Abstract
Bone fracture healing is a dynamic process that relies on coordinated cellular interactions for effective tissue regeneration. We employ optimized spatial transcriptomics to delineate the locations and interactions of the involved cell types within a mouse femur fracture model on Day 0 before fracture and at Days 5 and 15 postfracture. We improve RNA quality significantly by optimizing our decalcification method using Morse’s solution, coupled with the use of the Visium CytAssist platform and integrated analyses through the Seurat, CARD, and Monocle packages. This approach allows us to accurately localize critical cell populations, such as periosteum progenitor cells, and identify pivotal transcription factors that regulate their activation and differentiation into chondrocytes or osteogenic cells. We particularly focus on the transformation from mesenchymal progenitor cells (MPCs) to regenerative MPCs (rMPCs), revealing how these cells recruit macrophages near the fracture line during early healing stages and their involvement in fracture healing. Furthermore, using CellChat, we explore potential receptor‒ligand pathways that mediate these cellular interactions. The spatial–temporal mapping and molecular characterization performed in this study substantially deepen our understanding of the cellular and molecular processes involved in fracture healing, highlighting spatial transcriptomics as a robust approach for elucidating the mechanisms governing bone regeneration.
Subject terms: Bone remodelling, Cell biology
Spatial transcriptomics reveals MPC-to-rMPC differentiation, macrophage recruitment, and key transcriptional networks during mouse bone fracture healing, highlighting cellular interactions in fracture repair.
Introduction
Bone fracture healing is a complex regenerative process that involves both endochondral and intramembranous ossification, which are crucial for skeletal development, growth and regeneration throughout life1. Following fracture injury, bone healing is initiated by a cascade of inflammatory responses2–4. This cascade activates mesenchymal stem and progenitor cells within the periosteum that subsequently differentiate into chondrocytes and osteoblasts, which are essential cell types for callus formation and fracture healing3.
Throughout the healing process, many different cell types with highly dynamic roles are involved. At the earliest stages of healing, neutrophils and macrophages play crucial roles in clearing hematomas and stimulating the proliferation and differentiation of periosteal mesenchymal stem cells, setting the stage for later phases of healing4. As the process progresses to soft callus formation, vascular cells and osteoclasts become involved, contributing to callus formation and remodeling5,6. These dynamic shifts in cellular roles are essential for successful bone repair and underscore the complexity of the healing process and the importance of detailed characterization to advance the field of bone regeneration7.
Recent advancements in single-cell RNA sequencing (scRNA-seq) have significantly increased our understanding of the cellular landscape during bone repair. This sequencing technique enables the detailed mapping of regulatory networks, signaling mechanisms, and the dynamic roles of all cell types involved8–11. However, scRNA-seq involves cell dissociation, which not only alters transcriptional profiles but also removes the spatial context necessary for fully understanding complex cell interactions and environmental influences12. To bridge this gap, spatial transcriptomics preserves in situ transcriptional profiles and enables the analysis of cellular interactions within their native tissue architecture13. This technology has revolutionized our understanding of both soft and hard tissue architectures. In soft tissues such as the heart and brain, spatial relationships and molecular pathways that underlie physiological functions and responses to diseases, such as acute ischemic stroke, have been elucidated by highlighting the spatial distribution of cell types and their molecular signatures to enable a more detailed assessment of cellular interactions14–16.
Applying spatial transcriptomics to hard tissues such as bone presents distinct challenges, primarily owing to decalcification, a necessary step prior to sequencing that severely degrades RNA and diminishes the resolution of spatial transcriptomic spots. Previous studies on intervertebral discs17 derived from 3-week-old mice revealed distinct cellular clusters and their key roles in disc homeostasis and degeneration. Another study on femurs18 from 10-week-old mice identified cellular components of the stem cell niche and cell‒cell communication in the bone marrow. However, the resolution of these spatial data is low, with only approximately 300 genes detected per spot. This contrasts sharply with the 3000-5000 genes per spot observed in soft tissues, such as brain14,15 or tumor19. Spatial spots in the transcriptomic data from mineralized tissues often present low counts of unique molecular identifiers (UMIs) and gene counts, which restrict the resolution and utility of the data. This highlights the ongoing need for improved methodologies, which can increase data quality and resolution, to study these challenging tissue environments.
Building on recent technological advancements20, we developed an enhanced spatial transcriptomic method tailored for musculoskeletal tissues requiring decalcification, which significantly improved data quality and resolution. This method overcomes the limitation of mRNA degradation in traditional methods, allowing the generation of detailed transcriptomic maps that reveal the spatial and temporal dynamics of cell subtype localization, function, and communication during bone fracture healing. Notably, we conducted high-quality spatial transcriptomic sequencing of long bone periosteal cells at different time points (Days 0, 5, and 15), enabling a more precise exploration of their temporal changes and roles in fracture repair. In our study, we intensively explored the critical transformation from mesenchymal progenitor cells (MPCs) to regenerative MPCs (rMPCs), a process pivotal for effective bone repair. Initially, we identified the transcriptional regulatory networks involved in the activation of MPCs into rMPCs, highlighting key transcription factors that drive this transition. This activation plays a crucial role in the early stages of bone healing, where activated MPCs recruit macrophages to the fracture site, significantly influencing inflammation and tissue remodeling. Furthermore, we employed CellChat to investigate the complex receptor‒ligand interactions that enable communication between these evolving cell populations. This innovative approach integrates high-dimensional spatial data with single-cell RNA sequencing, providing a detailed view of the cellular and molecular dynamics at play. By revealing the interactions and differentiation trajectories of various cell types within the healing bone, our study increases our understanding of the bone healing process and lays the groundwork for future research into the mechanisms underlying bone repair and regeneration.
Materials and Methods
Animal study
All the mice used were maintained in a specific pathogen-free environment. Wild-type (WT) C57BL/6J mice, Gli1-CreER mice and Rosa-tdTomato mice were originally obtained from Cyagen Biosciences. Gli1-CreER Rosa-tdTomato (Gli1ER/Td) mice were generated by breeding Gli1-CreER mice with Rosa-tdTomato mice. Male mice at 12 weeks of age were used for the animal experiments in the present study. All animal experiments were conducted in accordance with the guidelines for the housing and care of laboratory animals and performed following regulations approved by the Animal Care and Use Committee at China Medical University (NO. CMU2022132). We have complied with all relevant ethical regulations for animal use. In the fracture group mice, closed transverse fractures were made on the right femur using a blunt guillotine with a preinserted intramedullary pin for stabilization21.
Spatial transcriptomics
Spatial transcriptomics was performed using the Visium Spatial Gene Expression System (10x Genomics). At each time point (Day 0, Day 5, and Day 15), two femurs from different mice were used, providing biological replicates for spatial transcriptomic analysis. The Day 0 unfractured femurs served as baseline controls, providing a spatial transcriptomic reference for normal bone architecture and cellular composition, which allowed direct comparisons with postfracture samples. The Day 5 postfracture samples corresponded to the early fracture healing phase, characterized by the formation of granulation tissue and the initiation of chondrogenesis, whereas the Day 15 samples corresponded to the hard callus phase, where endochondral ossification progresses, leading to new bone formation. Femurs were collected at different time points, fixed in 4% paraformaldehyde (PFA) overnight at 4 °C, decalcified in Morse’s solution for 20 h at room temperature, and subsequently processed for paraffin embedding. A series of 6-μm-thick longitudinal sections were cut across the femur or the entire fracture callus from one side of the cortical bone to the other side of the cortical bone. For each sample, a central cross-section was selected to ensure maximal representation of the tissue architecture and was carefully mounted at the center of the superfrost slides. These samples were then gently dried in a controlled heating environment set at 60 °C for a period of three hours, ensuring optimal preparation for subsequent spatial transcriptomic analysis.
