Abstract
Set2 methylation of histone H3 at lysine 36 (K36) has recently been shown to be associated with RNA polymerase II (Pol II) elongation in Saccharomyces cerevisiae. However, whether this modification is conserved and associated with transcription elongation in other organisms is not known. Here we report the identification and characterization of the Set2 ortholog responsible for K36 methylation in the fission yeast Schizosaccharomyces pombe. We find that similar to the budding yeast enzyme, S. pombe Set2 is also a robust nucleosome-selective H3 methyltransferase that is specific for K36. Deletion of the S. pombe set2+ gene results in complete abolishment of K36 methylation as well as a slow-growth phenotype on plates containing synthetic medium. These results indicate that Set2 is the sole enzyme responsible for this modification in fission yeast and is important for cell growth under stressed conditions. Using the chromatin immunoprecipitation assay, we demonstrate that K36 methylation in S. pombe is associated with the transcribed regions of Pol II-regulated genes and is devoid in regions that are not transcribed by Pol II. Consistent with a role for Set2 in transcription elongation, we find that S. pombe Set2 associates with the hyperphosphorylated form of Pol II and can fully rescue K36 methylation and Pol II interaction in budding yeast cells deleted for Set2. These results, along with our finding that K36 methylation is highly conserved among eukaryotes, imply a conserved role for this modification in the transcription elongation process.
Covalent histone modifications represent a major mechanism by which cells regulate the structure and function of chromatin. A number of different posttranslational modifications are known to occur on histones, including acetylation, methylation, phosphorylation, ubiquitylation, and, more recently, sumoylation (7, 17, 36, 41). While the majority of these modifications are restricted to the flexible N- and C-terminal tail domains of these proteins, a significant number of these modifications have been identified in their highly structured globular domains (11, 53). The function of these modifications are not well understood, but it is becoming increasingly clear that they coordinate their effects in the form of a histone code to regulate the complex and diverse activities associated with DNA in chromatin (21, 46, 50).
A large body of work now shows that histone methylation plays a key role in the regulation of chromatin structure and function. In particular, studies show that the methylation of lysine and/or arginine residues regulates diverse cellular functions such as transcriptional repression and activation, heterochromatin formation, X inactivation, and polycomb-mediated gene silencing (10, 14, 19, 23, 26, 54). More recently, studies have revealed an unexpected role for histone methylation in the process of transcription elongation by RNA polymerase II (Pol II). In the budding yeast Saccharomyces cerevisiae, the histone methyltransferases Set1 and Set2, which catalyze H3 lysine 4 (K4) and lysine 36 (K36) methylation, respectively, have been found to be associated with the elongation-competent form of Pol II (13, 15). While Set1 association is dependent on the Kin28 kinase, which phosphorylates the serine 5 (Ser5) position of the C-terminal domain (CTD) of Pol II (13, 15, 24, 31), Set2 association and methylation is dependent on Ctk1, which phosphorylates the serine 2 position of the CTD (25, 27, 28, 39, 52). While the precise function of these enzyme associations with Pol II is still unclear, it is believed that K4 and K36 methylation function in the elongation process at different stages of the transcription elongation cycle (42, 43).
To date, the association of Set2 with elongating Pol II has only been demonstrated in S. cerevisiae. Whether this enzyme has a conserved role and associates with Pol II in other organisms is not known. In the fission yeast Schizosaccharomyces pombe, Set1-mediated H3 K4 methylation is preferentially enriched at the euchromatic loci, in particular at the regions containing open reading frames (33, 34). Moreover, a potential ortholog of S. cerevisiae Set2 has been identified in S. pombe (34). In this report, we characterize the fission yeast S. pombe Set2 (SpSet2) and find that this enzyme is a robust K36 methyltransferase that mediates nucleosome-selective methylation. Similar to what is found in budding yeast, K36 methylation in S. pombe is restricted to the coding region of active genes, and we show that the SpSet2 enzyme interacts with Pol II and restores K36 methylation in S. cerevisiae when the endogenous SET2 gene is deleted. These studies, and the fact that K36 methylation is conserved across eukaryotes, suggest a highly conserved role for K36 methylation in transcription.
MATERIALS AND METHODS
Yeast strains.
The S. pombe yeast strains used in these studies were SP1173 (wild type; h- leu1-32 his2 ura4 ade6-216) and SPK549 (set2Δ h+ leu1-32 ura4 set2::kanMX6 cen1::ura4 ade6-210). For the growth assays, S. pombe strains SPK131 (wild type; h- leu1-32 his2 ura4 Rint2::ura4 ade6-216) and SPK612 (set2Δ h- leul-32 his2 ura4 Rint2::ura4 set2:kanMX6 ade6-210) were used. The set2Δ strain was constructed by a PCR-based method using a kanMX6 module to replace the S. pombe set2+ gene as described (5). Deletion was confirmed by PCR and Southern blot analysis. SP1173 was used to set a genomic 3XFlag epitope tag in S. pombe set2+ as described (5), producing the SpSet2-3Flag strain SPK653 (h+ leu1-32 ura4 set2-3XFLAG-kan cen1::ura4 ade6-210). S. cerevisiae wild-type and set2Δ strains in the BY4742 background were obtained from Research Genetics.
Preparation of histones.
Histones were prepared as previously described (47). Briefly, nuclei were isolated by detergent lysis and low-speed centrifugation from 293T cells grown at 37°C in Dulbecco's modified Eagle's medium containing 5% fetal bovine serum. Histones were extracted from nuclei by either DNase I or acid extraction. Wild-type Tetrahymena thermophila was grown in enriched 1% proteose peptone, and macronuclear histones were isolated from vegetatively growing cells (47). Nuclei were isolated from wild-type S. cerevisiae cells grown in yeast extract-peptone-dextrose medium, and histones were acid extracted from isolated nuclei as described (47).
