Abstract
Introduction
Knee osteoarthritis (KOA) is highly prevalent, and platelet-rich plasma (PRP) therapy has emerged as a novel treatment option. Although PRP is promising, its effectiveness remains controversial. In Japan, PRP therapy is administered as a self-funded treatment in specialized clinics. The National Regenerative Medicine Database (NRMD) was established to support the evaluation of regenerative therapies. This study aimed to analyze patient characteristics and treatment outcomes of PRP therapy for KOA in our hospital registered in NRMD database and to identify factors that influence therapeutic effectiveness.
Methods
This retrospective observational study analyzed PRP treatment data for KOA in our hospital from January 2020 to May 2024 which was registered in the NRMD. Leukocyte-poor PRP (LP-PRP) was prepared using the MyCells kit, and patients received three injections at 3- to 5-week intervals. The eligibility criteria included chronic knee pain unresponsive to conservative treatment for KOA. Visual Analog Scale (VAS) for knee pain were collected at baseline and 3 and 6 months post-treatment. Statistical analyses included paired t-tests, logistic regression, and one-way analysis of variance (ANOVA).
Results
A total of 2068 patients (2815 knees) were included. The average age was 65.2 years, and there were more female than male patients. PRP therapy significantly improved the VAS scores from 53.5 at baseline to 35.8 at 6 months (P < 0.05). Patients with Kellgren-Lawrence (KL) grade 4 showed less improvement than those with KL grades 2 and 3. Blood neutrophil levels positively correlated with pain severity. VAS improvement negatively correlated with age and body mass index (BMI). No major adverse events were reported during the study period.
Conclusion
PRP therapy for KOA, as observed in the clinical setting in Japan, is a safe treatment option with significant pain reduction, particularly in patients with less severe deformity of KOA. However, its effectiveness decreases in severe deformity cases. These real-world clinical data provide valuable insights for refining the patient selection criteria and advancing KOA treatment strategies.
Keywords: Platelet rich plasma, Knee osteoarthritis, National regenerative medicine database in Japan, Real-world data
Abbreviations
- KOA
Knee osteoarthritis
- PRP
Platelet-rich plasma
- NRMD
National Regenerative Medicine Database
- LP-PRP
Leukocyte-poor PRP
- VAS
Visual Analog Scale
- KL
Kellgren-Lawrence
- BMI
Body mass index
- MHLW
Ministry of Health, Labour and Welfare
- AMED
Japan Agency for Medical Research and Development
- PMDA
Pharmaceuticals and Medical Devices Agency
- ANOVA
Analysis of Variance
- MA
Mechanical axis
- MCID
Minimal Clinically Important Difference
- FTA
Femorotibial angle
- RWD
Real-world data
- RCT
Randomized controlled trial
- AAOS
American Academy of Orthopaedic Surgeons
- OARSI
Osteoarthritis Research Society International
- ESSKA
European Society for Sports Traumatology, Knee Surgery and Arthroscopy
1. Introduction
The prevalence of knee osteoarthritis (KOA) is high [1], and various novel treatments are under development [2,3]. One such treatment is platelet-rich plasma (PRP) therapy [4]. This therapy involves using plasma rich in platelets, which contain various bioactive substances that act on the tissues within the joint, promoting the body's innate “self-repair capacity” and “anti-inflammatory effects [5]." Although numerous studies have been conducted on PRP therapy for KOA, its effectiveness remains a subject of ongoing debate [6]. In Japan, a legal amendment in 2015 allowed PRP therapy to be administered only in facilities that have undergone strict review and are registered with the Ministry of Health, Labor, and Welfare (MHLW) [7]. As PRP therapy is not covered by insurance, it is provided as a self-funded treatment. When registering with the MHLW, facilities can classify the treatment as either research or clinical practice, but most register it as “treatment.” To properly assess the effectiveness of PRP therapy, it is crucial to record real-world data (RWD) from clinical settings using a reliable database [8]. The Japanese Society for Regenerative Medicine established the National Regenerative Medicine Database (NRMD) at the request of the MHLW and the Japan Agency for Medical Research and Development (AMED). This database supports the research and development of regenerative medical products, covering all phases from clinical research to post-marketing surveillance [9].
One of the key benefits of registering cases treated with PRP in a database is that the data can be used as historical controls for comparison with other treatments. However, the lack of a control group makes it difficult to provide robust academic evidence to definitively demonstrate its efficacy. Despite this limitation, the database is highly useful for examining the backgrounds of patients receiving PRP therapy and offers valuable insights into patient demographics and characteristics. Additionally, the database enables comparisons between effective and ineffective cases among patients treated with PRP, allowing for the analysis of factors associated with symptom improvement. These investigations are crucial for identifying which patients are most likely to benefit from PRP therapy and which patients may not be suitable candidates for this treatment. These findings can help refine the criteria for selecting appropriate candidates for PRP therapy, thereby improving the overall effectiveness of its application [10].
Furthermore, healthcare systems and medical economics vary by country, and the positioning of PRP therapy for KOA and background of patients receiving PRP therapy are likely to differ across regions [11]. However, no database analyses on PRP therapy for KOA have been published in Japan. In this context, the current study aimed to analyze the patient background and treatment outcomes of cases in our hospital, which were registered in the NRMD database of PRP therapy for KOA with the goal of identifying the appropriate indications and limitations of PRP therapy in KOA. Our hypothesis is that by analyzing data of our hospital registered in the NRMD database, we will be able to identify suitable cases for PRP therapy in KOA, contributing to the future development of PRP therapy.
