Abstract
The farnesyltransferase inhibitor L-744,832 selectively blocks the transformed phenotype of cultured cells expressing a mutated H-ras gene and induces dramatic regression of mammary and salivary carcinomas in mouse mammary tumor virus (MMTV)–v-Ha-ras transgenic mice. To better understand how the farnesyltransferase inhibitors might be used in the treatment of human tumors, we have further explored the mechanisms by which L-744,832 induces tumor regression in a variety of transgenic mouse tumor models. We assessed whether L-744,832 induces apoptosis or alterations in cell cycle distribution and found that the tumor regression in MMTV–v-Ha-ras mice could be attributed entirely to elevation of apoptosis levels. In contrast, treatment with doxorubicin, which induces apoptosis in many tumor types, had a minimal effect on apoptosis in these tumors and resulted in a less dramatic tumor response. To determine whether functional p53 is required for L-744,832-induced apoptosis and the resultant tumor regression, MMTV–v-Ha-ras mice were interbred with p53−/− mice. Tumors in ras/p53−/− mice treated with L-744,832 regressed as efficiently as MMTV–v-Ha-ras tumors, although this response was found to be mediated by both the induction of apoptosis and an increase in G1 with a corresponding decrease in the S-phase fraction. MMTV–v-Ha-ras mice were also interbred with MMTV–c-myc mice to determine whether ras/myc tumors, which possess high levels of spontaneous apoptosis, have the potential to regress through a further increase in apoptosis levels. The ras/myc tumors were found to respond nearly as efficiently to L-744,832 treatment as the MMTV–v-Ha-ras tumors, although no induction of apoptosis was observed. Rather, the tumor regression in the ras/myc mice was found to be mediated by a large reduction in the S-phase fraction. In contrast, treatment of transgenic mice harboring an activated MMTV–c-neu gene did not result in tumor regression. These results demonstrate that a farnesyltransferase inhibitor can induce regression of v-Ha-ras-bearing tumors by multiple mechanisms, including the activation of a suppressed apoptotic pathway, which is largely p53 independent, or by cell cycle alterations, depending upon the presence of various other oncogenic genetic alterations.
Mutationally activated ras genes are the oncogenes most frequently found in human tumors (2). They have been identified in approximately 30% of all human cancers and are particularly prevalent in human pancreatic and colon carcinomas (90 and 50%, respectively) (5, 6). The Ras proteins, H-Ras, N-Ras, K-Ras4B, and K-ras4A, are low-molecular-weight GTP-binding proteins that function in the transduction of growth-proliferative signals from the membrane to the nucleus (2). Cycling of Ras between the active, GTP-bound and inactive, GDP-bound forms is accomplished by the proteins’ intrinsic GTPase activity and a number of accessory proteins. Mutations in ras that impair the GTPase activity result in constitutively active forms of the proteins.
Localization of the Ras proteins to the inner surface of the cell membrane is essential for their function (17, 23, 28, 55) and occurs following a series of posttranslational modifications (58). The first and obligatory step in this series is the addition of a 15-carbon isoprenoid, farnesyl, to the cysteine residue 4 amino acids from the C terminus of the protein, a reaction mediated by the enzyme farnesyl-protein transferase (FPTase). Subsequent to farnesylation, the three C-terminal amino acids are proteolytically cleaved and the now C-terminal cysteine is methylated. In H-Ras, N-Ras, and K-Ras4A, but not K-Ras4B, a palmitate is added to one or more upstream cysteine residues. Based on the demonstration that farnesylation is required for the transforming activity of oncogenic Ras, much effort has been directed toward the development of inhibitors of FPTase for use in the treatment of human cancer (14, 16).
Many strategies have been used to develop FPTase inhibitors (FTIs), including screening of natural products and rational design based upon the substrates of the farnesylation reaction. We and others have developed potent, cell-active inhibitors that are mimetics of the Ras CAAX motif (14, 16), the Ras C-terminal tetrapeptide that is the minimal protein substrate required for interaction with the enzyme (46, 47). As a class, these compounds are potent, nonsubstrate inhibitors of FPTase and are highly selective with respect to the related prenyltransferase geranylgeranyl-protein transferase type I. The CAAX peptidomimetics have been shown to inhibit the anchorage-dependent (25, 48) and anchorage-independent (7, 30–32, 42, 48) growth of Ras-transformed fibroblasts and human tumor cell lines. Additionally, these compounds cause the transformed morphology of cells in culture to revert (7, 24, 45). In vivo, the peptidomimetics block the growth of both transformed fibroblasts and human tumor cell lines in a nude mouse xenograft model (32, 42, 51).
We have recently demonstrated that daily treatment with L-744,832, a potent, cell-active FTI, causes dramatic regression of mammary and salivary tumors in mouse mammary tumor virus (MMTV)–v-Ha-ras transgenic mice (31). Although the mechanism of tumor response was not explored, the rapid regression was suggestive of a significant elevation in apoptosis. Ras activation has been found to be associated with decreased cellular susceptibility to apoptosis in a variety of in vitro and in vivo contexts (1, 21, 27, 35, 44), and the inhibition of Ras activity has been shown to reactivate the apoptotic response (1, 3, 34).
One important determinant of tumor cell apoptosis is the p53 tumor suppressor protein, a crucial component of the G1 cell cycle checkpoint. In response to DNA damage or certain cell cycle perturbations, p53 can induce either growth arrest in the G1 phase of the cell cycle or apoptosis (57). p53 has been shown to play a critical role in the apoptotic response of cancer cells to a variety of DNA-damaging agents, and functional inactivation of p53 can therefore lead to resistance to these agents (12, 36, 37). However, it should be noted that the extent to which DNA damage-induced apoptosis is p53 dependent can vary with the cellular context (39, 43, 50).
It remains unclear whether the apoptosis induced in response to Ras inactivation is p53 dependent. Since mutation of p53 is the most frequently observed genetic alteration in human cancer (20), it is of considerable clinical relevance to determine the mechanism by which tumors regress in response to FTIs and to determine whether FTIs will be efficacious against tumors lacking functional p53. It is similarly important to determine whether FTIs will be effective in tumors already possessing high spontaneous apoptosis rates, such as those overexpressing the c-myc oncogene in addition to activated ras (21). Answers to these questions may be important in defining the range of human tumors in which these agents will be efficacious. We therefore tested the effect of L-744,832 on the kinetics of tumor growth and on apoptosis and cell cycle changes in mice harboring the MMTV–v-Ha-ras transgene together with a homozygous mutation of the gene for the tumor suppressor p53 (9, 22) or a deregulated c-myc gene (21, 49). Treatment with L-744,832 caused tumor regression in both of the doubly transgenic strains. Furthermore, the mechanism of regression involved either an increase in the level of apoptosis or a change in cell cycle characteristics, or both, depending on the genetic alterations present in the tumor.
