Abstract
In an effort to understand the relationship between Vibrio and vibriophage populations, abundances of Vibrio spp. and viruses infecting Vibrio parahaemolyticus (VpVs) were monitored for a year in Pacific oysters and water collected from Ladysmith Harbor, British Columbia, Canada. Bacterial abundances were highly seasonal, whereas high titers of VpVs (0.5 × 104 to 11 × 104 viruses cm−3) occurred year round in oysters, even when V. parahaemolyticus was undetectable (<3 cells cm−3). Viruses were not detected (<10 ml−1) in the water column. Host-range studies demonstrated that 13 VpV strains could infect 62% of the V. parahaemolyticus strains from oysters (91 pairings) and 74% of the strains from sediments (65 pairings) but only 30% of the water-column strains (91 pairings). Ten viruses also infected more than one species among V. alginolyticus, V. natriegens, and V. vulnificus. As winter approached and potential hosts disappeared, the proportion of host strains that the viruses could infect decreased by ∼50% and, in the middle of winter, only 14% of the VpV community could be plated on summer host strains. Estimates of virus-induced mortality on V. parahaemolyticus indicated that other host species were required to sustain viral production during winter when the putative host species was undetectable. The present study shows that oysters are likely one of the major sources of viruses infecting V. parahaemolyticus in oysters and in the water column. Furthermore, seasonal shifts in patterns of host range provide strong evidence that the composition of the virus community changes during winter.
Viral infection of marine microbial communities (reviewed in references 17, 49, 63, and 64) has been associated with reduced primary production (23, 47, 50) and increased bacterial mortality through either lytic (21, 39, 42, 46, 62) or temperate (27, 28, 39, 59, 60) phage. Frequently, Vibrio spp. and vibriophage, which are common in seawater (4, 53) and easily culturable (16), have served as model systems in studies of host-virus interactions in the water column (see, for example, references 22, 33, 37, 43, 52, 54, and 61). Vibrio spp. are typically much more abundant in sediments (104 g−1) (41), plankton (109 g−1) (31), and shellfish (105 g−1) (1) than in the water column (∼10 ml−1) (5). In contrast, even though virus particles are extremely abundant in sediments (108 to 109 ml−1) (10, 15) and in the water column (ca. 107 to 108 ml−1) (64), vibriophage are most abundant in mollusks (105 to 108 g−1) (3, 14), relatively rare in the water column (∼2 ml−1) (34), and frequently undetectable in sediments.
The marine bacterium Vibrio parahaemolyticus is a gastrointestinal pathogen that is abundant (104 g−1) (12) in oysters. It can cause disease in humans that consume raw shellfish, and in 1997 it was responsible for a major outbreak of disease in British Columbia (6). High abundances of viruses that infect V. parahaemolyticus (VpVs; 106 g−1) (3) also occur in oysters. However, with the exception of a study showing transduction of the agarase gene between V. alginolyticus and V. parahaemolyticus (2), and host range studies using V. vulnificus (13, 14), little work has been done on vibriophage in oysters.
We examined seasonal changes in the abundances of V. parahaemolyticus, its phage, and total presumptive Vibrio spp. in oysters and the neighboring water column, as well as host range and the sensitivity of host and virus strains. We show that oysters are likely one of the major sources of viruses infecting V. parahaemolyticus in oysters and in the water column. Furthermore, seasonal shifts in patterns of host range provide strong evidence that the composition of the virus community changes during winter and that the persistence of these viruses in oysters during winter is supported by hosts other than V. parahaemolyticus. These results imply that there can be strong coupling between phage and host populations that occur in different environments and that a broad host range can explain the occurrence of phage populations during periods during which their hosts are apparently absent.
MATERIALS AND METHODS
Sample collection.
Oyster and seawater (250 ml) samples were collected from a commercial aquaculture bed in Ladysmith Harbour, British Columbia, Canada (49°00′N, 123°49′W) biweekly from June to September 2000 and approximately monthly thereafter until May 2001. Samples for plating efficiency tests (see below) were also collected in August 2003. Seawater samples were collected adjacent to the oysters, and all samples were transported on ice and analyzed within 24 h. Ten to twelve oysters were collected across the bed, shucked, and homogenized (55) before analysis, except for August 2003, when 10 oysters were analyzed separately and then pooled for further testing. The temperature within the oysters was determined, at the time of collection, using a hand-held thermometer, and water salinity was determined by using a hand-held refractometer (both Fisher Scientific).
TPV enumeration and isolation.
Oyster homogenates were vortexed and centrifuged (4,100 × g for 60 s) to sediment large debris, and the supernatants were subsampled. Although centrifugation may slightly reduce bacterial abundance (∼10% in test samples), filtration was not possible without prior clarification. Filtration was used because it is the accepted standard for water quality and because its has greater precision than other methods such as spread plating. Also, because filtration was used for water samples in the present study and others, it allowed direct comparison of abundance estimates from different environments. Abundance estimates of mesophilic total presumptive Vibrio spp. (TPV) were obtained by duplicate serial dilutions of water and oyster subsamples in 10 ml (final volume) of ultrafiltered (30-kDa cutoff; Millipore) (51), sterilized natural seawater that was diluted to 15 practical salinity units (psu) with reverse-osmosis water (Millipore). The dilutions were filtered onto 0.45-μm-pore-size cellulose membranes (47-mm HAWP; Millipore) which were laid, two per plate, onto TCBS agar (BD Gibco). The plates were incubated at 25°C for ∼24 h. Colonies were purified by serially restreaking three times onto marine Luria-Bertani agar plates (MLB; 0.5 g liter−1 each of Casamino Acids, peptone, and yeast extract, 0.3% [vol/vol] glycerol, and 1% [wt/vol] agar in 15 psu of ultrafiltrate base) and identified biochemically (API 20E; bioMérieux).
