Abstract
The transposons Tn5, Tn10, Tn611, and Tn5096 were characterized regarding transposition in Gordonia polyisoprenivorans strain VH2. No insertional mutants were obtained employing Tn5 or Tn10. The thermosensitive plasmid pCG79 harboring Tn611 integrated into the chromosome of G. polyisoprenivorans; however, the insertional mutants were fairly unstable und reverted frequently to the wild-type phenotype. In contrast, various stable mutants were obtained employing Tn5096-mediated transposon mutagenesis. Auxotrophic mutants, mutants defective or deregulated in carotenoid biosynthesis, and mutants defective in utilization of rubber and/or highly branched isoprenoid hydrocarbons were obtained by integration of plasmid pMA5096 harboring Tn5096 as a whole into the genome. From about 25,000 isolated mutants, the insertion loci of pMA5096 were subsequently mapped in 20 independent mutants in genes which could be related to the above-mentioned metabolic pathways or to putative regulation proteins. Analyses of the genotypes of pMA5096-mediated mutants defective in biodegradation of poly(cis-1,4-isoprene) did not reveal homologues to recently identified genes coding for enzymes catalyzing the initial cleavage of poly(cis-1,4-isoprene). One rubber-negative mutant was disrupted in mcr, encoding an α-methylacyl-coenzyme A racemase. This mutant was defective in degradation of poly(cis-1,4-isoprene) and also of highly branched isoprenoid hydrocarbons.
The taxon Gordonia belongs to the Corynebacterinae, a mycolic acid-containing suborder of actinomycetes. Members of the genus Gordonia exhibit various interesting metabolic capabilities and degrade many recalcitrant compounds such as phthalic acid esters, alkylpyridines, dibenzothiophene, natural and synthetic rubbers, and diverse xenobiotics (4). G. polyisoprenivorans is an ideal model organism to study the mechanisms underlying the biodegradation of poly(cis-1,4-isoprene), which is still only slightly understood (41). Despite the rising interest in members of this genus in recent years due to their unusual and diverse abilities to catalyze the biotransformation and biodegradation of various compounds, useful genetic tools applicable to members of this taxon are scarce. Bröker et al. (16) constructed mobilizable Gordonia-Escherichia coli shuttle vectors based on the oriV of the native megaplasmid pKB1 isolated from Gordonia westfalica DSM 44215T. In contrast to the related genera Mycobacterium and Rhodococcus, methods for identification of genes such as transposon-induced mutagenesis have not been described for Gordonia species. This study aimed to identify transposons suitable for generation of insertional mutant libraries of G. polyisoprenivorans.
Study of the literature revealed few candidates which might be suitable for transposon mutagenesis of G. polyisoprenivorans. (i) Plasmids suitable for transfer of foreign DNA into several members of the genus Gordonia by electroporation or conjugation have been reported (3). These plasmids are derivatives of plasmid pNC903, originally isolated from Rhodococcus rhodochrous (29). Sequence comparison related the origin of replication (oriV) of pNC903 to oriV of the mycobacterial plasmid pAL5000 (40), and the thermosensitive, transposable derivative pCG79 of plasmid pAL5000 was characterized with regard to replication and transposition in G. polyisoprenivorans VH2. Following in vitro mutagenesis of the pAL5000 derivative and Mycobacterium-E. coli shuttle plasmid pB4, two plasmids were isolated as thermosensitive replicons (22): pCG59 and pCG63 replicated at 30°C, but not at temperatures above 39°C. Insertion of the artificial IS6100 derivative Tn611 into plasmid pCG63 yielded vector pCG79 for generation of insertional mutant libraries of Mycobacterium smegmatis (23). Like other members of the IS6 family, IS6100 was found to transpose by a replicative mechanism. Thus, plasmid pCG79 integrates completely during transposition by duplication of one of the two copies of IS6100 (33). (ii) Tn5096 is an artificial derivative of IS493 originally isolated from Streptomyces lividans that confers apramycin resistance (35, 49). IS493 contains two open reading frames that are likely to encode proteins. Whereas ORFA has no known function and is not required for transposition, ORFB probably encodes the transposase mediating transposition in many species of Streptomyces (6). IS493 derivatives transpose randomly throughout the genome, although some sequence specificity for the target sites was observed (7). Thus, Tn5096 was cloned into pBluescript SK−, yielding pMA5096, and was tested for suitability for insertional mutagenesis in G. polyisoprenivorans. (iii) Besides pCG76 (Tn611) and pMA5096 (Tn5096), Tn5 and Tn10 were also examined for transposition in G. polyisoprenivorans.
MATERIALS AND METHODS
Bacterial strains, plasmids, and cultivation conditions.
The bacteria and plasmids used in this study are listed in Table 1. The mutants of Gordonia polyisoprenivorans strain VH2 obtained by transposon mutagenesis are listed in Tables 2, 3, and 4. If not otherwise explicitly mentioned, G. polyisoprenivorans was grown at 30°C on standard I complex nutrient broth (St-I, Merck, Darmstadt, Germany), whereas Escherichia coli was cultivated at 37°C in Luria Bertani broth (LB). For growth experiments with synthetic poly(cis-1,4-isoprene) (average molecular size, 800 kDa; Aldrich, Steinheim, Germany) as the sole carbon source, G. polyisoprenivorans was cultivated at 30°C on mineral salts medium (MSM) (45). Antibiotics were applied according to Sambrook et al. (44) or as indicated in the text. Carbon sources were added to MSM as indicated in the text. MSM-IR-sandwich agar plates for screening of mutants are described below. Liquid cultures were done in Erlenmeyer flasks and incubated on a horizontal rotary shaker. Solid media were prepared by addition of agar-agar (15 g/liter).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Escherichia coli | ||
| XL1-Blue | recA1 endA1 gyrA96 thi1 hsdR17 (rK− mK+) supE44 relA1 λ−lac [F′ proAB lacIqlacZΔM15 Tn10(Tcr)] | 17 |
| S17-1 | thi1 proA hsdR17 (rK− mK+) recA1 tra gene of plasmid RP4 integrated into the chromosome | 47 |
| SM10(λpir) | thi-1 thr leu tonA lacY sipE recA::RP-4-2Tc::Mu Kmr λpir | 37 |
| Gordonia polyisoprenivorans VH2 | Wild type, rubber degrading | DSM44266 (2) |
| Plasmids | ||
| pBluescript SK− | lacPOZ′, Apr | Stratagene, San Diego, Calif. |
| pCG79 | Thermosensitive oriV, containing Tn611, Kmr Smr | 23 |
| pCZA163 | Contains Tn5096, Apr Aprar | |
| pLOFkm | Contains Tn10, Apr Kmr, requires λpir E. coli strains for replication | 26 |
| pMA5096 | Contains Tn5096, Apr Aprar | This study |
| pSUP5011 | Contains Tn5, mobilizable, Apr Cmr Kmr | 47 |
TABLE 2.
