Abstract
Photocages are widely investigated for their bioimaging, controlled drug release, and cellular process modulation. Developing universal photoactive units can significantly expand the structural diversity of photocages. Herein, we developed a class of photoactive units based on the classic aldehyde-hydrazone moiety by conjugating several fluorescent aldehydes with various substituted phenylhydrazines. The resulting hydrazone-based photocages degraded upon exposure to light (365-450 nm), releasing fluorescent aldehydes. Fluorescent enhancements, redshifts or blueshifts were clearly observed during uncaging. For bio-application, morpholine-hydrazone based hybrid BW3MP, staurosporine-derived hybrids BW3STS and FY4STS were constructed. They exhibited high release efficiencies of MP (75.5%, BW3MP) and STSA (65.3%, BW3STS), respectively. BW3MP co-localized with a lysosome dye, exhibiting a Pearson correlation coefficient of 0.94. The biological activity of BW3STS was unmasked under irradiation, with a 4-fold increase in IC50. Our work developed photoactive units that hold significant promise for the constitution of general suitable drug delivery systems (DDSs).

Subject terms: Drug delivery, Target identification, Photobiology, Chemical tools
Photocages are crucial for applications in bioimaging and controlled drug release, yet expanding their structural diversity remains a challenge. Here, the authors introduce a class of hydrazone-based photocages that release fluorescent aldehydes upon light exposure, enabling precise sub-organelle imaging and efficient light-controlled drug delivery, with significant implications for biomedical research.
Introduction
Photocages have garnered significant attention owing to their ability to precisely control the spatiotemporal release of functional entities, thereby enabling non-invasive modulation of biological processes and activities1. The core of photocage design lies in the photoactive units, which undergo structural changes upon exposure to light, thereby “unlocking” or activating the target molecules. Typical photoactive units, including o-nitrobenzyl derivatives2, coumarin derivatives3, BODIPY4, xanthene derivatives5, and others6–8, have been developed to construct photocages with promising applications in bioimaging9, drug delivery10, and the manipulation of cellular processes11. Most of these photoactive units reported focus on improving uncaging performance with high efficiency, increasing water solubility, long wavelength irradiation, and stability, etc. via frequently used modification strategies including hydrophilic substituents12, D-A (Donor-Acceptor) regulation13, the incorporation of electrostatic interactions14, and the selection of different groups to improve photolytic efficiency15.
However, the development of photoactive units to enrich the structural diversity of photocages remains crucial16. Thus, great efforts have been devoted to developing photoactive units. For instance, Chen et al.6 reported the construction of a multi-organelle-targeting and function-activatable fluorescent probe, Sanger-Mito/Lys/ER, based on a photocage derived from Sanger’s reagent. Chaudhuri17 reported a bimane-based photocage that enables release of carboxylic and amino acids. Sebej18 discussed the use of fluorescein analog photoactive units, allowing for the controlled release. Situ et al.19 explored Blebbistatin (Bleb) photocage, capable of precisely releasing halogen ions. A fluorescent organic nanoparticle for the delivery of two anticancer drugs was developed by Ray’s group20. In addition, Wei et al.21 reported the development of photolabile compounds derived from Sanger’s reagent that cleave amide bonds upon photocleavage. Then, Zhou et al.22 developed a photocage called photoactivatable nanobody conjugate inducers of dimerization (PANCIDs). Although these studies have expanded the structural library of photoactive units, most of the mentioned scaffolds of fluorescent molecules are still limited to some specific structures. Therefore, a universal photocage design strategy, founded on innovative photoactive units compatible with a broad range of substrates, is urgently required to unlock the full potential of photocages in bio-applications23.
Herein, inspired from the hydrazone-based photosensors24 and oxime unit developed as a photoactive tag15, we hypothesized that hydrazones may have the potential for development as a general photoactive unit. Thereafter, we successfully developed five series of hydrazone photocages via a one-step reaction between phenylhydrazine derivatives and various aldehydes of dyes. These compounds exhibited photodegradation upon visible light irradiation, reinstating the intermolecular charge transfer (ICT) effect and restoring fluorescence of aldehydes. These uncaging processes were fully confirmed by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS) and cellular confocal imaging.
To further demonstrate the versatility of hydrazone-based photocages in biomedical applications, we designed and synthesized compounds BW3MP, FY4STS, and BW3STS by incorporating the 3-(4-morpholinyl)-1-propylamine moiety and the staurosporine derivative to the aldehyde-hydrazone framework. The designed BW3MP, FY4STS, and BW3STS could serve as carriers of functional molecules, enabling the controlled release of fluorophore and bioactive agents. Among them, BW3STS, using BW3 as a drug carrier, exhibited lower cytotoxicity and fluorescence intensity. Under 405 nm light irradiation, it decomposed to release STSA with an efficiency of 65.3%, restoring cytotoxicity against cancer cells, accompanied with fluorescent intensity significantly enhanced, thus achieving the visualized release of the drug.
Results
Design of hydrazone-based photocages
These photocages designed were shown in Fig.1a. We have chosen different aldehydes of dyes and phenylhydrazines to construct five series of photocages (PC1-2, FY1-4, CM1-3, TP1-2, BW1-3), which were synthesized by one-step reactions at room temperature. These aromatic aldehydes have excellent fluorescent properties25–28, and the phenylhydrazone moiety served as a fluorescence-quenched tag, locked in an off-state until triggered by light to release their corresponding fluorophore (Fig.1a).
Fig. 1. Design of five series hydrazone-based photocages.
a The photocages synthesized by one-step reactions and triggered by light to release their corresponding fluorophore, b design of BW3MP as a sub-organelle imaging tool, and design of FY4STS and BW3STS as prodrugs.
Next, we apply hydrazone-based unit for sub-organelle targeting, providing a foundation for targeted release in the future. BW3MP was synthesized by incorporating the 3-(4-morpholinyl)-1-propylamine moiety to the hydrazone framework (Fig.1b). Subsequently, BW3MP was investigated as a lysosome dye to validate its sub-organelle imaging.
Staurosporine, a natural alkaloid, has been recognized for its role in cancer treatment29,30. However, staurosporine has been excluded from potential clinical applications due to its lack of specificity31. Using staurosporine derivative (STSA) as a cargo to conjugate with hydrazone framework, we modified BW3 and FY4 respectively, resulting in FY4STS, and BW3STS as prodrugs of staurosporine (Fig.1b).
Uncaging process investigation
Absorption and emission analysis
It is known that hydrazones are inherently unstable under acidic conditions, undergoing hydrolysis to produce corresponding aldehydes32. Thereby, we firstly evaluated the pH stability of the PC1, FY1, CM1, CM2, CM3, and BW1. As shown in Supplementary Fig. 1, no hydrolysis or decomposition was observed at pH 1.2 and pH 7.4 in the absence of light, even after prolonged incubation (10 h), demonstrating good chemical stability of these compounds.