V. parahaemolyticus enumeration and isolation.
Abundances of V. parahaemolyticus were determined in oysters as outline by the U.S. Food and Drug Administration (55). Oyster homogenates were diluted 10-fold in 2% NaCl and inoculated into a series of alkaline peptone water tubes for an overnight most probable number (MPN) assay. Positive tubes were plated onto TCBS agar, and colonies of V. parahaemolyticus were confirmed biochemically (API 20E).
VpV enumeration and isolation.
Titers of VpVs (viruses ml−1) were determined in oyster and water samples by plaque assay (48) using MLB plates and top agar overlay (0.6% [wt/vol] agar). To confirm that the pooled oyster samples were representative of individuals, the VpV titers for 10 oysters were determined separately and then pooled. The pooled sample titer was slightly lower, but within one standard deviation of the mean obtained from the individuals. Duplicate parallel dilutions were performed by using the same diluent as for TPV counts. The host strain used for enumeration, clinical isolate 94Z944 E1-80 (British Columbia Centre for Disease Control), was determined to be the most permissive host in terms of total viral abundance in test assays and is referred to as the control strain. For most samples, one to three different viruses (indicated by plaque morphology) were cloned by three rounds of plaque purification. Viruses were then amplified by the plate-lysate method (45), 0.22 μm filtered, and maintained at 4°C in the dark.
Host range and plating efficiency assays.
Virus host ranges were determined with strains of V. parahaemolyticus and other Vibrio spp. isolated during the present study or obtained from other sources (Table 1). Portions (10 to 20 μl) of amplified virus stocks were spotted onto lawns of bacteria and monitored for zones of clearing for 1 to 7 days. Oyster homogenates from 21 February 2001 (the winter community) and from 28 August 2003 (the summer community) were used to compare the relative plating efficiencies among several summer strains of V. parahaemolyticus. Plaque assays and efficiencies were expressed relative to the control strain.
TABLE 1.
Sources of bacterial strains used to determine the host range of VpVsa
| Species | Strain(s) | Sourceb |
|---|---|---|
| Vibrio parahaemolyticus | C00-9a | Ladysmith Harbour (BC) |
| Vibrio parahaemolyticus | J00-5 to J00-9a | Jericho Pier (BC) |
| Vibrio parahaemolyticus | S00-1 to S01-31 | Strait of Georgia (BC) |
| Vibrio parahaemolyticus | V408-10 to V497-B1 | Ladysmith Harbour (BC) |
| Vibrio alginolyticus | ATCC17749 | ATCC |
| Vibrio alginolyticus | C00-6a | Ladysmith Harbour (BC) |
| Vibrio alginolyticus | J00-10a | Jericho Pier (BC) |
| Vibrio alginolyticus | PWH3a | Gulf of Mexico (TX) |
| Vibrio alginolyticus | S99-45/S00-4 | Strait of Georgia (BC) |
| Vibrio alginolyticus | V478-16/V497-C16 | Ladysmith Harbour (BC) |
| Vibrio fluvialus | S99-44 | Strait of Georgia (BC) |
| Vibrio natriegens | ATCC 14048 | ATCC |
| Vibrio vulnificus | S99-48 | Strait of Georgia (BC) |
| Vibrio vulnificus | S01-38 | Strait of Georgia (BC) |
Strains from Ladysmith Harbour were isolated during the course of this study. All other strains were isolated in our lab (8), except for the V. alginolyticus and V. natriegens American Type Culture Collection (ATCC) strains.
BC, British Columbia, Canada; TX, Texas.
Statistical analysis.
Undetectable values were assumed to equal the detection limits for calculations of mean abundances and for correlation analyses. Correlations were performed by using VassarStats (http://faculty.vassar.edu/lowry/VassarStats.html). Bacterial and viral abundances in the oysters were expressed per cubic centimeter of tissue homogenate.
RESULTS
Physical parameters.
Water temperatures (Fig. 1 and Table 2) varied from ca. 12 to 14°C in summer and decreased to near 4°C during winter, while salinity ranged from 20 to near 30 psu in summer and winter, respectively.
FIG. 1.
Temperature (⧫) and salinity (⋄) during May 2000 to May 2001.
TABLE 2.
Range of biological and physical parameters obtained from this study and othersa
| Parameter | Measurements
|
Locatione | Source or reference | |||
|---|---|---|---|---|---|---|
| Unit | Min | Mean | Max | |||
| Abundances (oyster) | ||||||
| Virus (VpVs) | Viruses cm−3 | 5.0 × 103 | 3.3 × 104 | 1.1 × 105 | Coastal BC | This study |
| Vibrio spp. | Cells cm−3 | <10 | 2.5 × 104 | 7.5 × 104 | Coastal BC | This study |
| V. parahaemolyticus | Cells cm−3 | <3.0 | 243 | 2.3 × 103 | Coastal BC | This study |
| Abundances (water) | ||||||
| Virus (VpVs) | Viruses liter−1 | <0.05 | 3.4 | 8.5 | Coastal BC | 8 |
| <1.0 | 16 | 18.0 | Coastal HI | 40 | ||
| 50 | 380 | 1.9 × 103 | Coastal FL/HI | 34 | ||
| Vibrio spp. | cells ml−1 | <1.0 | 710 | 5.4 × 103 | Coastal BC | This study |
| V. parahaemolyticus | cells ml−1 | <0.01 | 0.07 | 0.46 | Chesapeake Bay, US | 32 |
| 0.02 | 0.24 | 0.68 | Coastal US | 11 | ||
| <0.01 | 2.7 | 550 | Okayama, JP | 7 | ||
| <1.0 | 34 | 70 | Pacific NW, US | 36 | ||
| Physiology | ||||||
| Bacterial growth rateb | Day−1 | 0c | 0c | 0.33d | 56 | |
| Oyster grazing rate | Day −1 | 0.05 | 9 | |||
| Burst size | Viruses cell−1 | 5.0 | 130 | 350 | 24 | |
| Physical | ||||||
| Temp | °C | 4.0 | 9.4 | 14 | Coastal BC | This study |
| Salinity | psu | 20 | 24 | 31 | Coastal BC | This study |
If only one value is reported, that value is marked as the mean value. VpV, V. parahaemolyticus virus.