Genetic characterization of auxotrophic mutants of G. polyisoprenivorans obtained by pMA5096-mediated insertional mutagenesis
| Mutant | Insertion locus | Accession no. | Identical amino acids (%) | E value |
|---|---|---|---|---|
| A3-5-22 | argD, putative acetylornithine transaminase, Mycobacterium avium subsp. paratuberculosis k10 | AAS03681 | 108/157 (69) | 7e-46 |
| A16-03-05 | argF, putative ornithine-carbamoyltransferase, Mycobacterium tuberculosis CDC1551 | NP_336149 | 65/107 (60) | 1e-24 |
| A17-25-12 | argC, putative N-acetyl-gamma-glutamyl-phosphate reductase, Corynebacterium efficiens YS-314 | NP_738136 | 23/53 (43) | 0.18 |
| A29-56-30 | aspC, putative aspartate transaminase, Corynebacterium efficiens YS-314 | BAC19471 | 76/96 (79) | 2e-40 |
| A32-45-07 | Putative ino1, inositol-3-phosphate synthase, Nocardia farcinica IFM10152 | BAD60404 | 149/227 (66) | 4e-54 |
| A33-45-50 | Putative aminotransaminase, Streptomyces coelicolor A3(2) | NP_629136 | 21/33 (64) | 4.2 |
| A39-81-47 | aspC, putative aspartate transaminase, Nocardia farcinica IFM10152 | BAD60279 | 156/208 (75) | 8e-90 |
| B37-79-25 | Noncoding region between putative tetR regulators, Mesorhizobium loti MAFF303099 and putative ribulose-5-phosphate 4-epimerase, Rubrobacter xylanophilus DSM 9941 | ZP_00199572 | 39/83 (47) | 9e-9 |
| C8-69-39 | aspC, putative aspartate transaminase, Nocardia farcinica IFM10152 | BAD60279 | 50/65 (77) | 2e-20 |
TABLE 3.
Pigmentation mutants of G. polyisoprenivorans obtained by pMA5096-mediated insertional mutagenesis
| Mutant | Phenotype compared to wild type | Insertion locus | Accession no. | Identical amino acids (%) | E value |
|---|---|---|---|---|---|
| 38-33 | Brighter, yellow | Next to putative crtA, spheroidene monooxygenase, taxonomically undetermined marine bacterium strain P99-3 | BAC77674 | 37/90 (41) | 4e-13 |
| F1 | Dark orange, without light induction | Putative anti-anti-sigma regulatory factor, Geobacter sulfurreducens PCA; GSU1427 | NP_952479 | 17/54 (31) | 0.17 |
| Transcription initiation sigma factor crtS, Streptomyces setonii | S55033 | 31/68 (53) | 2e-8 | ||
| 34-07 | White | crtI, phytoene desaturase, Gordonia sp. strain TM 414 | BAC75676 | 107/201 (53) | 1e-49 |
TABLE 4.
Characterization of rubber-deficient mutants of G. polyisoprenivorans obtained by pMA5096-mediated insertional mutagenesis
| Mutant | Phenotypea | Insertion locus | Accession no. | Identical amino acids (%) | E value |
|---|---|---|---|---|---|
| A1-1-44 | IR+/−, GA− | Hypothetical protein, Corynebacterium efficiens YS-314 | BAB99514 | 15/32 (46) | 0.023 |
| A32-S | IR− | iscA, HesB-like protein, Mycobacterium avium subsp. paratuberculosis k10 | AAS04261 | 35/48 (72) | 1e-09 |
| A46-51-33 | IR+/− | mmsA, putative methylmalonate semialdehyde dehydrogenase, Mycobacterium avium subsp. paratuberculosis k10 | AAS06765 | 220/288 (76) | e-116 |
| B9-27-27 | IR+/−, GA− | Putative LuxR-family transcriptional regulator, Streptomyces avermitilis MA-4680 | BAC75042 | 42/129 (36) | 0.009 |
| Putative integral membrane protein, Streptomyces coelicolor A3(2) | CAB88464 | 29/63 (46) | 1e-04 | ||
| B31-72-50 | IR+/−, GA− | Putative recR, Mycobacterium avium subsp. paratuberculosis k10 | AAS02633 | 130/203 (64) | 2e-36 |
| D21-94-19 | IR−, GA−, Sq−, Ph− | mcr, putative α-methylacyl-CoA racemase, Nocardia farcinica IFM10152 | BAD60217 | 44/69 (63) | 3e-16 |
| K8-77-41 | IR− | Putative oxidoreductase Mycobacterium tuberculosis CDC1551 | NP_334806 | 159/384 (41) | 7e-67 |
| J38-58-40 | IR− | Putative Na+/H+ antiporter, Streptomyces coelicolor A3(2) | SCO5246 | 29/104 (27) | 0.007 |
GA, geranylacetone; Sq, squalene; Ph, phytol; −, negative; +/−, leaky.
Isolation, analysis, and manipulation of DNA.
Plasmid DNA was prepared from crude cell lysates by the alkaline extraction method (12). Before lysis, cells of G. polyisoprenivorans were incubated in the presence of lysozyme (2 mg/ml) for at least 2 h at 37°C. Total DNA of Gordonia was prepared as described by Ausubel et al. (5), modified as follows: cells of 50-ml cultures were harvested by centrifugation and suspended in a mixture of 8.5 ml TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and 1 ml lysozyme solution (10 mg/ml in TE buffer). After 2 h incubation at 37°C, 500 μl of a sodium dodecyl sulfate (SDS) solution (100 g/liter) and 50 μl of a proteinase K solution (20 mg/ml in TE buffer) were added and mixed gently. After additional incubation at 37°C for 1 h, 5 ml 5 M NaCl and 1.5 ml of a CTAB solution (100 g hexadecyltrimethyl ammonium bromide per liter of 0.7 M NaCl) were added, and the solution was incubated for 20 min at 65°C. DNA was restricted with restriction endonucleases (Gibco/BRL, Gaithersburg, MD) as mentioned in the text under the conditions recommended by the manufacturer. All other genetic procedures and manipulations were conducted as described by Sambrook et al. (44).
Transfer of transposon containing plasmids to G. polyisoprenivorans.
