Abstract
The lung pathogen Pneumocystis spp. is the causative agent of a type of pneumonia that can be fatal in people with defective immune systems, such as AIDS patients. Atovaquone, an analog of ubiquinone (coenzyme Q [CoQ]), inhibits mitochondrial electron transport and is effective in clearing mild to moderate cases of the infection. Purified rat-derived intact Pneumocystis carinii cells synthesize de novo four CoQ homologs, CoQ7, CoQ8, CoQ9, and CoQ10, as demonstrated by the incorporation of radiolabeled precursors of both the benzoquinone ring and the polyprenyl chain. A central step in CoQ biosynthesis is the condensation of p-hydroxybenzoic acid (PHBA) with a long-chain polyprenyl diphosphate molecule. In the present study, CoQ biosynthesis was evaluated by the incorporation of PHBA into completed CoQ molecules using P. carinii cell-free preparations. CoQ synthesis in whole-cell homogenates was not affected by the respiratory inhibitors antimycin A and dicyclohexylcarbodiimide but was diminished by atovaquone. Thus, atovaquone has inhibitory activity on both electron transport and CoQ synthesis in this pathogen. Furthermore, both the mitochondrial and microsomal fractions were shown to synthesize de novo all four P. carinii CoQ homologs. Interestingly, atovaquone inhibited microsomal CoQ synthesis, whereas it had no effect on mitochondrial CoQ synthesis. This is the first pathogenic eukaryotic microorganism in which biosynthesis of CoQ molecules from the initial PHBA:polyprenyl transferase reaction has been unambiguously shown to occur in two distinct compartments of the same cell.
Pneumocystis pneumonia (PcP) remains a major opportunistic infection among AIDS patients and can also occur in those undergoing immunosuppressive treatment for cancer and solid organ transplantation (29, 74). Pneumocystis is an unusual and ubiquitous fungal protist with several features unlike those of most other fungi (29, 30). There is no adequate cultivation method yet developed that provides sufficient numbers of pure organism preparations suitable for many types of experiments. Hence, data on direct biochemical analyses of Pneumocystis jirovecii or Pneumocystis carinii, which infect humans and laboratory rats, respectively, are not readily obtained.
Aggressive prophylaxis has decreased the number of acute PcP cases; however, AIDS patients and other patients with prolonged immunodeficient conditions can experience recurrent infections. The treatments of choice for PcP include the combination of trimethoprim with sulfamethoxazole, pentamidine, and trimetrexate, but some individuals cannot tolerate these drugs (39). An alternative drug used clinically for mild to moderate PcP is atovaquone, an analog of ubiquinone (coenzyme Q [CoQ]) (3, 11, 15, 17, 18, 22).
Ubiquinones and atovaquone.
Ubiquinone plays a pivotal role in inner mitochondrial membrane electron transport by moving electrons from complex I and complex II to complex III (cytochrome bc1 complex) or to the alternative oxidase pathway (6, 10, 70). Ubiquinone is also found in other cellular membranes (1, 10, 12, 55, 75). High concentrations have been reported in the Golgi complex, lysosomal, and other intracellular membranes (16, 27, 28, 50, 56, 70), where it can exceed that in the mitochondrion. Other cytoplasmic membranes in which CoQ has been found in rat liver include the nuclear envelope and peroxisomes (70). High concentrations of CoQ can be found in cell surface membranes (13), and CoQ was shown to serve as an electron and proton carrier in cell surface membranes of mammalian cells (5, 55, 61, 70). CoQ also serves as an important endogenous antioxidant (25, 47, 59, 70) and plays a role in development and longevity (20, 40, 52, 54, 71) in addition to several other functions now known to involve this ubiquitous molecule (70). Except for its role in cellular respiration, very little is known about the functions of CoQ in Pneumocystis spp.
Atovaquone binds competitively and tightly to the cytochrome bc1 complex and inhibits mitochondrial electron transport in the malarial parasite Plasmodium falciparum (15, 17, 18, 38). This parasite does not depend on oxidative phosphorylation for most of its ATP, but when electron transport is disrupted, a number of dehydrogenase enzyme activities are impaired because electrons originating from these enzymes pass through the electron transport chain. Plasmodium synthesizes pyrimidines de novo by a key enzyme, dihydroorotate dehydrogenase (DHOD), and it cannot utilize host pyrimidines. Therefore, a block of electron transport inhibits DHOD activity, which in turn inhibits pyrimidine production, which is lethal for Plasmodium. In contrast, Pneumocystis species are aerobic organisms (17) and have typical mitochondria with well-developed cristae (76). As in Plasmodium, atovaquone also binds to the P. carinii cytochrome bc1 complex. However, unlike Plasmodium, disruption of electron transport in P. carinii apparently reduces the transmembrane electrochemical proton gradient (proton-motive force) across the inner mitochondrial membrane and hence inhibits oxidative phosphorylation leading to reduction in cellular ATP (7). It has now been demonstrated that atovaquone has little effect on DHOD activity in P. carinii (23), but it reduces oxygen consumption (17), ATP levels (7), and cell proliferation (23).
Because atovaquone is a valuable alternative drug for malaria, PcP, and toxoplasmosis, the development of drug-resistant populations is of serious concern (35, 42, 58, 73). Increased cytochrome b mutations were detected in human-derived P. jirovecii from AIDS patients with prior exposure to atovaquone (35). Atovaquone-resistant strains of Plasmodium have been experimentally developed; in all cases reported, resistance was correlated with mutations in the cytochrome b gene (58, 63). The mutations are characterized by amino acid substitutions at the CoQ-binding sites in cytochrome b. These changes presumably result in conformational alterations that allow CoQ, but not atovaquone, to bind to the mutated cytochrome b protein or fit into the pocket in the inner mitochondrial membrane Qo site. Recently, the molecular basis for atovaquone resistance in P. jirovecii was modeled in the cytochrome bc1 complex of Saccharomyces cerevisiae (21, 38). This study showed that multiple mutations around the binding sites can lead to atovaquone resistance by decreasing the affinity for the drug by reducing the volume of the binding pocket. Atovaquone resistance may be more complex (73), as evidence is growing for multiple functions of CoQ (5, 20, 40, 51, 54, 55, 61, 70) and its presence at cellular sites other than the mitochondrion.
Ubiquinone biosynthesis.
In CoQ biosynthesis, the polyprenyl tail is formed from isopentenyl pyrophosphate (C5) units produced by the isoprenoid biosynthetic pathway, whereas the benzoquinone ring is derived from p-hydroxybenzoic acid (PHBA), which is produced from chorismic acid, a product of the shikimate pathway (48, 53) (Fig. 1). Vertebrates, which do not have this pathway, synthesize PHBA from dietary aromatic amino acids. A key step in CoQ synthesis is the condensation of PHBA with a polyprenyl diphosphate catalyzed by PHBA:polyprenyl transferase; then, the completed CoQ molecule is produced following a series of reactions that modify the ring.
FIG. 1.
CoQ synthesis and atovaquone. A. Generalized scheme of CoQ biosynthesis de novo. The benzoquinone ring is derived from chorismate, which is produced by the shikimic acid pathway. The polyprenyl moiety is derived from the isoprenoid pathway. After condensation of PHBA and a polyprenyl diphosphate, several modifications at the aromatic ring moiety occur, leading to the production of the completed CoQ molecule. B. Structure of the hydroxynaphthoquinone atovaquone (566C80), an analog of ubiquinone.
