Abstract
The genetic analysis of Candida albicans, the major fungal pathogen of humans, is hampered by its diploid genome, the absence of a normal sexual cycle, and a nonstandard codon usage. Although effective methods to study gene function have been developed in the past years, systems to control gene expression in C. albicans are limited. We have established a system that allows induction of gene expression in C. albicans by the addition of tetracycline (Tet). By fusing genetically modified versions of the reverse Tet repressor from Escherichia coli and the transcription activation domain of the Gal4 protein from Saccharomyces cerevisiae, a C. albicans-adapted reverse Tet-dependent transactivator (rtTA) was created that was expressed from the constitutive ADH1 or the opaque-specific OP4 promoter. To monitor Tet-inducible gene expression, the caGFP reporter gene was placed under the control of a Tet-dependent promoter, obtained by fusing a minimal promoter from C. albicans to seven copies of the Tet operator sequence. Fluorescence of the cells demonstrated that gene expression could be efficiently induced by the addition of doxycycline in yeast, hyphal, and opaque cells of C. albicans. The Tet-inducible gene expression system was then used to manipulate the behavior of the various growth forms of C. albicans. Tet-induced expression of a dominant-negative CDC42 allele resulted in growth arrest as large, multinucleate cells. Filamentous growth was efficiently inhibited under all tested hyphal-growth-promoting conditions by Tet-inducible expression of the NRG1 repressor. Tet-induced expression of the MTLa1 gene in opaque cells of an MTLα strain forced the cells to switch to the white phase, whereas Tet-induced expression of the MTLa2 transcription factor induced shmooing. When the ecaFLP gene, encoding the site-specific recombinase FLP, was placed under the control of the Tet-dependent promoter, Tet-inducible deletion of genes which were flanked by the FLP target sequences was achieved with high efficiency to generate conditional null mutants. In combination with the dominant selection marker caSAT1, the Tet-inducible gene expression system was also applied in C. albicans wild-type strains, including drug-resistant clinical isolates that overexpressed the MDR1, CDR1, and CDR2 multidrug efflux pumps. This system, therefore, allows a growth medium-independent, Tet-inducible expression and deletion of genes in C. albicans and provides a convenient, versatile new tool to study gene function and manipulate cellular behavior in this model pathogenic fungus.
The yeast Candida albicans is a member of the normal microflora on mucosal surfaces of the gastrointestinal and urogenitary tract in healthy persons, but it can also cause severe infections, especially in immunocompromised patients. The molecular analysis of C. albicans is hampered by its diploid genome, the absence of a normal sexual cycle, and a nonstandard codon usage. Nevertheless, techniques have been developed in the past years for efficient genetic manipulation of C. albicans, which has considerably advanced our knowledge about the biology and pathogenicity mechanisms of this fungus (4, 9).
Systems that allow researchers to experimentally control the expression of specific genes in an organism under study are highly valuable for analyzing gene function and also for manipulating the behavior of the organism. Several regulatable promoters are employed by investigators to induce or repress gene expression in C. albicans, e.g., the PCK1 promoter, the MAL2 promoter, and the MET3 promoter, which are repressed by glucose or methionine/cysteine (1, 8, 20). When a target gene is placed under the control of one of these promoters, its expression can be turned on or shut off by incubating the C. albicans cells in appropriate inducing or repressing growth media. For many purposes, however, it is desirable to control gene expression without the necessity of changing the growth medium, but simply by the addition of an inducing or repressing substance that itself does not affect metabolism. The tetracycline (Tet) system allows such a growth medium-independent control of gene expression by a small molecule that can easily diffuse into the cells. It is based on the tetracycline repressor protein (TetR) from Escherichia coli, which binds to its target sequence, the tet operator (tetO), in the promoter region of the tetracycline resistance genes to repress their expression in the absence of tetracycline. When present, tetracycline binds with high affinity to TetR, resulting in dissociation of the repressor from the promoter and expression of the tet genes (16). The bacterial Tet system has been adapted for use in eukaryotic cells by fusing the activation domain of a transcription factor to TetR, thereby turning it into a tetracycline-controlled transcriptional activator (tTA) (14). A gene that is placed under the control of a minimal promoter in which all activating sequences have been removed and replaced by the tetO sequence will be expressed in cells producing tTA, which in the absence of tetracycline binds to tetO to allow transcription. The addition of tetracycline to the cells results in dissociation of the activator from the promoter, thereby shutting off gene expression. This Tet-Off system has also been established in C. albicans and used to efficiently control the expression of specific genes (30, 33, 35).
The regulatable promoters mentioned above are usually used to turn down gene expression, especially of essential genes that cannot be deleted from the genome, to gain information about gene function by depletion of the gene product from the cells (8, 33). But of equal importance for addressing biological questions is the possibility of inducing the expression of specific genes under conditions in which they are normally not expressed. The Tet-Off system is less suitable for this purpose, because gene induction would require efficient removal of tetracycline from cells grown in the presence of the drug, which is not always feasible. In addition, to keep genes whose products have a toxic effect on the cells in a repressed state before induction, tetracycline would have to be present in sufficient concentrations all the time after the corresponding strains have been constructed. Remarkably, certain amino acid exchanges in TetR have been found to reverse its dependence on tetracycline, i.e., the reverse Tet repressor (rTetR) binds to tetO only in the presence of tetracycline, not in its absence (15). Fusion of rTetR to a transcription activation domain generated a reverse tetracycline-controlled transactivator (rtTA) which can be used to induce gene expression in a wide variety of eukaryotic cells by the addition of the tetracycline derivative doxycycline (Dox) (15). Since then, this Tet-On system has been further improved by the identification of novel rtTA mutants with reduced basal activity and increased Dox sensitivity (44).
In the present study, we have established such a Tet-inducible gene expression system for C. albicans and used it to investigate the consequences of expression of specific genes on the behavior of C. albicans yeast and hyphal cells as well as opaque cells, the mating-competent form of the fungus. Our results demonstrate that this C. albicans-adapted tetracycline-dependent gene expression system is a versatile tool with which to study gene function and manipulate cellular behavior in this important human fungal pathogen.
MATERIALS AND METHODS
Strains and growth conditions.
C. albicans strains used in this study are listed in Table 1. All strains were stored as frozen stocks in 15% glycerol at −80°C. Strain CAI4 and derivatives were propagated on synthetic dextrose (SD) agar plates containing 6.7 g of yeast nitrogen base without amino acids (BIO 101, Vista, Calif.), 20 g of glucose, 0.77 g of complete supplement medium without uracil (BIO 101), and 15 g of agar per liter. Strains WO-1, MTLa/Δmtlα, and their derivatives were subcultured separately in the white and opaque phases at room temperature on agar plates containing Lee's medium, pH 6.8 (2), and 5 μg ml−1 phloxine B, which selectively stains opaque colonies pink (38). The clinical isolates and their derivatives were propagated on YPD agar plates (20 g of peptone, 10 g of yeast extract, 20 g of glucose, 15 g of agar per liter). Strains were routinely grown in YPD liquid medium at 30°C. To support the growth of ura3 strains, 100 μg ml−1 uridine was added to the media. Filamentous growth was induced by growing the cells at 37°C on agar plates containing Lee's medium (19), synthetic low-ammonium dextrose (SLAD) medium (13), or 10% fetal calf serum (FCS). To induce hyphal growth in liquid media, cells from a YPD overnight culture were inoculated into Lee's medium, SLAD medium, or RPMI 1640 medium with 10% FCS and incubated at 37°C. Mycophenolic acid-resistant (MPAR) and -sensitive (MPAS) cells of strains SMC4TF23A and -B were distinguished by the size of the colonies produced after plating appropriate dilutions of the cultures on SD agar plates containing 1.5 μg ml−1 MPA (25).
TABLE 1.
