removal of MG132. The inhibition of protein degradation by MG132 is rapidly reversed upon inhibitor removal (31). We therefore compared the changes in hsp production and the cells’ resistance to 52°C at different times after washing to remove MG132. When the function of the proteasome was restored, yeast cells began to lose thermotolerance very rapidly (with an apparent half-life of 30 min), and by 2 h, their ability to survive at 52°C was similar to that of control cells (Fig. 6).
FIG. 6.
Effects of MG132 removal on thermotolerance and the level of hsps. After a 2-h incubation with 50 μM MG132, ise1 cells were washed with fresh medium to remove the inhibitor. Half of the cells were then resuspended in medium containing 50 μM MG132 (MG132), and the other were incubated with 0.1% DMSO alone (MG132 removed). These cells were incubated for an additional 2 h. At the indicated times, aliquots were taken and assayed for resistance to 52°C for 5 min. In parallel, the contents of hsp104 and Ydj1p in these aliquots were assayed by Western blotting as described for Fig. 5. Data are mean values ± SD from four independent experiments.
Surprisingly, after MG132 removal, there was no corresponding decrease in the levels of hsp104 and of Ydj1p (as determined by Western blotting). In fact, under this condition, production of these hsps continued at a rate similar to that in the cells maintained in the presence of MG132 (Fig. 6). Thus, after proteolysis was reinitiated, thermotolerance fell rapidly, while the content of hsps remained high and continued to increase for at least 2 h. Interestingly, a similar rapid loss of thermotolerance without a loss of hsps has been seen when heat-shocked yeast and bacterial cells are shifted back to the normal temperature (6, 33). These results indicate that blocking proteasome function leads to an increased cell resistance to high temperature not simply through the induction of hsps but also through some additional protection mechanism involving a short-lived component.
Rapid accumulation of trehalose upon inhibition of proteasome function.
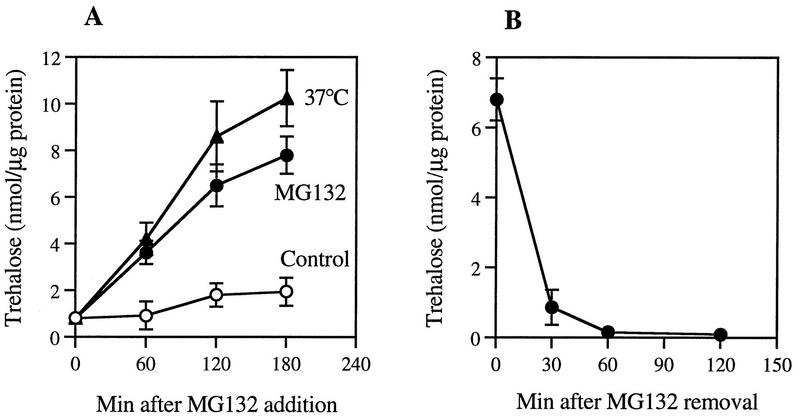
One other molecule that has been shown to accumulate during heat shock in yeast and other microorganisms is the nonreducing disaccharide trehalose (2, 10, 37). Moreover, its accumulation has been shown to correlate with cellular resistance to heat and dessication (2, 10, 37), and trehalose and hsp104 appear to have synergistic effect in protecting yeast cells from heat (13). As an attempt to identify additional mechanisms by which proteasome inhibitors promote thermotolerance, we tested whether MG132 may cause an accumulation of trehalose in yeast. Upon incubation of growing ise1 cells with 50 μM MG132 at 30°C, the level of trehalose in the cells increased markedly. After 3 h, a two- to threefold increase in its level was observed (Fig. 7A). A similar buildup of trehalose was seen when part of the culture was shifted from 30 to 37°C to induce heat shock, in accord with prior reports (10, 13). Furthermore, treatment of the cells with a highly specific and irreversible inhibitor of the proteasome, clasto-lactacystin β-lactone, at a concentration (20 μM) that inhibited protein breakdown by 40 to 50% (Fig. 4) caused an increase in the cellular level of trehalose similar to that seen with MG132 (Table 2).