Tissue preparation and sequencing procedures were carried out following standardized protocols. Initially, the slides were deparaffinized and subjected to hematoxylin/eosin (H&E) staining, followed by imaging using a Leica DMI8 whole-slide scanner at 10× resolution. For spatial gene expression analysis, we utilized the Visium CytAssist Spatial Gene Expression for FFPE kits (10× Genomics, PN-1000520). The Visium HD platform was used for the Day 0 samples, whereas the Visium platform was employed for the Day 5 and Day 15 samples, according to their respective protocols. The tissue slices were subjected to crosslinking treatment followed by probe hybridization and connection to the cross-linked sections. After the probes were connected, RNA from the tissue sections was digested via RNase with a tissue removal enzyme (10× Genomics, PN-3000387) to release the probes from the cells. These probes were then captured by the capture sequences on the Visium CytAssist Spatial Gene Expression slide using the Visium CytAssist instrument. The released probes were subsequently extended, eluted, and then transferred to new tubes for further use. Libraries were sequenced with paired-end dual-indexing (28 cycles Read 1, 10 cycles i7, 10 cycles i5, 50 cycles Read 2). The libraries were sequenced on an Illumina NovaSeq 6000 sequencer, targeting a sequencing depth of at least 100,000 reads per spot with a pair-end 150 bp (PE150) reading strategy. The sequencing was performed by CapitalBio Technology, Beijing, ensuring comprehensive coverage and analysis.
For data processing, alignment was conducted using the Space Ranger pipeline, with further analysis performed via the Seurat R package (v5.1.0)22. To ensure comparability between the Visium HD and standard Visium platforms, the HD data were analyzed using a bin size of 60 μm in the Space Ranger pipeline. This approach allowed for a consistent spatial resolution across datasets, enabling direct comparisons of gene expression patterns and cellular dynamics across different time points during fracture healing. The spatial raw count data were integrated into Seurat using the Load10×_Spatial function and normalized through the SCTransform function. Integration of the Day 0, 5, and 15 datasets was achieved using Harmony and Seurat wrappers. Clustering was executed at a resolution of 1.2 using the Seurat FindClusters function. Differential gene expression analysis, Gene Ontology (GO) enrichment analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis and gene set enrichment analysis (GSEA) were performed via the clusterProfiler package23,24. Trajectory analyses were performed via the Monocle package25, deconvolution was conducted via the CARD package26 with default parameters, and cellular interactions were examined with the CellChat R package27.
Immunohistochemistry (IHC)
For immunofluorescence staining of macrophages, fractured mouse femurs on Day 5 postfracture were fixed in 4% PFA, decalcified in 10% EDTA for 5 days, transferred to 30% sucrose in PBS overnight, and embedded in OCT compound (Thermo Fisher Scientific, Waltham, MA, USA) for frozen tissue sectioning at a thickness of 6 μm, aided by the use of cryofilm 2C (SECTION-LAB Co. Ltd., Hiroshima, Japan). A series of 6 μm-thick longitudinal sections were cut across the entire fracture callus from one side of the cortical bone to the other, with sections taken through the center of the callus used as representative samples. Sections were stained with H&E, and adjacent sections were incubated with a rat anti-F4/80 antibody (1:100; eBioscience, 14-4801-82) at 4 °C overnight, followed by incubation with an Alexa Fluor 488-conjugated goat anti-rat IgG secondary antibody (1:200; Abcam, ab150157). The sections were scanned with a Nikon Eclipse 90i fluorescence microscope. To quantify positive cells, the periosteum was divided into two regions: the distal region, located 2 mm away from the fracture line, and the “close to fracture line” region, within 2 mm of the fracture line. Within these regions, F4/80+ cells were counted and normalized to the area of the thickened periosteum.
Statistics and reproducibility
Statistical analyses were performed using GraphPad Prism 9.5.0 (GraphPad Software Inc., San Diego, CA). Two-tailed Student’s t tests were applied as appropriate. A p value of less than 0.05 was considered statistically significant. Bar graphs were generated, and the data are presented as the means ±standard deviations (SDs). At least three biological replicates were analyzed in each experimental group.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Spatial dynamics of cellular interactions in bone fracture healing
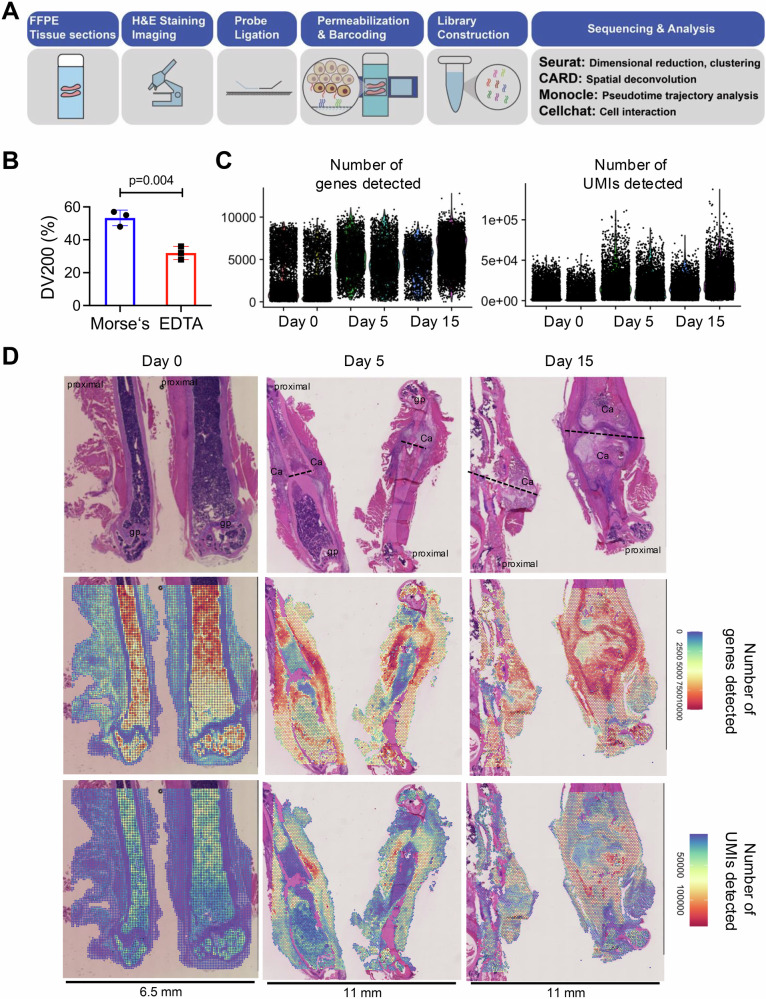
We generated fractures on the femurs of male mice at 12 weeks of age and then collected and processed the tissues for spatial transcriptomics on Day 0 before fracture and at Days 5 and 15 postfracture. We used the 10× Visium CytAssist platform and sampled two mice at each time point. Data analysis was conducted using a series of R packages, including the Seurat, Monocle, CARD and CellChat packages, as depicted in our experiment protocol flowchart (Fig. 1A). We employed an optimized decalcification method using Morse’s solution on the basis of prior findings to improve methods for determining RNA scope20. Here, we found that Morse’s method resulted in higher DV200 scores than did standard EDTA decalcification, indicating superior RNA preservation (Fig. 1B). This increased RNA quality translated into notably better sequencing quality per spot than the levels reported in the literature (Supplementary Table 1). These femur samples comprised a total of 24,213 spots, with an average of 16254 UMIs and 4131 genes per spot (Fig. 1C).
Fig. 1. Overview of the spatial transcriptomics workflow and quality control results for fracture healing in mouse femurs.
A Schematic representation of the spatial transcriptomics workflow using the CytAssist platform for formalin-fixed paraffin-embedded (FFPE) tissue sections. The process includes H&E staining for initial tissue imaging, followed by probe ligation, permeabilization, barcoding, and library construction, with data analysis performed via the Seurat, CARD, and Monocle software packages. B Bar graph showing the comparison of RNA quality between samples processed using Morse’s solution and EDTA decalcification methods, as indicated by DV200 percentages (a measure of RNA integrity). The data are presented as the means ± standard deviations (SDs). C Violin plots showing the number of detected genes (nFeature_Spatial) and the number of unique molecular identifiers (nCount_Spatial) per spatial spot on Day 0 before fracture and Days 5 and 15 after fracture. D Histological and spatial transcriptomic analysis of mouse femurs on Day 0 before fracture and at Days 5 and 15 after fracture. Upper panel: H&E staining images. Middle panels: spatial plots showing the number of detected genes (nFeature_Spatial) and the number of unique molecular identifiers (nCount_Spatial) per spatial spot on Day 5 post-fracture. The lower panels: spatial plots showing the number of unique molecular identifiers (UMI, nCount_Spatial) per spatial spot on Day 0 before fracture and Days 5 and 15 after fracture. The fracture lines are labeled with dashed lines, the proximal part of the femur is labeled, gp growth plate, Ca callus.