It was earlier shown that the major homolog in P. carinii is CoQ10, and CoQ9 was also present (14). Subsequent studies involving metabolic radiolabeling experiments demonstrated that four homologs, CoQ7, CoQ8, CoQ9, and CoQ10, are synthesized de novo by P. carinii (60). Unlike the situation with the hepatoma cell line HepG2 in which radiolabeled PHBA was apparently incorporated mainly or only into CoQ (4), P. carinii incorporated this precursor into ubiquinones as well as other compounds, such as fatty acids (60).
Interestingly, atovaquone not only reduced ATP levels in P. carinii, but the drug also inhibited CoQ biosynthesis in the organism (31). The results from experiments on intact organisms suggested that regulatory mechanisms operate between different cellular compartments that control CoQ synthesis in this organism. Thus, in the present study, CoQ synthesis was examined in P. carinii homogenates and subcellular fractions. We here report that CoQ synthesis of the four homologs was detected in both the microsomal and mitochondrial fractions, and we further demonstrate that atovaquone inhibited only microsomal CoQ synthesis.
MATERIALS AND METHODS
Organisms.
Pneumocystis carinii was isolated from the lungs of corticosteroid-immunosuppressed rats inoculated with cryopreserved organism preparations as previously described (32). Briefly, viral antibody-negative, P. carinii-free female Lewis rats (Harlan Sprague-Dawley, Indianapolis, IN) were immunosuppressed with methylprednisolone acetate (Depo-Medrol; Upjohn Co., Kalamazoo, MI) and were twice inoculated intratracheally with cryopreserved organisms. After 8 to 10 weeks of immunosuppression, moribund rats were sacrificed and their lungs were perfused, excised, and cut into small pieces. The P. carinii organisms were isolated by homogenization (Stomacher; Tekmar, Cincinnati, Ohio) using a HEPES-buffered solution containing the mucolytic agent glutathione, which causes the detachment of organisms from host cells and other P. carinii organisms (32). Purification involved sieving and a series of centrifugation steps at high and low speeds, followed by membrane microfiltration as described previously (32). The purity of these organism preparations (>95% to 100%) was previously quantified by various microscopic, biochemical, and immunochemical analyses (32, 60). In the present study, the final organism preparation was further analyzed for microbial contamination, since the P. carinii organisms used for these experiments were prepared under normal open laboratory bench conditions. The final organism preparation was suspended in 1 to 2 ml of a HEPES buffer solution containing 1.3 × 107 to 7.1 × 108 P. carinii organisms. Analyses for common fungi and bacteria were performed by transferring 1/50 and 1/200 volumes of the samples onto Sabouraud and Mueller-Hinton agar plates, respectively. No fungal colonies were detected after incubation overnight at room temperature (100% P. carinii purity; n = 6). Aliquots of the same preparations were tested for bacteria by incubation overnight at 37°C, and colony counts compared to P. carinii organisms were calculated. The average was 0.065 ± 0.033% bacteria (>99.9% P. carinii purity); the highest value observed was 0.19% bacteria (>99.8% P. carinii purity).
Rat lung controls.
The incorporation of PHBA into CoQ was examined in rat lungs. Cell-free homogenates of whole lungs from (i) normal, untreated rats and (ii) corticosteroid-immunosuppressed, uninoculated rats were prepared and incubated with radiolabeled PHBA under the same experimental procedures used for isolated, purified P. carinii organisms.
Extraction, purification, fractionation of lipids, and separation of CoQ homologs.
Total lipids were extracted by using a neutral solvent system, purified, and fractionated as described previously (14). The ubiquinones in the neutral lipid fraction were isolated by silica gel H one-dimensional thin-layer chromatography (TLC) using the solvent system petroleum ether-diethylether-acetic acid (80:20:1, vol/vol/vol).
A validated method developed by Tang et al. (65) for measuring CoQ in mouse tissues was adopted for the current study. To determine relative masses of CoQ homologs, total lipids (9.33 mg) were extracted from pooled samples of P. carinii organisms isolated and purified from four rats. A solution containing 0.9 ml n-propanol and 0.l ml H2O was added to the dried lipid sample and the mixture was vortex mixed. After centrifugation at 11,352 × g for 10 min, 100 μl of the supernatant was injected into the high-performance liquid chromatography (HPLC) apparatus equipped with an ESA model 582 solvent delivery module (Chelmsford, MA) and a C18 reversed-phase column (1.5 cm by 4.8 mm inner diameter; 5-μm particle size). Elution with the mobile phase (150 ml hexane, 15 ml isopropanol, 15 ml glacial acetic acid, 4.2 g sodium acetate, 820 ml methanol; filtered through a 0.2-μm membrane) was at 1.1 ml/min. HPLC runs were carried out at room temperature. Ubiquinone homologs in the injected material were first passed through an on-line electrochemical oxidation cell preceding the analytical column; the oxidized forms were then detected and quantified sequentially with a sensitive coulometric detector (ESA Coulochem II) according to methods described by Tang et al. (65, 66).
A mixture of authentic CoQ6, CoQ7, CoQ8, CoQ9, and CoQ10 standards was similarly analyzed to determine elution times of the homologs. To correct for molecular mass differences of the homologs, the detector was calibrated by analyzing 10 μg/ml each of authentic CoQ9 and CoQ10. Peak areas of each P. carinii CoQ homolog obtained in the analysis were adjusted to account for molecular mass and detector sensitivity.
In the metabolic radiolabeling experiments, the ubiquinone fraction was isolated by TLC and then dissolved in 200 μl of chloroform-methanol (1:2, vol/vol). CoQ homologs were isolated using a Beckman model 100A pump and 421 system controller (Beckman Instruments, Berkeley, CA) equipped with a Hypersil ODS C18 column (5-μm particle size). The P. carinii ubiquinone sample (20 μl) was injected with a mixture of cold authentic CoQ7, CoQ8, CoQ9, and CoQ10 as carrier. The mobile phase was hexane-methanol (1:9, vol/vol) at a flow rate of 0.8 ml/min. The eluate was monitored at A290 using a variable wavelength detector (Hitachi, Tokyo, Japan), CoQ homologs were collected, and their radioactivity was determined by liquid scintillation spectrometry.
Incorporation in vitro of PHBA into ubiquinones.
Organisms (108 to 109) were resuspended in 1 ml of a cold HEPES-buffered solution containing 0.85% NaCl, 25 mM HEPES, 10 mM MgCl2, 1 mM EDTA, and 1 mM EGTA; pH 7.4. The organisms were disrupted by two passages through a French pressure cell (Aminco, Urbana, IL) at 20,000 lb/in2 using a flow rate of 15 drops/min. Then, the preparation was further treated to three cycles of freezing in an acetone-dry ice bath (∼1 min) and thawing in a 37°C water bath (∼1 min). We analyzed the incorporation of PHBA into CoQ with only the polyprenyl diphosphate precursors and cofactors available in concentrated suspensions of the P. carinii homogenate preparations. Assay mixtures contained 0.8 ml of the whole-cell homogenate (representing 8.0 × 107 to 8.0 × 108 cells and containing 0.8 to 1.3 mg protein) and 0.2 ml of the same buffer solution containing PHBA and trace amounts of radiolabeled PHBA.
Optimal pH and temperature and the time course of the reaction were determined. Under these incubation conditions, the reaction was essentially over after 1 min with only a little additional radioactivity in CoQ after 15 min. All subsequent assays were performed using cell-free homogenate or subcellular fractions with 2.5 μCi, 5 μCi, or 10 μCi of [U-14C]PHBA (11.5 mCi/mmol; Sigma Chemical Co., St. Louis, MO) at 37°C for 1 min with or without atovaquone. Subsequent assays were performed using 5 μCi PHBA at 543 μM final concentration. The metabolic radiolabeling reaction was terminated by the addition of 3 ml of chloroform-methanol (1:2, vol/vol).
