Abstract
Pathogenic variants in the LRRK2 gene affecting catalytic domains are the most common genetic cause of Parkinson’s disease (PD). Nevertheless, LRRK2 variants at the armadillo (ARM) domain would indirectly affect the protein’s activity by interacting with RAB proteins. We present a family with PD recurrence segregating the new LRRK2 allele at the ARM domain, p.[Leu.119Pro;Leu488Pro]. Clinical exams were conducted on nine relatives. Neuropathology of the index case showed loss of substantia nigra neurons and Alzheimer’s disease-type lesions. In silico analysis of the p.[Leu.119Pro;Leu488Pro] LRRK2 variant predicted alterations in ARM tertiary structure and binding affinity. These predictions were supported by functional genomics using recombinant LRRK2WT and LRRK2Leu119Pro;Leu488Pro. We found increased interaction between LRRK2Leu119Pro;Leu488Pro and RAB8A, but not with RAB10. Additionally, docking studies revealed stronger affinity of LRRK2Leu119Pro;Leu488Pro for RAB8A (P < 0.0001) and allosteric properties beyond the mutated residues. We propose p.[Leu119Pro;Leu488Pro] as a cause of familial PD.
Subject terms: Computational biology and bioinformatics, Genetics, Diseases, Neurology
Introduction
Pathogenic missense variants in the leucine-rich repeat kinase 2 (LRRK2) gene are the most common genetic cause of familial and sporadic Parkinson’s disease (PD)1. Most PD-causing LRRK2 variations are clustered in the kinase and Roc-COR enzymatic domains. All these variants increase the LRRK2 kinase activity either directly or by compromising the GTPase function2. In addition, genetic variants of LRRK2 affecting scaffold domains of the protein have been identified as risk or protective factors for PD in different populations3.
The most well-validated physiological LRRK2 substrates are a subset of RAB proteins, which are small GTPases that regulate the secretory and endocytic pathways4,5. LRRK2 interacts with and phosphorylates RAB proteins through its armadillo (ARM) domain to regulate their activities, localisation, and effector binding6,7. In turn, RAB proteins phosphorylated by LRRK2 bind to its ARM domain, creating a feed-forward pathway that provides spatial control and activates LRRK2 kinase activity8. Therefore, LRRK2 missense variants located at the ARM domain would indirectly affect the kinase activity of the protein9,10.
Here, we present a family with a high recurrence of PD and the new LRRK2 variant p.[Leu119Pro;Leu488Pro] segregating with the disease, which is located in the ARM domain. This multidisciplinary study includes the family’s clinical, pathological, and genetic characterisation, as well as computational and experimental investigations to validate the impact of p.[Leu119Pro;Leu488Pro]. We provide evidence of the pathogenicity of this novel LRRK2 allele using both cellular models and docking studies, which suggested that p.[Leu119Pro;Leu488Pro] increases LRRK2 binding to RAB8A.
Results
Clinical and genetic findings
The index case (II-6) had a substantial family history with the father affected by PD and, later on, two brothers and a nephew (Fig. 1a). She was 57 years old when PD was diagnosed. Resting tremor (RT) in her right limbs was the first symptom. She had a good response to levodopa-carbidopa (LDCD), but seven years later, she developed dyskinesias and motor fluctuations. She does not have orthostatic hypotension or early urinary problems. Because oral medication could not control levodopa-induced complications, LDCD intestinal gel infusion (DUODOPA®, AbbVie) was started when she was 79 years old. At that time, off-medication UPDRS part III scored 38 with a Hoehn–Yahr (H&Y) stage of 3. This treatment resulted in much improvement in her quality of life. At 83, cognitive assessment using the SCales for Outcomes in Parkinson’s disease-COGnition (SCOPA-COG) scored 21/43 (score range: 0–43, higher scores reflecting better performance). Cognitive impairment progressed until she died at 86.
Fig. 1. Family tree, neuropathology and molecular findings of the PD family related to the novel LRRK2 allele p.[Leu119Pro; Leu488Pro] and GBA1 deletion.
a The family tree of the patients depicts the family history of PD. Squares symbolise males and circles females. All filled squares/circles indicate affected members, and the line above squares/circles indicates neurological assessment. LRRK2 and GBA genotypes are indicated. Age at diagnosis and current age are below the genotypes. The asterisk indicates an obligatory carrier (individual II.3). b Neuropathology of the index case: substantia nigra showed no Lewy bodies or neurites after immunostained with α-alpha-Synuclein (left). Hippocampus neurofibrillary tangles and neuropilic threads after immunostaining with α-Tau (AT 100) (right). Images were taken from optical sections that are representative of the group averages. Scale bar: 100 µm. c LRRK2 domains structure and the location of p.[Leu119Pro;Leu488Pro]. ARM: armadillo repeats, ANK: ankyrin repeats, LRR: leucine-rich repeats, Roc: Ras of complex proteins, COR: C-terminal of ROC, MAPKKK: kinase domain, WD40: WD40 repeats. d Sanger sequencing LRRK2 p.[Leu119Pro; Leu488Pro] chromatogram. Right: LRRK2 p.Leu488Pro/c.1463 T > C variant and left LRRK2 p.Leu119Pro/c.356 T > C variant. e GBA1 long PCR amplicons showing the deletion in the carriers.
Her brain was donated to the Hospital Fundación Alcorcon Brain Bank. Moderate cortical atrophy was observed at autopsy, being more intense in the middle gyrus of the frontal lobe and the operculum. The ventricular system showed mild dilatation in coronal sections of the cerebrum. No other cerebral alterations were observed except for a sieve-like state in the white matter and the basal ganglia. There was pallor of the substantia nigra (SN) and the locus ceruleus (LC) (Fig. S1a). Axial sections of the brainstem showed mild atrophy. The cerebellum showed no abnormality.
