Abstract
Small aortic annulus is recognized as a critical risk factor in transcatheter aortic valve replacement, predisposing patients to prosthesis-patient mismatch. This case series examines 3 distinct anatomical subtypes of small aortic annulus patients undergoing transcatheter aortic valve replacement: 1) tricuspid aortic valve morphology; 2) type 0 bicuspid aortic valve; and 3) failed surgical bioprosthesis. The analysis highlights subtype-specific technical challenges and procedural optimization strategies, emphasizing the importance of preprocedural imaging and prosthesis selection in mitigating complications.
Key Words: aortic stenosis, small aortic annulus, transcatheter aortic valve replacement
Visual Summary

Transcatheter aortic valve replacement (TAVR) has become a pivotal intervention for severe aortic stenosis in older patients. Small aortic annulus (SAA), defined as an annular area <400 mm2,1 constitutes a high-risk anatomical subgroup associated with substantially elevated risks of prosthesis-patient mismatch (PPM).2 Accumulating evidence supports the efficacy of TAVR in patients with severe aortic stenosis and small aortic annulus.2,3
Take-Home Messages
-
•
SAA, defined as an annular area <400 mm2, demonstrates significantly elevated risks of prosthesis-patient mismatch compared with normal aortic annulus.
-
•
TAVR strategy optimization in SAA aortic stenosis requires integration of preprocedural CT measurements, adjacent structures, and intra-TAVR balloon assessment to determine prosthesis size and implant depth.
-
•
Patients with SAA exhibit heterogeneous risk stratification across anatomical subtypes. Specific pathological features, including bicuspid aortic valve morphology and calcific leaflet fusion demonstrate elevated susceptibility to prosthesis-patient mismatch, necessitating individualized procedural strategies for these high-risk cohorts.
Emerging comparative studies suggest potential hemodynamic advantages of self-expanding valves in SAA patients, with CHOICE (A Comparison of Transcatheter Heart Valves in High Risk Patients With Severe Aortic Stenosis: The CHOICE Trial) reporting larger effective orifice areas (EOAs) and reduced severe PPM rates compared with balloon-expandable systems.4,5 Nevertheless, patients with SAA require precise individualized procedural strategies, as different clinical scenarios may be associated with this specific anatomical structure to minimize the occurrence of PPM.
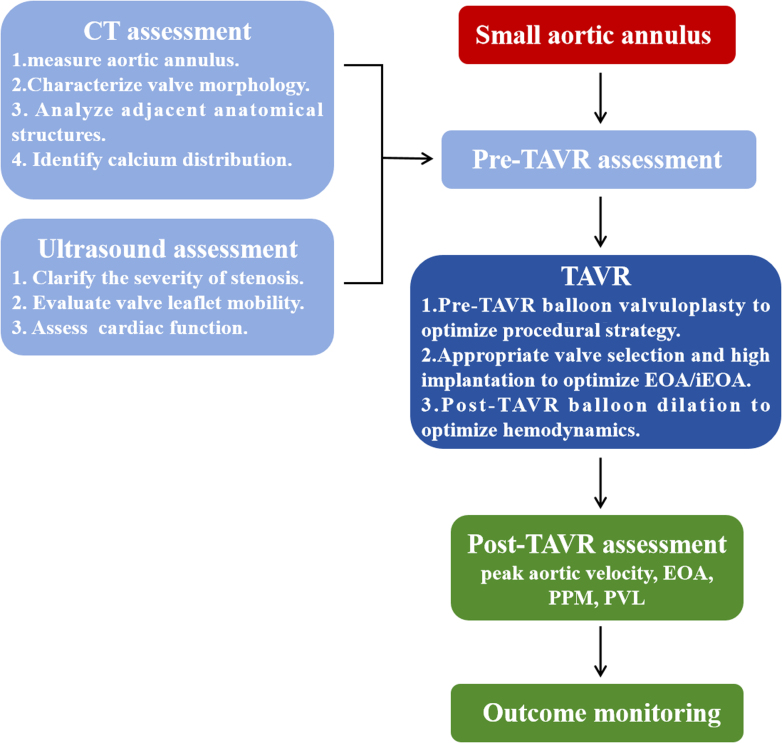
This clinical case series reports 3 cases of severe aortic stenosis with SAA demonstrating distinct anatomical configurations, highlighting the considerations for optimizing procedural strategies in different subtypes. We used a multimodality imaging assessment as the guiding framework to analyze these clinical scenarios. Through these cases, we underscore the critical role of anatomy-driven decision-making in TAVR procedural strategies.
