Abstract
The betaproteobacterium Mitsuaria chitosanitabida (formerly Matsuebacter chitosanotabidus) 3001 produces a chitosanase (ChoA) that is classified in glycosyl hydrolase family 80. While many chitosanase genes have been isolated from various bacteria to date, they show limited homology to the M. chitosanitabida 3001 chitosanase gene (choA). To investigate the phylogenetic distribution of chitosanases analogous to ChoA in nature, we identified 67 chitosan-degrading strains by screening and investigated their physiological and biological characteristics. We then searched for similarities to ChoA by Western blotting and Southern hybridization and selected 11 strains whose chitosanases showed the most similarity to ChoA. PCR amplification and sequencing of the chitosanase genes from these strains revealed high deduced amino acid sequence similarities to ChoA ranging from 77% to 99%. Analysis of the 16S rRNA gene sequences of the 11 selected strains indicated that they are widely distributed in the β and γ subclasses of Proteobacteria and the Flavobacterium group. These observations suggest that the ChoA-like chitosanases that belong to family 80 occur widely in a broad variety of bacteria.
Chitosan, a linear polymer composed of β-1,4-linked glucosamine (GlcN) residues with various numbers of N-acetylated residues, is a deacetylated derivative of chitin. The chitooligosaccharides produced by the enzymatic hydrolysis of chitosan are widely used in the food, agricultural, and pharmaceutical fields because of their various physiological activities.
Chitosanases (EC 3.2.1.132) are glycosyl hydrolases that catalyze the hydrolysis of the β-1,4-glycosidic linkage of chitosan and thereby produce glucosamine oligosaccharides. To date, many chitosanases have been found in a variety of microorganisms, including bacteria (11, 18, 26, 29, 30, 42, 43, 44, 48), fungi (6, 8, 9, 10, 16, 38, 50), plants (27), and viruses (40). The chitosanases that have been sequenced so far have been classified into four different families in the classification system of glycosyl hydrolases: families 8, 46, 75, and 80 (12, 13, 14). This classification of the chitosanases is based on the amino acid sequence similarity of their catalytic domains. Family 8 includes five chitosanases from bacterial organisms along with cellulase, licheninase, and endo-1,4-β-xylanase (17, 24, 45). Family 46 includes 18 chitosanases, 16 from bacterial organisms and 2 from Chlorella viruses (2, 3, 21, 22, 33, 36, 40, 46, 47). The 3-dimensional structures of the family-46 chitosanases from Streptomyces sp. strain N174 (20) and Bacillus circulans MH-K1 (31) and of the family-8 chitosanase from Bacillus sp. strain K17 (1) have been determined. The catalytic residues of the family-8 and -46 chitosanases are reported to be glutamic acid (Glu) and aspartic acid (Asp) (4). Family 75 includes 17 chitosanases, 14 and 3 of which are from fungi and bacteria, respectively (37, 49).
Prior to this study, only two bacterial chitosanases have been classified into family 80 (http://afmb.cnrs-mrs.fr/CAZY/). These show no significant nucleotide or amino acid sequence homology to the chitosanases in other families. We previously reported our identification of the chitosanase gene (choA) from Mitsuaria chitosanitabida (formerly Matsuebacter chitosanotabidus) (2a), which was then classified into family 80 (23, 28). Furthermore, we recently reported that Glu-121 and Glu-141 are the catalytically important residues of ChoA (35). We have also succeeded in functionally expressing chitosanase in the yeast Schizosaccharomyces pombe (34).
In the study reported here, we identified other chitosanases that can be classified into family 80 and investigated their phylogenetic distribution to determine how commonly this type of chitosanase occurs in nature.
MATERIALS AND METHODS
Materials.
Restriction enzymes were purchased from TaKaRa Biomedicals (Kyoto, Japan) and New England Biolabs. Chitosan was obtained from San-in Kensetsu (Shimane, Japan). Ampicillin and 2-mercaptoethanol were purchased from Wako Pure Chemical Industries (Osaka, Japan). All other reagents were of analytical grade.
Strains, plasmid, media, and culture conditions.
M. chitosanitabida 3001 and 67 other strains (strains 1 to 67), which were isolated from many different places in Japan, were used in this study. All were grown at 30°C with shaking in chitosan medium containing 0.5% colloidal chitosan, 0.2% K2HPO4, 0.1% KH2PO4, 0.07% MgSO4, 0.05% NaCl, 0.05% KCl, 0.01% CaCl2, and 0.05% yeast extract. The plasmid vector pT7 blue (TaKaRa Biomedicals) was used to clone the chitosanase genes into the cloning host Escherichia coli DH5α. All E. coli strains were grown at 37°C on LB medium containing appropriate antibiotics for the selection of the transformants.
Screening of chitosan-degrading bacteria from nature.
Samples collected from soil or water at various locations in Japan were suspended in 5 ml of distilled water, and particles were removed by sedimentation. The diluted supernatants were cultured in phosphate buffer with chitosan for 3 days, then plated onto a chitosan plate containing 0.5% colloidal chitosan, 0.2% K2HPO4, 0.1% KH2PO4, 0.07% MgSO4, 0.05% NaCl, 0.05% KCl, 0.01% CaCl2, 0.05% yeast extract, and 1.5% agar (pH 6.0), and incubated at 30°C to screen for chitosan-degrading bacteria, which were detected by their clear-zone-forming ability.
Physiological characteristics.