C. albicans strains used in this study
| Strain | Parent | Relevant genotype or characteristicsa | Reference or source |
|---|---|---|---|
| SC5314 | Wild-type strain | 12 | |
| CAI4 | SC5314 | ura3Δ::imm434/ura3Δ::imm434 | 10 |
| TETG25A and -B | CAI4 | ADH1/adh1::Ptet-caGFPb | This study |
| TETC36A and -B | CAI4 | ADH1/adh1::Ptet-CDC42D118Ab | This study |
| TETN42A and -B | CAI4 | ADH1/adh1::Ptet-NRG1b | This study |
| TETMa46A and -B | CAI4 | ADH1/adh1::Ptet-MTLa2b | This study |
| WO-1 | Wild-type strain, MTLα | 37 | |
| WUM5A | WO-1 | ura3Δ::FRT/ura3Δ::FRT | 42 |
| WTETG40A and -B | WUM5A | OP4/op4::Ptet-caGFPb | This study |
| WTETMa41A and -B | WUM5A | OP4/op4::Ptet-MTLa1b | This study |
| WTETMa43A and -B | WUM5A | OP4/op4::Ptet-MTLa2b | This study |
| MTLa/Δmtlα | CAI4 | MTLa/mtlαΔ | 17 |
| ΔmtlαTETG40A and -B | MTLa/Δmtlα | OP4/op4::Ptet-caGFPb | This study |
| ΔmtlαTETMa43A and -B | MTLa/Δmtlα | OP4/op4::Ptet-MTLa2b | This study |
| SMC4 | CAI4 | CDC42/cdc42Δ::FRT ACT1/act1::FRT-CDC42-MPAR-FRT | 25 |
| SMC4TF23A and -B | SMC4 | ADH1/adh1::Ptet-ecaFLPb | This study |
| SMC6 | CAI4 | cdc42Δ::FRT/cdc42Δ::FRT ACT1/actl::FRT-CDC42-MPAR-FRT | 25 |
| SMC6TF23A and -B | SMC6 | ADH1/adh1::Ptet-ecaFLPb | This study |
| F2 | Clinical isolate, fluconazole sensitive | 11 | |
| F2NIM1A and -B | F2 | ADH1/adh1::Ptet-caGFPb | This study |
| F5 | Clinical isolate, fluconazole resistant, MDR1 overexpression | 11 | |
| F5NIM1A and -B | F5 | ADH1/adh1::Ptet-caGFPb | This study |
| G2 | Clinical isolate, fluconazole sensitive | 11 | |
| G2NIM1A and -B | G2 | ADH1/adh1::Ptet-caGFPb | This study |
| G5 | Clinical isolate, fluconazole resistant, MDR1 overexpression | 11 | |
| G5NIM1A and -B | G5 | ADH1/adh1::Ptet-caGFPb | This study |
| DSY294 | Clinical isolate, fluconazole sensitive | 34 | |
| DSY294NIM1A and -B | DSY294 | ADH1/adh1::Ptet-caGFPb | This study |
| DSY296 | Clinical isolate, fluconazole resistant, CDR1 and CDR2 overexpression | 34 | |
| DSY296NIM1A and -B | DSY296 | ADH1/adh1::Ptet-caGFPb | This study |
Apart from the indicated features, all strains constructed in this study are identical to their parental strains.
The whole cassette containing rtTA under control of the ADH1 (or OP4) promoter, the URA3 (or caSAT1) selection marker, and the indicated target gene under control of the Tet-inducible promoter was inserted into one of the ADH1 or OP4 alleles.
Plasmid constructions. (i) Generation of a C. albicans-adapted reverse tet repressor gene.
Inspection of the nucleotide sequence of the rtTAS-M2 gene contained in plasmid pUHrT62-1 (44) showed that in addition to the amino acid exchanges that created rtTA-M2 a total of 19 CTG codons, which would be mistranslated as serine instead of leucine in C. albicans, had been introduced into the rtetR part. In contrast, the original tetR gene contains only one CTG codon. Therefore, we chose to introduce the five amino acid exchanges described by Urlinger et al. (44) into the original tetR gene and at the same time change the single CTG codon to a TTG codon by PCR-mediated, site-specific mutagenesis to produce a C. albicans-adapted reverse Tet repressor gene, cartetR. The tetR coding sequence without the stop codon was amplified from plasmid pASK75 (36) with the primer pair TETR4 (5′-atATGTCTAGATTAGATAAAAGTAAAGTGATTAACgGCGCATTAGAGtTGCTTAATGgGGTCGG-3′) and TETR2 (5′-ggctgctcgagGACCCACTTTCACATTTAAG-3′), digested at the XbaI and XhoI restriction sites (underlined) and cloned in pBluescript to create pTET17. The tetR start codon in primer TETR4 is highlighted in boldface, and the introduced nucleotide exchanges resulting in the serine-glycine substitution at amino acid position 12 (S12G), the glutamate-glycine substitution at amino acid position 19 (E19G), and the CTG-TTG codon substitution at nucleotide positions 46 to 48 are marked by lowercase letters. pTET17 then served as template for sequential rounds of inverse PCR and religation, using the primer pairs TETR5 (5′-TAAGGCGTCGAGCAAAGCCC-3′) and TETR6 (5′-cCaATTGAGATGTTAGATAGGC-3′), TETR7 (5′-TCTTCCAATACGCAACCTAA-3′) and TETR8 (5′-aCAAGAGCATCAAGTCGCTAA-3′), and TETR9 (5′-ATCAAATAATTCGATAGCTTG-3′) and TETR10 (5′-agaCAAGGTGCAGAGCCAGCCTT-3′), resulting in plasmid pTET20, which carries the cartetR gene encoding the amino acid substitutions S12G, E19G, A56P, D149E, and H179R, but without the start and stop codons.
(ii) Generation of a C. albicans-adapted reverse tetracycline-dependent trans-activator gene.
To generate the tetracycline-dependent transactivator, the cartetR gene was fused with the activation domain of the Saccharomyces cerevisiae GAL4 gene. For this purpose, a GAL4 fragment corresponding to amino acids 764 to 881 of Gal4p and including the stop codon (reverse sequence in boldface) was amplified from plasmid pCL41 (Clontech, Heidelberg, Germany) with the primer pair GAL1 (5′-GCCACTGACCCCGTCgaCTTTGTTTGGTGGCGCC-3′) and GAL2 (5′-atataggatccTTACTCTTTTTTTGGGTTTGGTGGGG-3′). The PCR product was digested at the introduced SalI and BamHI sites (underlined) and cloned in pBluescript to generate pGAL4AD1. This plasmid served as template to exchange the CTG codon at nucleotide positions 2506 to 2508 of GAL4 for a TTG codon by inverse PCR with the primer pair GAL3 (5′-TTACCATCATCAATTTTACTAGCC-3′) and GAL4 (5′-TAATTCAAAACCAtTGTCACCTGGTTGG-3′), resulting in plasmid pGAL4AD2. The SalI-BamHI caGAL4AD fragment from pGAL4AD2 was then cloned together with the XbaI-XhoI cartetR fragment from pTET20 in pBluescript to generate pTET21 containing the cartTA gene, a fusion of cartetR and caGAL4AD. The ACT1 transcription termination sequence (TACT1) was amplified from genomic DNA of C. albicans strain CAI4 with the primers ACT16 (5′-TTCTAAGAtctAAATTCTGGAAATCTGG-3′) and ACT17 (5′-atatactgcaGACATTTTATGATGGAATGAATGGG-3′). The PCR product was digested at the introduced BglII and PstI sites (underlined) and cloned behind the cartTA stop codon in the BamHI/PstI-digested pTET21 to create pTET22.
(iii) Construction of a tetracycline-inducible gene expression cassette.
An ADH1 promoter fragment was amplified from CAI4 genomic DNA with the primer pair ADH1 (5′-TGTCAAAGGATTCcgCGGTTGAGATGGAG-3′) and ADH2 (5′-TTTGTTCtAGACATAATTGTTTTTGTATTTGTTG-3′). The PCR product was digested at the SacII site introduced at position −868 and at the XbaI site introduced behind the start codon (reverse sequence in boldface) and fused with the XbaI-PstI fragment from pTET22, thereby placing a complete cartTA gene, including the regenerated start codon, under the control of the ADH1 promoter.
A fragment containing sequences from the ADH1 coding region (positions +321 to +1015) was amplified from CAI4 genomic DNA with the primer pair ADH5 (5′-TGAATTCTGTCgACAAGGTGCTGAACC-3′) and ADH6 (5′-CGTATCTACCCAAGATggTACCTTCTTCCATC-3′). The PCR product was digested at the introduced SalI and KpnI sites (underlined) and cloned together with a SacI-XhoI fragment from plasmid pUHC13-3 (14) containing seven copies of the tet operator sequence (tetO) into pBluescript to generate pTET1. To create the tetracycline-dependent promoter Ptet, a fragment from the upstream region of the OP4 gene (positions −375 to −8) was amplified from genomic DNA of C. albicans strain WO-1 with the primer pair OPS6 (5′-TCATTGTcgacTATTTATATTTGTATGTGTGTAGG-3′) and OPS9 (5′-CATAAACCAAATTGagcTCCAAACTTCTT-3′). The PCR product was digested at the introduced SalI and SacI sites (underlined) and fused to the SacI-KpnI fragment from pTET1 containing tetO and ADH1 sequences. The resulting fragment was combined in the vector pBluescript with the SacII-PstI fragment containing the PADH1-cartTA fusion and with a SalI-PstI fragment from pGFP41 (28) containing a C. albicans-adapted GFP reporter gene (caGFP) and the URA3 selection marker to generate plasmid pTET25 (Fig. 1A). The cassette contained in this plasmid can be integrated into one of the ADH1 alleles in the C. albicans genome by homologous recombination with the flanking ADH1 sequences, using the URA3 marker for selection of transformants. The cartTA gene will then be constitutively expressed from the ADH1 promoter, so that addition of doxycycline to the cells allows binding of rtTA to the tetO sequences, resulting in transcription of the caGFP gene.
