Abstract
The SET domain is an evolutionarily conserved domain found predominantly in histone methyltransferases (HMTs). The Neurospora crassa genome includes nine SET domain genes (set-1 through set-9) in addition to dim-5, which encodes a histone H3 lysine 9 HMT required for DNA methylation. We demonstrate that Neurospora set-2 encodes a histone H3 lysine 36 (K36) methyltransferase and that it is essential for normal growth and development. We used repeat induced point mutation to make a set-2 mutant (set-2RIP1) with multiple nonsense mutations. Western analyses revealed that the mutant lacks SET-2 protein and K36 methylation. An amino-terminal fragment that includes the AWS, SET, and post-SET domains of SET-2 proved sufficient for K36 HMT activity in vitro. Nucleosomes were better substrates than free histones. The set-2RIP1 mutant grows slowly, conidiates poorly, and is female sterile. Introducing the wild-type gene into the mutant complemented the defects, confirming that they resulted from loss of set-2 function. We replaced the wild-type histone H3 gene (hH3) with an allele producing a Lys to Leu substitution at position 36 and found that this hH3K36L mutant phenocopied the set-2RIP1 mutant, confirming that the observed defects in growth and development result from inability to methylate K36 of H3. Finally, we used chromatin immunoprecipitation to demonstrate that actively transcribed genes in Neurospora crassa are enriched for H3 methylated at lysines 4 and 36. Taken together, our results suggest that methylation of K36 in Neurospora crassa is essential for normal growth and development.
In eukaryotes DNA is wrapped around histones, which are subject to a variety of covalent modifications (e.g., methylation, acetylation, phosphorylation, and ubiquitylation) (51, 54). These modifications are believed to alter chromatin structure by influencing histone-DNA, histone-histone, and histone-nonhistone protein interactions (16, 55). Permutations of histone modifications constitute a “histone code” that can impact chromatin-templated processes, including DNA methylation, heterochromatin formation, transcription, and DNA replication and repair (18, 46). The SET [Su(var)3-9, Enhancer-of-zeste, Trithorax] domain histone methyltransferases (HMTs) constitute one group of evolutionarily conserved chromatin-modifying proteins (10, 12, 19, 23, 24, 40, 45, 60). A well-characterized example is the Neurospora crassa DIM-5 protein, which trimethylates lysine 9 of histone H3 and directs DNA methylation (48, 49, 58, 59). In addition to DIM-5, the N. crassa genome encodes nine other putative SET domain proteins (Fig. 1) (4). Our interest in determining the function and specificities of these putative methyltransferases prompted the current study.
FIG. 1.
Domain organization of DIM-5 and other putative SET proteins of Neurospora crassa. Ten SET domain genes were identified in N. crassa: dim-5 (NCU04402.2); set-1 (NCU01206.2); set-2 (NCU00269.2); set-3 (NCU01932.2); set-4 (NCU04389.2); set-5 (NCU06119.2); set-6 (NCU09495.2); set-7 (NCU07496.2); set-8 (NCU01973.2); and set-9 (NCU08733.2) (4). Domains, identified using SMART (26, 41), are abbreviated as follows: AWS (Associated With SET, SM00570); SET (SM00317); pS (post-SET, SM00508); WW (SM00456), CC (coiled coil); PreSET (SM00468); AT-hook (SM00384); PHD zinc finger (SM00249); JmjC (SM0058); RRM 1 (SM00360). The set-2RIP1 allele has predicted nonsense codons (indicated by asterisks) at positions 173, 178, 395, 410, 446, 485, 491, 553, 562, 595, 615, 617, 621, 639, 685, 723, and 787, and amino acid substitutions H235Y, S240L, H261Y, A365V, P378L, L393F, H416Y, R418W, L454F, R456C, T463I, H476Y, H479Y, L490F, T498I, T515I, H527Y, S532L, T582I, H593Y, S610F, H635Y, S636F, T645I, H675Y, H684Y, L692F, H742Y, A750V, S768L, R775C, P788S, T861I, and P862L. The sequence of set-2RIP1 is available at GenBank (accession number AY971375).
While some histone methylation, such as methylation of lysines 9 (K9) and 27 (K27) of histone H3, appears to be associated with condensed chromatin, other histone methylations, including methylation of lysines 4 (K4), 36 (K36), and 79 (K79) of histone H3, are hallmarks of euchromatic regions (12, 24, 45). Methylation of K4 and K36 of histone H3 is believed to be associated with transcription elongation (11, 13, 43). Saccharomyces cerevisiae Set2p, the first characterized K36-specific histone methyltransferase, preferentially associates with the hyperphosphorylated (elongating) form of RNA polymerase II (47). Genetic, biochemical, and drug-based (6-azauracil) analyses have revealed a role for this enzyme in the transcription elongation process (21, 27, 28). Curiously, this function is apparently nonessential, and no striking phenotypes have been reported for set2 mutants.
We identified an N. crassa homologue of Set2p and used repeat induced point mutation (RIP) (42) to generate a null allele of the set-2 gene. We show that SET-2 is a nucleosome-selective K36-specific methyltransferase that methylates histones associated with transcribed regions of active genes. Furthermore, we find that methylation of this residue is essential for normal growth and development in Neurospora crassa.
MATERIALS AND METHODS
Neurospora crassa strains and isolation of genomic DNA.
Neurospora crassa strains were grown, maintained, and crossed according to standard procedures (6). Strains used in this study are listed in Table 1. For isolation of genomic DNA, strains were grown with shaking in Vogel's minimal medium N with appropriate supplements at 32°C for 3 days. Genomic DNA for PCR and Southern analyses was isolated as previously described (29).
TABLE 1.