2. Methods
2.1. Study design
This study was a retrospective observational study, analyzing data in our hospital which were registered according to predefined registration rules. The flowchart of data selection and analysis for the study is presented in Fig. 1. A total of 2068 patients (2815 knees) were initially included. After applying the exclusion criteria, 516 patients (727 knees) with complete clinical outcome data and Kellgren-Lawrence (KL) grade-based KOA classification were included in the analysis of VAS improvement and patient characteristics.
Fig. 1.
Flowchart of data selection and analysis for the study KOA: Knee osteoarthritis, PRP: Platelet-rich plasma, NRMD: National Regenerative Medicine Database, VAS: Visual Analog Scale.
2.2. Database utilization
We used the NRMD, which was established by the Japanese Society for Regenerative Medicine with support from the MHLW, AMED, and Pharmaceuticals and Medical Devices Agency (PMDA). The analyzed data included patients with KOA treated at a specialized PRP outpatient clinic in our hospital with prospective and continuous registration in the NRMD from January 2020 to May 2024. Data were entered into the NRMD by third-party data entry specialist who were not involved in the care of the patients. All data entries and modifications were logged for record keeping. This study was approved by the Juntendo University Medical Ethics Committee (approval number: H19-0267).
2.3. PRP protocol
Leukocyte-poor PRP (LP-PRP) was prepared using the MyCells kit (MyCells Autologous Platelet Preparation System; Kaylight, Ramat-Hasharon, Israel). This Class III medical device has been approved by the PMDA in Japan and has 510(k) clearance from the FDA. For each injection, 6 mL of LP-PRP was obtained from 22 mL of autologous venous blood. After centrifuging for 7 min at 2000×g, the platelet-poor plasma was removed, and the remaining plasma was filtered to collect P2-Bβ PRP (leukocyte-poor PRP), based on the PAW classification system [12]. This preparation method allows approximately 2 billion platelets to be administered intraarticularly per injection. In this study, the average number of platelets administered per injection was 1.9195 × 10ˆ9 based on data from 1140 patients registered in the NRMD. The white blood cell count in LP-PRP was 0.31 ± 1.8 times that of whole blood, with a predominance of lymphocytes (77.1 %) in these patients. The injections were performed by orthopedic surgeons via percutaneous injection into the suprapatellar bursa, with or without ultrasound guidance, depending on the difficulty of the injection. Standard treatment involved three injections at 3- to 5-week intervals, and a combination with exercise therapy was recommended.
2.4. Indications for PRP therapy
The indications for PRP therapy were determined based on the treatment protocol registered with the Ministry of Health, Labor, and Welfare of Japan (PB3150023). Patients eligible for PRP therapy were those with chronic knee pain due to KOA that had persisted for more than six months and were resistant to conservative treatments. There were no restrictions on patient age, sex, or severity of knee deformity. Patients with active inflammatory diseases, infections, ongoing cancer treatment, platelet-related blood disorders, or an inability to communicate effectively were excluded from treatment.
2.5. Treatment follow-up
Changes in the visual analog scale (VAS) scores were observed and recorded at three time points: before treatment, three months after treatment initiation, and six months after treatment initiation. In patients with bilateral KOA, VAS scores for each knee were evaluated and recorded separately. In the analyses examining the relationship between VAS changes and various parameters, patients with low initial VAS scores were excluded. The study focused on cases with a VAS of 30 or higher, which is a common inclusion criterion in studies evaluating PRP therapy for KOA.
2.6. Data analysis
Cases where essential data for the analysis were missing were excluded from the relevant analysis; however, the decision to exclude cases was made individually for each analysis. Improvements in the scores before and after treatment were evaluated using paired t-tests. Comparisons between two groups were performed using Student's t-test. Spearman's rank correlation coefficient was used to calculate the correlation coefficients. For comparisons involving three or more groups, one-way analysis of variance with post hoc Tukey honest significant difference test was employed. All statistical analyses were performed using GraphPad Prism version 10.2.3. A significance level of 5 % was applied, and the data were presented as mean ± standard deviation (SD).
3. Results
3.1. Patient characteristics
During the study period, 2815 knees from 2068 patients were registered in the NRMD from our hospital (Fig. 1 and Table 1). Of all the patients, 36.1 % (747 cases) received PRP therapy for both knees. The average age of patients at the initiation of treatment was 65.2 ± 17.6 years of age. There were 1283 females and 785 males, with a higher proportion of female patients. The average age of females was 67.7 ± 12.0 years of age, while the average age of males was lower at 60.6 ± 23.8 years of age. The average body mass index (BMI) of females was 23.7 ± 3.9 kg/m2, and that of males was 25.4 ± 5.0 kg/m2. The number of right knees was 1441 and that of left knees was 1374. Primary KOA included 2079 knees, secondary KOA caused by trauma or other factors included 684 knees, and 52 knees were unavailable.
Table 1.