Rates correspond to published observations at the minimum, mean, and maximum temperatures listed for this study.
These temperatures (4.0/9.4°C) are usually reported as bacteriostatic for V. parahaemolyticus.
The reported rate of 0.33 day−1 was at a temperature of 15°C instead of the corresponding 14°C in this study.
BC, British Columbia, Canada; FL/HI, Florida and Hawaii; NW, Northwest; US, United States; JP, Japan.
Bacterial and viral abundances.
With the exception of TPV in oysters (with salinity), bacterial abundances (Fig. 2A) were significantly correlated with temperature and salinity (Table 3), with highest abundances in summer when temperatures were >10°C and salinities were <26 psu. The highest bacterial abundance was in June when the TPV within oysters was 7 × 104 cells cm−3, while in the water TPV peaked in early July at 5.4 × 103 cells ml−1. TPV were undetectable in oysters (<10 cells cm−3) and in the water (<1 cell ml−1) during winter. V. parahaemolyticus in oysters followed a pattern similar to that of TPV but peaked 3 weeks later at 2.3 × 103 cells cm−3 before it reached undetectable levels (<3 cells cm−3) by late September. The abundance of V. parahaemolyticus in oysters was better correlated to TPV abundance in the water (r = 0.71, P < 0.05) than in oysters (r = 0.48, P = 0.10) and was even better correlated (r = 0.84, P < 0.05) if the 3-week shift between maximum abundances was incorporated.
FIG. 2.
Bacterial and viral abundances from May 2000 to May 2001. Closed symbols indicate abundances within oysters, and open symbols indicate water-column abundances. (A) Bacterial abundances: water-column total presumptive Vibrio spp. (□), oyster total presumptive Vibrio spp. (▪), and oyster V. parahaemolyticus (•). (B) Viral abundance in the oyster (▴). Values are means of duplicate samples; error bars indicate the ranges of duplicates and are smaller than the width of the symbol if not visible.
TABLE 3.
Pearson correlation analysis of the physical and biological parameters measured in this studya
| Variable | Pearson correlation r (P)
|
|||||
|---|---|---|---|---|---|---|
| Water TPV | Oyster TPV | Oyster V. parahaemolyticus | Oyster VpV | Oyster temp | Water salinity | |
| Water TPV | 1 | 0.72* (<0.05) | 0.71* (<0.05) | −0.05 (0.87) | 0.85* (<0.05) | −0.79* (<0.05) |
| Oyster TPV | 1 | 0.48 (0.10) | −0.24 (0.45) | 0.79* (<0.05) | −0.36 (0.25) | |
| Oyster V. parahaemolyticus | 1 | −0.08 (0.82) | 0.58* (<0.05) | −0.66* (<0.05) | ||
| Oyster VpV | 1 | −0.22 (0.50) | 0.29 (0.36) | |||
| Oyster temp | 1 | −0.72* (<0.05) | ||||
| Water salinity | 1 | |||||
In contrast to bacteria, viruses in oysters were always detectable, and there was no seasonal pattern to their abundance (Fig. 2B). Titers ranged from 5.0 × 103 to 1.1 × 105 viruses cm−3, with an average of 3.3 × 104 viruses cm−3 (Table 2). Although viral abundance appeared to oscillate versus oyster TPV during summer, the correlation between the two parameters was not significant (r = −0.24, P = 0.45). In fact, viral abundance was not correlated to any of the measured parameters (Table 3). Viruses were always undetectable (<10 ml−1) in the water.
Host range.
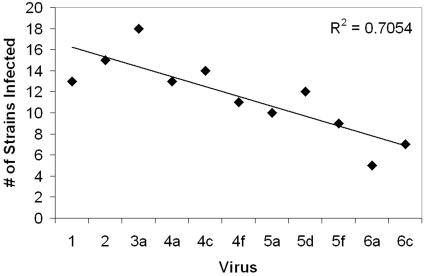
Viruses were able to infect most strains of V. parahaemolyticus from oysters and sediment (62 and 74% of the pairings, respectively) but infected fewer strains from the water (30% of the pairings; Table 4). Collectively, the 13 viral strains infected 17 of 19 strains of V. parahaemolyticus, with each virus infecting a median of 11 strains (range, 5 to 14 strains). On average, each strain of V. parahaemolyticus was sensitive to ca. 7 of the 13 viruses (53% coverage of the total pairings) and ranged from two strains that were immune to all 13 viruses to three strains that could be infected by 12 viruses. Ten of the viruses were not species specific and infected four of nine V. alginolyticus strains (the species closest to V. parahaemolyticus), one V. natriegens strain, and one of two V. vulnificus strains (Table 4). There was also a temporal trend in the patterns of infection (Fig. 3). The number of bacterial strains infected (i.e., sensitivity) by viruses when V. parahaemolyticus was present (summer) showed a significant decreasing trend (r2 = 0.71, P < 0.05) as the season progressed. Viruses infected from 12 to 18 (of 30) strains in June through August, 8 or less in September (the end of the period when V. parahaemolyticus was detectable), and 15 and 10 (Table 4) for the two viruses isolated during the winter.
TABLE 4.