Patient 1
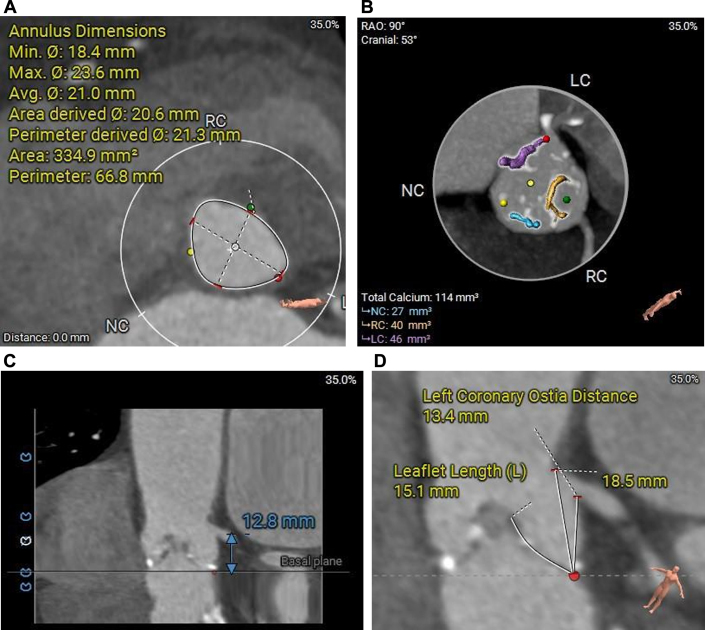
A 72-year-old woman with a history of asthma and coronary artery disease presented to our hospital with progressive angina. Her height was 1.64 m and weight 80 kg, yielding a calculated body surface area (BSA) of 1.87 m2. Transthoracic echocardiography (TTE) revealed severe aortic stenosis with a peak aortic valve velocity of 4.5 m/s, mean transvalvular gradient of 50 mm Hg and EOA of 0.78 cm2. The Society of Thoracic Surgeons (STS) risk score was 2.73%. Pre-TAVR computed tomography (CT) confirmed tricuspid aortic valve morphology and assessed annular dimensions, left ventricular outflow tract, sinus of Valsalva, sinotubular junction, ascending aorta, coronary ostial heights, and valvular calcification (Figure 1, Supplemental Figure 1, Table 1).
Figure 1.
Computed Tomography Assessment of the Aortic Root and Left Coronary Artery (Case 1)
(A) Short-axis view of annuls. (B) Calcium burden of aortic valve. (C) Height of left coronary artery ostial. (D) Length of left leaflet.
Table 1.