Patient characteristics.
| Total (N = 2068) | Female (N = 1283) | Male (N = 785) | |
|---|---|---|---|
| Age (y.o.) | 65.2 ± 17.6 | 67.7 ± 12.0 | 60.6 ± 23.8 |
| Height (cm) | 161.5 ± 9.7 | 156.4 ± 6.5 | 170.8 ± 7.4 |
| Body weight (kg) | 63.7 ± 13.6 | 57.9 ± 10.0 | 74.1 ± 13.1 |
| Body Mass index (kg/m2) | 24.3 ± 4.4 | 23.7 ± 3.9 | 25.4 ± 5.0 |
y.o., years old.
Based on the KL grade from standing plain radiographs, the severity of KOA was evaluated as follows: KL1/2/3/4 = 125/502/835/617 patients, with KL3 being the most common grade (Table 2). The femorotibial angle (FTA) was 179.4 ± 5.2°. Full-length standing lower limb radiograph were taken in 999 knees, the average mechanical axis (MA) deviation (percent MA) was 31.6 ± 24.2 %, indicating a prevalence of varus deformity. A history of intraarticular hyaluronic acid injections covered by insurance in Japan was confirmed in 1402 cases; however, information was unavailable for the remaining 1413 cases. The total number of hyaluronic acid injections administered to patients with a confirmed history is presented in Table 3. The most common frequency was up to five injections, which was reported in 615 cases (43.8 %). Subsequently, 297 patients underwent 6–10 treatments. Additionally, 190 patients (13.6 %) reported receiving >51 hyaluronic acid injections according to RWD based on patient self-reporting.
Table 2.
Patient characteristics (knees).
| Total (N = 2815) | Female (N = 1801) | Male (N = 1014) | |
|---|---|---|---|
| KL grade (KL 1/2/3/4) | 125/502/835/617 (N = 2079) | 62/283/568/451 (N = 1364) | 63/219/267/166 (N = 715) |
| FTA (degrees) | 179.4 ± 5.2 (N = 1386) | 179.3 ± 5.6 (N = 897) | 179.6 ± 4.5 (N = 489) |
| %MA (%) | 31.6 ± 24.2 (N = 999) | 31.5 ± 24.7 (N = 659) | 31.7 ± 23.1 (N = 340) |
| VAS (0) | 53.5 ± 26.0 (N = 2505) | 55.0 ± 25.9 (N = 1621) | 47.9 ± 25.6 (N = 884) |
| VAS (3 M) | 38.5 ± 27.0 (N = 1098) | 40.1 ± 27.3 (N = 747) | 35.2 ± 26.2 (N = 351) |
| VAS (6 M) | 35.8 ± 25.9 (N = 1257) | 36.9 ± 26.2 (N = 831) | 33.6 ± 25.1 (N = 426) |
| VAS improvement (6 M) | 15.8 ± 27.3 (N = 1155) | 17.1 ± 27.9 (N = 764) | 13.1 ± 26.1 (N = 391) |
KL, Kellgren-Lawrence; FTA, femorotibial angle; MA, mechanical axis; VAS, visual analog scale; VAS (0), baseline VAS; 3 M, three months; 6 M, six months.
Table 3.
History of intraarticular hyaluronic acid injections.
| Number of hyaluronic acid injections | Number of patients |
|---|---|
| 1–5 | 615 |
| 6–10 | 297 |
| 11–20 | 171 |
| 21–50 | 128 |
| 51–100 | 83 |
| >101 | 107 |
3.2. Clinical symptom changes after PRP therapy and analysis of influencing factors
The baseline VAS score before treatment was 53.5 ± 26.0 (Table 2). Although many clinical studies use a VAS score of 30 or higher as an inclusion criterion, 23.3 % (583 knees) of the patients receiving PRP treatment had a baseline VAS score of less than 30. At three months after the start of treatment, the VAS score significantly improved to 38.5 ± 27.0, and at six months, it further improved to 35.8 ± 25.9 (P < 0.05). It is difficult to assess pain improvement using VAS changes in patients with a low initial VAS score [13]. Therefore, the following analysis was conducted on 1922 knees with an initial VAS score of 30 or higher. Of these, both the baseline and six-month post-treatment VAS scores were available for 889 knees. The mean VAS improvement at six months for these 889 knees was 21.9 ± 26.0.
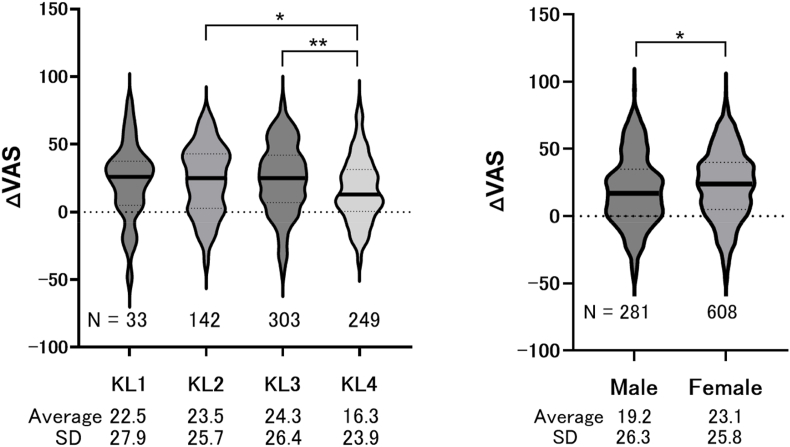
For 727 knees with confirmed KL grades of these 889 knees, VAS improvements were compared according to KL grade (Fig. 2). Table 4 summarizes the characteristics of the 516 patients (727 knees) with complete clinical outcome d1ata and KL grade-based KOA classification for the analysis of VAS improvement and patient characteristics. The mean age was 66.8 ± 10.6 years, with a BMI of 24.2 ± 3.7 kg/m2. Baseline VAS scores averaged 63.2 ± 18.0, with an improvement to 41.9 ± 25.4 at six months. No significant differences were observed in VAS improvement among KL grades 1–3, but KL grade 4 showed significantly less improvement compared to grades 2 and 3. Additionally, a comparison of VAS improvement by sex showed that women had a greater improvement (23.1 ± 25.8) than men (19.2 ± 26.3) (Fig. 2).
