Abstract
In the present study, glutaryl-7-amino cephalosporanic acid acylase from Pseudomonas sp. strain 130 (CA130) was mutated to improve its enzymatic activity and stability. Based on the crystal structure of CA130, two series of amino acid residues, one from those directly involved in catalytic function and another from those putatively involved in surface charge, were selected as targets for site-directed mutagenesis. In the first series of experiments, several key residues in the substrate-binding pocket were substituted, and the genes were expressed in Escherichia coli for activity screening. Two of the mutants constructed, Y151αF and Q50βN, showed two- to threefold-increased catalytic efficiency (kcat/Km) compared to wild-type CA130. Their Km values were decreased by ca. 50%, and the kcat values increased to 14.4 and 16.9 s−1, respectively. The ability of these mutants to hydrolyze adipoyl 6-amino penicillinic acid was also improved. In the second series of mutagenesis, several mutants with enhanced stabilities were identified. Among them, R121βA and K198βA had a 30 to 58% longer half-life than wild-type CA130, and K198βA and D286βA showed an alkaline shift of optimal pH by about 1.0 to 2.0 pH units. To construct an engineered enzyme with the properties of both increased activity and stability, the double mutant Q50βN/K198βA was expressed. This enzyme was purified and immobilized for catalytic analysis. The immobilized mutant enzyme showed a 34.2% increase in specific activity compared to the immobilized wild-type CA130.
Cephalosporin acylase (CA) (EC 3.5.1.11) is a commercially valuable enzyme, primarily due to its ability to hydrolyze cephalosporin C (CPC) and glutaryl 7-aminocephalosporanic (GL-7-ACA) to produce 7-ACA, an important starting material in the production of semisynthetic cephalosporin antibiotics. The current method used in the pharmaceutical industry to manufacture 7-ACA involves toxic chemical deacylation of CPC obtained by fermentation (6, 30). This process also utilizes many costly techniques in order to overcome environmental and ecological problems. In the past decade, enzymatic methods for deacylation have attracted more attention in the manufacturing of cephalosporin antibiotics, and several enzyme-based methods have been developed. Most common is the two-enzyme process that uses d-amino acid oxidase and CA to produce 7-ACA in sequential reactions (1). In addition, because most of the CAs studied thus far have a substrate preference of GL-7-ACA over CPC, great efforts have been made to obtain CA with high CPC affinity by screening for novel CA enzymes or by protein engineering to increase their CPC hydrolysis activity (10, 12, 13, 27, 28). The CA from Pseudomonas sp. strain N176 (N176) was reported to have the highest CPC/GL-7-ACA deacylation activity ratio with 4.0% activity to CPC compared to GL-7-ACA (3).
CAs have been categorized into five classes according to their gene structures, molecular masses, and enzymatic properties (5, 18, 19). The gene encoding a class I CA enzyme from Pseudomonas sp. strain 130 (AF085353) was cloned and expressed in Escherichia coli, and its enzyme, CA130, was characterized previously (11, 20, 21, 34-36). CA130 shows very high acylase activity toward GL-7-ACA but has only 2.3% activity toward CPC compared to that of GL-7-ACA. The crystal structures of three GL-7-ACA acylases have been independently identified by Kim et al. (PDB: 1FM2) (17), Fritz-Wolf et al. (PDB: 1GK0) (8), and our group (PDB: 1GHD). The analysis of the crystal structures indicated that CA belongs to the superfamily of the N-terminal nucleophile (Ntn) hydrolases, which is defined by SCOP (for structure classification of proteins) as containing a distinctive four-layer αββα structure motif and the N-terminal residue of the β chain serving as the nucleophile (2, 15).
The structures of CAD (CA from Pseudomonas diminuta) in complex with substrates GL-7-ACA (PDB: 1JVZ) or glutarate (PDB: 1JW0) have also been reported (16). The extensive interactions found between the glutaryl moiety of GL-7-ACA and residues that form the side chain pocket account for the substrate preference for GL-7-ACA over CPC. Site-directed mutagenesis studies have produced mutants with increased deacylation activity toward CPC. The deacylation activity of a triply mutated CAD toward CPC was increased by ca. 790%, so the activity ratio between substrates was increased to 16% of the activity of CAD toward GL-7-ACA (24). In a recent study, Fritz-Wolf et al. (8) reported that modifications to CA by site-directed mutagenesis could result in a single-step enzymatic production of 7-ACA from CPC.