The main microscopic findings were observed in the SN with substantial loss of dopaminergic neurons and gliosis (Fig. S1a). Neuronal loss in the locus coeruleus was less intense. Intense astrocytosis was observed in the rest of the subcortical grey nuclei. Lewy bodies were not observed in any part of the brain. There was slight transcortical gliosis in cortical grey matter and patchy laminar microvacuolization in cortical layers II and III. Inmunohistochemistry using α-AT100 and α-beta-amyloide (Aβ) showed Alzheimer's Disease Neuropathologic Changes, with neurofibrillary tangles (NFTs) and senile plaques ranked in A2, B2, C2 score11 (Figs. 1b and S1b, c). NFTs were noticed in the entorhinal cortex, closely related areas, and the hippocampus, corresponding to Braak stages III/IV. In addition, the hippocampus showed patchy neuronal loss, slight gliosis, mixed 3R and 4R Tau, granule-vacuolar degeneration and Hirano bodies. There were no immunoreactive inclusions with α-TAR-DNA-binding protein (TDP-43) or α-synuclein. These neuropathological findings force us to rule out a genetic PD.
Genetic analysis of the patient (II-6) using a custom NGS targeted panel detected two heterozygous rare single-nucleotide variants (SNVs) in the LRRK2 gene (Tables 1 and S1). The variants were aminoacidic non-conservative changes of leucine by proline at positions 119 (c.356 T > C/p.Leu119Pro, rs33995463) and 488 (c.1463 T > C/p.Leu488Pro, novel variant) (Fig. 1c). The 2 SNVs were confirmed by Sanger sequencing (Fig. 1d). p.Leu119Pro and p.Leu488Pro are located at the ARM domain (IPR016024) in evolutionary conserve positions (Fig. S2). These SNVs are rare in all populations (Table S2). To assess the frequency of these changes in populations of PD patients, we used data from the Accelerating Medicines Partnership Parkinson’s Disease (AMP-PD) group12. We found that p.Leu119Pro occurred with a frequency of 0.59 (17 alleles out of 2835), while p.Leu488Pro was not present in this population. Additionally, conflicting interpretations of pathogenicity were obtained using different baseline criteria (Tables S2 and S3 and Fig. S3). Due to the patient’s neuropathological findings, we interrogated genes for disorders with Tau pathology using a clinical exome sequencing (CES) panel (Table S4). No pathogenic variants were found in any of the genes.
Table 1.
Clinical and genotype data from affected and unaffected members of the Parkinson’s family
| FAMILY MEMBER | AGE AT DIAGNOSIS | YEARS OF EVOLUTION | RT | MF | D | PS | CI | NI | GENETIC FINDINGS |
|---|---|---|---|---|---|---|---|---|---|
| II.6 (†) | 57 | 29 | Yes | Yes | Yes | Yes& | Mild dementia |
DaTSCANΩ MRI: normal |
LRRK2: p.[Leu119Pro; p.Leu488Pro] GBA1: del exon4-exon 11 |
| II.3 (†) | 73 | 14 | Yes | No | No | – | Dementia | – | * |
| II.7 | NA | NA | No | No | No | No | NA | – | None |
| II.8 | 75 | 11 | Yes | No | No | No | No | DaTSCANΩ | LRRK2: p.[Leu119Pro; p.Leu488Pro] |
| III.4 | 57 | 7 | Yes | Yes | Yes | Yes | Mild Cognitive Impairment |
DaTSCANΩ MRIΩ |
LRRK2: p.[Leu119Pro; p.Leu488Pro] GBA1: del exon4-exon 11 |
| III.1 | NA | NA | No | No | No | No | NA | – | None |
| III.6 | NA | NA | No | No | No | No | NA | – | GBA1: del exon4-exon 11 |
| III.9 | NA | NA | No | No | No | No | NA | – |
LRRK2: p.[Leu119Pro; p.Leu488Pro] GBA1: del exon4-exon 11 |
| III.10 | NA | NA | No | No | No | No | NA | – |
LRRK2: p.[Leu119Pro; p.Leu488Pro] GBA1: del exon4-exon 11 |
NA not applicable, RT resting tremor, MF motor fluctuation, D Dyskinesias, PS psychotic symptoms, NI neuroimaging studies, DaTScan single photon emission computed tomography with Ioflupane I123 injection, DaTScanΩ showed a decrease in the uptake of the radiotracer in the putamen, MRI magnetic resonance imaging, MRIΩ small hyperintense lesions in white matter
&After pharmacological treatment CI cognitive impairment
*Obligatory carrier of LRRK2: p.[Leu.119Pro; p.Leu488Pro] and GBA: del exon4-exon 11
The index case nephew (III-4) was 57-years-old when RT in his right limb started. PD was diagnosed with an excellent response to LDCD, but dyskinesias and motor fluctuations developed four years after the diagnosis. When we saw the patient, off-medication UPDRS part III scored 40, and he had a stage 3 of H&Y. Because of uncontrolled motor complications, he was treated with deep brain stimulation in both subthalamic nuclei. Dyskinesia and motor fluctuations improved greatly, but dysarthria and gait freezing did not. Mild cognitive impairment was present since he was 62-years-old. Genetic analysis revealed that this patient was a carrier of both variants in LRRK2. The index’s brother (II-8) developed PD at an older age and did not experience severe motor complications or cognitive decline.
As we found evidence of a β-Glucocerebrosidase (GBA1) gene gross deletion in II-6 and III-4 patients’ samples, we amplified the entire GBA1 gene using long-PCR as previously described. Both patients had a two-band pattern corresponding to the GBA1 gene (8 Kb) and a smaller band of 6 Kb. Patient (II-8) did not carry the GBA1 deletion (Fig. 1e).
Sanger sequencing of the smaller band revealed a recombination event of GBA1 with its pseudogene GBAP1. In addition, we located the 5’ recombination point by Sanger sequencing; the breaking point localised at the intron 2/10 of the GBA1 gene in a thymine repeat area (Fig. S4). Since exon 11 of the GBA1 and GBAP1 genes are nearly identical, we could not detect the downstream recombination point. Nevertheless, we identified GBAP1 exon 11 and the rs421016, rs368060 and rs1135675 SNVs in exon ten found in GBAP1 (Fig. S4).
Genetic testing by Sanger sequencing was performed after written informed consent in 6 additional relatives of the PD index case. They were patient II-8, four at-risk asymptomatic individuals (children III-9, 59-years-old, and III-10, 55-years-old), and the brother’s children (III-1, 66-years-old, and III-6, 55-years-old), and her unaffected husband (II-7). Table 1 shows clinical features and the genetic results. The affected brother (II-8) carried the LRRK2 SNVs. The two probands’ children were carriers of the LRRK2 SNVs and the GBA1 deletion. This segregation analysis confirmed that p.[Leu119Pro; Leu488Pro] are in cis. On the other hand, the two brothers’ children were not carriers of LRRK2 SNVs, but did carry other variants, including GBA1 deletion in III-1. Thus, genetic testing showed that all affected relatives were carriers of p.[Leu119Pro;Leu488Pro], while some asymptomatic relatives carried all or one of the rare variants found in the index case. These findings suggest that p.[Leu119Pro; Leu488Pro] in LRRK2 is not fully penetrant in this family, at least in individuals younger than 60.