MATERIALS AND METHODS
Animals.
MMTV–v-Ha-ras, MMTV–c-neu, and MMTV–c-myc mice in an inbred FVB genetic background were purchased from Charles River Laboratories. These mice contain a mutationally activated H-ras or c-neu gene or a wild type c-myc gene, respectively, under the control of the MMTV promoter-enhancer (41, 49). TSG-p53 transgenic mice (9), containing a homozygous mutation in the p53 tumor suppressor gene, were purchased from GenPharm International and maintained in a hybrid CB6F1 genetic background (C57BL6 × BALB/c). Therefore, the mice used in some of the studies were in an inbred background (FVB; ras/myc and c-neu mice used to generate the data in Fig. 3 and Table 3), while mice in a genetically mixed background (FVB × BALB/c × C57BL6) were used in all other studies. All animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (42a), and experimental protocols were reviewed by the Merck and University of Texas Health Science Center at San Antonio Animal Care and Use Committees.
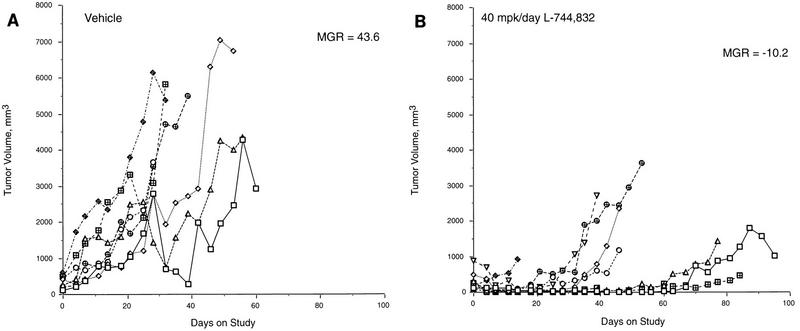
FIG. 3.
Tumor growth curves for ras/myc mice. Mice were treated daily with the vehicle or with L-744,832 at 40 mg kg−1. Total tumor volume was measured twice weekly and is plotted versus time. The MGR calculated 14 days after initiation of treatment is indicated for each group.
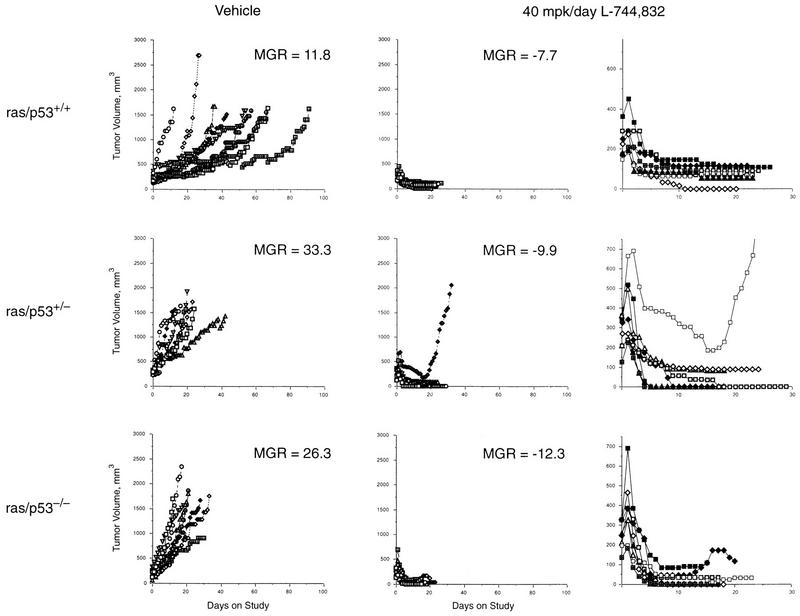
TABLE 3.
Effect of L-744,832 on the growth of tumors in transgenic mice
| Mouse genotype and compounda | No. of animals | MGR ± SEM (mm3/day)b | P valuec |
|---|---|---|---|
| ras/p53+/+ | |||
| Vehicle | 9 | 11.8 ± 5.6 | |
| L-744,832 | 7 | −7.7 ± 1.1 | <0.005 |
| ras/p53+/− | |||
| Vehicle | 7 | 33.3 ± 6.4 | |
| L-744,832 | 8 | −9.9 ± 2.3 | <0.001 |
| ras/p53−/− | |||
| Vehicle | 8 | 26.3 ± 4.9 | |
| L-744,832 | 8 | −12.3 ± 1.9 | <0.001 |
| ras/myc | |||
| Vehicle | 7 | 43.6 ± 12.7 | |
| L-744,832 | 9 | −10.2 ± 5.0 | <0.005 |
| c-neu | |||
| Vehicle | 14 | 25.6 ± 5.4 | |
| L-744,832 | 13 | 15.5 ± 4.2 | >0.05 |
Mice were treated with daily subcutaneous doses of the vehicle or of L-744,832 at 40 mg kg−1.
MGRs of the primary tumor (ras/p53 mice) or all tumors (ras/myc and c-neu mice) 14 days following the initiation of treatment are shown. Values are averages of MGRs for all animals in a treatment group.
The two-sided Student t test was used for comparison with the mean MGR of the vehicle control, except for the ras/p53+/+ mice, for which the Wilcoxon rank-sum test was used. The biweight estimate (40) of 6.2 ± 0.7 mm3/day is a more robust estimate of the average growth rate of the ras/p53+/+ vehicle group than is the MGR.
Female MMTV–v-Ha-ras mice were mated with male MMTV–c-myc mice. Offspring carrying both transgenes were identified by PCR analysis of tail DNA. Primers used for the detection of the MMTV–v-Ha-ras transgene were 5′-CAGGGACCAGCAAGACATC-3′ (5′ sense primer) and 5′-CCCTGAACCACGCATCAAC-3′ (3′ antisense primer). Those used for detection of the c-myc transgene were 5′-GGTGATAGTCCCTTCACATC-3′ (5′ sense primer) and 5′-GTGCCACCTGACGTCTAAGA-3′ (3′ antisense primer). All four primers were added at a concentration of 100 μM each to a standard PCR mixture containing 5% deionized formamide. Following initial denaturation at 99°C, reactions were run for 30 cycles of 58°C for 0.5 min, 72°C for 1 min, and 94°C for 0.5 min.