Sequence analysis (Set2 homology search).
Database searches and protein sequence identifications were performed with BLAST (3) and PSI-BLAST (4). Sequences were aligned and the phylogeny tree was calculated using the neighbor-joining method in the program Clustal X (20). The resulting dendrogram was displayed using the tree drawing program NJplot (35).
Cloning of S. pombe Set2 and generation of expression constructs.
Using primers specific to the open reading frame of set2+ in S. pombe (S. pombe GeneDB ID SPAC29B12.02c), full-length set2+ with a C-terminal Flag epitope tag inserted just before the stop codon was PCR amplified from genomic DNA. The resulting product was cloned into either the pCAL-n (Stratagene) bacterial or the PN823 yeast expression plasmid. The SpSet2 coding region was sequenced for accuracy. The resulting SpSet2-PN823 (SpSet2-Flag) plasmid, which is driven by the ADH1 promoter, was transformed into the S. cerevisiae set2Δ strain BY4742. As a control, the PN823 plasmid without the open reading frame of set2+ (empty vector) and ScSet2-Flag were transformed into wild-type and set2Δ BY4742 strains. Wild-type full-length ScSet2-Flag bacterial and yeast expression constructs have been described previously (48). Transformants were selected on synthetic complete medium (SC) plates lacking uracil.
Expression of recombinant SpSet2.
Plasmids expressing either SpSet2-Flag or empty vector were transformed into Escherichia coli BL21(DE3) cells. Five-ml cell cultures were grown to an optical density at 600 nm (OD600) of 0.8 to 1.0 in Luria Broth (LB) medium supplemented with ampicillin (100 μg/ml), followed by addition of 1 mM isopropylthiogalactopyranoside (IPTG) for 3 h at 30°C. Harvested cells were resuspended in 600 μl lysis buffer (50 mM Tris-HCl, pH 8.0, 0.1% Triton X-1000, 350 mM NaCl, 10% glycerol, 1 mg/ml lysozyme, 1 mM phenylmethylsulfonyl fluoride, and 2 μg/ml each leupeptin, aprotinin, and pepstatin). Lysates were prepared by sonication as previously described (48).
In vitro histone methyltransferase assays.
Histone methylation assays were performed as previously described with minor modifications (48). Briefly, 1 μl of bacterial lysate was incubated with either 1.25 μg recombinant H3, 5 μg chicken core histones, 5 μg chicken oligonucleosomes, or 5 μg H3 synthetic peptide along with 1 μCi S-adenosyl-l-[methyl-3H]methionine ([3H]SAM, 69.8 Ci/mmol, Amersham Biosciences) in methyltransferase buffer (final concentration 50 mM Tris, pH 9.0, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, and 2 μg/ml each leupeptin, pepstatin, and aprotinin) for 30 min at 30°C in a total volume of 10 μl; 2 μl of the reaction was spotted on p81 Whatman paper, while the remainder was analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) followed by Coomassie staining and fluorography. Identical reactions were performed in parallel using nonradiolabeled SAM (40 μM, Sigma) and analyzed by SDS-PAGE followed by Western blotting with the anti-Me2K36 antibody.
Nuclear and whole-cell lysate extractions.
For nuclei extractions, wild-type and set2Δ S. pombe strains were grown in 1 liter yeast extract supplemented with adenine (YEA) to a final OD600 between 2.0 and 2.5 prior to harvesting. Transformed S. cerevisiae strains were grown to a final OD600 between 2.0 and 2.5 in 200 ml SC lacking uracil prior to harvesting. Nuclei were extracted by Dounce homogenization from these cell pellets as previously described (12). Yeast whole-cell extracts were prepared from 20 ml cultures grown to a final OD600 between 2.5 and 3.0 as described (8) and only differed in the breaking buffer used for cell disruption (50 mM Tris, pH 8.0, 300 mM NaCl, 1 mM Mg-acetate, 1 mM Imidazole, 0.1% NP-40, 0.5 mM EDTA, 10% glycerol, 2 mM PMSF, phosphatase inhibitor cocktail I [5 μl, Sigma], and 2 μg/ml each pepstatin, aprotinin, and leupeptin).
Electrophoresis and immunoblot analyses.
SDS-PAGE and Western blot analyses were performed using procedures and reagents from Amersham Biosciences. The anti-H3 mono-K36Me (anti-Me1K36), tri-K36Me (anti-Me3K36), and C terminus of H3 (anti-H3 C-term) rabbit polyclonal antibodies were obtained from Abcam and used at dilutions of 1:1,000, 1:10,000, and 1:20,000, respectively. All other histone modification-detecting antibodies (rabbit) were obtained from Upstate Biotechnology Inc. and used at the following dilutions: 1:3,500 to 1:5,000 for di-K36Me (anti-Me2K36), 1:20,000 for di-K4Me (anti-Me2K4), 1:10,000 for K9Ac (anti-Ac K9), and 1:40,000 for tri-K4Me (anti-Me3K4). Mouse monoclonal anti-Flag antibody (M2; Sigma) was used at 1 μg/ml. Anti-polymerase CTD antibodies 8WG16 (Unmod CTD) and H14 (Ser5 phosphorylation) were used at dilutions of 1:500 and 1:10,000 to 1:30,000, respectively. The immunoglobulin M H14 antibody was detected using horseradish peroxidase-conjugated donkey anti-mouse immunoglobulin M at 1:5,000 (Jackson ImmunoResearch Laboratories). Typically, 20 to 50 μg of whole-cell extracts or 20 to 100 μg nuclei were resolved on SDS-PAGE gels (8% for RNA Pol II and Flag blots or 13 to 15% for histone modification blots), followed by transfer to polyvinylidene difluoride membranes and immunoblot analyses.
Flag immunoprecipitations.