FIG. 7.
Effect of MG132 on the cellular content of trehalose. (A) Exponentially growing ise1 cells were treated with or without MG132 (50 μM) or incubated at 37°C. At indicated times, aliquots of cells were collected and their trehalose contents were measured. (B) After 2 h of incubation with MG132 (50 μM), the inhibitor was removed by washing cells with fresh medium. These cells were then resuspended in the medium without MG132 and further incubated for 2 h. At the given times, cells were collected and trehalose contents were measured. Data are mean values ± SD from three independent experiments.
TABLE 2.
Effects of different types of proteasome inhibitors on the cellular content of trehalosea
| Inhibitor | Trehalose (nmol/μg of protein) |
|---|---|
| None (0.1% DMSO) | 1.76 ± 0.38 |
| MG132 (50 μM) | 4.62 ± 1.74 |
| β-Lactone (20 μM) | 3.46 ± 1.18 |
Exponentially growing ise1 cells at 30°C were treated with MG132 (50 μM), β-lactone (20 μM), or 0.1% DMSO (control) for 2 h, and then cellular trehalose contents were measured as described in Materials and Methods. Data presented are mean values ± SD from three independent experiments.
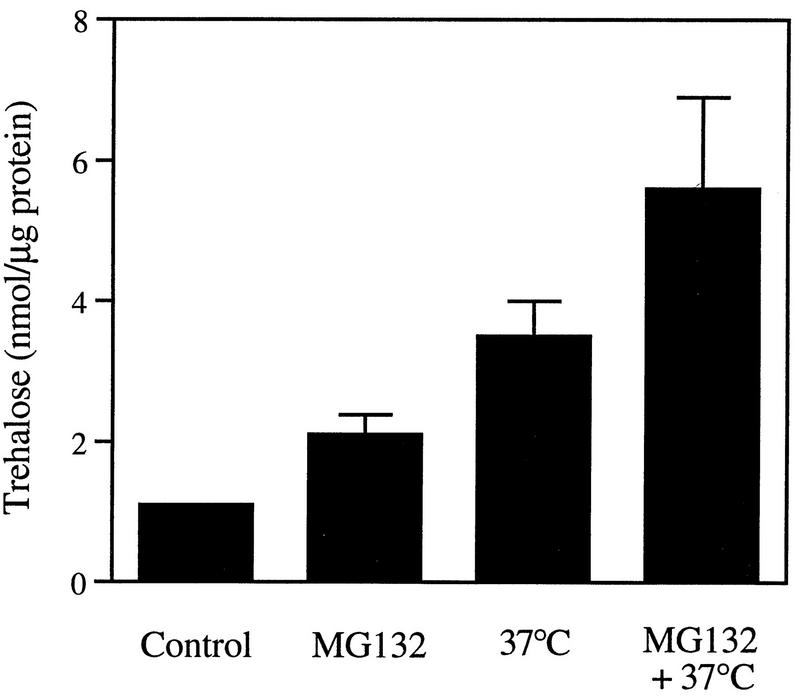
These observations with the β-lactone confirmed that the buildup of the disaccharide was a specific consequence of the inhibition of proteasome function and not any nonspecific effect of MG132. Moreover, when the reversible inhibitor MG132 was removed from the medium, the cellular level of trehalose decreased very quickly, and after 1 h, almost no trehalose was detected (Fig. 7B). Thus, its content fell (unlike that of hsps) under conditions where thermotolerance also decreased rapidly (Fig. 6). In addition, when cells were exposed to MG132 and 37°C together, the content of trehalose increased to higher levels than in cells exposed only to 37°C or incubated only with MG132 at 30°C (Fig. 8). In fact, increasing temperature and MG132 had either additive or synergistic effects (depending on the experiment) in causing accumulation of trehalose.
FIG. 8.
Effect of combined exposure to MG132 and 37°C on the cellular content of trehalose. Exponentially growing ise1 cells were preincubated for 2 h with 50 μM MG132 or with 0.1% DMSO (control). The cultures were then divided in half, and one half was incubated at 37°C for an additional 30 min. After collecting cells by centrifugation, we measured the cellular level of trehalose. Data presented are the mean values ± SD from three independent experiments.