To examine the ability of transposons to integrate into the G. polyisoprenivorans genome, the transposon-carrying plasmids were transferred to G. polyisoprenivorans. Plasmids pLOFKm and pSUP5011 were transferred by conjugation from E. coli strain SM10(λpir) or S17-1, respectively (3). For selection of potential transposon-induced mutants, mating mixtures were plated on MSM agar plates containing 0.2% (wt/vol) sodium acetate and kanamycin (50 μg/ml). Plasmids pCG76 and pMA5096 were transferred by electrotransformation to G. polyisoprenivorans according to a previously described protocol (3).
Screening for mutants defective in poly(cis-1,4-isoprene) utilization.
Mutants were characterized for their ability to degrade rubber on MSM-IR-sandwich agar plates. MSM agar plates were overlaid with 3 ml of a 2% (wt/vol) solution of poly(cis-1,4-isoprene) (IR) in chloroform. After evaporation of the solvent, the remaining IR film was covered with a layer of about 1 to 2 mm of MSM agar. Mutants were transferred by toothpicks through the top MSM agar layer into the IR film to provide cells with direct contact to the solid substrate.
Mapping of transposon insertions.
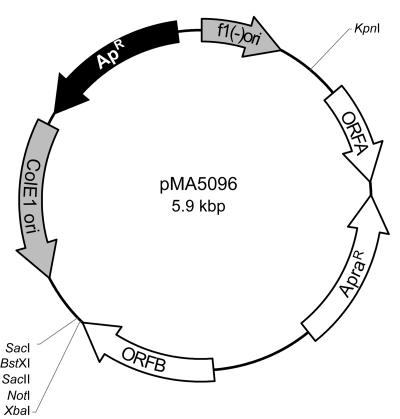
To map insertions of pMA5096 (Fig. 1) in G. polyisoprenivorans strain VH2 mutants, total genomic DNA was digested with ApaI and transferred to E. coli after religation. The resulting plasmids were used as the template for sequencing together with 5-IRD800-labeled oligonucleotides (MWG-Biotech, Ebersberg, Germany) Tn5096up 5′-ACGACCGCTCGCGCTGATCCA-3′, Tn5096down 5′-CCCGCGTCACGGTCCTCGTCGTCC-3′, Usp 5′-AGGGTTTTCCCAGTCACGACGTT-3′, and Rsp 5′-GAGCGGATAACAATTTCACACAGG-3′ as primers. The DNA sequences of the insertion loci were characterized by BLASTX computational analysis (1).
FIG. 1.
Molecular organization of transposable plasmid pMA5096 comprising Tn5096. Unique restriction sites and relevant structural genes: Apr, ampicillin resistance gene; Aprar, apramycin resistance gene; ORFA, unknown function; ORFB, transposase encoded by Tn5095; ColE1 ori, plasmid origin of replication for E. coli used in the absence of helper phage; f1(−) ori, f1 filamentous phage origin of replication. Arrows indicate the 5′ to 3′ direction of the coding regions.
DNA sequence analysis.
DNA sequences were determined with IRD800-labeled primers by using the SequiTherm EXCEL II Long-Read L-C kit and a Li-COR model 4200 sequencer (LI-COR Biosciences, Lincoln, NE).
Lipid analysis.
Fatty acid analysis of total lipid extracts of whole cells was done by gas chromatography (52) in combination with mass spectrometry (GC/MS). For this, 10 to 15 mg of lyophilized cells were subjected to methanolysis for 4.5 h at 100°C in the presence of 15% (vol/vol) sulfuric acid suspended in methanol and chloroform. The resulting fatty acid methyl esters were analyzed by GC/MS on a series 68901 GC system equipped with a series 5973 electron ionization mass selective detector (Hewlett Packard, Waldbronn, Germany). A 3-μl portion of the organic phase was analyzed after splitless injection onto a BP21 capillary column (50 m by 0.22 mm; film thickness of 250 nm) (SGE) using helium (constant flow of 0.6 ml min−) as the carrier gas. The temperatures of the injector and detector were 250 and 240°C, respectively. The following temperature program was applied: 120°C for 5 min, increase of 3°C min−1 to 180°C, and increase of 10°C min−1 to 220°C for 31 min. Data were evaluated by using the National Institute of Standards and Technology Mass Spectral Search program (50).
RESULTS AND DISCUSSION
Four transposons were examined for the ability to generate transposon insertion mutants of G. polyisoprenivorans strain VH2.
Tn5 and Tn10.
No kanamycin-resistant colonies of G. polyisoprenivorans were obtained upon conjugational transfer of pLOFKm or pSUP5011. Since DNA transfer by conjugation was demonstrated to occur between E. coli S17-1 and G. polyisoprenivorans with recipient transfer frequencies of up to 5 × 10−6 (3), and since both transposons originate from gram-negative bacteria, the transposases are probably not properly expressed in G. polyisoprenivorans. Tn5 and Tn10 were not suitable for in vivo construction of insertional mutant libraries of this strain. Therefore, transposons occurring in gram-positive bacteria with high G+C contents were investigated for their applicability in G. polyisoprenivorans.
Transposition mediated by pCG79.
The thermosensitive plasmid pCG79 was transferred to G. polyisoprenivorans strain VH2 by electroporation, and transformants were selected at 30°C on St-I agar plates containing kanamycin (50 μg/ml) and streptomycin (20 μg/ml). Plasmid DNA was isolated from 10 randomly chosen transformants and their restriction patterns were analyzed. All transformants harbored pCG79, indicating autonomous replication of this plasmid in G. polyisoprenivorans at 30°C. For selection of transposon-induced mutants, transformants were grown in St-I medium with kanamycin and streptomycin for 48 h at 30°C, the temperature was shifted to 40°C for 24 h, and the cultures were then plated at appropriate dilutions on MSM agar plates containing 0.2% (wt/vol) sodium acetate and both antibiotics. After 5 to 6 days, kanamycin- and streptomycin-resistant colonies appeared at 40°C. Plasmid pCG79 could not be isolated from 17 randomly chosen mutants, indicating loss of the vector or integration of pCG79 or Tn611 into the genome.
The end product of Tn611 transposition was previously shown to be a cointegrate molecule resulting from integration of the transposon-carrying vector into the chromosome and one additional copy of the IS6100 sequence (23). Southern hybridization employing PstI-restricted genomic DNA of these 17 mutants and digoxigenin-labeled DNA of complete pCG79 DNA as the probe revealed integration of pCG79 into the genome of VH2 at random sites (data not shown). Similar results were obtained with integrational mutants of M. smegmatis (23).
Approximately 25,000 mutants were screened for defects in utilization of poly(cis-1,4-isoprene) on MSM-IR sandwich agar plates. After 2 weeks of incubation at 30°C, 19 mutants were identified that exhibited a phenotype distinguishable from that of the wild type, because cells did not propagate along the IR interlayer, indicating a defect in rubber utilization. The putative mutants were then cultivated in liquid MSM containing 0.5% (wt/vol) poly(cis-1,4-isoprene) to confirm a phenotype in rubber utilization. All mutants started growth on IR with a delay of approximately 24 to 48 h; however, after this lag phase cell densities similar to those with the wild type were obtained. The mutations were rather unstable, and some cells reverted to the wild-type phenotype during the initial phase of incubation.
