Abstract
Propionate is a very abundant carbon source in soil, and many microorganisms are able to use this as the sole carbon source. Nevertheless, propionate not only serves as a carbon source for filamentous fungi but also acts as a preservative when added to glucose containing media. To solve this contradiction between carbon source and preservative effect, propionate metabolism of Aspergillus nidulans was studied and revealed the methylcitrate cycle as the responsible pathway. Methylisocitrate lyase is one of the key enzymes of that cycle. It catalyzes the cleavage of methylisocitrate into succinate and pyruvate and completes the α-oxidation of propionate. Previously, methylisocitrate lyase was shown to be highly specific for the substrate (2R,3S)-2-methylisocitrate. Here, the identification of the genomic sequence of the corresponding gene and the generation of deletion mutants is reported. Deletion mutants did not grow on propionate as sole carbon and energy source and were severely inhibited during growth on alternative carbon sources, when propionate was present. The strongest inhibitory effect was observed, when glycerol was the main carbon source, followed by glucose and acetate. In addition, asexual conidiation was strongly impaired in the presence of propionate. These effects might be caused by competitive inhibition of the NADP-dependent isocitrate dehydrogenase, because the Ki of (2R,3S)-2-methylisocitrate, the product of the methylcitrate cycle, on NADP-dependent isocitrate dehydrogenase was determined as 1.55 μM. Other isomers had no effect on enzymatic activity. Therefore, methylisocitrate was identified as a potential toxic compound for cellular metabolism.
Propionate is a common carbon source in soil (9) and can be metabolized by a variety of microorganisms. Aerobic metabolism of propionate generally starts with the activation of propionate to the corresponding coenzyme A (CoA) ester propionyl-CoA as summarized in reference 32. One of the major pathways is that of the coenzyme B12-dependent methylmalonyl-CoA pathway leading to the citric acid cycle intermediate succinyl-CoA. The other main pathway is the α-oxidation of propionate to pyruvate via the methylcitrate cycle. This pathway is found in bacteria, as well as in fungi, and involves a condensation of propionyl-CoA and oxaloacetate to methylcitrate (7, 17, 32). This reaction is catalyzed by a cycle specific methylcitrate synthase. The next step is the dehydration of methylcitrate to methylaconitate via a specific methylcitrate dehydratase. The rehydration of methylaconitate to methylisocitrate was shown at least in Escherichia coli and Salmonella enterica serovar Typhimurium to be catalyzed by the citric acid cycle aconitase AcnB (8, 16). The last pathway-specific reaction, catalyzed by a methylisocitrate lyase, is the cleavage of methylisocitrate into pyruvate and succinate. Methylisocitrate lyases have been well characterized, especially from bacterial but also from fungal sources (6, 13, 14, 22, 27, 28). However, only one gene sequence coding for a fungal methylisocitrate lyase has been published. It was shown that the so-called nonfunctional isocitrate lyase 2 from Saccharomyces cerevisiae possesses methylisocitrate lyase activity (22). The protein sequence showed a high degree of similarity to isocitrate lyases from other sources but solely exhibited activity toward methylisocitrate. Extracts of cells, which carried a deletion of the ICL2 gene, showed a drastic reduction of methylisocitrate lyase activity compared to the wild type, when grown at the carbon sources threonine or ethanol. Unfortunately, no growth experiments had been performed to study the effect of the mutation on growth in the presence of propionate. Due to the high sequence similarity of this methylisocitrate lyase to isocitrate lyases, it was not possible to predict whether other uncharacterized fungal proteins display isocitrate lyase or methylisocitrate lyase activity. Although the specific methylisocitrate lyase from Aspergillus nidulans was purified and characterized (6), it was difficult at that time to gain access to the genome database from A. nidulans. Therefore, it was not possible to identify the corresponding gene and to study the phenotype of a deletion mutant.
In the food and feed industry, propionate is commonly used as a preservative against molds. This is surprising because of the ability of filamentous fungi to use propionate as the sole carbon source. Nevertheless, cometabolism of glucose and propionate strongly affects the growth rate in a manner, which is strictly dependent on the amount of propionate present (5, 7). It has been shown that propionyl-CoA possesses a severe negative effect on the pyruvate dehydrogenase complex from various organisms (2, 3, 5, 24). Deletion of methylcitrate synthase led to an inability of A. nidulans to remove propionyl-CoA, causing a strong accumulation of this compound. Therefore, the negative effect of propionyl-CoA on growth could at least in part be explained by a blockage of the pyruvate dehydrogenase complex. In addition to the growth-inhibitory effect of propionyl-CoA, it also disturbed formation of polyketides, most likely via competition of propionyl-CoA with the natural substrates acetyl- and malonyl-CoA for the respective binding sites (5, 7, 36, 37).
In the present study the main interest was the investigation of the phenotypic effects caused by the accumulation of methylisocitrate. Previously, it was shown that NADP-dependent isocitrate dehydrogenase from bovine heart mitochondria and rat liver cytosol is strongly inhibited by α-threo-methylisocitrate with a Ki of <1 μM (1, 25). NADP-dependent isocitrate dehydrogenase also exists in A. nidulans, and the cytosolic, peroxisomal, and mitochondrial enzymes are all produced from a single gene (31). The exact function of this enzyme has not yet been proven. However, a general function may be the shuttle of reducing equivalents between NADP in different cellular compartments, because the compartmental membranes are impermeable for pyrimidine nucleotides and other small molecules. There are good indications that the mitochondrial enzyme is involved in the NADPH-dependent synthesis of glutamate from α-ketoglutarate (23). The peroxisomal enzyme might provide NADPH for the degradation of unsaturated fatty acids and the cytoplasmic enzyme provides NADPH for anabolic processes and for the reduction of glutathione and thioredoxin (19, 31).
By creating a mutant with a deletion of the gene coding for methylisocitrate lyase, it was possible to study the effect of intracellularly generated methylisocitrate on growth and development with a focus on NADP-dependent isocitrate dehydrogenase.
MATERIALS AND METHODS
Identification and deletion of the methylisocitrate lyase coding region.
The gene coding for methylisocitrate lyase was identified from N-terminal sequencing of the purified protein and a subsequent BLAST search against the A. nidulans genome as described in Results.
In all transformation steps for generation of the deletion construct, E. coli MRF' XL1-Blue (MBI Fermentas, St. Leon-Rot, Germany) was used. The coding region including 1,154 bp upstream of the ATG start codon and 1,415 bp downstream of the TGA stop codon was amplified by DyNAzyme EXT DNA polymerase (BioCat, Heidelberg, Germany). The oligonucleotides used were MICLup2Pst (5′-CTA CGC TGC AGG CAC TCA TGA AG-3′; the PstI restriction site is in boldface) and AnMICLnest_down (5′-GGC AAT TCA CCG TCA AGG AC-3′). As template DNA genomic DNA from A. nidulans RMS011 was used. The resulting PCR product was cloned into the PCR2.1 vector (Invitrogen, Karlsruhe, Germany). Positive clones were analyzed by PstI restriction, releasing a fragment of 4,452 bp, which contained the mclA gene, the whole upstream region, and 1,287 bp of the downstream region (which possessed an internal PstI restriction site). The fragment was subcloned into a previously PstI-restricted pUC19 vector (Invitrogen). In order to exclude the coding region from the construct and for introduction of a NotI restriction site, an inverse PCR on the vector was performed with a proofreading polymerase (Accuzyme polymerase; Bioline, Luckenwalde, Germany). Oligonucleotides were NotMICLup (5′-CCG CGT GGA GTA CTG GAA GG-3′; reads toward the upstream region) and NotMICLdown (5′-CCG CTT TGA TGT GAG CGT TCG-3′; reads toward the downstream region), which both contained a half NotI restriction site (indicated in boldface). The resulting PCR product was eluted from an agarose gel and phosphorylated with polynucleotide kinase as described by the manufacturer (New England Biolabs, Frankfurt, Germany). The phosphorylated product was self-ligated with T4 DNA ligase (NEB) and yielded a vector with a pUC19 backbone, 1,131-bp upstream region, and 1,269-bp downstream region, both regions separated by a newly generated NotI restriction site. In this NotI site the argB gene from vector pAlcArg (7) was subcloned, leading to the final deletion construct. The deletion part was removed from the pUC-backbone by PstI restriction and used for transformation of A. nidulans RMS011 (Table 1).