These observations together strongly suggest that trehalose is the short-lived metabolite which is essential for thermotolerance induced upon exposure to proteasome inhibitors: (i) it has thermoprotective effects, (ii) it accumulates when protein breakdown is inhibited, (iii) its cellular content, unlike that of hsps, decreases rapidly after MG132 removal (when proteolysis is reinitiated), and (iv) its level is closely correlated with thermotolerance when cells are exposed to the inhibitor and 37°C together.
DISCUSSION
Mechanism of induction of hsps and thermotolerance.
A common feature of the diverse conditions that elicit the heat shock response is that they cause damage to cell proteins. The present findings provide further strong evidence that the accumulation of such abnormal proteins signals this response (1, 17, 22, 40). We found that inhibition of proteasome function by MG132 or the β-lactone, which prevents the rapid degradation of abnormal proteins, causes induction of all four hsps tested and a dramatic increase in thermotolerance. The magnitude of the increase in cell survival at 52°C was directly proportional to the degree of inhibition of protein breakdown and its duration (Fig. 3). Similar effects were seen in studies with a mutant strain in which one of the peptidase activities of the proteasome is inactivated (data not shown). Furthermore, no such effects were seen with protease inhibitor that did not block protein breakdown by the proteasome (e.g., inhibitor of the vacuolar proteases) (Fig. 4). Also, high temperatures, which should cause damage to cell proteins, and MG132, which reduces their degradation, had synergistic effects in promoting thermotolerance (Fig. 6).
In almost all eukaryotic and prokaryotic cells, the heat shock response is elicited by very similar stimuli. In Escherichia coli, inhibition of protein breakdown also causes induction of hsps (17), and in related studies, we have found that treatment of MDCK cells with MG132 or lactacystin leads to a rapid induction of multiple hsps and to thermotolerance (4). In addition, in several human cell lines, these inhibitors cause an induction of hsps via a specific activation of heat shock transcription factor 2 (34). A marked increase in hsp70 was also recently found in HepG2 cells treated with proteasome inhibitors (52). These inhibitors are now widely used by cell biologists, immunologists, and biochemists to analyze the functions of the proteasome in vivo. The present finding may complicate the interpretation of experiments using these inhibitors, especially in long-term studies of intact cells, where possible indirect effects due to induction of hsps clearly have to be considered.
Exactly how the proteasome inhibitors stimulate transcription of hsps is unclear. The simplest mechanism would be that they cause abnormal proteins to build up and saturate the cells’ degradative machinery, resulting in a failure of the cell to degrade a critical short-lived, positive regulator of transcription of hsps. A similar model has been shown to activate the transcription of hsps in E. coli (16). Their expression is regulated by a specific component of RNA polymerase, ς32. This positive regulator is normally degraded with a half-life of 2 to 3 min, but is stabilized manyfold during heat shock. The rapid degradation of ς32 requires both the FtsH protease and the molecular chaperones DnaK (an hsp70 homolog) and its cofactors DnaJ and GrpE. During heat shock, unfolded polypeptides accumulate and saturate the binding capacity of these chaperones, leading to reduced breakdown of ς32 and enhanced transcription of hsps (16). In eukaryotic cells, no such short-lived regulator of hsps transcription has yet been found, although such a regulator of heat shock transcription factor 2 appears to exist and to respond to the level of abnormal proteins (34).
Dissociation of thermotolerance from induction of hsps.