Gram staining was performed by using the Gram color kit from Merck. pH and temperature tolerance were determined using LB medium. Growth at various pH values ranging from pH 3 to 9 and at various temperatures ranging from 20 to 60°C was observed spectroscopically (optical density at 600 nm) over a period of 3 days. Urease activity, reduction of nitrate, indole production from tryptophan, and H2S production from cysteine were determined according to Smibert and Krieg (39). Other physiological and biochemical tests were performed as described by Cowan and Steel (7).
Analysis of isoprenoid quinones.
Quinone was extracted by using previously described methods (25). The extracted crude quinone was analyzed by normal-phase thin-layer chromatography using ubiquinone 10 as a standard. Normal-phase thin-layer chromatography was carried out on a Kiesel gel 60 F254 plate (Merck) with benzene-acetone (93:7, vol/vol). The UV-visualized band containing quinone was collected from the thin-layer chromatography plate and extracted with chloroform-methanol (1:1, vol/vol). The samples were then dried, and the precipitate was dissolved in ethanol. The purified quinone was further analyzed by high-performance liquid chromatography using ethanol as the solvent phase (15).
Western blot analysis for the detection of chitosanase.
Western blot analysis was undertaken to determine the cross-reactivity of various chitosanases with a ChoA-specific antibody (28). Cell extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12.5% acrylamide gel, performed as described by Laemmli (19), and the proteins were then transferred electrophoretically to a polyvinylidene difluoride membrane (Immobilon-PSQ; pore size, 0.45 μm; IPVH 304FO; Millipore). To immunolabel the chitosanases, the nitrocellulose membrane was incubated at room temperature with shaking in TBS-M buffer (20 mM Tris-HCl, 0.137 M NaCl, 0.1 M HCl, 0.25% Tween 20, and 5% dry milk) for at least 1 h. Afterwards, the membrane was rinsed several times in TBS buffer and then incubated for 1 h with the affinity-purified rabbit antiserum against ChoA. After several rinses in TBS buffer, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody, and the membrane-bound immunocomplexes were detected with an ECL system as recommended by the manufacturer (Amersham Pharmacia Biotech). The rabbit antibody specific for the chitosanases was custom-made by TaKaRa Biomedicals.
Southern hybridization analysis.
The total genomic DNAs of various chitosan-degrading bacteria were extracted by the cetyltrimethylammonium bromide method as described by Sambrook et al. (32) and digested with the BamHI restriction enzyme. The digested DNAs were then fractionated on a 0.7% agarose gel, denatured, neutralized, and transferred to a nylon membrane (Hybond-N; Amersham) by the capillary method. The Southern blot membranes were hybridized at 42°C for 10 h with the choA probe in a buffer containing 15 ml of Gold hybridization buffer, 0.07% NaCl, and 0.1% blocking agent. The membranes were then washed twice for 20 min at 42°C with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.4% sodium dodecyl sulfate and 6 M urea and twice for 20 min at room temperature with 20× SSC. The labeled bands were visualized by using the ECL system according to the manufacturer's recommendations (Amersham Pharmacia Biotech).
PCR amplification of chitosanase genes.
PCR amplification was performed by using a DNA thermal cycler (Perkin-Elmer/Cetus) with the three forward primers 5′-GGAACCTCTCCTACATTC-3′ (cho420-), 5′-CTGGTSACSGCSACCAAG-3′ (cho748-), and 5′-ACGGTCAATCAATGGCAG-3′ (cho844-) and the two reverse primers 5′-CATGTTCTTSGACCACTT-3′ (cho-1692) and 5′-CGCGGGTCGATGGCA-3′ (cho-1773), which were designed based on the nucleotide sequence of the choA gene of M. chitosanitabida 3001. The cho748- and cho-1692 primers have a mixed base, C+G (S). PCR amplification was performed with 0.5 μg genomic DNA in 50 μl of reaction buffer supplemented with final concentrations of 1.5 mM MgCl2, 50 μM each deoxynucleoside triphosphate, 0.1 μg of each synthesized primer, and 2.5 Uof Ex-Taq DNA polymerase (TaKaRa). The cycle program was as follows: 1 min at 94 to 96°C, 2 min at 45 to 58°C, and 3 min at 72°C (25 cycles).
PCR amplification of the 16S rRNA gene.
Genomic DNA was extracted from selected chitosan-degrading bacterial strains, and the 16S rRNA-coding region was PCR amplified using the two oligonucleotide primers 5′-ATCTGGTTGATCCTGCCAGT-3′ (positions 2 to 21 relative to E. coli 16S rRNA) and 5′-GGCTACCTTGTTACGACTT-3′ (positions 1510 to 1492 relative to E. coli 16S rRNA). The PCR program consisted of an initial denaturation step of 1 min at 95°C followed by 35 cycles of 94°C for 1 min, 48°C for 2 min, and 72°C for 3 min. The PCR products of the expected size were purified using a PCR product purification kit, then cloned into the pT7 blue plasmid vector and sequenced using primers 5′-CCAGCAGCCGCGGTAATAC-3′ (corresponding to the complementary nucleotide sequence 518 to 536 of E. coli 16S rRNA) and 5′-AAACTCAAAGGAATTGACGG-3′ (corresponding to the complementary nucleotide sequence 907 to 926 of E. coli 16S rRNA). Computer-assisted analysis and comparison of DNA sequences were performed using the BLAST program in the National Center for Biotechnology Information network service.
Phylogenetic analysis.
16S rRNA gene sequences determined in this study were compared with 16S rRNA gene sequences of related bacteria obtained from GenBank by using the neighbor-joining method with the CLUSTAL W program on the Web (http://www.ddbj.nig.ac.jp/search/clustalw-e.html). The phylogenetic tree was drawn by the Tree View program.
