Abstract
Inflammasomes are essential regulators of innate immunity, inflammation, and cellular apoptosis, and they have surfaced as significant modulators of cancer progression and regulation. Inflammasomes are macromolecular complexes assembled in response to damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). They induce inflammation via the oligomerization and activation of caspases. These cysteine proteases cleave the pro-inflammatory cytokines IL-1β and IL-18 into their physiologically active mature versions. Recent discoveries reveal that inflammasomes are implicated not only in infections but also in malignancies, suggesting a significant connection between inflammation and tumor development. This article emphasizes that inflammasomes cause pyroptosis in a variety of immune cells, such as dendritic cells, macrophages, T cells, and fibroblasts, in addition to tumor cells. The induction of CD8+ T cells allows inflammasomes to commence an immunological response against the tumor, successfully inhibiting its growth and progression. The inflammasome comprises four main types: NLRP1, NLRP3, NLRC4, and AIM2. Nevertheless, the inflammasomes are activated by infection, injury, or stimulation of host cells, thus triggering the inflammatory response. The essential roles of the NLRP1, NLRP3, NLRC4, and AIM2 inflammasomes are emphasized in both tumors and immune cells. Furthermore, the article provides an overview of inhibitors targeting various tumor inflammasome pathways currently in clinical trials. Here, in this review, we underscore the role of the inflammatory response in cancer progression and highlight the significance of inflammasomes in regulating immune cells within the tumor microenvironment. Targeting these inflammasomes offers novel strategies for cancers.
Keywords: Inflammasome, Immunotherapy, Tumor microenvironment, Immune cells
Introduction
Cancer is one of the most prevalent causes of death worldwide, and its prevalence is continuously increasing [1]. In the process of tumor development, inflammation is widely regarded as a crucial factor. Early in the mid-nineteenth century, researchers first discovered the connection between inflammation and cancer [2]. Inflammation plays a role in the progression of cancer and is closely associated with the efficacy of anticancer treatments [3]. Inflammation has the potential to improve the efficacy of the antitumor immune response by promoting the infiltration and activity of immune cells. The growth and development of malignancies can be regulated by the infiltration and activity of adaptive and innate immune cells [4]. These immune cells may inhibit the progression of tumors through direct killing of tumor cells [5], induction of apoptosis [6], and suppression of angiogenesis [7].
Macrophages, Natural Killer (NK) cells, and dendritic cells (DCs) are essential components of the innate immune system [8]. Innate immune cells serve as the primary defence against pathogens by identifying pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) via pattern recognition receptors (PRRs), therefore generating immune responses that restore tissue homeostasis. The inflammasome, a multiprotein complex, is pivotal to this process since it regulates immunological and inflammatory responses [9]. Four well-characterized canonical inflammasome types are NLRP1, NLRP3, NLRC4, and AIM2. Inflammasomes are triggered in response to infections, cellular damage, or stress signals, leading to the maturation and release of pro-inflammatory cytokines such as IL-1β and IL-18 [10]. These cytokines are crucial in activating host defences, encompassing the recruitment of immune cells and the enhancement of anti-pathogen responses.
We have found that tumor immunotherapy is emerging as a new therapy for cancer patients in recent years. Treatment strategies include mainly modifying the tumor microenvironment (TME) and the use of immune checkpoint inhibitors and the like. However, there is a limited understanding of interactions among multiple types of immune cells and molecules associated with immunotherapy. The inflammasome has consequently become a most scrutinized topic in this cutting-edge field. Recent advances in inflammasome biology, particularly the discovery of non-canonical pathways (caspase-4/11 activation by cytoplasmic lipopolysaccharide) and their tumor-specific roles, have redefined its therapeutic potential [11]. Emerging evidence highlights inflammasome-mediated pyroptosis as a novel immunogenic cell death mechanism, bridging innate and adaptive anti-tumor responses [12]. Recent studies have shown that the inflammasome is involved in the inflammatory process and may greatly promote the effect of immunotherapy [13, 14]. Activation of the inflammasome is tightly related to control of immune cells and processes such as cell pyroptosis [15], prompting the investigation of its potential to mediate in tumor immunotherapy.
Not only does incorporating the potential role of inflammasome offer a deeper understanding of immunotherapy mechanisms, but it also provides novel opportunities for future research and clinical practice of immunotherapy. However, it could enable the creation of breakthroughs in personalized treatment, higher response rates to treatment, and overcoming immunotherapy resistance. New and more effective therapeutic approaches for cancer patients are offered by it.
In the present review, we give a complete survey regarding the structures and activation mechanisms of four inflammasomes and the functions of inflammasomes in different immune cells and their interactions within the tumor. We further investigate the function of inflammasome activation in anti-tumor immunity and comment on inflammasome-targeted inhibitors in clinical trials and potential targets for future anti-tumor immunotherapy. While previous reviews have focused on canonical NLRP3 signaling, emerging evidence reveals subtype-specific roles: NLRP1 promotes metastasis in hormone-resistant breast cancers via IL-18-mediated angiogenesis, while AIM2 paradoxically suppresses glioblastoma growth through interferon-γ (IFN-γ)crosstalk [16]. Our work distinctively synthesizes these divergent mechanisms with translational therapeutics, including first-in-class NLRP3 inhibitors in Phase II trials for PD-1 refractory melanoma [17].
Inflammasome biology and activation
The inflammasome concept was first introduced by Dr. Jurg Tschopp in 2002 [18]. Since then, extensive research has been conducted to elucidate the structure, activation mechanisms, and functions of different types of inflammasomes. The inflammasome is an essential element of the innate immune system, tasked with triggering an immune response to diverse microbial infections and danger signals [19]. It is a multi-protein complex consisting of PRRs, such as NOD-like receptors (NLRs) or absent in melanoma 2 (AIM2)-like receptors, adaptor proteins, and caspase-1 [20]. After assembly, the inflammasome facilitates the oligomerization of pro-caspase-1 into its enzymatically active form, which cleaves precursor cytokines (pro-IL-1β and pro-IL-18) into their mature, bioactive isoforms [21]. Inflammasome governs pyroptosis, a type of cellular demise characterized by caspase-1/4/5/11-dependent inflammatory programmed cell death. Unlike apoptosis, which is a non-inflammatory planned cell death, pyroptosis is caused by gasdermin family protein-mediated membrane hole creation, which increases innate immune responses by causing cell swelling, lysis, and the production of pro-inflammatory cytokines such as IL-1β and IL-18 [22].
Moreover, inflammasomes are categorized into canonical and non-canonical pathways based on their activation mechanisms and effector caspases. Canonical inflammasomes (NLRP1, NLRP3, NLRC4, AIM2) rely on caspase-1 to cleave pro-IL-1β and pro-IL-18 into active cytokines, triggering pyroptosis via gasdermin D (GSDMD) [23]. In contrast, non-canonical inflammasomes are activated by caspase-4/5/11 in response to cytosolic lipopolysaccharide (LPS), directly cleaving GSDMD to induce pyroptosis without processing IL-1β/IL-18 [24, 25]. These pathways differ in structure and function: canonical inflammasomes require sensor proteins (NLRs, AIM2) and adaptor ASC for caspase-1 recruitment, while non-canonical pathways bypass ASC and utilize caspase-4/5/11 as both sensors and effectors [25] (Table 1).
Table 1.
Main differences between canonical and non-canonical inflammasomes
| Feature | Canonical inflammasomes | Non-canonical inflammasomes | Refs. |
|---|---|---|---|
| Key caspase | Caspase-1 | Caspase-4/5/11 | [20, 24, 25] |
| Activation trigger | PAMPs (bacterial toxins), DAMPs (ATP), K+ efflux, ROS | LPS, oxidized phospholipids (oxPAPC) | |
| Structural components |
NLRs (NLRP1/3, NLRC4) AIM2 |
Caspase-4/5/11 (direct LPS sensing) | |
| Cytokine processing | Cleaves pro-IL-1β/IL-18 | Does not directly process cytokines | |
| Pyroptosis mechanism | GSDMD cleavage pore formation | GSDMD cleavage pore formation | |
| Role in tumor immunity | Dual role (pro-tumorigenic via IL-1β; anti-tumor via pyroptosis) | Amplifies inflammation, promotes metastasis |
Overall, the inflammasome serves as a vital component of the innate immune system, essential for maintaining immune balance and safeguarding against infections and detrimental stimuli. To enhance comprehension of the inflammasome activation mechanism, we will provide a concise summary of the various categories of inflammasomes (Fig. 1). These activation mechanisms of canonical and non-canonical inflammasomes not only underpin innate immune responses but also critically shape the TME. Their ability to regulate cytokine release and pyroptosis in immune cells positions them as pivotal modulators of anti-tumor immunity, as elaborated in the following sections.
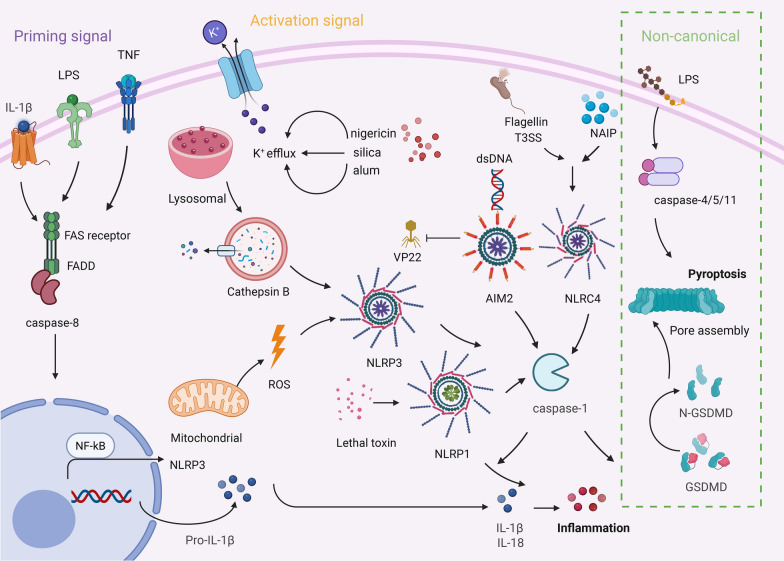
Fig. 1.
Activation mechanism of canonical and non-canonical inflammasome. Canonical inflammasomes are triggered by pattern recognition receptors (NLRP3, NLRC4, AIM2) by the detection of PAMPs or DAMPs. A two-step signaling process is involved: priming signal (the expression of NLRP3 and IL-1β precursor induced by TLR ligands or TNF-α through the NF-κB pathway) and an activation phase (K + efflux and lysosomal degradation triggering NLRP3 oligomerization), followed by the recruitment of the adaptor protein ASC and the activation of caspase-1. Subsequently, IL-1β/IL-18 precursors and the GSDMD were cleaved to liberate mature inflammatory mediators and initiate pyroptosis. Non-canonical inflammasomes are directly activated through the recognition of intracellular LPS by caspase-4/5/11, independent of ASC, leading to pyroptosis via GSDMD cleavage, while also indirectly activating the classical NLRP3 pathway through K+ efflux or mitochondrial ROS, thereby creating a cascade that amplifies the inflammatory response. The green box signifies the non-canonical inflammasome
Canonical inflammasomes
NLRP1
NLRP1 (also known as NALP1), initially identified as a protein containing NACHT, LRR, and PYD domains characteristic of the NLR family, plays a critical role in inflammasome activation [18]. While most NLRP1 is present in mammals, there is one NLRP1 gene in humans and several homologous NLRP1 genes (NLRP1a, NLRP1b, and NLRP1c) in mice [26]. Furthermore, NLRP1 can be activated through various mechanisms, including exposure to anthrax lethal toxin [26], Shigella flexneri [27], DPP9 (dipeptidyl peptidase) [28], viral infection [29], toxoplasma [30], and certain metabolic inhibitors. After activation, NLRP1 has the capacity to release pro-inflammatory cytokines, including IL-1β and IL-18 [18]. Overall, NLRP1 functions as a crucial sensor protein within the inflammasome, significantly contributing to the immune response against pathogens and the modulation of inflammatory processes.
NLRP3
NLRP3 is widely known as the most characterized and exploited inflammasome in the NLR protein family. It is located mainly in diverse immune cells, such as macrophages and DCs [31]. Moreover, NLRP3 is widely expressed and plays a critical role in the regulation of inflammation and immunity.
For the activation of the NLRP3 inflammasome, two signals are required, which are called priming signals and activating signals [32]. IL-1β/IL-1R1, lipopolysaccharide (LPS)/Toll like receptor 4 (TLR4), and tumor necrosis factor (TNF)/TNF receptor (TNFR) transmit the priming signal, and all these factors activate NF-kB [33]. The upstream regulators of NF-κB include Caspase-8, an apoptotic caspase, and Fas-associated protein with death domain, which in turn leads to the overexpression of NLRP3 and the production of pro-IL-1β [33, 34]. Simultaneously, priming signals promote the assembly of ASC and caspase-1 through various stimuli (such as K+ efflux [35], ROS production [36], release of lysosomal cathepsin B [37], and DAMPs, etc.), resulting in the activation of the NLRP3 inflammasome complex. The primary mode of activation is induced by K+ efflux. Mitochondrial dysfunction [38], disrupting signals leading to ROS production, can mediate NLRP3 activation. Lysosomal rupture results in the cytoplasmic release of cathepsin B, and the phagocytosis of particulate material activates the NLRP3 complex, forming a crystal structure upon engulfment by phagocytes. Common DAMPs include nigericin [39], silica [40], aluminum [41], etc. NLRP3 inflammasome activation induces the production of caspase-1, leading to the maturation and release of IL-1β and IL-18. Upon activation, caspase-1 cleaves the pore-forming protein GSDMD, resulting in its N-terminal region creating pores in the cell membrane [42]. This process promotes the release of IL-1β and IL-18, resulting in lytic types of pyroptosis and inflammation.