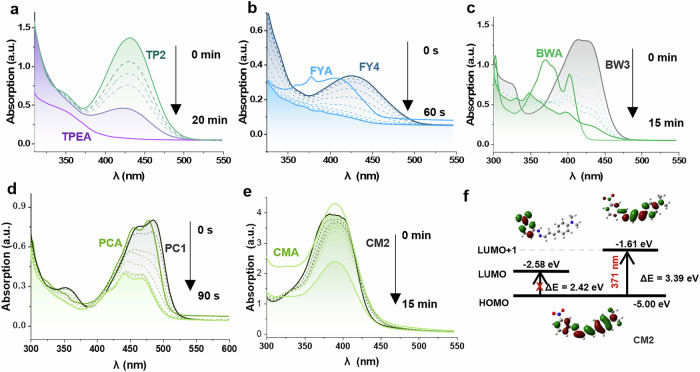
After that, the photolysis behaviors were monitored for targets under UV-Vis light irradiation by detecting the changes of their absorption spectra. As shown in Fig. 2a–d and Supplementary Fig. 2, the absorption bands of these compounds in DMSO all decreased during the uncaging, and the absorption bands of them gradually blueshifted to that of their corresponding aldehyde. These phenomena were consistent with the calculated results that the energy gaps (Eg) between Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO) for PCA (2.81 eV), FYA (3.19 eV), TPEA (3.53 eV) and BWA (3.42 eV) were slightly larger than those for PC, FY, BW and TP compounds (Supplementary Fig. 3 and Table S3). What is more, the absorption bands of PC, FY, and BW split into multiple characteristic absorption bands due to the presence of fully conjugated multiple phenyl rings in the aldehydes33 (Fig. 2b–d). These observations may suggest the occurrence of uncaging reaction, leading to the generation of the substrate aldehyde.
Fig. 2. The changes in absorbance of the photocages.
a–e The absorbance of TP2, FY4, BW3, PC1, CM2 (10 μM in DMSO, dark curves: initial absorption spectra, dashed curves: intermediate time spectra, light colored solid lines without tag: spectra at specific time points indicated on the right) over time under light irradiation compared to the absorbance of their related aldehydes TPEA, FYA, BWA, PCA and CMA (10 μM in DMSO, light colored solid lines with compound tag), f Frontier molecular orbital energy levels and electron density of HOMO/LUMO of CM2.
Exceptionally, for compounds CMA and CM1-3, their absorptions of λmax are almost identical (Fig. 2e and Supplementary Fig. 2). Notably, the absorption λmax of CM2 was not consistent with the calculated Eg of HOMO/LUMO. The transition from HOMO to LUMO in CM2, with Eg of 2.42 eV, was forbidden (Table S2 and Fig. 2f, f = 0.014, 99.3% of S0→S1). The predominant transition originated from the S0→S4 transition (f = 1.492), which was corresponding to the transition from the HOMO to the LUMO + 1 energy levels (Fig. 2f, 98.0%), with Eg of 3.39 eV (371 nm). Such a transition was in proximity to the absorption λmax of CM2 (385 nm).
Molar absorption coefficients (ε) of a range of compounds were determined to assess their photon absorption capability at specific irradiation wavelengths (Table 1). These results showed strong light absorption capabilities due to their large conjugated planes34, allowing for efficient harvesting of photonic energy from light. Among them, the CM series exhibited the highest molar absorption coefficients of 3.21 × 104 L·mol−1·cm−1 (CM2). In contrast, the FY series demonstrated the lowest values with a level of ~0.24 × 104 L·mol−1·cm−1 as shown in Tables 1 and S3.
Table 1.
Photophysical data of the compounds
| λ (nm) | ε (104L/(mol·cm)) | Excited State | f | Eg (eV) | Φu (×10−3) | Φd (×10−4) | |
|---|---|---|---|---|---|---|---|
| TP2 | 365 | 1.38 | S0→S5 | 0.7805 | 2.83 | 3.34 | 4.91 |
| FY4 | 405 | 0.21 | S0→S2 | 0.2416 | 3.67 | 287 | 202 |
| BW3 | 385 | 1.70 | S0→S3 | 0.8242 | 3.03 | 16.6 | 23.2 |
| CM2 | 365 | 3.21 | S0→S4 | 1.4917 | 2.88 | 1.49 | 4.32 |
| PC1 | 450 | 2.65 | S0→S1 | 0.5140 | 2.17 | 1.61 | 3.64 |
λ irradiation wavelength, ε molar absorption coefficients calculated at the absorption λmax of each compounds (TP2 at 430 nm, FY4 at 428 nm, BW3 at 414 nm, CM2 at 386 nm, PC1 at 494 nm), f oscillator strengths, Eg energy gap between HOMO and LUMO, Φu quantum yield of hydrazone degradation, Φd quantum yield of aldehyde release.
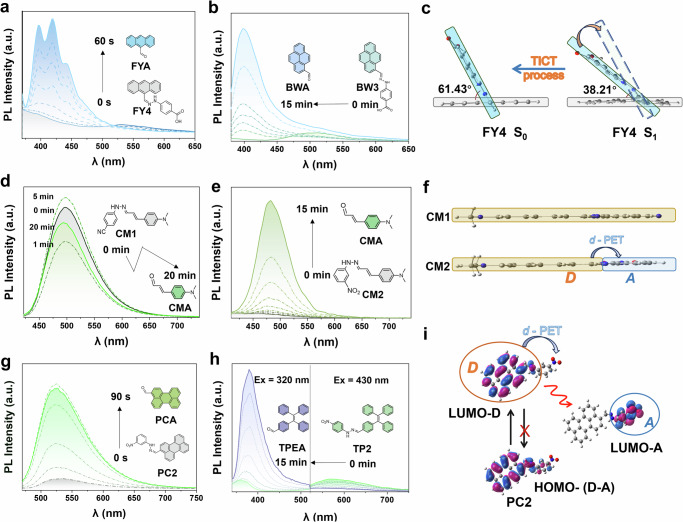
The photoluminescence (PL) fluorescence spectra are shown in Fig. 3 and Supplementary Fig. 4. Interestingly, despite the high ε of these compounds, their fluorescence intensity is relatively weak as compared with their corresponding substrate aldehyde. After uncaging, their noticeable fluorescent intensities enhancement, ranging from 6.5 to 20.3 folds of initial points could be observed with all the compounds except CM1. Among them, compounds of PC, FY, and CM series showed no significant shift in the maximum emission wavelength after uncaging process. The BW and TP series initially exhibited green or blue fluorescence emission. After uncaging, the emission spectra significantly blueshifted, with notably enhanced fluorescence intensity (e.g., 8.5 times for BW3, 13.5 times for TP2). The weak fluorescence of them was mainly attributed to the blockage of the intramolecular charge transfer (ICT) effect in the targets. Specifically, the substrate aldehyde emits fluorescence via the ICT effect. When additional functional groups are introduced, the ICT state becomes suppressed, causing energy dissipation through non-radiative transitions. After deprotection (cage removal), the ICT state is restored, thereby recovering their original fluorescence, which closely resembles that of their corresponding substrate aldehydes (Supplementary Fig. 5).
Fig. 3. PL spectra of major compounds for different irradiation times.
Gray curves: initial emission spectra; dashed: emission spectra at intermediate time points; colored solid lines: spectra at specific time points indicated on the right. a FY4 with 169.5 mW·cm−2 at 405 nm irradiation, λex = 365 nm, b BW3 with 166.0 mW·cm−2 at 385 nm irradiation, λex = 365 nm, c Dihedral angle changes between the benzene ring and anthracene ring in S0/S1 of FY4, and the TICT effect of FY4, d, e CM1 and CM2 with 164.3 mW·cm−2 at 365 nm irradiation, λex = 385 nm, f Comparative analysis of fluorescence mechanisms in CM1 and CM2, D: electron donor, A: electron acceptor, g PC2 with 171.2 mW·cm−2 at 450 nm irradiation, λex = 460 nm, h TP2 with 164.3 mW·cm−2 at 365 nm irradiation, λex = 320 and 430 nm, i Frontier molecular orbital energy levels and electron density distributions of HOMO/LUMO for PC2, along with the d-PET effect of PC2.