Neurospora crassa strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| N1 (74-OR8-1) | mat a | FGSC 988 |
| N39 | mat A; fl | FGSC 4317 |
| N40 | mat a; fl | FGSC 4347 |
| N150 (74-OR23-IV) | mat A | FGSC 2489 |
| N623 | mat A his-3; inl | FGSC 6103 |
| N1214 | amRIP8 | 37 |
| N1674 | mat A his-3; lys-1 am132 inl; [(am/hph/am)ec42pJ12]RIP | 15 |
| N2257 | ridRIP4 mat a his-3 | 9 |
| N2264 | his-3; dim-5 leu-2 pan-2 | 7 |
| N2836 | mat A his-3; mus-52Δ::hph; inl | This study |
| N2948 | mat A his-3+::Pccg-1-set-2+-sgfp+; inl | This study |
| N2949 | mat a his-3+::Pccg-1-set-2-sgfp+; set-2RIP1; inl | This study |
| N2950 | amRIP8; inl | This study |
| N2951 | set-2RIP1; amRIP8; inl | This study |
| N2952 | mat a; inl | This study |
| N2953 | mat a; inl | This study |
| N2954 | mat A; inl | This study |
| N2955 | mat A; inl | This study |
| N2956 | mat A; set-2RIP1; inl | This study |
| N2957 | mat A; set-2RIP1; inl | This study |
| N2958 | mat A; set-2RIP1; inl | This study |
| N2959 | mat A; set-2RIP1; inl | This study |
| N2960 | mat A; set-2RIP1; inl | This study |
| N2961 | his-3+::P-Flag; set-2RIP1; inl | This study |
| N2962 | his-3+::P-set-2+-Flag; set-2RIP1; inl | This study |
| N2970 | mat A his-3; hH3K36L; mus-52Δ::hph; inl | This study |
| N2975 | mat A his-3; hH3K36L; mus-52Δ::hph; inl | This study |
| N2971 | [(mat A his-3+::Pccg-1-set-2-sgfp+; set-2RIP1; inl) + (mat A his-3+::Pccg-1-set-2+-sgfp+; inl)] | This study |
| N2972 | [(mat A his-3+::Pccg-1-set-2-sgfp+; set-2RIP1; inl) + (mat A his-3+::Pccg-1-set-2+-sgfp+; inl)] | This study |
| N2973 | mat A his-3+::P-Flag; inl | This study |
| N2974 | mat A his-3+::P-set-2+-Flag; inl | This study |
Generation of set-2RIP1.
We used tBLASTn and BLASTp (1) to search the N. crassa genome sequence (http://www-genome.wi.mit.edu/annotation/fungi/neurospora/) with the S. cerevisiae SET2/YJL168C protein sequence (NCBI protein accession number NP_012367) as bait, and found one potential homologue, locus NCU00269.1 (58% similarity; BLASTp E-value = e−108). The predicted coding region of this gene was amplified with primers SET2-5 and SET2-6 (Table 2). Fragments were digested with XbaI and PacI and inserted into XbaI- and PacI-digested pMF272 (8) to yield pKA10. Plasmid pKA10 was linearized with DraI and inserted at the his-3 locus of N1674 by gene replacement as described (29).
TABLE 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| SET2-5 | 5′-GCTCTAGATGGAGGACGGCCATCACTCACCG-3′ |
| SET2-6 | 5′ CCTTAATTAAACGACTGACGGGCTCCTGTT 3′ |
| SET2-7 | 5′ AGCGTGGCAGTTCGTCGCCTCCCAACCCTC 3′ |
| SET2-8 | 5′ GTATAGGCGGTGATATGAACCATGCGTGCG 3′ |
| SET2-9 | 5′ TTTCATTGGCGGCAAAACGCAAACAGAGCG 3′ |
| SET2-10 | 5′ CAAAACGATTGCTTCAGGAATGGAGCAAAT 3′ |
| SET2-11 | 5′ AAGGAGAAGTGGAGGTCTCTTCCGGTTGAG 3′ |
| SET2-12 | 5′ GACGTGGAGGATGAGGGCGAGAATACTCCT 3′ |
| SET2-13 | 5′ ATTAGACGTGCTGAGAATGATAACTCAGGA 3′ |
| SET2-14 | 5′ CGGAATTCAGCGTGGCAGTTCGTCGCCTCCAA 3′ |
| SET2-15 | 5′ CGGGATCCACGACTGACGGGCTCCTGTTGTGC 3′ |
| SET2-16 | 5′ CGGGATCCCATGGTGGATGCAGCAGCAGCTGG 3′ |
| SET2-20 | 5′ CGGAATTCGTACTCTTCATCGTCCTCGGTAGC 3′ |
| SET2-21 | 5′ CGGGATCCCCATGGAGGACGGCCATCACTCA 3′ |
| 1219 | 5′ CGGGATCCCATATGGCCCGCACTAAGCAGACCGCC 3′ |
| 1220 | 5′ CGGAATCTTAGGACTTCTGGTAGCGACGAATCTC 3′ |
| 1586 | 5′ GTAACGCCAGGGTTTTCCCAGTCACGACGGGATCCTGAACTGCTATGGCTGCC 3′ |
| 1503 | 5′ CTGCCCTGGAGATTGTTGGAGCTCGGTATCGTGTGATCATGAACC 3′ |
| 1497 | 5′ GCCGAGCTCCAACAATCTCCAGGGCAGACG 3′ |
| 1498 | 5′ GCCGCTAGCTAGGAGTTTGTATCACGGTCA 3′ |
| 1496 | 5′ CCGTGATACAAACTCCTAGCTAGCTCACGCGACGTGACTTTG 3′ |
| 1505 | 5′ GCGGATAACAATTTCACACAGGAAACAGCGAATTCGAACAAGGTCCGTCTGTCTC 3′ |
| mtrAF | 5′ CAGAGCAGCAGGAGCAGT 3′ |
| mtrAR | 5′ CGACGCACTAGCAGTTGC 3′ |
| mtrBF | 5′ TCGTCTGCACGAGATCCG 3′ |
| mtrBR | 5′ CATGATGGCGTTTGGGTC 3′ |
| mtrCF | 5′ CCGTACCCAGAGTCAAAC 3′ |
| mtrCR | 5′ CATGATGGTGCCAGTGAG 3′ |
| mtrDF | 5′ GGCACCATCACGGATAAC 3′ |
| mtrDR | 5′ GACGGCACCAGTAACGGT 3′ |
| mtrEF | 5′ GTTTACGCTTTCGTCGGC 3′ |
| mtrER | 5′ GCAGAGCATGTTGAGGGC 3′ |
| mtrFF | 5′ TTCGTCATCGGCATGGGC 3′ |
| mtrFR | 5′ GCACTACCAATGCAGCAG 3′ |
| hH3AF | 5′ CCCAATAAGTTCAAACCG 3′ |
| hH3AR | 5′ TCCAGCCCAATTCCTCCG 3′ |
| hH3BF | 5′ GGGAGTTGCAAATCAACC 3′ |
| hH3BR | 5′ CTGCTTAGTGCGGGCCAT 3′ |
| hH3CF | 5′ ATGGCCCGCACTAAGCAG 3′ |
| hH3CR | 5′ TCCTGGGCAATCTCACGG 3′ |
Primary heterokaryotic His+ transformants were screened by Southern analysis to verify correct integration. Two transformants (N2971 and N2972) were crossed to N223 to derive homokaryotic duplication strains (N2947 and N2948), which were crossed to induce RIP. Southern analysis of six slow-growing progeny revealed polymorphisms caused by RIP. One of the mutants (N2949) was selected for further study. The mutated endogenous set-2RIP1 allele was amplified by PCR with primers SET2-7 and SET2-13, which recognize the flanks of the endogenous set-2 outside of the duplication (Table 2). The set-2RIP1 allele of N2949 was sequenced using primers SET2-5 through SET2-13 at the University of Oregon Genomics and Proteomics Facility. The second copy of set-2 gene (duplication at the his-3 locus) was removed by crossing N2949 with a his mutant (N623) and screening the His− progeny (lacking the duplication) for the set-2RIP1 allele by Southern analysis.