In silico and in vitro studies
Our in silico studies, based on the experimental structure of LRRK2 (PDB:8FO2), indicate that the p.[Leu119Pro; Leu488Pro] variant alters the secondary structure of LRRK2 (Fig. S3a). Using various tools of PyMOL (version 1.8)13, the analysis revealed slight differences in structural flexibility, leading to reduced atomic mobility (greater rigidity) and slight changes in the electrostatic potential surfaces between LRRK2WT and LRRK2Leu119Pro;Leu488Pro (Fig. S3b, c). DynaMut214 and DUET evaluated the impact of mutations on protein stability, predicted considerable deleterious effects for the p.[Leu119Pro;Leu488Pro] variant, with notable changes in folding free energy (ΔΔG Leu119-Leu488 → Pro119-Pro488 STABILITY: −1.89 Kcal/mol and −1.39 Kcal/mol, respectively) and increased protein instability (Fig. S3d). Additionally, Missense3D predicted structural damage due to introducing a buried proline and the buried/exposed switch, where the substitution caused a shift between the buried and exposed states of the target residue (Fig. S3d). SPPIDER further predicted the creation of new binding sites near position 119 and the loss of binding sites near position 488 in LRRK2Leu119Pro;Leu488Pro (Fig. S3e). Collectively, these in silico findings, combined with the low frequency of these SNVs would support the notion that the p.[Leu119Pro;Leu488Pro] allele is pathogenic.
We then performed experimental studies by overexpressing in HEK293 cells pDEST53-LRRK2WT and pDEST53-LRRK2Leu119Pro;Leu488Pro constructs (obtained by mutagenesis of the former). The two constructs express the LRRK2 open reading frame in fusion with the green fluorescence protein (GFP). We observed that recombinant LRRK2 localised in the cytoplasm homogeneously or formed aggregates. We found no allele-dependent differences in the expression pattern of the recombinant proteins (Fig. 2a). Since the ARM domain of LRRK2 interacts with RAB proteins15, we studied the effect of p.[Leu119Pro; Leu488Pro] upon this interaction. RAB10 and RAB8A were selected as candidates because they are well-characterised and there is evidence of their interaction with the ARM domain of LRRK28. Using proximity ligation assay (PLA), we quantified the interaction between recombinant LRRK2WT or LRRK2Leu119Pro;Leu488Pro with endogenous RAB10 or RAB8A. PLAs results revealed no differences between LRRK2WT or LRRK2Leu119Pro;Leu488Pro in their interaction with RAB10 (P = 0.4192) (Fig. 2b). When we tested the interaction with RAB8A, we found a significant increase in the number of interactions per cell for LRRK2Leu119Pro;Leu488Pro and RAB8A compared to LRRK2WT (P < 0.0001) (Fig. 2c). These results suggest that the LRRK2 variant p.[Leu119Pro;Leu488Pro] has differential effects on its substrates RAB8A and RAB10.
Fig. 2. Functional genomics study of recombinant LRRK2WT and LRRK2Leu119Pro;Leu488Pro shows increased binding to RAB8A.
a Representative images of recombinant LRRK2 proteins in fusion with GFP (left) and signal quantification for cytosolic or aggregate patterns. LRRK2WT: 70.78 ± 5.11 cytosolic and 29.22 ± 5.11 aggregate vs LRRK2Leu119Pro;Leu488Pro 70.58 ± 9.17 cytosolic and 29.42 ± 9.17 aggregate (Four independent experiments, at least 30 cells). Cochran–Mantel–Haenszel χ² test: P-value = 0.9975 b Representative images of proximity ligation assays using α-GFP (LRRK2WT and LRRK2Leu119Pro;Leu488Pro) and α-RAB10. On the right is the quantification of the interaction number of LRRK2-RAB10 (dots) per cell. c Representative images of proximity ligation assays using α-GFP (LRRK2WT and LRRK2Leu119Pro;Leu488Pro) and α-RAB8A. On the right, quantification of the interaction number of LRRK2-RAB8A (dots) per cell. b, c The box plot lines correspond from the bottom of the box to the top: 25th percentile, median, 75th percentile. The whiskers extend to the minimum and maximum values. Three independent experiments with four different images for each experiment. Shapiro test followed by Mann–Whitney U-test: ns not significant, ***P-value < 0.001.
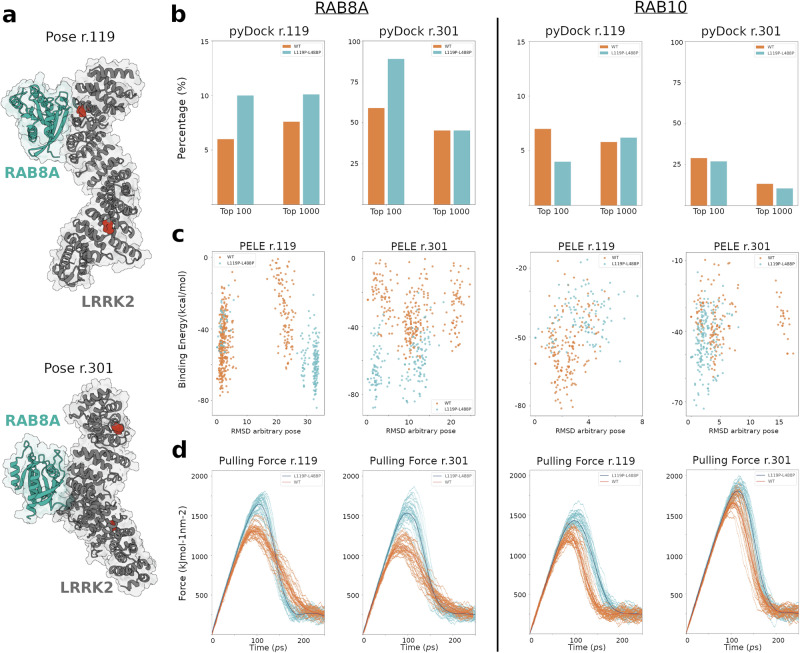
p.[Leu119Pro; Leu488Pro] increases potential docking poses and binding affinity between LRRK2 and RAB8A
To further investigate the effect of p.[Leu119Pro; Leu488Pro] on LRRK2 and its binding affinity for RAB8A and RAB10, we conducted a docking study. We first used Masif to compute, for each residue at the LRRK2 surface, its probability of being part of a protein–protein interface. For residue 119 of LRRK2, we found that Leu had a lower probability score than Pro (0.41 vs 0.55, respectively). At residue 488, we found a low score for both Leu and Pro (0.24 vs 0.20, respectively), suggesting that this residue does not participate in the LRRK2-RAB8A/RAB10 complex interface.