Pre-TAVR CT Measurements and CT AV Calcium Burden of 3 Cases
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Valve type | Tricuspid aortic valve | Type 0 bicuspid aortic valve | Failed surgical bioprosthesis |
| Annulus | Diameter (avg): 21.0 mm Diameter (min-max): 18.4-23.6 mm Area: 334.9 mm2 |
Diameter (avg): 23.0 mm Diameter (min-max): 20.4-25.7 mm Area: 396.5 mm2 |
Diameter (avg): 19.2 mm Diameter (min-max): 18.6-19.8 mm Area: 289.4 mm2 |
| LOVT | Diameter (avg): 20.0 mm Diameter (min-max): 15.1-24.9 mm |
Diameter (avg): 25.3 mm Diameter (min-max): 20.9-29.8 mm |
Diameter (avg): 21.5 mm Diameter (min-max): 17.0-26.0 mm |
| SoV | LCC: 26.9mm RCC: 25.5mm NCC: 25.8mm |
Diameter (avg): 26.0 mm Diameter (min-max): 23.0-28.9 mm |
LCC: 36.1 mm RCC: 34.0 mm NCC: 34.7 mm |
| STJ | Diameter (avg): 23.1 mm Diameter (min-max): 22.3-23.8 mm |
Diameter (avg): 23.1 mm Diameter (min-max): 22.3-23.8 mm |
Diameter (avg): 32.4 mm Diameter (min-max): 31.8-33.1 mm |
| AA | Diameter (avg): 31.0 mm Diameter (min-max): 29.8-32.3 mm |
Diameter (avg): 38.0 mm Diameter (min-max): 36.8-39.3 mm |
Diameter (avg): 38.4 mm Diameter (min-max): 37.6-39.2 mm |
| Coronary artery ostial height | LCA: 12.8mm RCA: 16.4mm |
LCA: 11.0mm RCA: 11.4mm |
LCA: 12.8 mm RCA: 17.1 mm |
| AV calcium | 392 mm3 | 208 mm3 | 0 mm3 |
AA = ascending aorta; AV = aortic valve; CT = computed tomography; LCA = left coronary artery; LCC = left coronary cusp; LVOT = left ventricular outflow tract; NCC = non-coronary cusp; RCC = right coronary cusp; SoV = sinus of valsalva; STJ = sino tubular junction; RCA = right coronary artery.
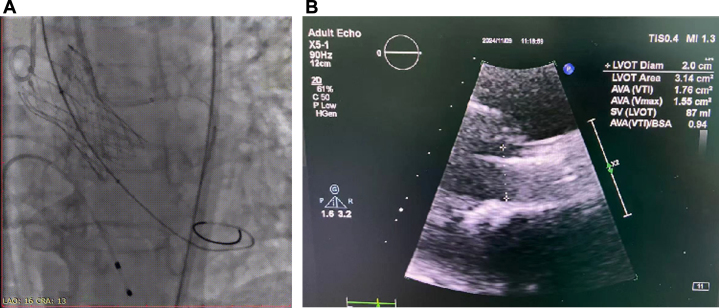
TAVR procedure was conducted under local anesthesia with conscious sedation. After placement of a proximal coronary protection device in the left main coronary artery, balloon valvuloplasty was performed using an 18-mm balloon. Following multidisciplinary heart team evaluation, a 21-mm TaurusElite transcatheter heart valve (Peijia Medical) was deployed at 2 mm below the annular plane, with subsequent post-balloon dilation using a 20-mm balloon (Figure 2A, Video 1).
Figure 2.
Aortogram and Transthoracic Echocardiography After Transcatheter Aortic Valve Replacement (Case 1)
(A) Aortogram of aortic root. (B) Transthoracic echocardiography of aortic valve. LVOT = left ventricular outflow tract.
Follow-up
Postprocedural electrocardiography revealed no new conduction abnormalities. TTE demonstrated a mild peak aortic velocity of 1.8 m/s, EOA of 1.76 cm2, and indexed effective orifice area (iEOA) of 0.94 cm2/m2 (Figure 2B). The patient was discharged on post-TAVR day 4 without complications.
Interpretation
The initial case report describes a patient with tricuspid aortic valve morphology and mild leaflet calcification, demonstrating the feasibility and safety of TAVR guided by multimodality imaging in a patient with SAA. Although small-sized prostheses (<21 mm) are used in 22% to 44% of cases in the United States and Europe,6 Asian populations exhibit significantly smaller annular diameters compared with their European counterparts. This distinct anatomical profile heightens the risks of PPM and coronary obstruction, underscoring the critical need for tailored procedural strategies in SAA patients. Our team emphasizes that synergistic analysis of multimodality imaging (integrating aortic annular dimensions, sinus of Valsalva morphology, and the distribution of calcification) not only mitigates misjudgment risks inherent to single imaging modalities but also enables comprehensive multidimensional assessment.