Protein content was quantified (41) using bovine serum albumin (Sigma) as standard, and incorporation was expressed as pmol PHBA incorporated into CoQ/mg protein in the incubation mixture/min. Statistical analysis and kinetic parameters were evaluated using Microsoft Excel 2000 and Grafit (version 4.0.12; Erithacus, Sigma), respectively.
Subcellular fractions.
Subcellular fractions were prepared by differential centrifugation. Whole-cell homogenates were centrifuged in a swinging bucket rotor at 600 × g for 10 min at 4°C, and then the supernate was spun at 6,500 × g for 20 min. The resultant pellet (mitochondria) was saved, and the supernatant fraction was spun at 10,000 × g for 30 min. The pellet was pooled with the pellet from the previous step, and this sample was designated the mitochondrial fraction. The postmitochondrial supernate was then subjected to centrifugation at 105,000 × g for 90 min in an SW 50.1 rotor (L8-70 ultracentrifuge; Beckman, Palo Alto, CA) at 4°C. The high-spin pellet was designated the microsomal fraction, and the supernate was designated the soluble fraction.
Intact organisms, whole-cell homogenates, and subcellular fractions were stained with the mitochondrial stains MitoTracker Green FM (MTG; 100 nM), 10-N-nonyl acridine orange (NAO; 50 nM), and tetramethylrhodamine (TMRE; 200 nM). MTG stains lipids characteristic of mitochondrial membranes (36), NAO has high affinity for the mitochondrial marker lipid cardiolipin (45, 46), and NAO and TMRE are voltage-sensitive dyes that detect transmembrane electrical potentials (24, 57). All stains were from Molecular Probes (Portland, OR). Stained P. carinii preparations were viewed using fluorescence, bright-field, and differential interference optics with a Nikon Optiphot microscope. A B-2A filter was used to view MTG- and NAO-treated specimens, and a G2A filter was used to view TMRE-treated specimens. Micrographs at 1,000× magnification were obtained using a Spot II camera (Diagnostics Instruments, Inc., Sterling Heights, MI).
Subcellular fractions were also analyzed for enzyme markers. The presence of mitochondria was detected by succinate dehydrogenase activity using 2.3 to 2.7 mg of protein in each fraction and 0.1 M morpholinepropanesulfonic acid as the buffer in the assay mixture (2). The microsomal fraction was monitored by glucose-6-phosphate dehydrogenase (G6PD) activities. The potassium salt of G6PD and 3.5 to 3.9 mg of protein were used to determine this enzyme activity (Calzyme Laboratories, San Luis Obispo, CA). Protease inhibitor cocktail (for use with fungal and yeast cells; Sigma) was added to cell lysates (1 μl cocktail/1 ml lysate) before performing both enzyme assays. The whole-cell homogenate and the mitochondrial, microsomal, and soluble fractions were analyzed for CoQ biosynthetic activity by the incorporation of PHBA into CoQ.
Compounds tested for effects on P. carinii CoQ synthesis.
Atovaquone and respiratory inhibitors were tested for their effects on the incorporation of radiolabeled PHBA into CoQ. Antimycin A and dicyclohexylcarbodiimide (DCCD) were from Sigma (St. Louis, MO). All compounds tested were first dissolved in either dimethyl sulfoxide or ethanol as the primary solvent. The concentration of solvent in the final incubation mixture was <0.2%.
RESULTS
Ubiquinone homologs in P. carinii.
In the present study, the four CoQ homologs were detected by using an HPLC apparatus equipped with a coulometric detector. The greater sensitivity afforded by this newer technology enabled us to quantify the relative masses of the four homologs (Fig. 2).
FIG. 2.
HPLC separation of P. carinii CoQ homologs. A. CoQ7, CoQ8, CoQ9, and CoQ10 were detected in P. carinii, and the peak areas that represented these homologs were integrated in this analysis, set at 5 μA full scale. CoQ6 was not detected in the chromatogram. The arrow denotes the elution time for authentic CoQ6. B. The four CoQ homologs in P. carinii were detected using 1-μA full scale, in which CoQ10 was too high for integration but CoQ7 is clearly evident in the tracing. CoQ6 was not detected even at this higher detector sensitivity. C. Authentic CoQ standards analyzed under similar HPLC conditions indicate the elution times of CoQ6, CoQ7, CoQ8, CoQ9, and CoQ10.
Assay and controls.
Mammals lack the shikimate pathway that leads to the formation of chorismate, aromatic amino acids, and PHBA, but they can synthesize CoQ from metabolizing dietary phenylalanine or tyrosine to PHBA (64). Only background levels of radioactivity were detected in CoQ when cell-free homogenates of lungs from normal, untreated rats and immunosuppressed, uninoculated rats were incubated with radiolabeled PHBA (data not shown). These results confirm the earlier report that incorporation of mevalonate or PHBA into CoQ was not detected using lung tissue from normal or corticosteroid-treated rats (60). Thus, PHBA incorporation into P. carinii CoQ could not have been catalyzed by potential rat lung enzyme contaminants.
Compared to intact P. carinii organisms, higher incorporation rates were observed with cell homogenates, indicating that transport of this water-soluble precursor by the organism was limited. After incubating intact organisms with radiolabeled PHBA for 48 h, incorporation into ubiquinones was only 58 fmol · mg protein−1 (60). In contrast, the incorporation of PHBA into total P. carinii CoQ using the cell-free system was on the order of nmol · mg protein−1 · min−1.
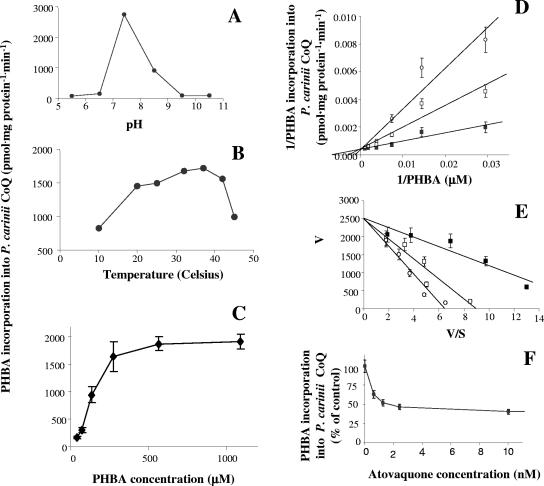
PHBA incorporation into the TLC-purified CoQ fraction and into individual homologs reflects the reactions from PHBA:polyprenyl transferase to completed CoQ molecules. The optimal pH for these reactions was within neutral ranges (Fig. 3A), and an optimal temperature of 37°C reflected the temperature of the rats from which the organisms were freshly isolated (Fig. 3B). Interestingly, there was a sharp increase between 10°C and 20°C, followed by a plateau before again increasing to 37°C.
FIG. 3.