Induction of hsps has been generally assumed to lead to thermotolerance, especially since most hsps are either molecular chaperones which can help prevent protein aggregation and promote refolding or components of the degradative system (e.g., ubiquitin and ubiquitin-conjugating enzymes) which help eliminate such irreversibly damaged polypeptides at high temperatures. Treatment with proteasome inhibitors, while inducing hsps, increased up to 100-fold cell survival at 52°C. Under these conditions, we also have found a marked increase in cellular resistance to other toxic insults (e.g., high concentration of ethanol or oxygen radicals) (data not shown). However, the cellular content of hsps did not correlate with thermotolerance, even though both responses appear to result from the same physiological signals. The dissociation of these two responses was most dramatic after removal of MG132 when the cells’ resistance to heat and oxygen radicals fell rapidly, while hsp production continued to increase (presumably due to the stability of hsps and their mRNAs). In addition, when yeast cells were exposed to both 37°C and MG132, these stimuli had synergistic effects in increasing cell survival, even though hsp content did not increase appreciably above levels seen with either stimulus alone. Thus, some component, in addition to hsps, is necessary for tolerance to heat.
This conclusion is consistent with several prior studies suggesting that heat shock-induced thermotolerance can be dissociated from hsp synthesis. For example, an increase in thermotolerance can be induced in yeast by incubation at 37°C even when protein synthesis is blocked (20) and in a yeast mutant which lacks the heat shock-specific transcription factor (48). In addition, upon down-shift of heat-shocked yeast or bacterial cells to 23°C, thermotolerance is lost within 1 to 2 h, even though the amounts of hsps do not fall (6, 33). Thus, proteasome inhibitors, like heat treatment, elicit two protective responses. First, there is an increase in expression of hsps, which presumably is important for the enhanced viability at 52°C. Accordingly, cycloheximide treatment blocked the ability of MG132 to increase thermotolerance (although alternative interpretations of cycloheximide’s effect may be possible). Second, an additional adaptation is essential for the increase in thermotolerance. This factor must be short-lived since the resistance to heat decreased to control level within 30 min after protein breakdown was reinitiated. Recently, a short-lived transcription factor, Hac1p, that is required for the unfolded protein response in the ER has been identified (9). Normally, Hac1p is rapidly degraded by the ubiquitin-proteasome pathway, but when abnormal proteins accumulate in the ER, a more stable transcription factor is produced by alternative splicing (9). Perhaps a similar short-lived regulator functions in the cytosol or nucleus and is critical for thermotolerance.
Possibly, the rapid fall in thermotolerance when the proteasome inhibitor was removed indicates that this state requires protein phosphorylation or some other reversible modification, which occurs when protein degradation decreases and abnormal proteins accumulate. Several protein kinases have been reported to be activated by heat shock (27, 30), and perhaps they are also activated when the proteasomes are inhibited. A membrane-associated protein kinase is activated by the accumulation of unfolded proteins in the ER and plays a role in the induction of ER chaperones (8, 35). Another possible way that protein phosphorylation might enhance thermotolerance is evident in E. coli, where upon heat shock, the major chaperones, DnaK and GroEL, undergo reversible phosphorylation, and this modification markedly enhances their affinity for unfolded proteins (46). Possibly, chaperone function is regulated similarly during heat shock in eukaryotic cells so as to enhance resistance to high temperatures.
Involvement of trehalose in the induced thermotolerance.
The present findings favor a simpler explanation, i.e., that the resistance to high temperatures requires a short-lived, small molecule, specifically, trehalose, which has thermoprotective effects and accumulates when protein breakdown is inhibited. Several conditions that induce the heat shock response in S. cerevisiae have been found to also cause a buildup of trehalose, in part by stimulating the expression of enzymes for trehalose biosynthesis (13, 23, 37). Furthermore, the time course of the accumulation of trehalose upon heat shock and the decline in its level following the return to the normal temperature paralleled the changes in thermotolerance (2). In the present study, we have shown that trehalose rapidly accumulates in cells, when the function of the proteasome is inhibited by treatment either with MG132 or with the specific, irreversible proteasome inhibitor, the β-lactone. It is noteworthy that very similar results were obtained with these structurally unrelated types of inhibitors. MG132 is a reversible peptide aldehyde that functions as a substrate analog, and the β-lactone is an irreversible inhibitor that covalently modifies 20S proteasome’s active site threonine and no other cell protein (14). Together these observations confirm that the buildup of trehalose was a specific consequence of the inhibition of proteasome function in the cells. Moreover, the cellular content of this disaccharide correlated closely with changes in viability at 52°C, unlike the cells’ content of hsps. Upon removal of the inhibitor and restoration of protein breakdown, the levels of trehalose fell dramatically within 30 min, as did cell resistance to high temperatures. In addition, heat shock (37°C) and MG132 had additive or synergistic effects in raising trehalose content and thermotolerance. Under these same conditions, the content of hsps did not correlate with thermotolerance.