To gain more insight into the protein–protein interface of LRRK2 in the region affected by p.[Leu119Pro;Leu488Pro] we used PyDock. We focused on the top 100 and 1000 pyDock poses to minimise the bias of false-positive docking poses. When evaluating the docking results for LRRK2-RAB8A (structure representation of the top energetic pose of the complex in Fig. 3a), the poses close to residue 119 showed increased number of docking poses for LRRK2Leu119Pro;Leu488Pro (10/100 and 101/1000) compared to LRRK2WT (6/100 and 76/1000) (Fig. 3b, left). No pose was found close to residue 488 for either LRRK2WT or LRRK2Leu119Pro;Leu488Pro. Importantly, we observe larger number of docking poses close to residue 301 (residue at top pyDock pose) for LRRK2Leu119Pro;Leu488Pro (89/100 and 453/1000) compared to LRRK2WT (59/100 and 453/1000), suggesting a potential allosteric effect on the LRRK2 structure in regions beyond the mutated residues. In this regard, the Masif probability score at residue 301 increased from 0.42 in LRRK2WT to 0.48 in LRRK2 Leu119Pro;Leu488Pro. Notably, the number of pyDock poses close to residues 119 and 301 for the LRRK2–RAB10 system was systematically lower, proposing a lower affinity of RAB10 in those two regions (Fig. 3b, right).
Fig. 3. Comparison of the effect of p.[Leu119Pro;Leu488Pro] on the interaction affinity of LRRK2-RAB8A or LRRK2-RAB10.
a Structural representation of the top energetic pose of the complex between LRRK2 (grey) and RAB8A (cyan), close to LRRK2 residues 119 (TOP) and 301-top pyDock (BOTTOM). The red dots highlight p.Leu119Pro and p.Leu488Pro LRRK2 SNVs. b Percentage of poses obtained from pyDock regarding RAB8A (left) and RAB10 (right) close to LRRK2 residues 119 and 301, for LRRK2WT (orange) and LRRK2Leu119Pro;Leu488Pro (blue). c Binding energy profiles obtained from PELE for poses regarding RAB8A (left) and RAB10 (right) close to LRRK2 residues 119 and 301 for LRRK2WT (orange) and LRRK2Leu119Pro;Leu488Pro (blue). d Steering MD results representing the pulling force for the LRRK2-RAB complex regarding RAB8A (left) and RAB10 (right) close to LRRK2 residues 119 and 301, for LRRK2WT (orange) and LRRK2Leu119Pro;Leu488Pro (blue).
To optimise the complex poses around LRRK2 residues 119 and 301 and obtain an energetic binding energy score, we used PELE16. For the pose close to residue 119, we found that LRRK2 Leu119Pro;Leu488Pro had a lower binding energy to RAB8A (more affinity) compared to LRRK2WT (−84.1 kcal/mol vs −77.2 kcal/mol, respectively). This difference was even more marked at residue 301, in which LRRK2Leu119Pro;Leu488Pro presented the lowest binding energy of −87.8 kcal/mol, compared to the -65 kcal/mol of LRRK2WT pose (Fig. 3c, left). These results suggest that p.[Leu119Pro;Leu488Pro] has an effect of increased interaction both locally (at residue 119) and allosterically (at residue 301). We did not explore the pose close to residue 488 due to no available pose within the top 1000 pyDock poses. In contrast, LRRK2 Leu119Pro;Leu488Pro-RAB10 showed higher binding energy (lower affinity) for the pose close to residue 119, compared to LRRK2WT. We noticed that for the pose close to residue 301, the LRRK2 Leu119Pro;Leu488Pro–RAB10 system showed lower binding energy. Nevertheless, we hypothesise that this difference would be negligible, given the low number of poses identified in the pyDock analysis.
We then used steered molecular dynamics to capture how p.[Leu119Pro;Leu488Pro] affect the force required to break the binding between LRRK2 and RAB8A (Fig. 3d, left). The average peak force to separate RAB8A from LRRK2 Leu119Pro;Leu488Pro was ~1600 kJ/mol nm2, which is higher than for LRRK2WT ( ~ 1250 kJ/mol nm2) close to residue 119. Similarly, the peak dissociation force at the position near residue 301 was higher for LRRK2 Leu119Pro;Leu488Pro (~1500 kJ/mol nm2) compared to LRRK2WT (~1100 kJ/mol nm2). These results further verify a stronger affinity between LRRK2 Leu119Pro;Leu488Pro and RAB8A, compared to LRRK2WT. RAB10 analyses showed an increase in the pulling force required for LRRK2Leu119Pro;Leu488Pro compared to LRRK2WT at the pyDock pose near residue 119 (difference of 350 kJ/mol nm2 for RAB8A vs ~300 kJ/mol nm2 for RAB10). However, this difference was marginal at the pyDock energy maxima position (Fig. 3c, right). Despite the force differences at residue 119, we consider that it will have a residual impact on the overall binding of LRRK2–RAB10, given the low number of PyDock poses.
Discussion
This study identified the novel LRRK2 variant allele p.[Leu119Pro;Leu488Pro], which affects the ARM domain, in a family with autosomal dominant PD. We further provided evidence of how this variant, located far from the catalytic domains of LRRK2, may cause the disease.