FIG. 1.
Structures of the DNA cassettes used to integrate Tet-inducible genes into the ADH1 or the OP4 locus of C. albicans. Bent arrows symbolize promoters (P), and the filled circles indicate the transcription termination sequence of the ACT1 gene (TACT1). The URA3 marker (gray arrows) contains its own promoter and termination sequences. The prefix ca indicates C. albicans-adapted versions of heterologous genes. Only relevant restriction sites used to construct the plasmids (see Materials and Methods) or to obtain the fragments used for transformation are shown. B, BamHI; Bg, BglII; EI, EcoRI; K, KpnI; Nc, NcoI; P, PstI; ScI, SacI; ScII, SacII; Sl, SalI; X, XbaI; Xh, XhoI.
(iv) Construction of additional cassettes with other tetracycline-inducible genes.
Several derivatives of pTET25 were generated to place genes other than caGFP under the control of the tetracycline-dependent promoter (Fig. 1). In pTET23 (Fig. 1H), the SalI-SalI fragment with the caGFP gene from pTET25 was replaced by a similar fragment from pSFL213 that contains the ecaFLP gene, encoding the site-specific recombinase FLP (40). In pTET42 (Fig. 1C), Ptet controls expression of the NRG1 gene. To facilitate insertion of the NRG1 and other open reading frames (ORFs) into the expression cassette, the ACT1 termination sequence was first amplified as a BamHI-XhoI fragment with the primers ACT29 (5′-CTAAGgatccAATTCTGGAAATCTGGAAATCTGG-3′) and ACT21 (5′-atatactcgagGACATTTTATGATGGAATGAATGGG-3′) and fused to the SalI-EcoRI fragment containing the 5′URA3 sequence. The BamHI-EcoRI fragment containing the TACT1 and caURA3 sequences was then cloned together with a SalI-BamHI fragment from pCdMNRG12 (41) containing the NRG1 ORF into the SalI/EcoRI-digested pTET25 to generate pTET42. In pTET36 (Fig. 1B), NRG1 was replaced by a dominant-negative allele of the CDC42 gene, CDC42D118A. The CDC42D118A allele was obtained by first amplifying the CDC42 coding region from CAI4 genomic DNA with the primer pair CDC9 (5′-ATCCAgtcgaCATGCAAACTATAAAATGTGTTG-3′) and CDC12 (5′-CTTCTAGTATCGggatCCTATAAAATAG-3′) and digesting the PCR product at the SalI site (underlined) introduced in front of the start codon (marked in boldface) and at an internal KpnI site at position +344. The C-terminal part of CDC42 was amplified with the primer pair CDC11 (5′-TGTCGGTACCCAAACTGcTTTACGAAACG-3′) and CDC12, thereby introducing the D118A substitution. The PCR product was digested at the natural KpnI site and at the BamHI site (underlined) introduced behind the stop codon (reverse sequence in boldface), ligated with the SalI-KpnI fragment containing the CDC42 N-terminal part, and substituted for the NRG1 ORF to generate pTET36. To insert the Ptet-caGFP fusion into the OP4 locus, the OP4 upstream sequence was amplified from strain WO-1 genomic DNA with the primers OPS15 (5′-CGCCACCACCTCCGcggTTTATTGAGGG-3′) and OPS16 (5′-tctagaCATTGTAAATTATTTATATTTGTATGTGTGTAGGAG-3′), digested at the SacII site introduced at position −720 and at the XbaI site introduced behind the start codon (reverse sequence in bold), and substituted for the ADH1 promoter fragment of pTET25. An XhoI-KpnI fragment containing OP4 downstream sequences was then amplified with the primers OPS3 (5′-CTTTAGTTAATGCTcgaGGTCAAGCTGCCTC-3′) and OPS4 (5′-CAACAAATTCAGGTacCTTGAAAGCTGCAAC-3′) and substituted for the 3′ADH1 fragment, resulting in pTET40 (Fig. 1D). To place the MTLa1 gene under the control of Ptet, a SalI-BamHI fragment containing the MTLa1 coding region (start and stop codons are marked in boldface) was amplified from CAI4 genomic DNA with the primers MATa1 (5′-CCTCGTTTTTTCgtcgaCAATGAACTCAGAAATAG-3′) and MATa2 (5′-ATTTCCAGTGGAtccATTGTGGCTAGG-3′), fused with the BamHI-EcoRI fragment from pTET42 containing TACT and 5′URA3 sequences, and substituted for the corresponding fragment with the caGFP gene in pTET40 to result in pTET41 (Fig. 1E). To place the MTLa2 gene under the control of Ptet, a SalI-BamHI fragment containing the MTLa2 coding region (start and stop codons are marked in boldface) was amplified from CAI4 genomic DNA with the primers MATa2-3 (5′-AAGACgtcgACCAATAATATGCCATATACC-3′) and MATa2-2 (5′-CAGAAggatcCTATTGAAAAACGTCCTCAG-3′) and substituted for the MTLa1 gene in pTET41 or for the NRG1 gene in pTET42, resulting in pTET43 and pTET46, respectively (Fig. 1F and G).
(v) Construction of a tetracycline-inducible gene cassette for use in C. albicans wild-type strains.
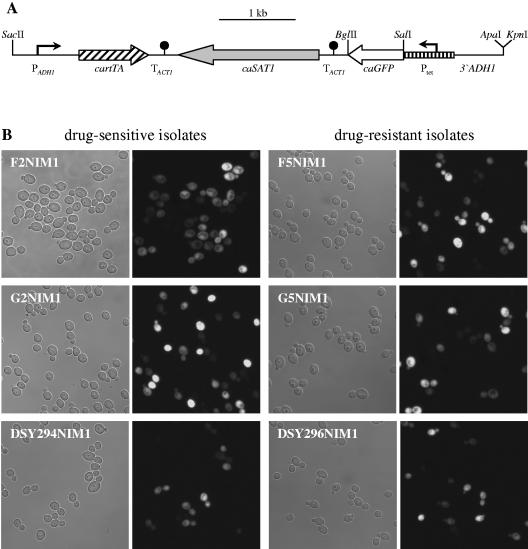
To allow more flexible use of the Tet-inducible gene expression cassette also in prototrophic C. albicans strains, the URA3 marker was replaced by the dominant caSAT1 selection marker (32) and additional unique restriction sites were introduced. To this end, a BglII-SalI fragment from pCBF1M4 (6) containing the ACT1 transcription termination sequence was first fused with an XhoI-PstI caSAT1 fragment from pSAT1 (32). The SalI site present within the intron of the caSAT1 marker then was destroyed by replacing the SalI-PstI fragment with an otherwise identical XhoI-PstI fragment (restriction sites are underlined), which was amplified with the primers SAT3 (5′-TTGTTcTCGAgATAATATTTCTCGTTTGGGATG-3′) and SAT2 (5′-CTAGTGATTTCTGCAGGACCACCTTTG-3′). The caGFP gene was amplified from the cassette with a primer binding in the OP4 upstream region and primer GFP24 (5′-AGTCTagatcTTTATTTGTATAGTTCATCCATGCC-3′), digested at the SalI site in front of the start codon and at the BglII site (underlined) introduced behind the stop codon (reverse sequence in boldface), and fused with the BglII-PstI TACT1-caSAT1 fragment. Finally, the 3′ADH1 fragment was amplified with a primer binding upstream of the NcoI site and primer ADH15 (5′-atataggtaccgggcCCGACAATCTTGATTGGGCATTTG-3′), which introduces KpnI and ApaI sites (underlined). The PCR product was digested with NcoI/KpnI and substituted for the NcoI-KpnI 3′ADH1 fragment present in the other cassettes. The final plasmid pNIM1 is shown in Fig. 8A.
FIG. 8.
Tetracycline-inducible gene expression in C. albicans wild-type cells. (A) Structure of the Tet-inducible gene cassette contained in plasmid pNIM1. Unique restriction sites that can be used to substitute other ORFs for caGFP and to excise the whole cassette from the vector backbone are indicated. (B) Expression of the tetracycline-inducible caGFP gene in matched drug-susceptible and -resistant clinical C. albicans isolates. Shown are phase-contrast and corresponding fluorescence micrographs of cells grown for 8 h in YPD medium at 30°C in the presence of 50 μg ml−1 doxycycline. Note that not all cells fluoresced at this time point, but did so during prolonged incubation. No fluorescence of the cells was seen in the absence of doxycycline (data not shown).
