Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) poses a significant global health challenge, disproportionately affecting women. Diabetic women with HFpEF represent a high-risk subgroup, particularly after experiencing ST-segment elevation myocardial infarction (STEMI), exhibiting increased mortality compared to men. While prolonged door-to-balloon (DTB) times, reflecting delayed reperfusion, are a critical factor in STEMI outcomes, they alone do not fully capture the observed outcome variability in diabetic women. Using an integrated clinical and pre-clinical approach this study aimed to investigate the relative contributions of metabolic dysfunction and coronary artery disease (CAD) in type 2 diabetes (T2D) to STEMI outcomes in women, beyond the impact of DTB time.
Methods
A retrospective case–control study analysed female STEMI patients undergoing primary percutaneous coronary intervention (pPCI, n = 40 T2D, n = 40 non-diabetic controls), comparing clinical characteristics, treatment strategies, and early outcomes. A preclinical model (female db/db mice) assessed cardiac function via echocardiography, Langendorff perfusions, and ischemia–reperfusion protocols. Metabolome of heart, liver, and skeletal muscle was assessed by 1H NMR spectroscopy.
Results
Our study reveals significantly higher mortality, impaired left ventricular function post-pPCI, and increased implantable cardioverter-defibrillator (ICD) implantation rates in diabetic STEMI patients, irrespective of DTB time, when compared to non-diabetic controls. Elevated inflammatory markers, acute hyperglycaemia and evidence of cardio-hepatic damage were identified in T2D patients. db/db mice exhibited analogous T2D-associated pathophysiology, including increased ischemia–reperfusion injury exacerbated by metabolic disturbances in the myocardium, liver, and skeletal muscle versus non-diabetic controls.
Conclusions
In diabetic women, multiple factors beyond reperfusion delays exacerbate acute myocardial injury. This necessitates the development of sex-specific strategies to manage the cardiovascular complications of diabetic HFpEF. The db/db mouse model provides a relevant preclinical tool for future research as it mimics human T2D-associated HFpEF and STEMI outcome.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-025-02771-z.
Keywords: Type 2 diabetes, STEMI, Female patients, db/db mice, HFpEF, Metabolism, Ischemia/reperfusion
Background
Heart failure with preserved ejection fraction (HFpEF) poses a significant and growing global health burden with increasing prevalence, largely due to aging populations as well as the rising incidence of contributing factors including obesity, hypertension and type 2 diabetes (T2D) [1, 2]. Women make up the majority of the HFpEF population, exhibiting relatively high burden of morbidity and mortality [3]. Phenotypic profile of HFpEF in women is defined by comorbidities, including older age, obesity, atrial fibrillation, inflammation, hypertension, hyperlipidaemia and T2D [3–6]. In women, T2D has been shown to elevate the risk of developing HFpEF by five times, compared to a 2.4-fold increase in men [7]. Furthermore, female sex is independently associated with the presence of diastolic dysfunction and worse clinical outcomes in HFpEF, highlighting that a sex‐specific approach is key to investigating the pathophysiology of HFpEF.
The intersection of coronary artery disease (CAD) and metabolic disorders in T2D increases the risk of acute coronary syndrome (ACS), but also significantly aggravates the course of myocardial infarction (MI) worsening patient prognosis [8]. Among the affected populations, female patients with T2D represent a particularly vulnerable group, exhibiting disproportionately poorer outcomes including increased mortality after ST-segment elevation myocardial infarction (STEMI) [8–13]. This heightened risk in women is potentially attributed to the cumulative burden of metabolic dysfunction, inflammation, oxidative stress and cardiac remodeling associated with T2D. However, in addition to biological factors, disparities in outcomes may also be due to poorer cardiovascular risk factor management in routine T2D care of women [14]. Although female T2D patients make up a significant proportion of patients with STEMI, clinical studies relatively rarely address differences in the STEMI outcomes in female patients with T2D [15].
Primary percutaneous coronary intervention (pPCI) is the gold standard for achieving rapid reperfusion post-STEMI. The critical nature of prompt intervention is underscored by the established relationship between Door-to-Balloon (DTB) reperfusion times and patient outcomes, where delays have been consistently associated with increased morbidity and mortality [16]. Generally, diabetic women experience delays in recognising and seeking treatment for STEMI, as they may present atypically with fatigue, nausea/vomiting, shortness of breath, or abdominal discomfort, rather than classic chest pain. Prolonged DTB times have been linked to higher mortality rates, regardless of how the patient presents [16].
Given the heterogeneous pathogenic mechanisms underlying HFpEF in diabetic women, it is unclear whether time to reperfusion (DTB) is the sole determinant of the poor STEMI outcome as in other patient populations. In this study we used an integrated clinical and pre-clinical (STEMI diabetic patients, db/db mouse model of T2D) approach to investigate whether the higher burden of co-morbidities interact synergistically with duration of the ischemic injury to exacerbate myocardial damage and impair function during reperfusion of female diabetic myocardium.
Methods
Study population
Population-based retrospective case–control study was conducted at the Department of Interventional Cardiology, Clinic for Invasive Cardiology, University Clinical Center Tuzla, Tuzla, Bosnia and Herzegovina. The study protocol was approved by the Ethics Committee of University Clinical Center Tuzla (Ref. 02-09/2-166-2/24) and all methods were performed in accordance with their guidelines and regulations.
Records of 1100 patients who underwent pPCI due to STEMI (ESC 2023 diagnosis criteria) [12] were screened for inclusion in the study. Demographic data, medical history, laboratory data, and clinical data during hospitalization were retrieved from hospital patient database. Patients with diabetes were identified as those with known (treated or not) T2D prior to admission. Our study involved 80 female patients (mean age 65) with STEMI who underwent cardiac catheterisation with pPCI between January 2019 and December 2023.
We compared baseline clinical characteristics, treatment strategy and outcomes among women with diabetes (n = 40, mean age 65 years), with the control arm consisting of age-matched female STEMI non-diabetic patients (n = 40). A special focus was on the impact of the treatment strategy on early mortality and cardiac function. The clinical characteristics of the patient cohort is shown in Tables 1, 2 and 3. Excluded were patients with previous MI, pre-diabetes, heart failure history, angioplasty in the last 3 months, heart failure or cardiogenic shock, severe renal failure (serum creatinine > 200 mmol/L, eGFR < 44 ml/min/1.73 m [2]), cancer and infectious diseases.
Table 1.
Patient characteristics at the time of hospitalization
| Control (n = 40) | Diabetic (n = 40) | P value | |
|---|---|---|---|
| Sex | Female | Female | |
| Age (years) | 65 ± 1.3 | 65 ± 1.3 | |
| BMI (kg/m2) | 29.3 ± 0.4 | *33 ± 0.5 | 0.0001 |
| HbA1c (%) | 5.4 ± 0.04 | *8.78 ± 0.3 | 0.0001 |
| Hypertension | 34 (85%) | 36 (90%) | |
| Obesity | 12 (15%) | *29 (36%) | 0.0003 |
| CKD | 3 (7.5%) | 9 (22%) | |
| Stage 1 | 1 | 3 | |
| Stage 2 | 1 | 3 | |
| Stage 3 | 1 | 3 | |
| OAD (non-SGLT2) | – | 36 | |
| SGLT2i | – | 4 | |
| Insulin | – | 24 |
Data are presented as mean ± SEM. Normality of data distribution was examined using Shapiro–Wilk’s normality test. Comparison between two groups was performed by Student’s t-test (Gaussian data distribution) or Mann–Whitney U test when data was non-normally distributed. Contingency analysis by chi-squared and Fisher’s exact test (two-sided). *p < 0.05 vs control. OAD-oral antidiabetic therapies including metformin, SGLT—sodium glucose co-transporter.