The pathophysiology of PD caused by LRRK2 variants in the catalytic domains is attributed to a gain of kinase function leading to hyperphosphorylation of substrates2. More recently, LRRK2 variants located at the scaffold ARM domain have been reported to cause an increase in LRRK2 kinase activity as well9,17. Indeed, the LRRK2 ARM domain is involved in the dimeric structure of the holoenzyme, which is needed for its kinase activity18. Therefore, evidence indicates that the LRRK2 ARM domain contributes to its kinase activity, and mutations in this domain may increase the potentially toxic function of the mutated protein. Regarding p.[Leu119Pro;Leu488Pro], this variant consists of two Leu-to-Pro substitutions at conserved positions of the ARM domain of LRRK2 that could affect the tertiary structure and change the binding affinity of LRRK2 and its partners. Our experimental results with recombinant LRRK2WT and LRRK2Leu119Pro;Leu488Pro proteins supported these predictions, indicating significant differences in their interactions with RAB8A, although this did not occur with RAB10. It could be proposed that p.[Leu119Pro;Leu488Pro] may specifically increase the interaction between LRRK2 and RAB8A, or affect the phosphorylation levels of RAB8A. Previous research has reported LRRK2 variant differences upon phosphorylation of RAB10 vs RAB12, suggesting pathophysiological variations among LRRK2 variants19.
RAB8A is linked to PINK120 and α-Synuclein21, PD-related proteins involved in dysfunctional mitochondrial dynamics and mitophagy. On the lysosomal side, although both RAB8A and RAB10 respond to lysosomal damage22–24, very recently these RAB proteins have been shown to have opposite roles in lysosomal biology25. These functional differences between RAB8A and RAB10 could be related to the increased interaction of LRRK2Leu119Pro;Leu488Pro with RAB8A and not RAB10.
From a three-dimensional viewpoint, our docking studies revealed a substantial increment in the affinity of LRRK2 ARM domain for RAB8A in the presence of p.[Leu119Pro; Leu488Pro] (P < 0.0001). This change in the affinity is attributed to both mutations together; however, the differential local effects were observed exclusively at residue 119. Of note, the allele LRRK2:p.Leu119Pro is overrepresented in Spanish patient cohorts and absent in both a Spanish control population26 and a clinical series of 105 early-onset PD patients (unpublished results). Furthermore, p.[Leu119Pro; Leu488Pro] may exhibit allosteric properties that influence LRRK2 structure in areas beyond the mutated residues, suggesting that this double variant allele could have a more pronounced effect on LRRK2 than p.Leu119Pro alone. Overall, our docking findings show that LRRK2Leu119Pro;Leu488Pro has a higher affinity for RAB8A, which may lead to a gain of function in LRRK2 and contribute to the disease expression. Moreover, our computational results also highlight the lack of effect on RAB10, which agrees with our PLA analyses.
We also found that the index case was heterozygous for a previously reported GBA1 gross deletion27 that we detected in II-6 and III-4 patients. This heterozygous GBA1 deletion may impact clinical progression, as carriers of the GBA1-deleted allele frequently exhibit cognitive impairment. In contrast, patient II-8, who is not a carrier of this variant, showed a milder disease progression. It is important to note that we found unaffected relatives under the age of 60 who carry p.[Leu119Pro; Leu488Pro]. They are the children of the index case whose current clinical examination reveals no considerable findings. Given the age-related penetrance of other LRRK2 variants, we propose that these would be asymptomatic carriers from whom we should follow up clinically. Finally, we found that Lewy bodies were absent in the index case, while AD-type tau lesions and amyloid plaques were present. This finding aligns with LRRK2-related pathology. On the one hand, 21–54% of PD cases with variants in LRRK2 do not present Lewy bodies; on the other hand, prominent AD-type tau pathology is observed in most of the brains of LRRK2-PD cases28.
A limitation of our study is the relatively small size of the family and the limited number of available meioses to statistically evaluate the pathogenicity of the LRRK2 and GBA1 variants within this family. Consequently, we could not reach sufficient LOD score values to confirm the linkage between PD and the LRRK2 variant allele. Nevertheless, we performed a comprehensive study combining clinical, genetic, functional genomics, and computational analyses. These approaches suggest the pathogenicity of p.[Leu119Pro;Leu488Pro]. Additionally, we focused our computational and functional studies on evaluating the differences between LRRK2WT and LRRK2Leu119Pro;Leu488Pro proteins. The biology of RAB proteins and their biochemical pathways has been studied in relation to their interaction with LRRK2. Further biochemical analysis of RAB proteins requires additional experiments. Still, this study deserves attention for its focus on rare variants affecting the ARM region of LRRK2 and their differential impact on interactions with RAB proteins.
In summary, we have identified and studied the novel LRRK2 variant p.[Leu119Pro; Leu488Pro], which we propose as a cause of familial PD. Further studies about the impact of other LRRK2 ARM domain disease-causing variants are of major interest now and will also be for therapeutic decision-making in precision medicine.
Methods
Clinical and neuropathological exams
We studied a Spanish Caucasian family with a known history of Parkinsonism in five members from three generations, segregating in an autosomal dominant manner. Neurological examination was performed in the Movement Disorders Clinic at the Hospital Fundacion Alcorcon in Madrid (Spain). Diagnosis of PD was made based on London Brain Bank criteria29. Clinical examinations of PD patients included the Unified Parkinson’s Disease Rating Scale (UPDRS)30, Hoehn & Yahr scale31 and cognitive assessment according to the Movement Disorders Society Task Force on PD dementia32. The study received approval from the Hospital’s Ethics Committees, and informed consent was obtained from all participants whose genomic DNA was analysed (PIC-217-19).
Neuropathological autopsy and microscopy studies of the index case were performed in formalin-fixed and paraffin-embedded blocks of the cerebral cortex and white matter, basal ganglia, midbrain, brain stem, and cerebellar cortex. Immunostaining of 4 μm-thick tissue sections was performed and counterstained lightly with Carazzi’s hematoxylin. The following primary antibodies were used: α-GFAP rabbit polyclonal (1:1000; DAKO), α-Tau AT100 mouse monoclonal (1:100; Invitrogen), α-alpha-Synuclein mouse monoclonal (1:40; Novocastra), α-beta-Amyloid mouse monoclonal (1:40; DAKO), α-Neurofilament mouse monoclonal (1:2000; Sternberger), α-Tau-3-repeat isoform RD3(1:3000; Millipore) and α-Tau-4 repeat isoform RD4 (1:100; Millipore). Primary antibody binding was detected by the labelled streptavidin-biotin method (DAKO; EnVision +System-HRP Labelled Polymer α-mouse) following the manufacturer’s protocols.