Crosses between MMTV–v-Ha-ras and TSG-p53 mice and analysis of the offspring were carried out as previously described (22). Briefly, offspring of the various genotypes were generated as littermates from common matings so that all ras/p53 animals in the study were of a mixed genetic background derived from FVB, C57BL6, and BALB/c mice. Offspring were screened by PCR analysis of tail DNA for their ras and p53 status.
Treatment of animals.
Animals were examined twice weekly for the presence of tumors and were placed on study when they developed one or more tumors, the largest having a volume of 50 to 450 mm3. For evaluation of tumor growth kinetics, tumor-bearing mice were randomly assigned to either a vehicle control or an L-744,832 treatment group. L-744,832, dissolved as previously described (31) in an aqueous solution containing NaCl to adjust the osmolarity and sodium citrate to adjust the pH, was administered subcutaneously at 40 mg kg−1 once daily for at least 14 days. For measurement of apoptosis and cell cycle parameters, animals were treated subcutaneously with L-744,832 at 40 mg kg−1 once daily for 2 days and sacrificed 24 h after the second dose. Alternatively, animals were treated with a single intraperitoneal administration of doxorubicin at 10 mg kg−1 and sacrificed 48 h later.
Data analysis.
Tumor growth was monitored by caliper measurements done two to seven times weekly (see individual figure legends), and the data were analyzed as described previously (8, 31). Tumor volume was calculated according to the formula (W2 × L)/2, where W (width) and L (length) are in millimeters and L ≥ W. The area under the curve was calculated according to the formula [(vol1 + vol2)/2] × (day2 − day1). The mean growth rate (MGR) was calculated according to the formula {(sum AUC) − [vol1 × (dayn − day1)]}/(dayn − day1)2, where AUC is the area under the curve.
Apoptosis analysis.
Tumor samples were analyzed for the number of apoptotic cells by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling (TUNEL) method as previously described (22). Positively stained cells were evaluated by light microscopy. Positive cells within a 10- by 10-mm grid in the eyepiece were counted in 10 ×450 fields. The total number of cells per field varied from 500 to 800. Percent apoptosis was calculated by assuming an average of 670 cells/field.
Flow cytometry.
Tumor samples were analyzed by propidium iodide staining and flow cytometry with an EPICS ELITE flow cytometer (Coulter Cytometry, Miami, Fla.) as previously described (22). Histograms were analyzed for cell cycle compartment by using MultiCycle PLUS version 3.0 (Phoenix Flow Systems, San Diego, Calif.). At least 50,000 events were collected to maximize the statistical validity of the compartmental analysis.
Statistical analysis.
MGRs were compared between groups by using a two-sided Student t test or, when a group’s MGRs were not normally distributed, a two-sided Wilcoxon rank sum test (19). Levels of apoptosis among the different treatment groups for each genotype were compared by a one-way analysis of variance on ln counts. Comparisons to a treatment control were made by using Dunnett’s test (10), and pairwise comparisons were made by using the Tukey-Kramer multiple-comparison procedure (18). Flow cytometrically determined proportions of cells in the different phases of the cell cycle were compared among the different treatment groups by multivariate randomization analysis using step-down rerandomization tests on rank-transformed data (54).
RESULTS
Induction of apoptosis by L-744,832.
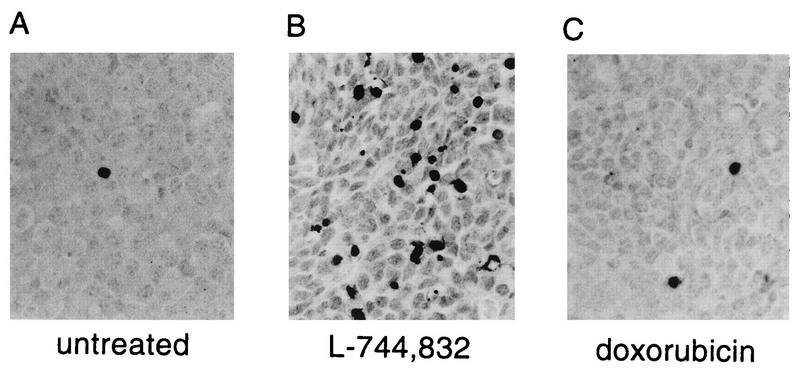
We have previously demonstrated that L-744,832 causes rapid regression of MMTV–v-Ha-ras tumors (31). It was of interest to determine whether the L-744,832-induced regression is mediated by elevated apoptosis, since ras activation has been associated with resistance to apoptosis (1, 21, 27, 35, 44). Tumor-bearing MMTV–v-Ha-ras mice were therefore treated once daily for 2 days with 40 mg of L-744,832 kg−1 and sacrificed 48 h after the first injection, during the period of maximal tumor shrinkage (see Fig. 2 and reference 31). Tumor tissue was taken at the time of sacrifice, and levels of apoptosis were quantitated by the TUNEL assay (13). As we have previously reported (21, 22), very low levels of spontaneous apoptosis were observed in untreated MMTV–v-Ha-ras tumors (Fig. 1A and Table 1). In contrast, L-744,832-treated tumors demonstrated markedly elevated apoptosis levels (Fig. 1B and Table 1).
FIG. 2.
Tumor growth curves for MMTV–v-Ha-ras × p53−/− mice. Mice with the indicated genotypes were treated daily with the vehicle or with L-744,832 at 40 mg kg−1. Primary tumor volume was measured daily and is plotted versus time. The MGR calculated 14 days after initiation of treatment is indicated for each group. The right column shows the growth curves for the L-744,832-treated tumors on an expanded scale.
FIG. 1.
Analysis of tumor apoptosis. Tumor cells undergoing apoptosis were detected on formalin-fixed and paraffin-embedded tissue sections by the TUNEL method (13). Shown are representative TUNEL-stained sections of a tumor from an untreated ras/p53+/+ mouse (A), a tumor from a ras/p53+/+ mouse treated with L-744,832 at 40 mg kg−1 (B), and a tumor from a ras/p53+/+ mouse treated with doxorubicin at 10 mg kg−1 (C).
TABLE 1.
