Abstract
The C-terminal domain (CTD) of mammalian RNA polymerase II consists of 52 repeats of the consensus hepta-peptide YSPTSPS, and links transcription to the processing of pre-mRNA. Although Pol II with a CTD shortened to five repeats (Pol II Δ5) is transcriptionally inactive on chromatin templates, it is not clear whether CTD is required for promoter recognition in vivo. Here, we demonstrate that in the context of chromatin, Pol II Δ5 can bind to the c-myc promoter with the same efficiency as wild type Pol II. However, Pol II Δ5 does not form a stable initiation complex, and does not transcribe promoter proximal sequences. Fluorescence recovery after photobleaching (FRAP) experiments with cells expressing enhanced green fluorescent protein (EGFP)-tagged Δ5 or wildtype Pol II revealed a single, highly mobile Pol II Δ5 fraction whereas wildtype Pol II yielded less mobile fractions. These data suggest that CTD is not required for promoter recognition, but rather for subsequent formation of a stable initiation complex and isomerization to an elongation competent complex.
INTRODUCTION
The large subunit of eukaryotic RNA polymerase II (Pol II LS) harbours a unique C-terminal domain (CTD) consisting of repeats of the consensus hepta-peptide sequence YSPTSPS (1). The consensus sequence is highly conserved across organisms, but the number of repeats appears to have increased through evolution (2).
The phosphorylation status of the CTD is essential for the regulation of transcription [reviewed in (3,4)]. In vivo, only the non-phosphorylated (IIA) form of Pol II can participate in the formation of a pre-initiation complex (PIC), while CTD phosphorylation is essential for transcriptional elongation (the IIO form) (3,4). The effectors of this regulation include several cyclin-dependent-, and stress-activated kinases, whose activities during certain stages of the transcription cycle may serve to regulate initiation, elongation and the binding of pre-mRNA processing factors to Pol II [for reviews see (5–8)]. The phosphorylation of non-engaged Pol II by kinases, such as ERK or CDK8/cyclin C may function to down regulate the transcription by preventing the formation of new PICs (9–11), suggesting that the CTD could be involved in controlling early steps of initiation. Phosphorylation of Pol II during the transition from initiation to elongation by CDK7/cyclin H of the general transcription factor TFIIH, and CDK9/cyclins T and K of the elongation factor P-TEFb, may relieve the inhibitory effects of the DSIF, NELF and Mediator complexes. Hence, Pol II with phosphorylated CTD (Pol II0) has a specific defect in the initiation on chromatin templates, while a polymerase with an unphosphorylated CTD can properly initiate. This leaves open the question whether an unphosphorylated CTD is required for promoter recognition, or if a CTD-deleted Pol II can recognize a chromatin-packaged promoter as well.
Here, we show that the recognition of the c-myc promoter by Pol II with a CTD truncated to five repeats (Pol II Δ5) is not affected in the context of chromatin, while subsequent steps like stable PIC formation and isomerization to an elongation competent complex are severely impaired. This is in agreement with our further observation that Pol II Δ5 is highly mobile in fluorescence recovery after photobleaching (FRAP) experiments and not stably associated with nuclear structures.
MATERIALS AND METHODS
Cell culture and cell lines
Cell lines were obtained by stable transfection of the Burkitt's lymphoma cell line Raji with DNA encoding the 8.1 kb HindIII–EcoRI c-myc gene locus on the episomal, self-replicating Epstein–Barr virus (EBV)-derived vector KH375, and selected with hygromycin. Subsequently, cells were transfected with LSmock, Pol II wt or Pol II Δ5 carrying a tet-off regulatable promoter (12), and selected with neomycin. HeLa cells were transfected with the plasmid pSV40-H2B-mRFP1 and stable single cell clones expressing H2B-MRFP were isolated. Positive cell clones were subsequently transfected with plasmids expressing α-amanitin resistant fusions of the large subunit of Pol II wt or Pol II Δ5 and enhanced green fluorescent protein (EGFP) (13) and selected with neomycin. If indicated, cells were treated with final concentrations of 2 µg/ml α-amanitin or 3 mM/ml sodium butyrate (SoB).