It has been suggested that trehalose and molecular chaperones, especially hsp104, function synergistically to enhance thermotolerance in yeast in stationary phase (13). Moreover, in vitro studies have shown that trehalose can stabilize certain proteins against heat inactivation (24), while the various chaperones can help prevent the aggregation of a damaged polypeptide and help refold or resolubilize denatured proteins (39). Thus, the expression of hsps and the accumulation of trehalose appear to be complementary protective responses. By simultaneously inducing both, the proteasome inhibitors appear to enhance cell resistance to heat and other toxic insults that irreversibly damage cell proteins and cause cell death.
Because induction of the heat shock response can protect tissues against a variety of toxic conditions, including anoxia and reperfusion injury, there has been appreciable interest in the possible applications of this response in medicine. Like heat treatment, the proteasome inhibitors can cause an induction of thermotolerance not only in yeast cells but also in mammalian cells, where these agents also cause induction of ER chaperones (4), which also help protect cells against anoxic injury. These findings together suggest that proteasome inhibitors may be an effective and relatively nontoxic way to elicit these protective responses, in contrast to other inducers of this response, such as high temperatures or incorporation of amino acid analogs or heavy metals, all of which can be highly damaging and therefore are probably not appropriate for therapeutic use in patients. Moreover, when the proteasome inhibitors are combined with these other stimuli, they have synergistic effects in protecting cells. Greater understanding of the molecular mechanisms by which these inhibitors promote resistance to high temperatures and other toxic insults may lead to practical applications of these inhibitors in medicine, agriculture, or biotechnology.
ACKNOWLEDGMENTS
We thank Proscript, Inc. (Cambridge, Mass.), and S. Omura for providing MG101, MG132, the β-lactone, and lactacystin and S. Lindquist, M. Douglas, K. A. Arndt, M. H. Hochstrasser, and J. C. Wang for supplying antibodies and yeast strains.
This study was supported by grants from the National Institute of Health (NIGMS), the Human Frontier Science Program, and Proscript, Inc.
REFERENCES
- 1.Ananthan J, Goldberg A L, Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986;232:522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- 2.Attfield P V. Trehalose accumulates in Saccharomyces cerevisiae during exposure to agents that induce heat shock response. FEBS Lett. 1987;225:259–263. doi: 10.1016/0014-5793(87)81170-5. [DOI] [PubMed] [Google Scholar]
- 3.Barnes C A, Johnston G C, Singer R A. Thermotolerance is independent of induction of the full spectrum of heat shock proteins and of cell cycle blockage in the yeast Saccharomyces cerevisiae. J Bacteriol. 1990;172:4352–4358. doi: 10.1128/jb.172.8.4352-4358.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Goldberg A L, Nigam S. Proteasome inhibition leads to a heat shock response, induction of endoplasmic reticulum chaperones and thermotolerance. J Biol Chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086. [DOI] [PubMed] [Google Scholar]
- 5.Caplan A J, Cyr D M, Douglas M G. Eukaryotic homologues of Escherichia coli dnaJ: a diverse protein family that functions with hsp70 stress proteins. Mol Biol Cell. 1993;4:555–563. doi: 10.1091/mbc.4.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavicchioli R, Watson K. Loss of heat-shock acquisition of thermotolerance in yeast is not correlated with loss of heat-shock proteins. FEBS Lett. 1986;207:149–152. doi: 10.1016/0014-5793(86)80030-8. [DOI] [PubMed] [Google Scholar]
- 7.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 8.Cox J S, Shamu C E, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 9.Cox J S, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- 10.De Virgilio C, Hottiger T, Dominguez J, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. I. Genetic evidence that trehalose is a thermoprotectant. Eur J Biochem. 1993;219:179–186. doi: 10.1111/j.1432-1033.1994.tb19928.x. [DOI] [PubMed] [Google Scholar]
- 11.Dick L R, Cruikshank A A, Destree A T, Grenier L, McCormack T A, Melandri F D, Nunes S L, Palombella V J, Parent L A, Plamondon L, Stein R L. Mechanistic studies on the inactivation of the proteasome by lactacystin in cultured cells. J Biol Chem. 1997;272:182–188. doi: 10.1074/jbc.272.1.182. [DOI] [PubMed] [Google Scholar]