Fig. 2.
Improvement of VAS and patient characteristics Box-and-violin plots showing VAS improvement according to (A) KL grade and (B) sex. VAS, visual analog scale; KL, Kellgren-Lawrence classification. ∗ indicates p < 0.05, and
∗∗ indicates p < 0.01.
Table 4.
Patient characteristics with clinical outcome data and Kellgren-Lawrence grading.
| Total (727 knees in 516 patients) | Female (505 knees in 348 patients) | Male (222 knees in 168 patients) | |
|---|---|---|---|
| Age (y.o.) | 66.8 ± 10.6 | 68.3 ± 10.3 | 63.8 ± 10.5 |
| Height (cm) | 161.1 ± 9.2 | 156.4 ± 6.3 | 170.7 ± 6.5 |
| Body weight (kg) | 63.1 ± 12.7 | 57.8 ± 8.6 | 73.8 ± 13.0 |
| Body Mass index (kg/m2) | 24.2 ± 3.7 | 23.7 ± 3.4 | 25.3 ± 4.0 |
| KL grade (KL 1/2/3/4) | 33/142/303/249 | 23/92/206/184 | 10/50/97/65 |
| VAS (0) | 63.2 ± 18.0 | 64.7 ± 18.1 | 59.7 ± 17.5 |
| VAS (6 M) | 41.9 ± 25.4 | 42.5 ± 25.4 | 40.3 ± 25.6 |
y.o., years old; KL, Kellgren-Lawrence; VAS, visual analog scale; VAS (0), baseline VAS; 6 M, six months.
Next, we analyzed the factors associated with VAS improvement. VAS improvement was negatively correlated with age (r = −0.76, p = 0.02) and BMI (r = −0.11, p < 0.05), whereas a positive correlation was found between percent MA (r = 0.13, p < 0.01) and the baseline VAS score (r = 0.32, p < 0.01) (Table 5). There was no correlation between the platelet count in the PRP and VAS improvement.
Table 5.
Correlations between improvement of VAS and patient characteristics.
| Responder (Mean ± SD) | Non-Responder (Mean ± SD) | p-value | Correlation with VAS Improvement (r) | Number of subjects | |
|---|---|---|---|---|---|
| Age (years) | 65.8 ± 11.4 | 68.1 ± 12.2 | 0.004 | −0.76 (p = 0.02) | 887 |
| Height (cm) | 160.6 ± 8.1 | 160.2 ± 9.4 | 0.54 | 0.05 (p = 0.41) | 852 |
| Body weight (kg) | 63.1 ± 12.7 | 63.1 ± 12.7 | 0.25 | −0.07 (p = 0.06) | 852 |
| BMI (kg/m2) | 24.0 ± 3.7 | 24.5 ± 3.8 | 0.059 | −0.11 (p < 0.05) | 852 |
| FTA(°) | 179.3 ± 5.41 | 180.0 ± 6.0 | 0.20 | −0.06 (p = 0.19) | 479 |
| %MA | 33.0 ± 27.3 | 27.9 ± 29.9 | 0.08 | 0.13 (p < 0.05) | 389 |
| VAS (0) (mm) | 65.4 ± 17.9 | 58.8 ± 17.7 | < 0.001 | 0.32 (p < 0.01) | 889 |
| PRP platelet ( × 109/mL) | 239.1 ± 53.4 | 240.8 ± 53.9 | 0.37 | 0.04 (p = 0.39) | 467 |
BMI, body mass index; FTA, femorotibial angle; MA, mechanical axis; VAS (0), baseline VAS; PRP, platelet-rich plasma.
Patients who experienced a VAS improvement of 15 or more, which is the Minimal Clinically Important Difference (MCID) for conservative treatment of KOA [14], were defined as “responders."
To further investigate the characteristics of responders and non-responders, we compared their baseline demographics and clinical parameters (Table 5). Responders had a significantly lower age (65.8 ± 11.4 years vs. 68.1 ± 12.2 years, p = 0.004) and tended to have a lower BMI (24.0 ± 3.7 kg/m2 vs. 24.5 ± 3.8 kg/m2, p = 0.059) compared to non-responders. Additionally, the baseline VAS score was significantly higher in responders (65.4 ± 17.9 vs. 58.8 ± 17.7, p < 0.001). Other factors, including height, body weight, FTA, and PRP platelet count, did not show significant differences between the two groups.