C. albicans transformation.
C. albicans strains were transformed by electroporation (18) with the following gel-purified, linear DNA fragments: the SacII-KpnI fragments from pTET25, pTET40, and pNIM1 containing the Ptet-caGFP fusion; the SacII-NcoI fragment from pTET36 containing the Ptet-CDC42D118A fusion; the SacII-KpnI fragment from pTET42 containing the Ptet-NRG1 fusion; the SacII-KpnI fragment from pTET41 containing the Ptet-MTLa1 fusion; the SacII-KpnI fragments from pTET43 and pTET46 containing the Ptet-MTLa2 fusion; and the SacII-KpnI fragment from pTET23 containing the Ptet-ecaFLP fusion. Uridine-prototrophic transformants were selected on SD agar plates, and nourseothricin-resistant transformants were selected on YPD agar plates containing 200 μg ml−1 nourseothricin (Werner Bioagents, Jena, Germany) as described previously (32). Single-copy integration of all constructs was confirmed by Southern hybridization.
Southern hybridization.
Genomic DNA from C. albicans strains was isolated as described previously (27). Ten micrograms of DNA was digested with appropriate restriction enzymes, separated on a 1% agarose gel, and, after ethidium bromide staining, transferred by vacuum blotting onto a nylon membrane and fixed by UV cross-linking. Southern hybridization with enhanced chemiluminescence-labeled probes was performed with the ECL labeling and detection kit provided by Amersham (Braunschweig, Germany) according to the instructions of the manufacturer.
Microscopy.
Phase-contrast and fluorescence micrographs of the cells were obtained using a Zeiss LSM 510 inverted confocal laser scanning microscope equipped with a Zeiss Axiovert 100 microscope. Imaging scans were acquired with an argon laser of 488-nm wavelength and corresponding filter settings for green fluorescent protein (GFP) and parallel transmission images. Observation was performed with a 63× immersion oil objective. For detection of nuclei, cells were fixed and stained with Hoechst dye 33258 and observed under UV light using a Zeiss Axiolab microscope equipped for epifluorescence microscopy with a 50-W mercury high-pressure bulb.
Nucleotide sequence accession number.
The sequence of the cassette contained in pNIM1 has been deposited in GenBank under accession no. QA090840.
RESULTS
Design of a tetracycline-inducible gene expression system for C. albicans.
To achieve a growth medium-independent induction of target genes in C. albicans by the addition of tetracycline, we designed an expression cassette containing the following components (see Materials and Methods for details of the construction): a gene encoding a reverse tetracycline-dependent transactivator (rtTA) under the control of the ADH1 promoter, an rtTA-dependent promoter controlling expression of the target gene, and the URA3 gene as a marker for the selection of transformants (Fig. 1A). The cartTA gene encodes a fusion protein consisting of the reverse Tet repressor from E. coli and the transcription activation domain of the Gal4 protein from Saccharomyces cerevisiae. It contains five amino acid exchanges that convert the original Tet repressor, which binds only in the absence of tetracycline to its target sequence, to the reverse Tet repressor, which binds only in the presence of doxycycline to the tet operator (44). In addition, all CTG codons, which would be mistranslated as serine instead of leucine due to the noncanonical C. albicans codon usage, were converted into the leucine codon TTG. The C. albicans-adapted cartTA gene was fused to the transcription termination sequence of the ACT1 gene and placed under the control of the ADH1 promoter. The rtTA-dependent promoter (Ptet) consists of the minimal promoter of the C. albicans OP4 gene, from which the upstream activating sequences described by Lockhart et al. (22) were removed, and seven copies of the tet operator sequence, tetO. The flanking ADH1 sequences allow integration of the whole construct into one of the ADH1 alleles in the C. albicans genome in a single transformation step. After integration, the cartTA gene should be constitutively expressed from the ADH1 promoter. Since binding of rtTA to the tet operator sequences requires doxycycline, expression of a target gene should be inducible upon addition of doxycycline to the growth medium.
Tetracycline-inducible expression of the GFP reporter gene in different morphological forms of C. albicans.
To monitor tetracycline-inducible expression of a target gene in individual C. albicans cells, we placed a C. albicans-adapted GFP reporter gene (caGFP) under the control of the Tet-dependent promoter. The whole cassette from plasmid pTET25 (Fig. 1A) was integrated into one of the ADH1 alleles of C. albicans strain CAI4, and two independent transformants, strains TETG25A and -B, were used for further analysis. These strains and the control strain SC5314 were grown to log phase in YPD medium at 30°C, conditions in which C. albicans grows in the yeast form, and doxycycline was added at different concentrations. We found that 50 μg ml−1 doxycycline resulted in efficient induction of the Tet-dependent promoter. Two hours after the addition of doxycycline many cells of the reporter strains exhibited a visible fluorescence, and both the fluorescence intensity of the cells and the number of fluorescent cells increased further during the next hours (Fig. 2A). No fluorescence of the reporter strains was seen in the absence of doxycycline, and doxycycline had no effect on the control strain SC5314.
FIG. 2.
Use of the caGFP reporter gene to monitor tetracycline-induced gene expression in C. albicans yeast, hyphal, and opaque cells. The figure shows phase-contrast (upper panels) and corresponding fluorescence (lower panels) micrographs of the cells. In all conditions, the two independently constructed strains of each pair (A and B) behaved identically, and only one of them is shown. (A) Strains TETG25A and -B and the control strain SC5314 were grown at 30°C in YPD medium in the absence (−Dox) or presence (+Dox) of 50 μg ml−1 doxycycline. Photos were taken at the indicated time points. (B) Hyphal growth of strains TETG25A and -B and SC5314 was induced by incubating the cells in RPMI medium with 10% FCS at 37°C without or with 50 μg ml−1 doxycycline as indicated. (C) Opaque cells of strains WTETG40A and -B and the control strain WO-1 were grown in Lee's medium at 25°C in the absence or presence of 50 μg ml−1 doxycycline and photos were taken at the indicated time points.
An advantage of a tetracycline-inducible gene expression system is that the promoter can be activated in any growth medium by simply adding the inducer. Under a variety of growth conditions C. albicans switches from yeast to hyphal growth. To test if doxycycline can induce gene expression in C. albicans hyphae, strains TETG25A and -B were cultivated in RPMI medium containing 10% serum, which strongly induces hyphal growth, in the presence or absence of 50 μg ml−1 doxycycline. In addition, the cells also were first grown for 4 hours in RPMI plus serum to induce hyphal formation before the addition of doxycycline. As can be seen in Fig. 2B, GFP expression was well inducible by doxycycline also in hyphal cells of C. albicans. No fluorescence of the hyphal cells was seen in the absence of the inducer, and doxycycline also had no effect on hyphae of the wild-type strain SC5314.
In addition to the yeast growth-hyphal growth switch, which is controlled by environmental conditions, C. albicans can also spontaneously switch from the normal rounded yeast form to an elongated cell type. This switching system was originally described in strain WO-1 and termed white-opaque switching because of the appearance of the colonies produced from the two types of cells on agar plates (37). It was later shown that opaque cells are the mating-competent form of C. albicans and that only strains which are homozygous for the mating type locus MTL can switch to the opaque form and mate with a partner of the opposite mating type (23, 26). The opaque cell phenotype is stable only at low temperatures, and at 37°C, the temperature of the human body, opaque cells rapidly switch back to the white phenotype. Because of the central role in mating of opaque cells, controlling gene expression in them would allow for studying the impact of specific genes on various aspects of this developmental program in C. albicans.
We used a ura3Δ mutant of the MTLα strain WO-1, strain WUM5A, to investigate expression of the Tet-inducible caGFP gene in opaque cells of C. albicans. Opaque cells of transformants carrying the cassette from pTET25 exhibited a fluorescent phenotype in the presence of Dox, but caGFP expression was reduced compared with induced cells of strains TETG25A and -B (data not shown). We observed that the ADH1 promoter was downregulated in opaque cells, presumably resulting in a lower expression of rtTA (data not shown). Therefore, we modified the system such that integration of the cassette was targeted to the OP4 locus and expression of the cartTA gene was driven from the opaque cell-specific OP4 promoter, which is highly active in opaque cells. Strain WUM5A was transformed with the cassette from plasmid pTET40 (Fig. 1D), and two independent transformants, strains WTETG40A and -B, were kept for further analysis. After the strains were switched to the opaque phase, we tested induction of caGFP expression by doxycycline during growth in Lee's medium at 25°C. As shown in Fig. 2C, GFP expression was well inducible by doxycycline in opaque cells of C. albicans. No fluorescence of opaque cells was seen in the absence of doxycycline, and doxycycline also had no effect on opaque cells of the wild-type strain WO-1. Tet-inducible gene expression in opaque cells was independent of the mating type, as doxycycline-induced caGFP expression was achieved with the same efficiency also in MTLa cells after integration of the cassette from pTETG40 into strain MTLa/Δmtlα to generate strains ΔmtlαTETG40A and -B (data not shown).
In summary, the results shown above demonstrated that the system developed in this study enabled an efficient induction of gene expression by the addition of doxycycline in yeast, hyphal, and opaque cells of C. albicans independently of the growth medium and allowed us to investigate the effect of expression of specific genes on the various morphological forms of the fungus.
Growth arrest by tetracycline-induced expression of a dominant-negative CDC42 allele.