In addition to activity levels, the stability of an enzyme is also an important parameter in using enzymes in various industrial bioreactors. Several strategies have been applied to engineer more stable enzymes (7, 14, 22, 25, 31). Among them, the protein engineering approach, which is generally achieved by changes to key residues by site-directed mutagenesis, is one of the most commonly used tools (4). A particularly successful example is enhancement of the stability of a penicillin G acylase (PGA) from Bacillus megaterium by site-directed mutagenesis (33). In that study, mutants were designed by modulating the three-dimensional structure of PGA. Mutants with selected polar residues substituted by Ala at the surface of PGA exhibited prolonged half-lives and enhanced stabilities in acidic or organic solvent environments.
CA130 shows its maximal specific activity in a neutral environment. In the reaction system catalyzed by CA, glutarate is produced, necessitating the addition of concentrated base solutions for pH control. This results in microalkaline environments for the enzymes (4), and both the activity and the half-life of CA130 may decrease quickly when the pH is greater than 8.0. It is therefore important to improve the stability of CA130 at alkaline pH in order to reduce the process costs in application. In an attempt to solve a similar problem, an E. coli PGA was obtained by oligonucleotide-directed random mutagenesis at a selected surface region with enhanced stability at alkaline pH (4).
We describe here progress in improving the stability and activity of CA130 toward GL-7-ACA by rational site-directed mutagenesis based on crystal structure analysis. Two series of mutants were constructed and screened. Several mutants were obtained with improved catalytic efficiency (decreased Km value and increased kcat value) toward GL-7-ACA and several mutants with higher stability than CA130.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli DH5α was used as the host strain to express the enzyme CA130. The plasmid pKKCA1 (Ampr, trc acy) (32), carrying the acy gene coding for CA130, was used for high level expression and site-directed mutagenesis. The plasmid pMFT7H6 (Ampr, T7, acy) (32) containing the natural or mutated acy gene was used to express enzymes in a large-scale fermentation for purification and immobilization (kindly provided by the laboratory of Enduo Wang).
Reagents.
7-ACA was provided by Zhejiang Haimen Pharmacy Co. GL-7-ACA, CPC, GL-6APA, and AD-6APA were synthesized in our lab (37). Oligonucleotides were synthesized by Sangon Co. Restriction endonucleases, T4 DNA ligase, and Pyrobest DNA polymerase were products of Takara Co. Protein molecular weight markers were purchased from Promega. DEAE-Sepharose CL-6B and Sephadex G75 were purchased from Pharmacia Biotech. EPI-30-IDA-Co was synthesized in our lab and used for enzyme immobilization (patent application 01818118.X [People's Republic of China]). All other reagents were AR grade.
DNA recombinant techniques.
All DNA manipulations were performed according to standard techniques. Site-directed mutagenesis was performed by using the method of megaprimer PCR (29) or overlap extension PCR (9) with plasmid pKKCA1 as the template. Mutants were confirmed by DNA sequencing (Genecore).
Enzyme purification.
Cells were collected and suspended in 100 ml of PB (50 mM sodium phosphate buffer [pH 7.0]) after growth for 14 to 16 h at 33°C. A crude cell extract was prepared by sonication and was centrifuged at 12,000 rpm for 15 min. The resultant supernatant was purified by 30 to 60% saturation ammonium sulfate in 4°C to get a protein sample that contains CA130. The dialyzed protein sample (dialysis buffer PB) was applied to DEAE-Sepharose CL-6B chromatography, which was pre-equilibrated with PB and then eluted with a linear gradient of 0 to 0.3 M NaCl in the same buffer. Fractions containing CA130 activity were collected and concentrated to a final volume of <1.0 ml and then subjected to Superdex G75 gel filtration chromatography. The column was eluted with PB (0.05 M NaCl). The final preparation of CA130 was concentrated by using an Amicon Ultra-15 centrifugal filter (Millipore) and assayed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Enzyme immobilization.
The transformant BL21(DE3)/pMFT7H6-QR (or wild-type CA130) was grown in 3 liters of LB medium (180 rpm, 33°C). IPTG (isopropyl-β-d-thiogalactopyranoside; final concentration, 0.5 mM) was added into the culture when the cell density (i.e., the optical density at 600 nm) reached 0.8 to 1.0. Cells were collected after 14 to 16 h. A crude cell extract was prepared with a French press. Enzymes were purified and immobilized by using the affinity immobilization carrier EPI-30-IDA-Co (produced by our laboratory [patented]) in one step.
Enzyme assay and characterization.
The activity and the kinetic parameters of CA130 were determined as described previously (35). One unit of enzyme activity is defined as the amount of enzyme that produces 1 μmol of 7-ACA or 6-APA per min at 37°C and pH 7.0. The optimal pH of enzyme was determined at 37°C in buffers with different pHs (pH 5 to 10).
Software.
The three-dimensional structure of CA130 was analyzed on a Silicon Graphics O2 workstation, running IRIX6.3, by using the Insight II program (ACCERLRY 1997, San Diego, CA). HBplus was used for calculating the H-bond between atoms and groups (23).