Nuclear run-on assay, S1 analysis
Isolation of nuclei, purification and hybridization of labelled RNA to membrane-bound oligonucleotides, the washing procedure of membranes including the digestion of single-stranded RNA with RNAse A, oligonucleotides complementary to the human antisense c-myc strand, in vitro transcribed T7 control RNA, as well as S1 analysis have been described in detail elsewhere (14).
Chromatin immunoprecipitation (ChIP) analysis
Cells were formaldehyde cross-linked and immunoprecipitated as described previously (14). Antibodies against HA-tag (3F10, Roche) and isotype control (Santa Cruz) were applied. Antibody/protein/DNA complexes were isolated by immunoprecipitation with blocked protein A positive Staph A cells. Following extensive washing, bound DNA fragments were eluted and analysed by subsequent PCR. Each reaction contained 3 µl of immunoprecipitated DNA, 1× Taq reaction buffer (Promega), 1.5 mM MgCl2, 50 ng of each primer, 1.7 U Taq polymerase (Promega) and 200 µM each dNTP (Boehringer Mannheim) (including 1 µCi [32P]dCTP) in a final reaction volume of 20 µl. PCRs were amplified for 1 cycle at 95°C for 5 min, annealing temperature of the primers for 5 min, 72°C for 3 min and 27 cycles at 95°C for 1 min, annealing temperature of the primers for 2 min, 72°C for 1.5 min. PCR products were separated by electrophoresis through a 6% polyacrylamide gel, and visualized by autoradiography. The following primers were used for the c-myc promoter region and insulin gene:
Myc-1: 5′-GGTCTGGACGGCTGAGGACCCCCG-3′;
Myc-2: 5′-CTCTCGCTGGAATTACTACAGCG3′;
Insulin 1: 5′-GCTGGTAGAGGGAGCAGATG-3′;
Insulin 2: 5′-CCCTGACTGTGTCCTCCTGT-3′.
FRAP analysis
FRAP analysis was performed on HeLa cells expressing H2B-MRFP, and Pol II wt-EGFP or Pol II Δ5-EGFP. Cells were seeded on round coverslips fitting in the temperature controlled (37°C) POC-Chamber (LaCon, Germany). Live cell microscopy was performed with a Zeiss LSM510 Meta confocal microscope equipped with 63×/1.4 NA Plan apochromat oil objective. An Ar laser (488 nm, 35 mW) and a HeNe laser (543 nm, 1 mW) were used to excite the fluorescent proteins. Image acquisition before and after bleaching was performed at low laser power (5%). For FRAP analysis a region of interest was selected and photobleached by an intense 488 nm Ar laser beam (set to 100%) for 5 s, after which confocal image series were recorded at 1 s time intervals for 1 min and thereafter at 5 s time intervals for ∼4 min. Mean fluorescence intensities of the bleached region were corrected for background and for total nuclear loss of fluorescence over the time course. FRAP data of at least eight nuclei were averaged and the mean curve as well as the standard deviations were plotted. As a control fixed specimen (4% paraformaldehyde, 10 min) of the same cell lines mounted in Vectashield (Vector, USA) were subjected to FRAP analysis and plotted as well.
RESULTS AND DISCUSSION
CTD is not required for recognition and binding to the c-myc promoter in vivo
A requirement for the CTD in the control of promoter escape and maturation of mRNA in vivo has been documented in detail before. Its role in promoter recognition, formation of a stable initiation complex and isomerization to an elongation competent complex, however, is still elusive. The c-myc promoter is an ideal tool to study this question, since Pol II is stalled immediately after the isomerization step proximal to the promoter. At this position Pol II is easily detectable in run-on transcription assays.