Table 2.
Patient plasma profile at the time of hospitalization
| Control (n = 40) | Diabetic (n = 40) | P value | |
|---|---|---|---|
| Hospital admission timepoint | |||
| Erythrocytes (× 109/L) | 4.25 ± 0.09 | 4.54 ± 0.22 | |
| Haemoglobin (g/L) | 129.2 ± 3.1 | 129.1 ± 2.4 | |
| Haematocrit (g/L) | 0.38 ± 0.008 | 0.38 ± 0.007 | |
| Thrombocytes (× 109/L) | 286.7 ± 19.6 | 270.1 ± 9.9 | |
| CRP (mg/L) | 3.6 ± 0.5 | 4.2 ± 0.6 | |
| Leukocytes (× 109/L) | 10.64 ± 0.7 | *11.66 ± 0.5 | 0.0131 |
| MCV (fL) | 89.71 ± 1.21 | *87.97 ± 0.78 | 0.0431 |
| Glucose (mmol/L) | 7.53 ± 0.2 | *14.18 ± 1.31 | 0.0001 |
| Urea (mmol/L) | 6.48 ± 0.4 | 7.45 ± 0.5 | |
| Creatinine (mmol/L) | 79.43 ± 3.5 | 93.83 ± 6.6 | |
| Potassium (mmol/L) | 3.75 ± 0.08 | *4.01 ± 0.09 | 0.049 |
| Na (mmol/L) | 139.7 ± 0.4 | *137.5 ± 0.6 | 0.049 |
| Ca (mmol/L) | 2.22 ± 0.05 | 2.34 ± 0.034 | |
| Mg (mmol/L) | 0.81 ± 0.01 | *0.74 ± 0.02 | 0.002 |
| P (mmol/L) | 0.97 ± 0.05 | 1.09 ± 0.06 | |
| AST (U/L) | 24.34 ± 1.8 | *30.06 ± 2.34 | 0.029 |
| ALT (U/L) | 23.50 ± 1.89 | *29.49 ± 2.16 | 0.0452 |
| Dyslipidaemia | 39 (97%) | 35 (87%) | |
| Cholesterol (mmol/L) | 5.7 ± 0.2 | 5.6 ± 1.6 | |
| Triglycerides (mmol/L) | 2.4 ± 0.2 | 2.5 ± 0.3 | |
| HDL (mmol/L) | 1.1 ± 0.05 | 1.2 ± 0.06 | |
| LDL (mmol/L) | 3.6 ± 0.2 | 3.4 ± 0.2 | |
Data are presented as mean ± SEM. Normality of data distribution was examined using Shapiro–Wilk’s normality test. Comparison between two groups was performed by Student’s t-test (Gaussian data distribution) or Mann–Whitney U test when data was nonnormally distributed. *p < 0.05 vs control. CRP—C reactive protein; MCV—Mean Corpuscular volume; Na—Sodium, Ca—Calcium; Mg—Magnesium; P—Phosphorus; AST—Aspartate aminotransferase; ALT—Alanine aminotransferase; HDL—High-density lipoprotein cholesterol; LDL—Low-density lipoprotein cholesterol.
Table 3.
Coronary angiography and percutaneous coronary intervention outcomes
| Control (n = 40) | Diabetic (n = 40) | P value | |
|---|---|---|---|
| Total stent length (mm) | 29.3 ± 2.2 | *21.6 ± 1.6 | 0.0075 |
| Stent diameter (mm) | 3.5 ± 0.08 | 3.3 ± 0.09 | |
| Total number of stents in pPCI | 38 (95%) | 36 (90%) | |
| CABG after index procedure | 1 | 3 | |
| ICD | 0 | *4 | 0.0402 |
| CRT | 0 | 1 | |
| Akinesis | 5 | *24 | 0.0001 |
| **Elective PCI after index procedure | 14 | 17 | |
| **Time to elective PCI (days) | 22 ± 5 | 27 ± 5 |
Data are presented as mean ± SEM. Normality of data distribution was examined using Shapiro–Wilk’s normality test. Comparison between two groups was performed by Student’s t-test (Gaussian data distribution) or Mann–Whitney U test when data was non-normally distributed. Contingency analysis by chi-squared and Fisher’s exact test (two-sided). *p < 0.05 vs control. CABG-coronary artery bypass CRT-cardiac resynchronization therapy, PCI-percutaneous coronary intervention, ICD- Implantable cardioverter defibrillator, **PCI- additional stent requirements for non-culprit lesions and **Time to PCI number of days required to carry out additional procedures.
Coronary angiography and pPCI procedure
The pPCI procedure was performed with the adoption of a standard radial approach that uses a 6F-guiding catheter. After bolus of heparin (100 U/Kg), 300 mg aspirin, and P2Y12 inhibitor in loading dose were given (clopidogrel 600 mg or prasugrel 60 mg), target lesion was crossed through 0.014-inch wires. Non-ionic, low-osmolality contrast agent was used in all patients. After angioplasty, all patients were admitted to the Department of Interventional Cardiology. In addition, they were given 100 mg aspirin and 75 mg clopidogrel or 10 mg prasugrel.
Concomitant medical treatment with β-blockers, angiotensin-converting enzyme inhibitors, and statins, were prescribed in accordance with the ESC Guideline for the management of ACS [17]. Door-to-balloon time was defined from patient anamnestic data (duration of the chest pain before entering in the catheterisation laboratory). Echocardiography was performed 4 days post-hospitalization just before discharge, or, in haemodynamically unstable patients, after the index procedure.
Antecubital venous blood samples for the laboratory analysis were collected at the beginning of the angiography procedure in the catheterisation laboratory, and on the third day of the hospitalization. Cardiovascular mortality in patients was defined as death related to myocardial ischemia and infarction, cardiogenic shock, heart failure, arrhythmia (ventricular fibrillation, ventricular tachycardia, or asystole), and cardiac arrest due to other or undetermined causes or cerebrovascular accident.
Animals
For all experiments in this study, 12-week female db/db mice (n = 12) were purchased from Charles River Laboratories (Italy) and aged until 20-weeks. 20-week lean (C57/Bl6) controls (n = 12) were purchased from Charles River Laboratories (UK). Animals were housed in individually ventilated cages and maintained under controlled temperature (22 ± 2 °C) on a 12:12 h light and dark cycles with access to standard chow diet (irradiated PicoLab® Mouse Diet 20 EXT, 5R58) and water ad libitum. Enrichment was provided in the form of tunnels and chew sticks. All animal experiments were in keeping with the UK Home Office Animals (Scientific Procedures) Act, 1986. At the end of the experimental model induction protocol, all animals were humanely sacrificed by phenobarbital I.P. injection.