Next-generation sequencing (NGS) panels and multiplex ligation-dependent probe amplification (MLPA) analysis
We designed a targeted NGS panel including the coding regions of 63 genes of PD, atypical Parkinsonism, Mendelian conditions that can cause Parkinsonism, and risk factors based on the evidence curated in the Mendelian Inheritance in Man (MIM) and the Movement Disorders Society Taskforce gene list33 (Table S1).
CES was performed on the index case. Based on fragmentation and capture, exonic regions were enriched using Agilent® Technologies (Santa Clara, CA, USA). Agilent Technologies' CES panel includes 5833 genes in total. This step was needed to discard gene variants related to tau pathologies34 (Table S4). The libraries obtained with the Nextera Rapid Captura Enrichment Kit (Illumina, San Diego, CA) were loaded on an Illumina MiniSeq system (Illumina) according to the manufacturer’s instructions.
Bioinformatics analysis of NGS data was conducted using a homemade pipeline developed at the Bioinformatics Unit of the Molecular Genetics Department at the Sant Joan de Déu Hospital (Barcelona, Spain) as described previously35. All variants selected (MAF < 1%) were confirmed by Sanger sequencing and categorised according to the international guidelines of the American College of Medical Genetics and Genomics (ACMG)36.
MLPA was used to identify copy number variations (CNVs) using the kit P051-D1 (MRC-Holland, Amsterdam, The Netherlands).
GBA1 deletion study
A GBA1 CNV study was performed using long PCR, as previously described37. Amplicons were visualised in a 1% agarose gel and purified using the PureLink™ PCR Purification Kit (ThermoFisher, Waltham, MA, USA) following the manufacturer’s protocol. The recovered DNA was Sanger sequenced.
Constructions of LRRK2 expression plasmids
The pDEST53-LRRK2WT plasmid was a gift from the Mark Cookson Lab (#25044, Addgene). To generate the pDEST53-LRRK2Leu119Pro;Leu488Pro plasmid, the fragment of interest was excised from pDEST53-LRRK2WT, cutting with XbaI (Thermo Fisher Scientific) and XhoI (Thermo Fisher Scientific) restriction enzymes. This fragment was cloned using T4 DNA ligase (Thermo Fisher Scientific) in the pBlueScript II SK plasmid (#212205, Addgene) previously digested with the same enzymes. Afterwards, we performed the double site-directed mutagenesis of the recombinant pBlueScript II SK by using the Q5® Site-Directed Mutagenesis kit (New England Biolabs). After each mutagenesis, Sanger sequencing with specific primers of the recombinant pBlueScript II SK mutant was needed to check the nucleotide change. The double mutant fragment containing the c.356 T > C and c.1463 T > C (for p.[Leu.119Pro; Leu488Pro]) nucleotide changes was excised with XbaI and XhoI and subcloned in pDEST53-LRRK2WT previously cut with the same enzymes. After ligation and selection, Sanger sequencing was carried out to check the LRRK2 open reading frame (ORF) sequence.
Functional impact study of the new LRRK2 allele
Transient transfection of constructs in the HEK293 cell line was performed using FuGENE® HD (Promega). After, cells were incubated at 37 °C for 48 h. The study of recombinant LRRK2 expression was in fixed cells with 4% paraformaldehyde and mounting medium Fluoromount-G with DAPI (4’,6-diamidino-2-phenylindole) (Thermo Fisher Scientific). To evaluate recombinant LRRK2 interactions with endogenous RAB8A and RAB10, we used Protein Ligation Assay (PLA, Duolink® In situ Detection Red Starter [Mouse/Rabbit] Kit, Sigma-Aldrich) and the following antibodies: mouse α-GFP (1:500; #ab1218), rabbit α-GFP (1:500; #ab6556), rabbit α-RAB8A (1:100; #ab188574) and mouse α-RAB10 (1:100; #SAB5300028).
We acquired the images using a Leica TCS SP8 X White Light Laser confocal microscope using a 63× oil immersion objective. Z-stacks were acquired every 0.1 µm along with the cell thickness. Image analysis was performed using maximum intensity projection in Image J/Fiji software (NIH, U.S.A. National Institutes of Health, Bethesda, MD, United States).
In silico protein structure preparation
We used PDB 5SZI for RAB8A and PDB 5LPN for RAB10, and computed the homology model of the LRRK2 ARM region (residues 12–650) using PDB 8FO2 as a template and the SWISS Homology model tool (https://swissmodel.expasy.org/). To obtain the LRRK2Leu119Pro-Leu488Pro variant structure, we first mutated the two residues using Maestro (Schrödinger Inc.) and equilibrated the system with a short molecular dynamics simulation. Briefly, using Gromacs v2023.3138 and the AMBER99sb force field, we solvated a periodic cubic box consisting of TIP3 water model and Na+ and Cl− ions at 0.1 M/L. Then, we ran a steepest descent minimisation followed by 200 pico-seconds of constant number-volume-temperature (NVT) equilibration step of the water solvent, both with the LRRK2-ARM domain and ions restrained. Afterwards, to heat the system to 300 K, we ran a series of 10–50 ps of constant number-pressure-temperature (NPT) MD simulations using different LRRK2 residues constraints, with force constants of 1000 kJ/mol nm2, 550 kJ/mol nm2, 300 kJ/mol nm2, 170 kJ/mol nm2, 90 kJ/mol nm2, 50 kJ/mol nm2, 30 kJ/mol nm2, 15 kJ/mol nm2, 10 kJ/mol nm2, and 5 kJ/mol nm2. We then set up a production MD of 60 ns, using a 2 fs time step (300 K, 1 bar, particle-mesh Ewald for electrostatic interactions). The last snapshot of the simulation was used as the reference structure for the LRRK2Leu119Pro;Leu488Pro ARM region of the protein. Importantly, such short molecular dynamics allow accommodating non-local structural changes that might result from the p.Leu119Pro and p.Leu488Pro, changing the affinity and docking profiles along the ARM region, and not only locally.