In case of compounds FY4 and BW3 with two conjugated planes, calculations of their excited state (in Fig. 3c and Supplementary Fig. 6) might suggest that the occurrence of a Twisted Intramolecular Charge Transfer (TICT) effect between two planes caused the fluorescence quenching35. For FY4, when a molecule returned from the excited state (S1) to the ground state (S0), a portion of the energy was consumed for molecular torsion, which resulted in vibrational relaxation, leading to energy loss. The calculated dihedral angle of FY4 changed from 38.21° to 61.43° upon transitioning from S1 to S0, as shown in Fig. 3c. Differently, the atoms of CM1 and CM2 were almost distributed within the same plane under the S1 and S0 states, so that the initial fluorescence of CM1 was relatively strong (Fig. 3d). However, the initial fluorescence of CM2 was very weak (Fig. 3e). The fluorescence quenching of CM2 might be due to a donor-excited photoinduced electron transfer (d-PET) effect (Fig. 3f) as well as TP2 and PC2 (Fig. 3g, h). These three targets are with electron-withdrawing groups (-NO2) on the benzene rings as electron acceptors. Taken PC2 as an example, the LUMO energy level of acceptor was situated between the HOMO and LUMO energy levels of the electron donor. The electron transferred to the electron acceptor so that it did not undergo a radiative transition (Fig. 3i).
UPLC-MS analysis
The solid evidence for confirming the uncaging processes was further provided by UPLC-MS, where we extracted the ion currents of the targets and the corresponding substrate aldehyde. Without exception, as the irradiation continued, the ion current of the targets gradually decreased until it disappeared, while the ion current of the substrate aldehyde gradually increased (Supplementary Figs. 7 and 8). These changes, together with the changes in the absorption (Fig. 2a–e and Supplementary Fig. 2) and emission spectra (Fig. 3 and Supplementary Fig. 4), unequivocally confirmed the realization of the degradation of hydrazones to aldehydes. In addition, the hydrazone degradation and aldehyde release quantum yield of them were listed in Table 1, indicating they could be efficiently utilized to trigger photoreactions, resulting in their uncaged processes. Notably, the uncaging products were fundamentally distinct from the conventional photoisomerization behavior of hydrazone-based photoswitches reported before36.
The properties of BW3MP and its photoreaction mechanism
We modified BW3 by attaching a morpholine unit to yield BW3MP. The photodegradation processes of BW3MP were also confirmed using fluorescence spectroscopy and UPLC-MS. After uncaging, the changes in absorption and emission spectra of BW3MP were similar to those of BW3, with the absorption spectra showing blue-shift and attenuation and the emission spectra exhibiting blue-shift and enhancement (Fig. 4a–c). Both BW3 and BW3MP underwent partial fluorescence quenching due to the blockage of the original ICT of BWA. However, quantum mechanical calculations revealed that the BW3 exhibited a tilted conformation (Fig. 4d), with the pyrene and the carboxyphenyl groups forming an angle, whereas the BW3MP displayed a nearly coplanar conformation between these two moieties (Fig. 4e). As a result, unlike the TICT quenching that was observed in BW3, BW3MP exhibited an a-PET effect (Fig. 4f), where the electron transitions between the Donor and Acceptor were existed37.
Fig. 4. The properties of BW3MP.
a Absorption spectra of BW3MP, where ε is the molar absorption coefficient, b, c Emission spectra of BW3MP in MeOH/H2O (25:75) and DMSO solution with 82.3 mW·cm−2 at 405 nm irradiation, λex = 410 nm. The dihedral angle changing and their energy of BW3 (d) and BW3MP (e). In both the S0 and S1 states of BW3MP, the pyrene and benzene rings remain coplanar (as calculated using Gaussian 09 software with IRC analysis at a step size of 6°). f a-PET effect of BW3MP.
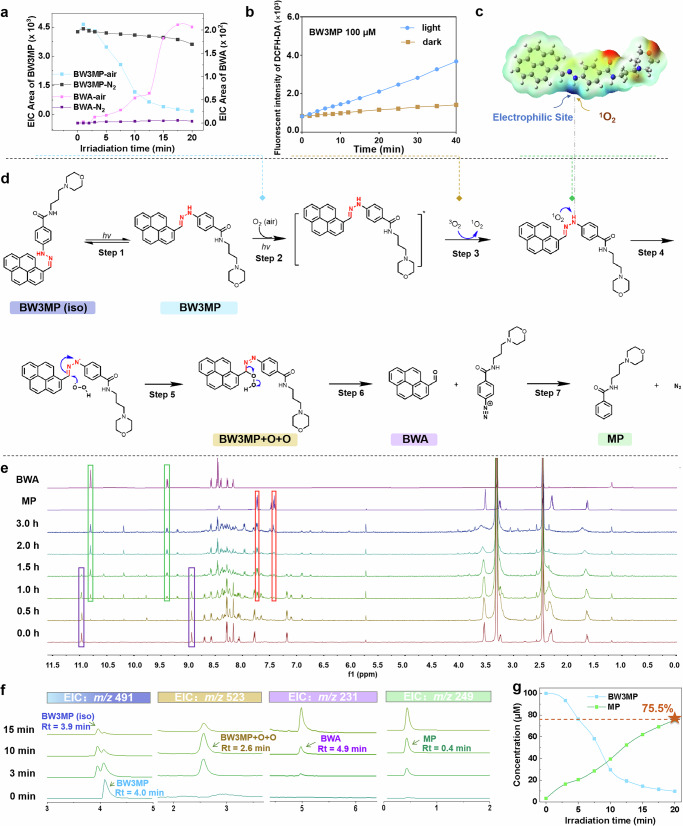
Subsequently, the uncaging mechanism of hydrazone-based photocages was also investigated, taking BW3MP as an example. Upon irradiation, BW3MP underwent isomerization, which may be based on the Z/E configuration transformation (step 1 in Fig. 5d), previously reported by Zhang et al.38. As shown in Fig. 5f, the ion peaks of N-(3-morpholinopropyl) benzamide (MP) and BWA gradually intensified, while the BW3MP peak weakened, implying that Step 1 (isomerization) and Step 2 (degradation) occur simultaneously. Notably, the BW3MP peak declined faster than its isomer, suggesting that BW3MP itself (rather than its isomer) predominantly participates in the degradation pathway. Next, the mass spectrometry captured a clear [M + 2O + H]+ of m/z 523.2323 at Rt = 2.6 min (Fig. 5f), indicating the involvement of oxygen. Thereafter, we also observed that BW3MP underwent almost no degradation and only exhibited isomerization under oxygen-free environment (Fig. 5a and Supplementary Fig. 9a), with the intensity of absorption and emission spectra slightly reducing (Supplementary Fig. 9b, c). The similar phenomena of some others were shown in Supplementary Fig. 10. Moreover, ROS detection in BW3MP using the DCFH-DA probe revealed a significant fluorescence enhancement under light irradiation (Fig. 5b), attributable to the irreversible oxidation of the probe by accumulated ROS. Notably, while the fluorescence signal continuously rises due to the irreversible nature of the probe’s oxidation, the actual ROS generation rate in the system may decrease as BW3MP is consumed during the reaction. In contrast, no notable fluorescence increase of DCFH-DA was observed in the dark, confirming the light-dependent generation of ROS by BW3MP. Based on these observations and similar mechanism reported by Han’s group15, we proposed a photo-oxidation process involving ROS (step 3). 1O2 exhibited nucleophilic reactivity, preferentially targeting the N-H position, which possessed the highest positive electrostatic potential from quantum mechanical calculations (Fig. 5c and Supplementary Fig. 3). Additionally, unlike the hydrolysis process, the photodegradation products were MP and BWA (Fig. 5d, f). Figure 5d displays the time-dependent 1H NMR evolution of BW3MP (1.5 mM in DMSO-d6) under irradiation, with direct comparisons to BWA and MP. BW3MP-specific proton signals (highlighted in purple boxes) progressively diminish with irradiation time, most notably the hydrazone N-H proton signal at 10.9 ppm, indicative of bond cleavage. Concurrently, characteristic proton signals of BWA and MP characteristic proton signals (marked by green and red boxes) intensify, confirming they were photodegradation products. Unassigned peaks in Fig. 5g likely originate from BW3MP isomer. Notably, the MP release rate of BW3MP was calculated to be 75.5% (Fig. 5g), which suggested that BW3MP could serve as visualizable photocages for the release of fluorophore and small molecules. Based on the computational results and experimental observations, we proposed the reaction mechanism as depicted in Fig. 5d, where 1O2 initially attacked the N-H (step 4), followed by [O-O-H] attacking the C=N bond from the other side (step 5), forming a peroxide intermediate. Subsequently, the compounds decomposed to release the aldehyde, forming an unstable intermediate with N≡N+- (step 6), and finally decomposed to release N2 (step 7).