Logistic regression analysis was performed with responder status as the dependent variable, and age, sex, BMI, KL grade, and baseline VAS score as explanatory variables. KL grade (OR = 0.58, 95 % confidence interval = 0.43–0.79) and baseline VAS score (OR = 1.03, 95 % confidence interval = 1.01–1.04) were identified as factors related to the effectiveness of PRP therapy (Table 6). Age, sex, and BMI, which were correlated with VAS improvement (Table 5), did not relate to the responder rate in this analysis (data not shown). A total of 535 knees (out of 889) were classified as responders, resulting in a response rate of 60.2 %.
Table 6.
Odds ratio for PRP-therapy responders.
| Odds ratio (adjusted) | 95 % CI |
p-value | ||
|---|---|---|---|---|
| lower | upper | |||
| Age | 0.977 | 0.953 | 1.002 | 0.074 |
| Sex | 0.835 | 0.511 | 1.365 | 0.472 |
| BMI | 0.995 | 0.932 | 1.062 | 0.883 |
| K-L grade | 0.582 | 0.428 | 0.793 | < 0.01 |
| %MA | 0.999 | 0.991 | 1.008 | 0.867 |
| VAS (0) | 1.029 | 1.014 | 1.043 | < 0.01 |
BMI, body mass index; FTA, femorotibial angle; MA, mechanical axis; VAS (0), baseline; PRP, platelet-rich plasma.
In terms of adverse events, there were no reports of serious complications in the cases registered in the NRMD and no instances of infection were observed.
3.3. Factors influencing pain at initial treatment
Finally, we analyzed the factors associated with the baseline VAS scores. It is important to note that this analysis does not investigate predictors of PRP treatment response, but rather the characteristics associated with greater pain intensity at the initial visit (Table 7). Higher age, BMI, and femorotibial angle (FTA) were associated with greater pain intensity. A negative correlation was found with the percent MA, indicating that the more pronounced the varus deformity, the greater the pain. In the blood test data, there was a negative correlation between pain and red blood cell count, whereas a positive correlation was observed with white blood cell and neutrophil counts, but there was no correlation with lymphocyte. These findings suggest that patients with severe inflammatory conditions experience more intense pain. No correlation was found between platelet count and baseline VAS scores.
Table 7.
Correlations between baseline VAS and patient characteristics.
| r | p-value | number of subjects | |
|---|---|---|---|
| Age | 0.23 | < 0.001 | 2503 |
| BMI | 0.12 | < 0.001 | 2387 |
| FTA | 0.21 | < 0.001 | 1327 |
| %MA (%) | −0.23 | < 0.001 | 972 |
| Red blood cells | −0.11 | < 0.001 | 1075 |
| White blood cells | 0.1 | < 0.002 | 1075 |
| Neutrophil | 0.08 | < 0.01 | 1075 |
| Lymphocyte | 0.05 | 0.14 | 1075 |
| Platelet | −0.002 | 0.93 | 1073 |
BMI, body mass index; FTA, femorotibial angle; MA, mechanical axis.
4. Discussion
In this study, we analyzed real-world clinical data on PRP therapy for KOA in the single university hospital, which is being performed as a self-funded treatment in Japan. We explored the relationship between patient characteristics, such as age, sex, and severity of deformity, and the effectiveness of PRP therapy. Our findings showed that PRP therapy was less effective in patients with KL grade 4, in whom joint space had disappeared. Additionally, the severity of pretreatment pain was correlated with older age, higher BMI, and varus deformity, and some of these factors were also associated with the effectiveness of PRP therapy. Patients with severe pain have higher neutrophil counts. These findings (insights) are the first to be published from the analysis of data from a single hospital registered in the NRMD and successfully elucidated RWD on PRP therapy for KOA in Japan.
Registering patients for disease treatment in public databases offers numerous benefits that significantly enhance medical research and patient care [15]. Patient safety is also greatly enhanced through public registries as they allow for continuous monitoring of adverse reactions, side effects, and long-term outcomes [16]. In this study, no cases of infection were observed when autologous peripheral blood-derived PRP was prepared on-site and administered intraarticularly. This information can be used as a reference for patients considering this treatment. Public databases offer invaluable RWD that reflect how treatments are performed in routine clinical practice rather than in the controlled environment of clinical trials. This insight is critical for understanding the safety and efficacy of treatments across diverse patient populations with different health conditions and helping healthcare professionals optimize treatment approaches in real-life scenarios.
The effectiveness of PRP therapy for KOA remains a topic of debate. Bennell et al. conducted a highly detailed randomized controlled trial (RCT) in Australia involving a large sample size comparing PRP therapy with saline injections [17]. Their findings showed no further improvement with PRP therapy compared with saline injection. In contrast, meta-analyses of RCTs comparing PRP with hyaluronic acid, corticosteroids, and saline have often reported significant improvements with PRP, although some studies have reported no such effects [18,19]. These conflicting results suggest that biases may have arisen from the selection of studies included in the meta-analyses, leaving the debate unresolved. To date, only one RCT from Japan has been reported. Yoshioka et al. conducted a study on KOA patients in Japan and found significant symptom improvement with PRP therapy compared to saline injection [20]. One of the differences between the Bennell and Yoshioka studies is the patient criteria: Bennell's study involved volunteers with knee pain persisting for one month, whereas Yoshioka's study targeted patients with knee pain lasting for at least three months. Additionally, there was a difference in the BMI of the patients, with Bennell's cohort having an average BMI of 29 and Yoshioka's cohort having an average BMI of 24. These differences in patient characteristics, along with variations in body size between the Australian and Japanese populations, may have influenced the study outcomes. Although there are numerous RCTs on PRP therapy for KOA, variations in patient characteristics and the type of PRP used in each study pose challenges in consolidating evidence [6]. Further investigations are necessary to clarify the role of PRP therapy in the treatment of KOA.