Effect of p.[Leu119Pro;Leu488Pro] on the docking between LRRK2 and RAB
We ran two orthogonal analyses to measure the effect of both p.Leu119Pro and p.Leu488Pro on the docking between LRRK2 and RAB8A or RAB10. We used the Masif site39, on the LRRK2-ARM structure, to generate a surface mesh, and compute its vertex-wise probability score that summarises the potential to interact with any other protein. We compare LRRK2WT and LRRK2Leu119Pro;Leu488Pro scores for the vertices around residues 119 and 488 (5 Ams radius). Additionally, we used pyDock40,41, a rigid docking software that generates around 92000 LRRK2-RAB complex poses (for both LRRK2WT and LRRK2Leu119Pro;Leu488Pro). We scored their binding affinity using a coarse-grained energy function. We then compared the number of LRRK2-RAB complexes within the top 100 and top 1000 energy scores, that are close (radius of 5A) to residue 119, residue 488, and residue 301 (top pyDock pose). This approach has previously shown that mutations at the interface and their effect on the number of top poses predicted by pyDock are related to missense variants41. Importantly, we run the same docking protocol between LRKK2 and RAB29 to compare its performance against a known crystal structure (PDB: 8ORF2). Among the top 60 pyDock energy-ranked conformations, we identified a pose with a DockQ score of 0.62, which is classified as medium quality according to CAPRI criteria. The ligand RMSD for this pose was 1.66 Å compared to the 8ORF2 PDB complex (Fig. S5), suggesting the reliability of pyDock for LRKK2-RAB systems.
Effect of p.[Leu119Pro;Leu488Pro] on interaction affinity between LRRK2 and RAB
To study interaction affinity, we first ran PELE16, a heuristic MonteCarlo (MC) sampling method to characterise intermolecular interactions. Each MC step involves multiple events, including protein backbone perturbation (using anisotropic network models), side chain sampling and minimisation, all using the OPLS2005 force field. In brief, we selected all pyDock poses close to residue 119 and residue 301 (top pyDock pose) for both LRRK2WT and LRRK2Leu119Pro;Leu488Pro. We excluded residue 488 for further analyses, given the low score from Masif and the low number of top 100 poses from pyDock. Then, we used PROPKA 3.0 to calculate the protonation state of titratable residues at pH 7.4 for every complex pose. Right after, we ran PELE with an equilibration protocol (moving RAB with a rotation factor of 0.01 rad and translation range of 0.02 Å for 40 PELE MC steps using 30 independent trajectories) to correct interface clashes derived from pyDock predictions. Next, we selected the top 10 poses from the equilibration step and ran a production protocol (rotation factor of 0.01 or 0.05 rad, translation range of 0.05 or 0.4 Å, for 40 PELE MC steps and 100 independent trajectories) and computed the whole complex and the protein–protein interaction energies. Thereafter, we compared the energy profiles between LRRK2WT and LRRK2Leu119Pro;Leu488Pro.
Additionally, we used Steered Molecular Dynamics (SMD) as a second modelling tool. In brief, SMD is a set of non-equilibrium pulling simulations that apply specific work with a constant velocity to the system. SMD allows the comparison of the required work/force to accomplish the dissociation between LRRK2 and RAB8A or RAB10 for both LRRK2WT and LRRK2Leu119Pro;Leu488Pro. We first selected the top energy pyDock pose close to residue 119, and the top pyDock pose from LRRK2WT to avoid biasing the results in favour of LRRK2Leu119Pro;Leu488Pro. We then mutated p.Leu119Pro and p.Leu488Pro using Maestro and energy-minimised the system following the aforementioned MD pipeline. Thereafter, we ran 30 replicas of SMD using an umbrella potential along the direction of the vector between the centre of mass of RAB and the interface residues from LRRK2. Concretely, we used a force constant of 1000 kJ mol−1 nm−2, a rate of change of 0.2 Å/ps, and restrained all the residues from LRRK2 during a total production time of 250 ps. We then compared the work profile between LRRK2WT and LRRK2Leu119Pro;Leu488Pro.
Statistical analysis
All data are expressed as means ± SD or box plots, showing the median. Box edges represent the 25th and 75th percentiles, and the whiskers extend to the minimum and maximum values. P-values are indicated by asterisks: *P-value < 0.05.
Cochran–Mantel–Haenszel χ² test was performed for potential differences in mean percentages of pDEST53-LRRK2WT and pDEST53-LRRK2Leu119Pro;Leu488Pro subcellular pattern localisation. The data’s normality was assessed by the Shapiro test, followed by the student's t-test, considering whether the variances were equal or different.
Statistical computing was done using RStudio software version 1.2.1335, and graphs were created with GraphPad Prism version 8.0.1.
Supplementary information
Acknowledgements
We thank the family for participating in this study. We are indebted to Jordi Pijuan and Teresa Periñan Tocino for their invaluable assistance with the in silico analyses and data regarding variant frequencies in PD populations, respectively. This study has been funded by Instituto de Salud Carlos III (ISCIII) through the project “PI22/00680” [J.H.] and co-funded by the European Union; the AGAUR/Generalitat de Catalunya and European Regional Development Fund (FEDER), (Grants SLT002/16/0030, 2021-SGR-01610 [F.P.]) and Juan de La Cierva JDC2022-048942-I from the Ministerio de Ciencia e Innovación. The funders played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript. Data on variant frequencies in PD populations were obtained in April 2025 from the AMP-PD. The CIBERER is an initiative of the Instituto de Salud Carlos III and the Department of Genetic Medicine of Sant Joan de Déu Children’s Hospital is part of the ‘Centre Daniel Bravo de Diagnòstic i Recerca de Malalties Minoritàries’.
Author contributions
L.V.-D. studied and interpreted the family clinical and genetics data. C.G. performed and interpreted the neuropathology study. A.P. performed genetic and cellular studies. M.O.-L. performed genetic studies. V.M. and V.G. performed and interpreted docking analysis. J.H. provided funding. J.H. and F.P. conceptualised work and wrote the first draft. All authors contributed, read and approved the final manuscript.
Data availability
All relevant data that support the findings of this study are presented in the main text and supplementary information. Other source data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Francesc Palau, Janet Hoenicka.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-025-00989-y.