Fig. 5. Photoreaction mechanism of photocages taking BW3MP as an example.
a Mass spectra peak area under air environments and oxygen-free environment, b Relative ROS levels of BW3MP (100 μM in DMSO, detected by fluorescence intensity of DCFH-DA fluorescent probe) with irradiation and non-irradiation, c Electron density of BW3MP, d The proposed uncaging mechanism for BW3MP, e Comparison of 1H NMR changes in BW3MP (1.5 mM in DMSO) during photodegradation with 1H NMR of BWA and MP. f EIC analysis of BW3MP and its uncaged products under different irradiation times, g Conversion rate of BW3MP, MP release rate was 75.5%.
Application for cell imaging and precise sub-organelle visualization
Confocal imaging of cells
Hydrazone units have been widely applied as key intermediates, linker of ADC drugs39 or pharmacophores in drug development40, fluorescent probes41, and molecular sensors42. In CCK-8 assay, the high cell viability was observed under either light or dark conditions, indicating that the photocages and their photoreaction products did not exhibit obvious cytotoxicity (Supplementary Fig. 11), which piqued our interest in designing low-damage cellular tools using these photocages. Next, MCF-7 cells were utilized for bioimaging investigation (Fig. 6a–d and Supplementary Figs. 12–14). In the case of BW3, fluorescence in cells showed significantly red-shifted under 405 nm excitation (Fig. 6a, b), while BW3 maintained its original fluorescence without irradiation (Fig. 6c, d). In addition, the fluorescence of BW3 could be maintained for no less than 10 min without decay, which may be an advantage over traditional dyes with quenched effect. Notably, BW3’s initial point image showed relatively high fluorescence intensity, which was distinctly different from their change of emission spectra. The phenomenon may be explained by the environmentally sensitive of TICT effect43. The cytoplasm, having a higher viscosity, hindered their planar rotation process, thereby partially obstructing TICT, which allowed for partial recovery of the fluorescence (Fig. 6f). Similarly, TICT effect of FY4 was also suppressed in cells, so that FY4 has a blue light emission at 0 s (Supplementary Fig. 9b). In contrast, TP2, PC1, and CM2, which based on PET quenching effect, were week emission and enhanced under irradiation in MCF-7 cells (Supplementary Figs. 9a, c, d and 14), which were in good agreement with the respective changes of PL spectra in DMSO, respectively. One the other hand, uncaged BW3 exhibited green emission in cells, which was different from the blue light emission in PL. It might be caused by typical excimer emission of BWA due to dimer formation in aqueous environment27. One piece of evidence was that BWA itself also emits green light in cell imaging (Supplementary Fig. 13). Another was that uncaged BW3 in MeOH/H2O emits a cyan light at 475 nm, which is longer wavelength than that in DMSO (Supplementary Fig. 5c and Fig. 6f). These phenomena are similar to the uncaging process of BW3MP (Fig. 4b). BW3MP initially emitted a bright cyan light in the cell images (Fig. 6e). During the uncaging process, the blue light gradually diminished, the green light remained constant, and there was a slight increase in red channel. The overlayed images showed the transition of luminescence from blue to green (Fig. 6g, h).
Fig. 6. Confocal imaging of cells treated with BW3 and BW3MP.
a Confocal imaging of cells treated with BW3 under irradiation. Overlay of four channels: red, blue, green and bright field cellular image (405 nm laser irradiation), b Fluorescence intensity (FI) changes across channels under irradiation, c Confocal imaging of MCF-7 cells treated with BW3 without irradiation Overlay of four channels: red, blue, green and bright field cellular image, d FI changes across channels without irradiation. e The TICT effect of BW3, and suppressed TICT in the intracellular environment. f PL spectra of BW3 in MeOH/H2O for different irradiation times (λex = 365 nm). g Confocal imaging of cells with BW3MP: Overlay of four channels: red, blue, green, and bright field cellular image (405 nm laser irradiation). h The fluorescence intensity of three channels (red, blue, and green) in (g).
Sub-organelle localization
As a demonstration of its application, BW3MP was selected for sub-organelle localization. A red lysosomal dye called Lyso-Tracker Red (C1046) was used to stain cells, serving to label the lysosomes within the cells, and then BW3MP was added to co-stain (Fig. 7a–d). As shown in Fig. 7a, b, we also observed the similar phenomenon of diminishing intensity at blue channel and enhanced red light. The faint red light at initial point in the red channel originates from Lyso-Tracker Red, while the blank control BW3MP almost did not emit red light at initial point (Figs. 5d and 7a). Through Pearson correlation coefficient analysis, a correlation coefficient of 0.94 was achieved between the location stained by the lysosomal dye and the position of BW3MP in the blue channel at starting time point. (Fig. 7c, d), which suggested that BW3MP and the dye were co-localized very well. The experimental results indicated that BW3MP may have the potential to serve as a fluorescent probe for lysosome targeting and dynamic visualization imaging.
Fig. 7. Colocalization of lysosomes using BW3MP, with Lyso-Tracker Red (C1046).
a Confocal imaging of cells with BW3MP and Lyso-Tracker Red (C1046), overlay of four channels: red, blue, green and bright field cellular image (405 nm laser irradiation), b The fluorescence intensity of the three channels (red, blue and green), c Correlation analysis of the blue channel and red channel at 0 s in the white square regions, d The correlation coefficient between the location stained by the lysosomal dye and the position of BW3MP in the white square regions.