These data revealed distinct cellular activities during the fracture healing process. On Day 0, gene expression was primarily concentrated within the bone marrow, reflecting the high metabolic activity of hematopoietic and stromal cells in the unfractured bone. As the healing process began, gene expression shifted toward the periosteal regions by Day 5, where an increase in the diversity of detected genes indicated active biological repair processes. Conversely, areas within the bone marrow near the fracture site presented reduced gene expression, likely due to early tissue damage and necrosis. By Day 15, gene expression was predominantly localized to the fracture callus, where active ossification was evident from the increased number of genes detected in areas undergoing new bone formation (Fig. 1D, Supplementary Fig. 1). These data provide a detailed and high-quality atlas of fracture healing, underscoring the significant improvements in spatial transcriptomic data quality enabled by our methodological refinements.
Defining the spatial clusters and cellular dynamics in bone healing
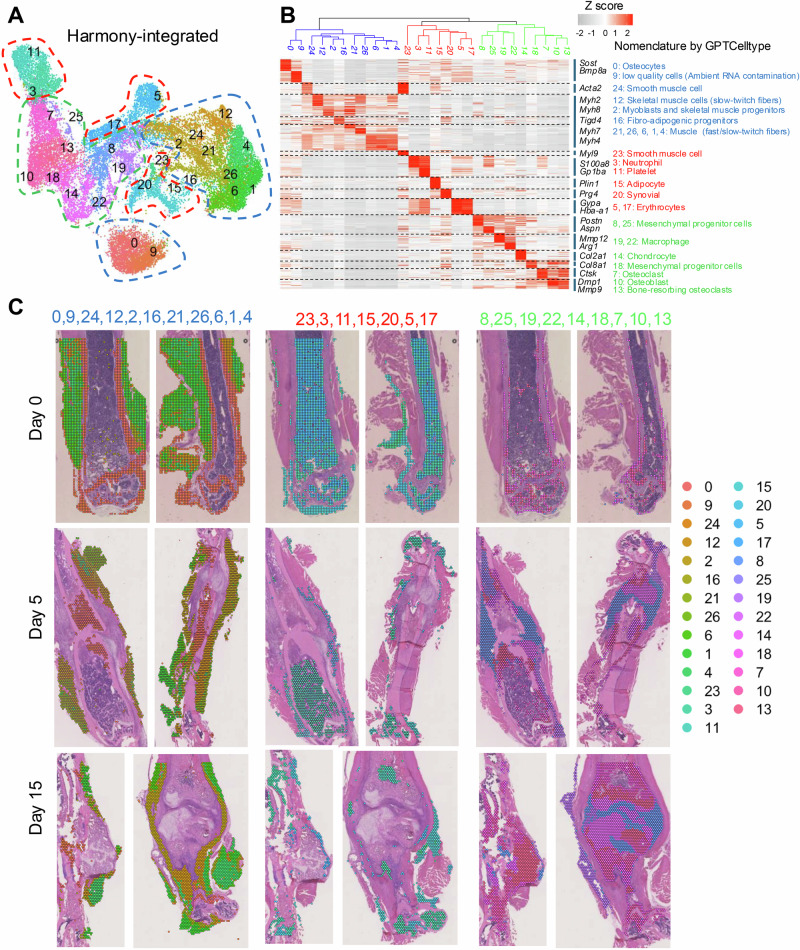
Using spatial transcriptomic analysis, our study revealed a complex network of cellular clusters within the fracture healing milieu. Data integration using Harmony, which incorporated a total of 24,213 spots across three time points (Day 0, Day 5, and Day 15), enabled the identification of 27 unique spatial clusters as projected by uniform manifold approximation and projection (UMAP) and spatial plots (Fig. 2A). This categorization was further substantiated by the hierarchy tree plot that categorized these clusters into three major groups, indicating distinct cellular functions within the healing bone (Fig. 2B, Supplementary Fig. 2A). The top 10 marker genes for each cluster are shown in Supplementary Table 2. For a refined classification of these clusters, we utilized the GPTCelltype package28, an advanced tool for predicting cell types on the basis of gene expression data from spatial transcriptomics. This approach was employed to analyze the top-expressed genes shown in Supplementary Table 2, and representative marker genes for each cluster are listed alongside the heatmap. Clusters related to bone and muscle tissues (Clusters 0, 9, 24, 12, 2, 16, 21, 26, 6, 1, and 4), labeled in blue, including osteocytes (Sost, Bmp8a), and various muscle-related groups (Myh2, Myh4), were predominantly mapped to the cortical bone and surrounding muscle tissues (Fig. 2C, left panel). Red-labeled clusters (Clusters 23, 3, 11, 15, 20, 5, and 17) associated with hematopoietic and inflammatory responses, such as neutrophils (S100a8, Ngp), platelets (Gp1ba, Gp5), and erythrocytes (Car1, Gypa), were concentrated in the bone marrow and at the fracture site, reflecting the critical immune response and wound healing processes active immediately postfracture. In addition to enriching the cellular landscape, adipocytes (Plin1, Cfd) are located between the muscle and periosteum and play roles in energy storage and local inflammation regulation. Synovial cells (Prg4, Htra4) are found near joints, where they contribute to joint lubrication (Fig. 2C, middle panel). Green-labeled clusters (Clusters 8, 25, 19, 22, 14, 18, 7, 10, and 13), including mesenchymal progenitor cells (Postn, Aspn), chondrocytes (Col2a1, Acan), macrophages (Mmp12, Arg1), and osteoclasts (Ctsk, Mmp9), were involved in the regeneration of tissue and became more prominent by Days 5 and 15, indicating their role in the stages of healing and regeneration around the forming callus (Fig. 2C, right panel).
Fig. 2. Analysis of spatial clusters and cellular dynamics in bone fracture healing.
A UMAP visualization of data integrated via Harmony, displaying 27 distinct spatial clusters, each colored differently to represent various cell populations within the fracture healing tissue. B Hierarchy heatmap plot displaying gene expression profiles across the identified spatial clusters. Clusters were nomenclatured via the GPTCelltype package. C Spatial plots depicting the distribution of major spatial clusters on Day 0, Day 5, and Day 15 of bone healing in a mouse femur fracture model.
Our spatial transcriptomic analysis across three time points (Days 0, 5, and 15 postfracture) revealed substantial changes in the distribution and frequency of various cellular clusters involved in bone fracture healing. Using UMAP visualizations (Supplementary Fig. 2B) and relative frequency data (Supplementary Fig. 2C), we observed dynamic shifts in cell populations that corresponded to the evolving healing environment. Initially, on Day 0, the cellular landscape was characterized predominantly by cortical and muscle-related cells. Postfracture, there was a noticeable shift in the proportions of certain muscle cell types, including a decrease in the proportion of fast-twitch skeletal muscle cells (Clusters 6, 1, 4), and increases in the proportions of slow-twitch skeletal muscle cells (Cluster 12), myoblasts and skeletal muscle progenitors (Cluster 2), and oxidative skeletal muscle cells (Cluster 26). This suggests an adaptive response in which muscle cell types that are more resilient or supportive of repair processes become more prevalent. Furthermore, the proportion of fibroadipogenic progenitors (Cluster 16) increased after fracture. These cells are crucial for their ability to produce an extracellular matrix and support angiogenesis, which are essential for supporting muscle repair and bone fracture healing29,30. The increased proportions of oxidative skeletal muscle cells and fibroadipogenic progenitors indicate a shift toward cell types actively involved in the repair and regeneration processes (Supplementary Fig. 3). The analysis also detailed the behavior of immune-related clusters, adipocytes, and synovial cells, collectively labeled in red in our spatial transcriptomic data. Unlike muscle and regeneration-related cells, these clusters did not exhibit significant changes in their relative proportions across the different time points, with the exception of erythrocytes (Clusters 5, 17), which exhibited a substantial decrease in proportion after fracture. This reduction may result from increased fibrosis following injury-induced necrosis (Supplementary Fig. 4). There were marked increases in the proportions of regeneration-related clusters postinjury, which were particularly noticeable by Day 5 for Clusters 8 and 25 (mesenchymal progenitor cells) and Day 15 for Clusters 7, 10, and 13 (osteogenic cells). These increases signify intensified regenerative activity as the healing process progresses, with the number of cells such as chondrocytes (Cluster 14) and mesenchymal progenitor cells (Clusters 8 and 25) increasing to facilitate new soft callus formation and initial stability (Supplementary Fig. 5). Further exploration of these cellular dynamics will increase our understanding of the fundamental mechanisms driving tissue regeneration and bone repair.