RESULTS AND DISCUSSION
Site-directed mutagenesis of key residues to improve activity.
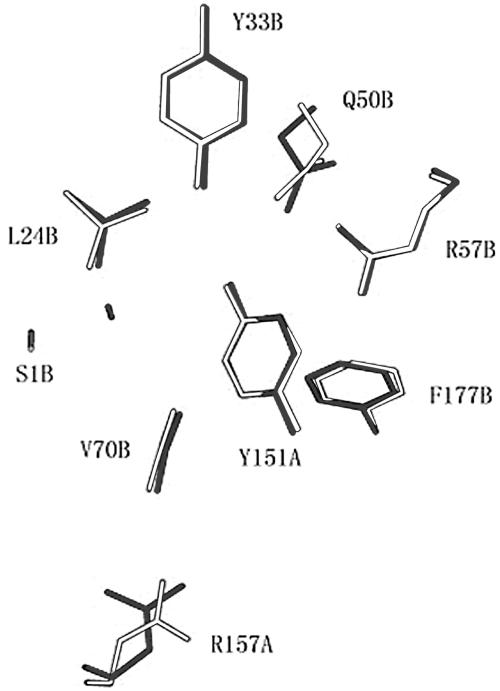
GL-7-ACA acylase CA130 from Pseudomonas sp. strain 130 is similar to CAD from P. diminuta, with a sequence identity of >90% (24), and both proteins belong to class I of the CA family. The analysis of crystal structures of GL-7-ACA acylase CAD and CAD complex with GL-7-ACA or glutarate has identified several residues that play important functions in enzyme catalysis (Tyr149α, Arg155α, Ser1β, Leu24β, Gln50β, Arg57β, Val70β, and Phe177β) in CAD (2). Superimposition of the structures of CA130 and CAD (Fig. 1) revealed that many functional amino acid residues have similar conformations, but the residues of R157α and Q50β have little overlap between CA130 and CAD even if they were located in same positions. The small degree of overlap of S1β at the active-site nucleophile implies that the sites might have slightly different positions in CA130 and in CAD.
FIG. 1.
Superimposed structures of 1GHD and 1FM2 in the GL-7ACA binding pocket. Residues from 1GHD are white; residues from 1FM2 are shaded. The labeled residues of Y151α, R157α, S1β, L24β, Y33β, Q50β, R57β, V70β, and F177β in 1GHD correspond to Y149α, R155α, S1β, L24β, Y33β, Q50β, R57β, V70β, and F177β, respectively, in 1FM2.
Significant efforts have been made to create mutated CA130 by site-directed mutagenesis. A series of mutated CA130 with key residues replaced were constructed. These residues included Tyr151α, Arg157α, Ser1β, Leu24β, Tyr33β, Gln50β, Arg57β, Val70β, and Phe177β residues of CA130. The mutated CA130s were then characterized for possible improved enzymatic activity toward GL-7-ACA. However, the assay revealed that, with the exception of two mutants, Q50βN and Y151αF, most of the mutated CA130s exhibited decreased specific activity to GL-7-ACA. When substituting Gln50β with Asn, the mutant enzyme Q50βN decreased its Km value for GL-7-ACA by ca. 50% (Table 1), a finding indicative of a strong bond with GL-7-ACA. The kcat value was also increased by ca. 42%, and the catalysis efficiency (kcat/Km) toward GL-7-ACA was improved by ∼3-fold. The Km value of another mutant enzyme, Y151αF, also decreased by ca. 50% compared to the wild-type CA130 and improved the catalysis efficiency by ∼2-fold. The total enzyme activities (per liter of culture medium) of Y151αF and Q50βN were 200 to 400 U more than wild-type CA130, and their specific activities were also improved by 22 and 48%, respectively.
TABLE 1.
Characterization of the engineered enzymesa
| Acylase | Substituted site(s) | Km (mM) | kcat (s−1) | Catalysis efficiency (kcat/Km [M−1/s−1]) | Total activity (U/liter) | Sp act (U/mg) |
|---|---|---|---|---|---|---|
| CA130 | Native acylase | 0.45 | 12.3 | 2.73 × 104 | 850 | 11.8 |
| Y151αF | Tyr151α→Phe | 0.24 | 14.4 | 6.00 × 104 | 1,050 | 14.4 |
| Q50βN | Gln50β→Asn | 0.22 | 17.9 | 8.14 × 104 | 1,250 | 17.5 |
| Y151αF/Q50βN | Tyr151α→Phe and Gln50β→Asn | 0.38 | 13.4 | 3.53 × 104 | 900 | 12.3 |
| R121βA | Arg121β→Ala | 0.45 | 12.2 | 2.71 × 104 | 840 | 11.6 |
| K198βA | Lys198β→Ala | 0.47 | 11.7 | 2.49 × 104 | 815 | 11.3 |
| D286βA | Asp286β→Ala | 0.41 | 12.7 | 3.10 × 104 | 870 | 12.0 |
| Q50βN/K198βA | Gln50β→Asn and Lys198β→Ala | 0.21 | 17.8 | 8.48 × 104 | 1,235 | 17.4 |
All of the enzymes for which activities were assayed for calculating the kinetic parameters were purified as described in Materials and Methods. Data are mean values of five determinations from independent experiments.