Complementation of set-2RIP1.
Primers SET2-14 and SET2-15 were used to amplify a 3.5-kb fragment that contains the promoter region (533 bp) and entire set-2 open reading frame (3043 bp). As a control, primers SET2-14 and SET2-16 were used to amplify a 536 bp fragment with the promoter region and the first codon. These fragments were digested with BamHI and EcoRI and cloned into pHT13, a Flag tag vector derived from pBM61, which encodes ten glycines followed by one Flag tag (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys) (29). The resulting plasmids, pKA27 and pKA28, were linearized with DraI and inserted at the his-3 locus of N2257 by gene replacement as described (9, 29). After Southern analysis to confirm correct integration of transforming DNA, two His+ transformants (N2973 for pKA27 and N2974 for pKA28) were selected for further study and crossed to the set-2RIP1 strain. Two representative His+ set-2RIP1 progeny from these crosses were used for further study: N2962, which has a Flag-tagged copy of set-2+ at the his-3 locus, and N2961, which has only the promoter region, the first codon, and a Flag tag at the his-3 locus.
Western blotting.
Isolation of nuclei and Western blotting were performed with ∼100 μg of nuclear protein as described previously (49). The following antibodies were used: anti-Set2 (made in rabbits by standard procedures using a purified N-terminal fragment of budding yeast Set2p containing amino acids 1 to 261), anti-H3 (Abcam ab4441), anti-H3K4me2 (Upstate 07-030), anti-H3K9me3 (49), anti-H3K27me3 (33), anti-H3K36me1 (Abcam ab9048), anti-H3K36me2 (Upstate 07-274), anti-H3K36me3 (47), anti-H3K79me2 (Upstate 07-366), and anti-H4K20me3 (Upstate 07-463). Histone modifications are represented according to a new nomenclature for modified histones (50). All antibodies were used at a dilution of 1:5,000. Antibody-peptide complexes were detected using horseradish peroxidase-conjugated goat antibody against rabbit immunoglobulin G and chemiluminescence (PIERCE).
Histone methyltransferase assays.
A 1.1-kb fragment coding for the first 372 amino acids of N. crassa SET-2 was amplified from DNA pools of an N. crassa conidial cDNA library (31) with primers SET2-20 and SET2-21, digested with EcoRI and BamHI and cloned in EcoRI- and BamHI-digested pGEX-5X-3 (Stratagene). Overexpression of the glutathione S-transferase (GST)-tagged protein was achieved in Escherichia coli using standard methods. Lysates containing tagged SET-2 proteins from uninduced or isopropylthiogalactopyranoside (IPTG)-induced bacterial strains were used for in vitro histone methyltransferase assays with either chicken core histones or oligonucleosomes as substrates and analyzed by filter binding, fluorography and Western blotting as described previously (47).
Histone H3 replacements.
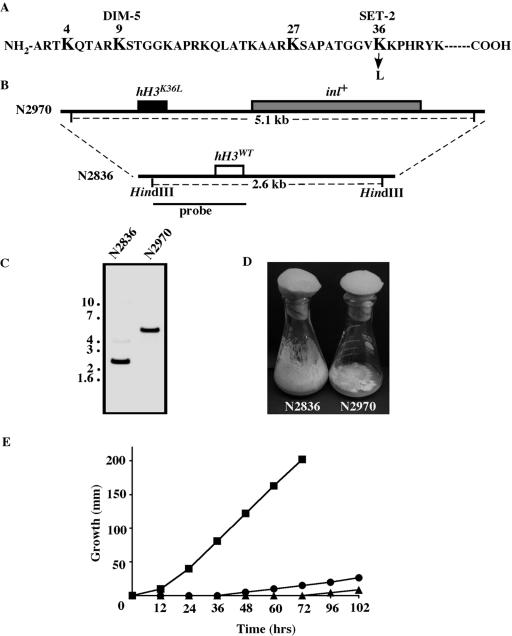
We adapted a general knockout strategy (H. V. Colot, G. Park, C. Ringelberg, S. Curilla, C. Crew, K. A. Borkovich, and J. C. Dunlap, personal communication) to replace the endogenous histone H3 gene with the K36L allele, as outlined in Fig. 5B. A plasmid containing the hH3K36L mutation (pKA37) was constructed as follows. Cosmid SV 31:9G (53) was used as template with primers 1496 and 1505 to amplify a downstream 1kb fragment of the histone H3 gene. Plasmid pEB22 containing the H3 coding region with the K36L mutation was used with primers 1586 and 1503 to amplify an upstream 2-kb fragment. The inl (myoinositol-1-phosphate synthase) plasmid pOKE1 was used as template with primers 1497 and 1498 to amplify a 2.6-kb fragment containing the wild-type inl+ gene. These three PCR products were mixed with BamHI and EcoRI linearized pRS416, and transformed into yeast strain PJ49-6A (17). Plasmid DNA was isolated from ura+ colonies and transformed into E. coli strain DH5αF′. Plasmid pKA37 was isolated from one of these transformants and the hH3K36L gene was sequenced with primers 1219 and 1220 to confirm the K36L mutation. Plasmid pKA37 was digested with BamHI and EcoRI and the 5.7-kb hH3K36L-inl fragment was purified and transformed into a mus-52 strain (N2836) by electroporation (29). Three of ten Inl+ transformants analyzed by Southern analysis were slow growing and appeared to have the correct hH3K36L replacements. Two transformants (N2970 and N2975) were used for further study.
FIG. 5.
hH3K36L allele confers phenotypes similar to the set-2RIP1 mutation. (A) Sequence of histone H3 amino-terminal tail. Lysines that are methylated are indicated in bold. (B) Strategy used for replacement of wild-type hH3 with hH3K36L. (C) Southern analysis to confirm replacement. DNA isolated from wild-type hH3 (N2836) and hH3K36L (N2970) was digested with HindIII. The blot was probed with a fragment indicated in B. The single ∼5.1-kb band indicates that the wild-type hH3 gene in N2836 was replaced by the mutated hH3K36L::inl+ allele. (D and E) Poor growth and conidiation of the hH3K36L strain. Wild-type (N150) and hH3K36L (N2970) strains were grown as described for Fig. 2. Linear growth of the wild type (N150: square) and two hH3K36L transformants (N2970 [triangles] and N2975 [circles]) were measured at 32°C in growth tubes.