Spatial dynamics of the periosteal region in fracture healing: differentiation of MPCs into rMPCs
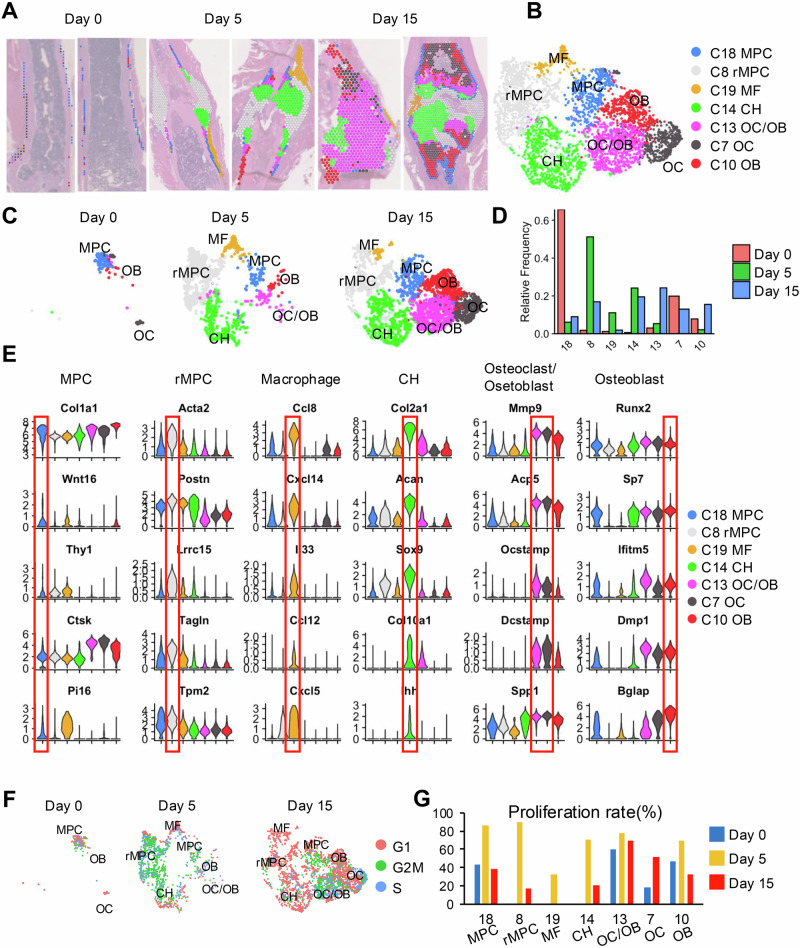
Next, we performed a detailed analysis of the cellular dynamics within the periosteal region, a critical area for bone regeneration. Using Loupe Cell software, we manually selected cell spots from the periosteal area (Supplementary Fig. 6), subsequently analyzing these cells on the basis of GPTCelltype nomenclature (Fig. 3A–C). On Day 0, the periosteal cell spots predominantly consisted of mesenchymal progenitors (Cluster 18) and a mixture of osteoblasts and osteoclasts (Clusters 7 and 10), reflecting the baseline state of the bone prior to injury. By Day 5, there was a notable shift in the cellular composition within the periosteal region, which was now dominated by a variety of chondrocytes (Cluster 14) and periosteum-derived cells that we termed regenerative mesenchymal progenitor cells (rMPC, Cluster 8) in this study. This change indicates the emergence of progenitor cells during regeneration. Notably, the proportion of macrophages (Cluster 19) within the periosteal area increased substantially by Day 5, which aligns with their role in mediating inflammation and tissue remodeling during the early stages of healing. By Day 15, osteoblasts (OBs) and osteoclasts (OCs) accounted for the majority of the cell population in the periosteal region, indicating advanced stages of bone repair where remodeling becomes predominant (Fig. 3D).
Fig. 3. Detailed analysis of periosteal region dynamics during bone healing.
A Histological sections of the fracture site on Day 0, Day 5, and Day 15 postfracture, stained to show the distribution and morphology of cells within the healing bone. The images illustrate changes in cell composition and structure at the fracture site over time, highlighted by spatial annotations that identify various cell types. B UMAP plots displaying the clustering of cell types on the basis of their gene expression profiles at various stages of healing. Each cluster is color-coded to represent different cell types, including mesenchymal progenitor cells (MPC), regenerative MPCs (rMPC), macrophages (MF), chondrocytes (CH), and osteoclasts/osteoblasts (OC/OB). C Panels displaying the distribution of specific cell types within the periosteum over the healing timeline. Each cell type is color-coded consistent with the UMAP plots, illustrating how the spatial organization within the periosteum evolves from Day 0 to Day 15. D Bar graphs showing the relative proportions of each cell cluster on Day 0, Day 5, and Day 15, reflecting the dynamic changes in periosteal cell populations during the healing process. E Violin plots depicting the expression patterns of key genes linked to periosteal cell function and bone healing across different cell types identified in the periosteum. F UMAP plots displaying the cell cycle status of periosteal cells on Day 0, Day 5, and Day 15. Each plot is color-coded to indicate different phases of the cell cycle (G1, S, and G2/M). G Bar charts illustrating the proliferation rates of different periosteal cell types on Day 0, Day 5, and Day 15, on the basis of cell cycle analysis, indicating that variations in cellular activity are crucial for the healing process.
We further explored the marker genes of periosteal stem cells using violin plots to display the distribution of gene expression across different spot areas (Fig. 3E). These plots highlighted that established markers such as Thy1, Ctsk, and Pi16 are highly expressed in the MPC population, underscoring their stem-like properties and pivotal roles in bone healing. Additionally, we utilized spatial expression mapping to illustrate the localization and expression of several key markers of periosteal progenitors reported in the literature and discovered that the expression patterns of Pdgfra, Ctsk, Ly6a, Pi16, and Edil3 are closely linked with periosteal regions essential for bone repair, providing new insights into the functional dynamics of periosteal stem cells during the healing process (Supplementary Figs. 7 and 8).
Our previous studies established fracture scRNA-seq databases and documented the appearance of proliferative progenitor cells (PPCs) early postfracture in the callus area, which are essential for fracture healing and stem cell differentiation3. In this study, we observed that the rMPC subpopulation specifically expressed markers of the PPC population, such as Acta2, Lrrc15, and Tagln (Fig. 3E), indicating myofibroblast characteristics that suggest a potent regenerative capacity. Cell cycle analysis (Fig. 3F, G) further confirmed the active proliferation capacity of rMPCs (Cluster 8), which was particularly evident on Day 5, with multiple periosteal cell types displaying heightened proliferative activity. This proliferation is indicative of the dynamic and critical nature of periosteal contributions to bone healing, emphasizing that this region acts not only as a structural boundary but also as an active participant in regeneration.
Given the critical role of Bmp2 in the initiation of fracture healing, we performed an in-depth analysis of its expression using single-cell RNA sequencing data (Supplementary Fig. 9). Bmp2 was predominantly expressed in regeneration-associated clusters, including mesenchymal progenitor cells (MPCs, Cluster 18), chondrocytes (CH, Cluster 14), and osteoblasts (OB, Cluster 10). Temporally, Bmp2 was expressed at low levels at baseline (Day 0) and was significantly upregulated on Day 5 postfracture, maintaining elevated levels on Day 15. This marked upregulation indicates that Bmp2 is actively involved in early- and mid-phase regenerative processes, particularly in rMPCs and osteogenic cells, underscoring its key regulatory role during the critical stages of fracture healing.