Application for drug release
Drug release of STSA
The efficient release of small molecules has sparked our further interest in designing hydrazone-based prodrugs. Two hybrids BW3STS and FY4STS were designed and synthesized by attaching staurosporine derivatives via one-step modification. As was shown in Fig. 8a, b, BW3STS exhibited an emission peak at 520 nm and an absorption peak at 420 nm. BW3STS followed a fluorescence quenching process driven by a-PET effect, which accounts for its initially weak fluorescence (Fig. 8d). As the irradiation time increases, both the absorption peak at 420 nm and the emission peak at 520 nm diminish to nearly disappearance, while the emission peak at 400 nm shows a significant enhancement. Additionally, we observed a gradual decrease in the BW3STS peak at m/z 870 (Rt = 4.7 min) in MS spectra, along with increases in the molecular ion peaks at 231 and 628 (Rt = 3.8 min), which correspond to the photodegradation products BWA and STSA (Supplementary Fig. 15). The release of BWA is mainly responsible for the notable increase in fluorescence. Similar phenomenon can also be observed in the photodegradation of FY4STS (Supplementary Figs. 16–18). The multiple peaks in the emission spectra during uncaging of FY4STS (Supplementary Fig. 16a) were similar to those observed for FYA (Fig. 3a), suggesting that the main emitter is likely to be FYA. Notably, the photodegradation yield of STSA reaches 65.3% (Fig. 8c) with BW3STS and 45.3% with FY4STS (Supplementary Fig. 16c).
Fig. 8. Photodegradation process of BW3STS prodrug.
a Emission and b absorbance of BW3STS in photodegradation processes with 81.8 mW·cm−2 at 405 nm irradiation, λex = 330 nm, c Conversion rate of BW3STS, and STSA release rate (65.3%) of BW3STS, d d-PET effect of BW3STS, e Cytotoxicity of STSA and BW3STS with irradiation and non-irradiation, error bars show cell viability of three independent measurements. f Confocal imaging with BW3STS. Overlay of four channels: red, blue, green, and bright field cellular image (laser irradiation: 120 mW·cm−2 intensity at 405 nm).
Then, we visually observed the release of BW3STS in cells. STSA itself has high toxicity in cells, with an IC50 of 0.9 ± 0.20 μM, while BW3STS demonstrated low toxicity in cells, with an IC50 of 17.2 ± 3.57 μM under non-irradiation conditions. Particularly, there was no significant toxicity to MCF-7 cells within the dosage range of 0–5 μM, indicating that BW3STS as a prodrug indeed can “cage” the toxicity of STSA. After 30 min irradiation, the toxicity increased 4-fold, with an IC50 reaching 4.8 ± 0.34 μM (Fig. 8e). This comparison also indicates that the drug was released under light and exhibited more pronounced biological activity. Cellular imaging allowed us to visualize the release process of BW3STS. Initially, the cell morphology was intact (in Fig. 8f), with clear outlines and exhibiting cyan fluorescence, suggesting that BW3STS has weak toxicity, while the fluorescence gradually shifted to yellow and intensified. The degradation process of FY4STS also accompanied similar phenomena of fluorescence enhancement and apoptosis in cells (Supplementary Fig. 17). These results suggest that hydrazone can be employed for the design as a linker, allowing for controllable, visualized, and efficient release of the biologically active STSA.
Discussion
In this study, we have developed a class of photocages with the aldehyde-hydrazone unit as a photoactive tag, where the hydrazone can be employed as a general tag to anchor with multiple aldehyde-based fluorescent probes. The majority of these hydrazone conjugates (FY, CM, BW, and PC) exhibited remarkable pH stability (pH 1.2-7.4), with degradation only upon light irradiation. Depending on the substituents, the fluorescence quenching of the caged compounds is based on various mechanisms such as TICT or PET, but the uncaging process of them released the fluorophore, restoring fluorescence, which is accompanied by evident phenomena such as redshifts, blueshifts, or enhancements in fluorescence. Next, we proposed that the photodegradation process follows a photo-oxidation reaction mechanism, as inferred from mass spectra results. Photolysis occurred more rapidly in aerobic environments, with the formation of a peroxide intermediate state during the process. The N-H bond was more susceptible to attack by O2 in the excited state, as supported by quantum mechanical calculations. Moreover, the detection of distinct fluorescence signal changes indicated that these photocages could function as fluorescence probes, potentially offering superior fluorescence brightness compared to conventional dyes that are subject to fluorescence quenching44.
Additionally, the hydrazone unit could be linked to a variety of bio-functional structure for different bio-applications. As a demonstration of their utility, BW3MP, FY4STS, and BW3STS successfully released fluorophores and small active molecules. Notably, the MP release rate of BW3MP was with high efficiency of 75.5%. Meanwhile, the BWA release rate was calculated about 25%, which may be due to the photo-instability of BWA45, as it underwent decomposition. Moreover, BW3MP achieved co-localized with a commercial lysosome dye, exhibiting a Pearson correlation coefficient of 0.94, confirming its potential as a sub-organelle localizer. The designed BW3STS successfully “caged” the toxicity of the staurosporine derivative. Under light exposure, the anticancer activity of BW3STS was enhanced 4-fold. The staurosporine derivative were released through photodegradation of the hydrazone unit, restoring its biological activity, demonstrating its potential as a photocage prodrug.
In summary, we have confirmed the feasibility and universality of this photoactive unit, facilitating the release of a variety of aldehyde-based fluorescent groups. Further research will focus on fine-tuning the substituents of these photocages for near-infrared responsiveness and enhanced bio-accessibility. We envision that hydrazone-based photocages will emerge as a valuable platform for bioimaging, subcellular localization, and drug delivery, overcoming the challenges of insufficient drug release efficiency in approved hydrazone-based ADC drugs46,47.
Methods
Materials
All starting materials were obtained from commercial suppliers and used without further purification. tetraphenylethylene aldehydes (TPEA), PCA, CMA, BWA, FYA, and the hydrazine and its derivatives used in the experiment were purchased from Accela ChemBio Co., Ltd. (China). Methanol was sourced from Xilong Scientific Co., Ltd. (China). Unless otherwise specified, all other reagents and solvents were of Analytical Reagent (AR) grade and were obtained from Shanghai Hushi Laboratory Equipment Co., Ltd. (China), used as received.
Thin-layer chromatography (TLC) and column chromatography
Reactions were magnetically stirred and monitored on a thin-layer chromatography (TLC) Silica gel 60 G254 plate from Shanghai Haohong Scientific Co., Ltd. The purification of compounds was performed with silica gel (200-300 mesh) column chromatography.
UPLC-MS
High-resolution mass spectra (HRMS) were obtained at the Mass Spectrometry Service Center of Fujian Institute of Microbiology on an Agilent 6545 Q-TOF LC-MS.