Chromatin immunoprecipitation.
Chromatin immunoprecipitation experiments were performed with wild-type (N150) germinating conidia as previously described (49) with minor modifications of the lysis buffer [50 mM HEPES-KOH, pH 7.5; 10 mM MgCl2; 150 mM KCl; 1 mM EDTA; 10% glycerol; 0.1% NP-40; 1 mM dithiothreitol; 1 mM phenylmethylsulfonyl fluoride; 1 mM Trichostatin A; 100 mM sodium butyrate; Complete Mini (Roche number 1836153) and 0.1% phosphatase inhibitor cocktail (Sigma P2850)] and immunoprecipitation wash buffer [25 mM HEPES-KOH, pH 7.5; 12.5 mM MgCl2; 10 mM EDTA; 10% glycerol; 0.1% NP-40; 1 mM dithiothreitol; 1 mM phenylmethylsulfonyl fluoride; 1 mM Trichostatin A; 100 mM sodium butyrate; Complete Mini (Roche number 1836153) and 0.1% phosphatase inhibitor cocktail (Sigma P2850)]. Antibodies used for immunoprecipitation were anti-H3K9me3 (49) and anti-H3K36me3 (47).
RESULTS
SET-2 is essential for normal development in Neurospora crassa.
We performed tBLASTn and BLASTp searches of the N. crassa genome sequence with a consensus SET domain (SM00317) as bait and found nine genes coding for putative SET proteins in addition to dim-5 (Fig. 1) (4). The set-2 (NCU00269.1) gene is located to the right of ro-2 (NCU00257.1) on LG IIIR (34). The 3,043-bp SET-2 open reading frame predicts a 954-amino-acid protein with a domain structure similar to that of S. cerevisiae Set2p, including the AWS (Associated With SET), SET, and post-SET domains (Fig. 1). The computer program PSORT (30) suggests that the protein would be nuclear (k = 83%).
To test the function of N. crassa SET-2, we took advantage of RIP, a genome defense mechanism that detects duplicated DNA sequences during the sexual phase, generates numerous polarized transition mutations (C→T) and usually results in methylation of the mutated DNA sequences (42). We placed a second copy of the set-2 gene at the his-3 locus, crossed this duplication strain and screened progeny by Southern hybridization to identify restriction fragment length polymorphisms resulting from RIP. Six mutant alleles were identified and one, set-2RIP1, was used for most of the subsequent experiments. This allele was expected to be nonfunctional because it contains a large number of mutations that would cause premature stops during translation and because it has many nonconservative changes throughout the predicted protein (Fig. 1).
We noticed several phenotypic defects in the mutants. All six grew slowly, and though they produced abundant aerial hyphae, they conidiated sparsely (Fig. 2A). Microscopic examination revealed that most aerial hyphae lacked the constrictions and budding observed in the wild type (data not shown). Crosses between wild-type and set-2RIP1 strains were infertile when the mutant was used as the female parent (Fig. 2B). Mutant progeny recovered from crosses in which the set-2RIP1 strain was the male parent showed reduced and variable growth rates compared to their wild-type siblings (Fig. 2C). These observations suggested that SET-2 is required for normal vegetative growth and sexual development in N. crassa.
FIG. 2.
SET-2 is essential for normal development in Neurospora crassa. (A) Poor conidiation in set-2RIP1 and complementation of mutation. The wild type (1; N2952), a set-2RIP1 strain (2; N2958), a set-2RIP1 derivative in which the mutation was complemented by a C-terminal Flag-tagged version of set-2+ incorporated at the his-3 locus (3; N2962), and a set-2RIP1 strain that has only a Flag tag at the his-3 locus (4; N2961) were grown at 32°C for 6 days on solid Vogel's medium N with 1.5% sucrose and supplemented with 0.2 mg/ml alanine and 0.05 mg/ml inositol. (B) The set-2RIP1 mutant is female sterile. Reciprocal crosses between the wild type (N1) and a set-2RIP1 strain (N2956) were accomplished using solid synthetic crossing medium with 0.1% sucrose as the carbon source and supplemented with 0.05 mg/ml inositol. The male parent was inoculated 4 days after the female parent. Photographs were taken after 18 and 58 days at 25°C. (C) Reduced and variable growth of set-2RIP1 strains. Linear growth at 32°C of wild-type (N150, N623, N2952, N2953, N2954, and N2955) and set-2RIP1 (N2949, N2956, N2957, N2958, N2959, and N2960) strains on the medium described in A were measured in race tubes (6, 38). Each value is an average of measurements from two tubes.
To verify that loss of SET-2 was responsible for the phenotypes, we reintroduced a functional copy of set-2 at the his-3 locus (Fig. 3A). Full complementation was observed; the complemented strain produced abundant conidia and was female fertile (Fig. 2A and data not shown).
FIG. 3.
set-2RIP1 mutant lacks SET-2 and H3 lysine 36 methylation. (A) Complementation of set-2RIP1. Western blot analyses were performed using crude nuclear protein from the wild-type (N150), a set-2RIP1 strain (N2956), and a set-2RIP1 strain that has been complemented with a Flag-tagged set-2+ at the his-3 locus (N2962). (B) Wild-type N. crassa has mono-, di-, and trimethyl-lysine 36. Western analysis of wild-type (N150) and set-2RIP1 (N2956) strains. (C) The dim-5 mutant has normal levels of lysine 36 methylation. Western analysis of nuclear proteins from wild-type (N150) and dim-5 (N2264) strains.
Characterization of SET-2.
We used an antibody developed against S. cerevisiae Set2p (see Materials and Methods) to look for cross-reacting proteins in N. crassa strains. The antibody recognizes the N terminus of S. cerevisiae Set2p (data not shown), which is highly conserved. We detected a protein of the expected size (106 kDa) in extracts from the wild type but not set-2RIP1 strains (Fig. 3A), consistent with our expectation that set-2RIP1 is a null allele and that Neurospora crassa lacks additional closely related proteins. The putative SET-2 protein was observed in the set-2RIP1 strain harboring a wild-type allele inserted at the his-3 locus (Fig. 3A).
As a first step to determine the specificity of SET-2, we analyzed histones from wild-type N. crassa with histone modification-specific antibodies. We found that lysines at positions 4, 27, 36, and 79 in histone H3 and at position 20 of histone H4 are methylated (Fig. 3). This is the first documentation of histone methylation in Neurospora crassa aside from that reported on lysines 4 and 9 of histone H3 (49). All three possible methylation states of K36, i.e., mono-, di-, and trimethylation, were detected in the wild-type strain (Fig. 3A and B). Methylation of K36 was specifically and completely lost in the set-2RIP1 mutant and was regained when the mutation was complemented (Fig. 3A and B). We conclude that the set-2 gene encodes a histone methyltransferase (HMT) responsible for all detectable K36 methylation in Neurospora crassa.