Periosteal region spot deconvolution and functional analysis
Owing to the resolution of the Visium platform being approximately 60 µm, each spot may contain between 2 and 10 single cells, necessitating initial deconvolution analysis to identify the proportions of different cell types within each spot. Leveraging our previously published single-cell transcriptomics atlases of fracture healing (Supplementary Fig. 10), we conducted a detailed deconvolution of the periosteal region spots at various time points (Fig. 4).
Fig. 4. Deconvolution analysis and pathway enrichment analysis of the periosteum region across fracture healing stages.
A Sequential histological sections from Day 0, Day 5, and Day 15 postfracture showing the periosteum region. Below each histological image, spatial plots and corresponding deconvolution results using the CARD package are presented, illustrating the inferred cellular composition within each spatial spot. B Heatmaps displaying the results of GSVA for different cell clusters identified in the periosteum across Days 0, 5, and 15. Each row represents a KEGG or Hallmark pathway. C SCENIC analysis results showing the activity of transcriptional regulators across different periosteal cell types and time points. This analysis reveals the regulatory landscapes that potentially guide the differentiation and function of cells in response to fracture.
On Day 0, spots within the periosteal region identified as mesenchymal progenitor cells (MPCs) through spatial transcriptomic nomenclature were further analyzed using the CARD deconvolution method. The results revealed that these spots predominantly consisted of osteoprogenitors (OsteoPs) and MPCs identified by scRNA-seq, corresponding to the cambium and fibrous layers, respectively. Thus, MPC spots correlate spatially with the two layers of the periosteum, both of which are critical for fracture repair31 (Fig. 4A).
Analysis on Day 5 highlighted spots within the red-framed area representing regenerative MPCs (rMPCs), where single-cell mapping predominantly revealed PPCs and muscle cells. These findings suggest that the rMPC spots exhibit myofibroblastic characteristics, potentially contributing to mechanical stability through muscle contraction. The blue-framed area, which marked the interface between the rMPCs and cartilage, was predominantly comprised of chondrocytes and hypertrophic chondrocytes, with a greater proportion of hypertrophic chondrocytes closer to the cortical bone region, which is consistent with the corresponding H&E staining results (Fig. 4A).
By Day 15, the focus was on the red-framed area at the interface of cartilage and osteoblasts, with deconvolution indicating a composition mainly of chondrocytes and osteoblasts and the presence of hypertrophic chondrocytes at the interface, aligning with the H&E staining results. The blue-framed area highlighted the woven bone region, which is primarily composed of osteoblasts, M2-type macrophages, and osteoclasts, illustrating the complex cellular dynamics during the later stages of bone healing. This analysis underscores the utility of deconvolution in resolving the cellular architecture within periosteal regions and elucidating their roles in different phases of bone repair (Fig. 4A).
GSVA (gene set variation analysis) was utilized to evaluate the enrichment of hallmark and KEGG pathways across different cell types and temporal phases within distinct spatial regions. The analysis revealed marked enrichment of pathways related to tissue injury and repair in both MPC and regenerative MPC (rMPC) cells, such as the hypoxia, TGF-beta, angiogenesis and Wnt signaling pathways, highlighting their involvement in the early stages of bone healing. Notably, pathways associated with DNA replication and the cell cycle were prominently activated in rMPCs on Day 5, indicating their active proliferative state.
Furthermore, metabolic pathways such as those for fructose, mannose, and galactose were most distinctly altered in chondrocytes, suggesting that these metabolic pathways play crucial roles in the formation of cartilaginous calli. Pathways related to postinjury inflammation were enriched in macrophages, whereas osteoclast/osteoblast (OC/OB) cells exhibited changes similar to those of MPCs and were particularly pronounced by Day 15, indicating ongoing bone remodeling and repair processes (Fig. 4B).
Next, a single-cell regulatory network inference and clustering (SCENIC) analysis32 was conducted to reveal the gene regulatory networks crucial for the spatial and temporal variations observed in different regions. The key transcriptional regulators for rMPCs included Meox2, En1, and Foxp1, suggesting their significant role in regulating rMPC function during repair. For chondrocytes, transcription factors such as Sox5, Sox8, and Sox9 have been identified, reinforcing their role in chondrogenesis. For OCs, the main transcriptional regulators were Prdm1, Spi1, and Vdr, highlighting the regulatory dynamics essential for osteoclast differentiation and activity. This analysis not only maps the dynamic cellular responses during bone healing but also highlights the complex molecular interplay that facilitates this process (Fig. 4C).
GSEA further revealed distinct functional shifts between MPCs and regenerative MPCs in response to bone fracture. MPCs, which are typically involved in maintaining bone integrity, presented enrichment of gene sets related to ossification and osteoblast differentiation. In contrast, rMPCs activated during repair processes presented enrichment of gene sets associated with the mechanical stress response, inflammation, and extracellular matrix organization, which are essential for effective tissue regeneration (Supplementary Fig. 11A). Dot plot analysis further confirmed the postfracture upregulation of genes linked to biomechanical stimulation (Ccn1, Piezo1, Yap1, and Taz) and angiogenesis (Hif1a, Emcn, Notch4, and Tie1), underscoring the adaptive response of the periosteum to healing (Supplementary Fig. 11B).
Deciphering spatial trajectories in fracture healing
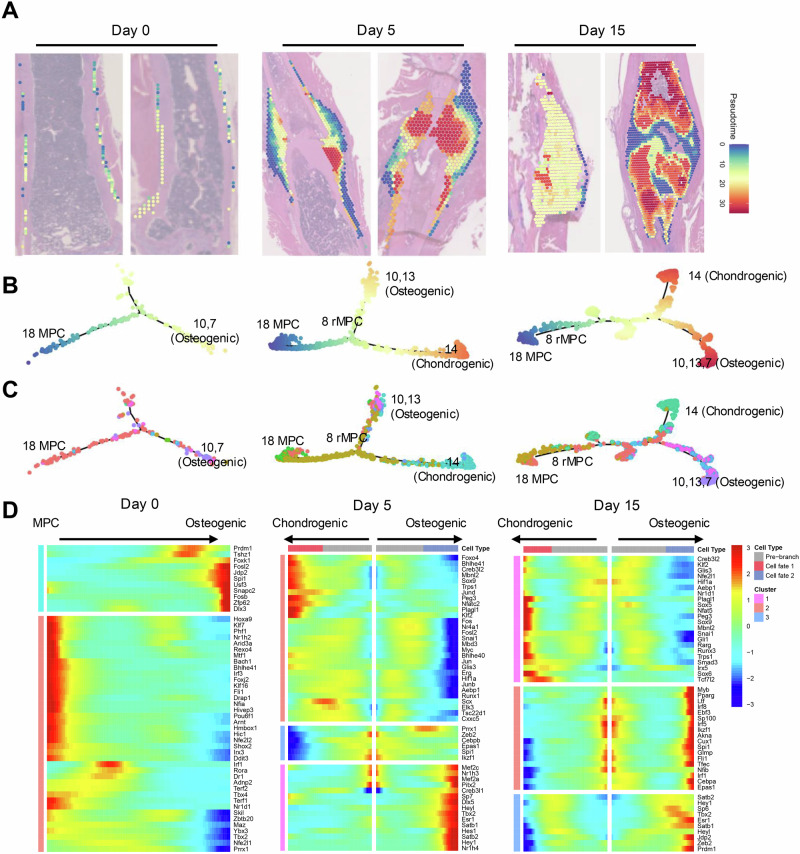
Spatial pseudotime analysis is crucial, as it highlights the dynamic progression of cellular activities during bone healing, linking spatial and temporal gene expression data to track the evolution of cell types from the injury response to tissue regeneration. We next utilized Monocle to perform pseudotime sequencing on spatially resolved spots, assigning a temporal progression to each spot on the basis of its gene expression profile. This pseudotime was then mapped to spatial positions, revealing early pseudotime positions for MPCs and later positions for chondrocytes and osteoblasts, indicating their respective roles in the early and late stages of bone healing (Fig. 5A).