NLRC4
NLRC4, often referred to as IPAF, Card12, and CLAN, is a constituent of the NLR family of intracellular receptor proteins. NLRC4 is chiefly linked to bacterial flagellin and the bacterial Type III secretion system (T3SS) [43, 44]. NLRC4 proteins can directly recruit and activate pro-caspase-1 independent of ASC [45]. It can interact with the NLR family apoptosis inhibitor protein (NAIP), activating downstream processes. It can bind to the NLR family inhibitor of NAIP, initiating downstream activation of NLRC4, leading to caspase-1 activation, and subsequently triggering the inflammatory response [45]. Additionally, NLRC4 enlists and activates caspase-8, instigating GSDMD-independent cell death in the presence of caspase-1 or GSDMD inhibition [45]. Pyroptosis not only triggers inflammation in the host but also captures dangerous microorganisms via the cell membrane pore, motivating the host to launch an immune response for the eradication of the pathogens [46].
AIM2
Absent in melanoma 2 (AIM2), which does not belong to the NLR family, is part of the HIN-200 family [47]. AIM2 is a protein composed of a Pyrin domain (PYD) and hematopoietic expression, interferon-inducible nature, and nuclear localization (HIN-200) domain [48]. The HIN-200 domain can bind to dsDNA, and AIM2 plays a crucial role in sensing abnormal dsDNA within the cell [49]. It can recognize dsDNA and recruit caspase-1 in an ASC-dependent fashion [50]. Active caspase-1 facilitates the maturation of precursor forms of IL-1β and IL-18 inside the cell, which are subsequently released into the extracellular space [51]. These two cytokines play essential regulatory roles in immune responses, particularly in the response to inflammation and infection. Viral-encoded protein (VP22) can inhibit the activation of AIM2, thereby suppressing viral proliferation [52]. The formation and activation of AIM2 inflammasomes constitute an important immune and inflammatory response of the immune system to the presence of abnormal dsDNA within cells. This mechanism contributes to clearing infections, maintaining cellular homeostasis, and guiding the immune system to mount appropriate responses.
Non-canonical inflammasomes
Non-canonical inflammasomes (caspase-4/5/11) have garnered increasing attention in tumor immunology in recent years. Unlike canonical inflammasomes, non-canonical inflammasomes directly activate caspase-4/5/11 by recognizing intracellular lipopolysaccharide (LPS), inducing pyroptosis and releasing IL-1α and IL-18, thereby modulating the TME [18]. Other minor activators such as oxPAPC [53], Leishmania lipophosphoglycan [54], and hemin can also serve as activators of non-canonical inflammasomes [55]. For example, in pancreatic cancer, non-canonical activation of caspase-11 promotes tumor cell death via a GSDMD-dependent pathway and enhances chemotherapeutic drug sensitivity [56]. Furthermore, crosstalk between non-canonical inflammasomes and canonical pathways synergistically regulates immune cell infiltration. Inhibitors targeting these pathways have demonstrated anti-tumor potential in melanoma models [57]. When the immune response fails to effectively clear infections and evolves into persistent inflammation, non-canonical inflammasomes can lead to diseases such as sepsis and pulmonary infections. The results of canonical and non-canonical inflammasome activation are analogous. Both processes encourage cells to activate inflammatory programs, including pyroptosis and the secretion of inflammatory cytokines. Future studies should further elucidate cell-specific mechanisms to optimize targeted therapeutic strategies.
Inflammasomes and cancer
In the TME, inflammasomes have multiple functions in either facilitating or suppressing tumor progression, contingent upon the precise kind of inflammasome implicated. Some inflammasomes have been reported to promote tumor growth by enhancing inflammation and contributing to immune evasion mechanisms employed by the tumor [58]. On the other hand, some inflammasomes can inhibit tumor growth by promoting immune responses, activating anti-tumor immune cells, and enhancing tumor cell death [59]. The role of inflammasomes in the TME exhibits highly context-dependent characteristics, governed by the interplay of tumor type, cellular origin, and crosstalk with oncogenic signaling pathways [60]. For instance, in colorectal cancer (CRC), NLRP3 drives inflammation-associated carcinogenesis via the IL-1β/NF-κB signaling axis while suppressing metastasis through GSDMD-mediated pyroptosis [61]. However, NLRP3 activation in breast cancer suppresses tumor progression by promoting immunogenic cell death and enhancing CD8⁺ T cell infiltration [62]. Cellular origin further dictates functional divergence: macrophage-derived NLRP3 recruits myeloid-derived suppressor cells (MDSCs) via IL-1β secretion to fuel pancreatic cancer growth, while dendritic cell-derived NLRP3 enhances antigen presentation and T-cell activation, thereby inhibiting tumor progression [63]. Additionally, inflammasomes interact dynamically with oncogenic pathways such as hypoxia, metabolic reprogramming, and epithelial-mesenchymal transition (EMT) [12]. Hypoxia-induced NLRP3 promotes glycolysis and angiogenesis in renal cell carcinoma (RCC) through metabolic reprogramming [64]. Besides, NLRP3 inhibition suppresses EMT in non-small cell lung cancer by blocking TGF-β signaling [65]. Clinically, therapeutic outcomes of inflammasome-targeted agents like the NLRP3 inhibitor MCC950 (an NLRP3 inhibitor) are context-dependent: it effectively reduces colitis-associated cancers but fails to inhibit pancreatic ductal adenocarcinoma, underscoring the necessity for precision-targeted therapies tailored to tumor-specific contexts [66]. These findings highlight the dual roles of inflammasomes in cancer biology and emphasize the importance of dissecting microenvironmental determinants to optimize therapeutic strategies.
Emerging clinical data highlight the interplay between inflammasome activation and systemic inflammatory biomarkers. In addition to IL-1β and IL-18, inflammasomes indirectly elevate C-reactive protein (CRP) levels by stimulating IL-6 secretion, which subsequently activates hepatic CRP production [67]. Elevated serum ferritin, a marker of inflammation and cellular damage, correlates with NLRP3-driven pyroptosis in tumor-associated macrophages and predicts poor prognosis in colorectal and breast cancers [68]. Furthermore, neutrophil-to-lymphocyte ratio (NLR) and erythrocyte sedimentation rate (ESR) have been linked to inflammasome activity in cancer patients, reflecting chronic inflammation and immune dysregulation [69]. These biomarkers provide clinically actionable thresholds for monitoring inflammasome-related therapeutic responses. For instance, in non-small cell lung cancer, pretreatment hyperferritinemia is associated with reduced efficacy of anti-PD-1 therapy, suggesting that inflammasome inhibition could be a potential adjuvant strategy [70]. Integrating these markers with inflammasome profiling could refine patient stratification and therapeutic targeting.
Inflammasomes exhibit a more pronounced regulatory role in the TME than in tumor cells themselves, primarily through their activation in immune and stromal compartments. For instance, NLRP3 activation in dendritic cells enhances CD8+ T cell priming, while cancer-associated fibroblasts (CAFs) upregulate NLRP3 to drive IL-1β-dependent immunosuppression and metastasis. Similarly, macrophage-specific NLRP3 signaling polarizes tumor-associated macrophages toward a pro-tumorigenic M2 phenotype, fostering angiogenesis and immune evasion. These findings underscore that inflammasomes shape tumor progression predominantly via TME-mediated immunomodulation rather than tumor cell-autonomous mechanisms. Here, we have summarized the expression levels of inflammasomes in different tumors, indicating whether they contribute to pro-tumorigenesis or anti-tumorigenesis (Table 2).
Table 2.
Tumor-promoting function or anti-tumor effect of the inflammasome
| Types of inflammasome | Types of cancer | Pro-tumorigenesis | Anti-tumorigenesis | Expression of inflammasome in cancer | Refs. |
|---|---|---|---|---|---|
| NLRP1 | Metastatic melanoma | √ | – | [58] | |
| Breast cancer | √ | ↑ | [104] | ||
| Lung adenocarcinoma | √ | ↓ | [77] | ||
| Prostate cancer | √ | ↑ | [78] | ||
| Gastric cancer | √ | ↑ | [79] | ||
| Colitis and Colitis-Associated Tumorigenesis | √ | – | [80] | ||
| NLRP3 | Gastric cancer | √ | ↑ | [79] | |
| Colorectal cancer | √ | ↓ | [61] | ||
| Head and neck cancer | √ | ↑ | [82] | ||
| Oral squamous cell carcinoma | √ | ↓ | [72] | ||
| Prostate cancer | √ | ↑ | [78] | ||
| NLRC4 | Prostate cancer | √ | ↑ | [78] | |
| Breast cancer | √ | – | [85] | ||
| AIM2 | Cutaneous squamous cell carcinoma | √ | ↑ | [95] | |
| Colorectal cancer | √ | – | [61] | ||
| Breast cancer | √ | – | [73] | ||
| Non-small-cell lung cancer | √ | ↑ | [96] | ||
| Hepatocellular carcinoma | √ | ↓ | [167] | ||
| Renal carcinoma | √ | ↓ | [192] | ||
| Caspase-11 | Breast cancer | √ | ↑ | [73] | |
| Caspase-4/5/11 | Colorectal cancer | √ | ↑ | [61] | |
| Caspase-4 | Pancreatic ductal adenocarcinoma | √ | ↓ | [137] |
√: Indicates a promoting (pro-tumorigenesis) or inhibitory (anti-tumorigenesis) role
↑/↓: Upregulated or downregulated expression in cancer compared to normal tissue
– No available data
Inflammasome abbreviations: NLRP1, NLRP3, NLRC4, AIM2
Recent research has emphasized the suppressive impact of inflammasomes on tumor development. For instance, the research reveals a protective role of NLRP1 in gastrointestinal inflammation and tumorigenesis. NLRP1b−/− mice exhibit significantly increased incidence, inflammation, and tumor burden, correlating with reduced levels of IL-1β and IL-18 [71]. This highlights the involvement of NLRP1 in colonic epithelial cell compartments, mitigating tumor development, with significant implications for host immune responses during IBD and cancer [71]. Furthermore, the significant downregulation of NLRP3 expression in oral squamous cell carcinoma cells markedly reduced caspase-1 cleavage and IL-1β production, inhibiting cell proliferation, migration, and invasion [72]. This led to heightened E-cadherin expression and diminished N-cadherin expression, ultimately resulting in a significant reduction in the proliferation of oral squamous cell carcinoma [72]. The expression of AIM2 suppresses the proliferation and tumorigenicity of human breast cancer cells [73]. AIM2 gene therapy inhibits the proliferation of breast cancers in an in situ tumor model [73]. AIM2 promotes apoptosis in tumor cells while simultaneously suppressing NF-κB activation, indicating its prospective utility as a therapeutic gene for breast cancer in the future [73]. Here, it is noteworthy that the precise mechanisms of inflammasome function in various cancer types require further investigation.
On the contrary, whereas inflammasomes may have pro-tumorigenic effects in certain situations, they have also been demonstrated to play a tumor-promoting role in the progression of certain cancers. The research indicates that NLRP1 facilitates melanoma proliferation by augmenting inflammasome activation and suppressing apoptotic pathways, highlighting its oncogenic function in cancer cells [74]. NLRP1 is extensively expressed in 83% of initial breast cancer tissues, with expression levels significantly exceeding those in surrounding non-cancerous tissues [75]. Moreover, NLRP1 enhances the proliferation, migration, invasion, and tumorigenicity of the breast cancer cell line MCF-7, and is positively associated with lymph node metastases, TNM staging, and Ki-67 levels, while facilitating EMT [75, 76]. Bioinformatic examination of lung adenocarcinoma (LUAD) data obtained from TCGA and GEO indicated that NLRP1 expression is markedly diminished in LUAD compared to normal lung tissue, and it correlates with pathology, T staging, and N staging [77]. Low expression of NLRP1 is an independent adverse prognostic factor in LUAD, and its expression level is positively correlated with immune cell infiltration in the TME, emphasizing the important role of NLRP1 in LUAD prognosis and immune regulation [77]. NLRP1 and NLRC4 expressions in prostate cancer are elevated compared to normal prostate tissues, and NLRP1 and NLRC4 expressions in prostate cancer are favorably correlated with IL-1β and IL-18 levels, also significantly positively correlated with Gleason score and T staging in the Cancer Genome Atlas data [78]. The release of proinflammatory cytokines by inflammasomes [78], seems to play a significant role in the onset and development of prostate cancer.
The most well-studied inflammasome is NLRP3, which controls both the tumor and the TME composition. NLRP1 and NLRP3 are both highly expressed in gastric cancer, and their high expression is closely related to lymph node metastasis, low survival, and increased immune infiltrating cells [79]. NLRP3 shows a stronger association with immune infiltration and gastric cancer prognosis [79]. In particular, its expression is markedly increased in human CRC tissues and positively associated with tumor size, invasion, lymph node metastases, venous invasion, nerve infiltration, and TNM staging [80]. Inhibition of migration and proliferation in CRC cells, both in vitro and in vivo, was found following the knockdown of NLRP3, alongside the reversal of the EMT process in vitro [81]. In head and neck cancer, the upregulation of NLRP3 levels promotes the progression of tumor [82]. An overexpression of NLRP3 facilitates the invasive progression of prostate carcinoma (PCa) cells, whereas the silencing of NLRP3 impedes this advancement via positively modulating caspase-1 [83]. Experimental evidence demonstrates that the overexpression or inhibition of caspase-1 can reverse the effects of NLRP3 in PCa cells, ultimately suppressing tumor growth in PCa [83]. The diminished expression of the NLRP3 protein correlates with susceptibility to Crohn's disease [84]. Obesity-related NLRC4 inflammasome activation and IL-1 signaling promote the progression of breast cancer by inducing infiltrative myeloid cells, activating IL-1β, and facilitating disease development via adipocyte-mediated VEGFA production and angiogenesis [85].
Emerging evidence highlights the critical role of pathogen-associated inflammasome activation in tumorigenesis. For instance, HPV-driven oral squamous cell carcinoma (OSCC) exhibits NLRP3 upregulation, wherein viral oncoproteins E6/E7 perpetuate NF-κB-mediated IL-1β secretion, fueling chronic inflammation and EMT [86, 87]. Similarly, in EBV-associated gastric cancer, the viral latent membrane protein 1 (LMP1) activates NLRP3 via TLR/NF-κB signaling, resulting in IL-18 release and immunosuppressive microenvironment formation [88]. These findings exemplify how microbial agents exploit inflammasomes to subvert innate immunity, linking persistent infection to cancer progression. Such mechanisms underscore the therapeutic potential of targeting pathogen-inflammasome crosstalk in infection-related malignancies.