Nucleotide sequencing.
The chitosanase gene fragments in the recombinant pT7 blue plasmid were used for sequencing. Sequencing was carried out using the dideoxy nucleotide chain termination method by using an ABI Prism 377 DNA sequencer (Perkin Elmer). Computer analysis of the nucleotide and deduced amino acid sequences using choA sequences was performed by employing DNASIS (Hitachi Software Engineering Co. Ltd., Yokohama, Japan).
Nucleotide sequence accession numbers.
The nucleotide sequences of all 16S rRNA and partial chitosanase genes reported in this article have been deposited in GenBank. The accession numbers of the 16S rRNA gene sequences are as follows: Chryseobacterium sp. strain 2, AB024308; Herbaspirillum sp. strain 9, AB024305; Mitsuaria sp. strain 12, AY856841; Mitsuaria sp. strain 13, AB024306; Stenotrophomonas sp. strain 22, AY856842; Herbaspirillum sp. strain 27, AY856843; Pseudomonas sp. strain 38, AY856844; Stenotrophomonas sp. strain 45, AY856845; Comamonas sp. strain 46, AY856846; Sphingobacterium sp. strain 62, AY856847; Mitsuaria sp. strain 67, AY856848. The accession numbers of the partial chitosanase gene sequences are as follows: Chryseobacterium sp. strain 2, AY856849; Herbaspirillum sp. strain 9, AY856850; Mitsuaria sp. strain 12, AY856851; Mitsuaria sp. strain 13, AY856852; Stenotrophomonas sp. strain 22, AY856853; Herbaspirillum sp. strain 27, AY856854; Pseudomonas sp. strain 38, AY856855; Stenotrophomonas sp. strain 45, AY856856; Comamonas sp. strain 46, AY856857; Sphingobacterium sp. strain 62, AY856858; Mitsuaria sp. strain 67, AY856859.
RESULTS
Physiological and biological characteristics of chitosan-degrading bacteria.
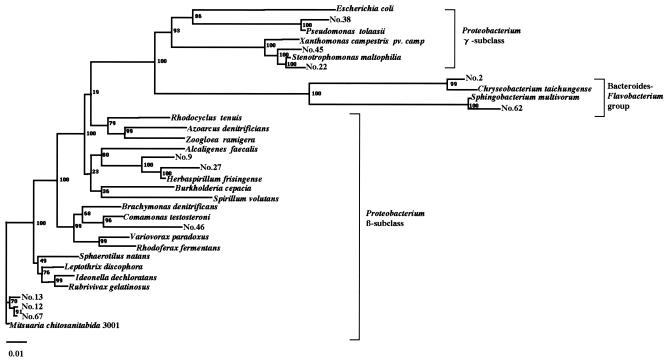
To identify additional chitosan-degrading bacteria, we screened 200 samples (120 soil samples, 60 samples of fresh water, and 20 samples of seawater) collected from many places in Japan by culturing them on chitosan-containing plates and searching for clear zones generated around the bacterial colonies (see Materials and Methods). This yielded 67 bacterial strains (38 from soil, 22 from fresh water, and 7 from seawater), numbered as strains 1 to 67, which were then tested for their physiological and biological properties (summarized in Table 1). All isolates were gram negative, and most had physiological and biological properties similar to those of M. chitosanitabida 3001, but some were different, especially strains 2 and 46, which produce menaquinone instead of ubiquinone. Taking these results together with those of later analysis of 16S rRNA gene sequence (see Fig. 3), we propose that some strains belong to the β and γ subclasses of Proteobacterium and the Flavobacterium group.
TABLE 1.
Comparison of physiological and biological characteristics of 11 isolated strains
| Characteristic | Resulta for strain:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 9 | 12 | 13 | 22 | 27 | 38 | 45 | 46 | 62 | 67 | M. chitosanitabida 3001 | |
| Gram staining | − | − | − | − | − | − | − | − | − | − | − | − |
| Nitrate reduction | − | + | + | + | + | + | + | + | + | + | + | + |
| Methyl red and V-P test | − | − | − | − | − | − | − | − | − | − | − | − |
| Indole production | + | − | − | − | − | − | − | − | − | − | − | − |
| O-F test | N | N | N | N | N | N | O | N | N | N | N | N |
| Oxidase production | + | + | + | + | + | + | − | + | + | + | + | + |
| Urease production | + | − | − | − | + | + | + | + | + | + | − | − |
| 2-Keto-gluconate production | + | − | − | − | − | − | − | − | − | − | − | − |
| 3-Keto lactose production | − | − | − | − | − | − | − | − | − | − | − | − |
| Dihydroxyacetone production | + | − | − | − | − | − | − | − | − | − | − | − |
| Catalase production | + | + | + | + | + | + | + | + | + | + | + | + |
| H2S production | − | − | − | − | − | − | + | − | − | − | − | − |
| Hydrolysis of Tween 20, 40, 60 | + | + | + | + | + | + | + | + | − | + | + | + |
| Highest temp for growth (°C) | 37 | 34 | 34 | 34 | 37 | 40 | 37 | 37 | 37 | 37 | 34 | 34 |
| pH for growth | 5-8 | 6-9 | 5-9 | 5-9 | 5-9 | 5-10 | 5-9 | 4-9 | 4-9 | 5-9 | 5-9 | 5-9 |
| Quinone type | MK-6 | UQ-8 | UQ-8 | UQ-8 | UQ-8 | UQ-8 | UQ-8 | UQ-8 | UQ-8 | MK-7 | UQ-8 | UQ-8 |
+, positive; −, negative. N, no action on carbohydrate; O, oxidation.