Fig. 5. Pseudotime analysis of cells involved in bone healing.
A Histological sections from Day 0, Day 5, and Day 15 postfracture, each overlaid with a pseudotime color gradient from blue to red. This gradient represents the inferred pseudotime from early to late cellular states within the periosteum on the basis of gene expression profiles. B Trajectory plots tracing the developmental pathways of periosteal cells, highlighting transitions from mesenchymal progenitor cells (MPCs) to osteogenic (Clusters 10, 13) and chondrogenic (Cluster 14) lineages. The color gradient indicates progression through pseudotime, depicting the evolutionary trajectory of these cells. C Trajectory plots showing cell clusters categorized by type, with colors representing distinct cellular identities, illustrating how different cell populations evolve through pseudotime during the healing process. D Heatmaps displaying the expression patterns of key transcription factors across different stages and cell types, from MPCs to differentiated osteogenic and chondrogenic cells. These heatmaps align transcriptional changes with pseudotime to reveal the regulatory landscape driving cellular differentiation.
Dimensionality reduction maps were generated to visualize the results, with colors representing pseudotime (Fig. 5B) and cell types (Fig. 5C) across different timepoints. On Day 0, MPCs differentiated toward an osteogenic lineage, whereas by Days 5 and 15, they differentiated into regenerative MPCs (rMPCs) and then progressed towards both chondrogenic and osteogenic fates. Branched expression analysis modeling (BEAM) analysis further revealed transcription factor activity along these differentiation pathways. On Day 0, the early upregulation of the transcription factors Prdm1 and Spi1 indicates the initiation of differentiation processes in quiescent periosteal MPCs toward osteogenic-related cells, setting the stage for subsequent bone formation and healing. On Days 5 and 15, factors such as Sox9, Nr4a1, and Hif1a were upregulated in the chondrogenic pathway, whereas Dlx5, Sp7, and Hey1 were upregulated in the osteogenic pathway (Fig. 5D).
The temporal expression patterns of the markers were also examined (Supplementary Fig. 12). The expression of rMPC markers, including Acta2, Lrrc15, and Tagln, initially increased and subsequently decreased along the differentiation pseudotime at Days 5 and 15, reflecting their dynamic roles in the early stages of fracture repair. Conversely, the expression of markers for chondrogenesis and osteogenesis, such as Acan and Alpl, continuously increased over time, consistent with the progression of tissue regeneration, as observed on Days 5 and 15. This multifaceted approach provides in-depth insights into the cellular dynamics and molecular mechanisms driving bone regeneration across different stages of healing.
Deciphering cell–cell interactions: spatial crosstalk in bone healing
Spatial transcriptomic data are particularly powerful for deciphering cellular crosstalk. We used CellChat software27 to analyze our spatial transcriptomic data of the fracture callus, where outgoing and incoming signal interaction strengths are plotted against one another as a measure of crosstalk strength. To clearly visualize the spatial interactions, certain cell clusters were merged according to their similarity: Clusters 1, 4, 6, 12, 21, and 26 were combined as skeletal muscle cells; Clusters 5 and 17 were combined as erythrocytes; Clusters 8 and 25 were combined as rMPCs; and Clusters 19 and 22 were combined as macrophages (Fig. 2B). We observed a high level of cellular communication activity within the areas of rMPCs and macrophages as fracture healing progressed (Fig. 6A), highlighting an active intercellular dialog critical for the healing process within these regions. This analysis was further substantiated by the scRNA-seq data, which revealed a consistent increase in the interactions among MPCs, PPCs, tissue repair-associated macrophages (MF2), OCs, and OBs concurrent with healing progression (Fig. 6B).
Fig. 6. Spatial cell communication analysis reveals signaling pathways and spatial dynamics of MPC-driven macrophage recruitment.
A Dot charts showing spatial transcriptomic (ST) analysis of interaction strengths among cell types at Days 0, 5, and 15. Each bubble’s size corresponds to the interaction strength. B Dot charts showing single-cell (SC) analysis of interaction strengths among cell types at Days 0, 5, and 10 postfracture. MPC mesenchymal progenitor cell, PPC proliferative progenitor cell, CH chondrocyte, HCH hypertrophic chondrocyte, EOB early osteoblast, OB osteoblast, Syn synovial cell, EC endothelial cell, MF1 inflammatory macrophage, MF2 tissue resident macrophage, OC osteoclast. C Spatial maps highlighting the colocalization of different cell types on Day 5. On Day 5, MPCs colocalized with macrophages. Insets provide magnified views of selected areas, illustrating the detailed spatial arrangement of these cells within the fracture site. D Distribution of tissue-resident macrophage scores calculated by the CARD package along the thickened periosteum from the fracture site, showing a decrease in the number of macrophages with increasing distance from the fracture (0–4 mm). R2 = 0.2962 indicates the strength of the trend. E Immunofluorescence images of macrophage distribution in fractured Gli1ER/Td mouse femurs on Day 5. Left panel: H&E staining. F Comparison of the macrophage density per square millimeter of the periosteum area near and distant from the fracture site. n = 3/group. The data are expressed as the means ± SDs and were analyzed by unpaired two-tailed t tests. G Bubble plot depicting the significance and probability of interactions between various cell types (MPC, OB, OC, MF2) across different days postfracture (D0, D5, D10). Each row represents a signaling pathway, and each column shows a specific cell type interaction, with color intensity indicating the communication probability and outlined bubbles marking statistically significant interactions. The signals in red are pathways involved in the MPC region, and the signals in blue are pathways involved in the OB region.
Given the limited resolution of Visium technology, which typically includes multiple cell types within a single spot, we employed the CARD26 deconvolution framework again to enhance our analysis. We integrated single-cell RNA sequencing data to accurately determine the proportions of different cell types within each spatial spot, with a special focus on the rMPC and macrophage areas. On the fifth day postfracture, the rMPC area exhibited distinct spatial arrangements. Detailed magnification revealed that at sites distant from the fracture line, within the identified MPC spot, the proportion of macrophages (purple color) was lower compared with that of MPCs (black color). Closer to the fracture line, however, there was a significant increase in the proportion of macrophages within the MPC spot, suggesting heightened cellular interactions in areas actively undergoing healing (Fig. 6C, D). To further validate these findings, we conducted in vivo experiments. We and others have reported that Gli1creERT2 effectively marks periosteal progenitor cells6,21,33. These cells proliferate significantly in the early stages of fracture healing and subsequently differentiate into chondrocytes and osteoblasts, actively participating in the repair process. This model aligns closely with the changes in periosteal progenitor cells that we have documented through both single-cell and spatial transcriptomics. Therefore, Gli1creERT2 genetically engineered mice could serve as an in vivo model for tracing periosteal progenitor cells. We administered tamoxifen to 3-month-old male Gli1ER/Td mice for five consecutive days, followed by femur fracture. Five days postfracture, we observed Gli1+ cell expansion along with thickening of the periosteum. Notably, in the periosteum near the fracture line, there was a significantly greater number of F4/80-stained macrophages than in areas farther from the fracture line (Fig. 6E, F). Interestingly, the majority of these macrophages were located in close proximity to Gli1+ periosteal progenitor cells.
To further investigate how cellular communication influences these spatial changes, we employed the CellChat method for higher-resolution analysis using single-cell RNA sequencing data. The bubble chart delineates specific pathway changes; within the MPC regions, there is a marked increase in Postn, Mif, Mdk, and Cxcl12 signaling, emphasizing the potential roles these interactions between MPCs and MF2 macrophages play across different stages of fracture healing (Fig. 6G). Concurrently, there was an increase in RANKL-mediated signaling among OBs, OCs, and MF2 macrophages, along with a notable increase in Spp1 signaling, both of which are predominantly associated with OB regions (Fig. 6G). Collectively, these findings provide important insights into the signaling communications essential for coordinating the complex cellular interactions that underpin the bone regeneration process.
Discussion
In our study, spatial transcriptomics was utilized to analyze the temporal and spatial dynamics of cell interactions and differentiation during bone fracture healing at three specific time points. This study increases our understanding of bone repair by elucidating the roles of different cell types in the progression from initial inflammatory responses to subsequent remodeling stages via the incorporation of critical spatial information to clarify cellular interactions. Importantly, our optimized technique to preserve adult mouse fracture tissue lays the framework for continued research on the fracture healing process.