Microbial regulation of inflammasomes in cancer
An important axis in tumor immunomodulation is the interaction between the microbiota and inflammasomes [89]. The NLRP3 inflammasome is activated in CRC cells by dysbiosis, especially the enrichment of pathobionts such as Fusobacterium nucleatum, which promotes IL-1β/IL-18-dependent tumor development and metastasis [89]. Conversely, commensal-derived butyrate inhibits NLRP3 activation in intestinal epithelial cells, restoring mucosal barrier integrity and suppressing colitis-associated carcinogenesis [90]. In the TME, microbial metabolites such as propionate engage the AIM2 inflammasome in dendritic cells, amplifying STING-dependent type I interferon responses and CD8+ T cell infiltration [48]. Notably, enriched cohorts show increased NLRP3-dependent anti-PD-1 responses in melanoma models, suggesting that microbiome-driven inflammasome activation may affect the effectiveness of immunotherapy [91, 92]. These findings position microbial-inflammasome crosstalk as a therapeutic target to reprogram tumor-immune dynamics.
In addition to the NLR family inflammasomes, AIM2 is linked to tumor proliferation. In CRC, AIM2 is typically downregulated [93]. The knockdown of AIM2 enhances CRC proliferation, motility, and EMT, whereas AIM2 overexpression has a contrary effect [93, 94]. AIM2 inhibits the expression of Gli1 and regulates CRC cell proliferation and migration in a Gli1-dependent manner [93]. In cutaneous squamous cell carcinoma (cSCC), AIM2 is upregulated at both mRNA and protein levels [95]. Knockdown of AIM2 leads to decreased cell survival, increased apoptosis, and reduced invasion and angiogenesis in cSCC cells [96, 97]. The results presented here indicate that AIM2 may represent a viable target for therapy for cSCC, impacting the invasive and metastatic growth of the tumor [97]. In NSCLC, elevated AIM2 expression correlates with unfavorable prognosis and enhances tumor cell growth and proliferation irrespective of inflammasome activity [96]. Knockdown of AIM2 enhances mitochondrial fusion, reduces cell proliferation, and influences the MAPK/ERK signaling pathway by regulating MFN2 and ROS production [97]. Low expression of AIM2 is highly correlated with an unfavorable prognosis in RCC [98]. Overexpression of AIM2 inhibits the proliferation, migration, and invasion of 786-O or OSRC-2 cells, and it may suppress the malignant characteristics of RCC through the enhanced induction of autophagy [97]. Aberrant expression of AIM2i-RGs in tumor tissues is closely associated with poor prognosis, and its role in immune regulation may involve tumor immune score, stromal score, CD8+ T cell abundance, and immune checkpoint-related markers [99]. The low expression of AIM2 in CRC was further confirmed by immunohistochemistry results [97].
In summary, the function of inflammasomes in tumor proliferation is dual. In specific cancer forms, inflammasomes can inhibit tumor incidence and progression. The unique role of every given inflammasome in certain contexts is contingent upon multiple elements, including the cancer type and the activation mechanism. After outlining the dual roles of inflammasomes in tumor progression, we now delve into how these mechanisms intersect with immune cell activity to orchestrate anti-tumor responses.
Inflammasomes synergize in anti-tumor immunity
Inflammation can facilitate the onset and advancement of cancer by promoting angiogenesis, enhancing cancer cell proliferation, facilitating tumor aggressiveness, suppressing immune responses, and altering the effectiveness of specific anti-cancer drugs [97]. The key to triggering a robust anti-tumor immune response lies in the active release of tumor cells, such as tumor-associated antigens (TAAs) and other intracellular components induced by processes like pyroptosis [100]. Specifically, by activating antigen-presenting cells, such as DC, it stimulates them to capture, process, and present antigens, activating T cells and initiating an immune response [101]. These mechanisms work together to create favorable conditions for the effective initiation of an immune response against tumor cells. However, inflammasomes can also exert anti-tumor effects. Emerging evidence underscores their paradoxical roles in promoting immune suppression and metastasis [102]. Notably, NLRP3 activation within tumor-associated macrophages recruits MDSCs, which dampen cytotoxic T-cell responses and foster an immunosuppressive microenvironment [103]. In breast cancer models, NLRP3-driven IL-1β secretion induces EMT, thus accelerating metastatic dissemination [104]. These findings highlight the tumor-dependent nature of inflammasome signaling and the necessity for cell-type-specific therapeutic targeting. Additionally, NLRP3 activation-induced pyroptosis of tumor cells releases DAMPs and tumor-associated antigens such as ATP and HMGB1 [105]. These chemicals modulate anti-tumor immune responses via two separate pathways: by influencing tumor cells and immune cells, respectively (Fig. 2).
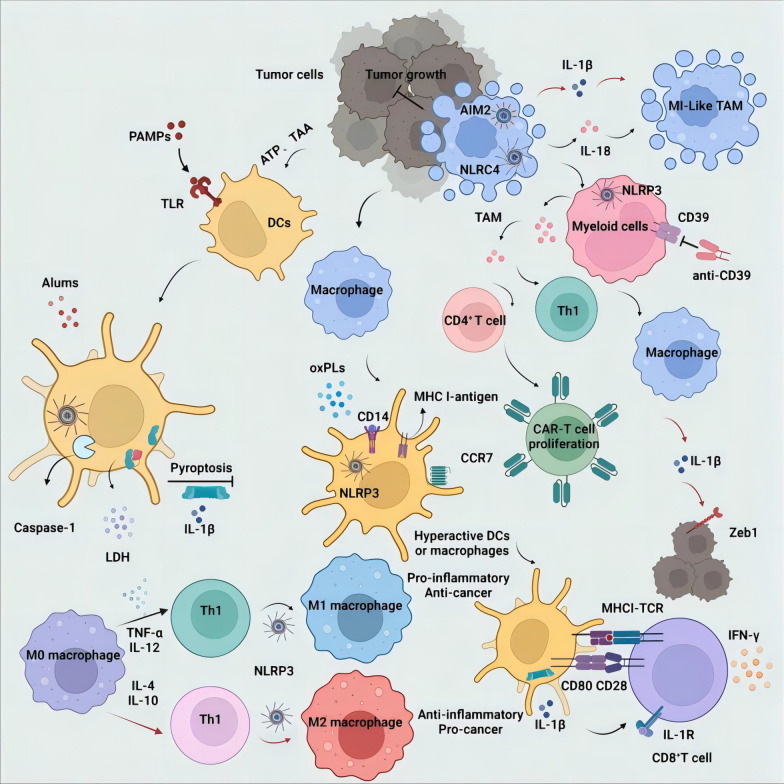
Fig. 2.
The impact of inflammasome activation on immune cells and anti-tumor immunity. Pro-tumor: Chemotherapy triggers NLRP3-mediated pyroptosis in tumor cells, releasing ATP and DAMPs that recruit immunosuppressive CD39+ myeloid cells and M2 macrophages, driving tumor progression via IL-1β-induced EMT and MDSC recruitment. Anti-tumor: NLRP3/AIM2 activation induces pyroptosis and IL-18 release, enhancing NK/CD8+ T cell cytotoxicity (via IFN-γ/PD-L1 suppression) and polarizing M1 macrophages to secrete IL-12 for Th1 activation. Spatial segregation and color-coding (red arrows: pro-tumor; black arrows: anti-tumor)
Mechanisms of pyroptosis evasion in tumor cells and immunotherapeutic implications
In the process of cancer development, tumor cells often have the ability to evade pyroptosis, facilitating their unrestricted growth and spread. Malignant tumor cells can avoid pyroptosis mainly due to functional loss mutations or epigenetic silencing of components in the inflammasome [106]. NLRP3 is presently the most thoroughly examined inflammasome. In some human tumors, the expression of the adaptor protein ASC of the NLRP3 inflammasome is regulated by methylation-induced silencing [107]. The epigenetic control of the NLRP3 inflammasome ASC in malignancies is affected by the presence of the CARD domain [108]. The inactivity of this protein in malignant malignancies is associated with their genesis and growth [109]. Additionally, inflammasomes exhibit a protective role in CRC-associated inflammation (CAC) [61]. By maintaining their components during acute and recurrent colitis and CAC, inflammasomes alleviate disease outcomes and tumor development [110]. At the same time, the lack of the ASC adaptor protein and Caspase-1 promotes disease in mouse models, and the NLRP3 gene in hematopoietic cells demonstrates that the inflammasomes act as attenuators in colitis and CAC [111]. However, it has been reported that studies have found that mice that are lacking NLRP3 or Caspase 1 are highly sensitive to azoxymethane/dextran sodium sulfate induced inflammation, resulting in an increase of the tumor burden in the colon to a significant extent [112]. Increasing IL-18 can prevent colitis associated tumors in IL18−/−, NLRP3−/− and Caspase 1−/− mice [113]. Preventing the development of colorectal tumors is largely dependent on IL-18 downstream.
The cell pyroptosis executor family, GSDM, is the only one in the downstream of the inflammasome [114]. GSDME, or DFNA5, belongs to the GSDM family [115]. GSDME inhibits malignancies, including melanoma, triple negative breast cancer, and CRC, in murine tumor models and human tumor specimens by converting apoptosis to pyroptosis [116]. NK cells during the pyroptosis process release cytotoxic granule enzyme-B onto GSDME in tumor cells, and subsequently lead to the holes forming on the cell membrane of tumor cells and ultimately lead to pyroptosis [117]. Simultaneously, tumor antigens and DAMPs are released [118]. The released chemicals elicit an in situ immunological response, thereby augmenting the cytotoxic effects of CD8+ T cells, NK cells, and natural killer T cells (NKT cells) [119]. This process contributes to strengthening the immune system’s attack against tumors.
The serum lactate dehydrogenase (LDH), serving as a marker for cell damage and pyroptosis, often signifies a poor prognosis in tumors. Cancer patients with elevated LDH levels, demonstrate diminished T cell infiltration and functionality inside the TME, along with a diminished response to checkpoint inhibitors [120]. Therefore, serum LDH is considered a potential biomarker for patients with impaired immune anti-tumor capabilities [121]. However, in patients with elevated LDH levels, it is still unclear whether pyroptosis of myeloid cells in the TME or draining lymph nodes (dLN) correlates with the attenuation of CD8+ T cell responses [120]. Hence, more accurate clinical markers are required to enhance the understanding of tumor cell pyroptosis in anti-tumor therapy.
Dendritic cell
Inflammasome activation triggers pyroptosis in both tumor and immune cells, but with divergent immunological consequences. In tumor cells, pyroptosis releases DAMPs and tumor-associated antigens, which recruit dendritic cells and enhance cross-presentation to CD8+ T cells, thereby amplifying anti-tumor immunity [122]. Conversely, pyroptosis in immune cells like dendritic cells can disrupt their survival and migration to lymph nodes, impairing their ability to prime T cells and inadvertently suppressing adaptive immune responses [56]. For instance, NLRP3 activation in dendritic cells reduces their CCR7-mediated migration to draining lymph nodes, limiting T-cell activation [123]. This duality underscores the need for cell-type-specific strategies when targeting inflammasomes for immunotherapy [124]. This suggests that GSDMD hole development and cell pyroptosis may be pivotal in modulating the activation of inflammatory dendritic cells in T cells.
Maintaining long-term vitality in DCs is crucial for achieving adaptive immunity. Studies indicate that Conventional Dendritic Cells (cDCs) exhibit greater tolerance under the stimulation of highly pathogenic pathogens or inflammasome activators such as nigericin, ATP, and FlaTox [125]. They can resist pyroptosis mediated by the inflammasome. The expression of crucial parts of the inflammasome system, such as NLRP3, ASC, caspase-1, and pro-IL-1β, is markedly diminished in cDCs [126]. The resistance of DCs to pyroptosis is linked to the transcription factors IRF8 in conventional DC type 1 (cDC1s) and IRF4 in conventional DC type 2 (cDC2s). These factors bind to the promoter sequences of inflammasome-related genes (NLRP3, AIM2), resulting in transcriptional suppression of these genes [127]. Therefore, they are identified as negative regulators of inflammasome activity within cDCs.
The role of inflammasomes is not only to stimulate cells to become hyperactive but also to trigger immune responses. Research has revealed that highly active DCs not only exhibit typical features of activated DCs, such as secreting TNF-α and IL-6, upregulating co-stimulatory molecules, and antigen presentation, but also undergo excessive migration to lymph nodes and provide IL-1β to CD8+ T cells [31]. When the Nlrp3 or Ccr7 genes are knocked out within DCs, this T cell stimulatory capacity is lost [31]. Interestingly, not all NLRP3 agonists demonstrate efficacy in anti-tumor immunity. Alum, for example, diminishes the functioning of dendritic cells, leading to inadequate activation of anti-tumor CD8+ T lymphocytes [31]. The conventional type 2 dendritic cell (cDC2) subset in humans is a primary DC subset, showing no pyroptosis in transcription and function concerning inflammasomes. Upon stimulation with ligands that moderately activate the inflammasome, cDC2 cells do not experience pyroptosis; rather, they produce IL-12 family cytokines and IL-1β [125]. This study presents potential therapeutic options for the treatment of inflammasome-dependent inflammatory illnesses and may introduce innovative strategies for adjuvant and vaccine development.
Macrophages
Macrophages can exert anti-tumor effects, and the activation of inflammasomes within macrophages can promote immune evasion by tumor cells [128]. Research indicates that lactic acid at the site of CRC serves not only as DAMPs to activate inflammasomes within macrophages but also can inhibit inflammasome activation through upregulation of ROS or the action of TGF-β [129]. Such regulatory mechanisms establish a complex interplay between inflammasome activity within macrophages and the occurrence and progression of tumors [130]. Monocyte-derived macrophages, when influenced by LPS or IFN-γ, can differentiate into two functional phenotypes: pro-inflammatory M1 and anti-inflammatory M2 [131]. The M1 phenotype exhibits phagocytic and antigen-presenting activities, produces Th1-activating cytokines [132], and demonstrates anti-cancer activity by mediating cytotoxic functions. In contrast, the M2 phenotype is believed to play a role in the establishment of a TME that is conducive to tumor survival and growth by supporting tumors, promoting angiogenesis, and suppressing the immune system.