Host range of VpVs isolated during summer 2000 and winter 2000 and 2001 versus co-occurring V. parahaemolyticus (from three environments) and related Vibrio spp.a
| Virus host or origin | Species | Strain | Date | Summer
|
Winter
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VpV LH1 (Jun-21) | VpV LH2 (Jul-27) | VpV LH3a (Aug-15) | VpV LH4a (Aug-29) | VpV LH4c (Aug-29) | VpV LH4f (Aug-29) | VpV LH5a (Sep-13) | VpV LH5d (Sep-13) | VpV LH5f (Sep-13) | VpV LH6a (Sep-26) | VpV LH6c (Sep-26) | VpV LH7a (Nov-16) | VpV LH8a (Jan-9) | ||||
| Oyster | V. parahaemolyticus | V408-10 | Jun-8 | + | + | − | + | + | − | + | + | + | − | − | + | + |
| V. parahaemolyticus | V416-7 | Jun-21 | + | + | + | + | + | + | − | + | + | + | + | + | + | |
| V. parahaemolyticus | V424-A3 | Jul-6 | + | + | + | + | + | − | + | + | − | + | − | − | − | |
| V. parahaemolyticus | V439-A1 | Jul-27 | + | + | + | + | + | + | + | + | + | + | − | + | + | |
| V. parahaemolyticus | V465-A1 | Aug-15 | + | + | + | − | + | + | + | + | + | − | + | + | + | |
| V. parahaemolyticus | V478-1 | Aug-29 | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| V. parahaemolyticus | V497-B1 | Sep-26 | − | − | + | + | + | − | − | + | − | − | − | − | − | |
| Water | V. parahaemolyticus | C00-9a | Sep-13 | − | + | + | + | + | − | + | − | − | − | + | + | + |
| V. parahaemolyticus | J00-5 | − | − | − | − | − | − | − | − | − | − | − | + | − | ||
| V. parahaemolyticus | J00-6a | − | − | + | − | − | − | − | − | − | − | + | + | − | ||
| V. parahaemolyticus | J00-7a | + | + | + | + | + | + | − | + | − | − | − | + | + | ||
| V. parahaemolyticus | J00-9a | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| V. parahaemolyticus | S00-1 | − | − | + | + | − | − | − | − | − | − | − | − | − | ||
| V. parahaemolyticus | S01-31 | + | − | − | − | + | + | − | − | + | − | − | − | − | ||
| Sediment | V. parahaemolyticus | S00-2 | + | − | + | + | + | + | − | − | − | − | − | + | − | |
| V. parahaemolyticus | S00-3 | + | + | + | + | + | + | + | + | + | + | − | + | + | ||
| V. parahaemolyticus | S00-5 | + | + | + | + | + | + | + | + | + | − | + | + | − | ||
| V. parahaemolyticus | S00-8 | + | + | + | + | + | + | + | + | − | − | − | − | − | ||
| V. parahaemolyticus | S01-20 | + | + | + | + | + | + | + | + | + | + | + | − | − | ||
| Other | V. alginolyticus | C00-6a | Jul-27 | + | + | + | − | − | − | − | − | − | − | − | + | + |
| V. alginolyticus | V478-16 | Aug-29 | − | + | + | − | − | − | − | − | − | − | − | − | − | |
| V. alginolyticus | V478-17 | Aug-29 | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| V. alginolyticus | V497-C16 | Sep-26 | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| V. alginolyticus | ATCC17749 | − | − | − | − | − | − | − | − | − | − | − | + | + | ||
| V. alginolyticus | S99-45 | − | + | + | − | − | + | + | + | + | − | + | − | − | ||
| V. alginolyticus | Three others | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| V. fluvialus | S99-44 | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| V. natriegens | ATCC14048 | − | − | − | − | − | − | − | − | − | − | − | + | − | ||
| V. vulnificus | S99-48 | − | + | + | − | − | − | − | − | − | − | − | + | + | ||
| V. vulnificus | S01-38 | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
+, Lytic infections; −, absence of lysis. Strains with accompanying dates were isolated from Ladysmith Harbour during this study; other strains are from different dates and sources (see Table 1).
FIG. 3.
Total number of bacterial strains infected (sensitivity) by each viral strain from oysters in summer. Viruses are organized in increasing isolation date and correspond to those in Table 4.
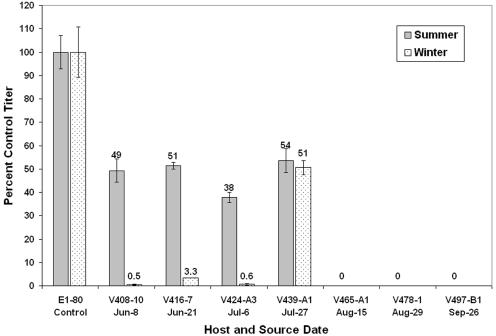
Plating efficiencies.
The efficiencies (Fig. 4) with which summer strains of V. parahaemolyticus recovered infectious viruses were tested with virus samples from summer and winter, when V. parahaemolyticus was undetectable. Bacterial strains from June and July recovered approximately half of the summer viral community relative to the control strain (E1-80), whereas only strain V439-A1, one of the most permissive of the summer (Table 4), recovered a substantial portion (∼51%) of the winter viral community relative to the control strain. Three summer strains of V. parahaemolyticus from June and July had very low plating efficiencies (∼0.5 to 3%) whereas the three strains obtained in August and September did not recover any of the >5 × 104 VpVs in either sample. Among the four strains that were sensitive, the mean recovery was just 14% of the winter community compared to 48% of the summer community.
FIG. 4.
Plating efficiency of summer versus winter oyster viral communities on strains of V. parahaemolyticus from oysters in summer. Host strains and source dates correspond to those in Table 4. Efficiencies are indicated on the figure for clarity and are relative to the control strain (E1-80). Values are means of duplicate samples; error bars indicate ranges of duplicates and are smaller than the width of the symbol if not visible.