TABLE 1.
A. nidulans strains used in this studya
| Strain | Genotype | Source or reference |
|---|---|---|
| A26 (mclA+) | biA1 | Fungal Genetics Stock Center, Kansas City, KS |
| RMS011 (mclA+) | pabaA1 yA2 ΔargB::trpCΔB trpC801 veA1 | 30 |
| SMI45 (mclA+) | pabaA1 yA2 wA3 veA1 | M. Krüger, Marburg, Germany |
| SMBA9 (ΔmclA) | pabaA1 yA2 ΔargB::trpCΔB ΔmclA::argB trpC801 veA1 | This study |
| SMBA10 (ΔmclA) | pabaA1 yA2 ΔargB::trpCΔB ΔmclA::argB trpC801 veA1 | This study |
| SRF200 (mclA+) | pyrG89 ΔargB::trpCΔB pyroA4 veA1 | 18 |
Methylisocitrate lyase-positive strains A26 and RMS011 were used as controls for excretion of methylisocitrate, growth inhibition, and induction of NADP-dependent isocitrate dehydrogenase.
Transformation of A. nidulans was performed by standard methods (34). Genomic DNA was isolated by standard procedures and subjected to XbaI restriction. A digoxigenin-labeled probe was amplified by PCR with digoxigenin-11-dUTP in the nucleotide mix and oligonucleotides NotMICLdown (see above) and MICLdown (5′-CTG CAG GCC GGC CAA GG-3′). This probe was specific for the downstream region. A Southern blot was performed on the restricted DNA, and bands were detected after hybridization with alkaline phosphatase-linked anti-digoxigenin Fab fragments (Roche Diagnostics, Mannheim, Germany) by use of CDPstar as described in the manufacturer's protocol (Roche Diagnostics).
Isolation of RNA, reverse transcription, and sequencing of cDNA.
Strain A26 (Table 1) was grown for 40 h on minimal medium containing 10 mM glucose and 100 mM propionate. The mycelium was harvested and frozen in liquid nitrogen, and ca. 0.1 g was ground to a fine powder. For RNA extraction, the RNeasy Plant Minikit (QIAGEN, Hilden, Germany) was used. An aliquot of the RNA was used as a template for first-strand cDNA synthesis with the sequence specific oligonucleotide cDNAmicl_down (5′-CAT ACA TAC ATT CGA ACG CTC AC-3′) and SuperScript II reverse transcriptase as described in the manufacturer's protocol (Invitrogen). Second-strand synthesis and amplification of the cDNA was performed by use of DyNAzyme EXT DNA polymerase and the sequence specific oligonucleotides cDNAmicl_down and cDNAmicl_up (5′-CAG TAC TCC ACG CCA GAC-3′). The resulting PCR product was cloned into the pDrive cloning vector (QIAGEN) and sequenced from both strands by SeqLab (Göttingen, Germany).
Phenotypic characterization of methylisocitrate lyase mutants and sexual crossing.
Growth experiments were performed as described earlier (5). Mutant strains were inoculated in replicate cultures, and mycelium was harvested after growth for the indicated time (result section). Carbon sources used for growth were glucose, sodium acetate, sodium propionate, and glycerol in the concentrations and combinations described for each experiment. For determination of growth inhibition, mycelium was harvested and dried for at least 12 h at 80°C and weighed. Biomass obtained from glucose, acetate, or glycerol as the sole carbon source was set as 100%, respectively.
The ability of the mutant strains to form conidia was determined from solid plates containing 2% agar and the indicated carbon sources. Spore suspensions were point inoculated, and plates were incubated for 3 days at 37°C (the growth time for plates containing propionate as sole carbon source was prolonged to 7 days).
Crossing of the yellow strain SMBA9 and the green strain SRF200, which carries an intact methylisocitrate lyase locus, was performed by standard procedures. In brief, both strains were inoculated on plates, which allowed both strains to grow. Agar blocks were removed from areas, where mycelium of both strains was found and transferred to an agar plate that lacked p-aminobenzoic acid and uracil. Only mycelium, which contained nuclei from both strains was able to grow on these plates. The plates were sealed and incubated for 10 days at 37°C, which induced the formation of cleistothecia. Mature cleistothecia were isolated and plated on nonselective media. A successful crossing event was monitored by green and yellow colonies in a 1:1 deviation. Single colonies were analyzed for their phenotypes on selective media. Pictures of the colonies were taken with a digital camera (Nikon Coolpix 995).
Purification of isocitrate lyase.
Isocitrate lyase from A. nidulans was purified from strain SMI45, which was grown for 40 h in minimal medium containing 100 mM acetate and 100 mM propionate as carbon sources. This composition of the medium induced both isocitrate lyase and methylisocitrate lyase activity (5). During the purification procedure fractions were checked for both activities.
Approximately 4 g of dry pressed mycelium was ground to a fine powder under liquid nitrogen and resuspended in 50 ml of buffer A (50 mM Tris-HCl, 2 mM MgCl2, 2 mM dithiothreitol [pH 8.0]). Cell debris was removed by centrifugation at 25,000 × g at 4°C for 20 min. The supernatant was subjected to fractionated (NH4)2SO4 precipitation from 0 to 40% and from 40 to 75% saturation. The pellet from the second precipitation was resolved in 5 ml of buffer A and loaded on a Phenyl-Sepharose column (bed size, 20 ml) previously equilibrated with buffer A containing 1 M (NH4)2SO4. Proteins were eluted with a decreasing (NH4)2SO4 gradient from 1 to 0 M. Isocitrate lyase containing fractions were pooled, concentrated, and desalted by use of centrifugal filter devices (30-kDa cutoff; Millipore, Schwalbach, Germany) and buffer A. The concentrated sample was loaded onto a ResourceQ column (bed size, 1 ml; Amersham Biosciences Europe, Freiburg, Germany) and eluted against a sodium chloride gradient from 0 to 0.2 M in buffer A. Enzyme-containing fractions were collected, desalted, and again subjected to chromatography on a ResourceQ column.
Purity of the active fractions was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (20). Further identification was made by peptide mass analysis. The purified protein band was excised from the gel and sent to the Ludwig-Maximilians-Universität München. The protein was digested with trypsin and peptides were subjected to matrix-assisted laser desorption ionization-time of flight analysis. The peptide masses were than compared to nonredundant protein databases.
Enzyme assays and determination of methylisocitrate from the growth medium.
The activities of isocitrate lyase and methylisocitrate lyase were determined as described before (5). The Km value of isocitrate lyase for the substrate d-isocitrate was identified by varying the substrate concentration of d,l-isocitrate in the assay from 1 to 0.1 mM, giving an effective substrate concentration of d-isocitrate in the range of 0.5 and 0.05 mM. The Km value for (2R,3S)-2-methylisocitrate was determined with stereoisomeric pure substrate (10) in a range of 1.5 and 0.25 mM.
Methylisocitrate lyase activity in mutant strains was defined with 0.5 mM methylisocitrate in the assay, which would have been sufficient for maximum activity of methylisocitrate lyase (Km = 31 μM) (6).