The polarization of macrophages influences the initiation, advancement, and management of numerous illnesses.TGR5 signaling has been identified as alleviating hepatic steatosis and inflammation, concurrently inhibiting the polarization of M1 macrophages in non-alcoholic steatohepatitis (NASH) mediated by NLRP3 [133]. Metformin, in regulating the AMPK/mTOR signaling pathway, inhibiting NLRP3 inflammasome activation, and promoting the polarization of M2 macrophages, has demonstrated an accelerating effect on wound healing [134]. The research findings provide new perspectives into the molecular mechanisms of metformin administration and its prospective therapeutic effects on wound healing. The activation of inflammasomes in macrophages can facilitate the polarization of M1 macrophages, therefore producing an anti-tumor immune response. tumor-associated macrophages in the TME primarily have an M2 phenotype. NLRP3 signaling, upon activation in macrophages, can stimulate CD4+ T cells, facilitating the development of Th2 cells, Th17 cells, and regulatory T cells [135]. It can also inhibit the production of Th1 responses and decrease the cytotoxic activation of CD8+ T cells [135]. Pancreatic ductal adenocarcinoma (PDA) tumor cells produce IL-1β, which is essential for forming the tumor microenvironment because it promotes the infiltration and activation of immunosuppressive cells and M2-polarized macrophages while also activating CD8+ cytotoxic T lymphocytes (CTLs) [136]. T lymphocytes, thereby inhibiting pancreatic tumor growth [137]. Since the activation of NLRP3 inflammasome can be activated under the influence of special cytokines, it can also regulate the polarization direction of macrophages, mediating anti-tumor immunity.
Fibroblasts
The generation and progression of cancer are highly dependent on the TME. Concerning Cancer Associated Fibroblasts (CAFs) expression, the progressiveness of breast cancer and patient prognosis are directly associated, and CAFs are significant in tumor stimulation via multiple processes [138]. Additionally, the evidence shows that CAFs have a vital role in orchestrating the immune cell recruitment to participate in inflammation in the TME [139]. This inflammatory response is an important contribution of the NLRP3 inflammasome. The NLRP3 inflammasome comprises a cluster of cytokines that also recognize DAMPs produced by stressed or injured cells [107]. Proinflammatory chemicals such as DAMPs are known to greatly activate the inflammasome, with particular note that tumors are rich sources of DAMPs and aid in inflammasome activation.
Inflammation studies have revealed that the inflammasome pathway is two-faced and both pro- and anti-tumorigenic, depending on the type of tumor. This pathway has been shown to play a role in tumor progression, metastasis, and immune evasion. The activation of the inflammasome in turn will lead to the synthesis of pro-inflammatory cytokines, IL-1β and IL-18, resulting in the survival and proliferation of tumor cells.
On the other hand, activation of the inflammasome can itself drive immune cell infiltration to facilitate tumor cell death. Thus, the effects of the inflammasome pathway across various tumor types are intricate and context-dependent. Therefore, further research is needed to comprehensively understand its role in cancer development and expand its potential as a therapeutic target. In fact, the upregulation of NLRP3 in CAFs has been shown in both mouse and human breast cancer [104]. Investigations also noted that fibroblasts can act as receptors of DAMPs, causing the inflammasome to be activated and releasing IL-1β [140]. Previous studies have demonstrated that silencing or reducing the expression of NLRP3 or IL1 in CAFs may inhibit CAF proliferation and breast cancer cell proliferation to an extent that prevents lung metastases [141]. These findings indicate that targeting the NLRP3 inflammasome pathway in CAFs could be a good therapeutic approach to inhibit breast cancer progression and metastasis. Furthermore, CAF secreted IL-1β has been shown to promote recruitment of MDSCs and to express the adhesion molecule, which contributes to tumor development and metastasis [142]. Activation of the NLRP3 inflammasome contributes to tumor development, which is supported by an analysis of CAFs. Interestingly, IL-1β expression is rather increased in tumor tissues, e.g., melanoma and breast cancer, however, anti-breast cancer effects have been seen in NLRP3 knockout animals [143, 144]. In this context, fibroblasts represent a key receptor for the inflammasome pathway and show the relevance of activating IL 1β in CAFs as a stimulus of the proinflammatory CAF phenotype.
The dual roles of NLRP3/IL-1β in CAFs are critically dependent on tumor type and experimental models. In breast cancer, NLRP3 activation within CAFs promotes metastasis through IL-1β-mediated recruitment of MDSCs, establishing an immunosuppressive TME [142]. Conversely, in CRC models, NLRP3 deficiency exacerbates colitis-associated carcinogenesis by impairing epithelial repair mechanisms [61]. This tumor-type specificity extends to therapeutic interventions: while NLRP3 inhibition with MCC950 suppresses pancreatic ductal adenocarcinoma progression, genetic NLRP3 ablation accelerates intestinal tumors [66]. Furthermore, cellular origin dictates functional outcomes—CAF-derived NLRP3 drives pro-tumorigenic inflammation, whereas dendritic cell-specific NLRP3 activation enhances anti-tumor CD8+ T cell responses in melanoma. These paradoxical effects underscore the necessity for precision targeting based on tumor microenvironmental context and cellular compartmentalization.
Our findings provide novel insights into the function of CAFs and the NLRP3 inflammasome in tumor growth and indicate that inhibition of this pathway in CAFs may have therapeutic potential in cancer treatment. However, it is important to study these underlying mechanisms further in greater detail and explore potential therapeutic strategies.
T cell
T lymphocytes are stimulated and collaborate with antigen presenting cells (APC) to induce an effective immune response against malignancies [145, 146]. The T Cell Receptor (TCR) pathway and the co-stimulatory pathway are two main signaling pathways that activate T cells, a type of immune cell crucial for the identification and eradication of tumor cells. When TCR on the T cell surfaces come into contact with a particular antigen with APCs, the TCR pathway is triggered, including a signaling process that causes the T cell to be activated and produce effector chemicals that can directly kill tumor cells [147]. Additionally, co-stimulating molecules such as CD28 on T cells and CD80/60 on APC bind to each other to provide a secondary signal of augmenting T cell activation and proliferation [148]. APCs, like dendritic cells, macrophages, and B cells, are responsible for presenting tumor antigens to T cells [149]. These tumor antigens are sequestered and processed by them, and presented on their surface in association with major histocompatibility complex (MHC) molecules. TCR recognizes the antigen/MHC complex, and the T cell responds to the activation, ensuing the immune response against tumor cells.
NK cells are an important part of the innate immune system, which can recognize and kill tumor cells without prior sensitization. In addition, it can directly lyse neoplastic cells and secrete cytokines that additionally strengthen the anti-tumor immune response. In recent years, utilizing T cells and NK cells for cancer therapy with the notion of growing the function and potency of these immune cells against tumors has been encouraging. Cancer therapeutic strategies include immune checkpoint inhibitors, chimeric antigen receptor (CAR) T cell therapy, and NK cell directed therapy. There are targeted therapies that can intervene in the activation and activity of T lymphocytes and NK cells, which can selectively eliminate tumor cells, yielding better outcomes for patients. This axis has recently been further verified by in vivo studies. For instance, NLRP3 activation via ATP enhanced IFN-γ CD8+ T cell infiltration and delayed melanoma progression in mice [123], while AIM2-dependent GSDMD signaling restored CD8+ T cell cytotoxicity in CRC models [150]. Clinically, MCC950 (an NLRP3 inhibitor) combined with anti-PD-1 increased tumor-infiltrating CD8+ T cells in a Phase I trial, underscoring translational potential.
The regulation of T cell activity by IL-1β is integral and it can play various regulatory roles in T cell differentiation, memory T cell development, and the augmentation of effector T cells which IL-1β does by activation of the transcription factors, such as signal transducer and activator of transcription (STAT), via myeloid differentiation main response 88 (MyD88) pathway [151, 152]. IL-1R is present on the CD4+ and CD8+ naive and memory T lymphocytes cell surfaces [153], and this activation mechanism allows transforming naive T cells into helper T cells, an essential phase of adaptive immune response. Once bound with IL-1β, the IL-1R will activate existing memory CD4+ T cells to augment their production of IFN-γ and other effector functions [154]. Moreover, IL1β is also able to promote the proliferation and differentiation of antigen-specific CD8+ T cells.
Moreover, it is found by research that IL-1β also encourages memory CD8+ T cell activation and stimulates memory CD8+ T cell generation in vivo in mice. The significance of this finding is that IL-1β plays a fundamental part in the generation and maintenance of T cell memory, an essential element of the immune system’s ability to maintain long-term protection against pathogen and tumor challenge. Accumulating in vivo evidence reinforces the role of inflammasomes in CD8+ T cell-mediated anti-tumor immunity. For example, NLRP3 activation in DCs enhances CD8+ T cell priming and tumor regression in murine models, while IL-18 deficient mice exhibit exacerbated colitis-associated carcinogenesis due to compromised CD8+ T cell responses [155, 156]. Critically, IL-1β overexpression in B16 melanoma models boosts CD8+ T cell cytotoxicity and suppresses tumor growth [122]. Moreover, NLRP3 ablation accelerates breast cancer progression by polarizing macrophages toward an immunosuppressive phenotype [104]. These in vivo findings validate that inflammasomes orchestrate CD8+ T cell activation to restrict tumorigenesis [157]. IL-1β acts upon T cells and therefore plays an important role in the regulation of the immune response, including the shaping of specific adaptive immunity. Knowing how IL-1β affects T cell function could help in the development of treatment. This pathway may be harnessed to enhance the tumor immune response and result in better cancer treatment outcomes.
Finally, it has also been observed that adjuvants such as alum induce IL-1β production [158]. CD8+ T cells do use IL-1β to organize immunological responses [153]. In a murine model, interestingly, IL-1β greatly increased the cytotoxic effect of CD8+ T cells and inhibited B16 melanoma progression [159]. Interestingly, IL1β is a tumor killer of Th1 cells, possibly resulting in immune suppression and tumorigenesis. These data show that the role of IL-1β in the TME is more complex, with both anti-tumor and pro-tumor activities contingent upon the conditions. In conclusion, IL-1β can be considered a novel immune adjuvant as it provides host recruitment of inflammatory cells to inflammatory sites and helps mature antigen-presenting cells. Also, it increases the ability of dendritic cells to augment T cell mediated immune responses. Here we present results which provide an intriguing possibility for IL-1β, as a target, for modulating immune responses in cancer and promoting anti-tumor immunity.
Other anti-tumor immunity
The NLRP3 inflammasome and the IL-1β pathway it regulates exhibit a dual function in tumor progression, serving as both facilitators and suppressors of cancer. For instance, NLRP3-driven IL-1β enhances immunosuppression in pancreatic ductal adenocarcinoma, whereas NLRP1 activation mitigates colitis-associated CRC by maintaining epithelial integrity [71, 160]. Multiple studies have documented increased levels of IL-1β in various cancers, including gastric cancer [161], head and neck squamous cell carcinoma [144], and breast cancer [162]. The expression of IL-1β is closely associated with tumor cell self-renewal and immunosuppression. Within the TME, IL-1β generated by MDSCs from the bone marrow can stimulate tumor angiogenesis. Furthermore, tumor-associated fibroblasts can identify DAMPs and activate the NLRP3 inflammasome, leading to the production of IL-1β, which promotes breast cancer cell proliferation and metastasis [104]. Nevertheless, IL-1β can also promote T lymphocyte-mediated tumor destruction and prompt the retreat of distant tumors. Research has demonstrated that introducing the IL-1β gene into a B16 melanoma model elevates the cytotoxicity of CD8+ T cells and facilitates tumor regression [163]. Intramuscular injection of IL-1β also significantly reduces the rate of lung metastasis in mice with Lewis lung carcinoma, demonstrating anti-metastatic effects [143]. Furthermore, transplanted DCs can release the inflammasome into the surrounding draining lymph nodes (DLNs) after several days, releasing IL-1β to exert their effects [31]. However, whether IL-1β can migrate into the TME and regulate the tumor immune response is still unclear.
Recent clinical trials targeting inflammasomes demonstrate promising synergy with established immunotherapies. The NLRP3 inhibitor IFM-2427 is currently in Phase I/II trials for advanced solid tumors, showing reduced IL-1β secretion and enhanced infiltration of CD8+ T cells in 65% of patients, with partial responses observed in PD-1-refractory melanoma [164]. Similarly, the IL-1β monoclonal antibody canakinumab reduced lung cancer incidence by 67% in high-risk patients. When combined with the PD-1 inhibitor pembrolizumab in NSCLC, it also improved progression-free survival [165]. In preclinical studies, the NLRP3/IL-1β pathway inhibitor OLT1177 (an oral NLRP3 inhibitor in clinical development) enhanced the efficacy of anti-PD-1 therapy in melanoma models, increasing tumor-infiltrating CD8+ T cells by 3.5-fold compared to anti-PD-1 monotherapy [166]. These strategies differ from conventional checkpoint inhibitors by simultaneously reprogramming immunosuppressive myeloid populations and activating innate-adaptive crosstalk [166]. A Phase Ib trial evaluating Dapansutrile, an oral NLRP3 inhibitor, in combination with nivolumab demonstrated a 38% objective response rate in refractory hepatocellular carcinoma, outperforming historical anti-PD-1 monotherapy controls [167].
Inflammasome inhibitors
Definitely, since the different regulatory functions exerted by the inflammasome pathway in malignant development, it is necessary to target the inflammasome in specific cell subpopulations to modulate this pathway. Targeting the inflammasome pathway with drugs that either inhibit or activate the inflammasome is also being developed by researchers, who acknowledge that such a pathway is important to provide anti-tumor therapy. By focusing on this targeting strategy, the immune response may be fine-tuned such that anti-tumor effects can be promoted while unintended pro-inflammatory consequences are minimized. These efforts constitute a continuous effort in the development of drugs to manipulate the inflammasome pathway, realizing its significance as a potential avenue for innovative and effective cancer treatment.