DISCUSSION
This study extends previous work by following the seasonal dynamics of Vibrio spp. and the viruses which infect them, both within and outside of oysters. When combined with data on host range and plating efficiency of V. parahaemolyticus for viruses in oyster and seawater samples, a comprehensive picture is provided of changes in the host and virus communities over several months. The results suggest that during summer viral production occurs in bacteria within the oysters, while during winter the virus population turns over in the absence of detectable hosts.
Summer viral production.
Two observations suggest that in June through August, when water temperatures were >10°C, conditions favored production of VpVs. First, high abundances within oysters of potential host cells (total presumptive Vibrio spp. and V. parahaemolyticus) and VpVs suggest high contact rates between viruses and hosts. Second, summer strains of V. parahaemolyticus from oysters were susceptible to most of the summer VpV strains.
Estimates of burst size and viral turnover time were used to infer whether V. parahaemolyticus could support VpV production within oysters. Burst size varies widely in V. parahaemolyticus (Table 2); however, if a typical estimate for marine bacteria of 50 viruses cell−1 (64) is used, lysis of ca. 660 cells cm−3 produces the average of 3.3 × 104 VpV cm−3 (Table 2). Many depuration and uptake studies have shown that bacteria and viruses are not accumulated by oysters but may persist at abundances well below those observed in the present study (reviewed in reference 44). Oysters only retain ∼5% of bacteria, whereas viruses should not be retained at all (9, 44). Therefore, bacteria and viruses in seawater and oysters are constantly exchanged. The few depuration studies that have examined rates of bacteriophage removal (2, 25, 26, 38) observed complete (>99%) turnover in ∼2 days, when phage are abundant, but persistence when abundances are <103 cm−3. Other studies that have examined RNA phage and eukaryotic RNA viruses have found slower depuration rates or persistence of high titers (reviewed in reference 44), although the reasons for this discrepancy have not been adequately addressed. Therefore, assuming the vibriophage population turned over every 2 days, ca. 136% of the average V. parahaemolyticus standing stock during summer (243 cells cm−3; Table 2) would have to be lysed daily by viruses in order to sustain the measured VpV titers within oysters. This is more than five times the daily bacterial production assuming a growth rate of 0.33 day−1, which is the maximum growth rate generally reported for V. parahaemolyticus in live shellfish at the maximal summer temperatures (Table 2). Only when virus abundance was lowest (5.0 × 103 cm−3) and bacterial abundance was highest (2.3 × 103 cm−3) could the estimated mortality of 55% of daily production be reasonable. Although V. parahaemolyticus can grow at 36 day−1 for 90 min when oysters are emerged during warm summer days, spikes in bacterial abundance are washed out ca. 1 to 2 h after the oysters are submerged (E. Buenaventura, K. Schallie, et al., Abstr. 2002 Fed. Food Safety Nutr. Meet., p. 76, 2002). Even if bursts of phage production occurred at these times, they would be washed out as well, and there is a limited window in the summer season (clear days, high air temperature) during which this would be applicable. Although these figures give us a sense of the capacity for VpV production within oysters, they must be interpreted with caution. Rates of depuration and retention observed by others are highly variable, presumably due to the myriad of physical conditions (e.g., types of systems, temperatures, etc.), mollusk species, and virus types that have been used in these studies. Furthermore, growth measurements have not been made for V. parahaemolyticus from the Pacific Northwest, and thus it may have higher growth rates at cooler temperatures than more southern strains. Consequently, if the depuration rates of VpVs were ∼2-fold slower and the growth rates of V. parahaemolyticus ∼3-fold higher (nearer 1 day−1), the production of VpVs could be supported by V. parahaemolyticus alone. However, given that other Vibrio spp. serve as hosts (Table 4) for VpVs, it is reasonable to argue that viral production during summer is sustained within oysters by multiple host species; however, this prospect is even more likely during colder periods when V. parahaemolyticus is at low levels or undetectable. Regardless of whether additional species were supporting VpV production, the results emphasize that viruses were significant agents of bacterial mortality within these oysters.
In contrast to within oysters, there appeared to be little production of VpVs within the water and sediment. Viruses infecting V. parahaemolyticus were undetectable (<10 ml−1) year round in water surrounding the oysters, although low abundances of VpVs have been detected in the coastal waters of British Columbia and elsewhere (Table 2). This suggests that seawater is not a source of VpV but can transmit VpVs among oysters (and other fauna). The low abundances of V. parahaemolyticus in seawater indicate that contact rates between VpVs and their hosts, and hence rates of lytic virus production, are also low. VpVs have not been detected in coastal British Columbia sediments (8); however, V. parahaemolyticus was common and very susceptible to infection by VpVs, indicating the potential for VpV production. It is possible that the V. parahaemolyticus strains obtained from the sediments were from the same population isolated from the oysters, given that both occurred in the same inlet where oyster aquaculture is extensive.
Overall, the low abundance of VpVs in seawater and the inability of oysters to preferentially concentrate any one type of phage is strong evidence that in summer V. parahaemolyticus and other bacteria within oysters produced high abundances of VpVs. Given the high production rates of V. parahaemolyticus and VpVs within oysters, which are continuously shed into the environment, the oysters serve as one of the major environmental sources of V. parahaemolyticus and VpVs for other locations (sediment, zooplankton, and mollusks) and seasons.
“Winter conundrum”: viral persistence.
Frequently, strong seasonal variations in viral abundance mirror those of total bacteria (27) or of specific hosts (58). In contrast, VpVs persisted in oysters during winter when V. parahaemolyticus was undetectable, similar to results from the Gulf of Mexico and the Pacific Northwest (3, 14). It is possible that VpVs simply persist in winter in the absence of viral production. Alternatively, viral production may occur within hosts in the viable but nonculturable state, or viral production may occur within non-Vibrio spp.