The concentration of methylisocitrate in the growth medium was tested by enzymatic methods after harvest of the mycelium. Samples of 50 to 100 μl were applied to a test containing NADH and lactate dehydrogenase. The assay was started by the addition of purified methylisocitrate lyase from E. coli, and the decrease in absorbance at 340 nm was monitored. Concentrations of methylisocitrate from the complete culture broth were calculated and referred to the mycelial dry weight.
NADP-dependent isocitrate dehydrogenase was assayed by monitoring the formation of NADPH in a slightly modified procedure as described in reference 1. The final assay with a volume of 1 ml contained: 50 mM Na-HEPES (pH 7.5), 2 mM d,l-isocitrate, 1 mM MnCl2 (or 2 mM MgCl2), 1 mM NADP, and crude extract. For determination of the Km value, all components were kept constant except the concentration of isocitrate, which was used in an effective concentration (d-isocitrate) between 1.0 and 0.025 mM. The inhibition constant of methylisocitrate was determined by preincubating the respective assay mixture with 25, 50, or 100 μM (2R,3S)-2-methylisocitrate and starting the reaction with an effective concentration of d-isocitrate in the range of 1.5 and 0.375 mM. The Ki was calculated from the factor of the increase of the Km with respect to the inhibitor concentration used.
Protein concentrations were determined by use of Bio-Rad protein assay concentrate (Bio-Rad Laboratories, Munich, Germany) as described in the manufacturer's protocol with bovine serum albumin as a standard.
RESULTS
Identification of the gene coding for methylisocitrate lyase.
Methylisocitrate lyase was purified from A. nidulans wild type as described in an earlier publication (6). After blotting of the purified enzyme on a polyvinylidene difluoride membrane (Millipore), the purified enzyme was subjected to N-terminal sequencing (kindly carried out by D. Linder, Justus-Liebig-University, Giessen, Germany) and revealed the putative peptide sequence SPSSLPPVQPP.
The exact interpretation of the sequence data was difficult because of the high amount of proline, which weakens the signal and leads to a high background. Nevertheless, a BLAST search against fungal proteins (http://www.ncbi.nlm.nih.gov/BLAST/) revealed a single hit on the hypothetical protein AN8755.2 (accession no. EAA60548) from the annotated A. nidulans genome (http://www.broad.mit.edu/annotation/fungi/aspergillus/index.html). Sequencing of the cDNA obtained with specific oligonucleotides confirmed the predicted sequence with an exception at the C-terminal end (accession no. AJ890109; protein ID no. CAI65406). At this site the sequence of the hypothetical protein is extended by 10 amino acids, which is mainly due to a wrong intron prediction at the C-terminal end. The protein consists of 604 amino acids with a molecular mass of 66.9 kDa, which is in good agreement with 66 ± 3 kDa as determined from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (6). However, a mitochondrial localization of the native enzyme was predicted because that seems to be the compartment in which the methylcitrate cycle takes place (7, 22). Therefore, the protein was scanned for a putative mitochondrial targeting sequence with the program MITOPROT (http://ihg.gsf.de/ihg/mitoprot.html). With a probability of 0.9992 (maximum = 1.0) the protein is transported to mitochondria with a cleavage site of the leader peptide at position 41 (MLRSIPRRVPRRLPIFTTTATAGGPSRLAQRAFTCGYLRM/SPSSL…), which is in perfect agreementwith the result from N-terminal sequencing. The native protein therefore has a molecular subunit size of 62.4 kDa, which is also consistent with that determined from gel electrophoresis.
The corresponding gene is located at the right arm of chromosome III and spans a region of 2,081 nucleotides (including five introns) on contig 1.161 (positions 60367 to 62448).
Prediction of a conserved sequence motif for fungal methylisocitrate lyases.
In previous publications, the crystal structure of isocitrate lyases from Mycobacterium tuberculosis (26) and from A. nidulans (4) had been determined. In addition, the structure of bacterial methylisocitrate lyases from E. coli and Salmonella enterica serovar Typhimurium had also been determined (14, 28). Interestingly, the sequence identity of the E. coli methylisocitrate lyase (PrpB) to the respective isocitrate lyase (AceA) was only 27%, but the structural identity was extremely high. Furthermore, a high sequence identity was found within the amino acids of the active site.
Isocitrate lyases contain conserved tryptophan, phenylalanine, and threonine residues, which are responsible for correct orientation of the glyoxylate moiety in the active site. In contrast, in bacterial methylisocitrate lyases the amino acids phenylalanine, leucine, and proline replaced these residues. Modeling of pyruvate into the active site revealed that these exchanges lead to a hydrophobic binding pocket, which gives more space for the additional methyl group of pyruvate (14).
An alignment of the methylisocitrate lyase from S. cerevisiae (ICL2) revealed that the first tryptophan residue from isocitrate lyases was still conserved but that phenylalanine and threonine were replaced by leucine and serine. Both changes may also provide more space for an additional methyl group. To check whether this is a general motif of fungal methylisocitrate lyases, a BLAST search against fungal databases was performed using the sequences from S. cerevisiae and A. nidulans as a template. The hypothetical methylisocitrate lyases from Neurospora crassa, Magnaporthe grisea, and Yarrowia lipolytica were identified by similarity and by their putative mitochondrial targeting sequence. An alignment of the residues, which are proposed to be involved in substrate binding of these methylisocitrate lyases against their isocitrate lyase counterparts, is shown in Fig. 1.
FIG. 1.
Partial alignment of fungal methylisocitrate lyases and isocitrate lyases. Putative active site residues are shown in boldface. Residues, which may specify the acceptance of the methyl group from methylisocitrate are in boldface and shaded. AnMICL, methylisocitrate lyase from A. nidulans (accession no. CAI65406); NcMICL, hypothetical methylisocitrate lyase from N. crassa (accession no. XP_331680); MgMICL, hypothetical methylisocitrate lyase from M. grisea (accession no. EAA47373); ScMICL, methylisocitrate lyase from S. cerevisiae (accession no. NP_015331); YlMICL, hypothetical methylisocitrate lyase from Y. lipolytica (Accession: XP_506117); IclAn, isocitrate lyase from A. nidulans (accession no. EAA62727); IclNc, isocitrate lyase from N. crassa (accession no. CAA44573); IclMg, isocitrate lyase from M. grisea (accession no. AAN28719); IclSc, isocitrate lyase from S. cerevisiae (accession no. NP_010987); IclYl, isocitrate lyase from Y. lipolytica (accession no. XP_501923).
From this sequence alignment we predict a sequence motif [GF(V/T)(L/M)QL(I/V)SLAG(L/V)H; amino acids providing the space for the methyl group are indicated in boldface] to be specific for fungal methylisocitrate lyases.
Deletion of the methylisocitrate lyase coding region and identification of mutant strains.