For Flag immunoprecipitations, 2.0 mg of each whole-cell extract was incubated with 12.5 μl of preequilibrated anti-Flag affinity beads (anti-Flag M2 agarose, Sigma) for 2 h at 4°C. After three washes in extraction buffer, the bead-bound proteins were analyzed by immunoblot analysis using the antibodies and dilutions indicated above.
Growth assays.
The effects of medium on cell growth were tested by growing wild-type and set2Δ strains (SPK131 and SPK612) in rich yeast extract supplemented with adenine (YEA) to a final OD600 of 0.8 and plating 1:10 serial dilutions of cells on either YEA or Edinburgh's minimal medium supplemented with amino acids (EMM). Plates were incubated at 30°C for 3 to 5 days.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed as previously described (52). Briefly, whole-cell extracts were prepared from formaldehyde-fixed wild-type and set2Δ S. pombe strains grown in 100 ml YEA medium to a final OD600 between 1.0 and 1.5. Extracts were sonicated to shear chromatin followed by immunoprecipitation using protein A-Sepharose (Amersham Biosciences) with anti-H3 di-K36Me (anti-Me2K36) at 3 μl/immunoprecipitation. Following washes and DNA elution, cross-links were reversed and DNA was extracted for amplification using standard PCR methods.
Specific regions in the promoter and coding regions of the following genes were amplified: ADE6, PMA1, and ACT1. For a control, we used a primer pair to the K region found in the mating type loci. Primer sequences are available upon request. The results represent the ratio of immunoprecipitated DNA to input DNA normalized to the immunoprecipitation/input ratio from the mating type locus-associated region (K region).
RESULTS
Histone H3 K36 methylation is highly conserved.
While K36 methylation has been demonstrated in budding yeast, its presence and relative abundance in other organisms has not been well established. To determine the conservation and relative abundance of K36 methylation in several diverse organisms, we isolated histones from budding yeast, Tetrahymena thermophila, chicken erythrocyte nuclei, and human 293T cells and probed them for K36 methylation using an antidimethyllysine 36 antiserum (anti-H3 Me2K36). For comparison and as a control, we used an antibody specific to dimethylation at histone H3 lysine 4 (anti-H3 Me2K4).
As shown in Fig. 1, we found that K36 dimethylation was present in all of the organisms analyzed, although the relative abundance varied between species. It is interesting that in Tetrahymena thermophila, K36 dimethylation appears to be less abundant compared to the levels of this modification found in yeast, chicken, and humans (Fig. 1). However, a possible reason for this observation may be an inability of the K36 dimethyl antibody to efficiently recognize Tetrahymena thermophila H3. In yeast, chicken, and humans, K36 is immediately preceded by the amino acid valine, while the predominant form of H3 in Tetrahymena thermophila (H3.1) contains an isoleucine that precedes K36 (GGVK36KPH versus GGIK36KPH). Thus, this amino acid substitution may decrease this antibody's ability to effectively recognize K36 methylation in the context of its surrounding residues in Tetrahymena thermophila. Nonetheless, mass spectrometry analysis confirms that K36 is indeed mono- and dimethylated in this organism (C. D. Allis and D. Hunt, personal communication), although the relative amounts of these methyl forms in Tetrahymena thermophila H3 are not known.
FIG. 1.
Conservation and abundance of histone H3 K36 methylation. We resolved 1 μg of Xenopus laevis recombinant histone H3 and 5 μg of total core histones from the species indicated on 15% SDS-PAGE gels, transferred to a polyvinylidene difluoride membrane, and probed with anti-Me2K36 and anti-Me2K4. Identical samples were examined in parallel by Coomassie staining to show histone loading. The asterisk indicates H3 breakdown products that are typically observed.
In addition to these results, previous studies have shown the existence of K36 methylation in humans, chicken, and sea urchin by protein sequencing (17). Furthermore, we and others have determined that K36 is also methylated in Drosophila melanogaster, Neurospora crassa, and Caenorhabditis elegans (B.D.S., unpublished results) (16). Thus, K36 methylation is found in a broad range of distinct eukaryotes.
With the finding that K36 methylation is highly conserved, we next asked whether Set2 homologs could be identified in these different organisms. Using the AWS (associated with SET), SET, and post-SET domains (amino acids 63 to 260) of the S. cerevisiae Set2 (ScSet2) protein as bait in a PSI-BLAST search, we found a significant number of proteins bearing similar sequence structures to Set2, and assembled them in a hierarchical family tree (Fig. 2). As documented in the figure, the ScSet2 protein was most similar to the S. pombe and Neurospora crassa (NCU00269.1) Set2 proteins (33% and 43% sequence identity, respectively) (34). While not as highly conserved, the domain structure of ScSet2 is found in a number of other proteins found in a variety of diverse organisms.
FIG. 2.
Conservation of Set2 proteins among eukaryotes. (A) Phylogenetic tree of Set2 and its putative homologs. Set2 proteins were identified in different species by using PSI-BLAST and then clustered into groups based on amino acid sequences using the phylogenetic analysis program Clustal X. ExPASy/SIB accession numbers: S. cerevisiae Sc_SET2, P46995; S. pombe Sp_SET2, O14026; N. crassa Nc-NCU00269.1, Q7RZU4; Homo sapiens Hs_HIF-1, Q9BYW2; Homo sapiens Hs_HSPC069, Q9NZW9; Drosophila melanogaster Dm_CG1716, Q9VYD1; Homo sapiens Hs_NSD1, Q96L73; Homo sapiens Hs_WHSC1L1, Q9BZ95; Homo sapiens Hs_WHSC1, O96028; C. elegans Ce_C43E11.3, Q8I7H3; and C. elegans Ce_K09F5.5, Q21404). The scale bar equals a distance of 0.05 amino acids. The asterisk indicates that this protein is also known as HYPB. (B) Schematic domain representation of Set2 proteins identified from the alignment in A. Protein names and lengths in amino acids are noted beneath each protein.