Characterizations of [U-14C]PHBA incorporation into CoQ using P. carinii whole-cell homogenates. Effects of pH and temperature on the incorporation in vitro of [U-14C]PHBA into P. carinii ubiquinones using whole-cell homogenates are shown. A. Effects of pH. Optimal pH was detected at pH 7.5. B. Effects of temperature. Incorporation was observed over a broad temperature range; optimal temperature was 37°C, which sharply declined at temperatures higher than 40°C. There was relatively high activity at 20°C, which is the optimal growth temperature reported for in vitro axenic cultivation of the organism (43). C. First-order kinetics was exhibited with increased substrate concentration. Values are means ± SEM; n = 4 separate experiments. D. Effects of atovaquone, shown in a double reciprocal plot demonstrating competitive inhibition. Filled squares, without atovaquone; open squares, with 2 nM atovaquone; open circles, with 10 nM atovaquone. Values are means ± SEM; n = 3 separate experiments. E. Eadie-Hofstee representation of the data shown in panel D, verifying competitive inhibition kinetics of atovaquone on the incorporation of PHBA into P. carinii ubiquinones. F. Maximal inhibition attained with atovaquone was 60% of untreated controls (40% inhibition); no further inhibition was observed at concentrations up to 10 μM. Values represent means ± SEM; n = 4.
The incorporation of PHBA into total P. carinii CoQ exhibited first-order kinetics (Fig. 3C). Although other ring compounds might serve as acceptors of the polyisoprenoid side chain (49, 53), in the present study we measured only the incorporation of PHBA into completed CoQ compounds. The PHBA incorporation kinetic data may reflect the limiting reaction rate in the pathway, which in other systems is the condensation reaction catalyzed by PHBA:polyprenyl transferase (70). The apparent Km for PHBA was 131 ± 10 μM. Although CoQ10 is present in the greatest mass (14), the incorporation rates of PHBA into the four individual homologs, CoQ7, CoQ8, CoQ9, and CoQ10, was 364 ± 17, 381 ± 12, 405 ± 15, and 431 ± 19 pmol · mg protein−1 · min−1 ± the standard error of the mean (SEM), respectively (n = 6). The relative specific activities of each homolog were calculated (it was highest for CoQ7): CoQ7 > CoQ8 > CoQ9 > CoQ10 (Table 1). Since the reaction was performed with excess PHBA (4× Km) without exogenous polyprenyl diphosphates in the reaction mix, the CoQ compounds formed in vitro reflect the endogenous pool of available polyisoprenoid precursors in P. carinii. Under these conditions, incorporation of PHBA into CoQ was essentially completed after 1 min of incubation. Incorporation (pmol/mg protein ± SEM; n = 3) into CoQ7, CoQ8, CoQ9, and CoQ10 after 1 min was 289 ± 22, 309 ± 26, 346 ± 27, and 367 ± 28, respectively. Incorporation increased by approximately 10% after 5 min and 30% after 15 min incubation and then decreased after 30 min.
TABLE 1.
Incorporation of [U-14C]PHBA into P. carinii CoQ homologs: relative specific activitiesa
| Parameter | CoQ7 | CoQ8 | CoQ9 | CoQ10 |
|---|---|---|---|---|
| % of total radioactivityb | 23.02 | 24.10 | 25.62 | 27.26 |
| % of total massc | 0.16 | 4.19 | 33.90 | 61.76 |
| Relative sp actd | 143.88 | 5.75 | 0.76 | 0.44 |
Whole P. carinii cell-homogenates were incubated with 543 μM cold PHBA and 10 μCi of [U-14C]PHBA. Lipids were extracted, and total ubiquinones were purified by TLC, and then each homolog was isolated by HPLC.
Radioactivity in each homolog divided by the sum of radioactivities in all four homologs.
Mass (HPLC peak area) of each homolog divided by the sum of masses of all four homolog.
Percentage of total radioactivity divided by percentage of total mass.
Incorporation of PHBA into P. carinii CoQ was independent of mitochondrial electron transport, proton-motive force, and oxidative phosphorylation. The respiratory inhibitor antimycin A, which blocks electron translocation from cytochrome b to c1 and hence ATP production, and the H+-ATPase pump inhibitor DCCD had no effect on P. carinii CoQ synthesis (Table 2).
TABLE 2.
Effects of cell respiration inhibitors on the incorporation of [U-14C]PHBA into P. carinii ubiquinonesa
| Inhibitor and concn | Range of radioactivities (dpm) (103) | Incorporation (pmol · mg protein−1 · min−1)b | % of control |
|---|---|---|---|
| Antimycin A (μg/ml) | |||
| 0 | 118-141 | 2,086 ± 136 | 100 |
| 10 | 127-132 | 2,013 ± 101 | 97 |
| 100 | 126-130 | 1,999 ± 58 | 96 |
| DCCD (μM) | |||
| 0 | 87-94 | 1,410 ± 31 | 100 |
| 100 | 88-91 | 1,291 ± 24 | 92 |
| 100 + 10 nM atovaquone | 31-48 | 564 ± 39 | 40 |
Whole-cell homogenates were incubated with 543 μM PHBA containing 5 μCi [U-14C]PHBA.
Mean ± SEM; n = 3.
Atovaquone inhibition of PHBA incorporation into total P. carinii CoQ.
Atovaquone strongly inhibited PHBA incorporation into P. carinii CoQ. It was previously observed that PHBA incorporation into CoQ in intact organisms was affected by atovaquone. There was a sharp decline at a low drug concentration (10 nM) followed by stimulation at a higher drug concentration and then a gradual decline. This response is consistent with the suggestion that CoQ biosynthetic rates in intact organisms are regulated by cross talk signals between different cellular compartments (31). Inhibition did not exceed 60%. This response was not observed using cell homogenates in the present study.
Kinetic analysis of experiments on whole-cell homogenates indicated that atovaquone was a strong competitive inhibitor, with a Ki value of 2.0 ± 0.2 nM compared to the Km of PHBA (131 μM) (Fig. 3D and E). However, as in intact cells, inhibition of P. carinii CoQ biosynthesis by atovaquone did not exceed 60% (Fig. 3F). This level of inhibition was attained with 10 nM atovaquone and remained unchanged by drug concentrations up to 10 μM.
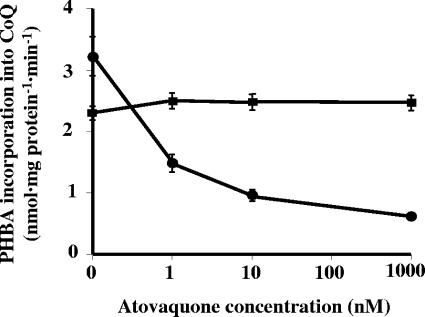
We next investigated whether atovaquone inhibited the synthesis of all CoQ homologs or only specific CoQ homologs and not other homologs. Thus, we analyzed the effect of the drug on the incorporation of PHBA into each CoQ homolog. Atovaquone inhibited the synthesis of all four homologs in a concentration-dependent manner (Fig. 4). Again, the maximal inhibition was approximately 60% of no-drug control. These results suggested that CoQ biosynthetic activity occurred in more than one cellular compartment, which we then tested by analyzing subcellular fractions.
FIG. 4.
Inhibition of PHBA incorporation into P. carinii CoQ homologs by atovaquone using whole-cell homogenates. Homologs in each set are arranged from left to right and correspond to CoQ7, CoQ8, CoQ9, and CoQ10, respectively.
Subcellular fractions.
The subcellular fractions obtained by differential centrifugation were characterized by light microscopy using fluorescence optics (Fig. 5). The mitochondrial fraction contained fluorescent bodies of approximately 1-μm diameter upon staining with MTG, NAO, and TMRE. This indicated that mitochondrial-specific lipids were detected and that a transmembrane potential was preserved in the isolated organelles. In contrast, the microsomal fraction did not contain fluorescent particles, indicating that it was devoid of mitochondria or mitochondrial membrane fragments. Low levels of nonspecific staining were observed, which is typically observed in other cells treated with these stains (24, 34).