PPM, associated with adverse long-term survival, functional recovery, heart failure readmissions, and reduced valve durability,2 remains a pivotal concern in SAA patients. Strategies such as supra-annular valve selection, controlled implantation depth, and post-dilatation have been validated to enhance post-TAVR hemodynamics.2 In this case, after balloon pre-dilatation assessment, a 21-mm valve was deployed at a 2-mm implantation depth, followed by post-dilatation. This strategy achieved an effective iEOA, successfully avoiding moderate-to-severe PPM (iEOA ≤0.85 cm2/m2), thereby demonstrating favorable procedural outcomes.
Small aortic root anatomy may elevate coronary obstruction risks. Consequently, meticulous evaluation of sinus dimensions and coronary ostial height is imperative during SAA-TAVR planning. For high-risk patients, strategies such as coronary protection device placement or BASILICA (Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction) may reduce obstruction risks. In this case, the elongated left coronary leaflet and small left coronary sinus conferred an elevated risk of coronary obstruction. Therefore, prophylactic protection of the left coronary artery was implemented before valve implantation.
Patient 2
A 64-year-old woman with coronary artery disease, diabetes mellitus, and hypertension presented with 6-month history of exertional dyspnea and was diagnosed with severe aortic stenosis. Her height was 1.65 m and weight 76 kg, yielding a calculated BSA of 1.82 m2. TTE demonstrated peak aortic velocity of 4.6 m/s, mean transvalvular gradient of 49 mm Hg, and EOA of 0.81 cm2. The STS mortality risk score was 2.63%. Preprocedural CT confirmed a Sievers Type 0 bicuspid aortic valve with small aortic annulus (Figure 3, Table 1).
Figure 3.
Computed Tomography Assessment of the Aortic Root and Right Coronary Artery (Case 2)
(A) Short-axis view of annulus. (B) Calcium burden of aortic valve. (C) Height of left coronary artery ostial. (D) Length of left leaflet.
The TAVR procedure was performed under local anesthesia with conscious sedation. Following predilation with an 18-mm balloon, a 21-mm TaurusElite valve (Peijia Medical) was implanted at 2-mm ventricular depth without post-dilation.
Follow-up
Postprocedural electrocardiography revealed no new conduction abnormalities. Post-TAVR TTE demonstrated absence of paravalvular leakage, with peak aortic velocity of 2.2 m/s, EOA of 1.69 cm2, and iEOA of 0.93 cm2/m2 (Figure 4). The patient was discharged on postoperative day 4.
Figure 4.
Aortogram and Transthoracic Echocardiography After Transcatheter Aortic Valve Replacement (Case 2)
(A) Aortogram of aortic root. (B) Transthoracic echocardiography of aortic valve.
Interpretation
The case 2 highlights the unique challenges of TAVR in a patient with type 0 bicuspid aortic valve (BAV) morphology and SAA. Although TAVR has demonstrated favorable outcomes in BAV populations, the characteristic anatomical features of BAV, including elliptical annuli, calcified raphe, and smaller supra-annular structures (Supplemental Figure 2), complicate preoperative hemodynamic assessment and exacerbate the risks of PPM. For borderline-sized BAV-SAA cases, prosthesis sizing should be guided by comprehensive aortic root anatomy rather than isolated annular diameter. Supra-annular anchoring is the predominant strategy in BAV, which necessitates preoperative estimation of iEOA using supra-annular metrics to ensure optimal outcomes.