Strikingly, in addition to the AWS, SET, post-SET, and WW domains, more complex eukaryotes have a large number of additional domains and sequences such as PHD fingers and HMG domains, indicating that these putative Set2 homologs may carry out additional chromatin-related functions. It is notable that the mouse homolog of human NSD1 (Nsd1) has been shown to mediate K36 methylation in vitro (37), suggesting that the other proteins listed in Fig. 2 may be bona fide K36-methylating homologs. However, it was not known whether any of these proteins methylate K36 and associate with Pol II.
S. pombe Set2 is a robust methyltransferase specific for K36.
To determine if the link between K36 methylation and transcription elongation might be conserved, we characterized the Set2 protein thought to be responsible for K36 methylation in S. pombe. We chose to focus on S. pombe because many proteins in this organism have been found to be more similar to their mammalian counterparts than to their complements in S. cerevisiae (44). In addition, the role of K36 methylation in this organism has not been investigated.
We first asked whether this protein is an active histone methyltransferase (HMT) and whether it catalyzes K36 methylation. To determine this, we cloned the S. pombe protein into a pCAL-n expression construct, expressed it in E. coli, and tested the recombinant protein in HMT assays using [3H]SAM as a cofactor. As shown in Fig. 3A, SpSet2 showed a robust HMT activity towards nucleosomal substrates and, to a lesser extent, free core histones in filter binding assays. In contrast, this enzyme showed little activity towards free histone H3 (Fig. 3A).
FIG. 3.
Set2 from Schizosaccharomyces pombe is a robust nucleosome-selective K36-specific methyltransferase. (A) Bacterial lysates containing recombinantly expressed SpSet2 (or vector-only control) were incubated with recombinant H3 (rH3), chicken core histones, or oligonucleosomes (Nuc.) and [3H]SAM. 3H incorporation was analyzed by the filter-binding assay and monitored by scintillation counting. (B) Reaction products from HMT assays using oligonucleosomes and SpSet2 were resolved on a 15% SDS-PAGE gel and examined by Coomassie staining (lower panel) and fluorography (upper panel). The asterisk indicates the H3 breakdown product that is typically observed. (C) Oligonucleosomes or recombinant H3 was incubated with SpSet2 and unlabeled SAM in an HMT assay followed by immunoblotting with the anti-Me2K36 antibody (upper panel). Parallel reactions were performed and examined by Coomassie staining to monitor loading (lower panel). (D) Filter-binding assays were performed as in A using bacterial lysates with or without SpSet2 and H3 peptides either unmodified (H3, 1-20 or H3, 27-45) or trimethylated at H3 K36 (H3, 27-45 K36 tri-Me) in the presence of [3H]SAM.
To determine the histone specificity of this methyltransferase, a portion of the HMT assays involving nucleosomal substrates were electrophoresed on a 15% SDS-PAGE gel and examined by fluorography. The results revealed that histone H3 was the only histone methylated (Fig. 3B). We next performed “cold” HMT assays with SpSet2 using unlabeled cofactor, followed by Western blot analysis with an antibody specific for K36 dimethylation to determine if SpSet2 was specific for K36. The results showed a significant immunoreactivity towards K36 dimethylation in the presence of SpSet2 (Fig. 3C). In contrast, no immunoreactivity was witnessed after these HMT assays with antibodies directed against either H3 lysine 79 dimethylation or K4 dimethylation (data not shown).
To further verify the site specificity of SpSet2, we examined H3 synthetic peptides that were either unmodified or trimethylated at K36 in filter binding assays. Although the overall level of activity towards H3 peptides was low (compare 3H incorporation levels between panels A and D), it was still sufficient to determine whether this activity could be blocked with a K36-methylated peptide. As shown in Fig. 3D, SpSet2 was able to methylate an H3 peptide of residues 27 to 45, but not that of an H3 N-terminal peptide (residues 1 to 20). Importantly, a matched residues 27 to 45 peptide that was trimethylated at K36 was not a substrate (Fig. 3D). These data demonstrate that SpSet2 is a robust nucleosome-selective HMT specific for K36 methylation.
Set2 and K36 methylation are associated with transcription elongation in S. pombe.
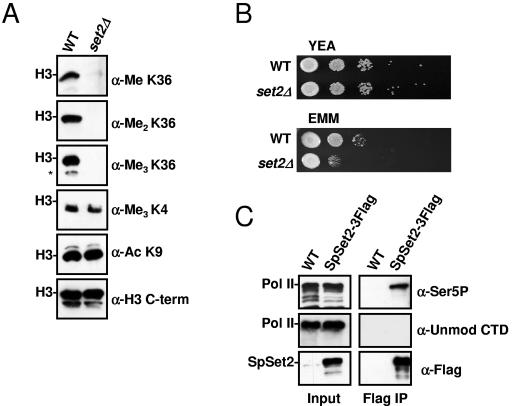
We next asked whether SpSet2 is responsible for in vivo K36 methylation in S. pombe and whether it associates with elongating Pol II. To address the first point, we deleted the set2+ gene from S. pombe and used these cells, along with the wild-type control, to generate purified nuclei for subsequent Western blot analyses. As shown in Fig. 4A, deletion of set2+ resulted in a complete abolishment of K36 methylation (mono-, di-, and trimethylation), but not K4 methylation or H3 K9 acetylation, in bulk histones, indicating that SpSet2 is the sole enzyme in fission yeast responsible for this modification. We also examined the set2 deletion (set2Δ) strain for growth defects and found that while set2Δ cells grew normally on rich YEA medium, they showed a strong growth defect in synthetic medium (EMM), which is nutrient depleted compared to YEA (Fig. 4B). These data reveal an important role for Set2 in cell growth under deprived nutrient or stressed conditions. Similar slow-growth phenotypes on minimal medium have been described for the deletion of other factors involved in transcription and translation (2, 45).