Tumor apoptosisa
| Tumor type and mouse genotype | Mean % of cells undergoing apoptosis ± SEM (no. of tumors analyzed)
|
||
|---|---|---|---|
| Untreated | L-744,832 | Doxorubicin | |
| Salivary | |||
| ras/p53+/+ | 0.28 ± 0.08 (16) | 4.5 ± 1.0 (5)b | 0.46 ± 0.16 (5) |
| ras/p53−/− | 0.32 ± 0.05 (23) | 2.5 ± 0.7 (9)b | NDc |
| Mammary | |||
| MMTV–v-Ha-ras | 0.12 ± 0.02 (14) | ND | |
| ras/myc | 1.6 ± 0.2 (15) | 2.3 ± 0.2 (13) | |
Tumor-bearing mice were left untreated or treated with L-744,832 at 40 mg kg−1 once daily for 2 days or once with doxorubicin at 10 mg kg−1 and sacrificed 48 h after the initial injection. Tumor cells undergoing apoptosis were detected on formalin-fixed and paraffin-embedded sections by the TUNEL method (13). Positively stained cells were evaluated by light microscopy and quantitated as described in Materials and Methods.
Statistically significantly different from the value for the corresponding untreated control (P < 0.01).
ND, not done.
Doxorubicin is a classic cytotoxic agent which is known to efficiently induce apoptosis in a number of in vitro and in vivo systems (37, 38). We have previously demonstrated that doxorubicin at the maximum tolerated dose slowed the rate of tumor growth in the MMTV–v-Ha-ras model but did not cause tumor regression as did L-744,832 (31). To determine whether less efficient induction of apoptosis is responsible for the less dramatic tumor response to doxorubicin, tumor-bearing MMTV–v-Ha-ras mice were sacrificed 48 h after a single treatment with 10 mg of doxorubicin kg−1 for TUNEL analysis of tumor apoptosis. Surprisingly, only a very modest increase in tumor apoptosis was seen in the MMTV–v-Ha-ras mice following treatment with doxorubicin (Fig. 1C and Table 1). Comparably low apoptosis levels were observed when tumors were analyzed 14 or 24 h following doxorubicin administration, indicating that the peak of apoptotic activity was not simply missed at 48 h (data not shown).
In addition to analysis of apoptosis, the DNA content of tumor cells from MMTV–v-Ha-ras mice was analyzed by flow cytometry to determine whether L-744,832 also alters the cell cycle distribution of tumor cell populations. As previously demonstrated (22), MMTV–v-Ha-ras tumors exhibit a relatively high proportion of cells in G1 and low S-phase values. Following treatment with L-744,832, there was virtually no effect on the cell cycle distribution of tumor cells (Table 2). Thus, the rapid tumor reduction seen in ras mice following L-744,832 treatment appears to be mediated through the activation of an apoptotic pathway and is not influenced by cell cycle redistribution. In contrast, treatment with doxorubicin nearly halts cell proliferation in ras/p53+/+ tumors, as evidenced by a further increase in an already high G1 value and a near absence of cells in S phase (Table 2). Thus, in these tumors doxorubicin inhibits tumor growth but fails to effectively induce apoptosis, and thus, a reduction in tumor size is not generally seen (31).
TABLE 2.
Tumor cell cycle characteristicsa
| Tumor type and mouse genotype | Treat- mentb | No. of tumors | Mean % of cells ± SEM in:
|
||
|---|---|---|---|---|---|
| G1 phase | S phase | G2/M phase | |||
| Salivary | |||||
| ras/p53+/+ | None | 15 | 93.8 ± 0.9 | 4.5 ± 0.6 | 1.7 ± 0.3 |
| L-744,832 | 4 | 95.4 ± 1.8 | 3.4 ± 1.4 | 1.2 ± 0.5 | |
| Doxorubicin | 6 | 97.8 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.4 | |
| ras/p53−/− | None | 18 | 84.8 ± 1.5 | 10.5 ± 1.4 | 4.7 ± 0.7 |
| L-744,832 | 7 | 94.5 ± 2.2 | 2.7 ± 0.8 | 2.7 ± 1.4 | |
| Mammary | |||||
| MMTV–v-Ha-ras | None | 8 | 96.3 ± 0.8 | 2.3 ± 0.4 | 1.4 ± 0.6 |
| ras/myc | None | 15 | 88.8 ± 0.9 | 8.5 ± 0.7 | 2.7 ± 0.2 |
| L-744,832 | 12 | 92.4 ± 1.5 | 5.9 ± 1.3 | 1.7 ± 0.3 | |
Mice were treated as described in Table 1, footnote a, and Materials and Methods. G1-, S-, and G2/M-phase fractions based on flow cytometry analysis of propidium iodide-stained tumor cells are shown for salivary and mammary tumors.
L-744,832 was administered at 40 mg kg−1/day, and doxorubicin was administered at 10 mg kg−1.
Tumor response to L-744,832 does not require p53.
The activity of a wide range of chemotherapeutic agents in many cell types has been shown to be p53 dependent (53), and this activity is frequently mediated by p53-dependent apoptosis (12, 36, 38). In light of the fact that MMTV–v-Ha-ras tumors regress in response to L-744,832 through the induction of apoptosis, it was of interest to determine whether this response was dependent upon the p53 status of the tumors. This issue is of clinical relevance in beginning to define the types of tumors which will respond to an FTI, since p53 is functionally inactivated in approximately half of all human tumors (20). MMTV–v-Ha-ras mice were therefore interbred with p53−/− mice to yield progeny that contain the MMTV–v-Ha-ras transgene but have different p53 genotypes, i.e., ras/p53+/+, ras/p53+/−, and ras/p53−/−. Mice from each genotype were randomly assigned to either a vehicle control group or a treatment group receiving L-744,832 at 40 mg kg−1/day when they developed a palpable tumor having a volume of 50 to 450 mm3. For animals having multiple tumors at the onset of treatment, the largest tumor was defined as the primary tumor. Consistent with previously published data (22), ras/p53+/+ and ras/p53+/− mice developed both mammary and salivary tumors, with the majority being mammary in origin (17 primary mammary and 2 primary salivary tumors in the ras/p53+/+ mice and 13 primary mammary and 7 primary salivary tumors in the ras/p53+/− mice). In contrast, the majority of tumors which appeared in the ras/p53−/− mice were salivary in origin (11 primary salivary and 6 primary mammary tumors).