FIG. 3.
Phylogenetic relationship of M. chitosanitabida 3001 with the 11 selected isolates and their related strains based on their 16S rRNA gene sequences. Bar, 1 nucleotide substitution per 100 nucleotides in the 16S rRNA gene sequence. The numbers at the nodes of the tree indicate bootstrap values (percentages) for each node of 1,000 bootstrap resamplings. The sequences used for the comparison with the 16S rRNA genes of the isolates were obtained from GenBank. The origins and accession numbers of the sequences are as follows: Escherichia coli, J01859; Pseudomonas sp. strain 38, AY856844; Pseudomonas tolaasii, AF255336; Xanthomonas campestris pv. campestris, AF000946; Stenotrophomonas sp. strain 45, AY856845; Stenotrophomonas maltophilia, AJ131903; Stenotrophomonas sp. strain 22, AY856842; Chryseobacterium sp. strain 2, AB024308; Chryseobacterium taichungense, AJ843132; Sphingobacterium multivorum, AB020205; Sphingobacterium sp. strain 62, AY856847; Rhodocyclus tenuis, D16208; Azoarcus denitrificans, L33694; Zoogloea ramigera, D14257; Alcaligenes faecalis, D88008; Herbaspirillum sp. strain 9, AB024305; Herbaspirillum sp. strain 27, AY856843; Herbaspirillum frisingense, AJ238359; Burkholderia cepacia, X87275; Spirillum volutans, M34131; Brachymonas denitrificans, D14320; Comamonas testosteroni, AB064318; Comamonas sp. strain 46, AY856846; Variovorax paradoxus, D88006; Rhodoferax fermentans, D16212; Sphaerotilus natans, Z18534; Leptothrix discophora, Z18533; Ideonella dechloratans, X72724; Rubrivivax gelatinosus, AB016167; Mitsuaria sp. strain 13, AB024306; Mitsuaria chitosanitabida 3001, AB024307; Mitsuaria sp. strain 67, AY856848; Mitsuaria sp. strain 12, AY856841.
Western blot analysis.
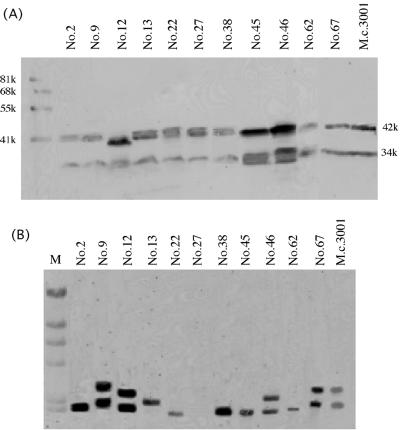
To determine the similarities between the chitosanases of the new chitosan-degrading bacterial strains and M. chitosanitabida 3001 ChoA, the isolates were subjected to Western blot analysis using a ChoA-specific antibody. Eleven isolates (strains 2, 9, 12, 13, 22, 27, 38, 45, 46, 62, and 67) showed the same band pattern as M. chitosanitabida 3001 ChoA, namely, a 34,000-molecular-weight (34K) and a 42K band (Fig. 1A). The other isolates showed four different band patterns (summarized in Table 2). These results suggest that the 11 isolates that showed the same signal pattern as M. chitosanitabida 3001 may produce chitosanases that are similar to ChoA.
FIG. 1.
Western blot and Southern hybridization analyses against isolates. (A) Each isolate was grown in PYS medium overnight and then cultured five more days in chitosan liquid medium. The precipitate was separated from the culture medium by centrifugation, and the intracellular chitosanase was detected by Western blot analysis using a ChoA-specific antibody and a horseradish peroxidase-conjugated secondary antibody. (B) Southern hybridization analysis was performed using total BamHI-digested genomic DNA from each strain and the chitosanase gene (choA) from M. chitosanitabida 3001 as the probe. M, λ HindIII-digested DNA.
TABLE 2.
Band patterns showing the reactivities of the chitosanases from new chitosan-degrading bacteria with an anti-ChoA antibody
| Signal(s) detected | Strains |
|---|---|
| 34K, 42K | M. chitosanitabida 3001; strains 2, 9, 12, 13, 22, 27, 38, 45, 46, 62, 67 |
| 34K | Strains 4, 7, 11, 14-17, 19-21, 26, 32, 35, 39, 59, 65 |
| 42K | Strains 29, 34, 37, 41-44, 47, 50-55, 58, 60, 61, 63 |
| Signals of different sizes | Strains 8, 23-25, 28, 30, 31, 40, 56, 57, 64, 66 |
| No signal | Strains 1, 3, 5, 6, 10, 18, 33, 36, 48, 49 |
Southern hybridization analysis.
To determine whether these 11 isolates have chitosanase genes that are similar to the choA gene of M. chitosanitabida 3001, Southern hybridization analysis using the choA gene as a probe was carried out. The probe used in this experiment contained the whole open reading frame of choA. The signal patterns detected are shown in Fig. 1B. Of the 11 isolates, only strains 9, 12, 46, and 67 had signal patterns similar to that of M. chitosanitabida 3001. Strains 2, 22, 38, and 45 showed similar signal patterns among themselves, while strains 13 and 62 yielded very different signals. Strain 27 did not give any signals. These observations suggest that at least four of the isolates that have an anti-ChoA antibody-reactive chitosanase have a chitosanase gene that is also similar to choA.