Absence of SET-2 did not noticeably impact methylation of residues other than K36 of histone H3 and did not affect DNA methylation (Fig. 3 and data not shown). Methylation of lysine residues 4, 9, 27, and 79 of H3 and lysine 20 of H4 were unaffected in set-2RIP1 (Fig. 3). Conversely, the dim-5 mutant, which lacks K9 methylation (49), showed normal levels of K36 methylation (Fig. 3C).
In order to further explore the enzymatic specificity of SET-2, we cloned a cDNA fragment coding for the first 372 amino acids (includes the AWS, SET, and post-SET domains) to generate a GST-tagged fusion protein and used this recombinant protein for in vitro studies. We found that the recombinant protein specifically methylated histone H3 at lysine 36 (Fig. 4). Although the recombinant protein was active on purified histones, it was significantly more active on nucleosomes, consistent with previous observation that the budding yeast Set2p is a nucleosome-selective HMT (Fig. 4) (47).
FIG. 4.
SET-2 preferentially methylates H3 in nucleosomes. (A) Lysates containing SET-2 from uninduced (U-SET-2) or IPTG-induced (I-SET-2) bacterial expression strains were used in HMT assays with chicken core histones or oligonucleosomes as substrates and 3H-labeled S-adenosyl-methionine (3H AdoMet). 3H incorporation was analyzed by the filter-binding assay (47). (B) Reaction products from HMT assays containing recombinant SET-2 from uninduced (U-SET-2) or IPTG-induced (I-SET-2) bacterial lysates, with chicken core histones or oligonucleosomes as substrates and 3H-labeled S-adenosyl-methionine were resolved on a sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis gel and examined by Coomassie staining (lower panel) and fluorography (upper panel). Asterisks indicate partial H3 breakdown product. (C) Reaction products from HMT assays containing recombinant SET-2 from uninduced (U-SET-2) or IPTG-induced (I-SET-2) bacterial lysates, with oligonucleosomes as substrates and AdoMet, were resolved on a sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis gel and examined by Coomassie staining (lower panel) and Western blotting (upper panel).
Mutation of K36 in histone H3 phenocopies the set-2RIP1 mutant.
It was formally possible that the phenotypes of the set-2RIP1 mutant might result from the effects of SET-2 on nonhistone proteins. To investigate this possibility, we took advantage of the recent finding that mus-52 mutants of Neurospora crassa preferentially integrate transforming DNA by homologous recombination (32), which makes gene replacements practical in this organism. We therefore replaced the single endogenous histone H3 gene with an allele harboring a lysine to leucine substitution at position 36 [hH3K36L], as described in Materials and Methods. Replacement of wild-type hH3 with hH3K36L was confirmed by Southern analysis (Fig. 5C). The hH3K36L mutant displayed slow growth and poor conidiation (Fig. 5D and 5E). It was also female sterile (data not shown).
In an attempt to obtain homokaryotic mutants, two transformants (N2970 and N2975) were crossed to a strain harboring a wild-type copy of histone H3 at the his-3 locus. We were unable to segregate the hH3K36L mutation at the endogenous locus from the wild-type copy at the his-3 locus suggesting that this residue is essential (data not shown). These observations argue that K36 of histone H3 is indeed the substrate of SET-2 that is critical for the normal growth and development of the fungus.
Chromatin associated with actively transcribed genes is enriched for methylated K4 and K36 in histone H3.
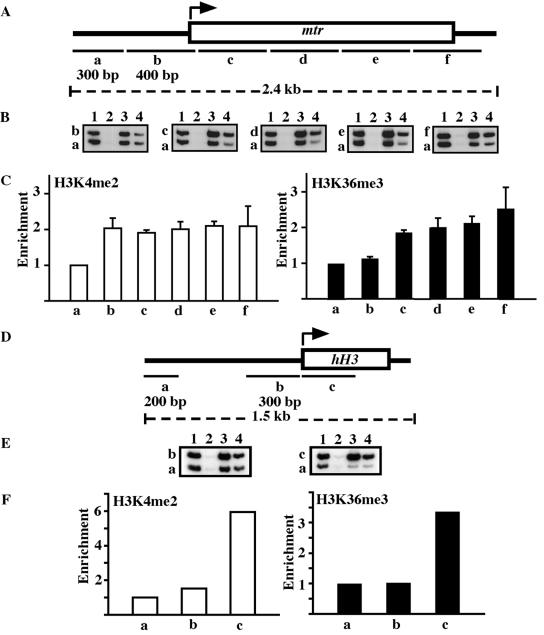
To investigate whether the distribution of K36 methylation on Neurospora crassa genes is similar to that reported for yeast (21), we performed chromatin immunoprecipitation assays on two actively transcribed genes, hH3 and mtr (methyltryptophan resistant) (34). We used antibodies specific for trimethyl-K36 and dimethyl-K4 (control) and primers designed to amplify 200- to 400-bp fragments in the upstream and coding regions of these genes (Fig. 6A and 6D). Unlike trimethylation of H3 K9, which is known to be enriched in genomic regions where DNA is methylated, K4 methylation is enriched in regions lacking DNA methylation (49). With both genes, we observed at least a twofold enrichment of both K4 and K36 methylation in the coding regions of these genes relative to their upstream regions (Fig. 6, region a). These findings suggest K4 and K36 methylations are enriched in actively transcribed regions.
FIG. 6.
H3 lysine 36 methylation is enriched in coding regions of actively transcribed genes. Chromatin immunoprecipitation analysis of regions across the mtr and hH3 genes. (A) Primers (Table 2) were designed to amplify 300-bp (a) or 400-bp (b, c, d, e, and f) fragments across a 2.4-kb region of the mtr gene. (B) PCRs were carried out to amplify region a with either b, c, d, e, or f. Samples are total input (1), no antibody (2), anti-H3K4me2 (3), and anti-H3K36me3 (4). (C) Relative enrichment of H3K4me2 and H3K36me3 at different regions across mtr. Relative enrichment values were calculated by dividing the ratio of band intensity for immunoprecipitated DNA/a region with ratio of intensities for total input/a region. Signal intensities are averages of PCRs from two independent chromatin immunoprecipitation experiments. All enrichments are standardized to that of region a. (D) Primers (Table 2) were designed to amplify 200-bp (a) or 300-bp (b and c) fragments across a 1.5-kb region of the hH3 gene. (E) PCRs were carried out to amplify region a with either b or c. (F) Relative enrichment of H3K4me2 and H3K36me3 at different regions across hH3.