References
- 1.Kluss, J. H., Mamais, A. & Cookson, M. R. LRRK2 links genetic and sporadic Parkinson’s disease. Biochem. Soc. Trans.47, 651–661 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito, G., Tomita, T. & Utsunomiya-Tate, N. LRRK2-mediated phosphorylation and thermal stability of Rab12 are regulated by bound nucleotides. Biochem. Biophys. Res. Commun.667, 43–49 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Sosero, Y. L. & Gan-Or, Z. LRRK2 and Parkinson’s disease: from genetics to targeted therapy. Ann. Clin. Transl. Neurol.10, 850–864 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steger, M. et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife5, e12813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeffer, S. R. LRRK2 phosphorylation of Rab GTPases in Parkinson’s disease. FEBS Lett.597, 811–818 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Steger, M. et al. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife6, e31012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waschbusch, D. et al. Structural basis for Rab8a recruitment of RILPL2 via LRRK2 phosphorylation of switch 2. Structure28, 406–417 e406 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vides, E. G. et al. A feed-forward pathway drives LRRK2 kinase membrane recruitment and activation. Elife11, e79771 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coku, I. et al. Functional analyses of two novel LRRK2 pathogenic variants in familial Parkinson’s disease. Mov. Disord.37, 1761–1767 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchand, A., Drouyer, M., Sarchione, A., Chartier-Harlin, M. C. & Taymans, J. M. LRRK2 phosphorylation, more than an epiphenomenon. Front Neurosci.14, 527 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyman, B. T. et al. National Institute on aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement8, 1–13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwaki, H. et al. Accelerating medicines partnership: Parkinson’s disease. Genetic resource. Mov. Disord.36, 1795–1804 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrödinger, L. L. C. The PyMOL Molecular Graphics System, Version 1.8 (Scientific Research, 2015).
- 14.Rodrigues, C. H. M., Pires, D. E. V. & Ascher, D. B. DynaMut2: Assessing changes in stability and flexibility upon single and multiple point missense mutations. Protein Sci.30, 60–69 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath, E., Waschbusch, D., Baker, B. M. & Khan, A. R. LRRK2 binds to the Rab32 subfamily in a GTP-dependent manner via its armadillo domain. Small GTPases12, 133–146 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrelli, K. W., Vitalis, A., Alcantara, R. & Guallar, V. PELE: protein energy landscape exploration. A novel Monte Carlo based technique. J. Chem. Theory Comput.1, 1304–1311 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Melachroinou, K. et al. Activation of FADD-dependent neuronal death pathways as a predictor of pathogenicity for LRRK2 mutations. PLoS One11, e0166053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marku, A. et al. The LRRK2 N-terminal domain influences vesicle trafficking: impact of the E193K variant. Sci. Rep.10, 3799 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalogeropulou, A. F. et al. Impact of 100 LRRK2 variants linked to Parkinson’s disease on kinase activity and microtubule binding. Biochem. J.479, 1759–1783 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieweg, S. et al. PINK1-dependent phosphorylation of Serine111 within the SF3 motif of Rab GTPases impairs effector interactions and LRRK2-mediated phosphorylation at Threonine72. Biochem. J.477, 1651–1668 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin, G. et al. alpha-Synuclein interacts with the switch region of Rab8a in a Ser129 phosphorylation-dependent manner. Neurobiol. Dis.70, 149–161 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Bonet-Ponce, L. et al. LRRK2 mediates tubulation and vesicle sorting from lysosomes. Sci. Adv.6, eabb2454 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eguchi, T. et al. LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc. Natl. Acad. Sci. USA115, E9115–E9124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamais, A. et al. Mutations in LRRK2 linked to Parkinson disease sequester Rab8a to damaged lysosomes and regulate transferrin-mediated iron uptake in microglia. PLoS Biol.19, e3001480 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamais, A. et al. The LRRK2 kinase substrates RAB8a and RAB10 contribute complementary but distinct disease-relevant phenotypes in human neurons. Stem Cell Rep.19, 163–173 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandres-Ciga, S. et al. Genome-wide assessment of Parkinson’s disease in a Southern Spanish population. Neurobiol. Aging45, 213 e213–213 e219 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichinose, Y. et al. Neuroimaging, genetic, and enzymatic study in a Japanese family with a GBA gross deletion. Parkinsonism Relat. Disord.61, 57–63 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Henderson, M. X., Sengupta, M., Trojanowski, J. Q. & Lee, V. M. Y. Alzheimer’s disease tau is a prominent pathology in LRRK2 Parkinson’s disease. Acta Neuropathol. Commun.7, 183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes, A. J., Daniel, S. E., Kilford, L. & Lees, A. J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry55, 181–184 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fahn, S. & Elton, R. L. Unified Parkinson’s disease rating scale (Macmillan Healthcare Information, Flor-ham Park, 1987).
- 31.Hoehn, M. M. & Yahr, M. D. Parkinsonism: onset, progression and mortality. Neurology17, 427–442 (1967). [DOI] [PubMed] [Google Scholar]
- 32.Emre, M., et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord.22, 1689–1707 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Marras, C. et al. Nomenclature of genetic movement disorders: Recommendations of the International Parkinson and Movement Disorder Society task force. Mov. Disord.32, 724–725 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Tacik, P., Sanchez-Contreras, M., Rademakers, R., Dickson, D. W. & Wszolek, Z. K. Genetic disorders with tau pathology: a review of the literature and report of two patients with tauopathy and positive family histories. Neurodegener. Dis.16, 12–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Natera-de Benito, D. et al. Common pathophysiology for ANXA11 disorders caused by aspartate 40 variants. Ann. Clin. Transl. Neurol.10, 408–425 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med17, 405–424 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siebert, M. et al. Novel mutations in the glucocerebrosidase gene of Brazilian patients with Gaucher disease. JIMD Rep.9, 7–16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham, M. et al. GROMACS 2023.3 Source Code (GROMACS, 2023).
- 39.Gainza, P. et al. Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat. Methods17, 184–192 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Cheng, T. M., Blundell, T. L. & Fernandez-Recio, J. pyDock: electrostatics and desolvation for effective scoring of rigid-body protein–protein docking. Proteins68, 503–515 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Barradas-Bautista, D. & Fernandez-Recio, J. Docking-based modeling of protein–protein interfaces for extensive structural and functional characterization of missense mutations. PLoS One12, e0183643 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data that support the findings of this study are presented in the main text and supplementary information. Other source data are available from the corresponding author upon reasonable request.