It has also been indicated that both macrophage AIM2 and DCs NLRP3 can contribute to tumorigenesis [168]. This highlights the importance of characterizing the function of the inflammasome in the different cell subsets of the TME. Nevertheless, little is currently known about the triggering of inflammasomes in specific TME subsets. Understanding what subsets of inflammasome contribute to tumor development in particular sets of tumors may also be helpful for the development of more effective therapeutic agents focused on the inflammasome in different types of tumors. Finally, we briefly summarized some tumor-targeted inflammasomes in the clinical trials (Fig. 3). These medicines have the potential to modulate the inflammasome pathway, and are useful for promoting the development of individualized and targeted cancer therapy. While inflammasome inhibitors hold promise for cancer therapy, their systemic suppression of immune pathways raises critical safety concerns. For example, NLRP3 inhibitors like MCC950 disrupt IL-1β/IL-18 signaling, which is essential for host defense against pathogens [169]. Preclinical studies report increased susceptibility to bacterial and fungal infections following NLRP3 blockade [170]. Similarly, clinical trials of IL-1β antagonists in cardiovascular diseases revealed a dose-dependent rise in fatal infections, underscoring the need for targeted delivery systems to minimize off-tissue effects [171]. Furthermore, AIM2 inflammasome inhibition may compromise antiviral immunity, as AIM2 is critical for detecting cytosolic DNA from viruses like HSV-1 [172]. These risks necessitate careful risk–benefit stratification in clinical applications. However, further research and clinical trials are needed to fully assess their effectiveness and safety for such tumor subtypes.
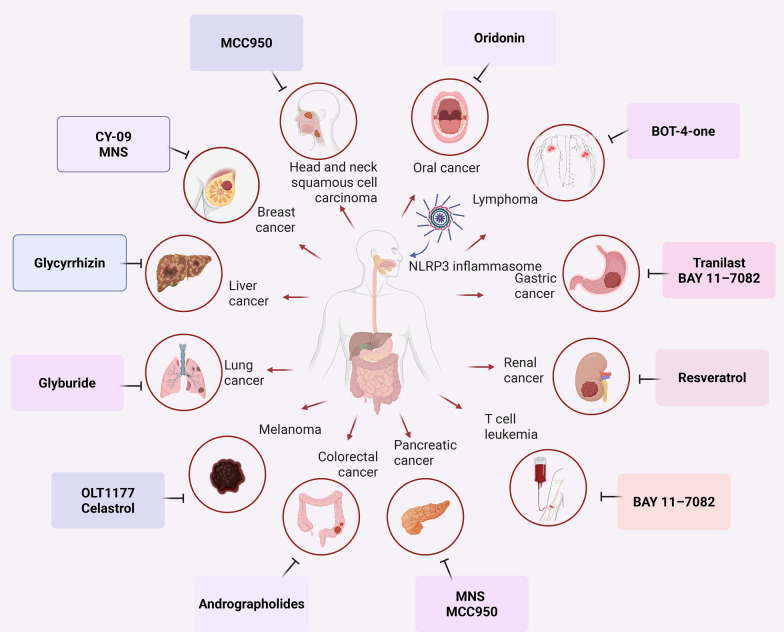
Fig. 3.
Inhibitors of various tumor inflammasomes in the clinical trials. Red arrows: The inflammasome pathway promotes cancer; black arrows: inflammasome inhibitor drugs have been reported to treat these cancers
NLRP3 inflammasome inhibitors have been formulated for the management of non-malignant tumors and have demonstrated encouraging outcomes in cancer therapy. One such inhibitor is MCC950 (also known as CRID3), which specifically targets NLRP3 without affecting other inflammasome receptors [173]. MCC950 interacts with the Walker B motif in the NACHT domain, obstructing ATP hydrolysis and limiting NLRP3 activation [174]. This particular blockage has been confirmed in both the wild-type form and mutant NLRP3. Research has shown that MCC950 suppresses the cancer of the pancreas and head and neck squamous cell carcinoma [175, 176]. Another inhibitor, CY-09, targets the NACHT domain akin to MCC950 and obstructs NLRP3-mediated tumor drug resistance and the suppression of EMT [177]. Tranilast is an inhibitor targeting the NACHT domain while also inhibiting signaling pathways such as TGF-β, MAPK, and NF-κB [178, 179]. Research has demonstrated that IL-1β displays significant anti-tumor efficacy against NSCLC, gastric cancer, and various other tumor cell types, resulting in reduced tumor proliferation and fibrosis [180, 181]. Other inhibitors that target the NACHT domain include 3,4-ethylene-β-nitrosostyrene (MNS) and oridolin [182]. Aridin, which has significant anti-tumor activity, acts through the PI3K/Akt pathway [183]. NLRP3 inhibitors show potential for the advancement of tailored therapeutics in cancer treatment. Still, additional investigation is necessary to thoroughly assess the effectiveness and safety of these inhibitors in specific subtypes of tumors through further research and clinical trials.
BAY 117082 has been demonstrated to trigger apoptosis in gastric cancer cells. To perform this action, it blocks the NF-κB signaling pathway, an important pathway in cancer cell survival and proliferation. Furthermore, BAY 117082 can successfully trigger apoptosis in gastric cancer cells by inhibiting this pathway and thus cause their death [184]. Additionally, MNS can significantly inhibit tumor cell invasion, migration, and proliferation in pancreatic cancer and breast cancer. These effects demonstrate that MNS may have a therapeutic use for treating cells as such. The underlying mechanism of MNS and its clinical potential for treating pancreatic and breast cancer will need to be further studied [185]. Inhibiting NLRP3, we have found that glyburide reduces inflammation-related lung tumorigenesis [186]. The inflammatory pathway that includes NLRP3 is crucial. Glyburide can effectively reduce inflammation and subsequently inhibit lung tumorigenesis by inhibiting NLRP3. The discovery highlights the potential of blocking the inflammasome pathway as a new approach to creating anti-tumor medications. As shown previously, BAY 117082 kills T cell leukemia cells through the inhibition of the NF-κB pathway [184, 187]. It is widely known that the NF-κB pathway plays an essential role in promoting cell survival and proliferation in cancerous cells. BAY 117082 can induce apoptosis and thus lead to the cell death of T cell leukemia cells via inhibition of this pathway. These include the potential inhibitors and mechanisms of their action and may be valuable to identify when developing anti-tumor therapeutics that target the inflammasome [188, 189]. However, more research is needed to determine if they work and are safe in different cancer types before they can be considered as a potential therapy.
Resveratrol has shown that inhibition of NLRP3 expression in kidney tumor cells, implying that the NACHT domain could be a major site for inhibitory activity [189]. A further potential pathway to targeting the inflammasome pathway is OLT1177. This inhibitor can break the IL-1β/IL-6/STAT3 signaling pathway to reduce the immunosuppressive capability of MDSCs, and inhibit tumor cell proliferation [190]. The anti-tumor impact of OLT1177 plus PD1 has been shown as augmented in synergy [191]. In addition to inhibiting kidney tumor cells, resveratrol has shown inhibitory effects on NLRP3 inflammasome activation in many other situations. For example, it showed potential to limit myocardial damage and diminish the systemic inflammatory response by doxorubicin, a chemotherapy drug that, among other toxic effects, is cardiotoxic. It has been shown that resveratrol also has efficacy against radiation therapy induced inflammation of the intestinal mucosa. The results presented herein demonstrate that resveratrol displays profound anti-inflammatory activity, which may be useful as a therapeutic agent for various inflammatory diseases, including renal carcinoma cells [192]. The proliferation and survival of lymphoma cells have been inhibited by BOT-4-one [193]. Investigated so far as an anti-cancer agent, particularly against lymphoma, which is a malignancy of the lymphatic system, this chemical is sought to be used. BOT-4-one also has the potential to inhibit the growth and survival of lymphoma cells, which may be useful in treating this type of cancer. However, further research and analysis are necessary to understand the mechanisms of action and potential therapeutic applications of BOT-4-one in lymphoma treatment. In addition, triptolide has been shown to inhibit the migratory and invasive activity of melanoma cells [194]. Melanoma cells are efficiently inhibited from migrating and invading through targeting the melanoma derived NLRP3/IL-1β pathway from macrophages by triptolide. It is highly influential in the advancement and spread of melanoma. In doing so, triptolide can specifically knock down this pathway and block the migratory and invasive capacity of melanoma cells. Result suggests that triptolide can be a potential therapeutic alternative for melanoma, one of the most aggressive forms of skin cancer.
Emerging evidence suggests that pharmacological interventions such as NSAIDs (aspirin) suppress NLRP3 inflammasome activation by reducing COX-prostaglandin E2 (PGE2) production, thereby attenuating tumor-associated inflammation [195]. Conversely, corticosteroids like dexamethasone inhibit inflammasome assembly by downregulating NF-κB dependent priming signals in murine melanoma models [196]. Antibiotics such as vancomycin alter gut microbiota composition, indirectly modulating NLRP3 activity in CRC via microbiota-derived metabolites [197]. These findings highlight the therapeutic potential of targeting inflammasomes through existing drugs. However, there has been limited exploration of AIM2 inhibitors relative to NLRP3, only two drugs with a non-specific inhibitory effect have been identified. As a good candidate characterized for its wide range of anti-inflammatory activity, methylene blue can selectively target NLRP3, NLRC4, AIM2, and non-classical inflammasomes [198]. Other non-specific inhibitors, such as andrographolide, inhibit translocation of AIM2 to the nucleus, reduce DNA damage, and decrease the inflammatory response in irradiated lung tissue [199]. The therapeutic effects of several compounds, including compounds such as glycyrrhizin acid [200], andrographolide [201], and methylene blue on breast cancer [202], colon cancer, and liver cancer have been shown. To date, however, there are few specific inhibitors available against NLRC4. There are two identified inhibitors of the inflammasome that are also effective against NLRC4, albeit with weaker specificity. Inhibition of NLRC4 and NLRP3 activation by sulforaphane reduces inflammation [203]. As a broad-spectrum inflammasome inhibitor, methylene blue has the ability to inhibit NLRC4, NLRP3, AIM2, and non-classical inflammasomes. In animal studies, methylene blue administration significantly improved survival rates in mice induced by lipopolysaccharide (LPS) by inhibiting inflammasome activation. It’s noteworthy that several NLRP3 inhibitors target the NACHT domain, which is also expressed in the NLRC4 inflammasome. Although several inhibitors of NLRP3 have been developed, only a small number of medications have been utilized in clinical studies. The area of targeted drug development for inflammasome activation necessitates additional research and attention. Further studies are needed to identify more specific inhibitors against NLRC4 and explore their therapeutic potential in various inflammatory and disease conditions.
Comparative analysis of inflammasome inhibitors
Recent advances in NLRP3-targeted therapies highlight both promises and challenges. For instance, MCC950, a potent NLRP3 inhibitor, synergizes with anti-PD1 in melanoma by reducing myeloid-derived suppressor cell infiltration [174]. However, its clinical translation is limited by poor oral bioavailability and off-target effects on non-immune cells. In contrast, Tranilast, an anti-fibrotic drug repurposed for NLRP3 inhibition, shows efficacy in pancreatic cancer models by suppressing TGF-β signaling, yet its dose-dependent toxicity necessitates careful optimization [204]. Notably, CY-09 emerges as a promising candidate due to its specificity in blocking NLRP3 ATPase activity, effectively reversing EMT in breast cancer [205]. These findings underscore the need for isoform-selective inhibitors and biomarker-driven patient stratification.
Specific inhibitors targeting NLRC4 are currently limited. Nevertheless, there are a few inflammasome inhibitors that have demonstrated effectiveness against NLRC4, although with weaker specificity. One such inhibitor is sulforaphane, capable of inhibiting both NLRC4 and NLRP3 activation, resulting in a reduction in inflammatory responses [203]. Methylene blue, acting as a broad-spectrum inflammasome inhibitor, has been shown to block the activation of NLRC4, NLRP3, AIM2, and non-classical inflammasomes. The administration of methylene blue in animal studies substantially increased the survival rates of mice that were induced by LPS by inhibiting inflammasome activation [198]. Although numerous inhibitors of NLRP3 and NLRP4 have been created, only a small number of medications have been implemented in clinical trials [198]. The development of targeted drugs for inflammasome activation is an active area of research, with increasing attention being directed towards studying the inflammasome pathways. Further research is imperative to identify more specific inhibitors against NLRC4 and explore their therapeutic potential in various inflammatory and disease conditions.
Recent advances in targeting inflammasomes have yielded promising inhibitors, though clinical translation remains challenging. MCC950 (CRID3), a selective NLRP3 inhibitor, demonstrated efficacy in preclinical models of pancreatic cancer and head/neck squamous cell carcinoma by suppressing IL-1β and tumor growth [206, 207]. However, Phase I trials revealed dose-dependent hepatotoxicity, limiting its therapeutic window [208]. Similarly, CY-09, another NLRP3-targeting compound, showed reduced tumor drug resistance in murine models, but its poor bioavailability and off-target effects on NLRC4 highlight the need for structural optimization [205]. Tranilast, an NLRP3/TGF-β dual inhibitor, reduced fibrosis in NSCLC patients but caused gastrointestinal side effects in 22% of participants [178]. Oltipraz, an AIM2 suppressor, is under evaluation for colitis-associated cancer yet its nonspecific inhibition of NF-κB raises concerns about immunosuppression [209]. While methylene blue broadly inhibits AIM2/NLRC4, its neurotoxicity at high doses underscores the importance of isoform-specific designs [210]. Furthermore, we systematically summarized pharmacologic agents targeting inflammasome pathways (including NLRP3, AIM2, and NLRC4 subtypes) along with their molecular mechanisms such as ATPase inhibition, structural domain blockade, and signaling pathway modulation, which demonstrate therapeutic potential across multiple malignancies including pancreatic, colorectal, breast, and melanoma cancers (Table 3). These findings emphasize the dual challenge of balancing efficacy and safety while addressing inflammasome redundancy in tumors.
Table 3.