Through analysis of the crystal structure of the CAD complex with GL-7-ACA, it has been revealed that R57β and Y149α are both involved in forming direct hydrogen bonds with the glutarate moiety of GL-7-ACA. The Q50β residue, forming hydrogen bonds with R57β and Y149α, may be involved in the binding of GL-7-ACA. We therefore replaced Q50β with other residues to test the influence of Q50β on GL-7-ACA binding. After the Q50β residue was mutated to N50β residue, the hydrogen bonds may still be formed between N50β and the two residues R57β and Y151α in the mutated CA130. Asn has a similar molecular structure but with a shorter side chain than Gln, so the substitution results in the mutant N50β containing a substrate-binding space larger than that created by Q50β in the structure of CA130, inducing the glutarate moiety of GL-7-ACA to bind into the pocket more deeply and effectively. The substitution of residue Tyr151α with Phe is not critical for catalytic function but creates noticeable effects on substrate binding. Without the hydroxyl group of Tyr, the mutant Y151αF still possesses comparable GL-7-ACA deacylation activity and seems able to bind GL-7-ACA even more effectively.
Both mutants, Y151αF and Q50βN, exhibited higher total activity after fermentation and higher specific activity after purification than did CA130. These results suggested that the mutants may be useful in the production of 7-ACA. However, unexpectedly, the double mutation (Y151αF/Q50βN) did not create a synergistic benefit of the two individual mutations but instead exhibited catalytic ability and substrate binding comparable to the wild-type CA130. It is possible that the double mutation (Y151αF/Q50βN) also might negatively influence substrate binding sites. Changes in polar (OH of Y151α) and charged (NH3+ of Q50β) regions of individual residues will interfere with the necessary flexible conformation of substrate binding sites.
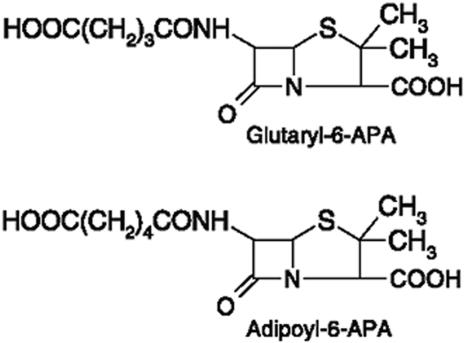
Although no significant increase in their activity toward CPC was observed, we found that Y151αF and Q50βN both have improved specific activity toward two substrate analogs, AD-6-APA and GL-6-APA (Table 2). AD-6-APA and GL-6-APA, synthesized in our laboratory, were used to screen CAs able to hydrolyze CPC and GL-7-ACA, respectively (37) (Fig. 2). AD-6-APA has a similar side chain to CPC, except that AD-6-APA lacks an extra NH2. The fact that Q50βN mutant had not improved the specific activity to CPC indicated that the binding sites of CA130 to accommodate the extra NH2 was a key to improve specific activity to CPC. Obviously, the Y151αF and Q50βN mutant cannot accommodate the extra NH2 of CPC, too. The side chain of AD-6-APA is larger than GL-7-ACA's. These results indicated that the two mutants have improved activity toward the substrate with the bigger side chain. It is also possible that the mutation enlarged the size of substrate binding pocket and so is therefore able to accommodate a larger hexyl moiety.
TABLE 2.
Substrate specificity of the engineered enzymesa
| Enzyme | % Enzyme activity
|
|||
|---|---|---|---|---|
| GL-7ACA | GL-6APA | AD-6APA | CPC | |
| C130 | 100 | 90.6 | 58.6 | 2.3 |
| Y151αF | 114 | 99.8 | 70.6 | 2.3 |
| Q50βN | 142 | 130 | 87.2 | 2.5 |
| Y151αF/Q50βN | 106 | 97.0 | 66.3 | 2.3 |
The enzyme activity of wild-type CA130 toward GL-7ACA was used as a control. Data are expressed as the percentage of enzyme activity relative to the control.
FIG. 2.
Molecular formulas of the substrate analogs: GL-6-APA and AD-6-APA.