Chitosanase sequence.
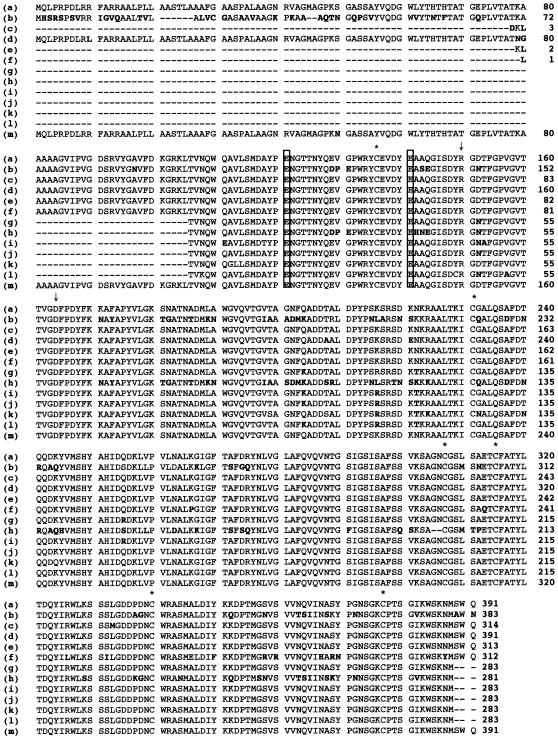
We amplified the chitosanase genes from all 11 isolates using five primers (three forward and two reverse) designed on the basis of the choA sequence. This generated six forward-reverse primer sets. Varied denaturation and annealing temperatures were employed with these primer sets. DNA fragments of approximately 1.4 kb were successfully amplified from isolates 2 and 67. A DNA fragment of about 0.85 kb was amplified from the other isolates. These fragments were purified and ligated with the pT7 blue vector, and their nucleotide sequences were determined. When the deduced amino acid sequences were aligned with the ChoA sequence (Fig. 2), all the sequenced fragments showed high (more than 95%) identity to ChoA, except for strain 27, the one we could not detect by Southern blot analysis (Fig. 1B), which had 77% identity at the amino acid level.
FIG. 2.
Comparison of the partial amino acid sequences of the chitosanases from the 11 selected isolates, Sphingobacterium multivorum, and M. chitosanitabida 3001. The amino acid sequences of the 11 selected isolates were deduced from the nucleotide sequences of the PCR-amplified DNA fragments. Boldface indicates amino acid residues different from those of the ChoA sequence. The two putative catalytic amino acid residues of ChoA are boxed. Asterisks mark the six cysteine residues of ChoA. The Arg-150 and Asp-164 residues, which are important for the catalytic activity of ChoA, are indicated by vertical arrows. Strains and GenBank accession numbers are as follows: (a) Mitsuaria chitosanitabida 3001, AB010493; (b) Sphingobacterium multivorum, AB030253; (c) Flavobacterium sp. strain 2, AY856849; (d) Herbaspirillum sp. strain 9, AY856850; (e) Mitsuaria sp. strain 12, AY856851; (f) Mitsuaria sp. strain 13, AY856852; (g) Stenotrophomonas sp. strain 22, AY856853; (h) Herbaspirillum sp. strain 27, AY856854; (i) Pseudomonas sp. strain 38, AY856855; (j) Stenotrophomonas sp. strain 45, AY856856; (k) Comamonas sp. strain 46, AY856857; (l) Sphingobacterium sp. strain 62, AY856858; (m) Mitsuaria sp. strain 67, AY856859.
16S rRNA gene sequence analysis.
To determine the phylogenetic relationships between the 11 selected chitosan-degrading bacterial strains and M. chitosanitabida 3001, we determined the almost-complete 16S rRNA gene sequences of these strains and subjected them to BLAST searching (http://www.ncbi.nlm.nih.gov/BLAST/). This revealed that strains 12, 13, and 67 appear to belong to the genus Mitsuaria, since their levels of 16S rRNA gene homology with Mitsuaria chitosanitabida 3001 were 99.4%, 98.4%, and 99.6%, respectively. Strain 2 appears to belong to the genus Chryseobacterium, since its 16S rRNA gene homology with Chryseobacterium taichungense was 97.3%. Moreover, strains 9 and 27 may be Herbaspirillum spp., given their 96.7% and 96.2% levels of homology, respectively, to the 16S rRNA gene of Herbaspirillum frisingense, while strains 22 and 45 may be Stenotrophomonas spp. (since they show 98.0% and 97.3% homology, respectively, to the Stenotrophomonas maltophilia 16S rRNA gene). Strain 38 may be a Pseudomonas sp. (96.7% homology with Pseudomonas tolaasii), strain 46 may be a Comamonas sp. (97.4% homology with Comamonas testosteroni), and strain 62 may be a Sphingobacterium sp. (97.0% homology with Sphingobacterium multivorum). Phylogenetic analysis of all 11 selected isolates and the related bacteria was carried out based on their 16S rRNA gene sequences (Fig. 3).
