Abstract
Background
This study evaluated the effects of three surface treatments—concentrated H2SO4, sodium borohydride (NaBH4), and their combination—on the shear bond strengths between polyether ether ketone (PEEK) and both composite resins and poly(methyl methacrylate) (PMMA).
Methods
PEEK specimens (n = 160) were randomly assigned to composite resin and PMMA groups, each of which was subdivided into normal and aging groups (n = 40), each comprising samples that were pristine (untreated) or treated with dimethyl sulfoxide (DMSO), NaBH4, or concentrated H2SO4 or sequentially treated with NaBH4 and concentrated H2SO4 (n = 8). The shear bond strength (SBS) of the normal and aged specimens were measured. For the normal and treated specimens, the surface and cross-sectional morphologies were analyzed using scanning electron microscopy (SEM), the chemical bond modifications were investigated using X-ray photoelectron and attenuated total reflectance Fourier-transform infrared spectroscopies (XPS and ATR-FTIR, respectively), the mechanical strengths were measured using three-point bending tests, and the cytotoxicities were evaluated using cell-counting kit-8 (CCK-8) assays and 4′,6-diamidino-2-phenylindole (DAPI) staining.
Results
NaBH4 etched the PEEK surface, generating a fibrous texture, while concentrated H2SO4 produced a surface possessing variously sized pores. Sequential treatment with NaBH4 and concentrated H2SO4 produced dense, permeating pores. XPS confirmed that NaBH4 reduced carbonyl groups to hydroxyl groups on the PEEK surface. ATR-FTIR spectroscopy confirmed that the silane coupling agent grafted onto the PEEK surface, forming Si–O–C bonds. NaBH4 and concentrated H2SO4 both strengthened the shear bonding between the PEEK and both the composite resins and PMMA (P < 0.05). The specimens sequentially treated with NaBH4 and 98% concentrated H2SO4 possessed the strongest shear bonding (P < 0.05). The aged specimens sequentially treated with NaBH4 and concentrated H2SO4 retained very strong shear bonds. CCK-8 cytotoxicity assays and DAPI staining confirmed that these surface treatments meet oral biocompatibility standards.
Conclusion
NaBH4 introduced chemical bonds to the PEEK surface, while concentrated-H2SO4-induced sulfonation enhanced the micromechanical interlocking between the PEEK and both the composite resins and PMMA. These combined physicochemical modifications significantly strengthened the adhesion between the PEEK and both the composite resins and PMMA and effectively strengthened PEEK’s bonding.
Keywords: Polyether ether ketone, Composite resin, Poly(methyl methacrylate), Sulfonation, SOdium Borohydride, Shear bond strength
Background
Polyether ether ketone (PEEK), an organic macromolecule formed by polymerizing monomers, comprises two ether bonds, one carbonyl group, and three benzene rings and is a semicrystalline thermoplastic high-temperature polymer [1]. Because PEEK exhibits excellent high-temperature resistance and chemical stability, it is used in a diverse range of dental applications, such as dental implants, implant abutments, removable denture frameworks, crowns, posts, prostheses, orthodontic archwires, and periodontal splints [2–6]. However, PEEK is primarily used for fixed dentures and removable denture frameworks [7].
In fixed dentures, despite its numerous excellent properties, PEEK is grayish white, which is substantially different from the natural tooth color [8, 9]. To overcome PEEK’s aesthetic drawbacks, a layer of composite resin is applied as a veneer to the surface of PEEK-based crowns in the anterior region of the mouth [10]. Because adhesives must be used to bond PEEK-based fixed restorations to abutment teeth, bonding must be strengthened between PEEK and resin adhesives for prolonging restorations’ lifespans [11]. In removable denture frameworks, strong bonding between the PEEK framework and polymethyl methacrylate (PMMA) helps to extend the lifespans of removable dentures [12].
Because PEEK is inert, conventional methods often fail to ideally bond PEEK with both composite resins and PMMA [13–15]. Currently, sandblasting, laser ablation, and concentrated H2SO4 are used to roughen PEEK surfaces, which increases the bonding contact area and, thereby, strengthens bonds between PEEK and both composite resins and PMMA [16–18]. Notably, however, these physical modifications do not introduce chemical bonds at the interface between PEEK and other materials [19]. Previously, we found that sodium borohydride (NaBH4) reduced the PEEK molecular chain’s carbonyl groups to hydroxyl groups, introducing interfacial hydroxyl functional groups that were subsequently silanized to chemical bonds [20]. Therefore, this dual physicochemical modification significantly strengthened PEEK’s shear bonding.
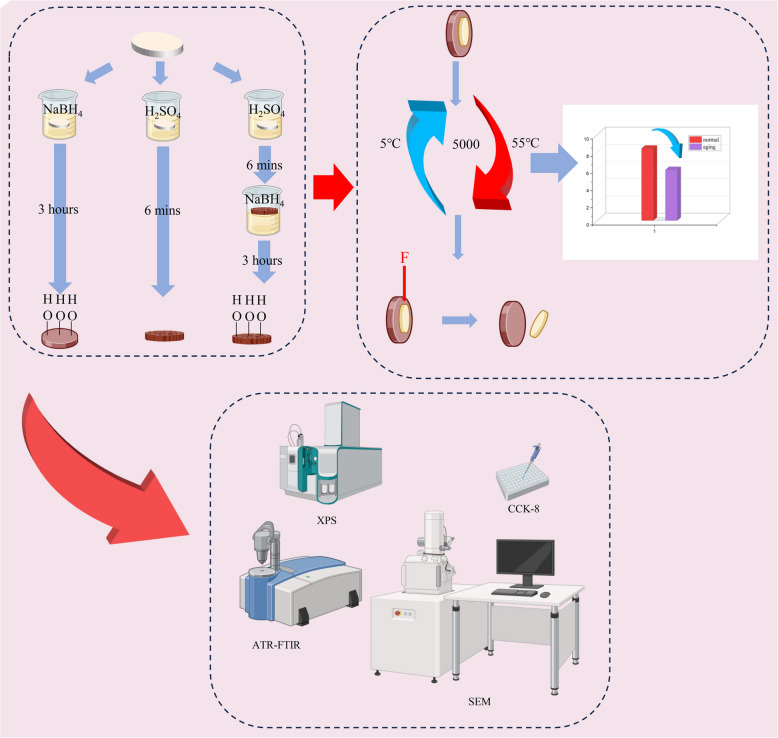
In this study, we used concentrated-H2SO4-induced sulfonation and NaBH4, both individually and sequentially, to modify PEEK surfaces and investigated the effects of the chemical bonding and structural changes on the bonding strengths between PEEK and both composite resins and PMMA. Additionally, to provide scientific evidence for the practical application of PEEK-based materials in biomedicine, the effects of the surface modifications on PEEK’s biocompatibility were investigated. For this study, the null hypotheses are that the different surface treatments do not strengthen the bonding between PEEK and either composite resins or PMMA and do reduce PEEK’s mechanical strength and biocompatibility (Fig. 1).
Fig. 1.
Schematic diagram of this study
Materials and methods
PEEK specimens (Shenzhen Zhihang Plastic & Metal Products Co., Ltd., Shenzhen, China), fluid resin (DenFil™ Flow, Vericom Co., Ltd., Cheonan City, South Korea), PMMA powder(New Century Dental, Type II, Shanghai, China), PMMA liquid(New Century Dental, Type II, Shanghai, China), silane coupling agent KH-570 (Kangjin New Material Technology Co., Ltd., KANG CHEMICAL, Dongguan, China), DMSO (Shanghai Mecklin Biochemical Technology Co., Ltd., Shanghai, China), concentrated H2SO4 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), NaBH4 (Sinopharm Chemical Reagent Co., Ltd., Beijing, China), anhydrous ethanol (Sinopharm Chemical Reagent Co., Ltd., Beijing, China), superhard gypsum(Die Stone, Heraeus, Bunkyo Ward, Tokyo), UV curing lamp(Demi™ Ultra, Kerr Corporation, Orange, USA), scanning electron microscope (Nova™ NanoSEM, FEI, Hillsboro, USA), X-ray photoelectron spectroscopy (XPS) (Thermo Scientific™ K-Alpha™, Waltham, USA), infrared spectrometer (Nicolet 200SXV, Waltham, USA), electronic universal testing machine (SANS–CMT4503, Shenzhen SANS Co., Ltd., Shenzhen, China), thermal cycling instrument, CCK-8 reagent kit (Shanghai Biyuntian Biotechnology Co., Ltd., shanghai, China), microplate reader (Cytation5, BioTek, Winooski, USA), 4',6-diamidino-2-phenylindole (DAPI) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China).