FIG. 4.
Set2 is responsible for mediating global K36 methylation in S. pombe. (A) S. pombe nuclear extracts prepared from wild-type (WT) and set2Δ strains were probed with antibodies against H3 K36 mono-, di-, and trimethylation. An antibody specific for the C terminus of H3 was used as a loading control. Antibodies specific for H3 K9 acetylation and H3 K4 trimethylation were used as additional controls. The asterisk indicates H3 breakdown products that were observed. (B) A slow-growth phenotype develops in the absence of S. pombe set2+ under nutrient-deprived conditions. Wild-type or set2Δ cells were spotted at a serial dilution of 1:10 and grown at 30°C on rich medium (YEA) for 3 days or minimal medium (EMM) for 5 days before being photographed. (C) SpSet2 interacts with the hyperphosphorylated form of Pol II. Whole-cell extracts (WCE) prepared from wild-type or genomically tagged SpSet2 (SpSet2-3Flag) were immunoprecipitated with anti-Flag antibody followed by immunoblotting with antibodies directed against unmodified CTD (8WG16, anti-unmod CTD), Ser5-phosphorylated CTD (H14, anti-Ser5P), or Flag (anti-Flag). The locations of Pol II and SpSet2-3Flag are indicated. The input whole-cell extracts were also examined by immunoblot analysis to monitor the presence of Pol II and SpSet2-3Flag.
To determine if the SpSet2 enzyme would be associated with Pol II, we tagged SpSet2 at its C terminus with a triple Flag epitope (SpSet2-3Flag) and then used this epitope to perform coimmunoprecipitation experiments to monitor the association of unmodified or hyperphosphorylated Pol II. The different forms of Pol II were monitored using antibodies 8WG16 and H14, which recognize unmodified and Ser5 phosphorylated CTD, respectively. As shown in Fig. 4C, immunoprecipitation of SpSet2-3Flag resulted in strong immunoreactivity of the Ser5-phosphorylated CTD form of Pol II. No unmodified Pol II could be detected in these immunoprecipitates, although unmodified Pol II could be readily detected in the input extracts. These data demonstrate that SpSet2 is associated with the elongating form of Pol II in S. pombe. This result is also consistent with the finding that a region in the C terminus of the SpSet2 protein contains similarity (17/42% identity/similarity) to a region in ScSet2 that was found to mediate association of Set2 with the phosphorylated polymerase (22).
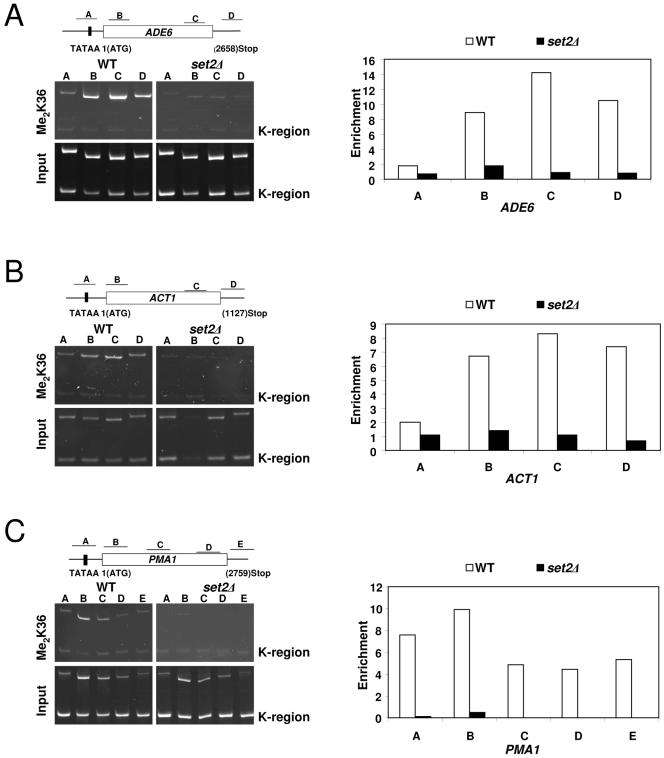
Next, we asked whether K36 methylation is associated with the transcribed regions of active genes in S. pombe. To address this, we used a K36 dimethylation-specific antiserum in ChIP assays to examine the abundance and distribution of this modification over genes. Consistent with observations in budding yeast (25, 39, 52), we found that K36 methylation was highly enriched over the transcribed regions of several active genes tested (Fig. 5). In contrast, nontranscribed regions of telomeric and mating type loci were found to be devoid of this methyl mark (data not shown and Fig. 5). These data strongly suggest that K36 methylation, mediated by SpSet2, is associated with the elongation process in S. pombe.
FIG. 5.
Set2-mediated K36 methylation is preferentially associated with the transcribed regions of active S. pombe genes. Left panels A to C: Chromatin immunoprecipitation assays were used to monitor the location of K36 dimethylation on actively transcribed genes (ADE6, ACT1, and PMA1) in wild-type and set2Δ strains using an H3 Me2K36-specific antibody. DNA from enriched precipitates (IP) were isolated and used in PCRs with promoter- and coding region-specific primer pairs for the indicated genes. A DNA fragment from the silent mating type loci (K region) of S. pombe known to lack modifications associated with active genes (H3 K4 methylation and H3 K14 acetylation) (34) was used as a control to normalize and calculate the relative enrichment of gene sequences in immunoprecipitated samples. Right panels: Quantification of the ChIP results shown in A to C. Relative enrichment values shown on the y axes were calculated by dividing the ratio of band intensities for immunoprecipitated DNA K region with the ratio of intensities for the input DNA K region. Gels and graphs are representative experiments from three independent repeats.