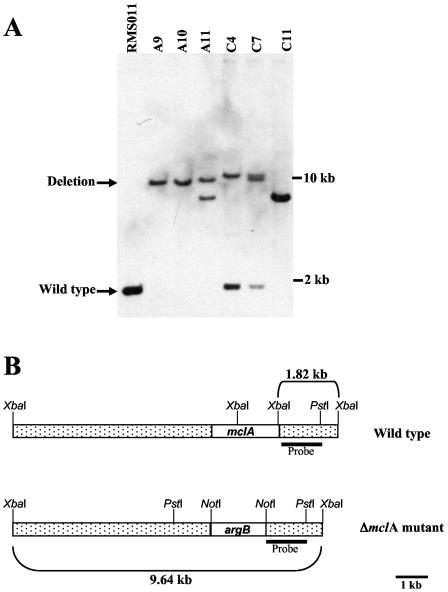
The coding region of the methylisocitrate lyase gene (mclA) with flanking regions was amplified from genomic DNA of strain RMS011 (Table 1). Thereby, a PstI restriction site ca. 1.15 kb upstream of the start ATG was introduced and, additionally, the endogenous PstI restriction site ca. 1.29 kb downstream of the stop codon was included. This enabled the subcloning into pUC19 vector. The coding region of the mclA gene was removed by PCR, generating a NotI restriction site in which the argB gene from A. nidulans (coding for carbamoyl transferase and leading to arginine prototrophy of transformants) was cloned. In this construct, a 1.13-kb upstream and a 1.27-kb downstream fragment of the mclA flanking regions surrounded the argB gene. For transformation of A. nidulans strain, RMS011 the vector backbone of the deletion construct was removed by PstI restriction, and only the deletion part was used further. Transformation of protoplasts was performed as described elsewhere (34). Genomic DNA from transformants was isolated, XbaI restricted, and subjected to Southern analysis using a digoxigenin-labeled probe against the downstream fragment. The wild type was supposed to yield a 1.8-kb fragment, whereas this fragment was expected to shift to 9.6 kb in case of a homologous integration of the deletion construct into the mclA locus. Transformants SMBA9 and SMBA10 exactly showed the expected pattern, whereas others seem to posses either tandem and/or ectopic integrations (Fig. 2).
FIG. 2.
Southern analysis of transformants. (A) Southern blot of different transformants in comparison to the original mclA+ strain RMS011. Strains A9 and A10 show the expected shift from 1.8 to 9.6 kb. (B) Diagram of the wild type and a transformant with a homologous integration of the deletion construct.
Phenotypic characterization of methylisocitrate lyase deletion mutants.
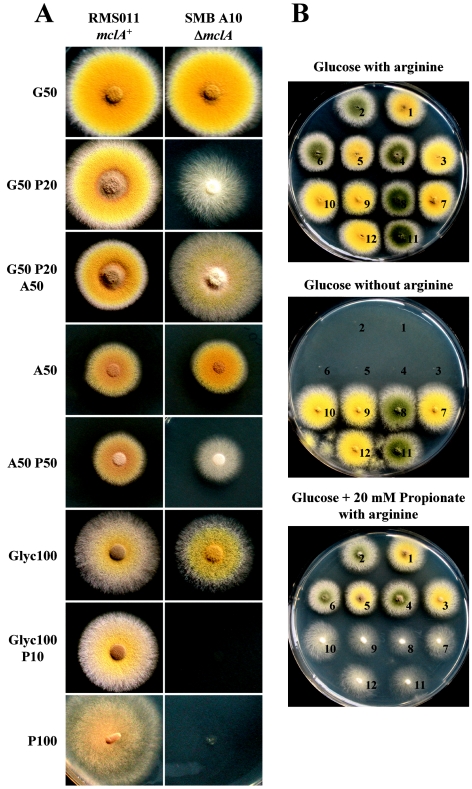
In order to study the phenotypes caused by a deletion of the mclA gene, growth experiments were performed on solid and liquid media. Liquid media were used to investigate a potential growth inhibitory effect by determination of the dried biomass, whereas solid agar plates were used to study the effect of propionate on asexual development. The effects of different carbon sources on development are illustrated in Fig. 3A. With propionate as the sole carbon and energy source the deletion strains did not produce any visible biomass or conidia, not even after incubation for more than 5 days. Therefore, we conclude that the methylisocitrate lyase is essential for the utilization of propionate. In contrast, no phenotype was visible, when glucose or acetate was used as sole carbon sources. This finding implies that methylisocitrate lyase is specifically involved in the methylcitrate cycle and not required for the citric acid or glyoxylate cycle. Addition of 20 mM propionate to glucose medium totally abolished the formation of conidia. However, a microscopic investigation of the colonies showed that development of conidiophores, including metulae and phialides was not disturbed (data not shown). Nevertheless, an extra addition of 50 mM acetate led to a slight restoration of conidiation. This is in agreement with the competition of acetate and propionate for the activation to the corresponding CoA ester as shown previously (5). Furthermore, when acetate was the main carbon source, propionate only showed a strong reduction of conidiation when used in the same or even in a higher concentration.
FIG. 3.
Phenotypes of A. nidulans strains grown on different carbon sources. (A) The mclA+ strain RMS011 and the mclA deletion strain SMBA10 were point inoculated onto media containing different carbon sources (G, glucose; P, propionate; A, acetate; Glyc, glycerol; numbers denote millimolar concentrations of carbon sources). The mutant strain is severely affected in growth and conidiation upon the addition of propionate. Propionate alone does not serve as a carbon source for the mutant strain. For further explanations refer to the text. (B) Progenies of a sexual cross of the deletion strain SMBA9 and the green mclA+ strain SRF200. Arginine auxotrophic and prototrophic strains were selected and point inoculated onto glucose medium with or without arginine and with an addition of propionate. All strains show the expected phenotypes (see also panel A, G50 and G50 P20).
A strong negative effect on biomass formation caused by the addition of propionate was observed when strains were grown in liquid media. It has been shown that propionate possesses some inhibitory effect on growth of an A. nidulans wild-type strains when glucose was the main carbon source (7). A 40% reduction of biomass was observed when 50 mM propionate was added, and a 60% reduction was observed in the presence of 100 mM propionate. On the other hand, the addition of various amounts of propionate had no effect on biomass formation when acetate was the main carbon source. Compared to that the methylisocitrate lyase mutants were severely affected in growth when glucose-propionate or acetate-propionate served as carbon sources. The strongest inhibitory effect was noticed when glycerol was the main carbon source (Table 2). This insinuates a severe inhibition of mitochondrial metabolism because glycerol is, like glucose, metabolized via the citric acid cycle without the gain of energy from cytoplasmic glycolysis.
TABLE 2.
Growth inhibition of two independent methylisocitrate lyase mutants from replicate culturesa
| Carbon source (concn [mM]) | Growth time (h) | Residual growth (%) | Inhibition (%) |
|---|---|---|---|
| Glucose (50) | 23 | 100 | 0 |
| Glucose (50), propionate (20) | 23 | 46 ± 2 | 54 |
| Glucose (50), propionate (50) | 23 | 20 ± 2 | 80 |
| Glucose (50), propionate (100) | 23 | 3.5 ± 1 | 96.5 |
| Glucose (50), acetate (100) | 23 | 95 ± 4 | 5 |
| Glucose (50), acetate (100), propionate (100) | 23 | 25 ± 1 | 75 |
| Acetate (100) | 30 | 100 | 0 |
| Acetate (100), propionate (20) | 30 | 90 ± 1 | 10 |
| Acetate (100), propionate (50) | 30 | 51 ± 2 | 49 |
| Glycerol (50) | 32 | 100 | 0 |
| Glycerol (50), propionate (5) | 32 | 3 ± 1 | 97 |
| Glycerol (50), propionate (20) | 32 | 0 | 100 |
The yield obtained with the sole carbon source glucose, acetate, or glycerol, respectively, was set to 100%. Numbers in parentheses denote the millimolar concentration of the respective carbon source.
Confirmation of mutant phenotype by sexual crossing.