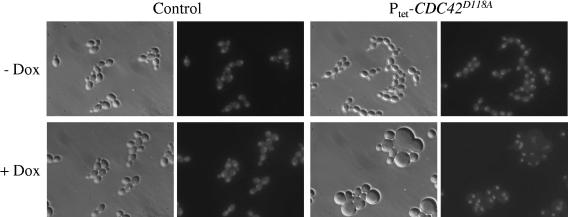
The ability to induce expression of an otherwise tightly repressed gene is especially important when studying the effect of gene products that have a toxic effect on the cells. The dominant-negative CDC42D118A allele encodes a mutated form of the small GTPase Cdc42p that is locked in the inactive GDP-bound form, and its overexpression in C. albicans causes growth arrest as large, multinucleate cells (45). To test whether this phenotype could also be achieved by tetracycline-inducible expression of the CDC42D118A allele, the D118A mutation was introduced into the CDC42 gene and the mutated CDC42 ORF was placed under the control of the tetracycline-dependent promoter in plasmid pTET36 (Fig. 1B). Two independent transformants of strain CAI4 carrying the Ptet-CDC42D118A fusion, strains TET36A and -B, were grown in YPD medium in the presence or absence of doxycycline. As shown in Fig. 3, doxycycline-induced expression of the CDC42D118A allele caused formation of grossly enlarged round cells, often containing two or more nuclei. In contrast, in the absence of doxycycline the strains carrying the Ptet-CDC42D118A fusion grew as normal budding yeast cells and could not be distinguished from the wild-type strain SC5314. These results demonstrated that the Tet-inducible gene expression system is useful for a controlled expression of toxic genes and for monitoring their effect on cell morphology.
FIG. 3.
Tetracycline-induced expression of the dominant-negative CDC42D118A allele produces enlarged, multinucleate cells. Strains SC5314 (control) and TETC36A and -B carrying the Ptet-CDC42D118A fusion were grown for 8 h at 30°C in YPD medium in the absence (−Dox) or presence (+Dox) of 50 μg ml−1 doxycycline. Shown are phase-contrast (left panels) and fluorescence (right panels) micrographs of cells stained with Hoechst dye to visualize nuclei.
Inhibition of hyphal growth by tetracycline-induced expression of the NRG1 repressor.
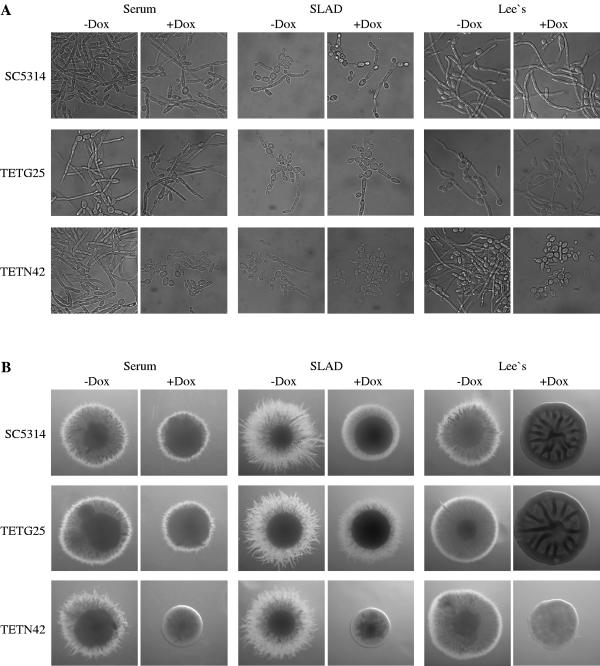
The NRG1 gene encodes a repressor that suppresses hyphal growth of C. albicans. Under hyphal-growth-inducing conditions NRG1 expression is downregulated to allow the morphogenetic switch to occur (7, 29). Constitutive expression of the NRG1 gene either from the ACT1 promoter (7) or from a Tet-repressible promoter (35) has been shown to inhibit hyphal formation and lock C. albicans in the yeast form. We tested whether filamentous growth of C. albicans could also be blocked by Tet-inducible expression of the NRG1 repressor. For this purpose, the NRG1 ORF was placed under the control of the Tet-inducible promoter in plasmid pTET42 (Fig. 1C) and the construct was integrated into the genome of strain CAI4. Two independent transformants, strains TETN42A and -B, were used to investigate the effect of doxycycline-induced NRG1 expression on morphogenesis in various liquid and solid hyphal-growth-inducing media. As shown in Fig. 4, in the absence of doxycycline the strains carrying the Ptet-NRG1 fusion formed hyphae as well as the wild-type strain SC5314 or a control strain carrying the Ptet-caGFP fusion. Doxycycline efficiently repressed hyphal growth of strains TETN42A and -B under all conditions tested. In liquid media, doxycycline had no effect on morphogenesis of the control strains, demonstrating that the inhibition of hyphal growth was caused by the induction of NRG1 expression. However, on solid media we observed a partial inhibition of filamentous growth by 50 μg ml−1 doxycycline also in the control strains. This inhibition was not seen at lower doxycycline concentrations (up to 30 μg ml−1), but at these concentrations doxycycline also did not completely block hyphal formation in the strains carrying the Ptet-NRG1 fusion, as the colonies exhibited some residual filamentation (data not shown).
FIG. 4.
Tetracycline-induced expression of the NRG1 gene inhibits hyphal formation in C. albicans. (A) Strains TETN42A and -B, containing the Ptet-NRG1 fusion, and the control strains TETG25A and -B, carrying the Ptet-caGFP fusion, or the wild-type strain SC5314 were grown for 8 h at 37°C in different hyphal-growth-inducing liquid media in the absence or presence of 50 μg ml−1 doxycycline. (B) The same strains were grown for 3 days (serum) or 5 days (SLAD, Lee's medium) at 37°C on the indicated hyphal-growth-inducing solid media without or with 50 μg ml−1 doxycycline, and individual representative colonies were photographed.
Tetracycline-induced expression of the MTLa1 gene forces opaque MTLα cells to switch to the white phase.
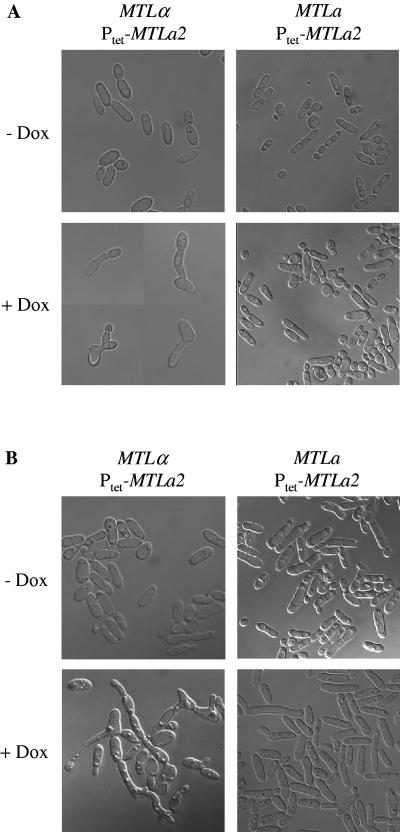
As noted above, C. albicans strains that are heterozygous at the mating type locus cannot switch to the opaque form because switching is suppressed by the a1/α2 repressor (26). MTLα strains like WO-1 are able to switch to the opaque form because they do not contain the MTLa1 gene and cannot produce the repressor. It was therefore interesting to investigate the effect of forced MTLa1 expression in opaque cells of strain WO-1. The MTLa1 ORF was placed under the control of the inducible Tet promoter in plasmid pTET41 (Fig. 1E), and the construct was integrated into the OP4 locus of strain WUM5A, generating the two independent transformants WTETMa41A and -B. When opaque cells of these strains were grown in the presence of doxycycline, they were unable to maintain the elongated-cell form and appeared as round-to-oval yeasts when observed by phase-contrast microscopy, suggesting that MTLa1 expression caused the opaque cells to switch to the white phenotype (Fig. 5A). This assumption was confirmed by plating the cells on Lee's agar containing phloxine B, which stains opaque colonies pink, thus allowing an easy discrimination of the two types of cells. Opaque cells grown in the absence of doxycycline maintained their morphology and produced no or only a few white colonies after plating. In contrast, when the strains were incubated in the presence of doxycycline and then plated, they produced white colonies, the proportion of which increased over time (Fig. 5B). However, even after prolonged incubation in the presence of doxycycline, many cells still produced opaque colonies, although few elongated opaque cells were detected by microscopy at the later time points, suggesting that despite their morphological appearance some cells had not stably switched to the white phase and could revert to the opaque morphology when MTLa1 expression was no longer induced during subsequent growth on the agar plates. The wild-type strain WO-1 or a control strain expressing caGFP instead of MTLa1 showed only background levels of opaque-to-white switching, irrespective of the presence or absence of doxycycline (data not shown). These results demonstrated that the a1/α2 repressor not only inhibits the switching of white cells to the opaque phase but also, when artificially produced in opaque cells, forces these cells to switch back to the white phase.