FIG. 5.
Light microscopy of P. carinii subcellular fractions treated with MitoTracker Green FM. Bar, 5 μm. A. Intact cells viewed under fluorescence optics. The mitochondria within cells and in the plane of focus are seen exhibiting intense fluorescence. B. The mitochondrial fraction showing particles similar to the size of mitochondria in intact cells was isolated and contained mitochondrial-specific lipids. C. The postmitochondrial fraction does not contain objects exhibiting fluorescence, demonstrating that this subcellular fraction is devoid of mitochondria and mitochondrial fragments.
Marker enzymes for mitochondria, microsomes, and the cytosol were analyzed. The mitochondrial fraction exhibited succinate dehydrogenase activity, whereas this enzyme was not detected in the microsomal and soluble fractions (Fig. 6A). Glucose-6-phosphate dehydrogenase was detected in the postmitochondrial but not the mitochondrial fraction (Fig. 6B). This enzyme activity was predominantly found in the microsomal fraction, although some activity was also detected in the soluble fraction.
FIG. 6.
Marker enzymes for mitochondrial and microsomal fractions. Open diamonds, mitochondrial fraction; open circles, microsomal fraction; X, soluble fraction. A. Succinate dehydrogenase activity was detected in the P. carinii mitochondrial fraction but not in the microsomal or soluble fractions. B. Glucose-6-phosphate dehydrogenase activity was predominantly found in the P. carinii microsomal fraction. A low level of enzyme activity was detected in the soluble fraction; no activity was detected in the mitochondrial fraction.
Subcellular localization of CoQ synthesis and effects of atovaquone on CoQ synthesis.
Incorporation of PHBA into CoQ was examined in subcellular fractions. Rates of incorporation (pmol · mg protein−1 · min−1 ± SEM; n = 3) were higher in the mitochondrial and microsomal fractions (2,456 ± 225 and 3,329 ± 180, respectively) compared to that in whole-cell homogenates (1,766 ± 201). The soluble fraction exhibited very low activity (25 ± 6).
We then addressed the question of whether certain CoQ homologs were synthesized in the mitochondria and others were synthesized in microsomes. Thus, we measured the incorporation of radiolabeled PHBA into individual homologs in these two subcellular fractions. PHBA was incorporated into all four homologs in both the P. carinii mitochondrial and microsomal fractions (Table 3). The rates for incorporation of PHBA into all CoQ homologs (pmol · mg protein−1 · min−1) were higher in the microsomal fraction. The radioactivity in each homolog reflected that observed in whole cells, i.e., radioactivity was progressively slightly higher as CoQ polyprenyl chain length increased. Relative specific activities of each homolog in the two subcellular fractions were not determined in the present study.
TABLE 3.
Incorporation of [U-14C]PHBA into individual CoQ homologs synthesized in the mitochondrial and microsomal fractions of P. cariniia
| CoQ homolog | Incorporation of [U-14C]PHBA into CoQ (pmol · mg protein−1 · min−1)
|
|
|---|---|---|
| Mitochondria | Microsomes | |
| CoQ7 | 518 ± 10 | 624 ± 52 |
| CoQ8 | 574 ± 55 | 736 ± 61 |
| CoQ9 | 660 ± 27 | 870 ± 18 |
| CoQ10 | 732 ± 30 | 894 ± 78 |
Mitochondrial and microsomal fractions containing 0.125 to 0.250 mg of protein were incubated under conditions similar to those used for assays on whole-cell homogenates. Values are means ± SEM; n = 3.
The mitochondrial and microsomal fractions were then compared for the efficacy of atovaquone on inhibiting PHBA incorporation into ubiquinones synthesized in these two cell compartments. The drug was effective in inhibiting CoQ biosynthesis in the microsomal fraction in a concentration-dependent fashion (Fig. 7). In contrast, atovaquone had no effect on the incorporation of PHBA into CoQ in the mitochondrial fraction.
FIG. 7.
Effects of atovaquone on [U-14C]PHBA incorporation into CoQ in P. carinii mitochondrial and microsomal fractions. Circles, mitochondrial fraction; squares, microsomal fraction. Incorporation into the microsomal fraction was inhibited, whereas the drug had no effect on CoQ synthesis in the mitochondrial fraction. Each point represents the mean ± SEM; n = 3.
DISCUSSION
The effect of temperature on the rate of PHBA incorporation into CoQ showed the highest activity at 37°C. However, elevated activity at 20°C was also observed. As growth of P. carinii in an axenic culture system was reported to be optimal at 20°C (44), this observation is consistent with the ability of isolated organisms to adjust to a lower temperature after removal from the mammalian host. These results also suggest the possibility that the organism might not be strict endosymbionts (endoparasites) and can normally live in an environment outside mammals.
Subcellular fractionation and experiments on intracellular compartments have not been previously performed on Pneumocystis, although the cyst wall and the cell surface membrane of P. carinii have been isolated and direct biochemical analyses of the glycoproteins have been described (8, 9). Thus, the present report is the first direct examination of the biochemistry and function of subcellular fractions in this unusual organism. Two enzymes, succinic dehydrogenase and G6PDH, have now been demonstrated in P. carinii. Glucose-6-phosphatase activity was also examined and was found highest in the soluble fraction (data not shown).
Localization of CoQ synthesis has not been studied in most organisms. Exceptions are mammalian tissues such as the rat liver, where it was first shown to occur in mitochondrial membranes (48). Following the discovery that CoQ is found in other cellular membranes, the endoplasmic reticulum (ER)-Golgi system was identified as another cellular compartment where CoQ biosynthesis occurs (27, 28, 51, 68, 69). Localization of CoQ synthesis in the plant cell ER-Golgi was also reported in spinach (62) and Arabidopsis thaliana (26), which is consistent with our data. Recently, the peroxisome was identified as a site for CoQ synthesis in rat liver (67).
In the present study, we showed that atovaquone is a strong competitive inhibitor of microsomal CoQ synthesis (Ki = 2 nM), which is on the same order of magnitude that the drug competitively inhibits the mitochondrial bc1 complex (Ki = 9 nM) isolated from S. cerevisiae (37). However, the mechanism of action of atovaquone on P. carinii microsomal CoQ synthesis is consistent with negative product feedback inhibition of the ubiquinone biosynthetic pathway. There is evidence that a high concentration of a specific CoQ homolog inhibits the incorporation of PHBA into that homolog in P. carinii (33; M. Basselin and E. S. Kaneshiro, unpublished data), which is in agreement with this hypothesis. Also, several other ubiquinone analog compounds exhibited similar inhibitory activities on P. carinii CoQ synthesis (E. S. Kaneshiro, M. Basselin, and O. Kayser, unpublished data). These observations demonstrate that high-affinity binding to cytochrome b is not the only biological activity of atovaquone against the pathogen.
The cell surface membrane electrical potential of animal cells is generated by pumps that extrude Na+, whereas cells of all other organisms are believed to extrude H+ to maintain the electrochemical gradient (19). It was shown that the transmembrane electrical potential at the cell surface of P. carinii is generated and maintained by proton ATPase pumps (72), and a P. carinii P-type proton ATPase gene was cloned and characterized (43). Thus, a CoQ-based electron transport system might participate in maintaining this H+ electrochemical gradient, providing the energy for cell surface membrane functions such as nutrient uptake and ion translocation.