Given the strong similarities found between the budding and fission yeast Set2 proteins, we finally asked if the fission yeast K36-methylating enzyme could complement the loss of Set2 in budding yeast cells. To examine this, we cloned the S. pombe set2+ gene, containing a C-terminal Flag tag, into a budding yeast expression construct (under the control of the ADH1 promoter) and expressed this protein in set2Δ cells. As a control, a similar expression construct containing the budding yeast SET2 gene was included. As shown in Fig. 6, full-length SpSet2 could be readily detected by Western blot analysis using an anti-Flag antibody. The S. pombe protein runs with a slower migration compared to the budding yeast Set2, as this protein is slightly larger than its budding yeast counterpart.
FIG. 6.
SpSet2 rescues K36 methylation in S. cerevisiae. Whole-cell extracts (WCEs) were prepared from S. cerevisiae wild-type and set2Δ strains that were transformed with an empty vector or the indicated Set2-Flag constructs. These extracts were immunoprecipitated with anti-Flag antibody followed by immunoblot analysis using antibodies directed against unmodified CTD (8WG16, anti-unmod CTD), Ser5-phosphorylated CTD (H14, anti-Ser5P), or Flag (anti-Flag). The locations of Pol II and Set2-Flag proteins are indicated. All input extracts showed equivalent levels of Pol II (data not shown). Nuclear extracts prepared from the same strains were immunoblotted with an antibody directed against K36 dimethylation or an antibody specific for K4 dimethylation (used as a loading control). The asterisk indicates H3 breakdown products that were observed.
We then purified nuclei from these strains and examined the levels of K36 methylation on bulk histones. Significantly, we found that the S. pombe Set2 protein could restore K36 methylation in set2Δ cells. This result suggests that SpSet2 forms a stable interaction with Pol II in budding yeast. To examine this idea further, we performed similar coimmunoprecipitation studies as described above and found that SpSet2 efficiently associates with the elongating form of Pol II, similar to its budding yeast counterpart. This result shows that these enzymes are interchangeable, thereby supporting the notion that Set2 and K36 methylation is functionally conserved.
DISCUSSION
Like the conservation found between histone protein sequences among eukaryotes, their covalent modifications are also highly conserved (17). Yet, a looming question has been whether these modifications perform the same functions in all of these different organisms or have distinct functions that have arisen through evolutionary change. To date, several sites of histone methylation have been associated with active transcription. These include the methylation of H3 at lysines 4, 36, and 79 (13, 15, 25, 29, 33, 42, 49). In S. cerevisiae, the enzymes responsible for K4 and K36 methylation have been found associated with the elongating form of Pol II (15, 42). This intriguing observation implies a novel role for histone methylation in the elongation phase of transcription. However, whether these enzymes are conserved and have similar functions in organisms outside of budding yeast has not been fully investigated.
In this report, we characterize the fission yeast enzyme responsible for K36 methylation and provide evidence that this modification in S. pombe is coupled with the transcription elongation process. Given budding yeast is evolutionarily distinct from fission yeast, this result suggests that Set2 has a conserved function in transcriptional regulation. While our study has focused on K36 methylation, several studies have characterized Set1 homologs responsible for K4 methylation outside of budding yeast (8, 9, 30, 32, 51). Although the link between K4 methylation and transcription elongation in these organisms is not well defined, there are a number of similarities found between Set1 and K4 methylation among eukaryotes that suggest a conserved role for this modification similar to what is found for Set2/K36 methylation. First, Set1-mediated K4 methylation is associated primarily with euchromatic regions in all organisms, with a distribution pattern on genes in metazoans and in S. pombe that is very similar to what is observed in budding yeast (40). Second, comparative studies of human and budding and fission yeast Set1 have shown that these proteins have nearly identical complex compositions, and in the case of S. pombe, Set1's methylation is dependent on the ubiquitin-conjugating enzyme Rad6 (38). Additionally, a Set1 homolog in humans, MLL2, has been shown to associate with the Ser5-phosphorylated form of Pol II, similar to the budding yeast counterpart (18). Collectively, these data suggest that the functional conservation of K4 and K36 methylation in Pol II-mediated transcription is highly conserved.
It is significant that while S. pombe has only one Set2-like homolog, our BLAST searches revealed that most multicellular organisms have a number of K36-methylating enzymes (Fig. 2 and data not shown). Although the functions of these other enzymes are not known, it is intriguing to speculate whether all of these putative K36-methylating enzymes could be associated with the elongating polymerase in their respective organisms, or if some of these Set2-like proteins are involved in other biological processes outside of Pol II-coupled transcription. While it will take in-depth characterization of each putative Set2 homolog to determine their role(s) in chromatin regulation, one clue to suggest that K36 methylation in more complex eukaryotes may have distinct functions other than a role with Pol II is the fact that only one Set2 homolog from any given species appears to contain a prototypical SRI (Set2 Rpb1 interacting) domain, which is the domain required for Set2 to mediate its association with the phosphorylated CTD (22). Given that not all Set2 homologs contain this domain, we speculate that K36 methylation will have a broad range of activities in chromatin in addition to a conserved role with the transcribing polymerase.
In summary, we demonstrate that SpSet2 is a true ortholog of the budding yeast Set2 enzyme and that this enzyme and K36 methylation are linked to the transcription elongation process in S. pombe. While our characterization studies are limited to S. pombe, an accompanying paper shows similar findings for the Neurospora crassa Set2 homolog (1). Furthermore, a link between K36 methylation and CTD phosphorylation has been suggested in Caenorhabditis elegans (16), and recent evidence shows a correlation of K36 methylation with active genes in metazoans (6). Taken together, these results indicate a highly conserved role for K36 methylation in transcriptional regulation.
Acknowledgments
This work was supported by an NIH grant (GM68088) to B.D.S., American Heart Association grant (0465506U) to B.D.S, National Cancer Institute intramural grant to S.I.S.G., and an NIH minority supplement to S.A.M. S.A.M. is a predoctoral Ford fellow and B.D.S. is a Pew Scholar in the Biomedical Sciences.