Biochemical analysis
Tissues (liver, skeletal muscle, heart) were rapidly collected upon termination and snap frozen in liquid N2 using Wollenberger tongs [18]. The frozen heart weight was normalised to tibia length to assess the degree of cardiac hypertrophy (heart weight:tibia length). Blood samples taken from the thoracic cavity at the time of sacrifice were centrifuged at 3000 rpm to obtain plasma. All samples were stored at − 80 °C until biochemical analysis. Plasma samples were analysed by the Clinical Biochemistry Laboratory at Addenbrookes Hospital, Cambridge.
In vivo assessment of left ventricular diastolic and systolic function
M-mode and Doppler echocardiography was performed in 20-week db/db and lean control mice. Anaesthesia was induced with 4% isoflurane and maintained at 1.5–2% for the duration of the procedure. Before assessment of cardiac function, fur was removed from the chest area to allow accurate assessment of cardiac function and mice were allowed to stabilize for at least 10 min. Body temperature was maintained at 37°C. During echocardiography, heart rate was measured from electrocardiogram. Echocardiography images were recorded using a Vevo-3100 imaging system with a 40-MHz linear probe (VisualSonics, Toronto, Canada).
Morphological measurements were taken in the parasternal short axis view at the level of the papillary muscles and ejection fraction was calculated from M-mode images. Diastolic transmitral left ventricle (LV) inflow images were acquired from apical four-chamber views using pulsed-wave doppler to calculate early (E) and late (atrial, A) peak filling blood flow velocities and E-wave deceleration time. Tissue doppler imaging in apical four-chamber view at the mitral annulus allowed measurements of early (e’) and atrial (a’) peak tissue velocities. The E/e’ ratio could then be calculated. Analysis was performed using VevoLab 5.5.1 software.
Langendorff heart perfusions
Mice were administered terminal anaesthesia via intra-peritoneal pentobarbitone injection. Beating hearts were rapidly excised, cannulated, and perfused in isovolumic Langendorff mode at 80 mmHg pressure maintained by a St. Thomas Hospital peristaltic pump controller feedback system (AD Instruments, UK), with Krebs–Henseleit (KH) buffer continuously gassed with 95% O2/5% CO2 (pH 7.4, 37 °C) containing (in mM): NaCl (116), KCl (4.7), MgSO4.7H2O (1.2), KH2PO4 (1.2), NaHCO3 (25), CaCl2 (1.4), and glucose (11) [19]. Cardiac function was assessed using a fluid-filled cling-film balloon inserted into the LV connected via a line to a pressure transducer and a Powerlab system (AD Instruments). The volume of the intraventricular balloon was adjusted using a 1.0 mL syringe to achieve an initial LV diastolic pressure of 4–9 mmHg. Functional parameters were recorded using LabChart software v.7 (AD Instruments) throughout the experiment. LV developed pressure (LVDP) was calculated from the difference between systolic and diastolic pressure [18–27].
Ischemia reperfusion protocol
After 20 min of equilibration, Langendorff crystalloid KH buffer perfused hearts were subject to 20 min of global normothermic (37 °C) ischemia and 2 h of reperfusion [18, 19, 22, 24–27]. At the end of the protocol, hearts were perfused for 1 min with 1% triphenyltetrazolium chloride (TTC) in PBS followed by 10 min incubation at 37 °C in 1% TTC. Hearts were sectioned into 1 mm slices (mouse heart gauge, Zivic instruments, USA), imaged and infarct size was quantified using Fiji-ImageJ Software [18, 25].
Metabolomic analysis using 1H nuclear magnetic resonance (NMR) spectroscopy
Snap frozen and pulverized tissue [liver, skeletal muscle (gastrocnemius and soleus), heart] was analysed using 1H NMR high resolution spectroscopy as previously described [18, 28]. Pulverised tissue samples (~ 45 mg) were subject to methanol/water/chloroform phase extraction. 1H NMR spectra were acquired using a vertical-bore, ultra-shielded Bruker 14.1. Tesla (600 MHz) spectrometer with a BBO probe at 298 K using the Bruker noesygppr1d pulse sequence. Acquisition parameters were 128 scans, 4 dummy scans and 20.8 ppm sweep width, acquisition time of 2.6 s, pre-scan delay of 4 s, 90° flip angle and experiment duration of 14.4 min per sample. TopSpin (version 4.0.5) software was used for data acquisition and for metabolite quantification. FIDs were multiplied by a line broadening factor of 0.3 Hz and Fourier-transformed, phase and automatic baseline-correction were applied. Chemical shifts were referenced to the TSP signal. Metabolite peaks of interest were initially integrated automatically using a pre-written integration region text file and then manually adjusted where required. Assignment of metabolites to their respective peaks was carried out based on previously obtained in-house data, confirmed by chemical shift and using Chenomx NMR Profiler Version 8.1 (Chenomx, Canada). Peak areas were normalized to the total metabolite peak area. Quantification of glycogen was performed by 1H magnetic resonance, giving a measure of the concentration of glucose monomers that are present in the observed peak. Being a large macromolecule, with possible differences in the mobility of glycosyl units, glycogen has been reported to be fully visible by MRS. The modified dual-phase Folch extraction method used for separating aqueous and lipid metabolites was not optimized for the extraction of glycogen, however all samples underwent the same extraction procedure allowing between treatment group comparison [18].
Statistical analysis
Data are presented as mean ± SEM unless otherwise stated. Normality of data distribution was examined using Shapiro–Wilk’s normality test. Comparison between two groups was performed by Student’s t-test (Gaussian data distribution) or Mann–Whitney U test when data was non-normally distributed, and an unequal variance t-test was performed in the absence of equal variance between groups. Two-way analysis of variance (ANOVA) with Tukey’s correction for multiple comparison and one-way ANOVA using Tukey’s correction was used for multiple comparisons where applicable. Qualitative variables were compared with Parson’s χ2 test. Contingency analysis was performed by chi squared and Fisher’s exact test (two-sided). Based on a comparison between two groups by t-test, a sample size of: n = 12/group for was shown to have > 90% power to detect differences in heart weight means at P < 0.05, n = 7/group was shown to have > 90% power to detect difference between E/A means at P < 0.05; 5 mice/group was shown to have > 90% power to detect difference between infarct size as well as phosphocreatine means with a significance level of P < 0.05. Statistical analysis was performed using GraphPad Prism (v10.2.2) software. Differences were considered significant when P < 0.05.
Results
Higher mortality and poorer functional outcome in STEMI type 2 diabetic patients
Forty patients (mean age 65 ± 1.3) were present in type 2 diabetes STEMI group and 40 patients without diabetes (mean age 65 ± 1.3) were in the control arm. Baseline characteristics are shown in Table 1. T2D patients were significantly obese (BMI 33 ± 0.5 kg/m2 vs 29 ± 0.4 kg/m2, P = 0.0001) but were comparable to control group in terms of hypertension and plasma lipid profile (Tables 1 and 2). At hospital admission, as well as three days post-admission, T2D patients had significantly altered plasma parameters versus non-diabetic patients (Table 2, Supplementary Table 1). T2D patients had significantly elevated plasma glucose as acute hyperglycaemia is common in STEMI patients [29].