Specimen grouping
This study employed a strictly controlled grouping design (Table 1). A total of 160 PEEK specimens were numbered using a random number table and divided into two main experimental groups: PEEK-composite resin bonding group (n = 80) and PEEK-PMMA bonding group (n = 80). Each group was further stratified into normal (n = 40) and aged subgroups (n = 40) using block randomization, with the aged subgroup undergoing 5000 thermal cycles (5–55 °C) to simulate six months of clinical service. Each subgroup contained five treatment subgroups (n = 8) as follows: P-PEEK group: no treatment; D-PEEK group: heated in DMSO solution at 120 °C for 3 h; B-PEEK group: heated in DMSO solution containing NaBH4 at 120 °C for 3 h; S-PEEK group: sulfonated in 98% concentrated H2SO4 for 6 min; BS-PEEK group: sulfonated in 98% concentrated H2SO4 for 6 min, then heated in DMSO solution containing NaBH4 at 120 °C for 3 h. DMSO was used as the solvent for NaBH4 to investigate whether DMSO itself affects the PEEK surface, thus the D-PEEK group was set to exclude solvent interference. To ensure experimental consistency, PEEK specimens were polished sequentially with 400, 800, and 1500 grit sandpaper before surface treatment, and ultrasonic cleaned and dried after treatment (Table 2).
Table 1.
Grouping of PEEK Specimens
| Composite resin (n = 80) | Normal group (n = 40) | P-PEEK (n = 8) |
| D-PEEK (n = 8) | ||
| B-PEEK (n = 8) | ||
| S-PEEK (n = 8) | ||
| SB-PEEK (n = 8) | ||
| Aging group (n = 40) | P-PEEK (n = 8) | |
| D-PEEK (n = 8) | ||
| B-PEEK (n = 8) | ||
| S-PEEK (n = 8) | ||
| SB-PEEK (n = 8) | ||
| PMMA (n = 80) | Normal group (n = 40) | P-PEEK (n = 8) |
| D-PEEK (n = 8) | ||
| B-PEEK (n = 8) | ||
| S-PEEK (n = 8) | ||
| SB-PEEK (n = 8) | ||
| Aging group (n = 40) | P-PEEK (n = 8) | |
| D-PEEK (n = 8) | ||
| B-PEEK (n = 8) | ||
| S-PEEK (n = 8) | ||
| SB-PEEK (n = 8) |
Table 2.
Chemical bond content before and after surface treatment for P-PEEK, D-PEEK, B-PEEK, S-PEEK, and SB-PEEK
| Spectra | Chemical bonds | P-PEEK | D-PEEK | B-PEEK | S-PEEK | SB-PEEK |
|---|---|---|---|---|---|---|
| C1 s | C = O | 3.27% | 3.68% | 1.21% | 2.94% | 1.06% |
| C-O | 6.51% | 5.82% | 10.25% | 6.23% | 12.37% | |
| C-H | 90.22% | 90.5% | 88.54% | 91.83% | 86.57% | |
| O1 s | O = C | 26.43% | 28.68% | 17.88% | 26.56% | 18.63% |
| O-C | 73.57% | 71.32% | 78.6% | 73.44% | 76.73% | |
| O–H | 0 | 0 | 3.52% | 0 | 4.64% |
Silanization of specimens
The silane coupling solution was prepared using an optimized protocol: Deionized water and anhydrous ethanol were precisely mixed at 1:19 (v/v) in a glass beaker, followed by addition of 2 wt% KH-570 silane coupling agent. Based on the silane hydrolysis kinetics theory proposed by Plueddemann [21], the system pH was carefully adjusted to 4–5 using lactic acid—a critical parameter ensuring optimal methoxy group (-OCH₃) hydrolysis to reactive silanols (Si–OH) with a half-life of 15–20 min. This pH range achieves complete hydrolysis while preventing solution gelation from rapid condensation under strongly acidic conditions (pH < 3). The solution was magnetically stirred at 500 rpm for 30 min (25 ± 1 °C) to complete hydrolysis, after which pretreated PEEK specimens were immersed and stirred for additional 30 min to ensure sufficient contact with active silanol species. Finally, samples were cured in a 60 °C forced-air oven for 2 h to form stable Si–O-C covalent bonds at the interface through condensation between silanol groups and PEEK surface hydroxyls.
Scanning electron microscopy (SEM) testing
Surface morphology of PEEK samples treated by the five methods was observed using a scanning electron microscope. The PEEK samples were sputter-coated with gold and observed for surface and cross-sectional morphology at an acceleration voltage of 10–20 kV.
X-ray photoelectron spectroscopy (XPS)
Changes in the types and quantities of surface functional groups on PEEK before and after modification were measured using an X-ray photoelectron spectroscopy (Thermo Scientific™ K-Alpha™, USA). In this experiment, monochromatic Al Kα radiation (1486.6 eV) was used as the X-ray source, and the C1 s peak at 284.8 eV was used to calibrate the contaminant carbon peak in the air.
Attenuated total reflectance fourier-transform infrared spectroscopic analysis
To detect whether the silane coupling agent was grafted onto the PEEK surface, ATR-FTIR was used to analyze the Si–O-C chemical bond. The spectra of surface-modified PEEK were collected on a Nicolet 200SXV infrared spectrometer equipped with an ATR attachment. The spectra were collected in the 4000 cm⁻1 to 400 cm⁻1 wavelength range with a resolution of 4 cm⁻1, and 16 scans were performed for each sample.
Mechanical property testing
The mechanical properties of PEEK strips were assessed using a three-point bending test to evaluate the impact of surface treatment. According to GB/T 9341–2008 standards, the PEEK strip dimensions were 80 × 10 × 4 mm, with 5 specimens per group. The width and thickness of each strip were accurately measured using a caliper, and the average value from three measurements was taken for the calculation. A loading rate of 2 mm/min and a span of 60 mm were set for testing. The bending strength and modulus were calculated using the formulas below, and the results were averaged over the five specimens in each group (Fig. 2).
| 1 |
Fig. 2.
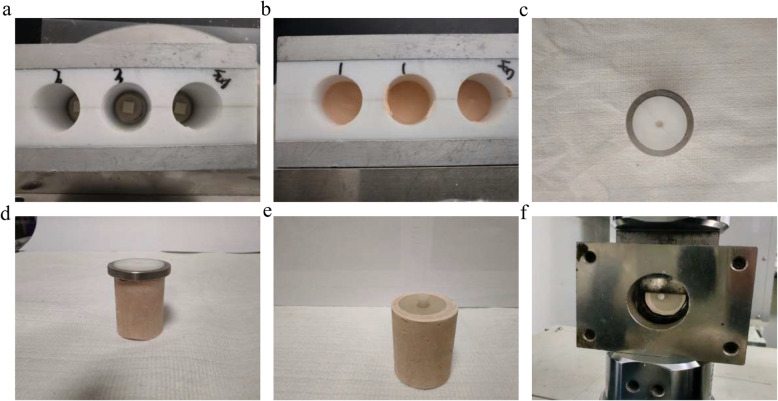
Shear Bond Strength Bonding Specimen Preparation Process. a Specimen placed on the central bottom of the mold; b Pouring superhard gypsum; c PTFE bonding mold; d Bonding resin rod to the PEEK specimen surface; e Finished bonding specimen; f Shear bond strength testing of the bonding specimen
P: Bending Strength (MPa)
F: Applied load (N)
L: Support span (mm)
B: Specimen width (mm).
H: Specimen thickness (mm).
| 2 |
E: Bending Modulus (GPa)
: Stress variation (MPa)
: Strain variation
Bonding strength testing
Preparation of bonding specimens
All PEEK specimens were immersed in the prepared silane coupling agent solution and stirred for 30 min, then dried in a vacuum oven at 60 °C. The specimens were bonded to composite resin and PMMA to prepare the bonding specimens, as described in Figs. 1, 2, and 3.
Fig. 3.

Bonding specimen failure mode diagram
A cylindrical mold (diameter = 3 mm, height = 4 mm) was placed on the PEEK specimen. The composite resin specimen preparation process is as follows: the composite resin was injected into a PTFE mold, forming a resin rod with a diameter of 3 mm and height of 4 mm. The resin rod was cured by UV light for 40 s, then carefully removed from the mold after 30 min, and the surrounding resin was cured with UV light for 20 s at each side. The PMMA specimen preparation process is as follows: the PMMA powder and liquid were mixed in the appropriate ratio, stirred evenly, and then injected into a PTFE mold to form a PMMA rod with a diameter of 3 mm and height of 4 mm. The mold was carefully removed after 30 min. The prepared bonding samples were stored in a 37 °C water bath for 24 h.
Aging treatment
Half of the prepared bonding specimens underwent 5000 thermal cycles between (5 °C-55 °C), with a dwell time of 30 s in each temperature chamber.
Shear bond strength
All bonding specimens were subjected to shear strength testing using a universal testing machine, with the loading head aligned and tightly attached to the bonding surface. The loading head moved at a speed of 1 mm/min, and the force (F) was recorded when the resin rod detached. Shear bond strength (SBS) was calculated using the following formula:
| 3 |
F: Maximum load at failure (N)
S: Bonding area (mm2)
SBS: Shear bond strength (MPa)
Failure mode analysis
The failure mode of the specimens after shear bond strength testing was analyzed (Fig. 3). Failure modes were classified as follows:
①Interface failure: complete separation of composite resin from PEEK or PMMA from PEEK;
②Cohesive failure: more than 50% of the composite resin and PMMA internal layer area is damaged;
③Mixed failure: less than 50% of the composite resin and PMMA internal layer area is damaged [22].