The site-directed mutagenesis of the key residues in CAD was also previously performed by Kim and Kim (15). Their results showed that some active site residues were critical not only for catalysis but also for posttranslational modification. These authors also obtained a triple mutant (Q50βM/Y149αK/F177βG) that expressed ∼8-fold-higher catalysis activity toward CPC compared to wild-type CAD but no mutant with improved activity to GL-7-ACA (24).
Site-directed mutagenesis of surface residues to improve stability.
The next series of mutations were designed to enhance the stability of CA130, by using a method previously described by Yang et al. (33) based on structure analysis. The principle of this method is to change the isoelectric point (IEP) of the protein. According to Russell and Fersht (26), the IEP of a protein is related to its surface charge; any change to the surface charge will affect the protein's IEP and stability. An analysis of a Ser protease family revealed that an increasing content of Ala in the surface of an enzyme was correlated with an increased IEP. Because of this, six surface-charged residues that were located far from the active site (not in the middle of any key secondary structure and not involved in interactions with other residues) were selected and replaced by Ala.
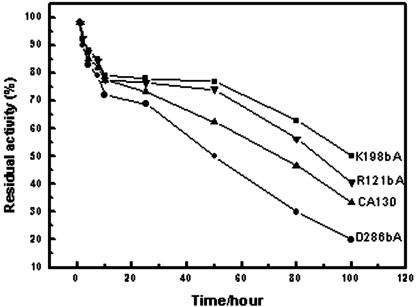
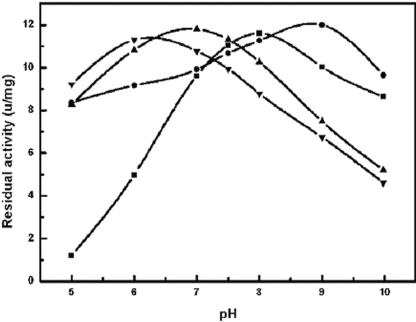
Three enzymes from the mutants constructed—Arg121βAla, Lys198βAla and Asp286βAla—showed improved stability with respect to half-life and optimal pH (Fig. 3 and Fig. 4). In contrast, the Km and kcat values toward GL-7-ACA were similar to wild type. The analysis of the expression level and specific activities of the mutated enzymes suggested that the substitutions had no effects on their activity to GL-7-ACA. The half-life of CA130 (at pH 7.0 and 37°C) is ca. 68.1 h, whereas the half-lives of R121βA, K198βA, and D286βA were changed to 88.3, 107.5, and 53.9 h, respectively. Compared to CA130, R121βA and K198βA extended their half-lives by ca. 30 to 58%. The mutations also affected the pH-dependent activity profile. K198βA and D286βA shifted their optimal pH to alkaline by about 1.0 to 2.0 pH units. R121βA shifted its optimal pH to 6.2. No obvious change was found between the wild-type and mutants with respect to their temperature-dependent activity profile.
FIG. 3.
Time-dependent enzymatic activity of CA130 (▴), K198βA (▪), R121βA (▾), and D286βA (•) stored at 37°C at pH 7.0. The residual enzyme activity was assayed as described previously (16). The half-lives of R121βA and K198βA increased by ca. 30 to 58% compared to wild-type CA130, however, the half-life of D286βA was decreased by about 21%. Each datum point represents mean values from five individual experiments. Similar results were obtained in each replicate.
FIG. 4.
pH activity profiles of CA130 (▴), K198βA (▪), R121βA (▾), and D286βA (•). The optimal pH of K198βA and R121βA shifted the profile to the alkaline region by about 1.0 to 2.0 pH units, whereas the optimal pH of D286βA shifted downward to the basic region by about 0.8 pH units. Three independent measurements were taken for each datum point. The data are mean values of replicated experiments.
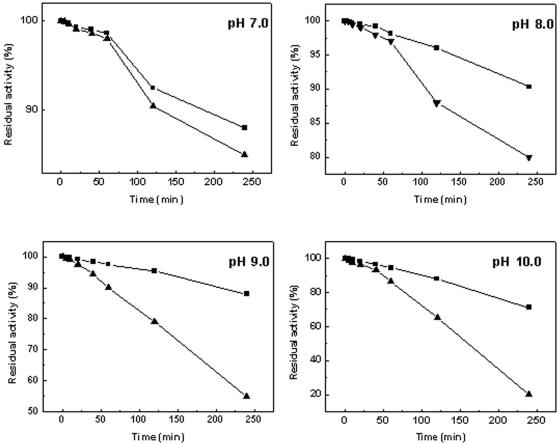
We were interested in K198βA for its prolonged half-life and increased optimal pH. To further investigate the stability of K198βA, K198βA and CA130 were stored for 4 h at 37°C at pH 7.0, 8.0, 9.0, and 10.0 and then assayed for their residual activity (Fig. 5). Although both the wild-type and the mutated enzymes lost activity concurrent with increasing pH, it was apparent that K198βA retained a higher activity compared to wild-type CA130 under the same treatment. This phenomenon was more obvious in the higher pH range.