One mutant was characterized regarding excision of pCG79 in more detail. After 11 days incubation at 30°C, approximately 50% of the cells of this mutant culture grown on rubber contained extrachromosomal pGC79 DNA. Therefore, pCG79-mediated insertional mutants were not stable at 30°C. At least two types of recombination events affect the stability of IS6100 cointegrates. Recombination between two copies of IS6100 may result in loss of the integrated plasmid, leaving one copy of the insertion sequence as a footprint at the original site of cointegration. Reversion of a mutation may also occur by recombination of the direct repeats of target DNA which are generated during transposition, yielding precise excision of the cointegrate. The frequency of excision of IS6100 cointegrates in amino acid-auxotrophic mutants of Streptomyces avermitilis yielding the wild-type phenotype ranged from 10−3 to 10−9 (54). Since G. polyisoprenivorans VH2 does not grow at 40°C with poly(cis-1,4-isoprene) as a carbon source, excision of the thermosensitive vector pCG79 could not be prevented, and transposon-induced mutagenesis employing pCG79 is hardly applicable to this strain.
Transposition of Tn5096 in G. polyisoprenivorans.
Since our attempts to generate stable transposon-induced mutants applying the above-mentioned plasmids failed, transposon Tn5096 was investigated for its ability to transpose in G. polyisoprenivorans. Therefore, a KpnI-XbaI DNA fragment comprising all the genes of Tn5096 was inserted into KpnI- and XbaI-digested pBluescript SK− plasmid DNA, yielding plasmid pMA5096.
When pMA5096 was introduced into cells of G. polyisoprenivorans by electroporation, apramycin-resistant colonies were observed after 5 to 6 days. Total genomic DNA was isolated from five clones, restricted with KpnI, and examined by Southern hybridization employing Tn5096 as the probe. Every mutant exhibited a single DNA fragment, varying in size, which hybridized with Tn5096, while DNA fragments of the wild type did not hybridize (data not shown). Therefore, the apramycin-resistant clones of G. polyisoprenivorans represented transposon-induced mutants.
For three randomly chosen mutants, the insertion loci of Tn5096 were determined. Total genomic DNA was digested with KpnI, ligated to KpnI-linearized pBluescript SK− DNA, and the ligation mixtures were transformed into E. coli XL1-Blue. Surprisingly, each recombinant ampicillin- and apramycin-resistant clone obtained from these ligation mixtures of genomic DNA from the three G. polyisoprenivorans mutants harbored only one KpnI restriction fragment. However, the sizes of the single KpnI fragments varied depending on the mutant. Therefore, Tn5096 had mediated integration of the entire plasmid pMA5096. To verify this assumption and to map the insertion sites of pMA5096, DNA regions adjacent to the inverted repeats in religated KpnI restriction fragments recovered from the mutants were sequenced. BLASTX analysis revealed that plasmid pMA5096 had inserted into different sites of the genome in all three mutants.
Therefore, pMA5096 is suitable for mapping genes in G. polyisoprenivorans VH2 by integrational mutagenesis. About 25,000 transposon mutants were then generated with plasmid pMA5096. Mutants were selected on St-I agar plate containing apramycin (50 μg/ml) and subsequently characterized for their ability to degrade rubber, squalene, acetonylacetone, and hexadecane and to grow prototrophically.
Auxotrophic mutants.
The insertion loci of pMA5096 were mapped in nine auxotrophic mutants as mentioned above (Table 2). The wild-type phenotype of six auxotrophic mutants which were defective in amino acid metabolism, as revealed by determination of pMA5096 insertion loci, was restored by supplementation of MSM with an amino acid mixture (vitamin-free casein hydrolysate, Merck, Darmstadt, Germany). Supplementation of single amino acids revealed that mutants A3-5-22, A16-03-05, and A17-25-12 required arginine, whereas mutants A29-56-30, A39-81-47, and C8-69-39 required a mixture of tryptophan, phenylalanine, and aspartate. Therefore, the genotypes corresponded to the observed phenotypes. In total, 0.4% of all mutants exhibited an auxotrophic phenotype. This proportion was congruent with values described previously for transposition of Tn5096 in Streptomyces griseofuscus (48).
Mutants altered in carotenoid biosynthesis.
Various mutants exhibiting a colony pigmentation deviating from the orange color of the wild-type VH2 were also obtained (Table 3). Some mutants grew as white colonies and were unable to synthesize carotenoids; the phenotype of these mutants was therefore referred to as carotenoid negative. Colonies of some other mutants were less intensively pigmented and therefore exhibited a carotenoid-leaky phenotype. A third group of mutants noticeably overproduced carotenoids, as indicated by the dark orange color of their colonies; the phenotype of these mutants was therefore referred to as carotenoid overproducing. These mutants also turned orange in the dark, whereas the coloration of wild-type colonies required exposure to light. Therefore, these VH2 mutants were probably deregulated in carotenoid biosynthesis.
The integration loci were mapped in mutants 34-07, 38-45, and F1 (Table 3). In the carotenoid-negative mutant 34-07, pMA5096 was mapped in a putative phytoene desaturase gene (crtI). Typically, crtI genes are clustered with genes involved in early steps of carotenoid biosynthesis. CrtI catalyzes a three- or four-step desaturation of phytoene to neurosporene and lycopene (25). Downstream of and colinear with crtI, a putative isopentenyl diphosphate isomerase gene (idi) was identified in the genome of strain VH2. In the carotenoid-leaky mutant 38-33, pMA5096 was mapped in the immediate neighborhood of a putative spheroidene monooxygenase gene (crtA). Spheroidene monooxygenases are involved in later steps of carotenoid biosynthesis; they catalyze the conversion of spheroidene to spheroidenone (20) and of spheroidene-OH to spheroidenone-OH (51) and, in Rhodospirillum rubrum, of spirilloxanthin to 2-ketospirilloxanthin as well (39). In the carotenoid-overproducing mutant F1, pMA5096 was mapped upstream of an ORF putatively encoding a transcription initiation factor. Several reasons may explain overproduction of carotenoids in these mutants (7, 36). Further analyses will be required.
Mutants defective in poly(cis-1,4-isoprene) utilization.