Cytotoxicity testing
Cell-counting kit-8 cytotoxicity testing
PEEK specimens from P-PEEK, D-PEEK, B-PEEK, S-PEEK, and SB-PEEK were fabricated into 10 mm × 5 mm × 3 mm pieces. After polishing, the specimens were sequentially sonicated in acetone, anhydrous ethanol, and deionized water for 30 min, dried, and then irradiated on both sides with UV light for 30 min. The extract was prepared according to the national standard GB/T 16886.12, incubated at 37 °C and 5% CO2 for 24 h, filtered through a filter, and stored at 4 °C. On days 1, 4, and 7 after changing the extract, 100 μL of DMEM and 10 μL of CCK-8 reagent were added to each well. Absorbance (OD) values were measured at 450 nm using a microplate reader after 1 h, and the relative growth rate (RGR) was calculated using the following formula:
| 4 |
RGR: relative growth rate
ODsample: Optical Density of Sample
ODblank: Optical Density of Blank
4′,6-diamidino-2-phenylindole staining
Both sides of the PEEK specimens were irradiated with UV light for 30 min for disinfection. L929 cells in the logarithmic growth phase were inoculated into 24-well plates at a density of 2 × 104 cells per well, and incubated at 37 °C with 5% CO2 for 24 h. After fixation with paraformaldehyde for 20 min, PBS washing was performed, and the cells were stained with DAPI for 15 min. Fluorescence microscopy was used for observation.
Statistical analysis
The data were analyzed using SPSS 22.0 software. Due to non-normal distribution and unequal variance, the Kruskal–Wallis H test was employed to assess group differences, followed by pairwise comparisons with the Mann–Whitney U test using Bonferroni correction. The chi-square test was used to analyze failure mode data, with the significance level set at P < 0.05.
Results
Scanning electron microscopic analysis
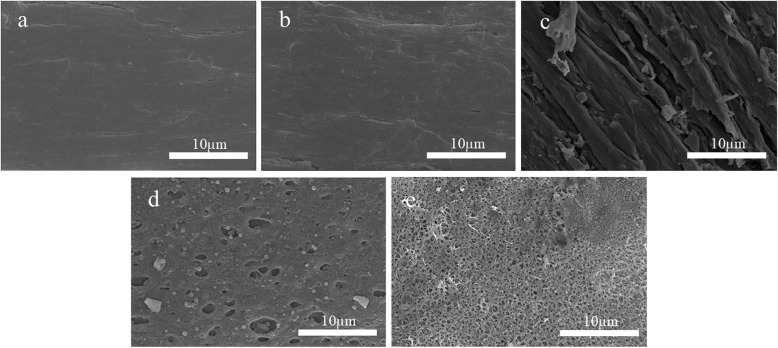
Figure 4 shows the front-facing scanning electron microscopy (SEM) images of the surface-treated PEEK specimens. In Fig. 4a and 4b, the PEEK surfaces are relatively smooth. In Fig. 4c, the NaBH4-treated PEEK surface possesses a fibrous etched structure. In Fig. 4d, the concentrated-H2SO4-treated PEEK surface possesses dense micro/nanopores, which diameters range from 200 nm to 4 µm. In Fig. 4e, the PEEK surface sequentially treated with concentrated H2SO4 and NaBH4 possesses dense, penetrating 3D-mesh-like micro/nanopores, which diameters range from 50 nm to 5 µm.
Fig. 4.
SEM images of PEEK specimens after surface treatment by five methods (a) SEM of P-PEEK, b SEM of D-PEEK, c SEM of B-PEEK, d SEM of S-PEEK, e SEM of SB-PEEK
Because concentrated H2SO4 and NaBH4 both etched the PEEK surface, the morphologies and depths of the surface etchings were investigated using cross-sectional SEM, as shown in Fig. 5. The cross-sections in Fig. 5a and b show cutting lines without any etching. The cross-section of the NaBH4-treated PEEK (Fig. 5c) shows approximately 50–100 µm deep etching. Figure 5d shows the cross-section of the concentrated-H2SO4-treated PEEK. The surface was slightly swollen, revealing more prominent etching approximately 50–80 µm deep. Figure 5e shows the cross-section of the PEEK sequentially treated with concentrated H2SO4 and NaBH4. Compared with the other samples, the sequentially treated PEEK sample showed more obvious etching, approximately 80–100 µm deep.
Fig. 5.
Cross-sectional SEM images of PEEK specimens after surface treatment by five methods (a) cross-sectional SEM of P-PEEK, b cross-sectional SEM of D-PEEK, c cross-sectional SEM of B-PEEK, d cross-sectional SEM of S-PEEK, e cross-sectional SEM of SB-PEEK
XPS
The X-ray photoelectron spectroscopy (XPS) spectra were corrected based on the reference C 1 s peak at 284.8 eV, corresponding to air-contamination-related carbon. Figure 6 presents the C 1 s and O 1 s spectra of the pristine PEEK and the PEEK samples treated with dimethyl sulfoxide (DMSO), NaBH4, or concentrated H2SO4 or sequentially treated with NaBH4 and concentrated H2SO4 (P-, D-, B-, S-, and SB-PEEK, respectively). The C 1 s spectrum primarily fits C–H, C–O, and C = O peaks; the O 1 s spectrum fits O–H, O–C, and O = C peaks; the S 1 s spectrum primarily fits S–C, S–O, and S = O peaks. For the normal and treated PEEK samples, the relative contents of the chemical bonds were calculated based on the areas under these peaks (Table. 2).
Fig. 6.
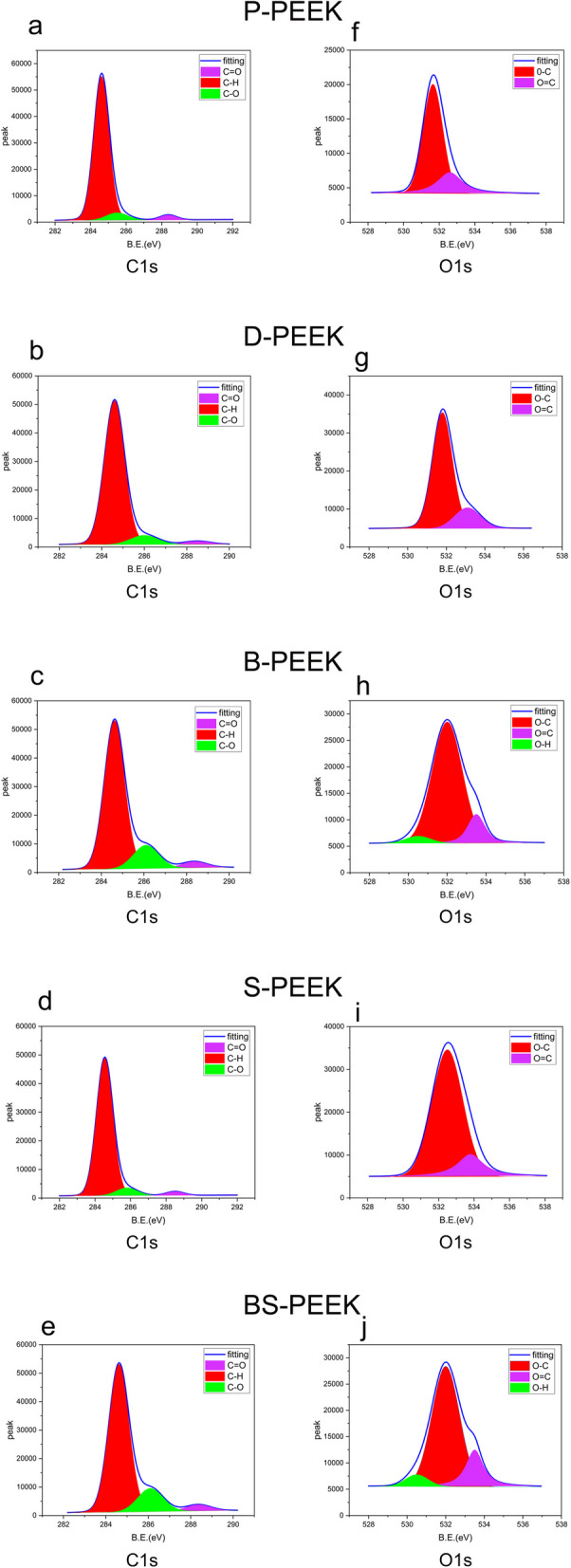
XPS spectra of PEEK specimens after surface treatments (a-e) represent the C1 spectra of P-PEEK, D-PEEK, B-PEEK, S-PEEK, and SB-PEEK, f-j represent the O1 spectra of the same specimens
In the C 1 s spectrum (Fig. 6), a C = O peak appears at 288.7 eV, and the relative C = O contents in P-, D-, and S-PEEK remain unchanged, ranging from approximately 2.94 to 3.68%. In B- and SB-PEEK, the relative C = O contents substantially decreased from approximately 1.06 to 1.21%, respectively. A C–O peak appears at 286 eV, and the relative C–O contents ranged from approximately 10.25 to 12.37% in B- and SB-PEEK, respectively, and from approximately 5.82 to 6.51% in P-, D-, and S-PEEK, indicating that C = O groups were gradually reduced to –OH groups on PEEK surfaces. The O 1 s spectrum supports this finding: a O = C peak appeared at 533.5 eV, and the relative O = C contents ranged from 26.43 to 28.68% in P-, D-, and S-PEEK and from 17.88 to 18.63% in B- and SB-PEEK, respectively. An O–H peak appears at 530 eV, and the relative O–H contents ranged from 3.52 to 4.64%, indicating that on PEEK surfaces, carbonyl groups were reduced to hydroxyl groups, generating O–H bonds. The C 1 s and O 1 s spectra suggest that DMSO did not alter the chemical bonds on PEEK’s surface and, therefore, can be used as a solvent.