DISCUSSION
The chitosanases that have been sequenced to date are classified into four different families in the classification system of the glycosyl hydrolases: families 8, 46, 75, and 80 (12, 13, 14). Recently, the chitosanse of Streptomyces griseus HUT 6037 was found to fall into a new glycosyl hydrolase family, family 5 (41). Prior to this study, family 80 contained only two chitosanases, those from M. chitosanitabida 3001 (28) and Sphingobacterium multivorum (23). In this study, we searched for additional bacteria that produce chitosanases resembling the family-80-type ChoA of M. chitosanitabida 3001. Almost all isolates have ubiquinone-8, which is the major quinone compound of members of the β subclass of the Proteobacteria (5), but isolates 2 and 62 have menaquinone-6 and -7, respectively. Menaquinone is known to be the major quinone component of the Flavobacterium group. This is supported by 16S rRNA gene analysis of these isolates, which shows that strain 2 belongs to the genus Chryseobacterium while strain 62 belongs to the genus Sphingobacterium.
Western blot analysis with a ChoA-specific antibody revealed that the intracellular chitosanase of M. chitosanitabida 3001 exhibited two bands: a 34 and a 42K band. The 42K band is ChoA attached to its signal polypeptide, while the 34K band is the mature form of excreted ChoA. Eleven of the 67 isolates showed identical signal patterns, suggesting that they produce chitosanases similar to ChoA and bear similarly sized signal polypeptides.
Southern hybridization analysis using choA as the probe revealed that of the 11 isolates identified by Western blot analysis, strains 9, 12, 46, and 67 showed the same signal as M. chitosanitabida 3001. Apart from strain 27, which did not give a signal at all, the remaining isolates showed signals of different sizes. Thus, it appears that the chitosanase gene of strain 27 may have low homology to choA compared to the others. This is supported by the deduced amino acid sequence of the strain 27 chitosanase, which showed only 77% homology to ChoA, while the chitosanases of the other 10 isolates showed more than 95% identity with ChoA. Alignment of the deduced amino acid sequences with that of choA revealed that the two glutamic acid residues (Glu-121 and Glu-141) reported to be putative catalytic residues for M. chitosanitabida 3001 ChoA (35) are conserved in all the chitosanases sequenced. Moreover, all six cysteine residues, as well as the Arg-150 and Asp-164 residues, which are important for the catalytic activity of ChoA, are conserved (35). Phylogenetic analysis using the 16S rRNA gene sequences of the 11 selected isolates then showed that choA-like genes are widely distributed in the β and γ subclasses of Proteobacteria and in the Flavobacterium group in nature.
In conclusion, we characterized 11 newly isolated strains that possess family-80-type chitosanases. Our analysis reveals that these chitosanases are widely distributed in the β and γ subclasses of the Proteobacteria and in the Flavobacterium group in nature. This wide distribution suggests that family-80 chitosanases occur commonly in nature.
Acknowledgments
This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Adachi, W., Y. Sakihama, S. Shimizu, T. Sunami, T. Fukazawa, M. Suzuki, R. Yatsunami, S. Nakamura, and A. Takenaka. 2004. Crystal structure of family GH-8 chitosanase with subclass II specificity from Bacillus sp. K17. J. Mol. Biol. 343:785-795. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama, K., T. Fujita, K. Kuroshima, T. Sakane, A. Yokota, and R. Takata. 1999. Purification and gene cloning of a chitosanase from Bacillus ehimensis EAG1. J. Biosci. Bioeng. 87:383-385. [DOI] [PubMed] [Google Scholar]
- 2a.Amakata, D., Y. Matsuo, K. Shimono, J. K. Park, C. S. Yun, H. Matsuda, A. Yokota, and M. Kawamukai. Mitsuaria chitosanitabida gen. nov., sp. nov., an aerobic, chitosanase-producing member of the ‘Betaproteobacteria.’ Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 3.Ando, A., K. Noguchi, M. Yanagi, H. Shinoyama, Y. Kagawa, H. Hirata, M. Yabuki, and T. Fujii. 1992. Primary structure of chitosanase produced by Bacillus circulans MH-K1. J. Gen. Appl. Microbiol. 38:135-144. [Google Scholar]
- 4.Boucher, I., T. Fukamizo, Y. Honda, G. E. Wilick, W. A. Neugebauer, and R. Brzezinski. 1995. Site-directed mutagenesis of evolutionary conserved carboxylic amino acids in the chitosanase from Streptomyces sp. N174 reveals two residues essential for catalysis. J. Biol. Chem. 270:31077-31082. [DOI] [PubMed] [Google Scholar]
- 5.Busse, H. J., T. El-Banna, H. Oyaizu, and G. Auling. 1992. Identification of xenobiotic-degrading isolates from the beta subclass of the Proteobacteria by a polyphasic approach including 16S rRNA partial sequencing. Int. J. Syst. Bacteriol. 42:19-26. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, C. Y., and Y. K. Li 2000. An Aspergillus chitosanase with potential for large-scale preparation of chitosan oligosaccharides. Biotechnol. Appl. Biochem. 32:197-203. [DOI] [PubMed] [Google Scholar]
- 7.Cowan, S. T., and K. J. Steel. 1965. Manual for the identification of medical bacteria. Cambridge University Press, London, United Kingdom.