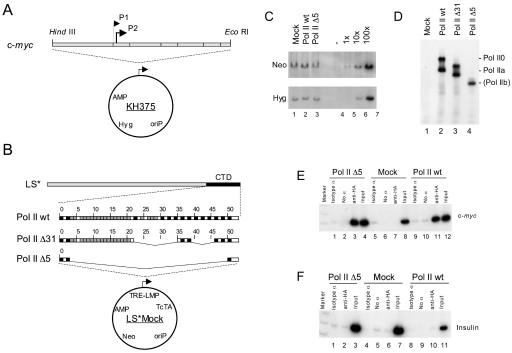
We have previously reconstituted transcriptional regulation of the c-myc gene on stably transfected episomal vectors in the B cell line, Raji. The episomal c-myc establishes a chromatin structure undistinguishable from the structure of an endogenous c-myc to the nucleosomes positioned upstream and downstream of the c-myc promoter. Importantly, episomes carry a stalled Pol II immediately downstream of the major c-myc P2 promoter as it is observed for the endogenous c-myc (14–18). We used Raji cells with a reconstituted c-myc chromatin on episome KH375 (Figure 1A), and introduced an additional episome carrying a tetracycline-regulatable, α-amanitin resistant large subunit of Pol II into these cells (Figure 1B). Cell lines were established carrying only the vector (mock), Pol II with the complete CTD (Pol II wt), or with internal deletions of 21 (Pol II Δ31; deletion of 23–36 + 39–47) and 47 repeats (Pol II Δ5; deletion of 4–50) (12,19). The copy number of episomes as determined by Southern analysis turned out to be similar for the c-myc and Pol II constructs in all cell lines (Figure 1C). Similar levels of the large subunit of Pol II were induced in all cell lines after removal of tetracycline (Figure 1D). Pol II wt and Pol II Δ31 displayed both the hyperphosphorylated (Pol II0) and hypophosphorylated (Pol IIa) forms while Pol II Δ5 migrated as a single small band in gel electrophoresis. Pol II Δ31 behaved like Pol II wt and is not further shown in the following experiments.
Figure 1.
Efficient binding of Pol II Δ5 to the c-myc promoter. (A) Raji cell lines carrying the episomal c-myc gene locus, and in addition (B) either LS*Mock (vector control), Pol II wt, or Pol II Δ5 were established. (C) The copy number of constructs was determined by Southern analysis with specific probes for the neomycin and hygromycin resistance genes. 1×, 10× and 100× correspond to 1, 10 and 100 copies/genome, respectively. (D) Proper expression of Pol II wt and Pol II Δ5 after removal of tetracycline was controlled by western blot analysis using anti-HA antibodies. Pol II Δ31 served as size control. (E) ChIP analysis revealed similar binding of Pol II Δ5 and Pol II wt to the c-myc promoter (lanes 3 and 11, respectively), while binding was not detectable in the Mock cell line (lane 7), or (F) to a non-transcribed region in the insulin gene.
ChIP experiments were performed to measure the binding of the Pol II wt and Pol II Δ5 to the c-myc promoter. Both RNA polymerases cross-linked to the c-myc promoter with the same high efficiency (Figure 1E, lanes 3 and 11). No cross-linking to the c-myc promoter could be observed in the mock cell line (lane 7), or to the promoter of the insulin gene, which is not transcribed in B cells (Figure 1F, lanes 2 and 10). Cross-linking of E2F to the c-myc promoter (14) was seen in all cell lines and is therefore not affected by the expression of Pol II wt, and Pol II Δ5 (data not shown). We conclude that Pol II Δ5 binds and cross-links to the c-myc promoter with the same efficiency as Pol II wt, and that the CTD has neither a positive or negative effect on c-myc promoter recognition. Whether the hypo- and hyperphosphorylated Pol II0 can recognize the c-myc promoter with different efficiencies, as reported previously for the adenovirus major late promoter (20), cannot be analysed in this assay, and remains unclear.