FIG. 5.
Tetracycline-induced expression of the MTLa1 gene forces MTLα opaque cells to switch to the white phase. Opaque cells of strains WTETMa41A and -B carrying the Ptet-MTLa1 fusion were grown in Lee's medium at 25°C in the absence or presence of 50 μg ml−1 doxycycline. (A) Phase-contrast micrographs of the cells grown for the indicated times in the presence of doxycycline. (B) Percentages of white colonies produced after plating the cells at the indicated times on Lee's agar plates containing phloxine B. Similar results were obtained in repeat experiments, although the proportion of white colonies varied.
Induction of shmoo formation by tetracycline-induced MTLa2 expression in opaque MTLα cells.
In contrast to S. cerevisiae, where relief from α2-mediated repression is sufficient for expression of a-specific genes in MATa cells, C. albicans requires a transcriptional activator encoded by the MTLa2 gene to express a-specific genes in MTLa cells (43). To investigate how expression of the MTLa2 gene in MTLα opaque cells would affect their behavior, the MTLa2 gene was placed under the control of the Tet-inducible promoter in plasmid pTET43 (Fig. 1F) and the construct was integrated into the genome of strain WUM5A. Two independent transformants, strains WTETMa43A and -B, were switched to the opaque phase, and MTLa2 expression was induced in the presence of doxycycline. As can be seen in Fig. 6A (left panels) the cells maintained the elongated opaque morphology, but many of them formed projections resembling the shmoos produced by MTLa or MTLα cells in response to the presence of a mating partner or its pheromone (3, 21, 24, 26, 31). No such shmoos were seen in the absence of doxycycline, demonstrating that their formation was caused by MTLa2 expression. Even longer shmoos were formed when the cells were grown on solid medium containing doxycycline (Fig. 6B, left). In contrast, Tet-induced MTLa2 expression did not cause shmooing in the MTLa strains ΔmtlαTETMa43A and -B, which were obtained by integrating the same construct from pTET43 into strain MTLa/Δmtlα (Fig. 6, right panels). In addition, by integrating the Ptet-MTLa2 fusion into the ADH1 locus of strain CAI4 to allow Tet-inducible expression (Fig. 1G), we showed that MTLa2 expression did not induce shmooing in heterozygous MTLa/α cells (data not shown). A possible explanation for the induction of shmooing by Tet-induced MTLa2 expression in MTLα cells is that these engineered cells express both a-specific and α-specific genes, because they contain the transcriptional activators α1 and a2, but not the a1/α2 repressor. Expression of both types of pheromones and their cognate receptors could then induce shmooing by autocrine signaling.
FIG. 6.
Tetracycline-induced expression of the MTLa2 gene induces shmooing in MTLα opaque cells. (A) Opaque cells of strains WTETMa43A and -B (MTLα, left panels) and ΔmtlαTETMa43A and -B (MTLa, right panels) carrying the Ptet-MTLa2 fusion were grown for 24 h in Lee's medium at 25°C in the absence or presence of 50 μg ml−1 doxycycline. Four examples of induced WTETMa43 cells producing shmoos with apical reversion to budding growth are shown (lower left). (B) The same strains were grown for 3 days at room temperature on Lee's agar plates without or with 50 μg ml−1 doxycycline, and cells from the colonies were observed by phase-contrast microscopy.
Generation of conditional C. albicans mutants by tetracycline-induced gene deletion.
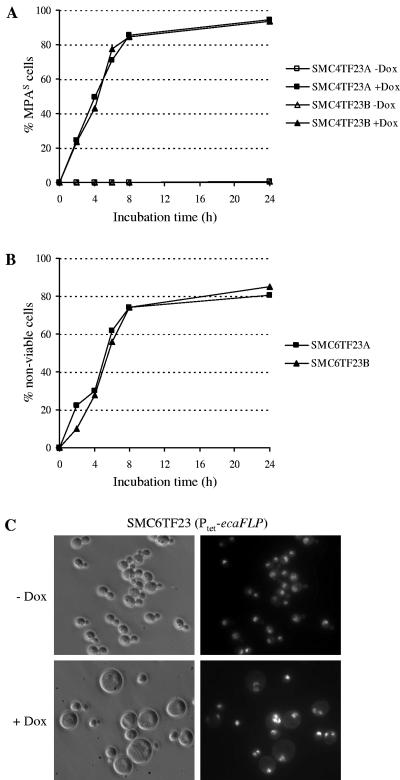
By expressing a C. albicans-adapted ecaFLP gene, encoding the site-specific recombinase FLP, from the inducible SAP2 promoter, it was previously demonstrated that essential genes which were flanked by direct repeats of the FLP target sequence could be efficiently deleted from the C. albicans genome to generate conditional lethal mutants (25). Activation of the SAP2 promoter required passage of the cells in a SAP2-inducing medium to allow FLP-mediated excision of the target gene. The null mutants thereby generated were then reinoculated into different fresh media to study the effect of gene deletion on the phenotype of the cells. Using the system established in the present study, we tested whether expression of the ecaFLP gene from the Tet-dependent promoter would enable deletion of an essential gene by addition of doxycycline to the cells and observation of the phenotypic consequences. The Ptet-ecaFLP fusion from plasmid pTET23 (Fig. 1H) was integrated into strains SMC4 and SMC6, which carried an ectopic copy of the essential CDC42 gene, together with an MPA resistance marker, between flanking FRT sites in a heterozygous CDC42/cdc42Δ (SMC4) or a homozygous cdc42Δ/cdc42Δ (SMC6) background. In the heterozygous mutant background, deletion of the FRT-CDC42-MPAR-FRT cassette can be detected by the appearance of MPA-sensitive cells because the cells still contain one of the wild-type CDC42 alleles and remain viable. Two independent transformants of strain SMC4, strains SMC4TF23A and -B, were grown in the absence or presence of doxycycline, and the percentage of MPAS cells in the populations was monitored over time (Fig. 7A). Eight hours after the addition of doxycycline about 85% of the cells had become MPA sensitive, demonstrating that the deletable CDC42 copy was efficiently excised from the genome in the majority of the population. The percentage of MPAS cells slightly increased further during prolonged incubation in the presence of doxycycline, but did not reach 100%. Without doxycycline addition all cells remained MPA resistant, demonstrating that no excision occurred in the absence of the inducer.
FIG. 7.
Tetracycline-induced deletion of the essential CDC42 gene. (A) Time course of tetracycline-induced, FLP-mediated excision of the FRT-CDC42-MPAR-FRT cassette in strains SMC4TF23A and -B carrying the Ptet-ecaFLP fusion and the deletable FRT-CDC42-MPAR-FRT cassette in a heterozygous CDC42/cdc42Δ background. The strains were grown at 30°C in YPD medium in the absence or presence of 50 μg ml−1 doxycycline, and excision of the FRT-CDC42-MPAR-FRT cassette was monitored as the percentage of MPAS cells in the population. (B) Generation of nonviable cells after doxycycline-induced excision of the single, deletable CDC42 copy in strains SMC6TF23A and -B. The cells were grown at 30°C in YPD medium to an optical density of 0.1, and then the cultures were divided in two equal halves, to one of which 50 μg ml−1 doxycycline was added. Appropriate dilutions of the cultures were plated at the indicated times, and the percentage of nonviable cells in the doxycycline-induced cultures was calculated as [1 -CFU (with Dox)/CFU (without Dox)] × 100. (C) Phenotype of cdc42 null mutants generated by FLP-mediated excision of the the FRT-CDC42-MPAR-FRT cassette in a homozygous cdc42Δ/cdc42Δ background. Strains SMC6TF23A and -B were grown for 8 h at 30°C in YPD medium in the absence (−) or presence (+) of50 μg ml−1 doxycycline. Shown are phase-contrast (left panels) and fluorescence (right panels) micrographs of cells stained with Hoechst dye to visualize nuclei.
We then investigated the effect of doxycycline-induced CDC42 deletion in the conditional null mutants. The efficiency of excision of the FRT-CDC42-MPAR-FRT cassette could not be monitored by the appearance of MPAS cells in this case, since the resulting null mutants are nonviable (25). Therefore, we estimated the percentage of null mutants in the population at various times after induction of ecaFLP expression by determining the number of CFU in cultures of strains SMC6TF23A and -B grown in the presence and absence of doxycycline (Fig. 7B). The percentage of nonviable cells generated by FLP-mediated CDC42 deletion was slightly lower than the percentage of MPAS cells in the heterozygous background, presumably because the remaining viable cells continue to grow during the incubation period whereas the null mutants cannot proliferate any more. Nevertheless, 8 h after doxycycline addition about 75% of the cells in the population were nonviable, and their percentage slightly increased further during prolonged incubation, indicating that the effect of CDC42 deletion should be observable in the majority of the cells. Indeed, when the cells were observed by microscopy, most of them exhibited the previously described phenotype of cdc42 null mutants, i.e., they formed grossly enlarged, round, multinucleate cells (Fig. 7C). These results demonstrate that the Tet-inducible gene expression system is also useful to create conditional lethal C. albicans mutants in which essential genes can be excised from the genome by the addition of doxycycline and to study the effect of gene deletion on the phenotype of the cells.