DISCUSSION
In addition to DIM-5, a K9 HMT (48), N. crassa is predicted to have nine SET domain proteins (Fig. 1) (4). We showed that lysines at positions 4, 27, 36, and 79 of histone H3 and position 20 of histone H4 are also methylated and identified the gene (set-2) and corresponding protein (SET-2) responsible for all methylation of K36, including mono-, di-, and trimethylation. We generated a null allele of the gene, set-2RIP1, and found that strains with this allele are slow growing, conidiate sparsely, are female sterile, and show loss of methylation at K36. Methylation of lysines 4, 9, 27, and 79 in histone H3 and lysine 20 of histone H4 was unaffected by the set-2 mutation. Introduction of a wild-type allele into the set-2RIP1 strain rescued all mutant phenotypes. A recombinant protein consisting of the first 372 amino acids of SET-2, including the AWS, SET, and post-SET domains, was capable of methylating K36 in vitro, and preferred nucleosome substrates over free histones. We also replaced the single endogenous histone H3 gene with an allele coding for H3 with a K36L substitution and found that this mutation mimics the phenotypes of the set-2RIP1 mutant. Finally, we showed that the coding regions of actively transcribed genes are enriched in H3 methylated at K4 and K36.
The observation that set-2RIP1 strains produce sparse conidia, grow slowly, and are female sterile suggests that methylation of K36 is required for the normal expression of genes involved in asexual and sexual differentiation pathways. Differentiation of vegetative hyphae into conidiophores and formation of conidia requires numerous genes in N. crassa, including the regulatory genes fl (encodes a binuclear zinc cluster protein that is similar to the Gal4 class of transcription factors) and rco-1 (a homolog of S. cerevisiae Tup1) (4). Strains with mutations in fl are aconidial, while rco-1 strains are defective in maturation of conidia, grow abnormally slowly, and are female sterile (2, 57). The switch from vegetative growth and conidiation to sexual development is complex and is thought to involve a number of components in addition to the mating type genes (4, 35). While the variable growth rates of set-2RIP1 siblings are reminiscent of the situation with N. crassa dim-5 and hpo mutants (7, 48), no mechanistic link is obvious.
The C terminus of Neurospora crassa SET-2 includes a WW domain (44), as well as a recently described phosphorylated C-terminal domain (CTD)-interacting domain (SRI domain) that has been shown to mediate the interaction of Set2p to RNA polymerase II during transcription elongation in budding yeast (20). The S. cerevisiae Δset2 mutant shows growth alterations in the presence of 6-azauracil, suggesting that it has defects in transcription elongation (21, 27, 28). Curiously, when Set2p is tagged with the LexA DNA binding domain and transformed into yeast cells along with a CYC1-lexAop-lacZ construct, it reduces β-galactosidase activity, which suggests that it can also function as a transcriptional repressor (47).
Chromatin immunoprecipitation experiments revealed that K36 methylation and Set2p itself associate with the coding region of genes (21, 39, 56). In chicken, high levels of di- and trimethylated K36 are found in active genes compared to inactive genes (3). In Schizosaccharomyces pombe, deletion of the set2 gene results in a loss of K36 methylation and slow growth under low nutrient conditions (Morris et al., accompanying paper [29a]). SpSET2 also associates with the hyperphosphorylated form of polymerase II and K36 methylation is associated with the transcribed regions of polymerase II-regulated genes (Morris et al., accompanying paper), as in budding yeast, chicken, and Neurospora crassa.
The presence of mono-, di-, and trimethyl-K36 in wild-type Neurospora crassa and their absence in the set-2RIP1 mutant indicates that SET-2 can add up to three methyl groups on lysine 36. It has been proposed that the ability to add three methyl groups to substrate lysines is a characteristic of many histone methyltransferases that contain a specific conserved phenylalanine in the SET domain (5). SET-2 includes this residue at position 296 (NCBI protein accession number EAA28504) (5).
Our observation that replacement of wild-type histone H3 with an allele with a substitution at residue 36, hH3K36L, affected vegetative growth, sporulation, and sexual development, like mutation of set-2, demonstrated that histone H3 is the critical target of SET-2. Although similar phenotypes have not been observed in yeasts, it is interesting that replacement of K36 with an arginine in S. cerevisiae reduces repression of the ΔUAS gal4::cat reporter, which was also observed when SET2 was deleted (25). In Caenorhabditis elegans, mutants for the Tousled-like kinase (TLK-1) are arrested in development at the 100-cell stage, remain undifferentiated, and have reduced levels of phosphorylated RNA polymerase II (serine 2) and K36 methylation (14). In mice, it has been proposed that the methylation of H3-K36 and H4-K20 by the Nuclear receptor-binding SET Domain-containing protein (NSD1) is essential for early postimplantation development (36). Mutations in this gene cause the Soto’s syndrome in humans, a disorder characterized by overgrowth of neural tissues, heart defects, advanced bone age, developmental delays, and increased risk of cancers (22, 52). In metazoans, active genes contain higher levels of di- and trimethylated K36 compared to inactive genes (3). Taken together, these studies suggest that methylation of K36 of histone H3 is an evolutionarily conserved modification essential for normal growth and development in eukaryotes.
Acknowledgments
We thank Emanuela Berge for generating pEB22, Hisashi Tamaru for generating pHT13, Michael Freitag for construction of N2836 and advice with the hH3 replacements, T. Jenuwein for the anti-H3K27me3, and P. B. Singh for anti-H3K9me3. We also thank Michael Freitag, Kristina Smith, and Gregory Kothe for helpful discussions and comments on the manuscript.