Type 0 BAV-SAA patients often exhibit coronary ostia positioned farther from the sinus centerline, amplifying coronary obstruction risks. Consequently, proactive coronary protection strategies should be prioritized in high-risk anatomies. In this case, our team prioritized balloon valvuloplasty with an 18-mm balloon to evaluate leaflet compliance, revealing transient coronary flow compromise during rapid ventricular pacing (Video 2). Based on this dynamic response, the procedural team elected to downsize to a 21-mm prosthesis and deliberately avoided post-dilatation to minimize leaflet displacement forces.
Patient 3
A 91-year-old man with a 20-year history of hypertension was admitted for severe dyspnea, having previously undergone surgical aortic valve replacement in 2004. His height was 1.70 m and weight 51 kg, yielding a calculated BSA of 1.55 m2. TTE revealed a peak aortic velocity of 3.9 m/s, mean transvalvular gradient of 39 mm Hg and EOA of 0.9 cm2. Given advanced age and compromised cardiac function, the STS mortality risk score was 15.6%. Preprocedural CT demonstrated thickened prosthetic leaflets (Figure 5, Table 1).
Figure 5.
Computed Tomography Assessment of the Aortic Root (Case 3)
(A to D) Short-axis view of aortic root (0-8 mm).
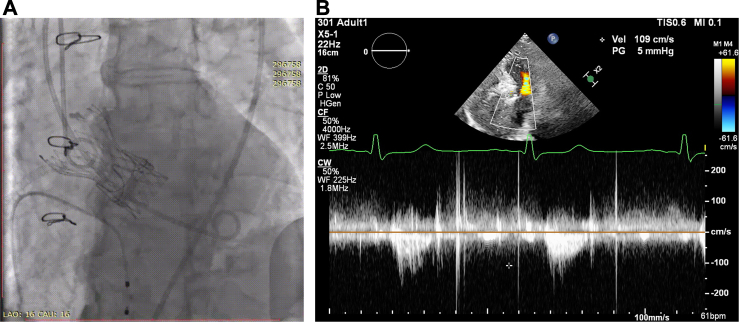
The multidisciplinary team performed balloon aortic valvuloplasty using a 20-mm balloon, followed by implantation of a 21-mm TaurusElite valve (Peijia Medical) at 7-mm ventricular depth. Post-deployment imaging confirmed adequate valve expansion without paravalvular leakage (Video 3).
Follow-up
Post-TAVR TTE demonstrated a peak aortic velocity of 1.09 m/s, EOA of 2.3 cm2, and iEOA of 1.48 cm2/m2 (Figure 6). The patient exhibited marked symptomatic improvement and was discharged on postoperative day 2.
Visual Summary.
Multimodal Imaging Assessment Protocol for TAVR in SAA-AS
Figure 6.
Aortogram and Transthoracic Echocardiography After Transcatheter Aortic Valve Replacement (Case 3)
(A) Aortogram of aortic root. (B) Transthoracic echocardiography of aortic valve.
Interpretation
The final case illustrates a valve-in-valve TAVR procedure. The metallic stent structure of the preexisting surgical bioprosthesis resulted in reduced available annular area, while simultaneously providing structural protection against neovalve-induced vascular compression. Compared with patients with native SAA, patients with prior surgical aortic valve replacement typically achieve superior outcomes with balloon valvuloplasty, allowing direct utilization of the prosthetic annular area to estimate postoperative iEOA. In such scenarios, utilization of larger balloon sizes during aortic valvuloplasty is recommended to achieve sufficient annular expansion for optimal hemodynamic performance. Patients with prior surgical valves often exhibit proportionally larger aortic root anatomies, correlating with lower incidence of PPM compared with native SAA valve recipients.When the predicted iEOA is suboptimal, balloon fracturing technique may be considered to augment the valvular orifice area.