Molecules modulating inflammasome function and their mechanisms in cancer
| Interventions | Target inflammasome | Mechanism of action | Cancer type | Clinical status | NCT numbers | Refs. |
|---|---|---|---|---|---|---|
| MCC950 | NLRP3 | Inhibition of ASC oligomerization | Head and neck squamous cell carcinoma | Preclinical | N/A | [144] |
| MCC950 | NLRP3 | Inhibits ATPase activity of NLRP3 | Pancreatic, HNSCC | N/A | N/A | [169] |
| CY-09 | NLRP3 | Binds NLRP3 NACHT domain, blocks activation | Colorectal, NSCLC | Preclinical | N/A | [177] |
| Tranilast | NLRP3 | Suppresses NLRP3/IL-1β/TGF-β signaling | Breast, gastric | N/A | N/A | [178, 179] |
| OLT1177 | NLRP3 | Inhibits NLRP3-ASC interaction | Melanoma, breast | Phase II | N/A | [191] |
| Andrographolide | AIM2 | Reduces AIM2 nuclear translocation | Radiation-induced lung injury | Phase II | NCT01993472 | [201] |
| Sulforaphane | NLRC4/NLRP3 | Inhibits NLRC4 oligomerization | Colorectal | Preclinical | NCT01265953 | [203] |
| Sulforaphane | NLRC4/NLRP3 | Inhibits NLRC4 oligomerization | prostate | Preclinical | NCT01228084 | [203] |
| Resveratrol | NLRP3 | Suppresses NLRP3 via SIRT1/AMPK pathways | Renal, melanoma | N/A | N/A | [189] |
| BAY 11-7082 | NLRP3 | Inhibits NF-κB/NLRP3 axis | Gastric, leukemia | Preclinical | N/A | [184] |
| Methylene Blue | NLRP3/NLRC4/AIM2 | Broad-spectrum inflammasome inhibition | Breast | Preclinical | NCT02084784 | [198] |
| Methylene Blue | NLRP3/NLRC4/AIM2 | Broad-spectrum inflammasome inhibition | colorectal | Complete | NCT01694966 | [198] |
| Triptolide | NLRP3 | Inhibits NLRP3/IL-1β in macrophages | Melanoma | Preclinical | N/A | [194] |
Conclusions
Comprehensive summaries of the mechanisms that activate both classical and non-classical inflammasome pathways are provided in this review. We also investigate the synergistic role of inflammasomes in immune cells for antitumor activity. Lastly, we examine inhibitors that are presently in clinical trials and target tumor inflammasome pathways.
Inflammasomes are multiprotein complexes that activate the production of pro-inflammatory cytokines, including IL-1β and IL-18, which are essential for modulating innate immune responses. Inflammasome activation occurs in two stages: priming, in which immune cells are sensitized by the upregulation of inflammasome components and cytokines via PRRs such as TLRs, and activation, where sensors detect danger signals (PAMPs or DAMPs), triggering an inflammatory response. The activation of inflammasomes recruits additional immune cells to coordinate a robust immune response, as immune cells, such as macrophages, dendritic cells, and T cells, express them.
Inflammasomes can exert both pro-tumor and anti-tumor effects. The dual roles of inflammasomes in cancer progression stem from their cell-type-specific signaling networks and dynamic interactions within the TME. For instance, NLRP3 exhibits pro-tumor effects in breast cancer by fostering immunosuppressive myeloid cell infiltration, whereas it exerts tumor-suppressive functions in colitis-associated CRC through IL-18-mediated epithelial repair. This dichotomy can be exploited therapeutically by developing cell-selective inflammasome inhibitors or combining inflammasome modulation with immune checkpoint blockade. Recent advances in single-cell transcriptomics now enable precise mapping of inflammasome activation states across immune cell subsets, paving the way for biomarker-driven personalized therapies. They can promote tumor progression by creating an immunosuppressive environment, but also enhance anti-tumor immunity by activating cytotoxic immune cells. Inflammasome inhibitors targeting various components, such as sensors, ASC, caspase-1, and GSDMD, are currently in clinical trials. These inhibitors have the potential to enhance anti-tumor immune responses and modulate the TME.
Inflammasomes exhibit cancer-type-specific duality, necessitating precision in therapeutic targeting. NLRP1 promotes melanoma proliferation via anti-apoptotic pathways, yet suppresses gastrointestinal tumorigenesis through IL-18-mediated epithelial protection. NLRP3 accelerates head/neck squamous carcinoma via caspase-1-dependent EMT, while its absence exacerbates colitis-associated carcinogenesis. AIM2 demonstrates tumor-suppressive activity in breast cancer via NF-κB inhibition, paradoxically enhancing cutaneous squamous carcinoma through angiogenesis promotion. Clinical trials with NLRP3 inhibitors underscore the imperative for subtype-stratified approaches. Future research must delineate tumor-microenvironment-specific inflammasome interactomes to optimize immunotherapy regimens.
In summary, understanding the activation mechanisms of inflammasomes and the collaborative roles of immune cells is crucial for developing effective cancer immunotherapies. Recent single-cell RNA sequencing studies reveal that inflammasome activation is spatially heterogeneous within tumors. For example, NLRP1 is upregulated in lung adenocarcinoma-associated fibroblasts, correlating with poor prognosis, while AIM2 silencing in renal carcinoma suppresses metastasis via autophagy induction. Such spatial and cellular specificity underscores the need for cell-type-targeted therapies, a paradigm shift from systemic inflammasome inhibition. Although NLRP3 is the main focus for inflammasome inhibitors, other components of inflammasomes are also important and could offer additional therapeutic possibilities. By enhancing efficacy and addressing drug resistance, inflammasome inhibitors may serve to augment existing cancer therapies, including immunotherapies, chemotherapy, and targeted treatments. Future research may uncover new inflammasome targets, thereby broadening treatment approaches and improving patient outcomes. The burgeoning field of inflammasome immunotherapy presents significant potential for more personalized and effective cancer treatments.
Acknowledgements
The authors recognize the utilization of BioRender for the creation of the schematics in Figs. 1, 2, 3.
Author contributions
LH, XC and RL wrote the manuscript. DN, WB, LY, XD, JT, YL and HZ collected information and prepared figures and tables. All authors edited and approved the final version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82203377 to Yanwei Lu; 82473238 to Haibo Zhang), Zhejiang Natural Science Foundation of China (Grant number: LQ22H160036, to Yanwei Lu; LY24H160022, to Haibo Zhang).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Luanluan Huang, Xiaoyan Chen, and Ruiqi Liu have contributed equally to this work.
Contributor Information
Jianming Tang, Email: 15900792812@163.com.
Haibo Zhang, Email: zhbdoctor@163.com.
Yanwei Lu, Email: 309960266@qq.com.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.McGettigan SE, Debes GF. Immunoregulation by antibody secreting cells in inflammation, infection, and cancer. Immunol Rev. 2021;303(1):103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age. Nat Rev Clin Oncol. 2021;18(5):261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heras-Murillo I, Adán-Barrientos I, Galán M, Wculek SK, Sancho D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat Rev Clin Oncol. 2024;21(4):257–77. [DOI] [PubMed] [Google Scholar]
- 5.Hu A, Sun L, Lin H, Liao Y, Yang H, Mao Y. Harnessing innate immune pathways for therapeutic advancement in cancer. Signal Transduct Target Ther. 2024;9(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Wu S, Zheng X, et al. Immune suppressed tumor microenvironment by exosomes derived from gastric cancer cells via modulating immune functions. Sci Rep. 2020;10(1):14749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. 2019;25(18):5449–57. [DOI] [PubMed] [Google Scholar]
- 8.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5(2):112–24. [DOI] [PubMed] [Google Scholar]
- 9.Ohto U. Activation and regulation mechanisms of NOD-like receptors based on structural biology. Front Immunol. 2022;13: 953530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5. [DOI] [PubMed] [Google Scholar]
- 11.Wei X, Xie F, Zhou X, et al. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19(9):971–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh V, Ubaid S, Kashif M, et al. Role of inflammasomes in cancer immunity: mechanisms and therapeutic potential. J Exp Clin Cancer Res. 2025;44(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Shi X, Qiu M, et al. Hydrogen sulfide plays an important role by influencing NLRP3 inflammasome. Int J Biol Sci. 2020;16(14):2752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey Sarkar R, Sinha S, Biswas N. Manipulation of inflammasome: a promising approach towards immunotherapy of lung cancer. Int Rev Immunol. 2021;40(3):171–82. [DOI] [PubMed] [Google Scholar]
- 15.Lee G, Ahn H, Lee E, Lee GS. The role of NLRP3 inflammasomes in trained immunity. Front Biosci (Landmark Ed). 2023;28(9):210. [DOI] [PubMed] [Google Scholar]
- 16.Arzuk E, Birim D, Armağan G. Correction: celecoxib inhibits NLRP1 inflammasome pathway in MDA-MB-231 cells. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(11):9205–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson BE, O’Brien S, Sheth RA, et al. Phase I study of BMS-986299, an NLRP3 agonist, as monotherapy and in combination with nivolumab and ipilimumab in patients with advanced solid tumors. J Immunother Cancer. 2025;13(1): e010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Huang H, Liu B, et al. Inflammasomes as therapeutic targets in human diseases. Signal Transduct Target Ther. 2021;6(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–20. [DOI] [PubMed] [Google Scholar]
- 21.Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4(3):198–208. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya K. Inflammasome-associated cell death: pyroptosis, apoptosis, and physiological implications. Microbiol Immunol. 2020;64(4):252–69. [DOI] [PubMed] [Google Scholar]
- 23.Guo Q, Wu Y, Hou Y, et al. Cytokine secretion and pyroptosis of thyroid follicular cells mediated by enhanced NLRP3, NLRP1, NLRC4, and AIM2 inflammasomes are associated with autoimmune thyroiditis. Front Immunol. 2018;9:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi YS. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology. 2017;152(2):207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi X, Sun Q, Hou Y, et al. Recognition and maturation of IL-18 by caspase-4 noncanonical inflammasome. Nature. 2023;624(7991):442–50. [DOI] [PubMed] [Google Scholar]
- 26.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38(2):240–4. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi T, Ogawa M, Sanada T, et al. The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell Host Microbe. 2013;13(5):570–83. [DOI] [PubMed] [Google Scholar]
- 28.Gai K, Okondo MC, Rao SD, et al. DPP8/9 inhibitors are universal activators of functional NLRP1 alleles. Cell Death Dis. 2019;10(8):587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng CH, Li TQ, Zhang W, Zhao Q, Wang Y. Targeting inflammasome activation in viral infection: a therapeutic solution. Viruses. 2023;15(7):1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Zhu J, Cao Y, Shen J, Yu L. Insight into inflammasome signaling: implications for toxoplasma gondii infection. Front Immunol. 2020;11: 583193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhivaki D, Borriello F, Chow OA, et al. Inflammasomes within hyperactive murine dendritic cells stimulate long-lived t cell-mediated anti-tumor immunity. Cell Rep. 2020;33(7): 108381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Luo L, Xu X, et al. Acetylation is required for full activation of the NLRP3 inflammasome. Nat Commun. 2023;14(1):8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. 2021;18(5):1141–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D, Zhong Z, Karin M. NF-κB: a double-edged sword controlling inflammation. Biomedicines. 2022;10(6):1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z, Chen ZM, Wu X, Zhang L, Cao Y, Zhou P. Distinct molecular mechanisms underlying potassium efflux for NLRP3 inflammasome activation. Front Immunol. 2020;11: 609441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao S, Chen F, Yin Q, Wang D, Han W, Zhang Y. Reactive oxygen species interact with NLRP3 inflammasomes and are involved in the inflammation of sepsis: from mechanism to treatment of progression. Front Physiol. 2020;11: 571810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Branco LM, Amaral MP, Boekhoff H, et al. Lysosomal cathepsins act in concert with Gasdermin-D during NAIP/NLRC4-dependent IL-1β secretion. Cell Death Dis. 2022;13(12):1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra SR, Mahapatra KK, Behera BP, et al. Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics. Int J Biochem Cell Biol. 2021;136: 106013. [DOI] [PubMed] [Google Scholar]
- 39.Tezcan G, Garanina EE, Alsaadi M, et al. Therapeutic potential of pharmacological targeting NLRP3 inflammasome complex in cancer. Front Immunol. 2020;11: 607881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou H, Zhang Q, Huang W, et al. NLRP3 inflammasome mediates silica-induced lung epithelial injury and aberrant regeneration in lung stem/progenitor cell-derived organotypic models. Int J Biol Sci. 2023;19(6):1875–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu X, Hao W, Liu Z, et al. Aluminum induces neuroinflammation via P2X7 receptor activating NLRP3 inflammasome pathway. Ecotoxicol Environ Saf. 2023;249: 114373. [DOI] [PubMed] [Google Scholar]
- 42.Karmakar M, Minns M, Greenberg EN, et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun. 2020;11(1):2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, Yang J, Shi J, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477(7366):596–600. [DOI] [PubMed] [Google Scholar]
- 44.Minns MS, Liboro K, Lima TS, et al. NLRP3 selectively drives IL-1β secretion by Pseudomonasaeruginosa infected neutrophils and regulates corneal disease severity. Nat Commun. 2023;14(1):5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477(7366):592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213(10):2113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li YK, Chen JG, Wang F. The emerging roles of absent in melanoma 2 (AIM2) inflammasome in central nervous system disorders. Neurochem Int. 2021;149: 105122. [DOI] [PubMed] [Google Scholar]
- 48.Kumari P, Russo AJ, Shivcharan S, Rathinam VA. AIM2 in health and disease: inflammasome and beyond. Immunol Rev. 2020;297(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma BR, Karki R, Kanneganti TD. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur J Immunol. 2019;49(11):1998–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma M, de Alba E. Assembly mechanism of the inflammasome sensor AIM2 revealed by single molecule analysis. Nat Commun. 2023;14(1):7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pierini R, Juruj C, Perret M, et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19(10):1709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120(Pt 5):772–81. [DOI] [PubMed] [Google Scholar]