In order to confirm that the observed phenotypes were due to a deletion of the genomic locus coding for methylisocitrate lyase and were not caused by other secondary effects, the mutant strain SMBA9 was crossed with strain SRF200. The transformant strain SMBA9 was prototroph for arginine because the argB gene was used as a selection marker during transformation. Nevertheless, the original copy of argB in this strain was still deleted. Strain SRF200 also carried a deleted argB locus. Due to that constellation, all arginine prototrophic progenies of the sexual crossing were supposed to carry a deleted methylisocitrate lyase gene and should show a sporulation defect in the presence of propionate. Because of the different conidial colors of the two strains (yellow for SMBA9 and green for SRF200), ascospores deriving from a crossing event of the two partners were easily identified by the deviation of green and yellow spore color of the offsprings from a single cleistothecium. For crossing, the carbon source solely consisted of glucose. Under this condition, no phenotype in cleistothecia formation was observed. A total of 24 arginine prototrophic and arginine auxotrophic colonies with green or yellow conidia were randomly selected and transferred to agar plates with or without the addition of propionate. Figure 3B shows a selection of six arginine prototrophic (ΔmclA) and six arginine auxotrophic (mclA+) strains, which displayed the expected phenotypes in the presence or absence of propionate, which verifies the phenotypes of the original transformed strains.
Ability of isocitrate lyase to cleave methylisocitrate.
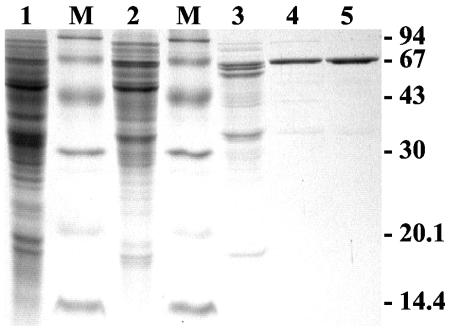
Isocitrate lyase is supposed to be localized within the glyoxisomes (33). Based on this assumption, it is conceivable that methylisocitrate could be shuttled from mitochondria to other compartments via tricarboxylic acid transporters and cleaved to pyruvate and succinate by isocitrate lyase. Therefore, we purified the isocitrate lyase to homogeneity from strain SMI45 (see Fig. 4 and Table 3), which was grown on a medium that contained both acetate and propionate as carbon sources. This was done in order to visualize the separation of isocitrate lyase activity from methylisocitrate lyase activity. As shown in Table 3 the purification factor for methylisocitrate lyase never exceeded a value of 2, whereas that of isocitrate lyase was 57-fold. The purified enzyme was subjected to peptide mass analysis by matrix-assisted laser desorption ionization-time of flight (kindly performed by the Zentrallabor für Proteinanalytik, Ludwig-Maximilians-Universität München, Munich, Germany). Peptide masses showed a sequence coverage of 57%, resembling an unambiguous identification of the protein as isocitrate lyase. The Km values for the substrates isocitrate and methylisocitrate were determined as 0.35 and 3.33 mM, respectively. The virtual Vmax with the substrate methylisocitrate was 1.34 U mg−1 (turnover number, 1.34 s−1), whereas that for isocitrate was 8.60 U mg−1 (turnover number, 8.63 s−1). The catalytic efficiency with methylisocitrate was calculated as 4.02 × 102 s−1 M−1 and with isocitrate 2.47 × 104 s−1 M−1. Therefore, the enzyme exhibits a 61-fold higher efficiency with isocitrate, and the overall activity with methylisocitrate is quite low. When 0.25 mM methylisocitrate was used as a substrate for purified isocitrate lyase, the activity with 1 mM d,l-isocitrate was 150 times higher (Table 3, group A). In addition, crude extracts of the methylisocitrate lyase mutants were checked for isocitrate lyase and methylisocitrate lyase activity. The activity of the latter was hardly detectable and never exceeded 3% of the activity of isocitrate lyase, which shows the deletion of the gene coding for methylisocitrate lyase. In addition, the production of isocitrate lyase from the genomic acuD gene is not sufficient to replace methylisocitrate lyase. An overproduction of isocitrate lyase might attenuate the methylisocitrate lyase mutant phenotype, but due to the different compartmental localization of both enzymes a complete restoration of the wild-type phenotype is unlikely to occur.
FIG. 4.
Purification of isocitrate lyase from A. nidulans SMI45. Lane 1, crude extract (17.8 μg); lane M, molecular mass standard; lane 2, 45 to 75% (NH4)2SO4 precipitate (13 μg); lane 3, Phenyl-Sepharose (4 μg); lane 4, First ResourceQ (1.1 μg); lane 5, Second ResourceQ (1.2 μg).
TABLE 3.
Purification protocol of isocitrate lyase from strain SMI45
| Substrate group and purification stepa | Activity (U) | Protein (mg) | Sp. act. (U/mg) | Purification factor | Yield (%) | Ratio (Icl/Micl) |
|---|---|---|---|---|---|---|
| Group A | ||||||
| Crude extract | 29.0 | 199.8 | 0.15 | 1 | 100 | 3:1 |
| 45 to 75% (NH4)2SO4 | 25.9 | 81.12 | 0.32 | 2.1 | 89.3 | 3.2:1 |
| Phenyl-Sepharose | 12.6 | 4.76 | 2.75 | 18.3 | 43.4 | 39.3:1 |
| ResourceQ (1) | 6.7 | 0.90 | 7.44 | 49.6 | 23.1 | 149:1 |
| ResourceQ (2) | 1.2 | 0.14 | 8.60 | 57.3 | 4.1 | 151:1 |
| Group B | ||||||
| Crude extract | 10.9 | 199.8 | 0.05 | 1 | 100 | |
| 45 to 75% (NH4)2SO4 | 8.2 | 81.12 | 0.10 | 2.0 | 75.2 | |
| Phenyl-Sepharose | 0.33 | 4.76 | 0.07 | 1.4 | 3.0 | |
| ResourceQ (1) | 0.04 | 0.90 | 0.05 | 1 | 0.4 | |
| ResourceQ (2) | 0.01 | 0.14 | 0.057 | 1.1 | 0.01 |
Group A, determination of activity with 1 mM threo-d,l-isocitrate as the substrate. The ratio (last column) compares the specific activities of isocitrate lyase (Icl) and methylisocitrate lyase (Micl). Group B, activity with 0.25 mM (2R,3S)-2-methylisocitrate as the substrate.
Excretion of methylisocitrate to the growth medium.
The levels of methylcitrate cycle enzymes are quite low when no propionate or other propionyl-CoA-generating carbon sources are available. In the presence of both propionate and an alternative carbon source, some induction of genes coding for enzymes of the methylcitrate cycle was observed (5). Therefore, it was supposed that methylisocitrate might be produced by the mutant strains and eventually excreted to the growth medium. After the harvest of mycelium for the determination of biomass formation, samples from the medium were removed and checked for the presence of methylisocitrate. As shown in Table 4, the methylisocitrate lyase mutants secrete methylisocitrate to the growth medium, whereas the amount was dependent on growth time and the concentration of propionate used. The competition of acetate and propionate is visualized by the different amounts of methylisocitrate excreted when the concentration of acetate was varied but that of propionate was kept constant. The higher the ratio of propionate to acetate, the higher the amount of methylisocitrate excreted. In comparison, no excretion of methylisocitrate was detectable in case of wild-type strains (data not shown). Therefore, it is obvious that methylisocitrate lyase is essential for complete metabolism of propionate.
TABLE 4.
Excretion of methylisocitrate to the growth mediuma
| Carbon source (mM) | Methylisocitrate (μmol/g of dried mycelium) |
|---|---|
| Glucose (50) | <4 |
| Glucose (50), propionate (10) | 41.5 ± 3 |
| Glucose (50), propionate (20) | 93.3 ± 5 |
| Glucose (50), propionate (50) | 119.2 ± 4 |
| Acetate (50) | <4 |
| Acetate (50), propionate (20) | 293 ± 10 |
| Acetate (100), propionate (20) | 157 ± 15 |
| Acetate (50), propionate (50) | 425 ± 22 |
| Acetate (100), propionate (50) | 287 ± 13 |
| Glycerol (50) | <2 |
| Glycerol (50), propionate (20) | No biomass |
The individual methylisocitrate lyase mutants (SMBA9 and SMBA10) were incubated for the periods used for determination of biomass formation (23 h when glucose was the main carbon source, 30 h with acetate, and 32 h with glycerol). The calculated amount of methylisocitrate from the growth medium was related to the amount of dried mycelium. All concentrations of carbon sources are shown in parentheses.