- 8.Eom, T. K., and K. M. Lee. 2003. Characteristics of chitosanases from Aspergillus fumigatus KB-1. Arch. Pharm. Res. 26:1036-1041. [DOI] [PubMed] [Google Scholar]
- 9.Fenton, D. M., and D. E. Eveleigh. 1981. Purification and mode of action of a chitosanase from Penicillium islandium. J. Gen. Microbiol. 126:151-165. [Google Scholar]
- 10.Grenier, J., N. Benhamou, and A. Asselin. 1991. Colloidal gold-complexed chitosanase: a new probe for ultrastructural localization of chitosan in fungi. J. Gen. Microbiol. 137:2007-2015. [Google Scholar]
- 11.Hedges, A., and R. S. Wolfe. 1974. Extracellular enzyme from Myxobacter AL-1 that exhibits both β-1,4-glucanase and chitosanase activities. J. Bacteriol. 120:844-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrissat, B. 1991. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 280:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrissat, B., and A. Bairoch. 1993. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 293:781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henrissat, B., and A. Bairoch. 1996. Updating the sequence-based classification of glycosyl hydrolases. Biochem. J. 316:695-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kainou, T., K. Okada, K. Suzuki, T. Nakagawa, H. Matsuda, and M. Kawamukai. 2001. Dimer formation of octaprenyl-diphosphate synthase (IspB) is essential for chain length determination of ubiquinone. J. Biol. Chem. 276:7876-7883. [DOI] [PubMed] [Google Scholar]
- 16.Kim, S. Y., D. H. Shon, and K. H. Lee. 1998. Purification and characteristics of two types of chitosanases from Aspergillus fumigatus KH-94. J. Microbiol. Biotechnol. 8:568-574. [Google Scholar]
- 17.Kimoto, H., H. Kusaoke, I. Yamamoto, Y. Fujii, T. Onodera, and A. Taketo. 2002. Biochemical and genetic properties of Paenibacillus glycosyl hydrolase having chitosanase activity and discoidin domain. J. Biol. Chem. 277:14695-14702. [DOI] [PubMed] [Google Scholar]
- 18.Kurakake, M., S. Yo-u, K. Nakagawa, M. Sugihara, and T. Komaki. 2000. Properties of chitosanase from Bacillus cereus S1. Curr. Microbiol. 40:6-9. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Marcotte, E. M., A. F. Monzingo, S. R. Ernst, R. Brzezinski, and J. D. Robertus. 1996. X-ray structure of an anti-fungal chitosanase from Streptomyces N174. Nat. Struct. Biol. 3:155-162. [DOI] [PubMed] [Google Scholar]
- 21.Masson, J. Y., I. Boucher, W. A. Neugebauer, D. Ramotar, and R. Brzezinski. 1995. A new chitosanase gene from a Nocardioides sp. is a third member of glycosyl hydrolase family 46. Microbiology 141:2629-2635. [DOI] [PubMed] [Google Scholar]
- 22.Masson, J. Y., F. Denis, and R. Brzezinski. 1994. Primary sequence of the chitosanse from Streptomyces sp. strain N174 and comparison with other endoglycosidases. Gene 140:103-107. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda, Y., Y. Iida, T. Shinogi, K. Kakutani, T. Nonomura, and H. Toyodai. 2001. In vitro suppression of mycelial growth of Fusarium oxysporum by extracellular chitosanase of Sphingobacterium multivorum and cloning of the chitosanase gene csnSM1. J. Gen. Plant Pathol. 67:318-324. [Google Scholar]
- 24.Mitsutomi, M., M. Isono, A. Uchiyama, N. Nikaidou, T. Ikegami, and T. Watanabe. 1998. Chitosanase activity of the enzyme previously reported as β-1,3-1,4-glucanase from Bacillus circulans WL-12. Biosci. Biotechnol. Biochem. 62:2107-2114. [DOI] [PubMed] [Google Scholar]
- 25.Okada, K., T. Kainou, K. Tanaka, T. Nakagawa, H. Matsuda, and M. Kawamukai. 1998. Molecular cloning and mutational analysis of the ddsA gene encoding decaprenyl diphosphate synthase from Gluconobacter suboxydans. Eur. J. Biochem. 255:52-59. [DOI] [PubMed] [Google Scholar]
- 26.Okajima, S., A. Ando, H. Shinoyama, and T. Fujii. 1994. Purification and characterization of an extracellular chitosanase produced by Amycolatopsis sp. CsO-2. J. Ferment. Bioeng. 77:617-620. [Google Scholar]
- 27.Osswald, W. F., J. P. Shapiro, H. Doostdar, R. E. McDonald, R. P. Niedz, C. J. Nairn, C. J. Hearn, and R. T. Mayer. 1994. Identification and characterization of acidic hydrolases with chitinase and chitosanase activities from sweet orange callus tissue. Plant Cell Physiol. 35:811-820. [DOI] [PubMed] [Google Scholar]
- 28.Park, J. K., K. Shimono, N. Ochiai, K. Shigeru, M. Kurita, Y. Ohta, K. Tanaka, H. Matsuda, and M. Kawamukai. 1999. Purification, characterization, and gene analysis of a chitosanase (ChoA) from Matsuebacter chitosanotabidus 3001. J. Bacteriol. 181:6642-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelletier, A., and J. Sygusch. 1990. Purification and characterization of three chitosanase activities from Bacillus megaterium P1. Appl. Environ. Microbiol. 56:844-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivas, L. A., V. Parro, M. Moreno-Paz, and R. P. Mellado. 2000. The Bacillus subtilis 168 csn gene encodes a chitosanase with similar properties to a Streptomyces enzyme. Microbiology 146:2929-2936. [DOI] [PubMed] [Google Scholar]