The first explanation requires that there is minimal decay or dilution of viruses within oysters and assumes that VpV production is absent given that V. parahaemolyticus and related Vibrio spp. are undetectable during winter (Fig. 2) (7, 31, 35, 65). Marine bacteriophage can be stored for several months with minimal decay in natural seawater at 4°C; hence, it is conceivable that, in the absence of depuration, the viruses could persist during the winter. Although mollusk pumping rates are lower during cold periods (9), they continue. Consequently, without production, VpV abundances within the oysters should decrease until they are equal (undetectable) with the surrounding seawater (2, 44).
The second explanation requires that viral production occurred during winter within VNBC V. parahaemolyticus. V. parahaemolyticus overwinters in sediments and zooplankton and is generally undetectable, or at very low levels (∼10−3 MPN ml−1), in mollusks and seawater during cold months (18, 20, 30, 31, 57). V. parahaemolyticus (and related Vibrio spp.) may enter the viable but nonculturable state within ∼2 months at 4°C (7, 29). However, it is not known whether viral replication can occur in these cells. It is possible that “summer strains” (mesophiles) of V. parahaemolyticus may be displaced by “winter strains” (psychrophiles) that are not culturable on the standard nutrient-rich medium at 25°C and yet can allow infection and viral production.
The third explanation is that VpVs infect other species that sustain viral production over the winter. V. parahaemolyticus comprised ≤14% of the cells belonging to Vibrio spp. and presumably a much smaller portion of the total bacteria within oysters. The high titers of VpV (104 to 105 cm−3), combined with a host range that extends to other species (Table 4), provide strong evidence that high abundances of VpVs persist within oysters during the winter because of viral production sustained by other species. This is consistent with the calculations above that indicated production of VpV in summer may also be supported by species other than V. parahaemolyticus. In winter, the virus community is less able to infect summer host strains (Fig. 3), as is shown by the plating efficiency data (Fig. 4). These data clearly demonstrate that the viral community composition has changed. Other examples of seasonal changes in viral communities that infect specific hosts include viruses infecting Synechococcus spp., which have greater resistance to UV radiation in summer than in winter (19). Therefore, winter VpV production was not supported by summer bacterial strains. Ultimately, the system is probably a dynamic mixture of altered host culturability and viral exploitation of cross-species infection.
Through the examination of seasonal changes in the abundances of bacteria and the viruses that infect them, the present study has shown that bacteria within oysters are likely one of the major environmental sources of viruses infecting V. parahaemolyticus. Also, seasonal shifts in patterns of host range provide strong evidence that a significant shift in the virus community occurs during winter, and although these viruses can infect many strains of V. parahaemolyticus, the persistence of these viruses in oysters during winter is likely supported by other hosts.
Acknowledgments
We thank M. Kelly for providing some host strains and J. Lawrence for helpful discussions. We also thank the staff of the CFIA Burnaby Laboratory, in particular Danielle (Oakes) Jorgensen and Kathleen Felton, for help in processing the MPN and API assays.
This study was supported by grants to A.M.C. and C.A.S. from the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Arias, C. R., M. C. Macian, R. Aznar, E. Garay, and M. J. Pujalte. 1999. Low incidence of Vibrio vulnificus among vibrio isolates from sea water and shellfish of the Western Mediterranean coast. J. Appl. Microbiol. 86:125-134. [DOI] [PubMed] [Google Scholar]
- 2.Baross, J. A., J. Liston, and R. Y. Morita. 1974. Some implications of genetic exchange among marine vibrios, including Vibrio parahaemolyticus, naturally occurring in the Pacific Oyster, p. 129-137. In T. Fujino, G. Sakaguchi, R. Sakazaki, and Y. Takeda (ed.), International Symposium on Vibrio parahaemolyticus: Proceedings of the Second U.S.-Japan Conference on Toxic Micro-Organisms. United States-Japan Cooperative Program on Development and Utilization of Natural Resources, Tokyo, Japan.
- 3.Baross, J. A., J. Liston, and R. Y. Morita. 1978. Incidence of Vibrio parahaemolyticus bacteriophages and other Vibrio bacteriophages in marine samples. Appl. Environ. Microbiol. 36:492-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Børsheim, K. Y. 1993. Native marine bacteriophages. FEMS Microbiol. Ecol. 102:141-159. [Google Scholar]
- 5.Cavallo, R. A., and L. Stabili. 2004. Culturable vibrios biodiversity in the Northern Ionian Sea (Italian coasts). Sci. Mar. 68:23-29. [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1998. Outbreak of Vibrio parahaemolyticus infections associated with eating raw oysters-Pacific Northwest, 1997. JAMA 280:126-127. [PubMed] [Google Scholar]
- 7.Chowdhury, M. A. R., H. Yamanaka, S. Miyoshi, and S. Shinoda. 1990. Ecology and seasonal distribution of Vibrio parahaemolyticus in aquatic environments of a temperate region. FEMS Microbiol. Ecol. 74:1-9. [Google Scholar]
- 8.Comeau, A. M. 2004. Distribution and diversity of Vibrio parahaemolyticus viruses (VpVs) and their hosts. Ph.D. thesis. University of British Columbia, Vancouver, British Columbia, Canada.
- 9.Dame, R. F. 1996. Ecology of marine bivalves: an ecosystem approach. CRC Press, Inc., Boca Raton, Fla.