FIG. 5.
Time-dependent pH stabilities of CA130 and K198βA. Enzymes are stored at 37°C at pH 7.0, 8.0, 9.0, and 10.0. The relative residual activities of CA130 (▴) and K198βA (▪) were determined after 0, 5, 10, 20, 40, 60, 120, and 240 min. Three independent measurements were taken for each datum point. The data are mean values of replicated experiments.
In the biocatalyst system in which GL-7-ACA is converted into 7-ACA by GL-7-ACA acylase, a base must be added to stabilize the pH, which may result in local alkaline conditions and cause enzyme inactivation. The prolonged half-life of K198βA and its ability to endure more alkaline solutions will make it more applicable for industry.
Fermentation and immobilization of double-mutant Q50N/K198Aβ.
Considering the benefits of Q50N and K198Aβ, a double-mutant Q50βN/K198βA was constructed. As expected, the mutant was found to possess the improved catalysis efficiency of Q50βN (Table 1) and stability of K198βA simultaneously. The time-dependent enzymatic activity and pH-dependent enzymatic activity of Q50βN/K198βA are the same as that of K198βA (data not shown).
To test the possible application of Q50βN/K198βA in the production of 7-ACA, the mutated acy gene of CA130 was cloned into an expression vector pMFT7H6 and transformed into E. coli BL21(DE3). The pMFT7H6 (Pt7, RBS, Tt7) is a His6 tag fusion expression vector, and the protein produced can be one step purified and immobilized by affinity chromatography. Compared to immobilized wild-type CA130, the immobilized enzyme with the double mutation exhibited increased total activity and specific activity (Table 3). The total activity of 1 liter of culture was increased by ca. 43%. The specific activity (units per 1 g of carrier) after immobilization was improved by about 34%.
TABLE 3.
Fermentation and immobilization of CA130 and Q50βN/K198βA expressed in pMFT 7H6a
| Immobilized enzyme | Total activity (U/liter) | Sp act (U/g) |
|---|---|---|
| CA130 | 1,220 | 252.5 |
| Q50βN/K198βA | 1,750 | 339.0 |
Data are expressed as the means of at least three independent experiments.
Improving the specific activity of CA130 will be highly valuable in industrial applications. To our knowledge, a CA130 with a high specific activity and with stability in conditions of alkaline pH is not currently available. In the present study, the site-directed mutants of CA130 were designed to enhance activity and stability. We succeeded in acquiring a double mutant (Q50βN/K198βA) that possesses the combined benefits of the two individual mutants. The high activity of the mutant Q50βN indicates that the shorter side chain at position 50 may increase the space available to accommodate the substrate for binding to the active sites of C130. The prolonged half-life and increased optimal pH in the mutant K198βA suggested that the substitution of surface-charged residues can be used to successfully improve stability for industrial application. The study of site-directed mutants of CA130 is significant for understanding the related mechanism of catalysis and can provide a guide for using protein engineering to reconstruct enzymes for industrial application.
Acknowledgments
This study was supported by the National High Technology Development Program of China (grant 2003AA235020) and the National Natural Science Foundation of China (grant 30125002).