Discussion
Aortic stenosis with concomitant SAA constitutes a distinct subgroup in TAVR populations. European multicenter registry data demonstrate that approximately 12.7% of TAVR recipients meet SAA diagnostic criteria (annular area <400 mm2).7 As TAVR indications expand to younger, lower-risk aortic stenosis patients with longer life expectancy, it has been a critical consideration to enhance long-term valve performance in making procedural strategies. PPM, which results from as discordance between EOA and BSA, is associated with accelerated structural valve deterioration, increased heart failure readmissions, and elevated mortality in moderate-to-severe cases. SAA patients exhibit higher PPM rates post-TAVR (38.3% overall, 10.2% severe),7 underscoring the imperative for anatomically tailored procedural strategies to mitigate this complication.
Compared with European TAVR populations, Asian patients exhibit smaller aortic annular diameters, a higher prevalence of BAV morphology, and greater utilization of smaller surgical valve prostheses, collectively contributing to an elevated proportion of SAA cases in Asian cohorts. Current evidence suggests supra-annular valve designs and controlled high implantation (ventricular depth ≤2 mm) may improve hemodynamic outcomes.7 However, distinct valve morphologies predispose to differential complication profiles. Therefore, tailoring individualized procedural strategies based on anatomic phenotypes is critical, necessitating integration of multimodality imaging to optimize the EOA while ensuring procedural safety.
Conclusions
In aortic stenosis patients with SAA undergoing TAVR, multimodal imaging should be used to comprehensively evaluate the aortic annulus and adjacent structures. Tailored procedural strategies must be planned based on the specific anatomical characteristics of the valvular complex.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures and videos, please see the online version of this paper.
Appendix
Post-TAVR Aortic Root Angiography in Case 1
Pre-TAVR Aortic Valvuloplasty in Case 2 Demonstrating Bilateral Coronary Artery Flow Compromise
Post-TAVR Aortic Root Angiography in Case 3
References
- 1.VARC-3 Writing Committee, Généreux P., Piazza N., et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021;77(21):2717–2746. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Pibarot P., Magne J., Leipsic J., et al. Imaging for predicting and assessing prosthesis-patient mismatch after aortic valve replacement. JACC Cardiovasc Imaging. 2019;12(1):149–162. doi: 10.1016/j.jcmg.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Head S.J., Reardon M.J., Deeb G.M., et al. Computed tomography-based indexed aortic annulus size to predict prosthesis-patient mismatch. Circ Cardiovasc Interv. 2019;12(4) doi: 10.1161/CIRCINTERVENTIONS.118.007396. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Wahab M., Landt M., Neumann F.J., et al. 5-year outcomes after TAVR with balloon-expandable versus self-expanding valves: results from the CHOICE randomized clinical trial. JACC Cardiovasc Interv. 2020;13(9):1071–1082. doi: 10.1016/j.jcin.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Abdelghani M., Mankerious N., Allali A., et al. Bioprosthetic valve performance after transcatheter aortic valve replacement with self-expanding versus balloon-expandable valves in large versus small aortic valve annuli: insights from the CHOICE trial and the CHOICE-Extend Registry. JACC Cardiovasc Interv. 2018;11(24):2507–2518. doi: 10.1016/j.jcin.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 6.Freitas-Ferraz A.B., Tirado-Conte G., Dagenais F., et al. Aortic stenosis and small aortic annulus. Circulation. 2019;139(23):2685–2702. doi: 10.1161/CIRCULATIONAHA.118.038408. [DOI] [PubMed] [Google Scholar]
- 7.Voigtländer L., Kim W.K., Mauri V., et al. Transcatheter aortic valve implantation in patients with a small aortic annulus: performance of supra-, intra- and infra-annular transcatheter heart valves. Clin Res Cardiol. 2021;110(12):1957–1966. doi: 10.1007/s00392-021-01918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Post-TAVR Aortic Root Angiography in Case 1
Pre-TAVR Aortic Valvuloplasty in Case 2 Demonstrating Bilateral Coronary Artery Flow Compromise
Post-TAVR Aortic Root Angiography in Case 3