Tetracycline-inducible gene expression in C. albicans wild-type strains.
So far we relied on auxotrophic host strains to use the Tet-inducible gene expression system in C. albicans. However, it is desirable to be able to genetically manipulate not only C. albicans laboratory strains but also prototrophic wild-type strains with specific properties, for example, clinical isolates that have developed resistance to antifungal drugs. Therefore, we modified the system by replacing the URA3 marker with the recently developed dominant selection marker caSAT1, which confers resistance to nourseothricin (32). In addition, unique restriction sites were introduced into the cassette to enable convenient and flexible use (see Materials and Methods). In the resulting plasmid, pNIM1 (Fig. 8A), the caGFP ORF is expressed from the Tet-inducible promoter and is flanked by unique SalI and BglII restriction sites, which are also compatible with XhoI and BamHI, respectively, so that other ORFs can easily be substituted for caGFP in a single cloning step.
To test the usefulness of this cassette in different C. albicans wild-type strains, we transformed three matched pairs of drug-susceptible and multidrug-resistant clinical isolates. The resistant isolates F5 and G5 constitutively overexpress the MDR1 efflux pump, and the resistant isolate DSY296 constitutively overexpresses the CDR1 and CDR2 efflux pumps (11, 34) (Table 1). Expression of the multidrug efflux pumps did not affect the susceptibility of the strains to nourseothricin, indicating that this drug is not a substrate of these transporters, and nourseothricin-resistant transformants were obtained from all six parental strains with similar efficiencies. Twelve transformants of each strain were analyzed by Southern hybridization, and the majority (87.5%) had correctly integrated the cassette at the ADH1 locus (data not shown), demonstrating that the caSAT1 marker allowed efficient and specific insertion of the Tet-inducible gene expression cassette into the genome of C. albicans wild-type strains. Two independent transformants of each parental strain were tested for doxycycline-inducible expression of the caGFP reporter gene. All strains showed comparable levels of fluorescence, demonstrating that overexpression of the efflux pumps did not interfere with the inducibility of the Tet promoter by doxycycline (Fig. 8B). Induction of caGFP expression appeared to be somewhat delayed compared with the laboratory strain CAI, but all cells eventually exhibited a fluorescent phenotype. These results suggest that, in combination with the dominant caSAT1 marker, the Tet-inducible gene expression system is generally applicable in C. albicans wild-type strains.
DISCUSSION
In this work we have established a system that enables an inducible expression of target genes in C. albicans cells by a small-molecule inducer, doxycycline, independently of the growth medium. An important aspect of any regulatable gene expression system is a reasonably tight repression under noninducing conditions and good inducibility of the promoter. The Tet-inducible gene expression system presented here represents the result of empirical trials to optimize its various components (data not shown). For example, we tested several C. albicans core promoters which had been described in the literature (5, 22, 39) in combination with the tet operator for their performance in controlling expression of the ecaFLP reporter gene. In this context, the basal activity of the putative ADH1 and WH11 minimal promoters was too high, as it resulted in FLP activity in the absence of the transactivator. Only the OP4 minimal promoter produced satisfactory results, as no detectable expression of ecaFLP or any of the other genes tested in this study was observed in the absence of doxycycline. Similarly, we evaluated different putative strong promoters for a sufficiently high, constitutive expression of the transactivator. The ACT1 and TEF3 promoters were less efficient than the ADH1 promoter for rtTA-dependent, doxycycline-inducible expression of several target genes. Therefore, the ADH1 locus was chosen to integrate the cassette into the C. albicans genome and to drive expression of the transactivator from the ADH1 promoter. Only in opaque cells was the OP4 promoter used instead because it produced superior activity in this cell type. When setting up the system, we first used a C. albicans-adapted version of the original rtetR gene described by Gossen et al. (15) in combination with the GAL4 activation domain. However, the resulting transactivator activated the Tet promoter to some degree also in the absence of doxycycline and exhibited inefficient inducibility. In contrast, the C. albicans-adapted second-generation transactivator containing the amino acid exchanges described by Urlinger et al. (44), in combination with the other optimized components, allowed the establishment of the efficient inducible gene expression system presented in this work.
The Tet-inducible gene expression system is complementary to the Tet-Off system that was previously adapted for C. albicans (30) and which is especially useful to repress the expression of genes under conditions in which they are normally expressed. For example, the Tet-Off system was applied in a knockdown approach to study the function of essential C. albicans genes on a large scale (33). In addition, the Tet-Off system was used to shut down expression of the NRG1 repressor to release C. albicans from the block in hyphal formation caused by the constitutive expression of NRG1 from the Tet-controlled promoter (35). In contrast, the Tet-On system developed here allows an inducible expression of genes in conditions in which they are normally not expressed. This applies to genes that are not present in the wild-type C. albicans genome, for example, heterologous genes (e.g., the ecaFLP gene used to excise a specific DNA fragment from the genome), mutant alleles of certain genes (as exemplified here by the dominant-negative CDC42D118A allele), or genes that are absent from certain cell types (for example, the MTLa1 and MTLa2 genes in an MTLα strain) and to native genes like NRG1, whose expression is downregulated in certain conditions. When such genes are placed under the control of the Tet-inducible promoter, the introduced copy is not expressed in the absence of the inducer and the engineered strains behave like a wild-type strain. Addition of doxycycline to the cells allows the effect of forced gene expression to be studied under any desired condition. To test the efficiency of the system, we chose target genes that were expected to have a certain effect on the various morphological forms of C. albicans. We could efficiently induce the previously described generation of enlarged, multinucleate cells by expression of the dominant-negative CDC42D118A allele (45) or by FLP-mediated deletion of the wild-type CDC42 gene (25) and also the repression of hyphal formation by forced expression of the NRG1 gene (7, 35). In addition, we could induce cellular behaviors that were not described previously, for example, the switching of opaque cells to the white phase upon expression of the MTLa1 gene in opaque MTLα cells and the formation of shmoos by the same cells upon induced expression of the MTLa2 gene.
However, one should also point out some limitations of the system. The use of the caGFP reporter gene demonstrated that doxycycline did not induce gene expression synchronously in a culture of C. albicans cells, some cells responding earlier than others to the addition of doxycycline. Therefore, a phenotype may not be observed in all cells of a population at a given time point. Depending on the target gene, a sufficient level of gene expression may be obtained only after prolonged incubation or may not be achieved at all in some cells. For example, although Tet-induced expression of the FLP recombinase allowed deletion of the essential CDC42 gene in the majority of the population to generate nonviable mutants without the necessity of changing the growth medium, it should be noted that ecaFLP expression from the SAP2 promoter during passage of the cells in a SAP2-inducing medium was achieved with considerably higher efficiency, generating an almost pure population of null mutants (25). Last but not least, higher doxycycline concentrations (50 μg ml−1) were necessary for maximal induction of the Tet-inducible promoter than for efficient repression of the Tet-repressible promoter in C. albicans (usually 20 μg ml−1) (30, 35) and we observed that at 50 μg ml−1 doxycycline had an inhibitory effect on hyphal growth of C. albicans on solid media. Although this negative effect was not observed at lower doxycycline concentrations, induction of gene expression was also less efficient at these concentrations. It is therefore important to include control strains in all experiments and to test which concentration of doxycycline is optimal for the specific purpose of a study.
A considerable advantage of the Tet-inducible gene expression system established in this work is that all necessary components are contained in one cassette. This design relieves researchers from the dependence on specific strains, as in the Tet-Off system, which so far has been used only in preconstructed strains expressing a tetracycline-controlled transactivator (30, 33, 35). In combination with the dominant caSAT1 selection marker the Tet-inducible gene expression system can, therefore, be introduced into any C. albicans strain in a single transformation step. Since target gene ORFs can easily be amplified by PCR and substituted for the caGFP reporter gene in plasmid pNIM1, strains expressing a desired target gene in a doxycycline-inducible manner can be constructed very quickly. The cassette containing the caGFP reporter can be used to generate otherwise identical control strains and also to monitor induction of gene expression in the specific strain background, cell type, and experimental condition. In conclusion, the Tet-inducible gene expression system is a highly useful additional tool to study gene function in C. albicans and to manipulate the behavior of the various morphological forms of the organism.
Acknowledgments
We thank Ryan Raisner and Sandy Johnson for the gift of the MTLa/Δmtlα strain, Dominique Sanglard for strains DSY294 and DSY296, Arne Skerra for plasmid pASK75, and Wolfgang Hillen for plasmid pUHrT62-1 and for providing the nucleotide sequence of the rtTAS-M2 gene. Stefan Löffler is acknowledged for constructing some plasmids and strains in the initial stages of the project. Sequence data for Candida albicans was obtained from the Stanford Genome Technology Center website at http://www-sequence.stanford.edu/group/candida. Sequencing of Candida albicans was accomplished with the support of the NIDR and the Burroughs Wellcome Fund. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 630).