It cannot be ruled out that atovaquone might reduce Pneumocystis viability not only because it inhibits mitochondrial electron transport but also as the result of a combination of actions on the mitochondrial and cell surface membranes and on the biosynthetic processes occurring in the ER-Golgi system as well. The observation that atovaquone inhibits microsomal CoQ synthesis suggests that a block of this process in this cellular compartment might lead to depletion of cell surface membrane CoQ. The cell surface membrane would then become dysfunctional, with deleterious consequences for the pathogen. Thus, microsomal CoQ synthesis would be an attractive target for the development of chemotherapeutic approaches against PcP.
The structural or biochemical basis for the differential effects of atovaquone in the two cell fractions is not currently understood. Atovaquone clearly enters the Pneumocystis mitochondrion, since it binds to cytochrome b and oxygen consumption is effectively blocked (17). Thus, the refractive nature of P. carinii mitochondrial CoQ synthesis to atovaquone suggests that the drug does not have access to the specific site where the biosynthetic machinery is operating within this organelle. Alternatively, the CoQ biosynthetic machineries in the mitochondria and the ER-Golgi system may have important structural differences that govern the accessibility of atovaquone to CoQ synthetic reactions, such as PHBA:polyprenyl transferases. The mechanisms of CoQ synthesis and its inhibition by atovaquone in this organism need further investigations. For example, the differential inhibition of atovaquone we observed on subcellular fractions might be evaluated on PHBA:polyprenyl transferases isolated from mitochondria and microsomes. Such studies would show whether accessibility of atovaquone to the mitochondrial CoQ biosynthetic machinery is hindered or if there are important differences in the conformation of the enzyme proteins in different cell compartments.
Ubiquinone homolog biosynthesis and their subcellular localizations have been studied in only a few cell types and organisms (26, 52). This is the first demonstration that biosynthesis of four ubiquinone homologs from the condensation of PHBA with a polyprenyl diphosphate to completed CoQ molecules occurs in both the mitochondria and microsomes in a eukaryotic pathogenic microbe.
Acknowledgments
We thank Oliver Kayser for generously providing atovaquone, Salim Merali for some P. carinii preparations used in this study, Peter Tang for assistance with HPLC analysis, and Harry Rudney for valuable discussions.
This work is supported by grants R21 AI49145 and RO1 AI29316 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Barr, R., B. A. Branstetter, A. Rajnicek, F. L. Crane, and H. Low. 1991. Chloroquine-sensitive transplasmalemma electron transport in Tetrahymena pyriformis: a hypothesis for control of parasite protozoa through transmembrane redox. Biochim. Biophys. Acta 1058:261-268. [DOI] [PubMed] [Google Scholar]
- 2.Bonner, W. D. 1955. Succinic dehydrogenase. Methods Enzymol. 1:722-725. [Google Scholar]
- 3.Cirioni, O., A. Giacometti, and G. Scalise. 1997. In vitro activity of atovaquone, sulphamethoxazole and dapsone alone and combined with inhibitors of dihydrofolate reductase and macrolides against Pneumocystis carinii. J. Antimicrob. Chemother. 39:45-51. [DOI] [PubMed] [Google Scholar]
- 4.Córdoba-Pedregosa, M. del, C., J. M. Villalba, and F. J. Alcaín. 2005. Determination of coenzyme Q biosynthesis in cultured cells without the necessity for lipid extraction. Anal. Biochem. 336:60-63. [DOI] [PubMed] [Google Scholar]
- 5.Crane, F. L., I. L. Sun, and E. E. Sun. 1993. The essential functions of coenzyme Q. Clin. Investig. 71(8 Suppl.):zfpg>S55-S59. [DOI] [PubMed]
- 6.Crofts, A. R., S. Hong, N. Ugulava, B. Barquera, R. Gennis, M. Guergova-Kuras, and E. A. Berry. 1999. Pathways for proton release during ubihydroquinone oxidation by the bc1 complex. Proc. Natl. Acad. Sci. USA 96:1002-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cushion, M. T., M. Collins, B. Hazra, and E. S. Kaneshiro. 2000. The effects of atovaquone and diospyrin-based drugs on the ATP content of Pneumocystis carinii f. sp. carinii. Antimicrob. Agents Chemother. 44:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Stefano, J. A., M. T. Cushion, V. Puvanesarajay, and P. D. Walzer. 1990. Analysis of Pneumocystis carinii cyst wall. II. Sugar composition. J. Protozool. 37:436-441. [DOI] [PubMed] [Google Scholar]
- 9.De Stefano, J. A., J. D. Myers, D. Du Pont, J. M. Foy, S. A. Theus, and P. D. Walzer. 1998. Cell wall antigens of Pneumocystis carinii trophozoites and cysts: purification and carbohydrate analysis of these glycoproteins. J. Eukaryot. Microbiol. 45:334-343. [DOI] [PubMed] [Google Scholar]
- 10.Ding, H., F. Daldal, and P. L. Dutton. 1995. Ion pair formation between basic residues at 144 of the Cyt b polypeptide and the ubiquinones at the Qo site of the Cyt bc1 complex. Biochemistry 34:15997-16003. [DOI] [PubMed] [Google Scholar]
- 11.Dohn, M. N., P. T. Frame, R. P. Baughman, S. W. Lafon, A. G. Smulian, P. Caldwell, and M. D. Rogers. 1992. Open-label efficacy and safety trial of 42 days of 566C80 for Pneumocystis carinii pneumonia in AIDS patients. J. Eukaryot. Microbiol. 38:220S-221S. [PubMed] [Google Scholar]
- 12.Ellis, J. E. 1994. Coenzyme Q homologs in parasitic protozoa as targets for chemotherapeutic attack. Parasitol. Today 10:296-301. [DOI] [PubMed] [Google Scholar]
- 13.Ellis, J. E., K. D. R. Setchell, and E. S. Kaneshiro. 1994. Detection of ubiquinone in parasitic and free-living protozoa, including species devoid of mitochondria. Mol. Biochem. Parasitol. 65:213-224. [DOI] [PubMed] [Google Scholar]
- 14.Ellis, J. E., M. A. Wyder, L. Zhou, A. Gupta, H. Rudney, and E. S. Kaneshiro. 1996. Composition of Pneumocystis neutral lipids and identification of coenzyme Q10 as the major ubiquinone homolog in P. carinii carinii. J. Eukaryot. Microbiol. 43:165-170. [DOI] [PubMed] [Google Scholar]
- 15.Fry, M., and M. Pudney. 1992. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem. Pharmacol. 43:1545-1553. [DOI] [PubMed] [Google Scholar]
- 16.Gille, L., and H. Nohl. 2000. The existence of a lysosomal redox chain and the role of ubiquinone. Arch. Biochem. Biophys. 375:347-354. [DOI] [PubMed] [Google Scholar]