We thank C. D. Allis, D. F. Hunt, and E. Selker for communicating results prior to publication and M. Bayer for technical assistance. We also thank the Strahl and Grewal lab members and E. Selker for helpful discussions and reading of the manuscript.
REFERENCES
- 1.Adhvaryu, K. K., S. A. Morris, B. D. Strahl, and E. U. Selker. 2005. Methylation of histone H3 lysine 36 is required for normal development in Neurospora crassa. Eukaryot. Cell 4:1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyoshi, Y., J. Clayton, L. Phan, M. Yamamoto, A. G. Hinnebusch, Y. Watanabe, and K. Asano. 2001. Fission yeast homolog of murine Int-6 protein, encoded by mouse mammary tumor virus integration site, is associated with the conserved core subunits of eukaryotic translation initiation factor 3. J. Biol. Chem. 276:10056-10062. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie, 3rd, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943-951. [DOI] [PubMed] [Google Scholar]
- 6.Bannister, A. J., R. Schneider, F. A. Myers, A. W. Thorne, C. Crane-Robinson, and T. Kouzarides. 2005. Spatial distribution of di-and tri-methyl lysine 36 of histone H3 at active genes. J. Biol. Chem. [DOI] [PubMed]
- 7.Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. [DOI] [PubMed] [Google Scholar]
- 8.Briggs, S. D., M. Bryk, B. D. Strahl, W. L. Cheung, J. K. Davie, S. Y. Dent, F. Winston, and C. D. Allis. 2001. Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 15:3286-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd, K. N., and A. Shearn. 2003. ASH1, a Drosophila trithorax group protein, is required for methylation of lysine 4 residues on histone H3. Proc. Natl. Acad. Sci. USA 100:11535-11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, R., and Y. Zhang. 2004. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 14:155-164. [DOI] [PubMed] [Google Scholar]
- 11.Cosgrove, M. S., J. D. Boeke, and C. Wolberger. 2004. Regulated nucleosome mobility and the histone code. Nat. Struct. Mol. Biol. 11:1037-1043. [DOI] [PubMed] [Google Scholar]
- 12.Edmondson, D. G., M. M. Smith, and S. Y. Roth. 1996. Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev. 10:1247-1259. [DOI] [PubMed] [Google Scholar]
- 13.Gerber, M., and A. Shilatifard. 2003. Transcriptional elongation by RNA polymerase II and histone methylation. J. Biol. Chem. 278:26303-26306. [DOI] [PubMed] [Google Scholar]
- 14.Grewal, S. I., and J. C. Rice. 2004. Regulation of heterochromatin by histone methylation and small RNAs. Curr. Opin. Cell Biol. 16:230-238. [DOI] [PubMed] [Google Scholar]
- 15.Hampsey, M., and D. Reinberg. 2003. Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell 113:429-432. [DOI] [PubMed] [Google Scholar]
- 16.Han, Z., J. R. Saam, H. P. Adams, S. E. Mango, and J. M. Schumacher. 2003. The C. elegans Tousled-like kinase (TLK-1) has an essential role in transcription. Curr. Biol. 13:1921-1929. [DOI] [PubMed] [Google Scholar]
- 17.Holde, K. E. 1988. Chromatin. Springer-Verlag, New York, N.Y.
- 18.Hughes, C. M., O. Rozenblatt-Rosen, T. A. Milne, T. D. Copeland, S. S. Levine, J. C. Lee, D. N. Hayes, K. S. Shanmugam, A. Bhattacharjee, C. A. Biondi, G. F. Kay, N. K. Hayward, J. L. Hess, and M. Meyerson. 2004. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol. Cell 13:587-597. [DOI] [PubMed] [Google Scholar]
- 19.Iizuka, M., and M. M. Smith. 2003. Functional consequences of histone modifications. Curr. Opin. Genet. Dev. 13:154-160. [DOI] [PubMed] [Google Scholar]
- 20.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 21.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 22.Kizer, K. O., H. P. Phatnani, Y. Shibata, H. Hall, A. L. Greenleaf, and B. D. Strahl. 2005. A novel domain in Set2 mediates RNA Polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol. Biol. Cell 25:3305-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouzarides, T. 2002. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 12:198-209. [DOI] [PubMed] [Google Scholar]
- 24.Krogan, N. J., J. Dover, A. Wood, J. Schneider, J. Heidt, M. A. Boateng, K. Dean, O. W. Ryan, A. Golshani, M. Johnston, J. F. Greenblatt, and A. Shilatifard. 2003. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol. Cell 11:721-729. [DOI] [PubMed] [Google Scholar]
- 25.Krogan, N. J., M. Kim, A. Tong, A. Golshani, G. Cagney, V. Canadien, D. P. Richards, B. K. Beattie, A. Emili, C. Boone, A. Shilatifard, S. Buratowski, and J. Greenblatt. 2003. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 23:4207-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, D. Y., C. Teyssier, B. D. Strahl, and M. R. Stallcup. 2004. Role of protein methylation in regulation of transcription. Endocrinol. Rev. 26:637-651. [DOI] [PubMed] [Google Scholar]
- 27.Li, B., L. Howe, S. Anderson, J. R. Yates 3rd, and J. L. Workman. 2003. The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J. Biol. Chem. 278:8897-8903. [DOI] [PubMed] [Google Scholar]
- 28.Li, J., D. Moazed, and S. P. Gygi. 2002. Association of the histone methyltransferase Set2 with RNA polymerase II plays a role in transcription elongation. J. Biol. Chem. 277:49383-49388. [DOI] [PubMed] [Google Scholar]
- 29.Litt, M. D., M. Simpson, M. Gaszner, C. D. Allis, and G. Felsenfeld. 2001. Correlation between histone lysine methylation and developmental changes at the chicken beta-globin locus. Science 293:2453-2455. [DOI] [PubMed] [Google Scholar]