- 10.Danovaro, R., and M. Serresi. 2000. Viral density and virus-to-bacterium ratio in deep-sea sediments of the Eastern Mediterranean. Appl. Environ. Microbiol. 66:1857-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DePaola, A., L. H. Hopkins, J. T. Peeler, B. Wentz, and R. M. McPhearson. 1990. Incidence of Vibrio parahaemolyticus in United States coastal waters and oysters. Appl. Environ. Microbiol. 56:2299-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DePaola, A., C. A. Kaysner, J. Bowers, and D. W. Cook. 2000. Environmental investigations of Vibrio parahaemolyticus in oysters after outbreaks in Washington, Texas, and New York (1997 and 1998). Appl. Environ. Microbiol. 66:4649-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DePaola, A., S. McLeroy, and G. McManus. 1997. Distribution of Vibrio vulnificus phage in oyster tissues and other estuarine habitats. Appl. Environ. Microbiol. 63:2464-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DePaola, A., M. L. Motes, A. M. Chan, and C. A. Suttle. 1998. Phages infecting Vibrio vulnificus are abundant and diverse in oysters (Crassostrea virginica) collected from the Gulf of Mexico. Appl. Environ. Microbiol. 64:346-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake, L. A., K. H. Choi, A. G. E. Haskell, and F. C. Dobbs. 1998. Vertical profiles of virus-like particles and bacteria in the water column and sediments of Chesapeake Bay, USA. Aquat. Microb. Ecol. 16:17-25. [Google Scholar]
- 16.Eilers, H., J. Pernthaler, F. O. Glockner, and R. Amann. 2000. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microbiol. 66:3044-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. [DOI] [PubMed] [Google Scholar]
- 18.Fukushima, H., and R. Seki. 2004. Ecology of Vibrio vulnificus and Vibrio parahaemolyticus in brackish environments of the Sada River in Shimane Prefecture, Japan. FEMS Microbiol. Ecol. 48:221-229. [DOI] [PubMed] [Google Scholar]
- 19.Garza, D. R., and C. A. Suttle. 1998. The effect of cyanophages on the mortality of Synechococcus spp. and seasonal changes in the resistance of natural viral communities to UV radiation. Microb. Ecol. 36:281-292. [DOI] [PubMed] [Google Scholar]
- 20.Grimes, D. J. 1991. Ecology of estuarine bacteria capable of causing human disease: a review. Estuaries 14:345-360. [Google Scholar]
- 21.Heldal, M., and G. Bratbak. 1991. Production and decay of viruses in aquatic environments. Mar. Ecol. Prog. Ser. 72:205-212. [Google Scholar]
- 22.Hennes, K. P., C. A. Suttle, and A. M. Chan. 1995. Fluorescently labeled virus probes show that natural virus populations can control the structure of marine microbial communities. Appl. Environ. Microbiol. 61:3623-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewson, I., J. M. O'Neil, C. A. Heil, G. Bratbak, and W. C. Dennison. 2001. Effects of concentrated viral communities on photosynthesis and community composition of co-occurring benthic microalgae and phytoplankton. Aquat. Microb. Ecol. 25:1-10. [Google Scholar]
- 24.Hidaka, T., and A. Tokushige. 1978. Isolation and characterization of Vibrio parahaemolyticus bacteriophages in sea water. Mem. Fac. Fish. Kagoshima Univ. 27:79-90. [Google Scholar]
- 25.Hoff, J. C., and W. J. Beck. 1965. Studies on the behavior of a bacteriophage in the Pacific oyster (Crassostrea gigas), p. 69-82. In W. J. Beck, J. C. Hoff, and T. H. Ericksen (ed.), Proceedings of the 1964 Northwest Shellfish Sanitation Research and Planning Conference.
- 26.Hoff, J. C., W. Jakubowski, and W. J. Beck. 1966. Studies on bacteriophage accumulation and elimination by the Pacific oyster (Crassostrea gigas), p. 74-90. In W. J. Beck and J. C. Hoff (ed.), Proceedings of the 1965 Northwest Shellfish Sanitation Research and Planning Conference.
- 27.Jiang, S. C., and J. H. Paul. 1994. Seasonal and diel abundance of viruses and occurrence of lysogeny/bacteriocinogeny in the marine environment. Mar. Ecol. Prog. Ser. 104:163-172. [Google Scholar]
- 28.Jiang, S. C., and J. H. Paul. 1996. Occurrence of lysogenic bacteria in marine microbial communities as determined by prophage induction. Mar. Ecol. Prog. Ser. 142:27-48. [Google Scholar]
- 29.Jiang, X. P., and T. J. Chai. 1996. Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable, nonculturable cells. Appl. Environ. Microbiol. 62:1300-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneko, T., and R. R. Colwell. 1973. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J. Bacteriol. 113:24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko, T., and R. R. Colwell. 1978. The annual cycle of Vibrio parahaemolyticus in Chesapeake Bay. Microb. Ecol. 4:135-155. [DOI] [PubMed] [Google Scholar]
- 32.Kaper, J. B., E. F. Remmers, H. Lockman, and R. R. Colwell. 1981. Distribution of Vibrio parahaemolyticus in Chesapeake Bay during the summer season. Estuaries 4:321-327. [Google Scholar]
- 33.Kellogg, C. A., and J. H. Paul. 2002. Degree of ultraviolet radiation damage and repair capabilities are related to G+C content in marine vibriophages. Aquat. Microb. Ecol. 27:13-20. [Google Scholar]
- 34.Kellogg, C. A., J. B. Rose, S. C. Jiang, J. Thurmond, and J. H. Paul. 1995. Genetic diversity of related vibriophages isolated from marine environments around Florida and Hawaii, USA. Mar. Ecol. Prog. Ser. 120:89-98. [Google Scholar]