During the course of treatment, tumor growth was monitored by daily caliper measurements. Growth curves for all of the primary tumors are shown in Fig. 2. Across all three p53 genotypes, tumors in the vehicle treatment group exhibited a progressive increase in size. Consistent with previously published data (22), tumors of the ras/p53−/− genotype grew more rapidly than those arising in the ras/p53+/+ mice. The change in tumor volume can be normalized over the period of treatment to yield an MGR expressed in cubic millimeters per day. Thus, the MGR calculated 14 days after the initiation of treatment for tumors of the ras/p53+/+ genotype from mice treated with the vehicle was 11.8 mm3/day, which is statistically significantly different (P = 0.03) from that of tumors in mice with the ras/p53−/− genotype similarly treated with the vehicle (26.3 mm3/day) (Table 3). Tumors in mice with the ras/p53+/− genotype exhibited an MGR of 33.3 mm3/day, similar to that of the tumors of ras/p53−/− mice. This result is consistent with our previous observation that salivary tumors from ras/p53+/− mice commonly exhibit some degree of loss of the normal p53 allele (22).
In contrast to the tumor progression observed in animals in the vehicle group, tumors of mice in the L-744,832 treatment groups exhibited a rapid decrease in size, regardless of the p53 genotype (Fig. 2). This tumor regression is reflected by the negative MGR of tumors in L-744,832-treated mice with each of the genotypes (Table 3). Thus, the MGRs of tumors in mice with the ras/p53+/+, ras/p53+/−, and ras/p53−/− genotypes were −7.7, −9.9, and −12.3 mm3/day, respectively. All of these MGRs are highly significantly different from those of vehicle-treated control mice with the corresponding genotypes (Table 3). The primary tumor from one animal with the ras/p53+/− genotype failed to respond to treatment with L-744,832, exhibiting an initial slow decrease in size, followed by a rapid increase in size (Fig. 2). The reason for the lack of response of this tumor is unknown. Nevertheless, these data suggest that the tumor growth response to the FTI L-744,832 is independent of p53 functional status. This result is particularly encouraging given the high rate of p53 mutation in human tumors.
Consistent with the observed tumor regression was the finding that p53-deficient tumors were still capable of undergoing apoptosis in response to L-744,832 (Table 1). However, the induction of apoptosis in ras/p53−/− tumors was somewhat attenuated compared to that in ras/p53+/+ tumors (8-fold versus 16-fold, respectively; P = 0.07), suggesting that both p53-dependent and p53-independent apoptotic pathways may be activated in response to the FTI.
As previously demonstrated (22), MMTV–v-Ha-ras tumors deficient in p53 possess an elevated proliferation rate relative to ras/p53+/+ tumors, as evidenced by a decrease in the proportion of cells in G1 and a corresponding increase in the S-phase value (Table 2). Surprisingly, in tumors deficient in p53, L-744,832 restored the higher G1-phase and lower S-phase values seen in ras/p53+/+ tumors (Table 2), suggesting that even in the absence of an intact G1/S checkpoint, inhibition of Ras function can reduce tumor cell proliferation. Thus, L-744,832 both induces apoptosis and slows cell cycle transit in p53-deficient MMTV–v-Ha-ras tumors, consistent with the efficient reduction in tumor volume seen following treatment in this model.
Response of MMTV–v-Ha-ras/MMTV–c-myc tumors to L-744,832.
Since tumor regression in response to L-744,832 appeared to be mediated primarily by elevated apoptosis in the MMTV–v-Ha-ras mice, it was of interest to determine whether the presence of additional genetic alterations that result in elevated levels of spontaneous tumor cell apoptosis would impair the ability of tumors to respond to L-744,832. Tumors arising in double-transgenic mice possessing both an MMTV–v-Ha-ras and an MMTV–c-myc transgene exhibit comparable growth rates to MMTV–v-Ha-ras tumors (21). However, the dynamics of tumor growth are markedly different in that the ras/myc tumors have much higher levels of spontaneous apoptosis but also higher S-phase fractions. Therefore, to determine whether tumors whose growth properties are influenced by c-myc overexpression, as well as ras activation, would respond as dramatically to L-744,832 as the MMTV–v-Ha-ras tumors, MMTV–v-Ha-ras and MMTV–c-myc mice were interbred. Progeny that were positive for both transgenes were randomized to either a vehicle-treated group or a treatment group receiving L-744,832 at 40 mg kg−1/day when palpable tumors were detected. Tumor growth was monitored by caliper measurements done twice weekly. At the initiation of treatment, numerous mammary tumors were present in the majority of these mice. Therefore, the total volume of all of the tumors in an animal was recorded. As shown in Fig. 3A, tumors in all seven animals in the vehicle-treated group increased in size over the course of the treatment period. In contrast, tumors in eight of nine animals in the L-744,832 treatment group exhibited regression 14 days following the initiation of treatment (Fig. 3B). The MGRs of tumors in the vehicle and L-744,832 groups (43.6 and −10.2 mm3/day, respectively) were significantly different (P < 0.005) (Table 3). However, despite the initial dramatic tumor shrinkage, the response of ras/myc tumors proved to be less durable than that of tumors in MMTV–v-Ha-ras mice, since beyond the 14-day evaluation point, the total volume of tumors in ras/myc animals treated with L-744,832 increased.
We have previously found that levels of spontaneous apoptosis in ras/myc tumors are greater than 10-fold higher than the levels seen in MMTV–v-Ha-ras tumors (21). It was therefore of interest to determine whether L-744,832 further elevated apoptosis levels in the ras/myc tumors, in light of the fact that these tumors did shrink dramatically following L-744,832 treatment. However, only a modest increase in apoptotic levels was observed in these mice (Table 1), suggesting that L-744,832 promoted tumor reduction by both apoptotic and nonapoptotic mechanisms in these transgenic mice. The effect of L-744,832 on cell cycle distribution in the ras/myc tumors was therefore assessed as for the other tumor models. As previously reported (21), ras/myc tumors had substantially lower G1-phase and higher S-phase values than MMTV–v-Ha-ras tumors (Table 2), consistent with the known ability of c-myc to induce entry into S phase. As in the ras/p53−/− tumors, L-744,832 reduced the percentage of cells in S phase while increasing the G1 cell fraction in ras/myc tumors. Coupled with the very modest elevation of an already high apoptotic activity, this decrease in proliferation rate resulted in the tumor size reduction shown in Fig. 3.
MMTV–c-neu mammary tumors fail to respond to L-744,832.