- 53.Bolívar BE, Brown-Suedel AN, Rohrman BA, et al. Noncanonical roles of caspase-4 and caspase-5 in heme-driven IL-1β release and cell death. J Immunol. 2021;206(8):1878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Carvalho R, Andrade WA, Lima-Junior DS, et al. Leishmania lipophosphoglycan triggers caspase-11 and the non-canonical activation of the NLRP3 inflammasome. Cell Rep. 2019;26(2):429-437.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zanoni I, Tan Y, Di Gioia M, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352(6290):1232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Pan R, Ouyang Y, et al. Pyroptosis in health and disease: mechanisms, regulation and clinical perspective. Signal Transduct Target Ther. 2024;9(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grazia G, Vegetti C, Benigni F, et al. Synergistic anti-tumor activity and inhibition of angiogenesis by cotargeting of oncogenic and death receptor pathways in human melanoma. Cell Death Dis. 2014;5(10): e1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lillo S, Saleh M. Inflammasomes in cancer progression and anti-tumor immunity. Front Cell Dev Biol. 2022;10: 839041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karki R, Kanneganti TD. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer. 2019;19(4):197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keshavarz Shahbaz S, Koushki K, Ayati SH, Bland AR, Bezsonov EE, Sahebkar A. Inflammasomes and colorectal cancer. Cells. 2021;10(9):2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faria SS, Costantini S, de Lima V, et al. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J Biomed Sci. 2021;28(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caronni N, La Terza F, Frosio L, Ostuni R. IL-1β(+) macrophages and the control of pathogenic inflammation in cancer. Trends Immunol. 2025;46:403–15. [DOI] [PubMed] [Google Scholar]
- 64.Finucane OM, Sugrue J, Rubio-Araiz A, Guillot-Sestier MV, Lynch MA. The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1β-dependent manner in macrophages. Sci Rep. 2019;9(1):4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Yan X, Wang Y, et al. NLRP3 inflammasome inhibition attenuates silica-induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells. Exp Cell Res. 2018;362(2):489–97. [DOI] [PubMed] [Google Scholar]
- 66.Perera AP, Fernando R, Shinde T, et al. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8(1):8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L, Wan SC, Mao L, Huang CF, Bu LL, Sun ZJ. NLRP3 in tumor-associated macrophages predicts a poor prognosis and promotes tumor growth in head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2023;72(6):1647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knetki-Wróblewska M, Grzywna A, Krawczyk P, et al. Prognostic significance of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in second-line immunotherapy for patients with non-small cell lung cancer. Transl Lung Cancer Res. 2025;14(3):749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun (Lond). 2022;42(10):937–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams TM, Leeth RA, Rothschild DE, et al. The NLRP1 inflammasome attenuates colitis and colitis-associated tumorigenesis. J Immunol. 2015;194(7):3369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H, Luo Q, Feng X, Zhang R, Li J, Chen F. NLRP3 promotes tumor growth and metastasis in human oral squamous cell carcinoma. BMC Cancer. 2018;18(1):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen IF, Ou-Yang F, Hung JY, et al. AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol Cancer Ther. 2006;5(1):1–7. [DOI] [PubMed] [Google Scholar]
- 74.Zhai Z, Liu W, Kaur M, et al. NLRP1 promotes tumor growth by enhancing inflammasome activation and suppressing apoptosis in metastatic melanoma. Oncogene. 2017;36(27):3820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei Y, Huang H, Qiu Z, et al. NLRP1 overexpression is correlated with the tumorigenesis and proliferation of human breast tumor. Biomed Res Int. 2017;2017:4938473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Habibipour L, Sadeghi M, Raghibi A, Sanadgol N, Mohajeri Khorasani A, Mousavi P. The NLRP1 emerges as a promising therapeutic target and prognostic biomarker across multiple cancer types: a comprehensive pan-cancer analysis. Cancer Med. 2025;14(8): e70836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shen E, Han Y, Cai C, et al. Low expression of NLRP1 is associated with a poor prognosis and immune infiltration in lung adenocarcinoma patients. Aging (Albany NY). 2021;13(5):7570–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang K, Ke Z, Huang J, Zhang X. Expression and clinical value of NLRP1 and NLRC4 inflammasomes in prostate cancer. Oncol Lett. 2023;26(3):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang P, Gu Y, Yang J, et al. The prognostic value of NLRP1/NLRP3 and its relationship with immune infiltration in human gastric cancer. Aging (Albany NY). 2022;14(24):9980–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Łukaszewicz-Zając M, Mroczko B. Circulating biomarkers of colorectal cancer (CRC)-their utility in diagnosis and prognosis. J Clin Med. 2021;10(11):2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shao X, Lei Z, Zhou C. NLRP3 promotes colorectal cancer cell proliferation and metastasis via regulating epithelial mesenchymal transformation. Anticancer Agents Med Chem. 2020;20(7):820–7. [DOI] [PubMed] [Google Scholar]
- 82.Sheeja K, Lakshmi S. Nod-like receptor protein 3 inflammasome in head-and-neck cancer. J Cancer Res Ther. 2020;16(3):405–9. [DOI] [PubMed] [Google Scholar]
- 83.Xu Z, Wang H, Qin Z, et al. NLRP3 inflammasome promoted the malignant progression of prostate cancer via the activation of caspase-1. Cell Death Discov. 2021;7(1):399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32(3):379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kolb R, Phan L, Borcherding N, et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat Commun. 2016;7:13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tokuzen N, Nakashiro KI, Tojo S, Goda H, Kuribayashi N, Uchida D. Human papillomavirus-16 infection and p16 expression in oral squamous cell carcinoma. Oncol Lett. 2021;22(1):528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian R, Zhu Y, Yao J, et al. NLRP3 participates in the regulation of EMT in bleomycin-induced pulmonary fibrosis. Exp Cell Res. 2017;357(2):328–34. [DOI] [PubMed] [Google Scholar]
- 88.Huang X, Zhang M, Zhang Z. The role of LMP1 in Epstein-Barr virus-associated gastric cancer. Curr Cancer Drug Targets. 2024;24(2):127–41. [DOI] [PubMed] [Google Scholar]
- 89.Cortese N, Rigamonti A, Mantovani A, Marchesi F. The neuro-immune axis in cancer: relevance of the peripheral nervous system to the disease. Immunol Lett. 2020;227:60–5. [DOI] [PubMed] [Google Scholar]
- 90.Xu X, Huang Z, Huang Z, et al. Butyrate attenuates intestinal inflammation in Crohn’s disease by suppressing pyroptosis of intestinal epithelial cells via the cGSA-STING-NLRP3 axis. Int Immunopharmacol. 2024;143(Pt 2): 113305. [DOI] [PubMed] [Google Scholar]
- 91.Jalali AM, Mitchell KJ, Pompoco C, Poludasu S, Tran S, Ramana KV. Therapeutic significance of NLRP3 inflammasome in cancer: friend or foe. Int J Mol Sci. 2024;25(24):13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Theivanthiran B, Evans KS, DeVito NC, et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J Clin Invest. 2020;130(5):2570–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu M, Wang J, Li H, Zhang Z, Cheng Z. AIM2 inhibits colorectal cancer cell proliferation and migration through suppression of Gli1. Aging (Albany NY). 2020;13(1):1017–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang Y, Zhang M, Jin C, et al. Absent in melanoma 2 suppresses epithelial-mesenchymal transition via Akt and inflammasome pathways in human colorectal cancer cells. J Cell Biochem. 2019;120(10):17744–56. [DOI] [PubMed] [Google Scholar]
- 95.Fania L, Didona D, Di Pietro FR, et al. Cutaneous squamous cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2021;9(2):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qi M, Dai D, Liu J, et al. AIM2 promotes the development of non-small cell lung cancer by modulating mitochondrial dynamics. Oncogene. 2020;39(13):2707–23. [DOI] [PubMed] [Google Scholar]
- 97.Farshchian M, Nissinen L, Siljamäki E, et al. Tumor cell-specific AIM2 regulates growth and invasion of cutaneous squamous cell carcinoma. Oncotarget. 2017;8(28):45825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Q, Gao S, Shou Y, et al. AIM2 promotes renal cell carcinoma progression and sunitinib resistance through FOXO3a-ACSL4 axis-regulated ferroptosis. Int J Biol Sci. 2023;19(4):1266–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qin Y, Pan L, Qin T, et al. Pan-cancer analysis of AIM2 inflammasomes with potential implications for immunotherapy in human cancer: A bulk omics research and single cell sequencing validation. Front Immunol. 2022;13: 998266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He Z, Feng D, Zhang C, et al. Recent strategies for evoking immunogenic Pyroptosis in antitumor immunotherapy. J Control Release. 2024;366:375–94. [DOI] [PubMed] [Google Scholar]
- 101.Nguyen NT, Le XT, Lee WT, et al. STING-activating dendritic cell-targeted nanovaccines that evoke potent antigen cross-presentation for cancer immunotherapy. Bioact Mater. 2024;42:345–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hao Y, Baker D, Ten Dijke P. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20(11):2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22(5):550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ershaid N, Sharon Y, Doron H, et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat Commun. 2019;10(1):4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li LR, Chen L, Sun ZJ. Igniting hope: harnessing NLRP3 inflammasome-GSDMD-mediated pyroptosis for cancer immunotherapy. Life Sci. 2024;354: 122951. [DOI] [PubMed] [Google Scholar]
- 106.Zhang RN, Jing ZQ, Zhang L, Sun ZJ. Epigenetic regulation of pyroptosis in cancer: molecular pathogenesis and targeting strategies. Cancer Lett. 2023;575: 216413. [DOI] [PubMed] [Google Scholar]
- 107.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feng S, Wierzbowski MC, Hrovat-Schaale K, et al. Mechanisms of NLRP3 activation and inhibition elucidated by functional analysis of disease-associated variants. Nat Immunol. 2025;26(3):511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Winter RN, Rhee JG, Kyprianou N. Caspase-1 enhances the apoptotic response of prostate cancer cells to ionizing radiation. Anticancer Res. 2004;24(3a):1377–86. [PubMed] [Google Scholar]
- 110.Pandey A, Shen C, Man SM. Inflammasomes in colitis and colorectal cancer: mechanism of action and therapies. Yale J Biol Med. 2019;92(3):481–98. [PMC free article] [PubMed] [Google Scholar]
- 111.Allen IC, TeKippe EM, Woodford RM, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207(5):1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jha S, Srivastava SY, Brickey WJ, et al. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci. 2010;30(47):15811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185(8):4912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu Y, Jiang Y, Li S, et al. The Gasdermin D N-terminal fragment acts as a negative feedback system to inhibit inflammasome-mediated activation of Caspase-1/11. Proc Natl Acad Sci U S A. 2022;119(45): e2210809119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li YQ, Peng JJ, Peng J, Luo XJ. The deafness gene GSDME: its involvement in cell apoptosis, secondary necrosis, and cancers. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(9):1043–8. [DOI] [PubMed] [Google Scholar]
- 116.Jiang M, Qi L, Li L, Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu Y, Liu Y, Zong L, et al. The multifaceted roles of GSDME-mediated pyroptosis in cancer: therapeutic strategies and persisting obstacles. Cell Death Dis. 2023;14(12):836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fang Y, Tang Y, Huang B. Pyroptosis: a road to next-generation cancer immunotherapy. Semin Immunol. 2023;68: 101782. [DOI] [PubMed] [Google Scholar]
- 119.Kumar S, Singh SK, Rana B, Rana A. Tumor-infiltrating CD8(+) T cell antitumor efficacy and exhaustion: molecular insights. Drug Discov Today. 2021;26(4):951–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Van Wilpe S, Koornstra R, Den Brok M, et al. Lactate dehydrogenase: a marker of diminished antitumor immunity. Oncoimmunology. 2020;9(1):1731942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu Y, Lu C, Pan N, et al. Serum lactate dehydrogenase activities as systems biomarkers for 48 types of human diseases. Sci Rep. 2021;11(1):12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Orehek S, Ramuta TŽ, Lainšček D, et al. Cytokine-armed pyroptosis induces antitumor immunity against diverse types of tumors. Nat Commun. 2024;15(1):10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–8. [DOI] [PubMed] [Google Scholar]
- 124.Garcia-Marques J, Yang CP, Espinosa-Medina I, Mok K, Koyama M, Lee T. Unlimited genetic switches for cell-type-specific manipulation. Neuron. 2019;104(2):227-238.e7. [DOI] [PubMed] [Google Scholar]
- 125.Gao Y, Xu G, Ma L, et al. Icariside I specifically facilitates ATP or nigericin-induced NLRP3 inflammasome activation and causes idiosyncratic hepatotoxicity. Cell Commun Signal. 2021;19(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.León B. Type 2 conventional dendritic cell functional heterogeneity: ontogenically committed or environmentally plastic. Trends Immunol. 2025;46(2):104–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McDaniel MM, Kottyan LC, Singh H, Pasare C. Suppression of inflammasome activation by IRF8 and IRF4 in cDCs is critical for T cell priming. Cell Rep. 2020;31(5): 107604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spalinger MR, Scharl M. PTPN2 as a promoter of colon carcinoma via reduction of inflammasome activation. Mol Cell Oncol. 2018;5(4): e1465013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen Y, Yang B, Zhao J, et al. Exploiting lactic acid bacteria for colorectal cancer: a recent update. Crit Rev Food Sci Nutr. 2024;64(16):5433–49. [DOI] [PubMed] [Google Scholar]
- 130.Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30(1):36–50. [DOI] [PubMed] [Google Scholar]
- 131.Schulz D, Severin Y, Zanotelli V, Bodenmiller B. In-depth characterization of monocyte-derived macrophages using a mass cytometry-based phagocytosis assay. Sci Rep. 2019;9(1):1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mosser DM, Hamidzadeh K, Goncalves R. Macrophages and the maintenance of homeostasis. Cell Mol Immunol. 2021;18(3):579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shi Y, Su W, Zhang L, et al. TGR5 regulates macrophage inflammation in nonalcoholic steatohepatitis by modulating NLRP3 inflammasome activation. Front Immunol. 2020;11: 609060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qing L, Fu J, Wu P, Zhou Z, Yu F, Tang J. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am J Transl Res. 2019;11(2):655–68. [PMC free article] [PubMed] [Google Scholar]