Many of the approximately 25,000 mutants grew poorly or not at all on MSM-IR-sandwich agar plates. The colonies spread not at all or much less than the wild-type colonies along the polyisoprene interlayer. During a second screening of all mutants primarily identified on MSM-IR-sandwich agar plates, many candidates again exhibited the wild-type phenotype. Finally, six mutants remained which reproducibly did not grow in liquid poly(cis-1,4-isoprene) medium (Table 4) and therefore exhibited a rubber-negative phenotype. In addition, two mutants exhibiting a rubber-leaky phenotype were obtained. All mutants grew on acetate and hexadecane like wild-type G. polyisoprenivorans.
Mutant D21.
In this rubber-negative mutant, pMA5096 was mapped upstream of a gene putatively encoding an α-methylacyl coenzyme A racemase (Mcr). This mutant was also defective in utilization of methyl-branched isoprenoid compounds such as geranylacetone, phytol, and squalene, whereas it grew like the wild type on n-hexadecane, indicating that the Mcr homologue is specifically required for degradation of isoprenoid compounds in G. polyisoprenivorans. Recently, Sakai et al. (43) described an Mcr isolated from Mycobacterium sp. strain P101 and proposed a pathway for complete degradation of isoprenoid alkanes. Growth on methyl-branched alkanes such as pristane, phytane, and squalane was impaired in an mcr disruption mutant. Purified Mcr of Mycobacterium sp. strain P101 catalyzed the conversion of the (R) isomer of 2-methylpentadecanoyl- coenzyme A to the (S) isomer. During β-oxidation, the desaturation step of α-methylacyl- coenzyme A is stereospecific for the (S) configuration, and the (R) isomer was proposed to inhibit this reaction (43).
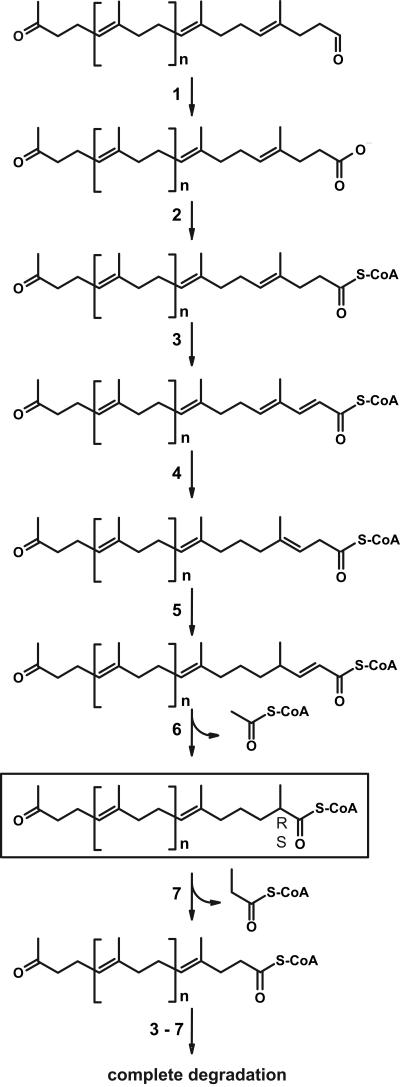
12-Oxo-4,8-dimethyltrideca-4,8-diene-1-al (ODMTD) was previously unequivocally identified as the major cleavage product of poly(cis-1,4-isoprene) in Xanthomonas sp. strain 35Y together with other structurally related metabolites differing from ODMTD only in the number of repetitive isoprene units between the terminal functions CHO-CH2O and OCH2-COCH3 (15). Degradation products with similar oligomer patterns also occurred in rubber-degrading cultures of Streptomyces coelicolor 1A and Streptomyces griseus 1D (13, 14). ODMTD and degradation products exhibiting additional isoprene units between terminal functions differ from isoprenoid alkanes such as pristane, phytane, and squalane mainly by the presence of carbon-carbon double bounds. An extension of the proposed β-oxidation pathway of pristane in Mycobacterium sp. strain P101 by including enzymatic steps analogous to degradation of fatty acids with cis-double bonds at even-numbered carbon atoms (Fig. 2, steps 4 and 5) may provide a reasonable pathway for degradation of the products resulting from cleavage of poly(cis-1,4-isoprene).
FIG. 2.
Proposed β-oxidation pathway for the degradation of intermediates resulting from enzymatic cleavage of poly(cis-1,4-isoprene), such as 12-oxo-4,8-dimethyltrideca-4,8-diene-1-al. One molecule of acetyl-CoA and one molecule of propionyl-CoA are released alternately during β-oxidation. Mcr is supposed to catalyze the conversion of the (R) isomer of a hypothetical 2-methyl-branched intermediate (marked with a frame) to the (S) isomer to enable desaturation during β-oxidation (modified according to Sakai et al. [43]).
The functional aldehyde group of the cleavage products of poly(cis-1,4-isoprene) may be oxidized to a carboxyl group (Fig. 2, step 1), which is then linked to coenzyme A (Fig. 2, step 2). We recently identified two genes coding for a putative heterodimeric molybdenum hydroxylase (OxiAB); presumably, these gene products are involved in oxidation of isoprenoid aldehydes in Streptomyces sp. strain K30 (42). Before acetyl-coenzyme A is separated during the first cycle of β-oxidation (step 6), two additional enzymatic steps analogous to degradation of unsaturated fatty acids (steps 4 and 5) are required to form the 2-enoyl-coenzyme A. Since only the (S) isomer of the resulting α-methylacyl-coenzyme A serves as a substrate for acyl-coenzyme A dehydrogenases, (R) isomers have to be racemized by Mcr. Subsequently, propionyl-coenzyme A is released after a second cycle of β-oxidation. Steps 3 to 7 are then repeated until complete degradation has occurred. Potentially, short-chain methyl-branched intermediates are also produced. These may be further degraded by MmsA (see below).
Mutant A46-51-33.
In this rubber-leaky mutant, pMA5096 was mapped in a gene coding for a protein exhibiting high similarities to putative methylmalonate semialdehyde dehydrogenases (MmsA, EC 1.2.1.27). MmsA catalyzes the oxidative decarboxylation of methylmalonate semialdehyde to propionyl-CoA during degradation of branched-chain amino acids via isobutyric acid. It also converts propanal to propionyl-CoA and malonate semialdehyde to acetyl-CoA (9, 21). Complete degradation of 2,4-dinitrotoluene by Burkholderia cepacia R34 also depends on MmsA (28). 2,4-Dinitrotoluene is converted by several enzymes to 2,4-dihydroxy-5-methyl-6-oxo-2,4-hexa-dienoic acid, which is than cleaved into pyruvate and methylmalonate semialdehyde. Thus, mutants defective in mmsA are only able to partially utilize 2,4-dinitrotoluene as a source of carbon and energy.