REFERENCES
- 1.Backen, A. C., I. D. Broadbent, R. W. Fetherston, J. D. Rosamond, N. F. Schnell, and M. J. Stark. 2000. Evaluation of the CaMAL2 promoter for regulated expression of genes in Candida albicans. Yeast 16:1121-1129. [DOI] [PubMed] [Google Scholar]
- 2.Bedell, G. W., and D. R. Soll. 1979. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect. Immun. 26:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, R. J., M. A. Uhl, M. G. Miller, and A. D. Johnson. 2003. Identification and characterization of a Candida albicans mating pheromone. Mol. Cell. Biol. 23:8189-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman, J., and P. E. Sudbery. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918-930. [DOI] [PubMed] [Google Scholar]
- 5.Bertram, G., R. K. Swoboda, G. W. Gooday, N. A. Gow, and A. J. Brown. 1996. Structure and regulation of the Candida albicans ADH1 gene encoding an immunogenic alcohol dehydrogenase. Yeast 12:115-127. [DOI] [PubMed] [Google Scholar]
- 6.Biswas, K., K. J. Rieger, and J. Morschhäuser. 2003. Functional characterization of CaCBF1, the Candida albicans homolog of centromere binding factor 1. Gene 323:43-55. [DOI] [PubMed] [Google Scholar]
- 7.Braun, B. R., D. Kadosh, and A. D. Johnson. 2001. NRG1, a repressor of filamentous growth in C. albicans, is down-regulated during filament induction. EMBO J. 20:4753-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Care, R. S., J. Trevethick, K. M. Binley, and P. E. Sudbery. 1999. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol. Microbiol. 34:792-798. [DOI] [PubMed] [Google Scholar]
- 9.De Backer, M. D., P. T. Magee, and J. Pla. 2000. Recent developments in molecular genetics of Candida albicans. Annu. Rev. Microbiol. 54:463-498. [DOI] [PubMed] [Google Scholar]
- 10.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franz, R., S. L. Kelly, D. C. Lamb, D. E. Kelly, M. Ruhnke, and J. Morschhäuser. 1998. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 42:3065-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 13.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 14.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gossen, M., S. Freundlieb, G. Bender, G. Müller, W. Hillen, and H. Bujard. 1995. Transcriptional activation by tetracyclines in mammalian cells. Science 268:1766-1769. [DOI] [PubMed] [Google Scholar]
- 16.Hillen, W., and C. Berens. 1994. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 48:345-369. [DOI] [PubMed] [Google Scholar]
- 17.Hull, C. M., R. M. Raisner, and A. D. Johnson. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307-310. [DOI] [PubMed] [Google Scholar]
- 18.Köhler, G. A., T. C. White, and N. Agabian. 1997. Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J. Bacteriol. 179:2331-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, K. L., H. R. Buckley, and C. C. Campbell. 1975. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 13:148-153. [DOI] [PubMed] [Google Scholar]
- 20.Leuker, C. E., A. Sonneborn, S. Delbrück, and J. F. Ernst. 1997. Sequence and promoter regulation of the PCK1 gene encoding phosphoenolpyruvate carboxykinase of the fungal pathogen Candida albicans. Gene 192:235-240. [DOI] [PubMed] [Google Scholar]
- 21.Lockhart, S. R., K. J. Daniels, R. Zhao, D. Wessels, and D. R. Soll. 2003. Cell biology of mating in Candida albicans. Eukaryot. Cell 2:49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockhart, S. R., M. Nguyen, T. Srikantha, and D. R. Soll. 1998. A MADS box protein consensus binding site is necessary and sufficient for activation of the opaque-phase-specific gene OP4 of Candida albicans. J. Bacteriol. 180:6607-6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lockhart, S. R., C. Pujol, K. J. Daniels, M. G. Miller, A. D. Johnson, M. A. Pfaller, and D. R. Soll. 2002. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162:737-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockhart, S. R., R. Zhao, K. J. Daniels, and D. R. Soll. 2003. Alpha-pheromone-induced “shmooing” and gene regulation require white-opaque switching during Candida albicans mating. Eukaryot. Cell 2:847-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michel, S., S. Ushinsky, B. Klebl, E. Leberer, D. Thomas, M. Whiteway, and J. Morschhäuser. 2002. Generation of conditional lethal Candida albicans mutants by inducible deletion of essential genes. Mol. Microbiol. 46:269-280. [DOI] [PubMed] [Google Scholar]
- 26.Miller, M. G., and A. D. Johnson. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293-302. [DOI] [PubMed] [Google Scholar]
- 27.Millon, L., A. Manteaux, G. Reboux, C. Drobacheff, M. Monod, T. Barale, and Y. Michel-Briand. 1994. Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus-positive patients: persistence of Candida albicans strains with the same genotype. J. Clin. Microbiol. 32:1115-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morschhäuser, J., S. Michel, and J. Hacker. 1998. Expression of a chromosomally integrated, single-copy GFP gene in Candida albicans, and its use as a reporter of gene regulation. Mol. Gen. Genet. 257:412-420. [DOI] [PubMed] [Google Scholar]
- 29.Murad, A. M., P. Leng, M. Straffon, J. Wishart, S. Macaskill, D. MacCallum, N. Schnell, D. Talibi, D. Marechal, F. Tekaia, C. d'Enfert, C. Gaillardin, F. C. Odds, and A. J. Brown. 2001. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 20:4742-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama, H., T. Mio, S. Nagahashi, M. Kokado, M. Arisawa, and Y. Aoki. 2000. Tetracycline-regulatable system to tightly control gene expression in the pathogenic fungus Candida albicans. Infect. Immun. 68:6712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panwar, S. L., M. Legrand, D. Dignard, M. Whiteway, and P. T. Magee. 2003. MFα1, the gene encoding the α mating pheromone of Candida albicans. Eukaryot. Cell 2:1350-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reuß, O., Å. Vik, R. Kolter, and J. Morschhäuser. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119-127. [DOI] [PubMed] [Google Scholar]
- 33.Roemer, T., B. Jiang, J. Davison, T. Ketela, K. Veillette, A. Breton, F. Tandia, A. Linteau, S. Sillaots, C. Marta, N. Martel, S. Veronneau, S. Lemieux, S. Kauffman, J. Becker, R. Storms, C. Boone, and H. Bussey. 2003. Large-scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol. Microbiol. 50:167-181. [DOI] [PubMed] [Google Scholar]
- 34.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saville, S. P., A. L. Lazzell, C. Monteagudo, and J. L. Lopez-Ribot. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skerra, A. 1994. Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene 151:131-135. [DOI] [PubMed] [Google Scholar]
- 37.Slutsky, B., M. Staebell, J. Anderson, L. Risen, M. Pfaller, and D. R. Soll. 1987. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J. Bacteriol. 169:189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soll, D. R., B. Morrow, and T. Srikantha. 1993. High-frequency phenotypic switching in Candida albicans. Trends Genet. 9:61-65. [DOI] [PubMed] [Google Scholar]
- 39.Srikantha, T., A. Chandrasekhar, and D. R. Soll. 1995. Functional analysis of the promoter of the phase-specific WH11 gene of Candida albicans. Mol. Cell. Biol. 15:1797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staib, P., M. Kretschmar, T. Nichterlein, H. Hof, and J. Morschhäuser. 2000. Differential activation of a Candida albicans virulence gene family during infection. Proc. Natl. Acad. Sci. USA 97:6102-6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staib, P., and J. Morschhäuser. 2005. Differential expression of the NRG1 repressor controls species-specific regulation of chlamydospore development in Candida albicans and Candida dubliniensis. Mol. Microbiol. 55:637-652. [DOI] [PubMed] [Google Scholar]
- 42.Strauß, A., S. Michel, and J. Morschhäuser. 2001. Analysis of phase-specific gene expression at the single-cell level in the white-opaque switching system of Candida albicans. J. Bacteriol. 183:3761-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsong, A. E., M. G. Miller, R. M. Raisner, and A. D. Johnson. 2003. Evolution of a combinatorial transcriptional circuit: a case study in yeasts. Cell 115:389-399. [DOI] [PubMed] [Google Scholar]
- 44.Urlinger, S., U. Baron, M. Thellmann, M. T. Hasan, H. Bujard, and W. Hillen. 2000. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. USA 97:7963-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ushinsky, S. C., D. Harcus, J. Ash, D. Dignard, A. Marcil, J. Morschhäuser, D. Y. Thomas, M. Whiteway, and E. Leberer. 2002. CDC42 is required for polarized growth in human pathogen Candida albicans. Eukaryot. Cell 1:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]