Synthesis
A mixture of aromatic aldehydes (2 mmol), any one of them, such as 9-anthre carboxaldehyde (FYA), 3-perylenecarboxaldehyde (PCA), cinnamaldehyde (CMA), 1-pyrenecarboxaldehyde (BWA) and TPE aldehydes (TPEA), was reacted with phenylhydrazine derivatives (6 mmol) in DCM solution (20 mL) and stirred in the nitrogen environment with proper reaction times under the protection from light. The crude products were purified by column chromatography on silica gel eluted with (DCM/MeOH = 15:1) to give the targets. The products were subsequently filtered, washed with DCM, and dried over anhydrous Na2SO4. All the steps of the synthesis were conducted at room temperature. The derivatives of 3-perylenecarboxaldehyde phenylhydrazone, 9-anthraldehyde phenylhydrazone, cinnamaldehyde hydrazone, 1-pyrenecarboxaldehyde hydrazone, and TPE aldehyde hydrazone were synthesized (Fig. 1a), which were named as PC1-PC2, FY1-FY4, CM1-CM3, BW1-BW3, and TP1-TP2, respectively. The HRMS results were shown in Figs. S1–S14 in Supplementary Data 4, and the NMR results were shown in Figs. S21–S48 in Supplementary Data 4.
The main synthetic method of BW3MP is as follows: 2 mmol of compound BW3, 1 mmol of EDCI, and 1 mmol of HOBt were dissolved in 10 mL of DCM, and then stirred at room temperature in the dark under the protection of nitrogen gas for 240 min, followed by the dropwise addition of 200 µL of 3-(4-Morpholino)-1-propylamine. For an additional 60 min, the reaction mixture was completed. The crude materials were filtered, washed with DCM, and dried over anhydrous Na2SO4. The HRMS results were shown in Fig. S15 in Supplementary Data 4, and the NMR results were shown in Figs. S49 and S50 in Supplementary Data 4.
To study the release rate, the reference compound MP was synthesized from benzoic anhydride and 3-(4-Morpholino)-1-propylamine as follows: 1 mmol of benzoic anhydride and 1 mmol of EDCI, and 1 mmol of HOBt were dissolved in 10 mL of DCM, and then stirred at room temperature in the dark under the protection of nitrogen gas for 240 min, followed by the dropwise addition of 200 µL of 3-(4-Morpholino)-1-propylamine. For an additional 60 min, the reaction mixture was completed. The crude materials were filtered, washed with DCM. The HRMS results were shown in Fig. S16 in Supplementary Data 4, and the NMR results were shown in Figs. S51 and S52 in Supplementary Data 4. The obtained MP was diluted with DMSO to different concentrations to prepare the standard curve.
The main synthetic method of BW3STS and FY4STS is as follows: 2 mmol of compound FY4 or BW3, 2 mmol of STSN, 1 mmol of HATU and 1 mmol of DIBG were dissolved in 10 mL of DCM, and then stirred at room temperature in the dark under the protection of nitrogen gas for no less than 240 min. The crude products were purified by column chromatography on silica gel eluted with (DCM/MeOH 15:1) to give the targets. STSA was synthesized with the similar way. The HRMS results were shown in Figs. S17–S20 in Supplementary Data 4, and the NMR results were shown in Figs. S53–S60 in Supplementary Data 4.
UV-Vis absorption and emission spectroscopy
UV-Vis absorption and Emission spectra, quantum yields, and all the fluorescence measurements were recorded using a Varioskan LUX 3020-80110.
Photochemical quantum yield determination
The quantum yield for the photoreaction of aldehyde-hydrazone cage was assessed in the following manner.
| 1 |
ΔM Number of staurosporine aldehyde molecules generated
Np Number of absorbed photons
The calculation of the number of aldehyde molecules generated was carried out using Eq. 2.
| 2 |
V Irradiated volume
NA Avogadro’s number
Δc Concentration increases of the substrate aldehyde
The number of absorbed photons (Np) can be evaluated from the measured the optical power absorbed by the test solution (Pa), irradiated time (ΔT) and the energy of a photon of 365 nm wavelength (Ep) according to Eq. 3.
| 3 |
Pa can be found by the following Eq. 4.
| 4 |
when imported Lambert-Beer law , the equation becomes.
| 5 |
A Corresponding absorbance of test solution at the irradiation wavelength
Ee Intensity of incident light at the irradiation wavelength
S Cross-sectional area of irradiation
ε Molar absorption coefficient
C Concentration increases of the aldehyde-hydrazone cage
L Optical path length
The energy of a photon Ep is calculated by Eq. 6.
| 6 |
h Planck’s constant
c Speed of light
λ Irradiation wavelength
The procedure for the measurement followed steps below
5.0 mL DMSO solution of our synthesized photocage (10 µM) in a flat-bottomed quartz tube was exposed to light irradiation at 365 nm under ambient condition. The inner diameter of the flat-bottomed tubes was measured by micrometer and calculated by the formula for circular area (2.270 cm2). The light source operated with a current stabilizer. The incident light intensity was measured with a power-meter (WATTCAS WP-TEC-1020SL, China).
A 0.5-μL portion of this solution was then subjected to UPLC-MS analysis following the photodegradation. The area of peaks from the TCI trace was integrated, and the concentrations of the product were quantified by pure aldehyde as reference (0.1, 1, 10, 100 μM).
Molar absorption coefficient measurements
Determination of the molar absorption coefficient: Initially, we calibrated the instrument using potassium dichromate standard according to European Pharmacopoeia 6.0, Chapter 2.2.2548 [Absorption spectrophotometry, ultraviolet and visible]. To prepare stock solutions of compounds TP2, CM2, FY4, PC1, and BW3, ~1.02 mg of each sample was accurately weighed using an analytical balance and dissolved in 1 mL of DMSO in a 5-mL centrifuge tube. Serial dilutions were subsequently prepared from these stock solutions. Appropriate concentrations were selected, and the absorption spectra of the solutions were recorded over the range of 300–800 nm using the Hitachi UH5300 UV-Vis spectrophotometer. Baseline correction was applied prior to data analysis. The molar absorption coefficients at various wavelengths were calculated from the absorbance measurements using the Beer-Lambert law:
where ε is the molar absorption coefficient (L·mol−1·cm−1), A is the absorbance, c is the concentration (mol/L), and l is the path length (cm).
Irradiation experiments
Irradiation experiments were done in 1.0-cm quartz fluorescence cuvette. Light source was operated by the photoreaction instrument (WP-TEC-1020SL), was purchased from WATTCAS (China).
NMR spectroscopy
NMR spectra of compounds PC1, TP2, FY1-4, BW2, and BW3MP were acquired on a JNM-ECZ600R/S1 600 MHz, and others were acquired on a Bruker AVANCE NEO 400 MHz spectrometer in DMSO-d6 at ambient temperature with tetramethylsilane (TMS) as an internal standard. NMR standards used were as follows: (1H NMR) DMSO-d6 = 2.50 ppm. (13C-NMR) DMSO-d6 = 39.52 ppm. All chemical shifts (δ) are reported in ppm relative to TMS. Spin multiplicities were reported as a singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), and broad (br) with coupling constant (J) reported in Hz.
In vitro detection of ROS
The production of ROS under laser irradiation was detected by DCFH-DA. The 96-well plate was added with 100 μL of PBS solution containing BW3MP (100 μM). Then the PBS solution was exposed to dark or 660 nm laser irradiation (150 mW·cm−2) for different durations ranging from 0 to 40 min. The fluorescence intensity of DCFH-DA (ROS levels) in PBS was detected using a multifunctional microplate reader (TECAN, M200 PRO, Switzerland) with excitation at 488 nm and emission at 529 nm.