Coronary angiography identified multivessel disease in both patient groups, mostly 3 vessel CAD (75% in diabetics vs 57% in controls, Fig. 1B) with predominant culprit lesion in right coronary artery (RCA) and left anterior descending (LAD) artery (Supplementary Table 2). Whilst both group of patients had comparable door-balloon time (Fig. 1C), T2D patients had significantly higher markers of myocardial damage such as circulating troponin (Fig. 1D) and plasma markers which are known to correlate with poorer ACS outcomes—increased plasma leukocytes, ALT and lower Mg (Table 2). We also found that 3 days post-STEMI, women with T2D maintain higher leukocyte count vs control group suggestive of enhanced inflammation (Supplementary Table 1).
Fig. 1.
STEMI outcome in diabetic and non-diabetic female patients. A Representative image of total LAD occlusion and restored coronary reperfusion post-stent placement in diabetic STEMI patient. B Number of vessels affected by CAD. C Onset (door) to balloon time. D Circulating troponin levels at the time of hospitalization. E In-hospital mortality. F Left ventricular ejection fraction 3 days post pPCI. G Door to balloon time significantly correlates with plasma troponin. Data are presented as mean ± SEM. Normality of data distribution was examined using Shapiro–Wilk’s normality test. Comparison between two groups was performed by Student’s t-test (Gaussian data distribution), or Mann–Whitney U test when data was non-normally distributed. *p < 0.05, n = 40 STEMI patients with diabetes, n = 40 STEMI non-diabetic controls
T2D patients had significantly higher post-STEMI mortality vs non-diabetic control group (Fig. 1E) accompanied by markedly increased akinesis of the infarcted region post pPCI (Table 3) leading to lower left ventricular ejection fraction (LVEF, Fig. 1F) and increased usage of implantable cardioverter defibrillators in T2D group (Table 3). Furthermore, there was a significant negative correlation between door-balloon time vs LVEF (Fig. 1G).
Whilst the total number of stents as well as stent diameter was comparable between groups (Table 3), total stent length was shorter in T2D group (Table 3) as they were implanted with shorter stents to reduce the subsequent complex revascularization risk. Furthermore, T2D patients required additional elective PCI (17 vs 14 in non-diabetic group, Table 3). This is indicative of additional requirements for stents in non-culprit lesions, and presence of multivessel disease.
Phenotypic features of female db/db mice mimic characteristics of women with T2D
20-week female db/db mice (Fig. 2A) developed a distinct diabetic phenotype characterised by obesity (Fig. 2B), cardiac hypertrophy (Fig. 2C, D), hyperinsulinaemia and marked hyperglycaemia (Fig. 2E, F). Plasma markers of renal function (urea, creatinine), liver function (ALT, AST) and free fatty acids were comparable between the groups (Supplementary Fig. 1).
Fig. 2.
Female db/db mouse morphological features. A Representative image of age-matched lean and db/db female mice. B Body weight measurements, C Index of hypertrophy (heart weight/tibia length), and D Heart weight, (n = 12 lean, n = 12 db/db). E Plasma insulin concentration and F Plasma glucose concentration, (n = 6 lean, n = 6 db/db). Data are presented as mean ± SEM. Normality of data distribution was examined using Shapiro–Wilk’s normality test. Comparison between two groups was performed by Student’s t-test (Gaussian data distribution), unequal variance t-test, or Mann–Whitney U test when data was non-normally distributed, *p < 0.05
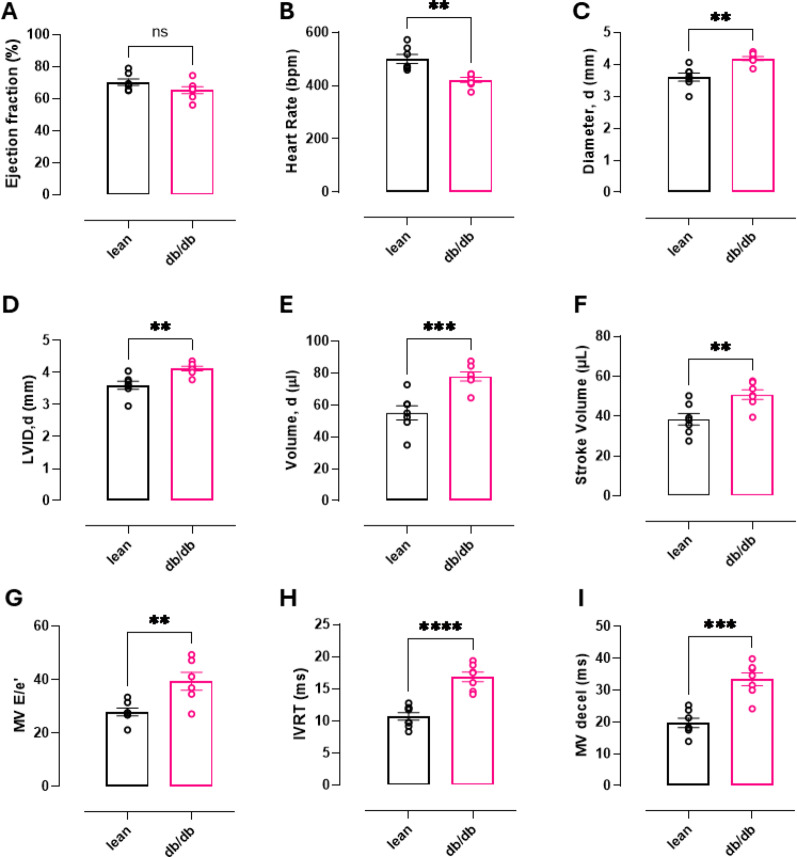
In addition to cardiac hypertrophy, echocardiographic assessment identified significant functional alterations (Fig. 3) including reduced heart rate, increased LV internal diameter (LVID), end diastolic volume, stroke volume, mitral valve (MV) E/e’, IVRT, and MV deceleration time. However, ejection fraction was comparable to lean controls suggestive of HFpEF with diastolic dysfunction rather than HFrEF phenotype.
Fig. 3.
Echocardiographic assessment of in vivo cardiac function. A–F Echocardiography measurements taken from parasternal short axis view: A Ejection fraction, B Heart rate, C Heart diameter in diastole, D LV internal diameter (LVID) in diastole, E End diastolic volume and F Stroke volume. G–I Assessment of diastolic function taken from apical four chamber view: G Ratio of mitral valve flow Doppler E wave to mitral annulus tissue Doppler e’ wave, H Isovolumetric relaxation time and I Mitral valve deceleration time. Data are presented as mean ± SEM. Normality of data distribution was examined using Shapiro–Wilk’s normality test. Comparison between two groups was performed by Student’s t-test (Gaussian data distribution), unequal variance t-test, or Mann–Whitney U test when data was non-normally distributed, *p < 0.05 (lean n = 7, db/db n = 7)
db/db mice have altered metabolic profile and increased susceptibility to ischemia/reperfusion injury
Development and progression of T2D in db/db mice leads to significant alterations in cardiac (Fig. 4A), liver and skeletal muscle metabolomic profile (Fig. 4B, C). Myocardial metabolomic profile of db/db mice is characterised by significantly decreased NADH and creatine as well as significantly elevated succinate and branched chain amino acids (leucine, valine and isoleucine).
Fig. 4.