REFERENCES
- 1.Anupama, P., and K. Harish. 1998. Recent trends in enzymatic conversion of cephalosporin C to 7-aminocephalosporinic acid. Crit. Rev. Biotechnol. 18:1-12. [Google Scholar]
- 2.Brannigan, J. A., G. Dodson, H. J. Duggleby, P. C. Moody, J. L. Smith, D. R. Tomchick, and A. G. Murzin. 1995. A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature 378:416-419. [DOI] [PubMed] [Google Scholar]
- 3.Charles, F. S., L. G. Otten, R. H. Cool, and W. J. Quax. 2003. Analysis of a substrate specificity switch residue of cephalosporin acylase. Biochem. Biophys. Res. Commun. 312:755-760. [DOI] [PubMed] [Google Scholar]
- 4.Del Rio, G., M. A. Rodriguez, M. A. Munguia, A. Lopez-Munguia, and X. Soberon. 1995. Mutant Escherichia coli penicillin acylase with enhanced stability at alkaline pH. Biotechnol. Bioeng. 48:141-148. [DOI] [PubMed] [Google Scholar]
- 5.Deshpande, B. S., S. S. Ambedkar, and V. K. Sudhakaran. 1994. Molecular biology of β-lactam acylase. World J. Microbiol. Biotechnol. 10:129-138. [DOI] [PubMed] [Google Scholar]
- 6.Diez, B., E. Mellado, R. Fouces, M. Rodriguez, and J. L. Barredo. 1996. Recombinant Acremonium chrysogenum strains for the industrial production of cephalosporin. Microbiologia 12:359-370. [PubMed] [Google Scholar]
- 7.Fersht, A. R., and L. Serrano. 1993. Principals of protein stability derived from protein engineering experiments. Curr. Opin. Struct. Biol. 3:75-83. [Google Scholar]
- 8.Fritz-Wolf, K., K. P. Koller, G. Lange, A. Liesum, K. Sauber, H. Schreuder, W. Aretz, and W. Kabsch. 2002. Structure-based prediction of modifications in glutarylamidase to allow single-step enzymatic production of 7-aminocephalosporanic acid from cephalosporin C. Protein Sci. 11:92-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 10.Honda, G., A. Matsuda, M. Zushi, S. Yamamoto, and K. Komatsu. 1997. Heterologous protein production in Acremonium chrysogenum: expression of bacterial cephalosporin C acylase and human thrombomodulin genes. Biosci. Biotechnol. Biochem. 61:948-955. [DOI] [PubMed] [Google Scholar]
- 11.Huang, X., R. Zeng, X. Ding, X. Mao, Y. Ding, Z. Rao, Y. Xie, W. Jiang, and G. Zhao. 2002. Affinity alkylation of the Trp-B4 residue of the beta-subunit of the glutaryl 7-aminocephalosporanic acid acylase of Pseudomonas sp. 130. J. Biol. Chem. 277:10256-10264. [DOI] [PubMed] [Google Scholar]
- 12.Ishii, Y., Y. Saito, T. Fujimura, H. Sasaki, Y. Noguchi, H. Yamada, M. Niwa, and K. Shimomura. 1995. High-level production, chemical modification and site-directed mutagenesis of a cephalosporin C acylase from Pseudomonas strain N176. Eur. J. Biochem. 230:773-778. [PubMed] [Google Scholar]
- 13.Isogai, T., M. Fukagawa, I. Aramori, M. Iwami, H. Kojo, T. Ono, Y. Ueda, M. Kohsaka, and H. Imanaka. 1991. Construction of a 7-aminocephalosporanic acid (7ACA) biosynthetic operon and direct production of 7ACA in Acremonium chrysogenum. Biotechnology 9:188-191. [DOI] [PubMed] [Google Scholar]
- 14.Kibanov, A. M. 2001. Improving enzymes by using them in organic solvents. Nature 409:241-246. [DOI] [PubMed] [Google Scholar]
- 15.Kim, S., and Y. Kim. 2001. Active site residues of cephalosporin acylase are critical not only for enzymatic catalysis but also for posttranslational modification. J. Biol. Chem. 276:48376-48381. [DOI] [PubMed] [Google Scholar]
- 16.Kim, Y., and W. G. Hol. 2001. Structure of cephalosporin acylase in complex with glutaryl-7-aminocephalosporanic acid and glutarate: insight into the basis of its substrate specificity. Chem. Biol. 8:1253-1264. [DOI] [PubMed] [Google Scholar]
- 17.Kim, Y., K. Yoon, Y. Khang, S. Turley, and W. G. Hol. 2000. The 2.0 Å crystal structure of cephalosporin acylase. Struct. Fold Des. 8:1059-1068. [DOI] [PubMed] [Google Scholar]
- 18.Lee, Y. H., T. S. Chang, H. J. Liu, and W. S. Chu. 1998. An acidic glutaryl-7-amino cephalosporanic acid acylase from Pseudomonas nitroreducens. Biotechnol. Appl. Biochem. 28(Pt. 2):113-118. [PubMed] [Google Scholar]
- 19.Li, Y., J. Chen, W. Jiang, X. Mao, G. Zhao, and E. Wang. 1999. In vivo posttranslational processing and subunit reconstitution of cephalosporin acylase from Pseudomonas sp. 130. Eur. J. Biochem. 262:713-719. [DOI] [PubMed] [Google Scholar]