In this study, the correlation between blood count data and pain levels in patients was analyzed, and it was revealed that lower red blood cell counts were associated with increased pain (Table 6). This suggests that patients with low red blood cell counts may have poor nutritional status or anemia, both of which could contribute to the intensification of KOA-related pain. Dong et al. investigated the role of eating habits in patients with chronic pain and concluded that dietary factors should be considered in pain management strategies [21]. This underscores the possibility that improving nutritional status may effectively reduce pain. Furthermore, this study found that higher neutrophil levels were correlated with greater pain, indicating that patients with elevated neutrophil counts and increased inflammatory responses tended to experience more severe pain (Table 6). Although the present study did not directly confirm the presence of inflammatory cytokines, it is plausible that patients with heightened inflammation may also have higher blood levels of these cytokines. If such inflammatory cytokines are present in PRP derived from the blood, the efficacy of PRP therapy could be reduced. Consequently, managing inflammation prior to PRP therapy may enhance treatment outcomes in patients with elevated inflammatory markers, although further studies are necessary.
Recommendations regarding PRP therapy for KOA vary considerably depending on the country or organization [19]. For instance, the American Academy of Orthopaedic Surgeons (AAOS) [22] and Osteoarthritis Research Society International (OARSI) do not recommend PRP therapy [23]. OARSI, in particular, categorizes PRP as “uncertain” due to the lack of consistent, high-quality evidence demonstrating its effectiveness in reducing pain or improving function in KOA patients. These guidelines emphasize the need for more rigorous studies before PRP is broadly recommended for the management of KOA. In contrast, the European Society for Sports Traumatology, Knee Surgery, and Arthroscopy (ESSKA) supports the use of PRP for the treatment of KOA [24]. According to ESSKA's consensus of ESSKA on injectable orthobiologics, clinical evidence from multiple studies, including high-level trials, supports the safety and effectiveness of PRP. PRP offers benefits over placebo, hyaluronic acid, and corticosteroid treatments. The ESSKA rates PRP highly, giving it a Grade A confidence level and considers it a viable first-line injectable treatment for KOA. The differences in these recommendations may reflect not only the varying levels of evidence available but also the different healthcare economic contexts and cultural factors in each region [25]. In any case, it is widely acknowledged that more high-quality clinical studies are needed to establish the true efficacy of PRP therapy in KOA treatment.
For PRP therapy in KOA, many facilities recommend limiting its use to patients with KL grades up to 3 [26]. In this study, the least improvement in VAS scores was observed in patients with KL grade 4, suggesting that PRP therapy may be most beneficial for patients with KL grades up to 3. However, in cases where patients face higher surgical risks due to underlying health conditions, for those who cannot undergo surgery due to caregiving responsibilities or work commitments, or are too young for joint replacement surgery, PRP may still be a reasonable palliative option, even in patients with KL grade 4 [27]. Weight loss is known to be effective in alleviating KOA symptoms, and this study also found that patients with a higher BMI experienced more pain [28]. Therefore, encouraging weight loss should be prioritized in patients with KOA. However, PRP therapy is not recommended for patients with severe obesity. This study also showed that older patients experienced less improvement in VAS scores (Table 4), although this effect diminished after adjusting for knee deformity severity (Table 5). This suggests that the higher severity of knee deformity in older patients could account for reduced pain improvement. Similarly, while men showed less improvement in VAS scores than women (Fig. 1), this trend disappeared in the logistic regression analysis (Table 4), indicating that sex may not play a significant role in determining the indications for PRP therapy. When counseling patients regarding PRP therapy for KOA, it is important to share these real-world findings. For patients who are likely to benefit from surgical treatment, surgery should be recommended to avoid delaying the optimal timing of intervention by continuing conservative treatments.
5. Limitations
This study had several limitations. First, because it was conducted based on treatment records rather than a structured research study, there was a significant amount of missing data. This was primarily due to omissions in documentation, data entry, or missed tests. Even among patients who continued to attend follow-up visits without dropping out, many were excluded from specific analyses because of these data gaps. Secondly, the lack of a control group made it impossible to definitively assess the effectiveness of PRP therapy within this study alone. However, because the data are part of a public database, they could potentially serve as historical control data in future studies comparing PRP therapy with other treatments. Thirdly, this was a single-center study; one advantage of using a public database such as the NRMD is that it allows for comparisons across multiple facilities using the same criteria. We anticipate that as more facilities contribute to NRMD, the data will support the development of safer and more effective treatments through broader, multicenter analyses. Fourthly, in Japan, medical treatments are generally provided under the universal health insurance system. PRP therapy, however, is conducted as part of private medical care outside the scope of the health insurance system, regardless of the treatment period. Consequently, there is a significant gap between PRP therapy, which is self-financed by patients, and existing treatment methods covered by insurance. Finally, it cannot be denied that the results of this study may include placebo effects stemming from the cost of receiving PRP therapy. Furthermore, in implementing PRP therapy, it is often combined with existing non-pharmacological and pharmacological treatments recognized under insurance-covered medical care. Particularly, in the process of undergoing PRP therapy, many patients revisit their approach to KOA by incorporating education and exercise therapy. However, the effectiveness of these combined approaches has not been thoroughly evaluated.