- 17.Gutteridge, W. 1991. 566C80, an antimalarial hydroxynaphthoquinone with broad spectrum: experimental activity against opportunistic parasitic infections of AIDS patients. J. Eukaryot. Microbiol. 38:141S-143S. [PubMed] [Google Scholar]
- 18.Haile, L. G., and J. F. Flaherty. 1993. Atovaquone: a review. Ann. Pharmacother. 27:1488-1494. [DOI] [PubMed] [Google Scholar]
- 19.Haines, T. H. 2001. Do sterols reduce proton and sodium leaks through lipid bilayers? Prog. Lipid Res. 40:299-324. [DOI] [PubMed] [Google Scholar]
- 20.Hihi, A. K., Y. Gao, and S. Hekimi. 2002. Ubiquinone is necessary for Caenorhabditis elegans development at mitochondrial and non-mitochondrial sites. J. Biol. Chem. 277:2202-2206. [DOI] [PubMed] [Google Scholar]
- 21.Hill, P., J. Kessl, N. Fisher, S. Meshnick, B. L. Trumpower, and B. Meunier. 2003. Recapitulation in Saccharomyces cerevisiae of cytochrome b mutations conferring resistance to atovaquone in Pneumocystis jiroveci. Antimicrob. Agents Chemother. 47:2725-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes, W. T. 1995. The role of atovaquone tablets in treating Pneumocystis carinii pneumonia. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:247-252. [DOI] [PubMed] [Google Scholar]
- 23.Ittarat, I., W. Asawamahasakda, M. S. Bartlett, J. W. Smith, and S. R. Meshnick. 1995. Effects of atovaquone and other inhibitors on Pneumocystis carinii dihydroorotate dehydrogenase. Antimicrob. Agents Chemother. 39:325-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson, J., M. R. Duchen, and S. J. R. Heales. 2002. Intracellular distribution of the fluorescent dye nonyl acridine orange responds to the mitochondrial membrane potential: implications for assays of cardiolipin and mitochondrial mass. J. Neurochem. 82:224-233. [DOI] [PubMed] [Google Scholar]
- 25.James, A. M., R. A. Smith, and M. P. Murphy. 2004. Antioxidant and prooxidant properties of mitochondrial coenzyme Q. Arch. Biochem. Biophys. 423:47-56. [DOI] [PubMed] [Google Scholar]
- 26.Jun, L., R. Saiki, K. Tatsumi, T. Nakagawa, and M. Kawamukai. 2004. Identification and subcellular localization of two solanesyl diphosphate synthases from Arabidopsis thaliana. Plant Cell Physiol. 45:1882-1888. [DOI] [PubMed] [Google Scholar]
- 27.Kalén, A., B. Norling, E. L. Appelkvist, and G. Dallner. 1987. Ubiquinone biosynthesis by microsomal fraction from rat liver. Biochim. Biophys. Acta 926:70-78. [DOI] [PubMed] [Google Scholar]
- 28.Kalén, A., E. L. Appelkvist, T. Choinacki, and G. Dallner. 1990. Nonaprenyl-4-hydroxybenzoate transferase, an enzyme involved in ubiquinone biosynthesis, in the endoplasmic reticulum-Golgi system of rat liver. J. Biol. Chem. 265:1158-1164. [PubMed] [Google Scholar]
- 29.Kaneshiro, E. S. 2002. Pneumocystis, p. 654-661. Encyclopedia of life sciences, vol. 15. Nature Publishing Group, London, England. [Online.] http://www.els.net. [Google Scholar]
- 30.Kaneshiro, E. S. 2002. Is Pneumocystis a plant? J. Eukaryot. Microbiol. 49:367-373. [DOI] [PubMed] [Google Scholar]
- 31.Kaneshiro, E. S., D. Sul, and B. Hazra. 2000. Effects of atovaquone and diospyrin-based drugs on ubiquinone biosynthesis in the opportunistic pathogen Pneumocystis carinii. Antimicrob. Agents Chemother. 44:14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaneshiro, E. S., M. A. Wyder, L. H. Zhou, J. E. Ellis, D. R. Voelker, and S. G. Langreth. 1993. Characterization of Pneumocystis carinii preparations developed for lipid analysis. J. Eukaryot. Microbiol. 40:805-815. [DOI] [PubMed] [Google Scholar]
- 33.Kaneshiro, E. S., M. Basselin, and S. M. Hunt. 2003. Evidence that biosynthesis of individual ubiquinone homologs in Pneumocystis carinii is under homolog-specific negative feedback (product) control. J. Eukaryot. Microbiol. 50:622-623. [DOI] [PubMed] [Google Scholar]
- 34.Kawai, F., M. Shoda, R. Harashima, Y. Sadaie, H. Hara, and K. Matsumoto. 2004. Cardiolipin domains in Bacillus subtilis Marburg membranes. J. Bacteriol. 186:1475-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kazanjian, P., W. Armstrong, P. A. Hossler, L. Huang, C. B. Beard, J. Carter, L. Crane, J. Duchn, W. Burman, J. Richardson, and S. R. Meshnick. 2001. Pneumocystis carinii cytochrome b mutations are associated with atovaquone exposure in patients with AIDS. J. Infect. Dis. 183:819-822. [DOI] [PubMed] [Google Scholar]
- 36.Keij, J. F., C. Bell-Prince, and J. A. Steinkamp. 2000. Staining of mitochondrial membranes with 10-nonyl acridine orange MitoFluor Green, and MitoTracker Green is affected by mitochondrial membrane potential altering drugs. Cytometry 39:203-210. [DOI] [PubMed] [Google Scholar]
- 37.Kessl, J. J., B. B. Lange, T. Merbitz-Zahradnik, K. Zwicker, P. Hill, B. Meunier, H. Palsdottir, C. Hunte, S. Meshnick, and B. L. Trumpower. 2003. Molecular basis for atovaquone binding to the cytochrome bc1 complex. J. Biol. Chem. 278:31312-31318. [DOI] [PubMed] [Google Scholar]
- 38.Kessl, J. J., P. Hill, B. B. Lange, S. R. Meshnick, B. Meunier, and B. L. Trumpower. 2004. Molecular basis for atovaquone resistance in Pneumocystis jirovecii modeled in the cytochrome bc1 complex of Saccharomyces cerevisiae. J. Biol. Chem. 279:2817-2824. [DOI] [PubMed] [Google Scholar]
- 39.Kovacs, J. A., V. J. Gill, S. Meshnick, and H. Masur. 2001. New insights into transmission, diagnosis, and drug treatment of Pneumocystis carinii pneumonia. JAMA. 286:2450-2460. [DOI] [PubMed] [Google Scholar]
- 40.Larsen, P. L., and C. F. Clarke. 2002. Extension of life-span in Caenorhabditis elegans by a diet lacking coenzyme Q. Science 295:120-123. [DOI] [PubMed] [Google Scholar]
- 41.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 42.McFadden, D. C., S. Tomavo, E. A. Berry, and J. C. Boothroyd. 2000. Characterization of cytochrome b from Toxoplasma gondii and Qo domain mutations as a mechanism of atovaquone resistance. Mol. Biochem. Parasitol. 108:1-12. [DOI] [PubMed] [Google Scholar]
- 43.Meade, J. C., and J. R. Stringer. 1995. Cloning and characterization of an ATPase gene from Pneumocystis carinii which closely resembles fungal H+ ATPases. J. Eukaryot. Microbiol. 42:298-307. [DOI] [PubMed] [Google Scholar]
- 44.Merali, S., U. Frevert, J. H. Williams, K. Chin, R. Bryan, and A. B. Clarkson, Jr. 1999. Continuous axenic cultivation of Pneumocystis carinii. Proc. Natl. Acad. Sci. USA 96:2402-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mileykovskaya, E., and W. Dowhan. 2000. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J. Bacteriol. 182:1172-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mileykovskaya, E., W. Dowhan, R. L. Birke, D. Zheng, L. Lutterodt, and T. H. Haines. 2001. Cardiolipin binds nonyl acridine orange by aggregating the dye at exposed hydrophobic domains on bilayer surfaces. FEBS Lett. 507:187-190. [DOI] [PubMed] [Google Scholar]