Attenuated total reflectance fourier-transform infrared spectroscopic analysis
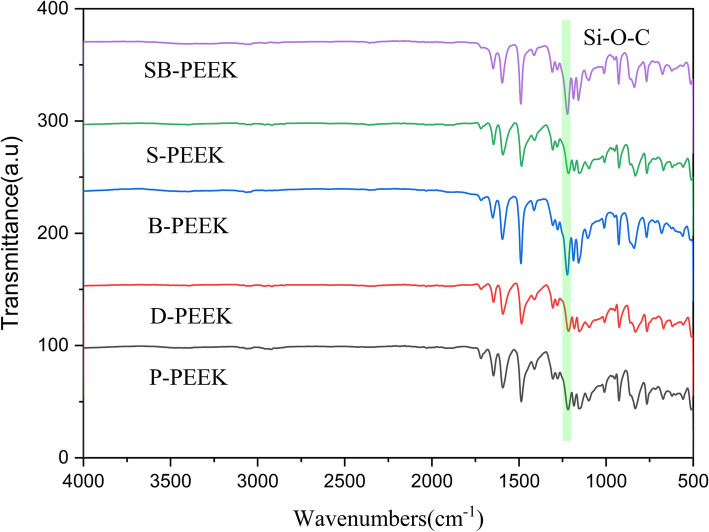
Figure 7 shows the attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectra of the silane-coupling-agent-coated PEEK surface. The peak at 1200 cm−1 corresponds to the Si–O–C bond. Clearly, the Si–O–C peak is substantially more intense for B- and SB-PEEK than for P-, D-, and S-PEEK because of the reaction between the B- and SB-PEEK surfaces’ hydroxyl groups and the silane coupling agent’s silanol group, forming Si–O–C bonds through dehydration condensation.
Fig. 7.
ATR-FTIR spectra of PEEK specimens treated with a silane coupling agent (a) P-PEEK ATR-FTIR, b D-PEEK ATR-FTIR, c B-PEEK ATR-FTIR, d S-PEEK ATR-FTIR, e SB-PEEK ATR-FTIR
Mechanical properties
Figure 8 shows the three-point bending test results for the PEEK specimens. No significant differences in the bending modulus or strength were observed between the control (P-PEEK) and experimental (D-, B-, S-, and SB-PEEK) groups (P > 0.05).
Fig. 8.
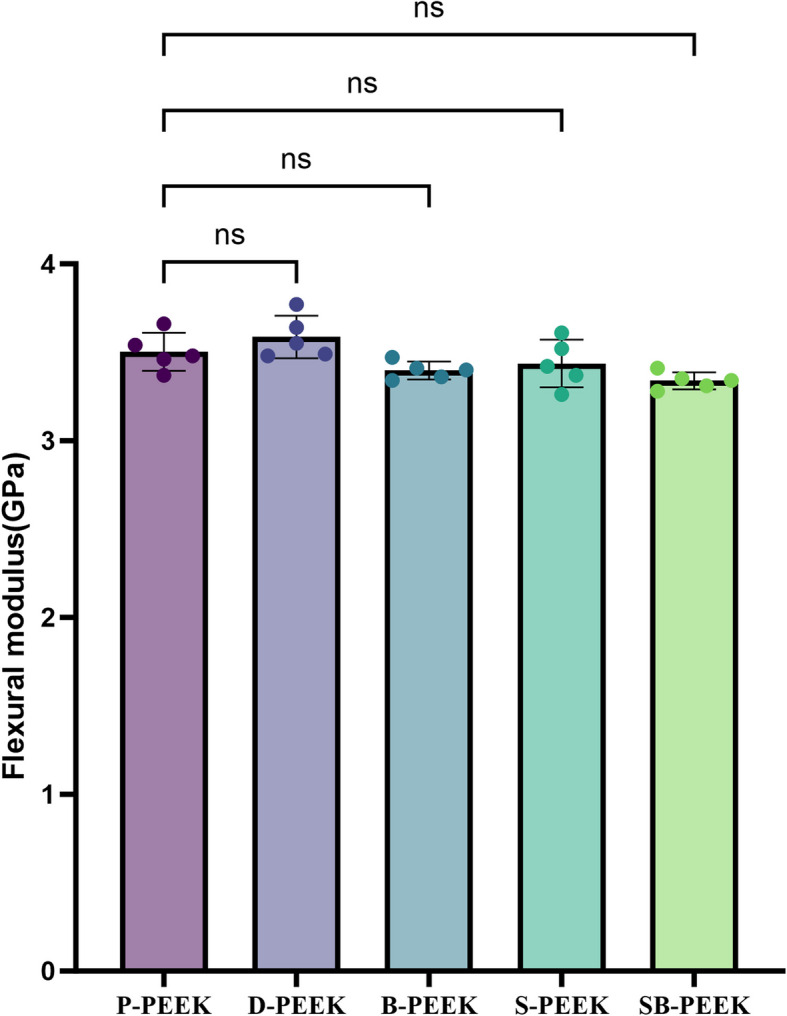
Three-point bending test results of PEEK specimens treated by five methods
Shear bond strengths of the normal and aged bonded peek specimens
Shear bond strengths of the normal bonded peek specimens
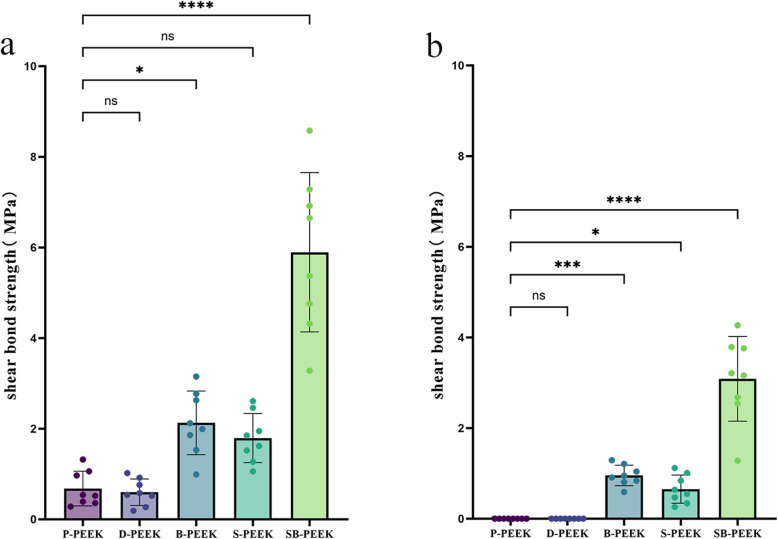
Figure 9a shows the shear bond strengths (SBSs) between PEEK and composite resins in the normal control (P-PEEK) and experimental (D-, B-, S-, and SB-PEEK) groups, which were 3.10 ± 0.25, 2.88 ± 0.56, 6.65 ± 0.98, 5.21 ± 0.72, and 10.34 ± 1.29 MPa, respectively. Figure 9b shows the SBSs between PEEK and PMMA in the control (P-PEEK) and experimental (D-, B-, S-, and SB-PEEK) groups, which were 1.45 ± 1.30, 1.43 ± 0.38, 3.74 ± 0.55, 3.41 ± 0.74, and 8.39 ± 1.11 MPa, respectively. Significant differences in SBSs were observed between the normal SB-, S-, and B-PEEK groups and the control (P-PEEK) group (P < 0.05), with SB-PEEK showing the strongest bonds. In the composite resin group, B-PEEK’s shear bond was significantly stronger than that of S-PEEK (P < 0.05). In the PMMA group, no significant difference in the SBS was observed between the B- and S-PEEK groups (P > 0.05).
Fig. 9.