- 31.Saito, J., A. Kita, Y. Higuchi, Y. Nagata, A. Ando, and K. Miki. 1999. Crystal structure of chitosanase from Bacillus circulans MH-K1 at 1.6-Å resolution and its substrate recognition mechanism. J. Biol. Chem. 274:30818-30825. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Seki, K., H. Kuriyama, T. Okuda, and Y. Uchida. 1997. Molecular cloning of the gene encoding chitosanase from Bacillus amyloliquefaciens UTK. Adv. Chitin Sci. 2:284-289. [Google Scholar]
- 34.Shimono, K., H. Matsuda, and M. Kawamukai. 2002. Functional expression of chitinase and chitosanase, and their effects on morphologies in the yeast Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 66:1143-1147. [DOI] [PubMed] [Google Scholar]
- 35.Shimono, K., K. Shigeru, A. Tsuchiya, N. Itou, Y. Ohta, K. Tanaka, T. Nakagawa, H. Matsuda, and M. Kawamukai. 2002. Two glutamic acids in chitosanase A from Matsuebacter chitosanotabidus 3001 are the catalytically important residues. J. Biochem. 131:87-96. [DOI] [PubMed] [Google Scholar]
- 36.Shimosaka, M., Y. Fukumori, X. Y. Zhang, N. J. He, R. Kodaira, and M. Okazaki. 2000. Molecular cloning and characterization of a chitosanase from the chitosanolytic bacterium Burkholderia gladioli strain CHB101. Appl. Microbiol. Biotechnol. 54:354-360. [DOI] [PubMed] [Google Scholar]
- 37.Shimosaka, M., M. Kumehara, X.-Y. Zhang, M. Nogawa, and M. Okazaki. 1996. Cloning and characterization of a chitosanase gene from the plant pathogenic fungus, Fusarium solani. J. Ferment. Bioeng. 82:426-431. [Google Scholar]
- 38.Shimosaka, M., M. Nogawa, Y. Ohno, and M. Okazaki. 1993. Chitosanase from the plant pathogenic fungus, Fusarium solani f. sp. phaseoli: purification and some properties. Biosci. Biotechnol. Biochem. 57:231-235. [DOI] [PubMed] [Google Scholar]
- 39.Smibert, R. M., and N. R. Krieg. 1994. Phenotypic characterization, p. 603-711. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 40.Sun, L., B. Adams, J. R. Gurnon, Y. Ye, and J. L. V. Etten. 1999. Characterization of two chitinase genes and one chitosanase gene encoded by chlorella virus PBCV-1. Virology 263:376-387. [DOI] [PubMed] [Google Scholar]
- 41.Tanabe, T., K. Morinaga, T. Fukamizo, and M. Mitsumomi. 2003. Novel chitosanase from Streptomyces griseus HUT 6037. Biosci. Biotechnol. Biochem. 67:354-364. [DOI] [PubMed] [Google Scholar]
- 42.Yabuki, M., A. Uchiyama, K. Suzuki, A. Ando, and T. Fujii. 1988. Purification and properties of chitosanase from Bacillus circulans MH52 K1. J. Gen. Appl. Microbiol. 34:255-270. [Google Scholar]
- 43.Yamasaki, Y., I. Fukumoto, N. Kumagai, Y. Ohta, T. Nakagawa, M. Kawamukai, and H. Matsuda. 1992. Continuous chitosan hydrolyzate production by immobilized chitosanolytic enzyme from Enterobacter sp. G-1. Biosci. Biotechnol. Biochem. 56:1546-1551. [DOI] [PubMed] [Google Scholar]
- 44.Yamasaki, Y., I. Hayashi, Y. Ohta, T. Nakagawa, M. Kawamukai, and H. Matsuda. 1993. Purification and mode of action of chitosanolytic enzyme from Enterobacter sp. G-1. Biosci. Biotechnol. Biochem. 57:444-449. [DOI] [PubMed] [Google Scholar]
- 45.Yatsunami, R., Y. Sakihama, M. Suzuki, T. Fukazawa, S. Shimizu, and T. Sunami. 2002. A novel chitosanase from Bacillus sp. strain K17: gene cloning and expression in Escherichia coli. Nucleic Acids Res. Suppl. 2:227-228. [DOI] [PubMed] [Google Scholar]
- 46.Yoon, H. G., H. Y. Kim, Y. H. Lim, H. K. Kim, D. H. Shin, B. S. Hong, and H. Y. Cho. 2000. Thermostable chitosanase from Bacillus sp. strain CK4: cloning and expression of the gene and characterization of the enzyme. Appl. Environ. Microbiol. 66:3727-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon, H. G., K. H. Lee, H. Y. Kim, H. K. Kim, D. H. Shin, B. S. Hong, and H. Y. Cho. 2002. Gene cloning and biochemical analysis of thermostable chitosanase(TCH-2) from Bacillus coagulans CK108. Biosci. Biotechnol. Biochem. 66:986-995. [DOI] [PubMed] [Google Scholar]
- 48.You, Y. J., K. J. Jo, Y. L. Jin, K. Y. Kim, J. H. Shim, Y. W. Kim, and R. D. Park. 2003. Characterization and kinetics of 45-kDa chitosanase from Bacillus sp. P16. Biosci. Biotechnol. Biochem. 67:1875-1882. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, X. Y., A. L. Dai, K. Kuroiwa, R. Kodaira, M. Nogawa, M. Shimosaka, and M. Okazaki. 2001. Cloning and characterization of a chitosanase gene from the koji mold Aspergillus oryzae strain IAM 2660. Biosci. Biotechnol. Biochem. 65:977-981. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, X. Y., A. L. Dai, X. K. Zhang, K. Kuroiwa, R. Kodaira, M. Shimosaka, and M. Okazaki. 2000. Purification and characterization of chitosanase and exo-β-d-glucosaminidase from a koji mold, Aspergillus oryzae IAM 2660. Biosci. Biotechnol. Biochem. 64:1896-1902. [DOI] [PubMed] [Google Scholar]