Fluorescence microscopy
Cell culture and imaging
Add 1 mL of culture medium and ~100 μL of the MCF-7 cell suspension, then incubate overnight in a cell culture incubator. The MCF-7 cell line, a human epithelial breast cancer cell line (ATCC® HTB-22TM), was originally derived in 1973 by Dr. Herbert Soule at the Michigan Cancer Foundation from the pleural effusion of a 69-year-old Caucasian female patient diagnosed with metastatic adenocarcinoma. The next day, wash the cells with PBS 1–2 times, replace the medium with 1 mL of fresh culture medium, add the test compound at a concentration of 1 μL/mL of medium, mix well, and incubate for 30 min. After incubation, wash the cells once with PBS and replace the medium with 1 mL of normal culture medium for continued incubation. Images were immediately acquired using a confocal microscope (Leica, STELLARIS 5, Germany), employing consistent acquisition parameters across all experimental groups. (Blue channel: λex: 405 nm; λem: 430–480 nm; Green channel: λex: 488 nm; λem: 500–530 nm; Red channel: λex: 638 nm; λem: 650–750 nm; Scale bar: 10 μm). For the cell imaging light irradiation experiments, the instrument’s built-in light source was directly applied at an intensity of 120 mW·cm−2.
Colocalization experiment
MCF-7 cells were cultured on a confocal dish for 24 h. Subsequently, the culture medium was replaced with a medium containing 10 μM BW3MP and 200 nM Lyso-Tracker Red (C1046) (Beyotime, China). After incubation for 30 min, these cells were washed with PBS and resuspended in fresh medium. Images were immediately acquired using a confocal microscope (Leica, STELLARIS 5, Germany), employing consistent acquisition parameters across all experimental groups.
CCK-8 assay
For the dark cytotoxicity
MCF-7 cells were seeded in flat-bottomed 96-well plates with the number of 7500 cells per well in the presence of 100 μL complete culture media for 24 h. The cell numbers were detected by hemocytometer count in cell suspension. After being washed with PBS three times, the MCF-7 cells were incubated with different concentrations (0, 0.1, 1, 10, 50, 100 μM) of FY4 and CM2. All stock solutions were prepared in DMSO (2 mM) and diluted with complete medium. After cultured for 24 h, the cells were washed with PBS 3 times. 10 μL Cell Counting Kit-8 (CCK-8) solution and 90 μL PBS were added per well simultaneously. After another 1-h culture, the absorbance at 450 nm was read by 96-well plate reader.
For the phototoxicity comparison between hydrazone-based photocages
MCF-7 cells were seeded in flat-bottomed 96-well plates with the number of 7500 cells per well in the presence of 100 μL complete culture media for 24 h. After washed with PBS three times, the MCF-7 cells were incubated with different concentrations (0, 0.1, 1, 10, 50, 100 μM) of FY4 and CM2, and (0, 0.01, 0.05, 0.1, 0.5, 1, 5, 10 μM) of BW3STS. All stock solutions were prepared in DMSO (2 mM) and diluted with complete medium. After cultured for 24 h, the cells were washed with PBS three times. Then the cells were get irradiated for 20 min. After cultured for 24 h, 10 μL Cell Counting Kit-8 (CCK-8) solution and 90 μL PBS were added per well simultaneously. After another 1-h culture, the absorbance at 450 nm was read by 96-well plate reader.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by Department of Science and Technology of Fujian Province (Grant Number 2024R1043); the National Natural Science Foundation of China (NSFC, Grant Numbers 81703555 and 81961138017).
Author contributions
Xia Wang: investigation, writing—original draft, software, formal analysis, analysis data, writing—review & editing. Jingming Zhou: writing—original draft, analysis data, writing—review & editing. Yu Cheng: investigation, funding acquisition. Hongmei Wu: investigation. Xuejing Su: investigation. Rundong Wang: investigation. Kaiyan Xiao: investigation. Weiwei Zhang: writing—review & editing. Zhong Chen: funding acquisition, supervision. Bing Xu: writing—review & editing. Yusheng Lu: analysis of data and funding acquisition. Hongqiang Qiu: supervision, funding acquisition. Lijun Xie: conceptualization, methodology, funding acquisition, supervision.
Data availability
The data supporting the findings of this study are available in the article and Supplementary Information or from the lead contact upon reasonable request. Cartesian coordinates (x, y, z) for all the compounds are provided in Suppelmentary Data 1. All figure data in the main text can be found in Supplementary Data 2, while the data in the Supplementary Information are provided in Supplementary Data 3. NMR and high-resolution mass spectrometry data are also included in Supplementary Data 4. Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, L.X. (Lijunxie8224@outlook.com).
Code availability
The codes supporting the computational studies of this study are available in the Supplementary Information or from the lead contact upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xia Wang, Jingming Zhou, Yu Cheng.
These authors jointly supervised this work: Yusheng Lu, Hongqiang Qiu, Lijun Xie.
Contributor Information
Yusheng Lu, Email: lu_yu_sheng@126.com.
Hongqiang Qiu, Email: hongqiangqiu@fjmu.edu.cn.
Lijun Xie, Email: Lijunxie8224@outlook.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-025-01561-8.
References
- 1.Li, Y. et al. Recent progress in studies of photocages. Smart Mol.1, e20220003 (2023). [Google Scholar]
- 2.Liu, T. et al. Photo-responsive polymers based on o-nitrobenzyl derivatives: from structural design to applications. Prog. Polym. Sci.146, 10.1016/j.progpolymsci.2023.101741 (2023).