Shear bond strength between PEEK and composite resin, and PEEK and PMMA (a) Shear bond strength between PEEK and composite resin; b Shear bond strength between PEEK and PMMA
Shear bond strengths of the aged bonded peek specimens
Figure 10a shows the SBSs between PEEK and composite resins in the aged control (P-PEEK) and experimental groups (D-, B-, S-, and SB-PEEK), which were 0.68 ± 0.38, 0.6 ± 0.29, 2.13 ± 0.70, 1.79 ± 0.54, and 5.89 ± 1.75 MPa, respectively. Aging significantly weakened the shear bonds. Figure 10b shows the SBSs between PEEK and PMMA in the corresponding aged specimens. Because the specimens in the P- and D-PEEK groups detached during aging, their SBSs were recorded as 0. The B-, S-, and SB-PEEK groups possessed SBSs of 0.96 ± 0.22, 0.65 ± 0.31, and 3.09 ± 0.93 MPa, respectively. Significant differences in SBSs were observed between the aged SB-, S-, and B-PEEK groups and the control (P-PEEK) group (P < 0.05), with SB-PEEK showing the strongest bonds.
Fig. 10.
Shear bond strength between PEEK and composite resin, and PEEK and PMMA after aging (a) Shear bond strength between PEEK and composite resin; b Shear bond strength between PEEK and PMMA
Failure mode analysis
Figure 11 shows the failure mode distributions of the SBSs between PEEK and both composite resins and PMMA. In the P- and D-PEEK groups (Fig. 11a and b, respectively), only interfacial failure occurred, while in the B-, S-, and SB-PEEK groups, mixed and cohesive failures both occurred. Chi-squared analysis revealed that in all the groups, the relationship between the SBS and failure mode was direct (P < 0.05).
Fig. 11.
Distribution of shear bond strength failure modes between PEEK and composite resin, and PEEK and PMMA (a) Shear bond strength failure mode distribution between PEEK and composite resin; b Shear bond strength failure mode distribution between PEEK and PMMA)
Cytotoxicity
CCK-8 cytotoxicity test
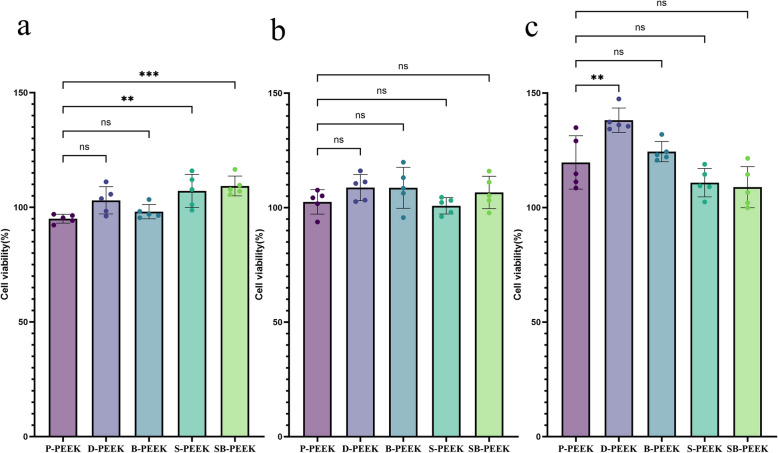
Figure 12 shows the cellular proliferation activities of L929 cells treated with PEEK specimens for 1, 4, and 7 days. These results indicate that for all the PEEK specimens (P-, D-, B-, S-, and SB-PEEK), cellular proliferation was between 90 and 135%, indicating that none of the PEEK specimens was cytotoxic to the cells. Therefore, the DMSO-, concentrated-H2SO4-, and NaBH4-treated PEEK specimens satisfied the biocompatibility requirements for practical application to oral materials.
Fig. 12.
CCK-8 cytotoxicity results for PEEK specimens treated by five methods after 1 day, 4 days, and 7 days
4′,6-diamidino-2-phenylindole staining analysis
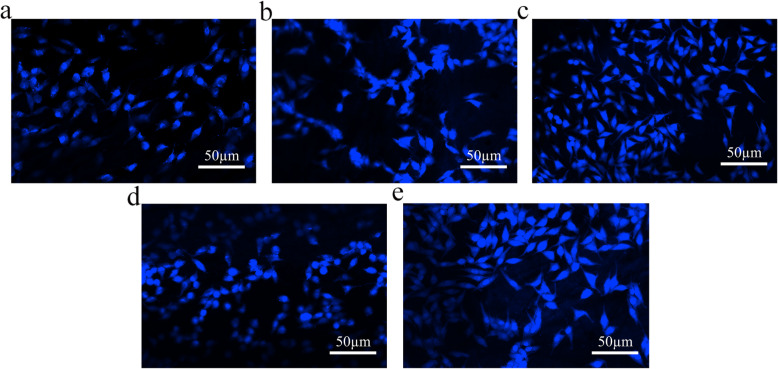
Figure 13 shows the 4′,6-diamidino-2-phenylindole (DAPI) staining results. L929 cells grew on all the materials, indicating that these materials obviously did not negatively impact cellular growth, which is consistent with the cell-counting kit-8 (CCK-8) assay results.
Fig. 13.
DAPI staining results for PEEK specimens treated by five methods
Discussion
Because of its excellent mechanical properties, chemical stability, and biocompatibility, PEEK, a semicrystalline thermoplastic polymer, has been widely applied in dentistry [23, 24]. However, the PEEK’s chemical inertness produces a low surface energy, hindering effective bonding between PEEK and other materials, which has become a critical issue in dental restorations and bonding applications [25, 26].
To overcome the PEEK surface’s inertness, research has mainly focused on altering the PEEK surface’s morphology through Physical modification methods, such as sandblasting, laser ablation, plasma treatment, and sulfonation, which mechanically roughen the PEEK surface, increasing the bonding contact area and enhancing the micromechanical interlocking effect [27–30]. Sandblasting is a simple, low-cost physical surface treatment. Although spraying aluminum oxide particles on the PEEK surface effectively roughens it and removes organic contaminants, it has a limited effect on strengthening PEEK’s bonding [31]. As a precise and efficient surface modification technique, localized high-energy laser ablation can cause carbonization and molecular chain crosslinking on the PEEK surface [32]. Several studies have shown that laser-ablated PEEK surfaces possess enhanced bonding; however, the precise control of the processing parameters is technically demanding, and surface carbonization may affect the material’s other physicochemical properties [33]. Plasma treatment is another effective physical surface modification method [34]. Plasma is an ionized gas generated by applying heat or electromagnetic fields to neutral gases. The plasma’s high-energy particles can break stable bonds and form active coatings on surfaces, which increase the material’s wettability and, thereby, strengthen its bonding [35]. However, the equipment’s and procedure’s complexities and high cost limit the widespread application of plasma treatment [18, 36]. Sulfonation is one of the most effective methods for physically modifying PEEK surfaces [37]. Concentrated H2SO4 penetrates the PEEK surface layer through strong ionization, dissolving low-molecular-weight PEEK chains and inducing swelling to form surface pores [38]. When the surface is rinsed with deionized water, the residual H2SO4 is removed, leaving behind variously sized pores, which substantially roughen the PEEK surface and increase bonding materials’ contact areas [39]. However, sulfonation’s surface modification mechanism is still primarily physical and does not introduce usable interfacial chemical bonds, hindering bond strengthening.
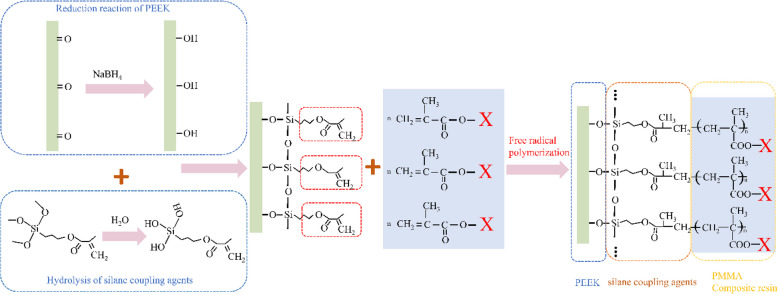
Physical modification of the PEEK surface can somewhat strengthen bonding; however, because of the lack of chemical bonding between PEEK and both composite resins and PMMA, the physically modified PEEK’s bonding is still not strong enough to satisfy clinical application requirements [40]. In our previous research, we introduced hydroxyl groups to the PEEK surface by sequentially treating it with NaBH4 and silane coupling agents to chemically bond PEEK and silicone rubbers. For silane coupling agents, the chemical formula is YSiX3, where Y represents an organic functional group possessing a structure similar to those of the composite resins and PMMA [41]. The structure of KH-570, used in this study, contains one methacryloxy group and three easily hydrolyzable methoxy groups [42]. Although PEEK exhibits inherent chemical inertness, surface treatments such as oxidation or plasma activation can introduce polar functional groups (e.g., -OH, -COOH) that provide reactive sites for subsequent chemical bonding with silane coupling agents [43]. The hydrolyzed trihydroxysilane (Si(OH)₃) form of γ-methacryloxypropyltrimethoxysilane (γ-MPS) undergoes condensation reactions with these surface functionalities, forming stable Si–O-C covalent bonds [34]. Furthermore, unreacted silanol groups can participate in additional dehydration condensation, creating a crosslinked Si–O-Si three-dimensional network that significantly enhances the coupling layer's stability and hydrolysis resistance. This reaction pathway is corroborated by characteristic FTIR absorption peaks in the 1000–1100 cm⁻1 region, corresponding to Si–O-C stretching vibrations. During bonding with resin materials (PMMA or composite resins), the methacryloyl group (-CH₂ = C(CH₃)COO-) of γ-MPS undergoes radical polymerization with residual C = C bonds in PMMA/Bis-GMA chains or resin monomers, establishing covalent bridges between PEEK and the organic matrix (Fig. 14) [44]. Our surface characterization results collectively demonstrate that silane coupling agents create stable covalent linkages at both interfaces—with the treated PEEK surface and the resin matrix—through a series of synergistic chemical reactions. This dual bonding mechanism not only enhances interfacial strength but also improves the overall chemical stability and long-term durability of the composite system. In this study, sulfonation, the most effective physical surface modification method, was used to modify the PEEK surface via a dual physicochemical treatment, where NaBH4 reduces the PEEK molecular chains’ carbonyl groups to hydroxyl groups, which are further chemically modified via silane coupling.