Spatial transcriptomics has the potential to revolutionize our understanding of bone homeostasis and regeneration, providing detailed insights into gene expression within bone architecture. This technique is used to reveal intricate cellular interactions in both soft and hard tissues and highlight the spatial location of microenvironments crucial for repair processes14,34,35. However, the application of spatial transcriptomics to bone is limited by RNA degradation from decalcification, typically yielding only approximately 300 genes per spot after EDTA treatment, whereas 3500 genes per spot are produced in undecalcified calvarial sutures of newborn mice18,36. Such low UMI and gene counts per spot significantly hinder single-cell clustering and the detection of differentially expressed genes, posing challenges for accurate data interpretation. To address these issues, we explored alternative decalcification methods, such as Morse’s solution20, which effectively preserves RNA integrity in RNA scope assays and enables the detection of nearly 5000 genes per spot in our femur or fracture samples. This improvement not only enhances the clustering and localization of cell types but also supports robust differential gene expression analysis, making Morse’s solution a viable method for spatial transcriptomics in hard tissues. Moreover, hard tissue sectioning poses another challenge—tissue detachment. Multiple processing steps, including several washes required by spatial transcriptomics, can exacerbate detachment issues in bone sections. Some degree of detachment was observed in our study, and future research could address this issue by testing substrates with enhanced adhesion. Currently, Superfrost slides and others recommended by 10× Genomics meet the basic requirements, but optimization can improve the technique.
The periosteum is characterized by its rich heterogeneity, serving as a crucial reservoir of stem cells and differentiated cells vital for bone growth and repair21,37–39. During the process of bone fracture healing, mesenchymal lineage cells within the periosteum play pivotal roles, engaging in complex regulatory mechanisms that facilitate tissue regeneration. Our previous scRNA-seq studies successfully revealed various types of periosteal mesenchymal cells and highlighted their diverse functions in the healing cascade. Building on these findings, spatial transcriptomics was employed in this study to further elucidate the precise spatial localization of these key cell populations. This advanced approach enabled us to map the locations of these critical cells within the periosteum, revealing their distinct roles and interactions during the dynamic process of bone healing. For example, periosteal MPCs, which highly express Pi16, also express markers such as Sca1 and Cd34, indicating strong clonogenic potential and multipotential differentiation. rMPCs, located near the fracture line within the thickened periosteum, not only exhibit high proliferative activity but also myofibroblast characteristics, which are crucial for mechanical stability in the repair of tissues such as skin. This study represents the first discovery of the spatial positions of these cells through spatial transcriptomics, highlighting the expression of mechanical signaling factors such as Ccn140, Yap/Taz41, and Piezo142 in the rMPC subgroup. Moreover, our findings on chondrocytes and osteoblasts within the callus align with the literature confirming the appearance of early cartilage on Day 5 postfracture in a hypoxic environment43 and the transition to a bone-rich callus by Day 15, with increased vascularization and skeletal regeneration.
Following fracture injury, MPCs proliferate and differentiate into chondrocytes and osteoblasts, forming a callus and facilitating bone healing, although the precise mechanisms remain incompletely understood1. This intricate process of bone repair hinges significantly on the timely differentiation of MPCs into rMPCs and chondrocytes37. Spatial transcriptomics has enabled us to perform pseudotemporal spatial analysis of MPCs, rMPCs, and differentiated cells, tracing the activation and differentiation pathways of uninjured MPCs that are distant from the fracture site. Upon receiving mechanical and inflammatory signals from an injury, signaling pathways such as the TGF-beta pathway are activated in resting MPCs. This activation engages transcription factors such as En144 and Meox245, which are pivotal in MPC differentiation into myofibroblasts and rMPCs, which impact both the intracellular and the extracellular matrix components. These transcription factors are crucial for downstream target activation, including the expression of Acta2. Acta2 is a characteristic marker of myofibroblasts46 and is also specifically expressed in reparative MPCs (rMPCs). Thus, it plays a pivotal role in defining the myofibroblast phenotype within the context of bone healing. This analysis, combined with single-cell pseudotemporal data, also revealed pathways associated with cartilage differentiation and extracellular matrix synthesis, alongside the upregulation of key transcription factors such as Sox9, Hif1a, and Sp7, which are essential for cartilage differentiation and bone repair. These findings reveal key signaling pathways and transcriptional regulators that coordinate the intricate cellular interactions underlying bone regeneration.
Spatial transcriptomics has advanced our ability to probe intercellular communication within the bone healing microenvironment, shedding light on the complex interplay that orchestrates the healing process. Although the resolution of currently employed spatial transcriptomic platforms such as Visium is not sufficient to detail communications between individual cells within each spot—which can contain 2–10 cells of potentially different types—the integration of computational deconvolution methods such as CARD has enabled us to infer probable cell types within these spots. We identified potential colocalizations of cell types within spots, revealing key interactions between MPCs and macrophages at the earliest stages of repair and between osteoblasts and osteoclasts at later stages. On Day 5 postfracture, our data revealed significant proximity between MPCs and macrophages, especially near the fracture line, suggesting that active MPCs might recruit macrophages in the initial stages of healing. This recruitment likely changes as healing progresses, with macrophages migrating closer to the fracture line, indicating their potential role in the activation and function of MPCs, which is consistent with previous studies reporting that macrophages are required for endochondral callus formation47. By Day 15, interactions between osteoblasts and osteoclasts are essential for successful bone regeneration. Our analysis suggested that OBs may regulate OC activity via Rankl signaling, which is known to be pivotal for bone remodeling and callus shaping. These findings underscore the dynamic nature of cellular interactions during bone repair and highlight potential targets for therapeutic intervention, such as Rankl48 and Spp149, to improve healing outcomes.
Despite these advancements in spatial transcriptomics, limitations in this technique remain. Challenges such as tissue detachment during processing and inherent limitations in resolution can affect the quality and interpretability of spatial transcriptomic data. We acknowledge these constraints and emphasize the need for methodological improvements to enhance resolution and data quality. Additionally, the robustness of our conclusions is somewhat limited by our sample size. Ideally, a third biological replicate would strengthen the validity of our findings and provide a more stable basis for statistical analysis. However, owing to resource constraints, this was not feasible within the scope of this study. Nevertheless, we utilized correlation analyses, heatmaps of differentially expressed genes (DEGs) across clusters, and split-view UMAP plots among different time points (Supplementary Fig. 13), which collectively demonstrated high precision between the two replicates at each time point, supporting the reliability and reproducibility of our findings. Furthermore, the current approach requires further validation in different models and human tissues to ensure its broader applicability in bone healing studies. As we move forward, it is essential to refine spatial transcriptomic techniques to overcome these limitations, aiming for higher resolution and more robust tissue preservation methods. Furthermore, the integration of spatial transcriptomics with other omics technologies could provide a more comprehensive view of bone healing and potentially reveal novel therapeutic targets for bone regeneration.
In conclusion, our study advances the understanding of the spatial and temporal dynamics underlying bone healing. The spatial transcriptomics data generated in this study provide a valuable resource for future investigations into the mechanisms of tissue regeneration and bone repair.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This study was funded by the National Key R&D Program of China (2021YFA1102600), the National Science Foundation of China (82103781, 82472433), and the Science Fund for Distinguished Young Scholars of Liaoning Province (2024JH3/10200034).
Author contributions
H.W., X.H., M.M. and T.D. contributed equally to this work and should be considered cofirst authors. L.Y., D.R., L.Q. and Y.W. designed and developed the experiments. H.W., X.H., M.M. and T.D. performed the main experiments. H.W., Y.Y. and L.Y. drafted the paper. M.M. and L.Y. performed the bioinformatic analysis. Y.Y., Y.Z. and L.Y. mentored the project and edited the paper. All the authors have read and agreed to the published version of the paper.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Simona Chera and Aylin Bircan. A peer review file is available.