6. Conclusion
This study investigated treatment outcomes and patient characteristics of PRP therapy for KOA in a single university hospital registered in the NRMD. The analysis revealed various characteristics of patients receiving self-funded PRP therapy in Japan, including age, sex, body composition, knee deformity severity, and pain level. Additionally, some of these patient characteristics influenced the effectiveness of PRP therapy. As PRP therapy for KOA is safe, with few adverse events, we hope to contribute to the future development of KOA treatments by sharing registered data with clinicians and researchers.
Ethics approval
The Juntendo University Medical Ethics Committee approved this study (approval number: H19-0267).
Data availability
Data supporting the findings of this study are available from the corresponding author, Y.S., upon reasonable request.
Funding
This study was funded entirely by Juntendo University and no funding was provided by any specific company.
Declaration of competing interest
None.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Yoshimura N., Muraki S., Oka H., Mabuchi A., En-Yo Y., Yoshida M., et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metabol. 2009;27:620–628. doi: 10.1007/s00774-009-0080-8. [Epub 2009 July 1]. PMID: 19568689. [DOI] [PubMed] [Google Scholar]
- 2.Emami A., Namdari H., Parvizpour F., Arabpour Z. Challenges in osteoarthritis treatment. Tissue Cell. 2023;80 doi: 10.1016/j.tice.2022.101992. [Epub 2022 November 28]. PMID: 36462384. [DOI] [PubMed] [Google Scholar]
- 3.Delanois R.E., Sax O.C., Chen Z., Cohen J.M., Callahan D.M., Mont M.A. Biologic therapies for the treatment of knee osteoarthritis: an updated systematic review. J Arthroplast. 2022;37:2480–2506. doi: 10.1016/j.arth.2022.05.031. [Epub 2022 May 21]. PMID: 35609847. [DOI] [PubMed] [Google Scholar]
- 4.Siddiq M.A.B., Clegg D., Jansen T.L., Rasker J.J. Emerging and new treatment options for knee osteoarthritis. Curr Rheumatol Rev. 2022;18:20–32. doi: 10.2174/1573397117666211116111738. PMID: 34784876. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Merchán E.C. Intra-articular platelet-rich plasma injections in knee osteoarthritis: a review of their current molecular mechanisms of action and their degree of efficacy. Int J Mol Sci. 2022;23:1301. doi: 10.3390/ijms23031301. PMID: 35163225, PMCID: PMC8836227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman E., Rao P., Abu-Farsakh H.N., Thonse C., Ali I., Upton A.E., et al. Systematic review of platelet-rich plasma in medical and surgical specialties: quality, evaluation, evidence, and enforcement. J Clin Med. 2024;13:4571. doi: 10.3390/jcm13154571. PMID: 39124838, PMCID: PMC11313071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arita A., Tobita M. Current status of platelet-rich plasma therapy under the act on the safety of regenerative medicine in Japan. Regen Ther. 2023;23:37–43. doi: 10.1016/j.reth.2023.03.001. PMID: 37063096, PMCID: PMC10090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez M., Jorquera C., de Dicastillo L.L., Fiz N., Knörr J., Beitia M., et al. Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study. Ther Adv Musculoskelet Dis. 2022;14 doi: 10.1177/1759720X221100304. PMID: 35721321, PMCID: PMC9201351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada K., Sato Y., Sugiyama D., Sawa Y. Establishment of the national consortium for regenerative medicine and national regenerative medicine database in Japan. Clin Ther. 2018;40:1076–1083. doi: 10.1016/j.clinthera.2018.05.008. [Epub 2018 June 27]. PMID: 29958729. [DOI] [PubMed] [Google Scholar]
- 10.Blonde L., Khunti K., Harris S.B., Meizinger C., Skolnik N.S. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35:1763–1774. doi: 10.1007/s12325-018-0805-y. [Epub 2018 October 24]. PMID: 30357570, PMCID: PMC6223979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Z., Chen W., Wei Z., Zhang Q., Tang G. Global trends and hotspots in the application of platelet-rich plasma in knee osteoarthritis: a bibliometric analysis from 2008 to 2022. Méd Sur. 2023;102 doi: 10.1097/MD.0000000000035854. PMID: 38013292, PMCID: PMC10681507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLong J.M., Russell R.P., Mazzocca A.D. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28:998–1009. doi: 10.1016/j.arthro.2012.04.148. PMID: 22738751. [DOI] [PubMed] [Google Scholar]
- 13.Allen K.D., Bierma-Zeinstra S.M.A., Foster N.E., Golightly Y.M., Hawker G. OARSI Clinical Trials Recommendations: Design and conduct of implementation trials of interventions for osteoarthritis. Osteoarthr Cartil. 2015;23:826–838. doi: 10.1016/j.joca.2015.02.772. PMID: 25952353. [DOI] [PubMed] [Google Scholar]