Fig. 14.
Schematic of silane coupling between PEEK and both composite resins and PMMA
The SEM images reveal that the NaBH4-treated PEEK surface appears fibrous. As a strong reducing agent, NaBH4 can break the aryl ether bonds, which, in turn, break PEEK’s molecular chains, reducing PEEK’s crystallinity and etching PEEK’s surface [45, 46]. This roughened surface increases the bonding contact area, enabling composite resins and PMMA to penetrate and mechanically interlock with PEEK’s fibrous structure. Additionally, on the PEEK surface, NaBH4 reduces the carbonyl groups to hydroxyl groups, which, because of the silane coupling agent, chemically bond with composite resins and PMMA. Concentrated H2SO4 only generates pores on PEEK’s surface, without forming any interfacial chemical bonds. The surface of the PEEK sequentially treated with concentrated H2SO4 and NaBH4 possesses loose, penetrating micro/nanopores. The sequentially treated PEEK formed the strongest shear bonds with composite resins and PMMA. Compared with NaBH4 alone, sequential treatment with concentrated H2SO4 and NaBH4 slightly swelled PEEK’s surface, widening the intermolecular spacing and enabling NaBH4 to cleave PEEK’s molecular chains and form loose pores [39]. The hydroxyl groups introduced to PEEK’s surface further strengthened PEEK’s physicochemical bonding. Moreover, in the normal (unaged) PEEK–composite resin bonding group, B-PEEK possessed significantly stronger shear bonds than S-PEEK (P < 0.05); however, in the normal PEEK–PMMA bonding group, no significant difference was observed between the SBSs of B- and S-PEEK (P > 0.05), which might be because liquid resin flows more easily than powdered PMMA mixed with liquid, enabling the resin to more effectively penetrate the NaBH4-etched fibrous structure. Additionally, because PMMA contains fewer double bonds than composite resins, it formed fewer crosslinks with the silane coupling agent, weakening the PEEK–PMMA interfacial bonding.
The decision to exclude commercial primers from our bonding protocol was based on comprehensive considerations. From a chemical bonding perspective, the dual functionality of KH-570-forming Si–O-C covalent bonds with PEEK surface hydroxyl groups through methoxysilane termini while copolymerizing with the resin matrix via methacryloxy groups—establishes a complete"chemical bridge"(Fig. 14). XPS analysis confirmed the formation of stable Si–O-C bonds on NaBH4-pretreated PEEK surfaces. Furthermore, this monolayer interface design prevents two potential issues associated with multi-step treatments: 1) interfacial stress concentration due to differential curing shrinkage rates between material layers [47]; and 2) chemical functional group redundancy leading to competitive polymerization between methacrylate groups in commercial primers and KH-570 [48]. We concur with the potential long-term stability advantages of MMA/UDMA systems, which will be systematically evaluated in subsequent aging experiments.
To simulate the temperature and moisture conditions of the oral environment, thermal cycling was employed for aging the bonding specimens over 5000 cycles, simulating six months of usage [49]. Thermal cycling affects bonding by possibly catalyzing further polymerization between the silane coupling agent and double-bond-bearing molecules in the composite resins and PMMA, thereby strengthening PEEK’s bonding; however, because PEEK, composite resins, and PMMA possess different thermal expansion coefficients, temperature changes cause cracks at the bonding interface, weaking PEEK’s bonding [50]. In this study, aging did not strengthen shear bonds between PEEK and both composite resins and PMMA. In fact, aging weakened these shear bonds. In the composite resin and PMMA groups, SB-PEEK’s shear bonds weakened from 10.34 and 8.39 to 5.89 and 3.09 MPa, respectively. The aged SB-PEEK group exhibited relatively large standard deviation in shear bond strength with composite resin after aging. We attribute this increased data variability to several factors: First, although the combined treatment markedly improves PEEK's surface roughness and chemical reactivity, the highly sensitive nature of these strong chemical reactions may introduce subtle inter-sample variations despite standardized protocols [51]. For instance, NaBH₄ as a strong reducing agent demonstrates sensitivity to temperature, reaction time and surface conditions, potentially leading to differences in etching depth and hydroxyl group introduction among specimens, ultimately affecting bonding consistency. Second, the treatment creates complex micro/nano-porous networks that enhance mechanical interlocking and chemical bonding, but the distribution and permeability of these structures may vary between samples [52]. During thermal cycling, such microstructural heterogeneity could lead to differential aging responses (e.g., moisture diffusion within pores, interfacial crack propagation), thereby increasing bond strength variability [53, 54]. Additionally, thermal stress effects may vary depending on interfacial structural differences among samples, influencing interface integrity and shear strength stability. These analyses suggest that while SB-PEEK demonstrates excellent bond strength, its relatively large standard deviation highlights the importance of modification consistency and long-term stability alongside performance enhancement. Future studies should focus on optimizing reaction parameters, standardizing procedures, employing automated processing to minimize human error, and increasing sample size to improve data reproducibility and statistical reliability.
For practical application to restorative materials, PEEK’s bonding must be strengthened while maintaining its mechanical strength [27]. To determine whether sulfonation, NaBH4, and their combination affect PEEK’s mechanical strength, we measured the three-point bending strength of each group and used SEM to measure the etching depths of the corresponding PEEK cross-sections. The three-point bending strength test results showed that sulfonation, NaBH4, and their combination did not reduce PEEK’s mechanical strength. The SEM images revealed that the etching depths of the NaBH4-, concentrated-H2SO4-, and sequentially treated PEEK were approximately 50–100, 50–80, and 80–100 µm, respectively, indicating that the surface treatments strengthened PEEK’s bonding without compromising its mechanical strength.
To evaluate the cytotoxicities of the surface-modified PEEK samples, we performed CCK-8 cellular proliferation assays and DAPI staining, both of which revealed that the sulfonated, NaBH4-treated, and sequentially treated PEEK samples were not cytotoxic to cells and, therefore, satisfy the requirements for oral clinical applications. The selection of L929 mouse fibroblasts for preliminary cytotoxicity assessment was based on multiple scientific rationales. As the gold-standard cell model recommended by ISO 10993–5, L929 cells offer significant technical advantages: their sensitivity to toxic substances and highly stable culture characteristics ensure reliable and reproducible experimental results [55, 56]. From a research design perspective, employing this standardized model during the initial screening phase of material development not only complies with international guidelines but also enables efficient and cost-effective preliminary safety evaluation [57]. It should be emphasized that while L929 cells effectively reflect basic cytotoxicity, their biological responses may differ from human oral microenvironment. Therefore, we have established a progressive validation system in our research protocol: following initial safety assessment, tissue-specific models including human osteoblasts (MG-63) and oral epithelial cells will be employed for functional verification. This simple-to-complex experimental strategy ensures both the efficiency and reliability of initial screening while establishing a solid foundation for subsequent clinical application research.
This study strictly adhered to the industry standard YY/T 0518–2009"Polymer-Based Adhesives for Dental Prostheses"during experimental design to ensure data reliability and reproducibility. It should be noted that while shear bond strength (SBS) testing, as recommended by ISO/TS 11405, is widely used for dental material evaluation, several methodological limitations warrant discussion [58]. First, uniaxial shear loading (1 mm/min) only reflects interfacial performance under a single mechanical condition, whereas clinical restorations experience complex multiaxial stresses (including combined shear, tensile and compressive loads), potentially leading to discrepancies between laboratory results and clinical performance. Second, the heterogeneous stress distribution in SBS testing may affect result accuracy. egarding aging protocols, while our 5000 thermal cycles (5–55 °C, 30 s dwell time) effectively simulate oral temperature variations (equivalent to 6 months of clinical service), two key limitations exist: 1) Failure to account for biofilm-induced interfacial degradation [59]; 2) Absence of dynamic mechanical loading [60]. A more comprehensive evaluation could be achieved by implementing the modified aging system for teeth (mAST) that combines multiple aging factors [61].