CTD is required for stable PIC formation and/or isomerization of Pol II at the c-myc promoter
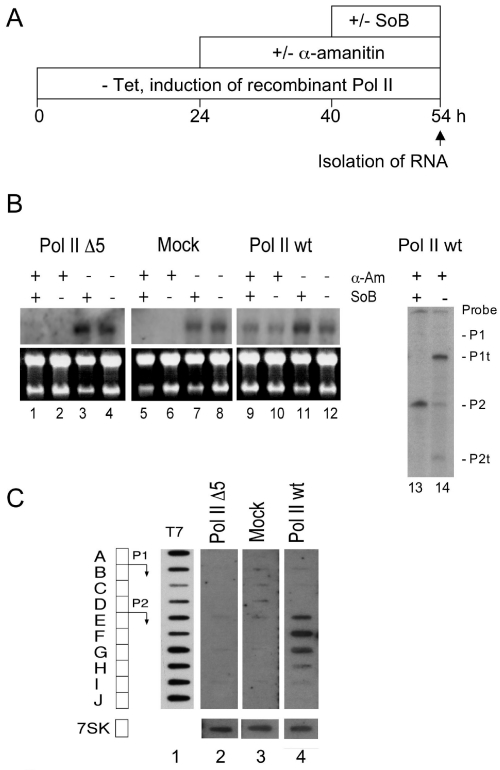
The episomal c-myc is induced after inhibition of histone deacetylase (HDAC) activity (14). We next tested whether Pol II wt and Pol II Δ5 can transcribe the c-myc gene after inhibition of HDAC activity by SoB. Cells were induced by removal of tetracycline, and treated with α-amanitin and SoB as indicated in Figure 2A. Expression of the episomal c-myc by SoB is induced in Pol II wt cells (Figure 2B, lane 9) but not in Mock and Pol II Δ5 cells (lanes 1 and 5). Note that transcription of the translocated, Ig-enhancer driven c-myc gene is repressed by SoB in Raji cells, leaving the signal for c-myc mRNA in lane 9 unchanged (14). However, induction of the episomal and repression of the translocated c-myc is distinguishable by S1 analysis. P1t and P2t mRNAs are derived from the translocated c-myc (lane 14) and P2 mRNA is derived from the episomal c-myc (lane 13).
Figure 2.
Transcription of the c-myc promoter by recombinant Pol II. (A) Tetracycline was washed out from cell lines at time point 0 h to allow expression of Pol II wt and Pol II Δ5. α-amanitin was added 24 h later to achieve quantitative inhibition of the endogenous Pol II. After 16 h, transcription of the episomal c-myc was induced by treatment of cells with SoB for 14 h. (B) northern analysis of c-myc RNA (left hand site), discrimination of endogenous and episomal c-myc RNA by S1 analysis (right hand site). P1t/P2t mark RNA from the endogenous, translocated c-myc gene (lane 14), P1/P2 RNA from the episomal c-myc (lane 13). (C) Nuclear run-on activity of Pol II Δ5 and Pol II wt at the episomal c-myc promoter. Cells were treated as described in (A), but nuclei were isolated 24 h after addition of α-amanitin, cells were not induced with SoB. (A–J) Long antisense oligonucleotides (50 nt) covering the c-myc promoter region; 7SK: antisense oligonucleotide for Pol III transcribed 7SK gene; T7: T7 RNA polymerase transcribed, uniformely labelled RNA of the c-myc promoter region.
Importantly, the failure of Pol II Δ5 to induce c-myc expression is not solely caused by its inability to support maturation of c-myc mRNA. Nuclear run-on experiments showed that Pol II Δ5 is defective in the initiation of transcription at the c-myc promoter and cannot transcribe promoter proximal sequences (Figure 2C, lane 2). In contrast, transcription by Pol II wt produced strong transcription signals on oligonucleotides E, F and G downstream of the c-myc P2 promoter (Figure 2C, lane 4). These signals were not detectable for Pol II Δ5 and mock cells (lanes 2 and 3). Since pausing of Pol II at the translocated c-myc promoter is abolished in Burkitt's lymphoma cells (21), including Raji cells (22), the detected run-on signals are almost derived exclusively from episomal c-myc alleles. From these data we conclude that Pol II Δ5 is defective either in the formation of a stable PIC, or in the execution of the subsequent isomerization step to an elongation competent complex. The strong cross-linking of Pol II Δ5 to the c-myc promoter argues that Pol II Δ5 is present at the c-myc promoter at least as frequently as Pol II wt, but is defective in initiating transcription.