It is notable that L-744,832 does not cause significant tumor regression in all transgenic mammary tumor models harboring an MMTV promoter-enhancer-regulated transgene. The c-neu gene is the rat homolog of the human gene for ErbB2. ErbB2 is highly homologous to the epidermal growth factor receptor and is thought to signal in part through the Ras pathway. Virtually all of the mammary tissue in female mice bearing the MMTV–c-neu transgene becomes malignant when the mice are approximately 6 months of age (41). Treatment of these tumor-bearing mice with L-744,832 at 40 mg kg−1/day did not have a significant effect on tumor growth. The MGRs (calculated 14 days after the initiation of treatment) of tumors from vehicle- and L-744,832-treated mice were 25.6 and 15.5 mm3/day, respectively (P > 0.05) (Table 3).
DISCUSSION
We have demonstrated that L-744,832, a potent and selective inhibitor of the enzyme FPTase (31), leads to the rapid regression of mammary and salivary tumors in MMTV–v-Ha-ras transgenic mice by inducing high levels of apoptosis. The induction of apoptosis in this setting is of particular significance, since a variety of studies have demonstrated that ras activation correlates with cellular resistance to apoptosis (1, 4, 35, 44). In fact, the MMTV–v-Ha-ras tumors exhibit only modest growth inhibition and a slight increase in apoptosis in response to doxorubicin, a DNA-damaging agent that efficiently induces apoptosis in a variety of cell types (56).
Apoptotic cell death in response to many commonly used cytotoxic agents has been shown to be p53 dependent (37, 38, 53), and thus the p53 status of human tumors is of considerable clinical relevance. In general, DNA-damaging agents induce p53-dependent apoptosis, while non-DNA-damaging agents often function in a p53-independent manner (53), although there are multiple exceptions to this generalization (39, 43, 50). To determine whether p53 mediates the apoptotic response of ras tumors to L-744,832, MMTV–v-Ha-ras mice were interbred with p53-deficient mice. Tumors arising in ras/p53−/− mice were found to regress at least as efficiently as ras/p53+/+ mouse tumors, indicating that the response is largely p53 independent. However, the induction of apoptosis was somewhat reduced in the p53-deficient mice, suggesting that there is some p53 dependence to the apoptotic response. This in vivo result is consistent with experiments with cultured cells which demonstrate that an FTI can induce p53-independent apoptosis in v-Ha-ras-transformed rodent fibroblasts when the cells are grown in an anchorage-independent manner (34).
If tumor response to L-744,832 is mediated primarily through the induction of apoptosis, one might predict that tumors with high spontaneous apoptosis levels would be less responsive to this agent. Overexpression of the c-myc proto-oncogene is associated both with increased proliferation, as well as increased susceptibility to apoptosis (1, 11), and we have previously demonstrated that tumors from MMTV–v-Ha-ras/MMTV–c-myc double transgenic mice exhibit markedly elevated levels of spontaneous apoptosis, compared to MMTV–v-Ha-ras mouse tumors (21). Here we show that ras/myc tumors also responded efficiently to L-744,832, although only a slight elevation in apoptosis was observed. Instead, L-744,832 appeared to induce regression in these tumors primarily through suppression of cell cycle transit.
These findings suggest that the effect of Ras inhibition on tumor response is affected by the presence of genetic alterations that abrogate growth control at the G1/S boundary. In tumors with an intact G1 checkpoint, inhibition of Ras relieves the suppression of apoptosis. However, in the presence of genetic alterations that impair the G1 checkpoint and therefore enhance Ras′ proliferative signals, such as p53 loss or c-myc overexpression, Ras inhibition by L-744,832 results in suppression of cell cycle transit. Interestingly, the mechanism of tumor response in the ras/p53−/− tumors was mediated by both an increase in apoptosis and a decrease in the tumor cell S-phase fraction.
While inhibition of farnesylation, and therefore function, of the Ras oncoprotein provided the initial focus for the development of FTIs, recent studies have suggested that Ras is not the only mediator of the biological effect of these compounds (45, 48). Many proteins have been identified as substrates of FPTase (15). Indeed, incorporation of mevalonate, a precursor in the isoprenoid biosynthetic pathway, was inhibited for approximately 20 proteins following treatment of cultured cells with an FTI (26). Furthermore, recent evidence suggests that the farnesylated protein RhoB, a member of the Rho family of proteins that regulate the actin cytoskeleton, may mediate the suppression of the transformed phenotype upon treatment with FTIs (33). Thus, inhibition of farnesylation of proteins in addition to Ras may also contribute to the change in the level of tumor apoptosis and cell cycle parameters observed following treatment with L-744,832.
It is unlikely that this effect of L-744,832 is due to nonspecific toxicity, since regression was not observed in tumors harboring a c-neu transgene controlled by the same promoter-enhancer sequences used in the ras and myc transgenes. Similarly, we failed to see regression of ocular melanomas arising in transgenic mice expressing the simian virus 40 T antigen under the control of the tyrosinase promoter (Tyr-Tag) following treatment with L-744,832 (data not shown). These results suggest that the ability of tumors to regress following treatment with L-744,832 is dependent upon the presence of an activated Ha-ras oncogene. The possibility that the tumors in the c-neu and Tyr-Tag mice harbor activated N- or K-ras genes cannot be ruled out. It has been shown that higher concentrations of an FTI are required to inhibit the anchorage-independent growth of human tumor cells harboring a mutant N- or K-ras gene than that of lines harboring a mutant H-ras gene (42). Alternatively, it is possible that Ras-mediated signal transduction pathways are simply not critical in either of these tumor types. It will therefore be important to test this class of agents in animal models in which tumor development is known to be dependent upon Ras signaling but in which the H-ras gene is not mutated.
Human cancers develop as a result of the progressive accumulation of genetic alterations in genes whose protein products play critical roles in cell proliferation (29, 52). Here we demonstrate that L-744,832 is effective in transgenic mouse tumors harboring multiple defined genetic alterations. This result is consistent with previous demonstrations of efficacy of FTIs in cell culture against human tumor cell lines which are known to contain multiple defined genetic alterations, including cell lines containing an activated ras gene and a mutated p53-encoding gene (42, 48). Indeed, the relatively long latency period and the stochastic manner in which tumors arise in the MMTV–v-Ha-ras oncomouse model suggest that these tumors harbor multiple genetic alterations (49), although the nature of the additional genetic changes is not known. Together, these data suggest that the FTIs might have broad clinical utility against human tumors. It is particularly encouraging that L-744,832 induces regression in tumors lacking functional p53, since mutation of p53 occurs with high frequency in human tumors and has been associated with resistance to the induction of apoptosis by a variety of forms of treatment, including chemotherapy and irradiation. Additionally, our data on the mechanism of action of L-744,832 suggest that an FTI used in combination with other classes of chemotherapeutics might offer additive or perhaps synergistic antitumor activity. Nonetheless, determination of the ultimate utility of FTIs against human cancers must await validation in clinical trials.