- 47.Mohora, M., E. Katona, and V. Dinu. 1999. Pro- and antioxidant functions of quinones in mammalian cells. Rom. J. Intern. Med. 37:3-14. [PubMed] [Google Scholar]
- 48.Momose, K., and H. Rudney. 1972. 3-Polyprenyl-4-hydroxybenzoate synthesis in the inner membrane of mitochondria from p-hydroxybenzoate and isopentenylpyrophosphate. A demonstration of isoprenoid synthesis in rat liver mitochondria. J. Biol. Chem. 247:3930-3940. [PubMed] [Google Scholar]
- 49.Nambudiri, A. M., D. Brockman, S. S. Alam, and H. Rudney. 1972. Alternate routes for ubiquinone biosynthesis in rats. Biochem. Biophys. Res. Commun. 76:282-288. [DOI] [PubMed] [Google Scholar]
- 50.Nohl, H., A. V. Kozlov, K. Staniek, and L. Gille. 2001. The multiple functions of coenzyme Q. Bioorg. Chem. 29:1-13. [DOI] [PubMed] [Google Scholar]
- 51.Nyquist, S. E., R. Barr, and D. J. Morre. 1970. Ubiquinone from rat liver Golgi apparatus fractions. Biochim. Biophys. Acta 208:532-534. [DOI] [PubMed] [Google Scholar]
- 52.Okada, K., K. Ohara, K. Yazaki, K. Nozaki, N. Uchida, M. Kawamukai, H. Nojiri, and H. Yamane. 2004. The AtPPT1 gene encoding 4-hydroxybenzoate polyprenyl diphosphate transferase in ubiquinone biosynthesis is required for embryo development in Arabidopsis thaliana. Plant Mol. Biol. 55:567-577. [DOI] [PubMed] [Google Scholar]
- 53.Olson, R. E., and H. Rudney. 1983. Biosynthesis of ubiquinone. Vitam. Horm. 40:1-43. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Aguilera, J. C., A. Gavilan, C. Asencio, and P. Navas. 2005. The role of ubiquinone in Caenorhabditis elegans longevity. Ageing Res. Rev. 4:41-53. [DOI] [PubMed] [Google Scholar]
- 55.Santos-Ocana, C., F. Cordoba, F. L. Crane, C. F. Clarke, and P. Navas. 1998. Genetic evidence for coenzyme Q requirement in plasma membrane transport. J. Bioenerget. Biomembr. 30:465-475. [DOI] [PubMed] [Google Scholar]
- 56.Sastry, P. S., J. Jayaraman, and T. Ramasarma. 1961. Distribution of coenzyme Q in rat liver cell fractions. Nature (London) 189:577. [DOI] [PubMed] [Google Scholar]
- 57.Scaduto, R. C., Jr., and L. W. Grotyohann. 1999. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 76:469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srivastava, I. K., J. M. Morrisey, E. Darrouzet, F. Daldal, and A. B. Vaidya. 1999. Resistance mutants reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol. Microbiol. 33:704-711. [DOI] [PubMed] [Google Scholar]
- 59.Stocker, R., V. W. Bowry, and B. Frei. 1991. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does α-tocopherol. Proc. Natl. Acad. Sci. USA 88:1646-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sul, D., and E. S. Kaneshiro. 2001. Pneumocystis carinii f. sp. carinii biosynthesizes de novo four homologs of ubiquinone. J. Eukaryot. Microbiol. 48:184-189. [DOI] [PubMed] [Google Scholar]
- 61.Sun, I. L., E. E. Sun, F. L. Crane, D. J. Morré, A. Lindgren, and H. Löw. 1992. Requirement for coenzyme Q in plasma membrane electron transport. Proc. Natl. Acad. Sci. USA 89:11126-11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swiezewska, E., G. Dallner, B. Andersson, and L. Ernster. 1993. Biosynthesis of ubiquinone and plastoquinone in the endoplasmic reticulum-Golgi membranes of spinach leaves. J. Biol. Chem. 268:1494-1499. [PubMed] [Google Scholar]
- 63.Syafruddin, D., J. E. Siregar, and S. Marzuki. 1999. Mutations in the cytochrome b gene of Plasmodium berghei conferring resistance to atovaquone. Mol. Biochem. Parasitol. 104:185-194. [DOI] [PubMed] [Google Scholar]
- 64.Szkopinska, A. 2000. Ubiquinone. Biosynthesis of quinone ring and its isoprenoid side chain. Intracellular localization. Acta Biochim. Pol. 47:469-480. [PubMed] [Google Scholar]
- 65.Tang, P. H., M. V. Miles, A. DeGrauw, A. Hershey, and A. Pesce. 2001. HPLC analysis of reduced and oxidized coenzyme Q10 in human plasma. Clin. Chem. 47:256-265. [PubMed] [Google Scholar]
- 66.Tang, P. H., M. V. Miles, L. Miles, J. Quinlan, B. Wong, A. Wenisch, and K. Bove. 2004. Measurement of reduced and oxidized coenzyme Q9 and coenzyme Q10 levels in mouse tissues by HPLC with coulometric detection. Clin. Chim. Acta 341:173-184. [DOI] [PubMed] [Google Scholar]
- 67.Teckle, M., M. Bentinger, T. Nordman, E.-L. Appelkvist, T. Chojnacki, and J. M. Olsson. 2002. Ubiquinone biosynthesis in rat liver peroxisomes. Biochem. Biophys. Res. Commun. 291:1128-1133. [DOI] [PubMed] [Google Scholar]
- 68.Teclebrhan, H., J. Olsson, E. Swiezewska, and G. Dallner. 1993. Biosynthesis of the side chain of ubiquinone: trans-prenyltransferase in rat liver microsomes. J. Biol. Chem. 268:23081-23086. [PubMed] [Google Scholar]
- 69.Teclebrhan, H., A. Jakobsson-Borin, U. Brunk, and G. Dallner. 1995. Relationship between the endoplasmic reticulum-Golgi membrane system and ubiquinone biosynthesis. Biochim. Biophys. Acta 1256:157-165. [DOI] [PubMed] [Google Scholar]
- 70.Turunen, M., J. Olsson, and G. Dallner. 2004. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta 1660:171-199. [DOI] [PubMed] [Google Scholar]
- 71.Uchida, N., K. Suzuki, R. Saiki, T. Kainou, K. Tanaka, H. Matsuda, and M. Kawamukai. 2000. Phenotypes of fission yeast defective in ubiquinone production due to disruption of the gene for p-hydroxybenzoate polyprenyl diphosphate transferase. J. Bacteriol. 182:6933-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.VanderHeyden, N., G. L. McLaughlin, and R. Docampo. 2000. Regulation of the plasma membrane potential in Pneumocystis carinii. FEMS Microbiol. Lett. 183:327-330. [DOI] [PubMed] [Google Scholar]
- 73.Walker, D. J., A. E. Wakefield, M. N. Dohn, R. F. Miller, R. P. Baughman, P. A. Hossler, M. S. Bartlett, J. W. Smith, P. Kazanjian, and S. R. Meshnick. 1998. Sequence polymorphism in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J. Infect. Dis. 178:1767-1775. [DOI] [PubMed] [Google Scholar]
- 74.Walzer, P. D., and M. T. Cushion (ed.). 2004. Pneumocystis carinii pneumonia, 3rd ed. Marcel Dekker, New York, N.Y.
- 75.Wanke, M., G. Dallner, and E. Swiezewska. 2000. Subcellular localization of plastoquinone and ubiquinone synthesis in spinach cells. Biochim. Biophys. Acta 1463:188-194. [DOI] [PubMed] [Google Scholar]
- 76.Yoshida, Y. 1989. Ultrastructural studies of Pneumocystis carinii. J. Protozool. 36:53-60. [DOI] [PubMed] [Google Scholar]