Nuclear mobility of EGFP-tagged Pol II wt versus -Pol II Δ5
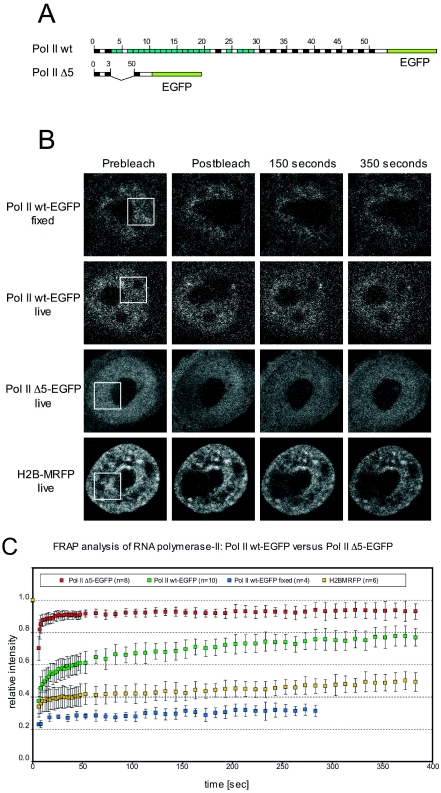
To study the nuclear mobility of Pol II wt and Pol II Δ5 in living cells, we fused EGFP to the carboxy-terminus of both molecules (Figure 3A), and expressed them under the control of the thymidine-kinase promoter in HeLa cells stably expressing a histone 2B-MRFP. HeLa cells expressing Pol II wt-EGFP turned out to be viable and could be cultured and expanded for months in the presence of α-amanitin. In contrast, Pol II Δ5-EGFP expressing cells could be cultured in the presence of α-amanitin for only three days before significant cell death was observed. Therefore, cells were selected with neomycin for 10 days before α-amanitin was added and FRAP experiments were performed. Cloning of EGFP to the carboxy-terminus of the large subunit of Pol II wt and Pol II Δ5 resulted in stable fusion proteins. Importantly, the stability of CTD is controlled by repeats 1–3 and repeat 52 (last repeat) in vivo (13,23). These repeats are present in Pol II Δ5. Moreover, we could recently show that fusion of EGFP to the C-terminus of Pol II mutants lacking repeats 1–3 and repeat 52 fully rescued the stability of CTD deletion mutants (23).
Figure 3.
FRAP analysis of EGFP-tagged Pol II. (A) Pol II wt and Pol II Δ5 were tagged with EGFP at the carboxy-terminus. (B) Pol II-EGFP and H2B-MRFP signals of exemplary nuclei subjected to FRAP analysis were shown: fixed Pol II wt-EGFP (panel 1); live Pol II wt-EGFP (panel 2), live Pol II Δ5-EGFP (panel 3) and live H2B-MRFP (panel 4). The images show the signal distribution before (‘Prebleach’), right after the bleaching (‘Postbleach’) and after a recovery period of 150 and 350 s. The squares in the Prebleach images indicate the bleached region of interest. (C) The plotted curves represent the mean relative intensities measured in the bleached regions over time. The averaged values of 4, 10, 8 and 6 nuclei (fixed Pol II wt-EGFP, live Pol II wt-EGFP, live Pol II Δ5-EGFP and live H2B-MRFP, respectively) and accordingly the standard deviations were plotted for each time point. Fixed samples served as positive controls and confirmed the effectiveness of the bleaching indicated by a drastic drop of the curve to ∼30% and a subsequent stay at this level (blue curve).
Both Pol II wt-EGFP and Pol II Δ5-EGFP displayed nuclear staining with the exclusion of the nucleoli (Figure 3B, panel 2 and 3). In Figure 3B panel 1 is an example of the EGFP signal of a fixed HeLa H2B-mRFP nucleus, stably transfected with Pol II wt-EGFP. Bleaching a region of interest results in a loss of EGFP fluorescence and an imprinted hole, observable over the whole time course and serving as a positive control for an effective bleaching event. Panel 4 in Figure 3B is an example of an monomeric red fluorescent protein (MRFP) signal of unfixed HeLa H2B-MRFP, Pol II Δ5-EGFP cells. Similar to the fixed cells, the MRFP signal shows only little recovery during the 350 s observation time. The mean intensities of such a bleached region calculated over the whole observation are plotted in Figure 3C. In case of the fixed control a loss of fluorescence to ∼30% is observed (Figure 3C, blue curve). The same result was obtained when performing FRAP analysis on fixed cells stably transfected withPol II Δ5-EGFP (data not shown).
FRAP analysis performed on living HeLa cells expressing Pol II wt-EGFP (exemplary nucleus shown in Figure 3B, panel 2) reveals a recovery curve reaching a plateau after ∼200 s (Figure 3C, green curve). The recovery behaviour of Pol II wt-EGFP is in accordance with data reporting several populations of Pol II with differing kinetics (24,25). At least three populations of Pol II exist, based on their differing kinetics. The largest fraction ought to be a free diffusing pool enabling the rapid recovery observable over the first 50 s. The other two fractions ought to be either bound but inactive, or bound and elongating polymerase-II-GFP molecules, which recover much slower and lead to the slightly accelerated recovery in the subsequent observation.