- 20.Li, Y., W. Jiang, Y. Yang, G. Zhao, and E. Wang. 1998. Overproduction and purification of glutaryl 7-amino cephalosporanic acid acylase. Protein Expr. Purif. 12:233-238. [DOI] [PubMed] [Google Scholar]
- 21.Mao, X., J. Zhang, Y. Li, Y. J. He, E. D. Wang, Y. L. Yang, W. H. Jiang, G. P. Zhao, and J. S. Chiao. 2002. Nucleotide sequence and protein sequence analysis of GL-7-ACA acylase from Pseudomonas sp. 130. Chinese J. Biotechnol. 18:45-50. [PubMed] [Google Scholar]
- 22.Matthews, B., H. Nicholson, and W. Becktel. 1987. Enhanced protein thermostability from site directed mutations that decrease the entropy of unfolding. Proc. Natl. Acad. Sci. USA 84:6663-6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald, I. K., and J. M. Thornton. 1994. Satisfying hydrogen bonding potential in proteins. J. Mol. Biol. 238:777-793. [DOI] [PubMed] [Google Scholar]
- 24.Oh, B., M. Kim, J. Yoon, K. Chung, Y. Shin, D. Lee, and Y. Kim. 2003. Deacylation activity of cephalosporin acylase to cephalosporin C is improved by changing the side chain conformations of active-site residues. Biochem. Biophys. Res. Commun. 310:19-27. [DOI] [PubMed] [Google Scholar]
- 25.Querol, E., J. A. Perez-Pons, and A. Mozo-Villarias. 1996. Analysis of protein conformational characteristics related to thermostability. Protein Eng. 9:265-271. [DOI] [PubMed] [Google Scholar]
- 26.Russell, A. J., and A. R. Fersht. 1987. Rational modification of enzyme catalysis by engineering surface charge. Nature 328:496-500. [DOI] [PubMed] [Google Scholar]
- 27.Saito, Y., T. Fujimura, Y. Ishii, Y. Noguchi, T. Miura, M. Niwa, and K. Shimomura. 1996. Oxidative modification of a cephalosporin C acylase from Pseudomonas sp. strain N176 and site-directed mutagenesis of the gene. Appl. Environ. Microbiol. 62:2919-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito, Y., Y. Ishii, T. Fujimura, H. Sasaki, Y. Noguchi, H. Yamada, M. Niwa, and K. Shimomura. 1996. Protein engineering of a cephalosporin C acylase from Pseudomonas strain N176. Ann. N. Y. Acad. Sci. 782:226-240. [DOI] [PubMed] [Google Scholar]
- 29.Sarkar, G., and S. S. Sommer. 1990. The “megaprimer” method of site-directed mutagenesis. BioTechniques 8:404-407. [PubMed] [Google Scholar]
- 30.Velasco, J., J. Luis-Adrio, M. Angel-Moreno, B. Diez, G. Soler, and J. L. Barredo. 2000. Environmentally safe production of 7-aminodeacetoxycephalosporanic acid (7-ADCA) using recombinant strains of Acremonium chrysogenum. Nat. Biotechnol. 18:857-861. [DOI] [PubMed] [Google Scholar]
- 31.Vogt, G., S. Woell, and P. Argos. 1997. Protein thermal stability, hydrogen bonds, and ion pairs. J. Mol. Biol. 269:631-643. [DOI] [PubMed] [Google Scholar]
- 32.Wang, E. D., Y. G. Zheng, Y. Li, W. H. Jiang, and Y. L. Yang. 2002. Expression of gene encoding GL-7ACA acylase in Escherichia coli. Acta Biochim. Biophys. Sinica 34:526-531. [PubMed] [Google Scholar]
- 33.Yang, S., L. Zhou, H. Tang, J. Pan, X. Wu, H. Huang, and Z. Yuan. 2002. Rational design of a more stable penicillin G acylase against organic cosolvent. J. Mol. Catalysis B Enzymatic 18:285-290. [Google Scholar]
- 34.Yang, Y. L., and D. F. Yun. 1991. Cloning of GL-7-ACA acylase from Pseudomonas sp. 130 and its expression in Escherichia coli. Chin. J. Biotechnol. 7:99-107. [PubMed] [Google Scholar]
- 35.Zhang, N., X. M. Ding, X. Huang, E. D. Wang, Y. L. Yang, G. P. Zhao, and W. H. Jiang. 2001. Mutagenesis of N-terminal amino acid residues in beta-subunit of glutaryl-7-amino-cephalosporanic acid acylase C130. Acta Biochim. Biophys. Sinica 33:671-676. [PubMed] [Google Scholar]
- 36.Zhang, W., X. Huang, G. Zhao, and W. Jiang. 2004. Affinity-labeled glutaryl-7-amino cephalosporanic acid acylase C130 can hydrolyze the inhibitor during crystallization. Biochem. Biophys. Res. Commun. 313:555-558. [DOI] [PubMed] [Google Scholar]
- 37.Zhu, S., Y. Yang, G. Zhao, and W. Jiang. 2003. A rapid and specific method to screen environmental microorganisms for cephalosporin acylase activity. J. Microbiol. Methods 54:131-135. [DOI] [PubMed] [Google Scholar]