Metabolomic profile of heart, skeletal muscle and liver determined by 1H NMR spectroscopy. Metabolomic profile of A Heart (n = 5/group), B Skeletal muscle (n = 6/group) and C Liver tissue (n = 7/group) from female db/db mice compared to lean controls, measured by 1H NMR spectroscopy. Each point represents the fold change (FC) of a metabolite plotted against the associated level of statistical significance for the change analysed by t-test (horizontal dashed line indicates p = 0.05 threshold)
Skeletal muscle metabolomic profile is severely altered with reduced energetic reserved (creatine, ATP, ADP, NAD), lipid and TCA cycle intermediates (Fig. 4B). db/db skeletal muscles show marked elevation of lactate, succinate, AMP as well as amino acids (glutamate, valine, leucine, isoleucine, Fig. 4B). T2D in db/db mice also caused altered liver metabolomic profile with altered levels of taurine, fumarate, acetyl carnitine and alanine (Fig. 4C).
Whilst baseline ex vivo cardiac function of Langendorff-perfused db/db hearts was comparable to lean controls (Fig. 5A), when subjected to 20-min ischemia db/db hearts show poorer functional recovery upon reperfusion (Fig. 5). Alongside significantly reduced LVDP (Fig. 5A), across reperfusion db/db hearts exhibit increased and irregular heart rate indicative of arrhythmogenesis (Fig. 5B). TTC staining (Fig. 5C) revealed that db/db hearts had significantly bigger infarct size compared to controls (Fig. 5D).
Fig. 5.
Female db/db mouse hearts have severely impaired post-ischemic recovery. A Left ventricular developed pressure (LVDP) during equilibration, ischemia and reperfusion. B Heart rate during equilibration, ischemia and reperfusion. C Representative image of lean and db/db TTC stained hearts D Infarct area quantification. Data are presented as mean ± SEM. Normality of data distribution was examined using Shapiro–Wilk’s normality test. Comparison between two groups was performed by Student’s t-test (Gaussian data distribution), unequal variance t-test or Mann–Whitney U test when data was non-normally distributed. *P < 0.05, (n = 5 lean, n = 5 db/db)
Discussion
Our study shows that, despite comparable time to reperfusion (DTB), diabetic female STEMI patients have poorer outcomes with higher mortality, reduced post-pPCI LVEF, increased plasma markers of cardiac damage and increased requirement for implantable cardioverter-defibrillator devices compared to non-diabetic controls (summarized in Fig. 6). We also identified blood marker parameters suggestive of significantly higher systemic inflammation, cardio-hepatic syndrome as well as lower circulating magnesium, a known independent predictor of electrocardiographic no-reflow and long-term mortality in diabetic women. Acute stress of STEMI resulted in hyperglycaemia in the diabetic group despite the use of glycaemic control medications.
Fig. 6.
Schematic summary of post-MI changes in female patients and mice with T2D
Comparable to diabetic female humans, female db/db mice exhibit obesity, hyperglycaemia and insulin resistance. Female db/db mice developed cardiac structural changes typical of HFpEF (myocardial hypertrophy, impaired diastolic function, LV stiffness). Furthermore, T2D led to significant perturbation of skeletal muscle, liver, and heart metabolism indicative of cardiometabolic syndrome. These metabolic comorbidities are known risk factors in the development of HFpEF. This cluster of multi-organ metabolic disturbances creates a complex network of pathways that exacerbate cardiac stress, contributing to the severe myocardial dysfunction post-I/R (poorer functional recovery and larger infarct size versus non-diabetic controls). This is the first study to combine both female T2D patients and db/db mice showing that T2D aggravates the course of myocardial infarction worsening the prognosis. Crucially, our study shows that in diabetic females longer DTB time is not the key determinant of poor STEMI recovery. Pre-clinical animal models of T2D studies seldom include female animals, leading to a gap in available robust data for the better understanding of the impact of biological sex in T2D cardiomyopathy [30]. Our study highlights female db/db mice as a feasible experimental model for the investigation of the impact of acute myocardial infarction in T2D with the outcome comparable to humans.
Coronary lesions in T2D patients typically appear long and diffuse, more likely in small-diameter arteries [31]. Consequently, lesion complexity could be relatively presented by the length and diameter of the stent. We observed no difference in diameter of stent and similar number of stents in pPCI between the groups. Furthermore, total stent length was significantly shorter in T2D patients. Given that the stent length significantly increases the thrombotic risk and the risk of stent-driven recurrent ischemic events [32], T2D STEMI patients were implanted with shorter stents, most often “spot stenting” to reduce the subsequent complex revascularization risk. Nevertheless, we have observed that T2D patients required further PCI or CABG on additional non-culprit lesions post-pPCI vs non-diabetics.
To what extent do the observed post-MI reductions in LVEF, higher mortality, and elevated troponin levels in diabetic women reflect a direct effect of T2D versus larger myocardial areas at risk or microvascular dysfunction? Our data, showing a similar burden of three-vessel coronary artery disease in diabetic and non-diabetic patients, refutes the hypothesis that a larger myocardial area at risk is the primary determinant of the observed disparities in outcome. There is also no significant difference in terms of blood vessels affected as both groups predominantly had lesions on the RCA and LAD. The comparable distribution of culprit lesions (LAD and RCA) in both groups, considering the LAD's supply to approximately 50% of the left ventricle, suggests no significant difference in myocardial involvement between diabetic and non-diabetic patients. Comparable revascularization strategies, including the number of stents used, stent diameter, and extent of additional procedures (PCI or CABG) on non-culprit vessels, were observed in both groups. This suggests that differences in the myocardial area at risk are not responsible for the increased incidence of reduced LVEF and mortality observed in the diabetic group. Whilst we acknowledge that diabetic HFpEF is characterised by underlying reduction in capillary density due to pericyte apoptosis, poorer endothelial dysfunction and increased oxidative stress [33], we can exclude the potential impact of microvascular obstruction on the diabetic group STEMI outcome as myocardial infarction without obstructive CAD (MINOCA) and spontaneous coronary artery dissection (SCAD) patients were not included [34, 35].
Our study has shown that despite comparable treatment window, STEMI patients with T2D had alterations in blood marker parameters which correlate with increased cardiac damage during MI: increased ALT, increased leukocytes and lower magnesium. Hepatic injury secondary to HF is well described, however, only limited data exist about the possible impact of ACS on the liver [36]. Here we have identified possible cardio-hepatic interaction in STEMI patients with T2D as they had higher circulating level of ALT on admission. Previous work has shown that greater severity of cardiac dysfunction was associated with worse elevation of liver enzymes including ALT [36, 37]. The combination of decreased LVEF and venous congestion was shown to be associated with elevated circulating liver enzymes suggesting a possible cardio-hepatic syndrome among patients with STEMI [37]. Elevated ALT was also independently associated with increased in-hospital all-cause mortality in patients with STEMI [37]. Furthermore, liver metabolomic profile of db/db mice has revealed that the development of T2D leads to impaired metabolism including depletion of lipid, amino acid and Krebs cycle intermediates as well as elevation of taurine.