This work is supported by N.I.H. grant GM35690 and N.S.F. grant MCB0131383 to E.U.S, N.I.H. grant GM68088 to B.D.S., an American Heart Association Postdoctoral Fellowship 0225370Z to K.K.A., and a Ford Foundation Fellowship award to S.A.M. B.D.S. is a Pew Scholar in the Biomedical Sciences.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey-Shrode, L., and D. J. Ebbole. 2004. The fluffy gene of Neurospora crassa is necessary and sufficient to induce conidiophore development. Genetics 166:1741-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannister, A. J., R. Schneider, F. A. Myers, A. W. Thorne, C. Crane-Robinson, and T. Kouzarides. 2005. Spatial distribution of di-and tri-methyl lysine 36 of histone H3 at active genes. J. Biol. Chem. 280:17732-17736. [DOI] [PubMed] [Google Scholar]
- 4.Borkovich, K. A., L. A. Alex, O. Yarden, M. Freitag, G. E. Turner, N. D. Read, S. Seiler, D. Bell-Pedersen, J. Paietta, N. Plesofsky, M. Plamann, M. Goodrich-Tanrikulu, U. Schulte, G. Mannhaupt, F. E. Nargang, A. Radford, C. Selitrennikoff, J. E. Galagan, J. C. Dunlap, J. J. Loros, D. Catcheside, H. Inoue, R. Aramayo, M. Polymenis, E. U. Selker, M. S. Sachs, G. A. Marzluf, I. Paulsen, R. Davis, D. J. Ebbole, A. Zelter, E. R. Kalkman, R. O'Rourke, F. Bowring, J. Yeadon, C. Ishii, K. Suzuki, W. Sakai, and R. Pratt. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 68:1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, R. E., M. Tachibana, H. Tamaru, K. M. Smith, D. Jia, X. Zhang, E. U. Selker, Y. Shinkai, and X. Cheng. 2004. In vitro and in vivo analyses of a Phe/Tyr switch controlling product specificity of histone lysine methyltransferases. J. Biol. Chem. 280:5563-5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, R. H. 2000. Neurospora: contributions of a model organism. Oxford University Press, New York, NY.
- 7.Freitag, M., P. C. Hickey, T. K. Khlafallah, N. D. Read, and E. U. Selker. 2004. HP1 is essential for DNA methylation in Neurospora. Mol. Cell 13:427-434. [DOI] [PubMed] [Google Scholar]
- 8.Freitag, M., P. C. Hickey, N. B. Raju, E. U. Selker, and N. D. Read. 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41:897-910. [DOI] [PubMed] [Google Scholar]
- 9.Freitag, M., R. L. Williams, G. O. Kothe, and E. U. Selker. 2002. A cytosine methyltransferase homologue is essential for repeat-induced point mutation in Neurospora crassa. Proc. Natl. Acad. Sci. USA 99:8802-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Cao, M., R. O'Sullivan, A. H. Peters, T. Jenuwein, and M. A. Blasco. 2004. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat. Genet. 36:94-99. [DOI] [PubMed] [Google Scholar]
- 11.Gerber, M., and A. Shilatifard. 2003. Transcriptional elongation control and histone methylation. J. Biol. Chem. 278:26303-26306. [DOI] [PubMed] [Google Scholar]
- 12.Grewal, S. I., and D. Moazed. 2003. Heterochromatin and epigenetic control of gene expression. Science 301:798-802. [DOI] [PubMed] [Google Scholar]
- 13.Hampsey, M., and D. Reinberg. 2003. Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell 113:429-432. [DOI] [PubMed] [Google Scholar]
- 14.Han, Z., J. R. Saam, H. P. Adams, S. E. Mango, and J. M. Schumacher. 2003. The C. elegans Tousled-like kinase (TLK-1) has an essential role in transcription. Curr. Biol. 13:1921-1929. [DOI] [PubMed] [Google Scholar]
- 15.Hays, S. M., J. Swanson, and E. U. Selker. 2002. Identification and characterization of the genes encoding the core histones and histone variants of Neurospora crassa. Genetics 160:961-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaenisch, R., and A. Bird. 2003. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33(Suppl.):245-254. [DOI] [PubMed] [Google Scholar]
- 17.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 19.Kanoh, J., S. Francesconi, A. Collura, V. Schramke, F. Ishikawa, G. Baldacci, and V. Geli. 2003. The fission yeast spSet1p is a histone H3-K4 methyltransferase that functions in telomere maintenance and DNA repair in an ATM kinase Rad3-dependent pathway. J. Mol. Biol. 326:1081-1094. [DOI] [PubMed] [Google Scholar]
- 20.Kizer, K. O., H. P. Phatnani, Y. Shibata, H. Hall, A. L. Greenleaf, and B. D. Strahl. 2005. A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol. Cell. Biol. 25:3305-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krogan, N. J., M. Kim, A. Tong, A. Golshani, G. Cagney, V. Canadien, D. P. Richards, B. K. Beattie, A. Emili, C. Boone, A. Shilatifard, S. Buratowski, and J. Greenblatt. 2003. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 23:4207-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurotaki, N., K. Imaizumi, N. Harada, M. Masuno, T. Kondoh, T. Nagai, H. Ohashi, K. Naritomi, M. Tsukahara, Y. Makita, T. Sugimoto, T. Sonoda, T. Hasegawa, Y. Chinen, H. A. Tomita Ha, A. Kinoshita, T. Mizuguchi, K. Yoshiura Ki, T. Ohta, T. Kishino, Y. Fukushima, N. Niikawa, and N. Matsumoto. 2002. Haploinsufficiency of NSD1 causes Sotos syndrome. Nat. Genet. 30:365-366. [DOI] [PubMed] [Google Scholar]
- 23.Lachner, M., and T. Jenuwein. 2002. The many faces of histone lysine methylation. Curr. Opin. Cell Biol. 14:286-298. [DOI] [PubMed] [Google Scholar]
- 24.Lachner, M., R. J. O'Sullivan, and T. Jenuwein. 2003. An epigenetic road map for histone lysine methylation. J. Cell Sci. 116:2117-2124. [DOI] [PubMed] [Google Scholar]
- 25.Landry, J., A. Sutton, T. Hesman, J. Min, R. M. Xu, M. Johnston, and R. Sternglanz. 2003. Set2-catalyzed methylation of histone H3 represses basal expression of GAL4 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23:5972-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letunic, I., R. R. Copley, S. Schmidt, F. D. Ciccarelli, T. Doerks, J. Schultz, C. P. Ponting, and P. Bork. 2004. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 32:D142-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, B., L. Howe, S. Anderson, J. R. Yates, 3rd, and J. L. Workman. 2003. The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J. Biol. Chem. 278:8897-8903. [DOI] [PubMed] [Google Scholar]
- 28.Li, J., D. Moazed, and S. P. Gygi. 2002. Association of the histone methyltransferase Set2 with RNA polymerase II plays a role in transcription elongation. J. Biol. Chem. 277:49383-49388. [DOI] [PubMed] [Google Scholar]
- 29.Margolin, B. S., M. Freitag, and E. U. Selker. 1997. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Newsl. 44:34-36. [Google Scholar]
- 29a.Morris, S. A., Y. Shibata, K. Noma, Y. Tsukamoto, E. Warren, B. Temple, S. I. S. Grewal, and B. D. Strahl. 2005. Histone H3 K36 methylation is associated with transcription elongation in Schizosaccharomyces pombe. Eukaryot. Cell 4:1446-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakai, K., and P. Horton. 1999. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 24:34-36. [DOI] [PubMed] [Google Scholar]
- 31.Nelson, M. A., S. Kang, E. L. Braun, M. E. Crawford, P. L. Dolan, P. M. Leonard, J. Mitchell, A. M. Armijo, L. Bean, E. Blueyes, T. Cushing, A. Errett, M. Fleharty, M. Gorman, K. Judson, R. Miller, J. Ortega, I. Pavlova, J. Perea, S. Todisco, R. Trujillo, J. Valentine, A. Wells, M. Werner-Washburne, and D. O. Natvig. 1997. Expressed sequences from conidial, mycelial, and sexual stages of Neurospora crassa. Fungal Genet. Biol. 21:348-363. [DOI] [PubMed] [Google Scholar]
- 32.Ninomiya, Y., K. Suzuki, C. Ishii, and H. Inoue. 2004. Highly efficient gene replacements in Neurospora strains deficient for nonhomologous end-joining. Proc. Natl. Acad. Sci. USA 101:12248-12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Burgos, L., A. H. Peters, S. Opravil, M. Kauer, K. Mechtler, and T. Jenuwein. 2004. Generation and characterization of methyl-lysine histone antibodies. Methods Enzymol. 376:234-254. [DOI] [PubMed] [Google Scholar]
- 34.Perkins, D. D., A. Radford, and M. S. Sachs. 2001. The Neurospora compendium; chromosomal loci. Academic Press, San Diego, Calif.