- 135.Daley D, Mani VR, Mohan N, et al. NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J Exp Med. 2017;214(6):1711–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sarantis P, Koustas E, Papadimitropoulou A, Papavassiliou AG, Karamouzis MV. Pancreatic ductal adenocarcinoma: treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol. 2020;12(2):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Tumor cell-derived IL1β promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Res. 2020;80(5):1088–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ye F, Liang Y, Wang Y, et al. Cancer-associated fibroblasts facilitate breast cancer progression through exosomal circTBPL1-mediated intercellular communication. Cell Death Dis. 2023;14(7):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hilmi M, Nicolle R, Bousquet C, Neuzillet C. Cancer-associated fibroblasts: accomplices in the tumor immune evasion. Cancers (Basel). 2020;12(10):2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou Y, Cao T, Li Z, et al. Fibroblasts in immune-mediated inflammatory diseases: The soil of inflammation. Clin Immunol. 2024;258: 109849. [DOI] [PubMed] [Google Scholar]
- 141.Zhang F, Guangchuan W, Chow R, et al. Multiplexed inhibition of immunosuppressive genes with Cas13d for on-demand combinatorial cancer immunotherapy. BioRxiv. 2023. 10.1101/2023.03.14.532668.38654827 [Google Scholar]
- 142.Lin Y, Cai Q, Chen Y, et al. CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase. Hepatology. 2022;75(1):28–42. [DOI] [PubMed] [Google Scholar]
- 143.Castaño Z, San Juan BP, Spiegel A, et al. IL-1β inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nat Cell Biol. 2018;20(9):1084–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gelfo V, Romaniello D, Mazzeschi M, et al. Roles of IL-1 in cancer: from tumor progression to resistance to targeted therapies. Int J Mol Sci. 2020;21(17):6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang YL, Yang F, Huang ZQ, et al. T cells, NK cells, and tumor-associated macrophages in cancer immunotherapy and the current state of the art of drug delivery systems. Front Immunol. 2023;14:1199173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Del Prete A, Salvi V, Soriani A, et al. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol. 2023;20(5):432–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Courtney AH, Lo WL, Weiss A. TCR signaling: mechanisms of initiation and propagation. Trends Biochem Sci. 2018;43(2):108–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Azuma M. Co-signal molecules in T-cell activation : historical overview and perspective. Adv Exp Med Biol. 2019;1189:3–23. [DOI] [PubMed] [Google Scholar]
- 149.Hilligan KL, Ronchese F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol Immunol. 2020;17(6):587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xi G, Gao J, Wan B, et al. GSDMD is required for effector CD8(+) T cell responses to lung cancer cells. Int Immunopharmacol. 2019;74: 105713. [DOI] [PubMed] [Google Scholar]
- 151.Kajino-Sakamoto R, Fujishita T, Taketo MM, Aoki M. Synthetic lethality between MyD88 loss and mutations in Wnt/β-catenin pathway in intestinal tumor epithelial cells. Oncogene. 2021;40(2):408–20. [DOI] [PubMed] [Google Scholar]
- 152.Recio C, Guerra B, Guerra-Rodríguez M, et al. Signal transducer and activator of transcription (STAT)-5: an opportunity for drug development in oncohematology. Oncogene. 2019;38(24):4657–68. [DOI] [PubMed] [Google Scholar]
- 153.Van Den Eeckhout B, Tavernier J, Gerlo S. Interleukin-1 as innate mediator of T cell immunity. Front Immunol. 2020;11: 621931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng R, Li Y, Chen D, et al. Changes of host immunity mediated by IFN-γ(+) CD8(+) T cells in children with adenovirus pneumonia in different severity of illness. Viruses. 2021;13(12):2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhivaki D, Kagan JC. NLRP3 inflammasomes that induce antitumor immunity. Trends Immunol. 2021;42(7):575–89. [DOI] [PubMed] [Google Scholar]
- 156.Nowarski R, Jackson R, Gagliani N, et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell. 2015;163(6):1444–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Deets KA, Vance RE. Inflammasomes and adaptive immune responses. Nat Immunol. 2021;22(4):412–22. [DOI] [PubMed] [Google Scholar]
- 158.Dang AT, Pasquali C, Ludigs K, Guarda G. OM-85 is an immunomodulator of interferon-β production and inflammasome activity. Sci Rep. 2017;7:43844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ji S, Lee J, Lee ES, Kim DH, Sin JI. B16 melanoma control by anti-PD-L1 requires CD8+ T cells and NK cells: application of anti-PD-L1 Abs and Trp2 peptide vaccines. Hum Vaccin Immunother. 2021;17(7):1910–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tengesdal IW, Dinarello CA, Marchetti C. NLRP3 and cancer: Pathogenesis and therapeutic opportunities. Pharmacol Ther. 2023;251: 108545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sultana Z, Bankura B, Pattanayak AK, et al. Association of Interleukin-1 beta and tumor necrosis factor-alpha genetic polymorphisms with gastric cancer in India. Environ Mol Mutagen. 2018;59(7):653–67. [DOI] [PubMed] [Google Scholar]
- 162.Nisar MA, Zheng Q, Saleem MZ, et al. IL-1β promotes vasculogenic mimicry of breast cancer cells through p38/MAPK and PI3K/Akt signaling pathways. Front Oncol. 2021;11: 618839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heinzerling L, Dummer R, Pavlovic J, Schultz J, Burg G, Moelling K. Tumor regression of human and murine melanoma after intratumoral injection of IL-12-encoding plasmid DNA in mice. Exp Dermatol. 2002;11(3):232–40. [DOI] [PubMed] [Google Scholar]
- 164.Li H, Guan Y, Liang B, et al. Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur J Pharmacol. 2022;928: 175091. [DOI] [PubMed] [Google Scholar]
- 165.Lee JM, Tsuboi M, Kim ES, Mok TS, Garrido P. Overcoming immunosuppression and pro-tumor inflammation in lung cancer with combined IL-1β and PD-1 inhibition. Future Oncol. 2022;18(27):3085–100. [DOI] [PubMed] [Google Scholar]
- 166.Blevins HM, Xu Y, Biby S, Zhang S. The NLRP3 inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front Aging Neurosci. 2022;14: 879021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Klück V, Jansen T, Janssen M, et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2020;2(5):e270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Deventer HW, Burgents JE, Wu QP, et al. The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res. 2010;70(24):10161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bakhshi S, Shamsi S. MCC950 in the treatment of NLRP3-mediated inflammatory diseases: latest evidence and therapeutic outcomes. Int Immunopharmacol. 2022;106: 108595. [DOI] [PubMed] [Google Scholar]
- 170.Ciarlo E, Heinonen T, Herderschee J, et al. Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo. Sci Rep. 2016;6:37944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qamar A, Rader DJ. Effect of interleukin 1β inhibition in cardiovascular disease. Curr Opin Lipidol. 2012;23(6):548–53. [DOI] [PubMed] [Google Scholar]
- 172.Rathinam VA, Jiang Z, Waggoner SN, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11(5):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Coll RC, Hill JR, Day CJ, et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15(6):556–9. [DOI] [PubMed] [Google Scholar]
- 175.Yaw A, Chan E, Yap J, Mai CW. The effects of NLRP3 inflammasome inhibition by MCC950 on LPS-induced pancreatic adenocarcinoma inflammation. J Cancer Res Clin Oncol. 2020;146(9):2219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen L, Huang CF, Li YC, et al. Blockage of the NLRP3 inflammasome by MCC950 improves anti-tumor immune responses in head and neck squamous cell carcinoma. Cell Mol Life Sci. 2018;75(11):2045–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zheng Q, Yao D, Cai Y, Zhou T. NLRP3 augmented resistance to gemcitabine in triple-negative breast cancer cells via EMT/IL-1β/Wnt/β-catenin signaling pathway. Biosci Rep. 2020;40(7):BSR20200730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang Y, Jiang H, Chen Y, et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med. 2018;10(4): e8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Darakhshan S, Pour AB. Tranilast: a review of its therapeutic applications. Pharmacol Res. 2015;91:15–28. [DOI] [PubMed] [Google Scholar]
- 180.Takahashi K, Menju T, Nishikawa S, et al. Tranilast inhibits TGF-β1-induced epithelial-mesenchymal transition and invasion/metastasis via the suppression of Smad4 in human lung cancer cell lines. Anticancer Res. 2020;40(6):3287–96. [DOI] [PubMed] [Google Scholar]
- 181.Saito H, Fushida S, Harada S, et al. Importance of human peritoneal mesothelial cells in the progression, fibrosis, and control of gastric cancer: inhibition of growth and fibrosis by tranilast. Gastric Cancer. 2018;21(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.He Y, Varadarajan S, Muñoz-Planillo R, Burberry A, Nakamura Y, Núñez G. 3,4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem. 2014;289(2):1142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.He H, Jiang H, Chen Y, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9(1):2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen L, Ruan Y, Wang X, et al. BAY 11–7082, a nuclear factor-κB inhibitor, induces apoptosis and S phase arrest in gastric cancer cells. J Gastroenterol. 2014;49(5):864–74. [DOI] [PubMed] [Google Scholar]
- 185.Chen IH, Chang FR, Wu YC, Kung PH, Wu CC. 3,4-Methylenedioxy-β-nitrostyrene inhibits adhesion and migration of human triple-negative breast cancer cells by suppressing β1 integrin function and surface protein disulfide isomerase. Biochimie. 2015;110:81–92. [DOI] [PubMed] [Google Scholar]
- 186.Li M, Liu H, Shao H, et al. Glyburide attenuates B(a)p and LPS-induced inflammation-related lung tumorigenesis in mice. Environ Toxicol. 2021;36(8):1713–22. [DOI] [PubMed] [Google Scholar]
- 187.Mori N, Yamada Y, Ikeda S, et al. Bay 11–7082 inhibits transcription factor NF-kappaB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood. 2002;100(5):1828–34. [DOI] [PubMed] [Google Scholar]
- 188.Liu H, Xu Y, Liang K, Liu R. Immune cells combined with NLRP3 inflammasome inhibitor exert better antitumor effect on pancreatic ductal adenocarcinoma. Front Oncol. 2020;10:1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tufekci KU, Eltutan BI, Isci KB, Genc S. Resveratrol inhibits NLRP3 inflammasome-induced pyroptosis and miR-155 expression in microglia through Sirt1/AMPK pathway. Neurotox Res. 2021;39(6):1812–29. [DOI] [PubMed] [Google Scholar]
- 190.Tengesdal IW, Dinarello A, Powers NE, et al. Tumor NLRP3-derived IL-1β drives the IL-6/STAT3 axis resulting in sustained MDSC-mediated immunosuppression. Front Immunol. 2021;12: 661323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tengesdal IW, Menon DR, Osborne DG, et al. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc Natl Acad Sci U S A. 2021;118(10): e2000915118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sun H, Cai H, Fu Y, et al. The protection effect of resveratrol against radiation-induced inflammatory bowel disease via NLRP-3 inflammasome repression in mice. Dose Response. 2020;18(2):1559325820931292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim BH, Min YS, Choi JS, et al. Benzoxathiol derivative BOT-4-one suppresses L540 lymphoma cell survival and proliferation via inhibition of JAK3/STAT3 signaling. Exp Mol Med. 2011;43(5):313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee HE, Lee JY, Yang G, et al. Inhibition of NLRP3 inflammasome in tumor microenvironment leads to suppression of metastatic potential of cancer cells. Sci Rep. 2019;9(1):12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pereira M, Liang J, Edwards-Hicks J, et al. Arachidonic acid inhibition of the NLRP3 inflammasome is a mechanism to explain the anti-inflammatory effects of fasting. Cell Rep. 2024;43(2): 113700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim AH, Lee Y, Kim E, Ji SC, Chung JY, Cho JY. Assessment of oral vancomycin-induced alterations in gut bacterial microbiota and metabolome of healthy men. Front Cell Infect Microbiol. 2021;11: 629438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ahn H, Kang SG, Yoon SI, et al. Methylene blue inhibits NLRP3, NLRC4, AIM2, and non-canonical inflammasome activation. Sci Rep. 2017;7(1):12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gao J, Peng S, Shan X, et al. Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death Dis. 2019;10(12):957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang J, Ren C, Bi W, Batu W. Glycyrrhizin mitigates acute lung injury by inhibiting the NLRP3 inflammasome in vitro and in vivo. J Ethnopharmacol. 2023;303: 115948. [DOI] [PubMed] [Google Scholar]
- 201.Xu S, Li X, Liu Y, Xia Y, Chang R, Zhang C. Inflammasome inhibitors: promising therapeutic approaches against cancer. J Hematol Oncol. 2019;12(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Taldaev A, Terekhov R, Nikitin I, et al. Methylene blue in anticancer photodynamic therapy: systematic review of preclinical studies. Front Pharmacol. 2023;14:1264961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Franke M, Bieber M, Kraft P, Weber A, Stoll G, Schuhmann MK. The NLRP3 inflammasome drives inflammation in ischemia/reperfusion injury after transient middle cerebral artery occlusion in mice. Brain Behav Immun. 2021;92:223–33. [DOI] [PubMed] [Google Scholar]
- 204.Massoud G, Parish M, Hazimeh D, et al. Unlocking the potential of tranilast: Targeting fibrotic signaling pathways for therapeutic benefit. Int Immunopharmacol. 2024;137: 112423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fan Y, Xue G, Chen Q, Lu Y, Dong R, Yuan H. CY-09 inhibits NLRP3 inflammasome activation to relieve pain via TRPA1. Comput Math Methods Med. 2021;2021:9806690. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 206.Tweedell RE, Kanneganti TD. Advances in inflammasome research: recent breakthroughs and future hurdles. Trends Mol Med. 2020;26(11):969–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jiao J, Zhao G, Wang Y, Ren P, Wu M. MCC950, a selective inhibitor of NLRP3 inflammasome, reduces the inflammatory response and improves neurological outcomes in mice model of spinal cord injury. Front Mol Biosci. 2020;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Walle LV, Stowe IB, Šácha P, et al. Correction: MCC950/CRID3 potently targets the NACHT domain of wild-type NLRP3 but not disease-associated mutants for inflammasome inhibition. PLoS Biol. 2019;17(10): e3000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Luo Y, Sun S, Zhang Y, et al. Effects of oltipraz on the glycolipid metabolism and the Nrf2/HO-1 pathway in type 2 diabetic mice. Drug Des Devel Ther. 2024;18:5685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lin ZH, Wang SY, Chen LL, et al. Methylene blue mitigates acute neuroinflammation after spinal cord injury through inhibiting NLRP3 inflammasome activation in microglia. Front Cell Neurosci. 2017;11:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.