Conclusion
This study investigated the effects of different surface treatments on the SBSs between PEEK and both composite resins and PMMA. Sequential treatment with H2SO4 and NaBH4 significantly strengthened the bonding between PEEK and both composite resins and PMMA. The proposed low-cost and convenient method for modifying PEEK surfaces could promote PEEK’s broader application in oral medicine, enabling more improvements and breakthroughs in clinical dental treatments.
Acknowledgements
Thanks to the technical support of the McDermid Lab at Jilin University.
Authors’ contributions
Yongcheng Ge: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ting Zhao: Writing – original draft, Investigation, Data curation, Conceptualization. Sizheng Fan: Writing – review & editing, Methodology, Investigation. Pengyuan Liu: Writing – review & editing, Methodology. Xiaoqiu Liu: Writing – review & editing, Validation, Resources, Funding acquisition, Conceptualization.
Funding
This work was supported by Key Research and Development Program of the Ministry of Science and Technology of China (2023YFB4605405, 2022YFB3704600), the Scientific and Technological Development Scheme of Jilin Province (No.YDZJ202402060 CXJD), and the Jilin University Teaching Reform Program (2023XZD106).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zol SM, Alauddin MS, Said Z, Mohd Ghazali MI, Hao-Ern L, Mohd Farid DA, Zahari NAH, Al-Khadim AHA, Abdul Aziz AH. Description of poly(aryl-ether-ketone) materials (PAEKs), polyetheretherketone (PEEK) and polyetherketoneketone (PEKK) for application as a dental material: a materials science review. Polymers (Basel). 2023;15(9):2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gama LT, Bezerra AP, Schimmel M, Rodrigues Garcia RCM, de Luca CG, Gonçalves T. Clinical performance of polymer frameworks in dental prostheses: a systematic review. J Prosthet Dent. 2024;131(4):579–90. [DOI] [PubMed] [Google Scholar]
- 3.Chokaree P, Poovarodom P, Chaijareenont P, Yavirach A, Rungsiyakull P. Biomaterials and clinical applications of customized healing abutment-a narrative review. J Funct Biomater. 2022;13(4):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Yang C, Sun C, Yan X, He J, Shi C, Liu C, Li D, Jiang T, Huang L. Fused deposition modeling PEEK implants for personalized surgical application: from clinical need to biofabrication. Int J Bioprint. 2022;8(4):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valenti C, Federici MI, Coniglio M, Betti P, Pancrazi GP, Tulli O, Masciotti F, Nanussi A, Pagano S. Mechanical and biological properties of polymer materials for oral appliances produced with additive 3D printing and subtractive CAD-CAM techniques compared to conventional methods: a systematic review and meta-analysis. Clin Oral Investig. 2024;28(7):396. [DOI] [PubMed] [Google Scholar]
- 6.Qian B, Ji K, Lu W, Wu G, Tan B, Jing J, Ji J. Polyetherketoneketone, a high-performance polymer for splinting mobile teeth: a clinical report. J Prosthet Dent. 2025;133(1):1–7. [DOI] [PubMed] [Google Scholar]
- 7.Freitas R, Duarte S, Feitosa S, Dutra V, Lin WS, Panariello BHD, Carreiro A. Physical, mechanical, and anti-biofilm formation properties of CAD-CAM milled or 3D printed denture base resins: in vitro analysis. J Prosthodont. 2023;32(S1):38–44. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Fang M, Zhao R, Liu H, Li K, Tian M, Niu L, Xie R, Bai S. Clinical applications of polyetheretherketone in removable dental prostheses: accuracy, characteristics, and performance. Polymers (Basel). 2022;14(21):4615. [DOI] [PMC free article] [PubMed]
- 9.Kiliç M, Dede D, Küçükekenci AS. Comparing the shear bond strength of veneering materials to the PAEKs after surface treatments. BMC Oral Health. 2023;23(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soares Machado P, Cadore Rodrigues AC, Chaves ET, Susin AH, Valandro LF, Pereira GKR, Rippe MP. Surface treatments and adhesives used to increase the bond strength between polyetheretherketone and resin-based dental materials: a scoping review. J Adhes Dent. 2022;24:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gama LT, Duque TM, Özcan M, Philippi AG, Mezzomo LAM, Gonçalves T. Adhesion to high-performance polymers applied in dentistry: a systematic review. Dent Mater. 2020;36(4):e93–108. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi S, Alqahtani NM, Al-Qarni MA, Alqahtani SM, Suleman G, Yaqoob A, Abdul Khader M, Elsir Elmahdi A, Chaturvedi M. Evaluation of the methods for determining accuracy of fit and precision of RPD framework in Digital (3D printed, milled) and conventional RPDs - a systematic review. BMC Oral Health. 2024;24(1):1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dondani JR, Iyer J, Tran SD. Surface treatments of PEEK for osseointegration to bone. Biomolecules. 2023;13(3):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H, Liu X, Wang J, Suo M, Zhang J, Sun T, Wang H, Liu C, Li Z. Strategies to improve the performance of polyetheretherketone (PEEK) as orthopedic implants: from surface modification to addition of bioactive materials. J Mater Chem B. 2024;12(19):4533–52. [DOI] [PubMed] [Google Scholar]
- 15.Hata K, Komagata Y, Nagamatsu Y, Masaki C, Hosokawa R, Ikeda H. Bond strength of sandblasted PEEK with dental methyl methacrylate-based cement or composite-based resin cement. Polymers (Basel). 2023;15(8):1830. [DOI] [PMC free article] [PubMed]
- 16.Luo C, Liu Y, Peng B, Chen M, Liu Z, Li Z, Kuang H, Gong B, Li Z, Sun H. PEEK for oral applications: recent advances in mechanical and adhesive properties. Polymers (Basel). 2023;15(2):386. [DOI] [PMC free article] [PubMed]
- 17.Pidhatika B, Widyaya VT, Nalam PC, Swasono YA, Ardhani R. Surface modifications of high-performance polymer polyetheretherketone (PEEK) to improve its biological performance in dentistry. Polymers (Basel). 2022;14(24):5526. [DOI] [PMC free article] [PubMed]
- 18.Han X, Sharma N, Spintzyk S, Zhou Y, Xu Z, Thieringer FM, Rupp F. Tailoring the biologic responses of 3D printed PEEK medical implants by plasma functionalization. Dent Mater. 2022;38(7):1083–98. [DOI] [PubMed] [Google Scholar]
- 19.Yu D, Lei X, Zhu H. Modification of polyetheretherketone (PEEK) physical features to improve osteointegration. J Zhejiang Univ Sci B. 2022;23(3):189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noiset O, Henneuse C, Schneider Y-J, Marchand-Brynaert J. Surface reduction of poly(aryl ether ether ketone) film: UV spectrophotometric, 3H radiochemical, and x-ray photoelectron spectroscopic assays of the hydroxyl functions. Macromolecules. 1997;30(3):540–8. [Google Scholar]
- 21.Plueddemann EP. Silane coupling agents. 2nd. Springer US; 1991.