- 14.Concoff A., Rosen J., Fu F., Bhandari M., Boyer K., Karlsson J., et al. A comparison of treatment effects for nonsurgical therapies and the minimum clinically important difference in knee osteoarthritis: a systematic review. JBJS Rev. 2019;7:e5. doi: 10.2106/JBJS.RVW.18.00150. PMID: 31415278, PMCID: PMC6727942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pop B., Fetica B., Blaga M.L., Trifa A.P., Achimas-Cadariu P., Vlad C.I., et al. The role of medical registries, potential applications and limitations. Med Pharm Rep. 2019;92:7–14. doi: 10.15386/cjmed-1015. [Epub 2019 January 15]. PMID: 30957080, PMCID: PMC6448488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellows B.K., Kuo K.L., Biltaji E., Singhal M., Jiao T., Cheng Y., et al. Real-world evidence in pain research: a review of data sources. J Pain Palliat Care Pharmacother. 2014;28:294–304. doi: 10.3109/15360288.2014.941131. [Epub 2014 August 19]. PMID: 25136897. [DOI] [PubMed] [Google Scholar]
- 17.Bennell K.L., Paterson K.L., Metcalf B.R., Duong V., Eyles J., Kasza J., et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. 2021;326:2021–2030. doi: 10.1001/jama.2021.19415. PMID: 34812863, PMCID: PMC8611484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao X., Yan L., Feng Y., Li X., Zhang K., Lv Z., et al. Efficacy and safety of corticosteroids, hyaluronic acid, and PRP and combination therapy for knee osteoarthritis: a systematic review and network meta-analysis. BMC Muscoskelet Disord. 2023;24:926. doi: 10.1186/s12891-023-06925-6. PMID: 38037038, PMCID: PMC10687893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa L.A.V., Lenza M., Irrgang J.J., Fu F.H., Ferretti M. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074–1086. doi: 10.1177/03635465211062243. [Epub 2022 March 22]. PMID: 35316112. [DOI] [PubMed] [Google Scholar]
- 20.Yoshioka T., Arai N., Sugaya H., Taniguchi Y., Kanamori A., Gosho M., et al. The effectiveness of leukocyte-poor platelet-rich plasma injections for symptomatic mild to moderate osteoarthritis of the knee with joint effusion or bone marrow lesions in a Japanese population: a randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2024;52:2493–2502. doi: 10.1177/03635465241263073. [Epub 2024 August 3]. PMID: 39097760. [DOI] [PubMed] [Google Scholar]
- 21.Dong H.J., Brain K., Olsson M., Dragioti E., Gerdle B., Ghafouri B. Eating habits and the desire to eat healthier among patients with chronic pain: a registry-based study. Sci Rep. 2024;14:4705. doi: 10.1038/s41598-024-55449-z. PMID: 38409442, PMCID: PMC10897138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubin J., Leucht P., Murray M., Pezold R. American Academy of Orthopaedic Surgeons technology overview summary: platelet-rich plasma (PRP) for knee osteoarthritis. J Am Acad Orthop Surg. 2024;32:296–301. doi: 10.5435/JAAOS-D-23-00957. [Epub 2024 January 30]. PMID: 38295392. [DOI] [PubMed] [Google Scholar]
- 23.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [Epub 2019 July 3]. PMID: 31278997. [DOI] [PubMed] [Google Scholar]
- 24.Laver L., Filardo G., Sanchez M., Magalon J., Tischer T., Abat F., et al. The use of injectable orthobiologics for knee osteoarthritis: a European ESSKA-ORBIT consensus. Part 1-Blood-derived products (platelet-rich plasma) Knee Surg Sports Traumatol Arthrosc. 2024;32:783–797. doi: 10.1002/ksa.12077. [Epub 2024 March 4]. PMID: 38436492. [DOI] [PubMed] [Google Scholar]
- 25.Al-Bannay H., Jarus T., Jongbloed L., Yazigi M., Dean E. Culture as a variable in health research: perspectives and caveats. Health Promot Int. 2014;29:549–557. doi: 10.1093/heapro/dat002. [Epub 2013 February 19]. PMID: 23424161. [DOI] [PubMed] [Google Scholar]
- 26.Kon E., de Girolamo L., Laver L., Andriolo L., Andia I., Bastos R., et al. Platelet-rich plasma injections for the management of knee osteoarthritis: the ESSKA-ICRS consensus. Recommendations using the rand/UCLA appropriateness method for different clinical scenarios. Knee Surg Sports Traumatol Arthrosc. 2024 doi: 10.1002/ksa.12320. Epub ahead of print. PMID: 38961773. [DOI] [PubMed] [Google Scholar]
- 27.Prost D., Bardot T., Baud A., Calvo A., Aumont S., Collado H., et al. Long term improvement of knee osteoarthritis after injection of single high/very high volume of very pure PRP: a retrospective analysis of patients optimally managed in dedicated centers. Regen Ther. 2024;25:203–212. doi: 10.1016/j.reth.2023.12.006. PMID: 38234679, PMCID: PMC10792744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gudbergsen H., Boesen M., Lohmander L.S., Christensen R., Henriksen M., Bartels E.M., et al. Weight loss is effective for symptomatic relief in obese subjects with knee osteoarthritis independently of joint damage severity assessed by high-field MRI and radiography. Osteoarthr Cartil. 2012;20:495–502. doi: 10.1016/j.joca.2012.02.639. [Epub 2012 March 5]. PMID: 22401872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author, Y.S., upon reasonable request.