- 35.Raju, N. B. 1992. Genetic control of the sexual cycle in Neurospora. Mycol. Res. 96:241-262. [Google Scholar]
- 36.Rayasam, G. V., O. Wendling, P. O. Angrand, M. Mark, K. Niederreither, L. Song, T. Lerouge, G. L. Hager, P. Chambon, and R. Losson. 2003. NSD1 is essential for early post-implantation development and has a catalytically active SET domain. EMBO J. 22:3153-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rountree, M. R., and E. U. Selker. 1997. DNA methylation inhibits elongation but not initiation of transcription in Neurospora crassa. Genes Dev. 11:2383-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan, F. J., G. W. Beadle, and E. L. Tatum. 1943. The tube method of measuring the growth rate of Neurospora. Am. J. Bot. 30:784-799. [Google Scholar]
- 39.Schaft, D., A. Roguev, K. M. Kotovic, A. Shevchenko, M. Sarov, K. M. Neugebauer, and A. F. Stewart. 2003. The histone 3 lysine 36 methyltransferase, SET2, is involved in transcriptional elongation. Nucleic Acids Res. 31:2475-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider, R., A. J. Bannister, and T. Kouzarides. 2002. Unsafe SETs: histone lysine methyltransferases and cancer. Trends Biochem. Sci. 27:396-402. [DOI] [PubMed] [Google Scholar]
- 41.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selker, E. U. 1990. Premeiotic instability of repeated sequences in Neurospora crassa. Annu. Rev. Genet. 24:579-613. [DOI] [PubMed] [Google Scholar]
- 43.Shilatifard, A. 2004. Transcriptional elongation control by RNA polymerase II: a new frontier. Biochim. Biophys. Acta 1677:79-86. [DOI] [PubMed] [Google Scholar]
- 44.Sims, R. J., 3rd, R. Belotserkovskaya, and D. Reinberg. 2004. Elongation by RNA polymerase II: the short and long of it. Genes Dev. 18:2437-2468. [DOI] [PubMed] [Google Scholar]
- 45.Sims, R. J., 3rd, K. Nishioka, and D. Reinberg. 2003. Histone lysine methylation: a signature for chromatin function. Trends Genet. 19:629-639. [DOI] [PubMed] [Google Scholar]
- 46.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 47.Strahl, B. D., P. A. Grant, S. D. Briggs, Z. W. Sun, J. R. Bone, J. A. Caldwell, S. Mollah, R. G. Cook, J. Shabanowitz, D. F. Hunt, and C. D. Allis. 2002. Set2 is a nucleosomal histone H3-selective methyltransferase that mediates transcriptional repression. Mol. Cell. Biol. 22:1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamaru, H., and E. U. Selker. 2001. A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414:277-283. [DOI] [PubMed] [Google Scholar]
- 49.Tamaru, H., X. Zhang, D. McMillen, P. B. Singh, J. Nakayama, S. I. Grewal, C. D. Allis, X. Cheng, and E. U. Selker. 2003. Trimethylated lysine 9 of histone H3 is a mark for DNA methylation in Neurospora crassa. Nat. Genet. 34:75-79. [DOI] [PubMed] [Google Scholar]
- 50.Turner, B. M. 2005. Reading signals on the nucleosome with a new nomenclature for modified histones. Nat. Struct. Mol. Biol. 12:110-112. [DOI] [PubMed] [Google Scholar]
- 51.van Holde, K. 1988. Chromatin. Springer Verlag, New York, NY.
- 52.Visser, R., and N. Matsumoto. 2003. Genetics of Sotos syndrome. Curr. Opin. Pediatr. 15:598-606. [DOI] [PubMed] [Google Scholar]
- 53.Vollmer, S. J., and C. Yanofsky. 1986. Efficient cloning of genes of Neurospora crassa. Proc. Natl. Acad. Sci. USA 83:4869-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolffe, A. P. 1998. Chromatin: structure and function. Academic Press, San Diego, Calif.
- 55.Wolffe, A. P., and J. J. Hayes. 1999. Chromatin disruption and modification. Nucleic Acids Res. 27:711-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao, T., H. Hall, K. O. Kizer, Y. Shibata, M. C. Hall, C. H. Borchers, and B. D. Strahl. 2003. Phosphorylation of RNA polymerase II CTD regulates H3 methylation in yeast. Genes Dev. 17:654-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamashiro, C. T., D. J. Ebbole, B. U. Lee, R. E. Brown, C. Bourland, L. Madi, and C. Yanofsky. 1996. Characterization of rco-1 of Neurospora crassa, a pleiotropic gene affecting growth and development that encodes a homolog of Tup1 of Saccharomyces cerevisiae. Mol. Cell. Biol. 16:6218-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, X., H. Tamaru, S. Khan, J. Horton, L. Keefe, E. Selker, and X. Cheng. 2002. Structure of the Neurospora SET domain protein DIM-5, a histone H3 lysine methyltransferase. Cell 111:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, X., Z. Yang, S. I. Khan, J. R. Horton, H. Tamaru, E. U. Selker, and X. Cheng. 2003. Structural basis for the product specificity of histone lysine methyltransferases. Mol. Cell 12:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, Y., and D. Reinberg. 2001. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 15:2343-2360. [DOI] [PubMed] [Google Scholar]