Regarding the inflammatory mechanisms involved in STEMI, the leukocyte count in T2D group was significantly higher on admission. Higher leukocyte count has been shown to correlate with STEMI size and is associated with the poor prognosis and mortality [38]. Sex differences also impact the inflammatory response after STEMI. Women were found to present with STEMI with higher lymphocyte counts [39]. On hospital admission, T2D STEMI patients also had lower circulating Mg. Lower circulating magnesium on admission in STEMI patients is an independent predictor of electrocardiographic no-reflow and long-term mortality as it has been shown to have pro-thrombolytic, pro-inflammatory and pro-atherogenic effects [40].
STEMI induces acute hyperglycaemic response, driven by cortisol and adrenaline release, which is significantly amplified in diabetic patients due to their compromised glucose control [41]. This stress-induced hyperglycaemia is associated with worse outcomes (increased mortality and morbidity). The effective management of acute hyperglycaemia is thus vital for improving outcomes in diabetic women presenting with STEMI. However, variations in clinical presentation and higher prevalence of comorbidities complicate treatment, underscoring the need for gender-specific considerations in therapeutic strategies. Persistent hyperglycaemia following STEMI poses long-term implications as well. It can hinder recovery, increase the risk of subsequent cardiovascular events, and exacerbate long-term complications associated with diabetes, making it essential to prioritise glycaemic control to improve overall prognosis [41].
In db/db diabetic cardiomyopathy, worse MI outcome including arrhythmia are in line with previously reported observations [9, 42–48]. A higher duration of ventricular tachycardia and the degeneration of ventricular tachycardia into ventricular fibrillation were historically observed upon reperfusion in db/db hearts due to chronically impaired E-C coupling [43]. This outcome is also in part caused by the loss of metabolic flexibility, energetic deficit and mitochondrial dysfunction [49]. Cardiac metabolomic profile of db/db mice is consistent with these observations as characterized by reduced creatine content. It has previously been shown that creatine is protective and anti-apoptotic during myocardial ischemia as it enhances energy reserve and cell survival [50]. T2D caused significant alteration in myocardial amino acid metabolism. Of note, branched chain amino acids (isoleucine, leucine, valine) were markedly elevated indicative of chronically impaired regulation leading to their accumulation [51].
db/db hearts were also characterized by elevated succinate in normoxia. It has been shown that individuals with T2D and obesity exhibit elevated levels of succinate in the circulation, urine, faeces, liver and retina [52]. We have previously shown in male db/db mice that the altered cardiac metabolism including elevated myocardial succinate is accompanied by the presence of dysregulated T-cell-mediated cardiac inflammation [53]. Given that succinate is not only oxidized but effluxed from the hearts [19], this normoxic succinate accumulation in db/db hearts could also act as a pro-inflammatory stress signal worsening the MI outcome observed in patients and mice [54, 55]. Succinate was shown to be the only metabolite significantly increased in coronary sinus blood in STEMI patients correlating with the extent of acute myocardial injury [56]. Furthermore, diabetic myocardium has been shown to be more susceptible to symptomless silent myocardial ischemia [57] due to underlying vascular disorders which could be driving the accumulation of myocardial succinate preceding the onset of MI. Moreover, diabetic myocardium exhibits Nai accumulation, and the ischemia-induced rise in intracellular Na⁺ is significantly greater in db/db hearts compared to controls [3, 43]. Elevated myocardial Nai was recently shown to cause pseudo-hypoxia and stabilization of HIF-1α despite normal tissue oxygenation, to decrease Gibb's free energy of ATP hydrolysis, increases the TCA cycle intermediates succinate and fumarate, decreases ETC activity at Complexes I, II and III, and causes a redox shift of CoQ to CoQH2[ 58]. Thus, myocardial Nai elevation could be responsible for the succinate accumulation in female db/db hearts.
Diabetic HFpEF is increasingly recognized as a multiorgan disorder rather than solely a cardiac dysfunction [59]. Given the metabolic crosstalk between organs, and to better understand mechanistic links between systemic metabolic dysfunction and cardiac performance, we investigated the metabolic signature of liver and skeletal muscles in female db/db mice. Skeletal muscle was found to have the most severe and extensive perturbation in metabolomic profile. Exercise intolerance in HFpEF is often linked to skeletal muscle dysfunction rather than cardiac output limitations alone. T2D was shown to lead to greater skeletal muscle atrophy and metabolic dysfunction which may be causing poorer exercise tolerance in HFpEF [60]. Decreased glycolysis and increased β-oxidation were previously shown to lead to increased branched-chain amino acid concentrations in T2D skeletal muscles [61].
T2D women with STEMI often face disparities in treatment they receive. They may receive less aggressive therapy compared to men leading to worse outcomes [15]. This reflects a disadvantage of women with T2D based on behavioural, treatment or societal aspects [15]. Diabetic women may delay seeking care due to social responsibilities, such as caregiving roles, or due to underestimating the severity of their symptoms. However, our study has shown comparable door-balloon time between T2D and non-diabetic patient groups. Women with T2D have higher prevalence of atypical STEMI symptoms (fatigue, nausea, shortness of breath) rather than a classic chest pain thus delaying diagnosis and timely intervention [8, 15]. These atypical symptoms are mostly secondary to peripheral diabetic neuropathy, which can, due to an increase oxidative stress, reduce perception of pain, nerve growth factors and mitochondrial dysfunction [62, 63].
This study is limited by its single-centre design, which may restrict the generalisability of our findings to other healthcare settings or diverse populations. Although our patient cohort is adequately sized to identify significant differences in outcomes, a larger, multi-centre cohort could yield more definitive conclusions. Additionally, our analysis did not include male patients or mice, preventing us from making any conclusions about potential inter-sex differences.
Furthermore, for the murine ischemia/reperfusion (I/R) experiments we used isolated Langendorff-perfused mouse heart model rather than surgically induced in vivo I/R. While the use of isolated perfused heart models allows for precise control of cardiac perfusion conditions, this experimental approach fails to replicate the complex systemic milieu present in vivo, particularly in the context of metabolic diseases such as obesity and diabetes thus limiting extrapolation to clinical settings. These conditions are characterized by altered circulating metabolites, hormones, and inflammatory mediators, all of which contribute to myocardial I/R injury outcome and cannot be reproduced ex vivo. Lack of these confounding factors impacts the I/R injury outcome. Furthermore, 20 min total global ischemia protocol produced small infarct size (< 5%) in control non-diabetic mice. This suggests the duration of ischaemia does not induce significant cardiomyocyte death and infarction in healthy hearts, thereby limiting the protocol utility in evaluating any future cardioprotective interventions. The absence of substantial infarction may impact the conclusions regarding the extent of myocardial injury. While the db/db model of T2D demonstrated increased susceptibility to arrhythmias, it remains unclear whether infarct development in db/db female mice occurs independently of arrhythmias.
Conclusions
Considering the rising prevalence of T2D and its associated cardiovascular risk, the impact of T2D on STEMI in women is of critical importance for public health. Understanding the interplay between T2D and STEMI in women can lead to better prevention strategies, earlier recognition of symptoms and more effective treatment options. Future research focusing on sex-specific strategies in T2D management is essential for improving cardiovascular disease outcomes in women.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Dr Nasima Kanwal and School of Physical and Chemical Sciences NMR Facility, Queen Mary University of London.