Data availability
The scRNA-seq data used in this study were previously generated3 and have been deposited in the Gene Expression Omnibus (GEO) database under the accession code GSE192630. The spatial transcriptomic data generated in this study have been deposited in the GEO database under the accession code GSE297119. The aligned spatial transcriptomic sequencing data generated in this study have been deposited in the Broad Institute Cell Portal under study numbers SCP2945 (Day 0), SCP2948 (Day 5) and SCP2626 (Day 15). The other data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
All code used in this study relies on publicly available packages and is accessible at https://github.com/CMUltyao/fx-visium.git. Detailed analytical workflows are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hanning Wang, Xuan He, Mingjie Ma, Tianxu Dou.
Contributor Information
Yan Yang, Email: yangyan_doctor@163.com.
Yue Zhu, Email: zhuyuedr@163.com.
Lutian Yao, Email: ltyao@cmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-025-08316-0.
References
- 1.Einhorn, T. A. & Gerstenfeld, L. C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol.11, 45–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claes, L., Recknagel, S. & Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol.8, 133–143 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Yao, L. et al. Activin A marks a novel progenitor cell population during fracture healing and reveals a therapeutic strategy. Elife12, e89822 (2023). [DOI] [PMC free article] [PubMed]
- 4.Kolar, P. et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. B. Rev.16, 427–434 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Matthews, B. G. et al. Analysis of aSMA-labeled progenitor cell commitment identified notch signaling as an important pathwayin fracture healing. J. Bone Min. Res.29, 1283–1294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi, Y. et al. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat. Commun.8, 2043 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loi, F. et al. Inflammation, fracture and bone repair. Bone86, 119–130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baryawno, N. et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell177, 1915–1932 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushita, Y. et al. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat. Commun.11, 332 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tikhonova, A. N. et al. The bone marrow microenvironment at single-cell resolution. Nature569, 222–228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong, L. et al. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. eLife9, e54695 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao, A., Barkley, D., Franca, G. S. & Yanai, I. Exploring tissue architecture using spatial transcriptomics. Nature596, 211–220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji, A. L. et al. Multimodal analysis of composition and spatial architecture in human squamous. Cell Carcinoma Cell182, 497–514 e422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han, B. et al. Integrating spatial and single-cell transcriptomics to characterize the molecular and cellular architecture of the ischemic mouse brain. Sci. Transl. Med.16, eadg1323 (2024). [DOI] [PubMed] [Google Scholar]
- 15.Ratz, M. et al. Clonal relations in the mouse brain revealed by single-cell and spatial transcriptomics. Nat. Neurosci.25, 285–294 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada, S. et al. Spatiotemporal transcriptome analysis reveals critical roles for mechano-sensing genes at the border zone in remodeling after myocardial infarction. Nat. Cardiovasc. Res.1, 1072–1083 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, Y. et al. Characterization of the nucleus pulposus progenitor cells via spatial transcriptomics. Adv. Sci.11, e2303752 (2024). [DOI] [PMC free article] [PubMed]
- 18.Xiao, X. et al. Spatial transcriptomic interrogation of the murine bone marrow signaling landscape. Bone Res.11, 59 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter, M. V., Moncada, R., Weiss, J. M., Yanai, I. & White, R. M. Spatially resolved transcriptomics reveals the architecture of the tumor-microenvironment interface. Nat. Commun.12, 6278 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Charleroy, C., Haseeb, A. & Lefebvre, V. Preparation of adult mouse skeletal tissue sections for RNA in situ hybridization. Methods Mol. Biol.2245, 85–92 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, L. et al. Periosteal mesenchymal progenitor dysfunction and extraskeletally-derived fibrosis contribute to atrophic fracture nonunion. J. Bone Min. Res34, 520–532 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuart, T. et al. Comprehensive integration of single-cell data. Cell177, 1888–1902.e1821 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu, S. et al. Using clusterProfiler to characterize multiomics data. Nat. Protoc.19, 3292–3320 (2024). [DOI] [PubMed] [Google Scholar]
- 25.Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature566, 496–502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma, Y. & Zhou, X. Spatially informed cell-type deconvolution for spatial transcriptomics. Nat. Biotechnol.40, 1349–1359 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun.12, 1088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou, W. & Ji, Z. Assessing GPT-4 for cell type annotation in single-cell RNA-seq analysis. Nat. Methods21, 1462–1465 (2024). [DOI] [PMC free article] [PubMed]
- 29.Yao, L. et al. Gli1 defines a subset of fibro-adipogenic progenitors that promote skeletal muscle regeneration with less fat accumulation. J. Bone Min. Res.36, 1159–1173 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Julien, A. et al. Direct contribution of skeletal muscle mesenchymal progenitors to bone repair. Nat. Commun.12, 2860 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, Y. L., Tang, X. T., Shu, H. S., Zou, W. & Zhou, B. O. Fibrous periosteum repairs bone fracture and maintains the healed bone throughout mouse adulthood. Dev. Cell59, 1192–1209.e1196 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods14, 1083–1086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeffery, E. C., Mann, T. L. A., Pool, J. A., Zhao, Z. & Morrison, S. J. Bone marrow and periosteal skeletal stem/progenitor cells make distinct contributions to bone maintenance and repair. Cell Stem Cell29, 1547–1561.e1546 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Arora, R. et al. Spatial transcriptomics reveals distinct and conserved tumor core and edge architectures that predict survival and targeted therapy response. Nat. Commun.14, 5029 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denisenko, E. et al. Spatial transcriptomics reveals discrete tumour microenvironments and autocrine loops within ovarian cancer subclones. Nat. Commun.15, 2860 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tower, R. J. et al. Spatial transcriptomics reveals a role for sensory nerves in preserving cranial suture patency through modulation of BMP/TGF-beta signaling. Proc Natl Acad. Sci. USA118, e2103087118 (2021). [DOI] [PMC free article] [PubMed]
- 37.Debnath, S. et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature562, 133–139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duchamp de Lageneste, O. et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun.9, 773 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, L. et al. Plasminogen regulates fracture repair by promoting the functions of periosteal mesenchymal progenitors. J. Bone Min. Res.36, 2229–2242 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lienau, J. et al. CYR61 (CCN1) protein expression during fracture healing in an ovine tibial model and its relation to the mechanical fixation stability. J. Orthop. Res.24, 254–262 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Kegelman, C. D. et al. YAP and TAZ promote periosteal osteoblast precursor expansion and differentiation for fracture repair. J. Bone Miner. Res.36, 143–157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu, Y. et al. Mechanosensitive Piezo1 is crucial for periosteal stem cell-mediated fracture healing. Int. J. Biol. Sci.18, 3961 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu, C. et al. The role of oxygen during fracture healing. Bone52, 220–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gyorfi, A. H. et al. Engrailed 1 coordinates cytoskeletal reorganization to induce myofibroblast differentiation. J. Exp. Med.218, e20201916 (2021). [DOI] [PMC free article] [PubMed]
- 45.Cunnington, R. H. et al. The Ski-Zeb2-Meox2 pathway provides a novel mechanism for regulation of the cardiac myofibroblast phenotype. J. Cell Sci.127, 40–49 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Lebœuf, M. et al. ENGRAILED-1 transcription factor has a paracrine neurotrophic activity on adult spinal α-motoneurons. EMBO Rep.24, e56525 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raggatt, L. J. et al. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am. J. Pathol.184, 3192–3204 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Ono, T. & Takayanagi, H. Osteoimmunology in bone fracture healing. Curr. Osteoporos. Rep.15, 367–375 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Wang, C. et al. Targeting angiogenesis for fracture nonunion treatment in inflammatory disease. Bone Res.9, 29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The scRNA-seq data used in this study were previously generated3 and have been deposited in the Gene Expression Omnibus (GEO) database under the accession code GSE192630. The spatial transcriptomic data generated in this study have been deposited in the GEO database under the accession code GSE297119. The aligned spatial transcriptomic sequencing data generated in this study have been deposited in the Broad Institute Cell Portal under study numbers SCP2945 (Day 0), SCP2948 (Day 5) and SCP2626 (Day 15). The other data that support the findings of this study are available from the corresponding author upon reasonable request.
All code used in this study relies on publicly available packages and is accessible at https://github.com/CMUltyao/fx-visium.git. Detailed analytical workflows are available from the corresponding author upon reasonable request.