Interestingly, mutant A46-51-33 exhibited reduced growth on MSM-IR-sandwich agar plates but did grow like the wild type in liquid poly(cis-1,4-isoprene) medium. Mutants of G. polyisoprenivorans which degrade rubber only partially are expected to exhibit a rubber-leaky phenotype for two reasons: (i) the carbon source is only partially converted into biomass and energy, and (ii) the cells require direct contact with the rubber substrate during degradation, and since poly(cis-1,4-isoprene) contained in MSM-IR-sandwich agar plates is solid and therefore immobile, fewer cells are able to establish contact with the rubber substrate. In contrast, cells can establish this contact much better in liquid cultures.
Mutant K8.
In this rubber-negative mutant, pMA5096 was mapped in a gene encoding an oxidoreductase. The deduced amino acid sequence exhibited homologies to probable mono- and dioxygenases and putative flavohemoproteins and presumably comprised a globin (pfam00042), an oxidoreductase flavin adenine dinucleotide-binding (pfam00970), and a 2-polyprenylphenol hydroxylase (UbiB, COG0543) domain; no signal peptide could be detected employing the software Signal P 3.0 (11). The function of this gene has to be assigned in further studies.
Mutants A1-1-44 and A32-S.
In the rubber-leaky mutant A1-1-44, pMA5096 was mapped in a gene whose translational product exhibited significant identities to hypothetical proteins exclusively present in Rhodococcus sp. strain RHA1, Corynebacterium glutamicum, and Corynebacterium efficiens. In the last, this ORF is colocated with a gene putatively encoding NifS, a cofactor of iron-sulfur (Fe-S) cluster biogenesis. In the rubber-negative mutant A32-S, pMA5096 was mapped in the promoter region of a gene coding for a protein highly homologous to HesB/IscA-like proteins of a variety of prokaryotes. Interestingly, a cobU homologue putatively encoding an adenosylcobinamide kinase was localized upstream of this gene. In mutant B31-72-50, a gene involved in cobalamin biosynthesis was also identified close to the insertion locus (see below). Therefore, a polar effect on cobU resulting from integration of pMA5096 may also affect rubber biodegradation by impairing vitamin B12 biosynthesis.
Krebs et al. (31) proposed a mechanistic scheme for NifS-directed Fe-S cluster assembly on IscA. More than 120 distinct types of Fe-S cluster-containing proteins belong to these most ancient, ubiquitous, and functionally diverse prosthetic groups and they are components of many proteins with redox, regulatory, or catalytic function (10). Although Fe-S-containing proteins have been the focus of extensive research, the process of cluster biosynthesis is not completely understood (30). Basic studies were carried out characterizing the organization and function of nitrogen-fixing (nif) genes in Azotobacter vinelandii. It was shown that the nifS and nifU gene products are essential for optimal assembly of Fe-S clusters in the nitrogenase proteins (18, 27). Homologues of both genes were later identified in A. vinelandii and E. coli as parts of widely conserved operons involved in general Fe-S cluster biosynthesis (55). IscA mediates iron delivery for assembly of Fe-S clusters on IscU in E. coli under limited free iron conditions (19) since iscA mutants failed to recruit intracellular iron during iron-limited conditions, resulting in decreased but not lethal biosynthesis of Fe-S clusters (53). A mutation in iscA may therefore only have an effect under conditions requiring large amounts of Fe-S cluster-containing enzymes.
The involvement of enzymes with redox functions is not unexpected in rubber degradation, and deficiencies in Fe-S cluster biogenesis could negatively affect rubber degradation in an iscA mutant. We recently described the identification of genes involved in early steps of poly(cis-1,4-isoprene) catabolism in Streptomyces sp. strain K30 (42). Downstream of the gene encoding the putative rubber-cleaving enzyme Lcp (latex-clearing protein), two genes coding for the subunits of a putative heterodimeric molybdenum hydroxylase (OxiAB) were identified in this bacterium. The amino acid sequence deduced from oxiA exhibited eight highly conserved cysteine residues with putative Fe-S cluster binding function. If OxiAB is responsible for further oxidation of isoprenoid aldehydes resulting from oxidative cleavage of poly(cis-1,4-isorene), mutants defective in Fe-S cluster assembly could exhibit reduced rubber-degrading activity if the situation is similar in G. polyisoprenivorans.
Mutant B31-72-50.
In this rubber-negative mutant, pMA5096 was mapped in a gene putatively encoding a RecR homologue. RecR together with RecF and RecO facilitates loading of RecA in the RecF pathway of homologous recombinational DNA repair in prokaryotes (32). RecR-deficient mutants of S. coelicolor exhibited increased sensitivity to DNA damage by UV irradiation and methyl methanesulfonate (38). Since rubber degradation is probably initiated by activated oxygen, reactive oxygen species may occur during rubber cleavage at higher concentrations than during degradation of other carbon sources. It is known that reactive oxygen species cause damage, in particular to DNA (34). However, a rubber-negative phenotype caused by RecR inactivation was surprising.
A putative cobyric acid synthase (EC6.3.510)-encoding gene (cobQ) was localized downstream of recR in G. polyisoprenivorans. CobQ catalyzes the four-step amidation sequence from cobyrinic acid a,c-diamide to the cobyric acid pentaamide intermediate in cobalamin (vitamin B12) biosynthesis (46). Cobalamin is required for the activity of several enzymes catalyzing intramolecular rearrangements of carbon-carbon bonds (8, 24) and may also be required during rubber biodegradation e.g., for rearrangement of the methyl groups. Insertion of pMA5096 in recR could cause a polar effect on cobQ expression, negatively affecting cobalamin biosynthesis in this mutant. Further experiments must clarify whether recR or cobQ is relevant for rubber biodegradation.
Mutant B9-27-27.
In this rubber-negative mutant, the amino acid sequence deduced from the DNA sequence of the insertion locus of pMA5096 revealed only low homologies to a putative LuxR family transcriptional regulator followed by a gene encoding a putative integral membrane protein. This mutant may be deficient in rubber degradation due to inactivation of a transcriptional regulator required for the induction of the genes involved.
Conclusions.