Abbreviations
- T2D
Type 2 diabetes
- CAD
Coronary artery disease
- ACS
Acute coronary syndrome
- MI
Myocardial infarction
- HF
Heart failure
- STEMI
ST segment elevation myocardial infarction
- pPCI
Primary percutaneous coronary intervention
- I/R
Ischemia reperfusion
- LVEF
Left ventricular ejection fraction
- LV
Left ventricle
- KH
Krebs–Henseleit
- LVDP
Left ventricle developed pressure
- TTC
Triphenyltetrazolium chloride
- NMR
Nuclear magnetic resonance
- SEM
Standard error of mean
- ANOVA
Analysis of variance
- RCA
Right coronary artery
- LAD
Left anterior descending
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- LVID
Left ventricle internal diameter
- MV
Mitral valve
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reserved ejection fraction
Author contributions
II, MY, EC. FC, HAP, DA performed the experiments. II, MY, EC, DA processed and analysed the data. II, MY, DA, HAP, MPM. wrote the main manuscript text. DA, MY prepared all the figures. All authors reviewed the manuscript.
Funding
Wellcome Trust Career Re-Entry Fellowship 221604/Z/20/Z (DA), Barts Charity grant G-002145 (DA). This work was supported by the Medical Research Council UK (MC_UU_00028/4) to MPM.
Availability of data and materials
All data deposited open access on dryad.com DOI: 10.5061/dryad.3n5tb2rts.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of University Clinical Centre Tuzla (Ref. 02-09/2-166-2/24) and all methods were performed in accordance with their guidelines and regulations. All animal experiments were in keeping with the UK Home Office Animals (Scientific Procedures), 1986.
Consent for publication
All authors consent the manuscript submission and publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ivana Iveljic and Megan Young contributed equally to this work.
References
- 1.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–71. 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20:685–95. [DOI] [PubMed] [Google Scholar]
- 3.Lambert R, et al. Intracellular Na+ concentration ([Na+]i) Is elevated in diabetic hearts due to enhanced Na+-glucose cotransport. J Am Heart Assoc. 2015;4:e002183. 10.1161/JAHA.115.002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sotomi Y, et al. Sex differences in heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10:e018574. 10.1161/JAHA.120.018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi K, et al. Effect of diabetes mellitus on the development of left ventricular contractile dysfunction in women with heart failure and preserved ejection fraction. Cardiovasc Diabetol. 2021;20:185. 10.1186/s12933-021-01379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abudureyimu M, et al. Heart failure with preserved ejection fraction (HFpEF) in type 2 diabetes mellitus: from pathophysiology to therapeutics. J Mol Cell Biol. 2022. 10.1093/jmcb/mjac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 8.Radomska E, Sadowski M, Kurzawski J, Gierlotka M, Polonski L. ST-segment elevation myocardial infarction in women with type 2 diabetes. Diabetes Care. 2013;36:3469–75. 10.2337/dc13-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol. 2006;290:H146-153. 10.1152/ajpheart.00583.2005. [DOI] [PubMed] [Google Scholar]
- 10.Murphy E, Amanakis G, Fillmore N, Parks RJ, Sun J. Sex differences in metabolic cardiomyopathy. Cardiovasc Res. 2017;113:370–7. 10.1093/cvr/cvx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fourny N, et al. Sex differences of the diabetic heart. Front Physiol. 2021;12:661297. 10.3389/fphys.2021.661297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson CA, Gullberg B, Merlo J, Rastam L, Lindblad U. Female advantage in AMI mortality is reversed in patients with type 2 diabetes in the Skaraborg project. Diabetes Care. 2005;28:2246–8. 10.2337/diacare.28.9.2246. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-de-Andres A, et al. Are there sex differences in the effect of type 2 diabetes in the incidence and outcomes of myocardial infarction? A matched-pair analysis using hospital discharge data. Cardiovasc Diabetol. 2021;20:81. 10.1186/s12933-021-01273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemens KK, Woodward M, Neal B, Zinman B. Sex disparities in cardiovascular outcome trials of populations with diabetes: a systematic review and meta-analysis. Diabetes Care. 2020;43:1157–63. 10.2337/dc19-2257. [DOI] [PubMed] [Google Scholar]
- 15.Regitz-Zagrosek V, Gebhard C. Gender medicine: effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat Rev Cardiol. 2023;20:236–47. 10.1038/s41569-022-00797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNamara RL, et al. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2006;47:2180–6. 10.1016/j.jacc.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 17.Byrne RA, et al. 2022 joint ESC/EACTS review of the 2018 guideline recommendations on the revascularization of left main coronary artery disease in patients at low surgical risk and anatomy suitable for PCI or CABG. Eur Heart J. 2023;44:4310–20. 10.1093/eurheartj/ehad476. [DOI] [PubMed] [Google Scholar]
- 18.Faulkes CG, et al. Naked mole-rats have distinctive cardiometabolic and genetic adaptations to their underground low-oxygen lifestyles. Nat Commun. 2024;15:2204. 10.1038/s41467-024-46470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prag HA, et al. Mechanism of succinate efflux upon reperfusion of the ischaemic heart. Cardiovasc Res. 2021;117:1188–201. 10.1093/cvr/cvaa148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aksentijevic D, et al. Intracellular sodium elevation reprograms cardiac metabolism. Nat Commun. 2020;11:4337. 10.1038/s41467-020-18160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aksentijevic D, et al. Myocardial creatine levels do not influence response to acute oxidative stress in isolated perfused heart. PLoS ONE. 2014;9:e109021. 10.1371/journal.pone.0109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chouchani ET, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–5. 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eykyn TR, et al. Multiple quantum filtered (23)Na NMR in the Langendorff perfused mouse heart: ratio of triple/double quantum filtered signals correlates with [Na]i. J Mol Cell Cardiol. 2015;86:95–101. 10.1016/j.yjmcc.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miljkovic JL, et al. Rapid and selective generation of H(2)S within mitochondria protects against cardiac ischemia-reperfusion injury. Redox Biol. 2022;55:102429. 10.1016/j.redox.2022.102429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prag HA, et al. Ischemia-selective cardioprotection by malonate for ischemia/reperfusion injury. Circ Res. 2022;131:528–41. 10.1161/CIRCRESAHA.121.320717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robb EL, et al. Selective superoxide generation within mitochondria by the targeted redox cycler MitoParaquat. Free Radic Biol Med. 2015;89:883–94. 10.1016/j.freeradbiomed.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Yin Z, et al. Structural basis for a complex I mutation that blocks pathological ROS production. Nat Commun. 2021;12:707. 10.1038/s41467-021-20942-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClements L, et al. Impact of reduced uterine perfusion pressure model of preeclampsia on metabolism of placenta, maternal and fetal hearts. Sci Rep. 2022;12:1111. 10.1038/s41598-022-05120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badiger S. Hyperglycaemia and myocardial infarction. Br J Med Pract. 2019;12: a010. [Google Scholar]