ACKNOWLEDGMENTS
We thank E. Scolnick for helpful discussions.
This work was supported in part by American Cancer Society grant DHP-150 to J.J.W.
REFERENCES
- 1.Arends M J, McGregor A H, Toft N J, Brown E J H, Wyllie A H. Susceptibility to apoptosis is differentially regulated by c-myc and mutated Ha-ras oncogenes and is associated with endonuclease availability. Br J Cancer. 1993;68:1127–1133. doi: 10.1038/bjc.1993.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 3.Bernhard E J, Kao G, Cox A D, Sebti S M, Hamilton A D, Muschel R J, McKenna W G. The farnesyltransferase inhibitor FTI-277 radiosensitizes H-ras-transformed rat embryo fibroblasts. Cancer Res. 1996;56:1727–1730. [PubMed] [Google Scholar]
- 4.Billadeau D, Jelinek D F, Shah N, LeBien T W, Ness B V. Introduction of an activated N-ras oncogene alters the growth characteristics of the interleukin 6-dependent myeloma cell line ANBL6. Cancer Res. 1995;55:3640–3646. [PubMed] [Google Scholar]
- 5.Bos J L. The ras gene family and human carcinogenesis. Mutat Res. 1988;195:255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- 6.Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 7.Cox A D, Garcia A M, Westwick J K, Kowalczyk J J, Lewis M D, Brenner D A, Der C J. The CAAX peptidomimetic compound B581 specifically blocks farnesylated, but not geranylgeranylated or myristylated, oncogenic Ras signaling and transformation. J Biol Chem. 1994;269:19203–19206. [PubMed] [Google Scholar]
- 8.Dexter D L, Diamond M, Creveling J, Chem S-F. Chemotherapy of mammary carcinomas arising in ras transgenic mice. Invest New Drugs. 1993;11:161–168. doi: 10.1007/BF00874150. [DOI] [PubMed] [Google Scholar]
- 9.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A M, Jr, Butel J S, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 10.Dunnett C W. A multiple comparison procedure for comparing several treatments with a control. J Am Statistical Assoc. 1955;50:1096–1121. [Google Scholar]
- 11.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 12.Fan S, El-Deiry W S, Bae I, Freeman J, Jondle D, Bhatia K, Fornace A J, Jr, Magrath I, Kohn K W, O’Connor P M. p53 gene mutations are associated with decreased sensitivity of human lymphoma cells to DNA damaging agents. Cancer Res. 1994;54:5824–5830. [PubMed] [Google Scholar]
- 13.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs J B. Ras C-terminal processing enzymes—new drug targets? Cell. 1991;65:1–4. doi: 10.1016/0092-8674(91)90352-y. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs J B, Oliff A. The potential of farnesyltransferase inhibitors as cancer chemotherapeutics. Annu Rev Pharmacol Toxicol. 1997;37:143–166. doi: 10.1146/annurev.pharmtox.37.1.143. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs J B, Oliff A, Kohl N E. Farnesyltransferase inhibitors: Ras research yields a potential cancer therapeutic. Cell. 1994;77:175–178. doi: 10.1016/0092-8674(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 17.Hancock J F, Magee A I, Childs J E, Marshall C J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg Y, Tamhane A C. Multiple comparison procedures. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 19.Hollander M, Wolfe D A. Nonparametric statistical methods. New York, N.Y: John Wiley & Sons, Inc.; 1973. [Google Scholar]
- 20.Hollstein M, Rice K, Greenblatt M S, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris C C. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 21.Hundley J E, Koester S K, Troyer D A, Hilsenbeck S G, Barrington R E, Windle J J. Differential regulation of cell cycle characteristics and apoptosis in MMTV-myc and MMTV-ras mouse mammary tumors. Cancer Res. 1997;57:600–603. [PubMed] [Google Scholar]
- 22.Hundley J E, Koester S K, Troyer D A, Hilsenbeck S G, Subler M A, Windle J J. Increased tumor proliferation and genomic instability without decreased apoptosis in MMTV-ras mice deficient in p53. Mol Cell Biol. 1997;17:723–731. doi: 10.1128/mcb.17.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson J H, Cochrane C G, Bourne J R, Solski P A, Buss J E, Der C J. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc Natl Acad Sci USA. 1990;87:3042–3046. doi: 10.1073/pnas.87.8.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James G L, Brown M S, Cobb M H, Goldstein J L. Benzodiazepine peptidomimetic BZA-5B interrupts the MAP kinase activation pathway in H-Ras-transformed Rat-1 cells, but not in untransformed cells. J Biol Chem. 1994;269:27705–27714. [PubMed] [Google Scholar]
- 25.James G L, Goldstein J L, Brown M S, Rawson T E, Somers T C, McDowell R S, Crowley C W, Lucas B K, Levinson A D, Marsters J J C. Benzodiazepine peptidomimetics: potent inhibitors of Ras farnesylation in animal cells. Science. 1993;260:1937–1942. doi: 10.1126/science.8316834. [DOI] [PubMed] [Google Scholar]
- 26.James G L, Goldstein J L, Pathak R K, Anderson R G W, Brown M S. PxF, a prenylated protein of peroxisomes. J Biol Chem. 1994;269:14182–14190. [PubMed] [Google Scholar]
- 27.Jansen B, Schlagbauer-Wadl H, Eichler H G, Wolff K, van Elsas A, Schrier P I, Pehamberger H. Activated N-ras contributes to the chemoresistance of human melanoma in severe combined immunodeficiency (SCID) mice by blocking apoptosis. Cancer Res. 1997;57:362–365. [PubMed] [Google Scholar]
- 28.Kato K, Cox A D, Hisaka M M, Graham S M, Buss J E, Der C J. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci USA. 1992;89:6403–6407. doi: 10.1073/pnas.89.14.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knudson A G. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohl N E, Mosser S D, deSolms S J, Giuliani E A, Pompliano D L, Graham S L, Smith R L, Scolnick E M, Oliff A, Gibbs J B. Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science. 1993;260:1934–1937. doi: 10.1126/science.8316833. [DOI] [PubMed] [Google Scholar]
- 31.Kohl N E, Omer C A, Conner M W, Anthony N J, Davide J P, deSolms S J, Giuliani E A, Gomez R P, Graham S L, Hamilton K, Handt L K, Hartmen G D, Koblan K S, Kral A M, Miller P J, Mosser S D, O’Neill T J, Rands E, Schaber M D, Gibbs J B, Oliff A. Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in ras transgenic mice. Nat Med. 1995;1:792–797. doi: 10.1038/nm0895-792. [DOI] [PubMed] [Google Scholar]