This study succeeded in establishing transposon-mediated insertional mutagenesis in G. polyisoprenivorans. Applying plasmid pMA5096 harboring Tn5096, mutants exhibiting defects in various metabolic pathways were identified and characterized. Mutants defective in poly(cis-1,4-isoprene) degradation were of particular interest because rubber biodegradation is still only slightly understood (41). These mutants shed some light on the steps involved in cleavage of the poly(cis-1,4-isoprene) backbone and on the degradation of the cleavage products. Interestingly, no mutants defective in genes for homologues to the two enzymes recently identified in Xanthomonas sp. strain 35Y (15) and in Streptomyces sp. strain K30 (42), which catalyze the initial poly(cis-1,4-isoprene)-cleaving reaction step, were identified among the insertional rubber-negative or -leaky mutants obtained. Considering the large number (25,000) of independent mutants which were analyzed in this study, it would be very surprising if such a mutant was overseen. This may indicate that G. polyisoprenivorans uses a different, third type of enzyme for cleavage of poly(cis-1,4-isoprene). This will be not surprising because G. polyisoprenivorans exhibits a phenotype for rubber degradation which is clearly different from that of the two other bacteria regarding clear-zone formation. Another explanation could be the occurrence of isoenzymes in G. polyisoprenivorans and that insertional inactivation of one gene homologue is compensated by the second gene. However, there is so far no evidence for the occurrence of redundant genes involved in rubber degradation in the few other microorganisms studied. Further detailed analysis of these mutants and additional studies now have to be done to completely reveal one of the last remaining terrae incognitae regarding the microbial degradation of abundant natural resources.
Acknowledgments
We are grateful for financial support provided by the Deutsche Bundesstiftung Umwelt (Osnabrück, Germany) in the context of an ICBIO project (AZ. 13072).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenskötter, M., D. Baumeister, M. M. Berekaa, G. Pötter, R. M. Kroppenstedt, A. Linos, and A. Steinbüchel. 2001. Taxonomic characterization of two rubber degrading bacteria belonging to the species Gordonia polyisoprenivorans and analysis of hyper variable regions of 16S rDNA sequences. FEMS Microbiol Lett. 205:277-282. [DOI] [PubMed] [Google Scholar]
- 3.Arenskötter, M., D. Baumeister, R. Kalscheuer, and A. Steinbüchel. 2003. Identification and application of plasmids suitable for transfer of foreign DNA to members of the genus Gordonia. Appl. Environ. Microbiol. 69:4971-4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arenskötter, M., D. Bröker, and A. Steinbüchel. 2004. Biology of the metabolically diverse genus Gordonia. Appl. Environ. Microbiol. 70:3195-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struni. 1987. Current protocols in molecular biology, vol. 1, 1st ed. John Wiley & Sons, New York, N.Y.
- 6.Baltz, R. H., D. R. Hahn, M. A. McHenney, and P. J. Solenberg. 1992. Transposition of Tn5096 and related transposons in Streptomyces species. Gene 115:61-65. [DOI] [PubMed] [Google Scholar]
- 7.Baltz, R. H., M. A. McHenney, and P. J. Solenberg. 1993. Properties of transposons derived from IS493 and application in streptomycetes, p. 51-56. In R. H. Balt et al. (ed.), Industrial microorganisms: basic and applied molecular genetics. American Society for Microbiology, Washington, D.C.
- 8.Banerjee, R., and M. Vlasie. 2001. Controlling the reactivity of radical intermediates by coenzyme B12-dependent methylmalonyl-CoA mutase. Biochem. Soc. T. 30:621-624. [DOI] [PubMed] [Google Scholar]
- 9.Bannerjee, D., L. E. Sanders, and J. R. Sokatch. 1970. Properties of purified methylmalonate semialdehyde dehydrogenase of Pseudomonas aeruginosa. J. Biol. Chem. 245:1828-1835. [PubMed] [Google Scholar]
- 10.Beinert, H., and P. J. Kiley. 1999. Fe-S proteins in sensing and regulatory functions. Curr. Opin. Chem. Biol. 3:152-157. [DOI] [PubMed] [Google Scholar]
- 11.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 12.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bode, H. B., A. Zeeck, K. Pluckhahn, and D. Jendrossek. 2000. Physiological and chemical investigations into microbial degradation of synthetic poly(cis-1,4-isoprene) Appl. Environ. Microbiol. 66:3680-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bode, H. B., K. Kerkhoff, and D. Jendrossek. 2001. Bacterial degradation of natural and synthetic rubber. Biomacromolecules 2:295-303. [DOI] [PubMed] [Google Scholar]
- 15.Braaz, R., P. Fischer, and D. Jendrossek. 2004. Novel type of heme-dependent oxygenase catalyzes oxidative cleavage of rubber (poly-cis-1,4-isoprene). Appl. Environ. Microbiol. 70:7388-7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bröker, D., M. Arenskötter, A. Legatzki, D. H. Nies, and A. Steinbüchel. 2004. Characterization of the 101-kilobase-pair megaplasmid pKB1, isolated from the rubber-degrading bacterium Gordonia westfalica Kb1. J. Bacteriol. 186:212-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bullock, W. O., J. M. Fernandez, and J. M. Stuart. 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques 5:376-379. [Google Scholar]
- 18.Dean, D. R., J. T. Bolin, and L. M. Zheng. 1993. Nitrogenase metalloclusters-structures, organization, and synthesis. J. Bacteriol. 175:6737-6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding, H., J. C. R. J. Clark, and B. Ding. 2004. IscA mediates iron delivery for assembly of iron-sulfur clusters in IscU under the limited accessible free iron conditions. J. Biol. Chem. 279:37499-37504. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Asua, G., H. P. Lang, R. J. Cogdell, and C. N. Hunter. 1998. Carotenoid diversity: a modular role for the phytoene desaturase step. Trends Plant Sci. 3:445-449. [Google Scholar]
- 21.Goodwin, G. W., P. M. Rougraff, E. J. Davis, and R. A. Harris. 1989. Purification and characterization of methylmalonate-semialdehyde dehydrogenase from rat liver. Identity to malonate-semialdehyde dehydrogenase. J. Biol. Chem. 264:14965-14971. [PubMed] [Google Scholar]
- 22.Guilhot, C., B. Gicquel, and C. Martín. 1992. Temperature sensitive mutants of the Mycobacterium plasmid pAL5000. FEMS Microbiol. Lett. 98:181-186. [DOI] [PubMed] [Google Scholar]
- 23.Guilhot, C., I. Otal, I. van Rompaey, C. Martín, and B. Gicquel. 1994. Efficient transposition in mycobacteria: construction of Mycobacterium smegmatis insertional mutant libraries. J. Bacteriol. 176:535-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halpern, J. 1985. Mechanism of coenzyme B12-dependent rearrangements. Science 227:869-875. [DOI] [PubMed] [Google Scholar]