In contrast, performing FRAP analysis on HeLa cells stably expressing Pol II Δ5-EGFP (exemplary nucleus shown in Figure 3B, panel 3) reveals a much faster recovery (Figure 3C, red curve). This argues for a high take off rate, and an increase of the free diffusing population of Pol II Δ5 correlating with the loss of 47/52 repeats of the CTD. We note that the bulk dynamics observed for the different polymerases may not reflect the situation at particular loci.
CONCLUSIONS
The finding that CTD is dispensable for the recognition of the c-myc promoter in the context of chromatin, but required for the isomerization of Pol II into an elongating enzyme underscores our notion that CTD is not only important for the control of RNA elongation, but also for the process of initiation. For this step, the CTD of Pol II probably exist must in its hypophosphorylated form. The inability of a CTD-deleted polymerase to initiate and to transcribe to promoter proximal pause sites may also be a safety mechanism. We could recently show that the integrity of Pol II in vivo can be controlled by a protease that cleaves the CTD from the large subunit (13,23). Removal of the CTD would not only disconnect such polymerases from the CTD-associated RNA-processing machinery, but also would prevent further rounds of transcription by such polymerases.
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgemeinschaft (SFB/Transregio5 and CR 59/22–21). Funding to pay the Open Access publication charges for this article was provided by GSF/BMBF.
Conflict of interest statement. None declared.
REFERENCES
- 1.Corden J.L., Cadena D.L., Ahearn J.M., Jr, Dahmus M.E. A unique structure at the carboxyl terminus of the largest subunit of eukaryotic RNA polymerase II. Proc. Natl Acad. Sci. USA. 1985;82:7934–7938. doi: 10.1073/pnas.82.23.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiller J.W., Hall B.D. Evolution of the RNA polymerase II C-terminal domain. Proc. Natl Acad. Sci. USA. 2002;99:6091–6096. doi: 10.1073/pnas.082646199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahmus M.E. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J. Biol. Chem. 1996;271:19009–19012. doi: 10.1074/jbc.271.32.19009. [DOI] [PubMed] [Google Scholar]
- 4.Palancade B., Bensaude O. Investigating RNA polymerase II carboxyl-terminal domain (CTD) phosphorylation. Eur. J. Biochem. 2003;270:3859–3870. doi: 10.1046/j.1432-1033.2003.03794.x. [DOI] [PubMed] [Google Scholar]
- 5.Bregman D.B., Pestell R.G., Kidd V.J. Cell cycle regulation and RNA polymerase II. Front Biosci. 2000;5:244–257. doi: 10.2741/bregman. [DOI] [PubMed] [Google Scholar]
- 6.Kobor M.S., Greenblatt J. Regulation of transcription elongation by phosphorylation. Biochim. Biophys. Acta. 2002;1577:261–275. doi: 10.1016/s0167-4781(02)00457-8. [DOI] [PubMed] [Google Scholar]
- 7.Oelgeschlager T. Regulation of RNA polymerase II activity by CTD phosphorylation and cell cycle control. J. Cell Physiol. 2002;190:160–169. doi: 10.1002/jcp.10058. [DOI] [PubMed] [Google Scholar]
- 8.Sims R.J., 3rd, Belotserkovskaya R., Reinberg D. Elongation by RNA polymerase II: the short and long of it. Genes Dev. 2004;18:2437–2468. doi: 10.1101/gad.1235904. [DOI] [PubMed] [Google Scholar]
- 9.Dubois M.F., Nguyen V.T., Dahmus M.E., Pages G., Pouyssegur J., Bensaude O. Enhanced phosphorylation of the C-terminal domain of RNA polymerase II upon serum stimulation of quiescent cells: possible involvement of MAP kinases. EMBO J. 1994;13:4787–4797. doi: 10.1002/j.1460-2075.1994.tb06804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hengartner C.J., Myer V.E., Liao S.M., Wilson C.J., Koh S.S., Young R.A. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol. Cell. 1998;2:43–53. doi: 10.1016/s1097-2765(00)80112-4. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet F., Vigneron M., Bensaude O., Dubois M.F. Transcription-independent phosphorylation of the RNA polymerase II C-terminal domain (CTD) involves ERK kinases (MEK1/2) Nucleic Acids Res. 1999;27:4399–4404. doi: 10.1093/nar/27.22.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meininghaus M., Chapman R.D., Horndasch M., Eick D. Conditional expression of RNA polymerase II in mammalian cells. Deletion of the carboxyl-terminal domain of the large subunit affects early steps in transcription. J. Biol. Chem. 2000;275:24375–24382. doi: 10.1074/jbc.M001883200. [DOI] [PubMed] [Google Scholar]