- 12.Dubiel W, Pratt G, Ferrell K, Rechsteiner M. Purification of an 11S regulator of the multicatalytic protease. J Biol Chem. 1992;267:22369–22377. [PubMed] [Google Scholar]
- 13.Elliot B, Haltiwanger R S, Futcher B. Synergy between trehalose and hsp104 for thermotolerance in Saccharomyces cerevisiae. Genetics. 1996;144:923–933. doi: 10.1093/genetics/144.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 15.Georgopoulos C, Welch W J. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 16.Georgopoulos C, Liberek K, Zylicz M, Ang D. Properties of the heat shock proteins of Escherichia coli and the autoregulation of the heat shock response. In: Morimoto R I, Tissieres A, Georgopoulos A, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 209–249. [Google Scholar]
- 17.Goff S A, Goldberg A L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985;41:587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg A L, Stein R, Adams J. New insights into proteasome function: from archaebacteria to drug development. Chem Biol. 1995;2:503–508. doi: 10.1016/1074-5521(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 19.Graham T R, Scott P A, Emr S D. Brefeldin A reversibly blocks early but not late protein transport steps in the yeast secretory pathway. EMBO J. 1993;12:869–877. doi: 10.1002/j.1460-2075.1993.tb05727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross C, Watson K. Heat shock protein synthesis and trehalose accumulation are not required for induced thermotolerance in depressed Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1996;220:766–772. doi: 10.1006/bbrc.1996.0478. [DOI] [PubMed] [Google Scholar]
- 21.Hendricks J P, Hartl F-U. Molecular chaperone functions of heat shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 22.Hightower L E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980;102:407–424. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- 23.Hottiger T, De Virgilio C, Bell W, Boller T, Wiemken A. The 70-kilodalton heat-shock proteins of the SSA subfamily negatively modulate heat-shock-induced accumulation of trehalose and promote recovery from heat stress in the yeast, Saccharomyces cerevisiae. Eur J Biochem. 1992;210:125–132. doi: 10.1111/j.1432-1033.1992.tb17399.x. [DOI] [PubMed] [Google Scholar]
- 24.Hottiger T, De Virgilio C, Hall M N, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur J Biochem. 1994;219:187–193. doi: 10.1111/j.1432-1033.1994.tb19929.x. [DOI] [PubMed] [Google Scholar]
- 25.Jensen T J, Loo M A, Pind S, Williams D B, Goldberg A L, Riordan J R. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell. 1995;83:129–135. doi: 10.1016/0092-8674(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 26.Jones E W. Tackling the protease problem in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:428–453. doi: 10.1016/0076-6879(91)94034-a. [DOI] [PubMed] [Google Scholar]
- 27.Kamada Y, Jung U S, Piotrowski J, Levin D E. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- 28.Kandror O, Busconi L, Sherman M Y, Goldberg A L. Rapid degradation of an abnormal protein in Escherichia coli involves the chaperone groEL and groES. J Biol Chem. 1994;269:23575–23582. [PubMed] [Google Scholar]
- 29.Kienle I, Burgert M, Holtzer H. Assay of trehalose with acid trehalase purified from Saccharomyces cerevisiae. Yeast. 1993;9:607–611. doi: 10.1002/yea.320090607. [DOI] [PubMed] [Google Scholar]
- 30.Kyriakis J M, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. BioEssays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 31.Lee D H, Goldberg A L. Selective inhibitors of the proteasome-dependent and vacuolar pathways in Saccharomyces cerevisiae. J Biol Chem. 1996;271:27280–27284. doi: 10.1074/jbc.271.44.27280. [DOI] [PubMed] [Google Scholar]
- 32.Lee D H, Sherman M Y, Goldberg A L. Involvement of the molecular chaperone Ydj1 in the ubiquitin-dependent degradation of short-lived and abnormal proteins in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4773–4781. doi: 10.1128/mcb.16.9.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackey B M, Derrick C. Heat shock protein synthesis and thermotolerance in Salmonella typhimurium. J Appl Bacteriol. 1990;69:373–383. doi: 10.1111/j.1365-2672.1990.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 34.Mathew, A., S. K. Mathur, and R. I. Morimoto. Personal communication.