- 30.Toedebusch R, Belenchia A, Pulakat L. Diabetic cardiomyopathy: impact of biological sex on disease development and molecular signatures. Front Physiol. 2018;9:453. 10.3389/fphys.2018.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanguineti F, et al. Chronic total coronary occlusion treated by percutaneous coronary intervention: long-term outcome in patients with and without diabetes. EuroIntervention. 2017;12:e1889–97. 10.4244/EIJ-D-15-00278. [DOI] [PubMed] [Google Scholar]
- 32.Kappetein AP, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg. 2013;43:1006–13. 10.1093/ejcts/ezt017. [DOI] [PubMed] [Google Scholar]
- 33.Tromp J, et al. Microvascular disease in patients with diabetes with heart failure and reduced ejection versus preserved ejection fraction. Diabetes Care. 2019;42:1792–9. 10.2337/dc18-2515. [DOI] [PubMed] [Google Scholar]
- 34.Pancholy SB, Shantha GP, Patel T, Cheskin LJ. Sex differences in short-term and long-term all-cause mortality among patients with ST-segment elevation myocardial infarction treated by primary percutaneous intervention: a meta-analysis. JAMA Intern Med. 2014;174:1822–30. 10.1001/jamainternmed.2014.4762. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhary R, et al. Sex-related differences in clinical outcomes among patients with myocardial infarction with nonobstructive coronary artery disease: a systematic review and meta-analysis. Int J Cardiol. 2022;369:1–4. 10.1016/j.ijcard.2022.07.050. [DOI] [PubMed] [Google Scholar]
- 36.Bannon L, et al. The cardio-hepatic relation in STEMI. J Pers Med. 2021. 10.3390/jpm11121241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, et al. Predictive value of elevated alanine aminotransferase for in-hospital mortality in patients with acute myocardial infarction. BMC Cardiovasc Disord. 2021;21:82. 10.1186/s12872-021-01903-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrari JP, et al. Correlation between leukocyte count and infarct size in ST segment elevation myocardial infarction. Arch Med Sci Atheroscler Dis. 2016;1:e44–8. 10.5114/amsad.2016.60759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Blokland IV, Groot HE, Hendriks T, Assa S, van der Harst P. Sex differences in leukocyte profile in ST-elevation myocardial infarction patients. Sci Rep. 2020;10:6851. 10.1038/s41598-020-63185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Annadatha A, et al. Serum Magnesium as a prognostic marker in acute coronary syndromes and its correlation with coronary prognostic index Medical. Sciences. 2023;27:e552639. [Google Scholar]
- 41.Karakasis P, et al. Prognostic value of stress hyperglycemia ratio in patients with acute myocardial infarction: a systematic review with Bayesian and frequentist meta-analysis. Trends Cardiovasc Med. 2024;34:453–65. 10.1016/j.tcm.2023.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–41. 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 43.Anzawa R, et al. Intracellular sodium increase and susceptibility to ischaemia in hearts from type 2 diabetic db/db mice. Diabetologia. 2006;49:598–606. 10.1007/s00125-005-0091-5. [DOI] [PubMed] [Google Scholar]
- 44.Anzawa R, et al. The role of Na+/H+ exchanger in Ca2+ overload and ischemic myocardial damage in hearts from type 2 diabetic db/db mice. Cardiovasc Diabetol. 2012;11:33. 10.1186/1475-2840-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou CC, et al. Mechanisms of ranolazine pretreatment in preventing ventricular tachyarrhythmias in diabetic db/db mice with acute regional ischemia-reperfusion injury. Sci Rep. 2020;10:20032. 10.1038/s41598-020-77014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng YW, et al. Hyperglycemia promotes myocardial dysfunction via the ERS-MAPK10 signaling pathway in db/db mice. Lab Invest. 2022;102:1192–202. 10.1038/s41374-022-00819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panagia M, Schneider JE, Brown B, Cole MA, Clarke K. Abnormal function and glucose metabolism in the type-2 diabetic db/db mouse heart. Can J Physiol Pharmacol. 2007;85:289–94. 10.1139/y07-028. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, et al. Diabetes exacerbates myocardial ischemia/reperfusion injury by down-regulation of MicroRNA and Up-regulation of O-GlcNAcylation. JACC Basic Transl Sci. 2018;3:350–62. 10.1016/j.jacbts.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marelli-Berg FM, Aksentijevic D. Immunometabolic cross-talk in the inflamed heart. Cell Stress. 2019;3:240–66. 10.15698/cst2019.08.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lygate CA, et al. Moderate elevation of intracellular creatine by targeting the creatine transporter protects mice from acute myocardial infarction. Cardiovasc Res. 2012;96:466–75. 10.1093/cvr/cvs272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernandez-Alvarez MI, et al. Early-onset and classical forms of type 2 diabetes show impaired expression of genes involved in muscle branched-chain amino acids metabolism. Sci Rep. 2017;7:13850. 10.1038/s41598-017-14120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Veledo S, Marsal-Beltran A, Vendrell J. Type 2 diabetes and succinate: unmasking an age-old molecule. Diabetologia. 2024;67:430–42. 10.1007/s00125-023-06063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson S, et al. Targeting T-cell mediated inflammation improves cardiac dysfunction and metabolic remodelling in type 2 diabetes. J Mol Cell Cardiol. 2022;173:210. [Google Scholar]
- 54.Milliken AS, Brookes PS. Amber alert: getting to the heart of succinate efflux in reperfusion injury. Cardiovasc Res. 2021;117:997–8. 10.1093/cvr/cvaa216. [DOI] [PubMed] [Google Scholar]
- 55.Milliken AS, Nadtochiy SM, Brookes PS. Inhibiting succinate release worsens cardiac reperfusion injury by enhancing mitochondrial reactive oxygen species generation. J Am Heart Assoc. 2022;11:e026135. 10.1161/JAHA.122.026135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohlhauer M, et al. Metabolomic profiling in acute ST-segment-elevation myocardial infarction identifies succinate as an early marker of human ischemia-reperfusion injury. J Am Heart Assoc. 2018. 10.1161/JAHA.117.007546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Draman MS, et al. A silent myocardial infarction in the diabetes outpatient clinic: case report and review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013:130058. 10.1530/EDM-13-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung YJ, et al. Elevated Na is a dynamic and reversible modulator of mitochondrial metabolism in the heart. Nat Commun. 2024;15:4277. 10.1038/s41467-024-48474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schiattarella GG, et al. Immunometabolic mechanisms of heart failure with preserved ejection fraction. Nat Cardiovasc Res. 2022;1:211–22. 10.1038/s44161-022-00032-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood N, et al. Skeletal muscle atrophy in heart failure with diabetes: from molecular mechanisms to clinical evidence. ESC Heart Fail. 2021;8:3–15. 10.1002/ehf2.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abi Akar E, et al. The analysis of the skeletal muscle metabolism is crucial for designing optimal exercise paradigms in type 2 diabetes mellitus. Mol Med. 2024;30:80. 10.1186/s10020-024-00850-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmitz T, et al. Do patients with diabetes with new onset acute myocardial infarction present with different symptoms than non-diabetic patients? Front Cardiovasc Med. 2024;11:1324451. 10.3389/fcvm.2024.1324451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Paschoal Oliveira, C., Simone Lopes Moreira, R., Guizzilini, S. & Batisa Santos, V. Preipheral neuropathy and clinical signs of acute coronary syndrome in patients with diabetes mellitus. Cotigare Enferm, 2017; 42017: 48491.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data deposited open access on dryad.com DOI: 10.5061/dryad.3n5tb2rts.