- 32.Kohl N E, Wilson F R, Mosser S D, Giuliani E A, deSolms S J, Conner M W, Anthony N J, Holtz W J, Gomez R P, Lee T-J, Smith R L, Graham S L, Hartmen G D, Gibbs J B, Oliff A. Farnesyltransferase inhibitors block the growth of ras-dependent tumors in nude mice. Proc Natl Acad Sci USA. 1994;91:9141–9145. doi: 10.1073/pnas.91.19.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lebowitz P F, Davide J P, Prendergast G C. Evidence that farnesyltransferase inhibitors suppress Ras transformation by interfering with Rho activity. Mol Cell Biol. 1995;15:6613–6622. doi: 10.1128/mcb.15.12.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebowitz P F, Sakamur D, Prendergast G C. Farnesyl transferase inhibitors induce apoptosis of ras-transformed cells denied substratum attachment. Cancer Res. 1997;57:708–713. [PubMed] [Google Scholar]
- 35.Lin H J, Eviner V, Prendergast G C, White E. Activated H-ras rescues E1A-induced apoptosis and cooperates with E1A to overcome p53-dependent growth arrest. Mol Cell Biol. 1995;15:4536–4544. doi: 10.1128/mcb.15.8.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowe S W. Cancer therapy and p53. Curr Opin Oncol. 1995;7:547–553. doi: 10.1097/00001622-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Lowe S W, Bodis S, McClatchey A, Remington L, Ruley H E, Fisher D E, Housman D E, Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- 38.Lowe S W, Ruley H E, Jacks T, Housman D E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 39.Malcomson R D G, Oren M, Wyllie A H, Harrison D J. p53-independent death and p53-induced protection against apoptosis in fibroblasts treated with chemotherapeutic drugs. Br J Cancer. 1995;72:952–957. doi: 10.1038/bjc.1995.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosteller F, Tukey J W. Data analysis and regression, a second course in statistics. Reading, Mass: Addison-Wesley Publishing Co.; 1977. [Google Scholar]
- 41.Muller W J, Sinn E, Pattengale P K, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 42.Nagasu T, Yoshimatsu K, Rowell C, Lewis M D, Garcia A M. Inhibition of human tumor xenograft growth by treatment with the farnesyl transferase inhibitor B956. Cancer Res. 1995;55:5310–5314. [PubMed] [Google Scholar]
- 42a.National Institutes of Health. Guide for the care and use of laboratory animals. Washington, D.C: U.S. Government Printing Office; 1985. [Google Scholar]
- 43.Nip J, Strom D K, Fee B E, Zambetti G, Cleveland J L, Hiebert S W. E2F-1 cooperates with topoisomerase II inhibition and DNA damage to selectively augment p53-independent apoptosis. Mol Cell Biol. 1997;17:1049–1056. doi: 10.1128/mcb.17.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nooter K, Boersma A W M, Oostrum R G, Burger H, Jochemsen A G, Stoter G. Constitutive expression of the c-H-ras oncogene inhibits doxorubicin-induced apoptosis and promotes cell survival in a rhabdomyosarcoma cell line. Br J Cancer. 1995;71:556–561. doi: 10.1038/bjc.1995.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prendergast G C, Davide J P, deSolms S J, Giuliani E A, Graham S L, Gibbs J B, Oliff A, Kohl N E. Farnesyltransferase inhibition causes morphological reversion of ras-transformed cells by a complex mechanism that involves regulation of the actin cytoskeleton. Mol Cell Biol. 1994;14:4193–4202. doi: 10.1128/mcb.14.6.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiss Y, Goldstein J L, Seabra M C, Casey P J, Brown M S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990;62:81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- 47.Schaber M D, O’Hara M B, Garsky V M, Mosser S D, Bergstrom J D, Moores S L, Marshall M S, Friedman P A, Dixon R A F, Gibbs J B. Polyisoprenylation of Ras in vitro by a farnesyl-protein transferase. J Biol Chem. 1990;265:14701–14704. [PubMed] [Google Scholar]
- 48.Sepp-Lorenzino L, Ma Z, Rands E, Kohl N E, Gibbs J B, Oliff A, Rosen N. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 1995;55:5302–5309. [PubMed] [Google Scholar]
- 49.Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987;49:465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 50.Strasser A, Harris A W, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 51.Sun J, Qian Y, Hamilton A D, Sebti S M. Ras CAAX peptidomimetic FTI 276 selectively blocks tumor growth in nude mice of a human lung carcinoma with K-ras mutation and p53 deletion. Cancer Res. 1995;55:4243–4247. [PubMed] [Google Scholar]
- 52.Vogelstein B, Kinzler K W. The multistep nature of cancer. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 53.Weinstein J N, Myers T G, O’Connor P M, Friend S H, Fornace A J, Jr, Kohn K W, Fojo T, Bates S E, Rubinstein L V, Anderson N L, Buolamwini J K, van Osdol W W, Monks A P, Scudiero D A, Sausville E A, Zaharevitz D W, Bunow B, Viswanadhan V N, Johnson G S, Wittes R E, Paull K D. An information-intensive approach to the molecular pharmacology of cancer. Science. 1997;275:343–349. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- 54.Westfall P H, Young S S. Resampling-based multiple testing. New York, N.Y: John Wiley & Sons, Inc.; 1993. [Google Scholar]
- 55.Willumsen B M, Norris K, Papageorge A G, Hubbert N L, Lowy D R. Harvey murine sarcoma virus p21 ras protein: biological and biochemical significance of the cysteine nearest the carboxy terminus. EMBO J. 1984;3:2581–2585. doi: 10.1002/j.1460-2075.1984.tb02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu G S, El-Deiry W S. Apoptotic death of tumor cells correlates with chemosensitivity, independent of p53 or Bcl-2. Clin Cancer Res. 1996;2:623–633. [PubMed] [Google Scholar]
- 57.Yonish-Rouach E. The p53 tumour suppressor gene: a mediator of a G1 growth arrest and of apoptosis. Experientia. 1996;52:1001–1007. doi: 10.1007/BF01920109. [DOI] [PubMed] [Google Scholar]
- 58.Zhang F L, Casey P J. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]