- 35.Mori K, Ma W, Gething M-J, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 36.Nagata K. Regulation of thermotolerance and ischemic tolerance. In: Feige U, Morimoto R I, Yahara I, Polla B S, editors. Stress-inducible cellular responses. Basel, Switzerland: Birkhauser; 1996. pp. 467–481. [Google Scholar]
- 37.Neves M-J, Francois J. On the mechanism by which a heat shock induces trehalose accumulation in Saccharomyces cerevisiae. Biochem J. 1992;288:859–864. doi: 10.1042/bj2880859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nitiss J, Wang J C. DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc Natl Acad Sci USA. 1988;85:7501–7505. doi: 10.1073/pnas.85.20.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsell D A, Lindquist S. Heat shock proteins and stress tolerance. In: Morimoto R I, Tissieres A, Georgopoulos A, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 457–494. [Google Scholar]
- 40.Parsell D A, Sauer R T. Induction of heat shock-like response by unfolded protein in Escherichia coli: dependence on protein level not protein degradation. Genes Dev. 1989;3:1226–1232. doi: 10.1101/gad.3.8.1226. [DOI] [PubMed] [Google Scholar]
- 41.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 42.Ruis H, Schüller C. Stress signaling in yeast. BioEssays. 1995;17:959–965. doi: 10.1002/bies.950171109. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez Y, Lindquist S. Hsp104 required for induced thermotolerance. Science. 1990;248:1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez Y, Parsell D A, Taulien J, Vogel J L, Craig E A, Lindquist S. Genetic evidence for a functional relationship between hsp104 and hsp70. J Bacteriol. 1993;175:6484–6491. doi: 10.1128/jb.175.20.6484-6491.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seufert W, Jentsch S. Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 1990;9:543–550. doi: 10.1002/j.1460-2075.1990.tb08141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherman M Y, Goldberg A L. Heat shock of Escherichia coli increases binding of dnaK (the hsp70 homolog) to polypeptides by promoting its phosphorylation. Proc Natl Acad Sci USA. 1993;90:8648–8652. doi: 10.1073/pnas.90.18.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherman M Y, Goldberg A L. Involvement of molecular chaperones in intracellular protein breakdown. In: Feige U, Morimoto R I, Yahara I, Polla B S, editors. Stress-inducible cellular responses. Basel, Switzerland: Birkhauser; 1996. pp. 57–78. [DOI] [PubMed] [Google Scholar]
- 48.Smith B J, Yaffe M P. Uncoupling thermotolerance from the induction of heat shock proteins. Proc Natl Acad Sci USA. 1991;88:11091–11094. doi: 10.1073/pnas.88.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Storz G, Polla B S. Transcriptional regulators of oxidative stress-inducible genes in prokaryotes and eukaryotes. In: Feige U, Morimoto R I, Yahara I, Polla B S, editors. Stress-inducible cellular responses. Basel, Switzerland: Birkhauser; 1996. pp. 239–254. [DOI] [PubMed] [Google Scholar]
- 50.Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welch W J. Mammalian stress response: cell physiology, structure/function of stress proteins and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- 52.Zhou M, Wu X, Ginsberg H N. Evidence that a rapidly turning over protein, normally degraded by proteasomes, regulates hsp72 gene transcription in HepG2 cells. J Biol Chem. 1996;271:24769. doi: 10.1074/jbc.271.40.24769. [DOI] [PubMed] [Google Scholar]