Effect of methylisocitrate on NADP-dependent isocitrate dehydrogenase.
NADP-dependent isocitrate dehydrogenase catalyzes the oxidative decarboxylation of isocitrate to α-ketoglutarate by the use of NADP as an acceptor of reducing equivalents. Investigations of NADP-dependent isocitrate dehydrogenases from mammalian cells have shown that this enzyme is severely inhibited upon the addition of d-threo-α-methylisocitrate (1, 25, 29). This resembles exactly the isomer of methylisocitrate, which is produced from the methylcitrate cycle and is additionally the sole substrate for methylisocitrate lyases (6, 10). Inhibition was competitive with respect to isocitrate. Nevertheless, methylisocitrate does not seem to resemble a natural occurring intermediate in mammalian cellular metabolism, because propionyl-CoA is metabolized via the methylmalonyl-CoA pathway.
A. nidulans also possesses NADP-dependent isocitrate dehydrogenases. The mitochondrial, peroxisomal, and cytoplasmic enzymes are all encoded by a single gene. Production of both the cytoplasmic and the peroxisomal isozymes was shown to be induced by acetate, propionate, butyrate, and Tween 80, whereas the mitochondrial enzyme was mainly present on glucose (31).
Until now, NADP-dependent isocitrate dehydrogenase from A. nidulans had neither been purified nor characterized with respect to the substrate specificity. Therefore, the activity of NADP-dependent isocitrate dehydrogenase was determined from methylisocitrate lyase mutant and wild-type strains when grown with different carbon sources. As a metal cofactor, manganese was used, although ca. 75 to 80% of the maximum activity could be obtained by replacement of the manganese with magnesium. Even in the absence of propionate the activity of the NADP-dependent isocitrate dehydrogenase in the methylisocitrate lyase deletion strains was slightly higher than that of wild-type control strains. The addition of propionate induced the activity, especially when glucose or glycerol was the main carbon source. By using acetate as the sole carbon source, NADP-dependent isocitrate dehydrogenase was strongly induced, and further addition of propionate only had a minor effect. Interestingly, maximum production of specific enzymatic activity was always lower with the wild type. An exception was the addition of acetate to glucose medium, where the wild type showed slightly higher values. This control especially showed that the high induction of NADP-dependent isocitrate dehydrogenase in the mutant strain was mainly due to the formation of methylisocitrate from propionate rather than to the addition of propionate itself (Table 5).
TABLE 5.
Activity of NADP-dependent isocitrate dehydrogenasea
| Carbon source (concn [mM]) | ΔmclA NADP-IDH (U/mg) | Factor of induction | WT NADP-IDH (U/mg) | Factor of induction | Ratio (ΔmclA/WT) |
|---|---|---|---|---|---|
| Glucose (50) | 0.12 | 1.0 | 0.082 | 1.0 | 1.5:1 |
| Glucose (50), propionate (20) | 0.87 | 7.3 | 0.22 | 2.7 | 4.0:1 |
| Glucose (50), propionate (50) | 1.10 | 9.2 | 0.25 | 3.0 | 4.4:1 |
| Glucose (50), acetate (20) | 0.20 | 1.6 | 0.31 | 3.8 | 1:1.6 |
| Glucose (50), acetate (50) | 0.28 | 2.2 | 0.40 | 4.9 | 1:1.4 |
| Acetate (100) | 1.05 | 1.0 | 0.67 | 1.0 | 1.6:1 |
| Acetate (100), propionate (20) | 1.27 | 1.2 | 0.66 | 1.0 | 1.9:1 |
| Acetate (100), propionate (50) | 1.23 | 1.2 | 0.66 | 1.0 | 1.9:1 |
| Glycerol (50) | 0.217 | 1.0 | 0.082 | 1.0 | 2.6:1 |
| Glycerol (50), propionate (5) | 1.56* | 7.2 | 0.29 | 3.5 | 5.4:1 |
Wild-type and methylisocitrate lyase mutant were incubated for the same time as indicated for the determination of growth inhibition. An exception is marked with an asterisk, where the mutant strain was incubated for 72 h in order to yield sufficient biomass. Numbers in parentheses indicate the molarity of the carbon sources. The factor of induction is calculated with respect to the value of the corresponding sole carbon source: glucose, acetate, or glycerol. Specific activities were calculated from three independent determinations, with an average error of <5%. IDH, isocitrate dehydrogenase.
The observed high production of NADP-dependent isocitrate dehydrogenase in the mutant strain and the significant excretion of methylisocitrate to the growth medium (see above) led to the assumption that methylisocitrate can pass the mitochondrial membranes. Therefore, methylisocitrate generated inside the mitochondria could also inhibit cytoplasmic enzymes.
To investigate whether the mitochondrial and cytoplasmic isozymes of NADP-dependent isocitrate dehydrogenase are inhibited in the same manner by methylisocitrate, in vitro assays were performed from crude extracts of cells either grown on glucose or acetate as sole carbon source. These carbon sources should lead mainly to the production of the mitochondrial and the cytoplasmic isoforms, respectively (31). The Km values for isocitrate in the absence of methylisocitrate were hardly distinguishable between both growth conditions, and also the Ki of methylisocitrate did not show any significant differences. The Km value for d-isocitrate was 0.032 ± 0.002 mM and shifted in the presence of 25 μM (2R,3S)-2-methylisocitrate to 0.525 mM (Ki = 1.62 μM). Upon addition of 50 μM methylisocitrate, the Km was changed to 1.085 mM (Ki = 1.52 μM), and an increase to 100 μM changed the Km to 2.075 mM (Ki = 1.56 μM), regardless of the growth condition the enzyme derived from. In all cases the Vmax of the specific activity was not affected, indicating a strictly competitive inhibition. Other isomers of methylisocitrate (10) in concentrations of up to 500 μM had no negative effect on NADP-dependent isocitrate dehydrogenase activity.
These data indicate that even low amounts of (2R,3S)-2-methylisocitrate produced from the methylcitrate cycle can act as efficient inhibitor of all isozymes of NADP-dependent isocitrate dehydrogenase. Furthermore, it is noteworthy that under conditions of methylisocitrate formation the specific activity of NADP-dependent isocitrate dehydrogenase from the mclA mutant in comparison to the wild type is increased. This implies that due to the inhibition by methylisocitrate the reduction of enzymatic activity is sensed, leading to an elevated production of NADP-dependent isocitrate dehydrogenase.
DISCUSSION
The data shown in this study clearly indicate that disruption of the methylisocitrate lyase leads to an inhibition of growth and reduced conidiation in the presence of propionate. Phenotypes observed for growth inhibition are similar to those observed for a deletion of methylcitrate synthase but differ in some points. A methylcitrate synthase deletion strain accumulates propionyl-CoA when propionate is present as a carbon source. A strong inhibition of the pyruvate dehydrogenase complex is observed, leading to the excretion of high amounts of pyruvate (millimolar range) and a reduction of the growth rate on carbon sources, for which the pyruvate dehydrogenase complex is required for the synthesis of acetyl-CoA. Using acetate as the main carbon source, only a slight inhibitory effect of the methylcitrate synthase mutant becomes visible upon the addition of propionate (50 mM acetate plus 50 mM propionate ≈ 20% inhibition), although propionyl-CoA is formed. This implied that the pyruvate dehydrogenase complex in particular is the target of propionyl-CoA (5, 7). Another effect of propionyl-CoA is the inhibition of secondary metabolism. The polyketides sterigmatocystin, ascoquinone A, and naphotopyrone are not produced under conditions in which propionyl-CoA accumulates. Nevertheless, conidiation in the presence of moderate concentrations of propionate (≈50 mM) is still possible, although conidia are not colored due to the inhibition of naphtopyrone synthesis (5, 36, 37).