- 25.Harada, J., K. V. P. Nagashima, S. Takaichi, N. Misawa, K. Matsuura, and K. Shimada. 2001. Phytoene desaturase, CRTI, of the purple photosynthetic bacterium, Rubriviva gelatinosus, produces both neurosporene and lycopene. Plant. Cell Physiol. 42:1112-1118. [DOI] [PubMed] [Google Scholar]
- 26.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in Gram-negative bacteria. Mol. Microbiol. 5:1561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson, M. R., V. L. Cash, M. C. Weiss, N. F. Laird, W. E. Newton, and D. R. Dean. 1989. Biochemical and genetic-analysis of the nifUSVWZM cluster from Azotobacter vinelandii. Mol. Gen. Genet. 219:49-57. [DOI] [PubMed] [Google Scholar]
- 28.Johnsons, G. R., R. K. Jain, and J. C. Spain. 2002. Origins of the 2,4-dinitrotoluene pathway. J. Bacteriol. 184:4219-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalscheuer, R., M. Arenskötter, and A. Steinbüchel. 1999. Establishment of a gene transfer system for Rhodococcus opacus PD630 based on electroporation and its application for recombinant biosynthesis of poly(3-hydroxyalkanoic acids). Appl. Microbiol. Biotechnol. 52:508-515. [DOI] [PubMed] [Google Scholar]
- 30.Kiley, P. J., and H. Beinert. 2003. The role of Fe-S proteins in sensing and regulation in bacteria. Curr. Opin. Microbiol. 6:181-185. [DOI] [PubMed] [Google Scholar]
- 31.Krebs, K., J. N. Agar, A. D. Smith, J. Frazzon, D. R. Dean, B. H. Huynh, and M. K. Johnson. 2001. IscA, an alternative scaffold for FE-S cluster biosynthesis. Biochemistry 40:14069-14080. [DOI] [PubMed] [Google Scholar]
- 32.Lee, B. I., K. H. Kim, S. J. Park, S. H. Eom, H. K. Song, and S. W. Suh. 2004. Ring-shaped architecture of RecR: implications for its role in homologous recombinational DNA repair. EMBO J. 23:2029-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín, C., J. Timm, J. Rauzier, R. Gómez-Lus, J. Davies, and B. Gicquel. 1990. Transposition of an antibiotic resistance element in mycobacteria. Nature (London). 345:739-743. [DOI] [PubMed] [Google Scholar]
- 34.Martinez, A., and R. Kolter. 1997. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J. Bacteriol. 179:5188-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McHenney, M. A., and R. H. Baltz. 1991. Transposition of Tn5096 from a temperature-sensitive transducible plasmid in Streptomyces spp. J. Bacteriol. 173:5578-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McHenney, M. A., and R. H. Baltz. 1996. Gene transfer and transposition mutagenesis in Streptomyces roseosporus: mapping of insertions that influence daptomycin or pigment production. Microbiology 142:2363-2373. [DOI] [PubMed] [Google Scholar]
- 37.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutants: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peláez, A. I., R. M. Ribas-Aparicio, A. Gómez, and M. R. Rodicio. 2001. Structural and functional characterization of the recR gene of Streptomyces. Mol. Genet. Genomics 265:663-672. [DOI] [PubMed] [Google Scholar]
- 39.Pinta, V., S. Ouchane, M. Picaud, S. Takaichi, C. Aster, and F. Reiss-Husson. 2003. Characterization of unusual hydroxyl- and ketocarotenoids in Rubrivivax gelatinosus: involvement of enzyme CrtF or CrtA. Arch. Microbiol. 179:354-362. [DOI] [PubMed] [Google Scholar]
- 40.Rauzier, J., J. Moniz-Pereira, and B. Gicquel-Sanzey. 1988. Complete nucleotide sequence of pAL5000, a plasmid from Mycobacterium fortuitum. Gene 71:315-321. [DOI] [PubMed] [Google Scholar]
- 41.Rose, K., and A. Steinbüchel. 2005. Biodegradation of natural rubber and related compounds: recent insights into a rare and hardly understood catabolic capability of microorganisms. Appl. Environ. Microbiol. 71:2803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose, K., K. B. Tenberge, and A. Steinbüchel. 2005. Identification and characterization of genes from Streptomyces sp. strain K30 responsible for clear zone formation on natural rubber latex and poly(cis-1,4-isoprene) rubber degradation. Biomacromolecules 6:180-188. [DOI] [PubMed] [Google Scholar]
- 43.Sakai, Y., H. Takahashi, Y. Wakasa, T. Kotani, H. Yurimoto, N. Miyachi, P. P. van Veldhoven, and N. Kato. 2004. Role of α-methylacyl coenzyme A racemase in the degradation of methyl-branched alkanes by Mycobacterium sp. strain P101. J. Bacteriol. 186:7214-7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 45.Schlegel, H. G., H. Kaltwasser, and G. Gottschalk. 1961. Ein Submersverfahren zur Kultur wasserstoffoxidierender Bakterien: Wachstumsphysiologische Untersuchungen. Arch. Mikrobiol. 38:209-222. [PubMed] [Google Scholar]
- 46.Scott, A. I., and C. A. Roessner. 2002. Biosynthesis of cobalamin (vitamin B12). Biochem. Soc. T. 30:613-620. [DOI] [PubMed] [Google Scholar]
- 47.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in Gram-negative bacteria. BioTechnology 1:784-791. [Google Scholar]
- 48.Solenberg, P. J., and R. H. Baltz. 1991. Transposition of Tn5096 and other IS493 derivatives in Streptomyces griseofuscus. J. Bacteriol. 173:1096-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solenberg, P. J., and S. G. Burgett. 1989. Method for selection of transposable DNA and characterization of a new insertion sequence, IS493, from Streptomyces lividans. J. Bacteriol. 171:4807-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein, S., A. Levitsky, O. Fateev, and G. Mallard. 1998. The NIST mass spectral search program, version 1.6d. National Institute of Standards and Technology, Gaithersburg, Md.
- 51.Takaichi, S. 1999. Carotenoids and carotenogenesis in anoxygenic photosynthetic bacteria, p. 39-69. In H. A. Frank et al. (ed.), The photochemistry of carotenoids. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 52.Timm, A., and A. Steinbüchel. 1990. Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl. Environ. Microbiol. 56:3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tokumoto, U., and Y. Takahashi. 2001. Genetic analysis of the isc operon in Escherichia coli involved in the biogenesis of cellular iron-sulfur proteins. J. Biochem. 130:63-71. [DOI] [PubMed] [Google Scholar]
- 54.Weaden, J., and P. Dyson. 1998. Transposon mutagenesis with IS6100 in the avermectin-producer Streptomyces avermitilis. Microbiology 144:1963-1970. [DOI] [PubMed] [Google Scholar]
- 55.Zheng, L. M., V. L. Cash, D. H. Flint, and D. R. Dean. 1998. Assembly of iron-sulfur clusters -identification of an iscSUA-hesBA-fdy gene cluster from Azotobacter vinelandii. J. Biol. Chem. 273:13264-13272. [DOI] [PubMed] [Google Scholar]