- 30.Milne, T. A., S. D. Briggs, H. W. Brock, M. E. Martin, D. Gibbs, C. D. Allis, and J. L. Hess. 2002. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol. Cell 10:1107-1117. [DOI] [PubMed] [Google Scholar]
- 31.Ng, H. H., F. Robert, R. A. Young, and K. Struhl. 2003. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11:709-719. [DOI] [PubMed] [Google Scholar]
- 32.Nishioka, K., S. Chuikov, K. Sarma, H. Erdjument-Bromage, C. D. Allis, P. Tempst, and D. Reinberg. 2002. Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation. Genes Dev. 16:479-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noma, K., C. D. Allis, and S. I. Grewal. 2001. Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science 293:1150-1155. [DOI] [PubMed] [Google Scholar]
- 34.Noma, K., and S. I. Grewal. 2002. Histone H3 lysine 4 methylation is mediated by Set1 and promotes maintenance of active chromatin states in fission yeast. Proc. Natl. Acad. Sci. USA 99(Suppl. 4):16438-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perriere, G., and M. Gouy. 1996. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78:364-369. [DOI] [PubMed] [Google Scholar]
- 36.Peterson, C. L., and M. A. Laniel. 2004. Histones and histone modifications. Curr. Biol. 14:R546-51. [DOI] [PubMed] [Google Scholar]
- 37.Rayasam, G. V., O. Wendling, P. O. Angrand, M. Mark, K. Niederreither, L. Song, T. Lerouge, G. L. Hager, P. Chambon, and R. Losson. 2003. NSD1 is essential for early post-implantation development and has a catalytically active SET domain. EMBO J. 22:3153-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roguev, A., D. Schaft, A. Shevchenko, R. Aasland, A. Shevchenko, and A. F. Stewart. 2003. High conservation of the Set1/Rad6 axis of histone 3 lysine 4 methylation in budding and fission yeasts. J. Biol. Chem. 278:8487-8493. [DOI] [PubMed] [Google Scholar]
- 39.Schaft, D., A. Roguev, K. M. Kotovic, A. Shevchenko, M. Sarov, A. Shevchenko, K. M. Neugebauer, and A. F. Stewart. 2003. The histone 3 lysine 36 methyltransferase, SET2, is involved in transcriptional elongation. Nucleic Acids Res. 31:2475-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider, R., A. J. Bannister, F. A. Myers, A. W. Thorne, C. Crane-Robinson, and T. Kouzarides. 2004. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat. Cell Biol. 6:73-77. [DOI] [PubMed] [Google Scholar]
- 41.Shiio, Y., and R. N. Eisenman. 2003. Histone sumoylation is associated with transcriptional repression. Proc. Natl. Acad. Sci. USA 100:13225-13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shilatifard, A. 2004. Transcriptional elongation control by RNA polymerase II: a new frontier. Biochim. Biophys. Acta 1677:79-86. [DOI] [PubMed] [Google Scholar]
- 43.Sims, R. J., 3rd, R. Belotserkovskaya, and D. Reinberg. 2004. Elongation by RNA polymerase II: the short and long of it. Genes Dev. 18:2437-2468. [DOI] [PubMed] [Google Scholar]
- 44.Sipiczki, M. 2000. Where does fission yeast sit on the tree of life? Genome Biol. 1:REVIEWS1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, K. N., L. Iwanejko, S. Loeillet, F. Fabre, and A. Nicolas. 1999. Disruption and functional analysis of seven ORFs on chromosome IV: YDL057w, YDL012c, YDL010w, YDL009c, YDL008w (APC11), YDL005c (MED2) and YDL003w (MCD1). Yeast 15:1255-1267. [DOI] [PubMed] [Google Scholar]
- 46.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 47.Strahl, B. D., S. D. Briggs, C. J. Brame, J. A. Caldwell, S. S. Koh, H. Ma, R. G. Cook, J. Shabanowitz, D. F. Hunt, M. R. Stallcup, and C. D. Allis. 2001. Methylation of histone H4 at arginine 3 occurs in vivo and is mediated by the nuclear receptor coactivator PRMT1. Curr. Biol. 11:996-1000. [DOI] [PubMed] [Google Scholar]
- 48.Strahl, B. D., P. A. Grant, S. D. Briggs, Z. W. Sun, J. R. Bone, J. A. Caldwell, S. Mollah, R. G. Cook, J. Shabanowitz, D. F. Hunt, and C. D. Allis. 2002. Set2 is a nucleosomal histone H3-selective methyltransferase that mediates transcriptional repression. Mol. Cell. Biol. 22:1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strahl, B. D., R. Ohba, R. G. Cook, and C. D. Allis. 1999. Methylation of histone H3 at lysine 4 is highly conserved and correlates with transcriptionally active nuclei in Tetrahymena. Proc. Natl. Acad. Sci. USA 96:14967-14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turner, B. M. 2000. Histone acetylation and an epigenetic code. Bioessays 22:836-845. [DOI] [PubMed] [Google Scholar]
- 51.Wysocka, J., M. P. Myers, C. D. Laherty, R. N. Eisenman, and W. Herr. 2003. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 17:896-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao, T., H. Hall, K. O. Kizer, Y. Shibata, M. C. Hall, C. H. Borchers, and B. D. Strahl. 2003. Phosphorylation of RNA polymerase II CTD regulates H3 methylation in yeast. Genes Dev. 17:654-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, L., E. E. Eugeni, M. R. Parthun, and M. A. Freitas. 2003. Identification of novel histone post-translational modifications by peptide mass fingerprinting. Chromosoma 112:77-86. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, Y., and D. Reinberg. 2001. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 15:2343-2360. [DOI] [PubMed] [Google Scholar]