- 3.Mukhtar, A. et al. Excited state complexes of coumarin derivatives. J. Fluoresc.32, 10.1007/s10895-021-02807-z (2022). [DOI] [PubMed]
- 4.Gomez, A. M. & Cristobal Lopez, J. Bringing color to sugars: the chemical assembly of carbohydrates to BODIPY dyes. Chem. Rec.21, 3112 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Sadeghpour, M. et al. Recent progress on the synthesis of henna-based dibenzoxanthenes. New J. Chem.45, 13669 (2021). [Google Scholar]
- 6.Jiang, L. et al. Sanger’s reagent as a new general phototrigger for organelle imaging. Sens. Actuator B Chem.398, 134742 (2024). [Google Scholar]
- 7.Wong, P. et al. Photocontrolled release of doxorubicin conjugated through a thioacetal photocage in folate-targeted nanodelivery systems. Bioconjugate Chem.28, 3016 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Paul, A. et al. Mitochondria-localized in situ generation of rhodamine photocage with fluorescence turn-on enabling cancer cell-specific drug delivery triggered by green light. Chem. Commun.56, 8412 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Shin, J. et al. Mitochondria-targeted nanotheranostic: harnessing single-laser-activated dual phototherapeutic processing for hypoxic tumor treatment. Matter4, 2508 (2021). [Google Scholar]
- 10.Shao, Q. et al. Design of a prodrug photocage for cancer cells detection and anticancer drug release. Talanta274, 10.1016/j.talanta.2024.126002 (2024). [DOI] [PubMed]
- 11.Ma, J. et al. Intracellular delivery and deep tissue penetration of nucleoside triphosphates using photocleavable covalently bound dendritic polycations. Chem. Sci.15, 6478 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao, W. et al. O-nitrobenzyl liposomes with dual-responsive release capabilities for drug delivery. J. Mol. Liq.334, 116016 (2021). [Google Scholar]
- 13.Peterson, J. A. et al. A family of BODIPY photocages cleaved by single photons of visible/near-IR light. J. Am. Chem. Soc.140, 7343 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Qin, Y. et al. Construction of covalent organic cages with aggregation-induced emission characteristics from metallacages for mimicking light-harvesting antenna. Angew. Chem. Int. Ed. 62, 10.1002/anie.202308210 (2023). [DOI] [PubMed]
- 15.Wang, L. S. et al. Oxime as a general photocage for the design of visible light photo-activatable fluorophores. Chem. Sci.12, 15572 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong, H. et al. Photo-controllable biochemistry: exploiting the photocages in phototherapeutic window. Chem. 9, 29 (2023). [Google Scholar]
- 17.Chaudhuri, A. et al. Bimane: a visible light induced fluorescent photoremovable protecting group for the single and dual release of carboxylic and amino acids. Org. Lett.19, 1598 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Sebej, P. et al. Fluorescein analogues as photoremovable protecting groups absorbing at ~520 nm. J. Org. Chem.78, 1833 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Situ, Z. et al. Boosting the release of leaving group from blebbistatin derivative photocages via enhancing intramolecular charge transfer and stabilizing cationic intermediate. J. Phys. Chem. Lett.14, 11580 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Ray et al. Visible light-responsive delivery of two anticancer drugs using single-component fluorescent organic nanoparticles. ACS Appl. Nano Mater.5, 7512 (2022). [Google Scholar]
- 21.Wei, T. et al. Sanger’s reagent sensitized photocleavage of amide bond for constructing photocages and regulation of biological functions. J. Am. Chem. Soc.142, 3806 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Zhou, C. et al. Photoactivatable nanobody conjugate dimerizer temporally resolves Tiam1-Rac1 signaling axis. Adv. Sci. 11, 10.1002/advs.202307549 (2024). [DOI] [PMC free article] [PubMed]
- 23.Vorobev, A. Y. & Moskalensky, A. E. Long-wavelength photoremovable protecting groups: on the way to in vivo application. Comput. Struct. Biotechnol. J.18, 27 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su, X. & Aprahamian, I. Hydrazone-based switches, metallo-assemblies and sensors. Chem. Soc. Rev.43, 1963 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Yang, J. et al. Synthesis, optical properties, determination and imaging in living cells and bamboo of cinnamaldehyde derivatives. Spectrochim. Acta A255, 119730 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Zhang, T., Huang, Y. & Chen, X. Tetraphenylethylene-based AIE nanoprobes for labeling lysosome by two-photon imaging in living cells. Spectrochim. Acta A306, 123630 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Feng, X. et al. Aggregation behaviour of pyrene-based luminescent materials, from molecular design and optical properties to application. Chem. Soc. Rev.52, 6715 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Krupka, O. & Hudhomme, P. Recent advances in applications of fluorescent perylenediimide and perylenemonoimide dyes in bioimaging, photothermal and photodynamic therapy. Int. J. Mol. Sci.24, 6308 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omura, S. et al. Staurosporine, a potentially important gift from a microorganism. ChemInform26, 535–548 (1995). [DOI] [PubMed]
- 30.Omura, S. et al. Staurosporine: new lease of life for parent compound of today’s novel and highly successful anti-cancer drugs. J. Antibiot.71, 688 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Park, B. S. et al. Staurosporine analogues from microbial and synthetic sources and their biological activities. Curr. Med. Chem.20, 3872 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Jeet, K. & Ronald, T. et al. Hydrolytic stability of hydrazones and oximes. Angew. Chem. Int. Ed.47, 7523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katoh, R., Kubota, H. & Sugawara, K. Absorption spectra of 1-methylnaphthalene, anthracene, pyrene, and perylene in the liquid phase. Chem. Phys. Lett. 844, 10.1016/j.cplett.2024.141263 (2024).
- 34.Alabugin, I. V., Gabriel, D. P. G. & Abdo, M. A. Hyperconjugation. Wires Comput. Mol. Sci.9, 1389 (2019). [Google Scholar]
- 35.Wang, C. et al. Twisted intramolecular charge transfer (TICT) and twists beyond TICT: from mechanisms to rational designs of bright and sensitive fluorophores. Chem. Soc. Rev.50, 12656 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Bala, I. et al. Multi-stage and multi-colour liquid crystal reflections using a chiral triptycene photoswitchable dopant. Nat. Chem.16, 2084–2090 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Niu, H. et al. Photoinduced electron transfer (PeT) based fluorescent probes for cellular imaging and disease therapy. Chem. Soc. Rev.52, 2322 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Zhang, Z. et al. Isomerization of bioactive acylhydrazones triggered by light or thiols. Nat. Chem.15, 1285 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Baah, S. et al. Antibody-drug conjugates—a tutorial review. Molecules26, 2943 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikolova-Mladenova, B. et al. Design, synthesis and cytotoxic activity of novel salicylaldehyde hydrazones against leukemia and breast cancer. Int. J. Mol. Sci. 24, 10.3390/ijms24087352 (2023). [DOI] [PMC free article] [PubMed]
- 41.Zhao, J. et al. A salicylaldehyde benzoyl hydrazone based near-infrared probe for copper(II) and its bioimaging applications. RSC Adv.12, 3073 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukherjee, S. & Betal, S. Sensing phenomena, extraction and recovery of Cu²+ followed by smart phone application using a luminescent pyrene based chemosensor. J. Lumin.204, 145 (2018). [Google Scholar]
- 43.Wang, C. et al. Quantitative design of bright fluorophores and AIEgens by the accurate prediction of twisted intramolecular charge transfer (TICT). Angew. Chem. Int. Ed. 59, 10.1002/anie.201916357 (2020). [DOI] [PubMed]
- 44.Li, Y. et al. Development and challenge of fluorescent probes for bioimaging applications: from visualization to diagnosis. Top. Curr. Chem. 380, 10.1007/s41061-022-00376-8 (2022). [DOI] [PubMed]
- 45.Ram, K. & Anastasio, C. Photochemistry of phenanthrene, pyrene, and fluoranthene in ice and snow. Atmos. Environ.43, 2252 (2009). [Google Scholar]
- 46.Tsuchikama, K. et al. Exploring the next generation of antibody-drug conjugates. Nat. Rev. Clin. Oncol.21, 203 (2024). [DOI] [PubMed] [Google Scholar]
- 47.Hamann, P. R. et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjugate Chem. 13, 10.1021/bc010021y (2002). [DOI] [PubMed]
- 48.European Pharmacopoeia 6.0. 2.2.25. Absorption spectrophotometry, ultraviolet and visible. (EDQM, 2008).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data supporting the findings of this study are available in the article and Supplementary Information or from the lead contact upon reasonable request. Cartesian coordinates (x, y, z) for all the compounds are provided in Suppelmentary Data 1. All figure data in the main text can be found in Supplementary Data 2, while the data in the Supplementary Information are provided in Supplementary Data 3. NMR and high-resolution mass spectrometry data are also included in Supplementary Data 4. Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, L.X. (Lijunxie8224@outlook.com).
The codes supporting the computational studies of this study are available in the Supplementary Information or from the lead contact upon reasonable request.