Methylisocitrate lyase mutants were inhibited in growth with all carbon sources tested when propionate was present. It was shown here that the NADP-dependent isocitrate dehydrogenase is severely affected in the presence of even low amounts of methylisocitrate. Due to the significant excretion of methylisocitrate to the growth medium, a concentration higher than 1.5 μM inside the cells (the Ki for the NADP-dependent isocitrate dehydrogenase) can be expected. Because of the obvious importance of the enzyme for many cellular processes, its inhibition might lead to a reduction of biomass formation and a decrease in the ability to produce conidia.
Nevertheless, NADP-dependent isocitrate dehydrogenase may not be the only target of methylisocitrate. Two other aspects should be mentioned: the first is a putative inhibition of aconitase. This enzyme is supposed not only to act in the citrate cycle but also in the methylcitrate cycle as shown for E. coli and S. enterica serovar Typhimurium (8, 16). It has also been proven that in eukaryotes aconitases are in general able to catalyze the reversible reaction from methylisocitrate to methyl-cis-aconitate (1, 21). Therefore, an accumulation of methylisocitrate might inhibit the aconitase, leading to an accumulation of citrate. This in turn can lead to a slowdown of glycolysis. Citrate is transported to the cytosol via a tricarboxylic acid transporter. Once in the cytosol, citrate acts to restrain further carbohydrate breakdown by direct inhibition of the phosphofructokinase (15, 35). In agreement with this assumption, a methylisocitrate lyase mutant, which was incubated for more than 1 week in a 100-ml culture containing 50 mM (5 mmol) glucose and 100 mM (10 mmol) propionate, was not able to consume glucose completely (data not shown). In contrast, the wild type and a methylcitrate synthase deletion strain (although the latter was also inhibited in growth) consumed the glucose in that period of time.
On the other hand, it is quite difficult to measure the inhibition of aconitase by methylisocitrate in vitro. In a coupled assay of the aconitase reaction from cis-aconitate to isocitrate with NADP-dependent isocitrate dehydrogenase, it cannot be ruled out that the observed inhibition derives from an inhibition of the aconitase or from the NADP-dependent isocitrate dehydrogenase as shown before. The fact that aconitases can also form methyl-cis-aconitate from methylisocitrate excludes the alternative standard method for determination of aconitase activity at 240 nm. In this assay the formation of cis-aconitate from citrate or isocitrate is determined. Unfortunately, methyl-cis-aconitate absorbs very similar at 240 nm as cis-aconitate. Therefore, detailed studies on the substrate specificity of purified aconitase from A. nidulans will be performed to clarify this question.
The second aspect is the trapping of oxaloacetate in methylisocitrate. Methylcitrate synthase possesses a very low Km for the substrates propionyl-CoA (1.7 μM) and oxaloacetate (<1 μM) (7). Therefore, it is able to compete for oxaloacetate with the citrate synthase. Once methylcitrate is formed it is converted to methylisocitrate, but the cleavage into the products pyruvate and succinate is not possible. This means that oxaloacetate has to be generated de novo, which might be very energy-consuming, especially when glycerol or acetate is the main substrate.
All of these possibilities together might explain not only the growth inhibition caused by propionate but also the inhibition of conidiation in the mutant strain. Conidia contain different nutrients, whereas one of the most important components is supposed to be trehalose (11, 12). Inhibition of the central metabolism by inhibition of aconitase (citrate accumulation and reduced glycolysis), trapping of oxaloacetate (which is needed for gluconeogenesis), and inhibition of the NADP-dependent isocitrate dehydrogenase might be sufficient to inhibit conidiation.
Nevertheless, the single mutants of NADP-dependent isocitrate dehydrogenase and aconitase have to be constructed in order to show whether the observed effects on conidiation and growth can be explained by a single gene defect or derive from a concerted action. Furthermore, a mutant with a deletion of the gene coding for methylcitrate dehydratase should be constructed, which is supposed to accumulate methylcitrate. A comparison of such a mutant with other mutants from the methylcitrate cycle (methylcitrate synthase and methylisocitrate lyase) will elucidate the effects of different intermediates of propionate catabolism on cellular metabolism.
Acknowledgments
This study was supported by a grant of the Deutsche Forschungsgemeinschaft (project BR 2216).
We thank Axel Brakhage for helpful discussions and Tilman Schlunk from the Zentrallabor für Proteinanalytik at the Ludwig-Maximilians-Universität München for identification of isocitrate lyase by peptide mass analysis. We also thank the anonymous reviewers for helpful suggestions for improving the manuscript.
REFERENCES
- 1.Beach, R. L., T. Aogaichi, and G. W. Plaut. 1977. Identification of d-threo-alpha-methylisocitrate as stereochemically specific substrate for bovine heart aconitase and inhibitor of TPN-linked isocitrate dehydrogenase. J. Biol. Chem. 252:2702-2709. [PubMed] [Google Scholar]
- 2.Brass, E. P. 1992. Interaction of carnitine and propionate with pyruvate oxidation by hepatocytes from clofibrate-treated rats: importance of coenzyme A availability. J. Nutr. 122:234-240. [DOI] [PubMed] [Google Scholar]
- 3.Brass, E. P., and R. A. Beyerinck. 1988. Effects of propionate and carnitine on the hepatic oxidation of short- and medium-chain-length fatty acids. Biochem. J. 250:819-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britton, K., S. Langridge, P. J. Baker, K. Weeradechapon, S. E. Sedelnikova, J. R. De Lucas, D. W. Rice, and G. Turner. 2000. The crystal structure and active site location of isocitrate lyase from the fungus Aspergillus nidulans. Structure Fold. Des. 8:349-362. [DOI] [PubMed] [Google Scholar]
- 5.Brock, M., and W. Buckel. 2004. On the mechanism of action of the antifungal agent propionate. Eur. J. Biochem. 271:3227-3241. [DOI] [PubMed] [Google Scholar]
- 6.Brock, M., D. Darley, S. Textor, and W. Buckel. 2001. 2-Methylisocitrate lyases from the bacterium Escherichia coli and the filamentous fungus Aspergillus nidulans: characterization and comparison of both enzymes. Eur. J. Biochem. 268:3577-3586. [DOI] [PubMed] [Google Scholar]
- 7.Brock, M., R. Fischer, D. Linder, and W. Buckel. 2000. Methylcitrate synthase from Aspergillus nidulans: implications for propionate as an antifungal agent. Mol. Microbiol. 35:961-973. [DOI] [PubMed] [Google Scholar]
- 8.Brock, M., C. Maerker, A. Schutz, U. Volker, and W. Buckel. 2002. Oxidation of propionate to pyruvate in Escherichia coli. Involvement of methylcitrate dehydratase and aconitase. Eur. J. Biochem. 269:6184-6194. [DOI] [PubMed] [Google Scholar]