- 35.Kelly, M. T. 1982. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl. Environ. Microbiol. 44:820-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly, M. T., and E. M. D. Stroh. 1988. Temporal relationship of Vibrio parahaemolyticus in patients and the environment. J. Clin. Microbiol. 26:1754-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long, R. A., and F. Azam. 2001. Antagonistic interactions among marine pelagic bacteria. Appl. Environ. Microbiol. 67:4975-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muniain-Mujika, I., R. Girones, G. Tofino-Quesada, M. Calvo, and F. Lucena. 2002. Depuration dynamics of viruses in shellfish. Int. J. Food Microbiol. 77:125-133. [DOI] [PubMed] [Google Scholar]
- 39.Ortmann, A. C., J. E. Lawrence, and C. A. Suttle. 2002. Lysogeny and lytic viral production during a bloom of the cyanobacterium Synechococcus spp. Microb. Ecol. 43:225-231. [DOI] [PubMed] [Google Scholar]
- 40.Paul, J. H., J. B. Rose, S. C. Jiang, P. London, X. T. Xhou, and C. Kellogg. 1997. Coliphage and indigenous phage in Mamala Bay, Oahu, Hawaii. Appl. Environ. Microbiol. 63:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeffer, C. S., M. F. Hite, and J. D. Oliver. 2003. Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. Appl. Environ. Microbiol. 69:3526-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proctor, L. M., and J. A. Fuhrman. 1990. Viral mortality of marine bacteria and cyanobacteria. Nature 343:60-62. [Google Scholar]
- 43.Proctor, L. M., A. Okubo, and J. A. Fuhrman. 1993. Calibrating estimates of phage-induced mortality in marine bacteria: ultrastructural studies of marine bacteriophage development from one-step growth experiments. Microb. Ecol. 25:161-182. [DOI] [PubMed] [Google Scholar]
- 44.Richards, G. P. 1988. Microbial purification of shellfish: a review of depuration and relaying. J. Food Prot. 51:218-251. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 46.Steward, G. F., J. Wikner, W. P. Cochlan, D. C. Smith, and F. Azam. 1992. Estimation of virus production in the sea. 2. Field results. Mar. Microb. Food Webs 6:79-90. [Google Scholar]
- 47.Suttle, C. A. 1993. Enumeration and isolation of viruses, p. 121-134. In P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. Cole (ed.), Handbook of methods in aquatic ecology. Lewis Publishers, Boca Raton, Fla.
- 48.Suttle, C. A. 1992. Inhibition of photosynthesis in phytoplankton by the submicron size fraction concentrated from seawater. Mar. Ecol. Prog. Ser. 87:105-112. [Google Scholar]
- 49.Suttle, C. A. 1994. The significance of viruses to mortality in aquatic microbial communities. Microb. Ecol. 28:237-243. [DOI] [PubMed] [Google Scholar]
- 50.Suttle, C. A., A. M. Chan, and M. T. Cottrell. 1990. Infection of phytoplankton by viruses and reduction of primary productivity. Nature 347:467-469. [Google Scholar]
- 51.Suttle, C. A., A. M. Chan, and M. T. Cottrell. 1991. Use of ultrafiltration to isolate viruses from seawater which are pathogens of marine phytoplankton. Appl. Environ. Microbiol. 57:721-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suttle, C. A., and F. Chen. 1992. Mechanisms and rates of decay of marine viruses in seawater. Appl. Environ. Microbiol. 58:3721-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson, F. L., T. Iida, and J. Swings. 2004. Biodiversity of vibrios. Microbiol. Mol. Biol. Rev. 68:403-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson, J. R., M. A. Randa, L. A. Marcelino, A. Tomita-Mitchell, E. Lim, and M. F. Polz. 2004. Diversity and dynamics of a North Atlantic coastal vibrio community. Appl. Environ. Microbiol. 70:4103-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.U.S. Food and Drug Administration. 1998. Bacteriological analytical manual. AOAC International, Gaithersburg, Md.
- 56.U.S. Food and Drug Administration. 1999. Parameter identification for a risk assessment on Vibrio parahaemolyticus in raw molluscan shellfish. Center for Food Safety and Applied Nutrition, College Park, Md.
- 57.Venkateswaran, K., C. Kiiyukia, K. Nakanishi, H. Nakano, O. Matsuda, and H. Hashimoto. 1990. The role of sinking particles in the overwintering process of Vibrio parahaemolyticus in a marine environment. FEMS Microbiol. Ecol. 73:159-166. [Google Scholar]
- 58.Waterbury, J. B., and F. W. Valois. 1993. Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl. Environ. Microbiol. 59:3393-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinbauer, M. G., and C. A. Suttle. 1999. Lysogeny and prophage induction in coastal and offshore bacterial communities. Aquat. Microb. Ecol. 18:217-225. [Google Scholar]
- 60.Weinbauer, M. G., and C. A. Suttle. 1996. Potential significance of lysogeny to bacteriophage production and bacterial mortality in coastal waters of the Gulf of Mexico. Appl. Environ. Microbiol. 62:4374-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinbauer, M. G., S. W. Wilhelm, C. A. Suttle, and D. R. Garza. 1997. Photoreactivation compensates for UV damage and restores infectivity to natural marine viral communities. Appl. Environ. Microbiol. 63:2200-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilhelm, S. W., S. M. Brigden, and C. A. Suttle. 2002. A dilution technique for the direct measurement of viral production: a comparison in stratified and tidally mixed coastal waters. Microb. Ecol. 43:168-173. [DOI] [PubMed] [Google Scholar]
- 63.Wilhelm, S. W., and C. A. Suttle. 1999. Viruses and nutrient cycles in the sea. Bioscience 49:781-788. [Google Scholar]
- 64.Wommack, K. E., and R. R. Colwell. 2000. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaccone, R., E. Crisafi, and L. Genovese. 1992. Ecology of vibrios in the Oliveri-Tindari Lagoon (Messina), 2-year study. Mar. Ecol. 13:149-161. [Google Scholar]