- 22.Peng TY, Shimoe S, Higo M, Kato M, Hirata I, Iwaguro S, Kaku M. Effect of laser engraving on shear bond strength of polyetheretherketone to indirect composite and denture-base resins. J Dent Sci. 2024;19(1):32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Zhang W, Yang M, Mengqi L, Zhou L, Liu Y, Liu L, Zheng Y. Comprehensive review of polyetheretherketone use in dentistry. J Prosthodont Res. 2025;69(2):215–32. [DOI] [PubMed]
- 24.Hao Y, Shi C, Zhang Y, Zou R, Dong S, Yang C, Niu L. The research status and future direction of polyetheretherketone in dental implant -a comprehensive review. Dent Mater J. 2024;43(5):609–20. [DOI] [PubMed] [Google Scholar]
- 25.Cassari L, Zamuner A, Messina GML, Marsotto M, Chen H, Gonnella G, Coward T, Battocchio C, Huang J, Iucci G, et al. Bioactive PEEK: surface enrichment of vitronectin-derived adhesive peptides. Biomolecules. 2023;13(2):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Deeb L, Almohareb T, Al Ahdal K, Maawadh A, Alshamrani AS, Alrahlah A. The impact of PEEK pretreatment using H2SO4, riboflavin, and aluminum trioxide on the extrusion bond strength to canal dentin luted with Polymethyl methacrylate and resin-based composite cement. Eur Rev Med Pharmacol Sci. 2023;27(20):9639–47. [DOI] [PubMed] [Google Scholar]
- 27.Adali U, Sütel M, Yassine J, Mao Z, Müller WD, Schwitalla AD. Influence of sandblasting and bonding on the shear bond strength between differently pigmented polyetheretherketone (PEEK) and veneering composite after artificial aging. Dent Mater. 2024;40(8):1123–7. [DOI] [PubMed] [Google Scholar]
- 28.Luo F, Mao R, Huang Y, Wang L, Lai Y, Zhu X, Fan Y, Wang K, Zhang X. Femtosecond laser optimization of PEEK: efficient bioactivity achieved by synergistic surface chemistry and structures. J Mater Chem B. 2022;10(36):7014–29. [DOI] [PubMed] [Google Scholar]
- 29.Chen T, Xu S, Chen X, Wang D, Liu C, Liu H. Effects of Nd: YAG LASER irradiation and O(2) plasma on the adhesive performance of poly-ether-ether-ketone (PEEK). J Mech Behav Biomed Mater. 2024;152: 106461. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Ma T, Liu X, Zhang X, Meng W, Wu J. Multifunctional surface of the nano-morphic PEEK implant with enhanced angiogenic, osteogenic and antibacterial properties. Regen Biomater. 2024;11:rbae067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shabib S. Use of Nd:YVO(4) laser, photodynamic therapy, sulfuric acid and sand blasting on improving bond integrity of PEEK to resin cement with adhesive. Photodiagnosis Photodyn Ther. 2022;39: 102865. [DOI] [PubMed] [Google Scholar]
- 32.Luo F, Li D, Huang Y, Mao R, Wang L, Lu J, Ge X, Fan Y, Zhang X, Chen Y, et al. Efficient osteogenic activity of PEEK surfaces achieved by femtosecond laser-hydroxylation. ACS Appl Mater Interfaces. 2023;15(31):37232–46. [DOI] [PubMed] [Google Scholar]
- 33.Henriques B, Fabris D, Mesquita-Guimarães J, Sousa AC, Hammes N, Souza JCM, Silva FS, Fredel MC. Influence of laser structuring of PEEK, PEEK-GF30 and PEEK-CF30 surfaces on the shear bond strength to a resin cement. J Mech Behav Biomed Mater. 2018;84:225–34. [DOI] [PubMed] [Google Scholar]
- 34.Ma T, Zhang J, Liu X, Sun S, Wu J. Effects of combined modification of sulfonation, oxygen plasma and silane on the bond strength of PEEK to resin. Dent Mater. 2024;40(4):e1–11. [DOI] [PubMed] [Google Scholar]
- 35.Primc G. Strategies for improved wettability of polyetheretherketone (PEEK) polymers by non-equilibrium plasma treatment. Polymers (Basel). 2022;14(23):5319. [DOI] [PMC free article] [PubMed]
- 36.Bötel F, Zimmermann T, Sütel M, Müller WD, Schwitalla AD. Influence of different low-pressure plasma process parameters on shear bond strength between veneering composites and PEEK materials. Dent Mater. 2018;34(9):e246–54. [DOI] [PubMed] [Google Scholar]
- 37.Yang C, Zhu K, Cheng M, Yuan X, Wang S, Zhang L, Zhang X, Wang Q. Graphene oxide-decorated microporous sulfonated polyetheretherketone for guiding osteoporotic bone regeneration. J Control Release. 2024;374:15–27. [DOI] [PubMed] [Google Scholar]
- 38.Escobar M, Souza JCM, Barra GMO, Fredel MC, Özcan M, Henriques B. On the synergistic effect of sulfonic functionalization and acidic adhesive conditioning to enhance the adhesion of PEEK to resin-matrix composites. Dent Mater. 2021;37(4):741–54. [DOI] [PubMed] [Google Scholar]
- 39.Xie M, Xiao GY, Song ZG, Lu YP. The formation process and mechanism of the 3D porous network on the sulfonated PEEK surface. ACS Appl Mater Interfaces. 2024;16(11):13585–96. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Bai S, Liu H, Li K, Zhong S, Li M, Chen L, Tian M, Niu L, Fang M. Effect of different surface treatments on PEEK-enamel bonds: bonding durability and mechanism. J Prosthet Dent. 2025;133(3):892.e1–892.e10. [DOI] [PubMed]
- 41.Asakura M, Aimu K, Hayashi T, Matsubara M, Mieki A, Ban S, Kawai T. Bonding characteristics of silane coupling agent and MMA-containing primer to various composite CAD/CAM blocks. Polymers (Basel). 2023;15(16):3396. [DOI] [PMC free article] [PubMed]
- 42.Chaijareenont P, Takahashi H, Nishiyama N, Arksornnukit M. Effects of silane coupling agents and solutions of different polarity on PMMA bonding to alumina. Dent Mater J. 2012;31(4):610–6. [DOI] [PubMed] [Google Scholar]
- 43.Noiset O, Schneider YJ, Marchand-Brynaert J. Adhesion and growth of CaCo2 cells on surface-modified PEEK substrata. J Biomater Sci Polym Ed. 2000;11(7):767–86. [DOI] [PubMed] [Google Scholar]
- 44.Kanie T, Arikawa H, Fujii K, Inoue K. Physical and mechanical properties of PMMA resins containing gamma-methacryloxypropyltrimethoxysilane. J Oral Rehabil. 2004;31(2):166–71. [DOI] [PubMed] [Google Scholar]
- 45.Wu WB, Huang JM. Electrochemical cleavage of aryl ethers promoted by sodium borohydride. J Org Chem. 2014;79(21):10189–95. [DOI] [PubMed] [Google Scholar]
- 46.Díez-Pascual AM, Martínez G, Gómez MA. Synthesis and characterization of poly(ether ether ketone) derivatives obtained by carbonyl reduction. Macromolecules. 2009;42(18):6885–92. [Google Scholar]
- 47.Lin D, Richard Liu C, Cheng GJ. Single-layer graphene oxide reinforced metal matrix composites by laser sintering: microstructure and mechanical property enhancement. Acta Mater. 2014;80:183–93. [Google Scholar]
- 48.Delgado AHS, Owji N, Ashley P, Young AM. Varying 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) level improves polymerisation kinetics and flexural strength in self-adhesive, remineralising composites. Dent Mater. 2021;37(9):1366–76. [DOI] [PubMed] [Google Scholar]
- 49.Gouda A, Sherif A, Wahba M, Morsi T. Effect of veneering material type and thickness ratio on flexural strength of bi-layered PEEK restorations before and after thermal cycling. Clin Oral Investig. 2023;27(6):2629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bähr N, Keul C, Edelhoff D, Eichberger M, Roos M, Gernet W, Stawarczyk B. Effect of different adhesives combined with two resin composite cements on shear bond strength to polymeric CAD/CAM materials. Dent Mater J. 2013;32(3):492–501. [DOI] [PubMed] [Google Scholar]
- 51.Qiu P, Bennani V, Cooper P, Dias G, Ratnayake J. Surface chemistry on PEEK surfaces: from enhanced biofunctionality to improved surface modifiability. Appl Mater Today. 2024;41: 102523. [Google Scholar]
- 52.Souza JCM, Raffaele-Esposito A, Carvalho O, Silva F, Özcan M, Henriques B. Surface modification of zirconia or lithium disilicate-reinforced glass ceramic by laser texturing to increase the adhesion of prosthetic surfaces to resin cements: an integrative review. Clin Oral Investig. 2023;27(7):3331–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.da Costa JA, Akhavan-Safar A, Marques EAS, Carbas RJC, da Silva LFM. The influence of cyclic ageing on the fatigue performance of bonded joints. Int J Fatigue. 2022;161: 106939. [Google Scholar]
- 54.Mohsen CA. Effect of surface roughness and thermal cycling on bond strength of C.P. titanium and Ti-6Al-4V alloy to ceramic. J Prosthodont Res. 2012;56(3):204–9. [DOI] [PubMed] [Google Scholar]
- 55.Kovrlija I, Menshikh K, Abreu H, Cochis A, Rimondini L, Marsan O, Rey C, Combes C, Locs J, Loca D. Challenging applicability of ISO 10993–5 for calcium phosphate biomaterials evaluation: Towards more accurate in vitro cytotoxicity assessment. Biomater Adv. 2024;160: 213866. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Zhou J, Pavanram P, Leeflang MA, Fockaert LI, Pouran B, Tümer N, Schröder KU, Mol JMC, Weinans H, et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018;67:378–92. [DOI] [PubMed] [Google Scholar]
- 57.Janjić K, Valentova A, Arellano S, Unterhuber A, Krause A, Oberoi G, Unger E, Tabrizi HAS, Schedle A. The impact of print orientation and graphene nanoplatelets on biaxial flexural strength and cytotoxicity of a 3D printable resin for occlusal splints. Dent Mater. 2024;40(11):1742–52. [DOI] [PubMed] [Google Scholar]
- 58.Staxrud F, Dahl JE. Silanising agents promote resin-composite repair. Int Dent J. 2015;65(6):311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y, Matin K, Shimada Y, Sadr A, Wang G, Tagami J, Feng X. Characteristics of biofilm-induced degradation at resin–dentin interfaces using multiple combinations of adhesives and resins. Dent Mater. 2021;37(8):1260–72. [DOI] [PubMed] [Google Scholar]
- 60.Szczesio-Wlodarczyk A, Fronczek M, Ranoszek-Soliwoda K, Grobelny J, Sokolowski J, Bociong K. 135 - the first step in standardizing an artificial aging protocol for dental composites. Dent Mater. 2023;39:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano DE. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24(1):90–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.