- 9.Conrad, R., and M. Klose. 1999. Anaerobic conversion of carbon dioxide to methane, acetate and propionate on washed rice roots. FEMS Microbiol. Ecol. 30:147-155. [DOI] [PubMed] [Google Scholar]
- 10.Darley, D. J., T. Selmer, W. Clegg, R. W. Harrington, W. Buckel, and B. T. Golding. 2003. Stereocontrolled synthesis of (2R,3S)-2methylisocitrate, a central intermediate in the methylcitrate cycle. Helvet. Chim. Acta 86:3991-3999. [Google Scholar]
- 11.Fillinger, S., M. K. Chaveroche, K. Shimizu, N. Keller, and C. d'Enfert. 2002. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 44:1001-1016. [DOI] [PubMed] [Google Scholar]
- 12.Fillinger, S., M. K. Chaveroche, P. van Dijck, R. de Vries, G. Ruijter, J. Thevelein, and C. d'Enfert. 2001. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology 147:1851-1862. [DOI] [PubMed] [Google Scholar]
- 13.Grimek, T. L., H. Holden, I. Rayment, and J. C. Escalante-Semerena. 2003. Residues C123 and D58 of the 2-methylisocitrate lyase (PrpB) enzyme of Salmonella enterica are essential for catalysis. J. Bacteriol. 185:4837-4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm, C., A. Evers, M. Brock, C. Maerker, G. Klebe, W. Buckel, and K. Reuter. 2003. Crystal structure of 2-methylisocitrate lyase (PrpB) from Escherichia coli and modeling of its ligand bound active centre. J. Mol. Biol. 328:609-621. [DOI] [PubMed] [Google Scholar]
- 15.Habison, A., C. P. Kubicek, and M. Rohr. 1983. Partial purification and regulatory properties of phosphofructokinase from Aspergillus niger. Biochem. J. 209:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horswill, A. R., and J. C. Escalante-Semerena. 2001. In vitro conversion of propionate to pyruvate by Salmonella enterica enzymes: 2-methylcitrate dehydratase (PrpD) and aconitase Enzymes catalyze the conversion of 2-methylcitrate to 2-methylisocitrate. Biochemistry 40:4703-4713. [DOI] [PubMed] [Google Scholar]
- 17.Horswill, A. R., and J. C. Escalante-Semerena. 1999. Salmonella typhimurium LT2 catabolizes propionate via the 2-methylcitric acid cycle. J. Bacteriol. 181:5615-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karos, M., and R. Fischer. 1996. hymA (hypha-like metulae), a new developmental mutant of Aspergillus nidulans. Microbiology 142(Pt. 11):3211-3218. [DOI] [PubMed] [Google Scholar]
- 19.Kelly, J. M., and M. J. Hynes. 1982. The regulation of NADP-linked isocitrate dehydrogenase in Aspergillus nidulans. J. Gen. Microbiol. 128(Pt. 1):23-28. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli, U. K. 1970. Cleavage of structural protein during assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 21.Lauble, H., and C. D. Stout. 1995. Steric and conformational features of the aconitase mechanism. Proteins 22:1-11. [DOI] [PubMed] [Google Scholar]
- 22.Luttik, M. A., P. Kotter, F. A. Salomons, I. J. van Der Klei, J. P. van Dijken, and J. T. Pronk. 2000. The Saccharomyces cerevisiae ICL2 gene encodes a mitochondrial 2-methylisocitrate lyase involved in propionyl-coenzyme A metabolism. J. Bacteriol. 182:7007-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macheda, M. L., M. J. Hynes, and M. A. Davis. 1999. The Aspergillus nidulans gltA gene encoding glutamate synthase is required for ammonium assimilation in the absence of NADP-glutamate dehydrogenase. Curr. Genet. 34:467-471. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama, K., and H. Kitamura. 1985. Mechanisms of growth inhibition by propionate and restoration of the growth by sodium bicarbonate or acetate in Rhodopseudomonas sphaeroides S. J. Biochem. 98:819-824. [DOI] [PubMed] [Google Scholar]
- 25.Plaut, G. W., R. L. Beach, and T. Aogaichi. 1975. Alpha-methylisocitrate. A selective inhibitor of TPN-linked isocitrate dehydrogenase from bovine heart and rat liver. J. Biol. Chem. 250:6351-6354. [PubMed] [Google Scholar]
- 26.Sharma, V., S. Sharma, K. Hoener zu Bentrup, J. D. McKinney, D. G. Russell, W. R. Jacobs, Jr., and J. C. Sacchettini. 2000. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat. Struct. Biol. 7:663-668. [DOI] [PubMed] [Google Scholar]
- 27.Simanshu, D. K., P. S. Satheshkumar, S. Parthasarathy, H. S. Savithri, and M. R. Murthy. 2002. Cloning, expression, purification and preliminary X-ray crystallographic studies of 2-methylisocitrate lyase from Salmonella typhimurium. Acta Crystallogr. D Biol. Crystallogr. 58:2159-2161. [DOI] [PubMed] [Google Scholar]
- 28.Simanshu, D. K., P. S. Satheshkumar, H. S. Savithri, and M. R. Murthy. 2003. Crystal structure of Salmonella typhimurium 2-methylisocitrate lyase (PrpB) and its complex with pyruvate and Mg2+. Biochem. Biophys. Res. Commun. 311:193-201. [DOI] [PubMed] [Google Scholar]
- 29.Smith, C. M., and G. W. Plaut. 1979. Activities of NAD-specific and NADP-specific isocitrate dehydrogenases in rat-liver mitochondria. Studies with d-threo-alpha-methylisocitrate. Eur. J. Biochem. 97:283-295. [DOI] [PubMed] [Google Scholar]
- 30.Stringer, M. A., R. A. Dean, T. C. Sewall, and W. E. Timberlake. 1991. Rodletless, a new Aspergillus developmental mutant induced by directed gene inactivation. Genes Dev. 5:1161-1171. [DOI] [PubMed] [Google Scholar]
- 31.Szewczyk, E., A. Andrianopoulos, M. A. Davis, and M. J. Hynes. 2001. A single gene produces mitochondrial, cytoplasmic, and peroxisomal NADP-dependent isocitrate dehydrogenase in Aspergillus nidulans. J. Biol. Chem. 276:37722-37729. [DOI] [PubMed] [Google Scholar]
- 32.Textor, S., V. F. Wendisch, A. A. De Graaf, U. Muller, M. I. Linder, D. Linder, and W. Buckel. 1997. Propionate oxidation in Escherichia coli: evidence for operation of a methylcitrate cycle in bacteria. Arch. Microbiol. 168:428-436. [DOI] [PubMed] [Google Scholar]
- 33.Valenciano, S., J. R. De Lucas, I. Van der Klei, M. Veenhuis, and F. Laborda. 1998. Characterization of Aspergillus nidulans peroxisomes by immunoelectron microscopy. Arch. Microbiol. 170:370-376. [DOI] [PubMed] [Google Scholar]
- 34.Weidner, G., C. d'Enfert, A. Koch, P. C. Mol, and A. A. Brakhage. 1998. Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5′-monophosphate decarboxylase. Curr. Genet. 33:378-385. [DOI] [PubMed] [Google Scholar]
- 35.Yoshino, M., and K. Murakami. 1982. AMP deaminase as a control system of glycolysis in yeast. Mechanism of the inhibition of glycolysis by fatty acid and citrate. J. Biol. Chem. 257:10644-10649. [PubMed] [Google Scholar]
- 36.Zhang, Y. Q., M. Brock, and N. P. Keller. 2004. Connection of propionyl-CoA metabolism to polyketide biosynthesis in Aspergillus nidulans. Genetics 168:785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, Y. Q., and N. P. Keller. 2004. Blockage of methylcitrate cycle inhibits polyketide production in Aspergillus nidulans. Mol. Microbiol. 52:541-550. [DOI] [PubMed] [Google Scholar]