- 13.Chapman R.D., Palancade B., Lang A., Bensaude O., Eick D. The last CTD repeat of the mammalian RNA polymerase II large subunit is important for its stability. Nucleic Acids Res. 2004;32:35–44. doi: 10.1093/nar/gkh172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert T., Wells J., Funk J.O., Pullner A., Raschke E.E., Stelzer G., Meisterernst M., Farnham P.J., Eick D. The chromatin structure of the dual c-myc promoter P1/P2 is regulated by separate elements. J. Biol. Chem. 2001;276:20482–20490. doi: 10.1074/jbc.M100265200. [DOI] [PubMed] [Google Scholar]
- 15.Strobl L.J., Eick D. Hold back of RNA polymerase II at the transcription start site mediates down-regulation of c-myc in vivo. EMBO J. 1992;11:3307–3314. doi: 10.1002/j.1460-2075.1992.tb05409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf D.A., Strobl L.J., Pullner A., Eick D. Variable pause positions of RNA polymerase II lie proximal to the c-myc promoter irrespective of transcriptional activity. Nucleic Acids Res. 1995;23:3373–3379. doi: 10.1093/nar/23.17.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pullner A., Mautner J., Albert T., Eick D. Nucleosomal structure of active and inactive c-myc genes. J. Biol. Chem. 1996;271:31452–31457. doi: 10.1074/jbc.271.49.31452. [DOI] [PubMed] [Google Scholar]
- 18.Albert T., Mautner J., Funk J.O., Hortnagel K., Pullner A., Eick D. Nucleosomal structures of c-myc promoters with transcriptionally engaged RNA polymerase II. Mol. Cell. Biol. 1997;17:4363–4371. doi: 10.1128/mcb.17.8.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartolomei M.S., Halden N.F., Cullen C.R., Corden J.L. Genetic analysis of the repetitive carboxyl-terminal domain of the largest subunit of mouse RNA polymerase II. Mol. Cell. Biol. 1988;8:330–339. doi: 10.1128/mcb.8.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H., Flores O., Weinmann R., Reinberg D. The nonphosphorylated form of RNA polymerase II preferentially associates with the preinitiation complex. Proc. Natl Acad. Sci. USA. 1991;88:10004–10008. doi: 10.1073/pnas.88.22.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strobl L.J., Kohlhuber F., Mautner J., Polack A., Eick D. Absence of a paused transcription complex from the c-myc P2 promoter of the translocation chromosome in Burkitt's lymphoma cells: implication for the c-myc P1/P2 promoter shift. Oncogene. 1993;8:1437–1447. [PubMed] [Google Scholar]
- 22.Eick D., Bornkamm G.W. Expression of normal and translocated c-myc alleles in Burkitt's lymphoma cells: evidence for different regulation. EMBO J. 1989;8:1965–1972. doi: 10.1002/j.1460-2075.1989.tb03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman R.D., Conrad M., Eick D. The role of mammalian RNA polymerase II CTD non-consensus repeats in CTD stability and cell proliferation. Mol. Cell. Biol. 2005;25:7665–7674. doi: 10.1128/MCB.25.17.7665-7674.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hieda M., Winstanley H., Maini P., Iborra F.J., Cook P.R. Different populations of RNA polymerase II in living mammalian cells. Chromosome Res. 2005;13:135–144. doi: 10.1007/s10577-005-7720-1. [DOI] [PubMed] [Google Scholar]
- 25.Kimura H., Sugaya K., Cook P.R. The transcription cycle of RNA polymerase II in living cells. J. Cell. Biol. 2002;159:777–782. doi: 10.1083/jcb.200206019. [DOI] [PMC free article] [PubMed] [Google Scholar]