Abstract
A recent study (D. C. Cooper, F. W. Picardal, A. Schimmelmann, and A. J. Coby, Appl. Environ. Microbiol. 69:3517-3525, 2003) has shown that NO3− and NO2− (NOx−) reduction by Shewanella putrefaciens 200 is inhibited in the presence of goethite. The hypothetical mechanism offered to explain this finding involved the formation of a Fe(III) (hydr)oxide coating on the cell via the surface-catalyzed, abiotic reaction between Fe2+ and NO2−. This coating could then inhibit reduction of NOx− by physically blocking transport into the cell. Although the data in the previous study were consistent with such an explanation, the hypothesis was largely speculative. In the current work, this hypothesis was tested and its environmental significance explored through a number of experiments. The inhibition of ∼3 mM NO3− reduction was observed during reduction of a variety of Fe(III) (hydr)oxides, including goethite, hematite, and an iron-bearing, natural sediment. Inhibition of oxygen and fumarate reduction was observed following treatment of cells with Fe2+ and NO2−, demonstrating that utilization of other soluble electron acceptors could also be inhibited. Previous adsorption of Fe2+ onto Paracoccus denitrificans inhibited NOx− reduction, showing that Fe(II) can reduce rates of soluble electron acceptor utilization by non-iron-reducing bacteria. NO2− was chemically reduced to N2O by goethite or cell-sorbed Fe2+, but not at appreciable rates by aqueous Fe2+. Transmission and scanning electron microscopy showed an electron-dense, Fe-enriched coating on cells treated with Fe2+ and NO2−. The formation and effects of such coatings underscore the complexity of the biogeochemical reactions that occur in the subsurface.
Dissimilatory reduction of ferric (hydr)oxide minerals has been documented for a large number of microorganisms in a wide range of environments (18, 19, 22, 26) and has become recognized as an important constituent of the global carbon and iron cycles (21, 23, 32, 44). Microbial reduction of Fe(III) and other metals has a profound effect on groundwater geochemistry and plays a key role in the fate of contaminant metals (5, 8, 13, 14, 20, 33) and organic compounds (16, 23, 24) in anoxic groundwater.
Nitrate has both natural and anthropogenic sources, but the extensive use of NO3− as an agricultural fertilizer threatens the quality of many groundwater systems (41). The use of denitrifying microorganisms to remediate NOx−-contaminated soils may be a solution for its removal in some environments (27). Since contaminant metals and NO3− may coexist in some iron-bearing sediments (12, 31), it is important to understand the complex, biogeochemical interactions that can occur during the microbial reduction of NOx− and Fe(III) (hydr)oxides. Shewanella putrefaciens 200 is a facultative anaerobe capable of utilizing NO3−, NO2−, and Fe(III), as well as O2, Mn(IV), trimethylamine N-oxide, thiosulfate, fumarate, and a number of other compounds as terminal electron acceptors for carbon metabolism (9, 29, 30). This makes S. putrefaciens an ideal organism for studying biogeochemical interactions during redox transformations of NO3−/NO2− and Fe(III)/Fe(II).
NOx− reduction by microorganisms typically precedes ferric (hydr)oxide reduction in experimental or subsurface systems, in part because NOx− tends to be more available to cells than the highly insoluble (at neutral pH) forms of Fe(III) (1, 9, 29, 30, 39). Recently, though, Cooper et al. reported that the reduction of NO3− and NO2− by S. putrefaciens 200 was inhibited by the presence of the solid-phase ferric (hydr)oxide goethite (α-FeOOH) (6). In addition, they observed that the presence of goethite in NOx−-reducing incubations resulted in greatly enhanced production of N2O. Cooper et al. speculated that the inhibition and enhanced N2O production resulted from reaction of small amounts of biogenic Fe(II) with NO2− to form an Fe (hydr)oxide coating on the cell surface that inhibited transport of soluble electron acceptors into the cell. Although the stoichiometry and products are subject to speculation, reaction 1 shows such a reaction between surface-sorbed Fe2+ (☰Fe2+) and NO2− that could lead to production of a solid Fe (hydr)oxide.
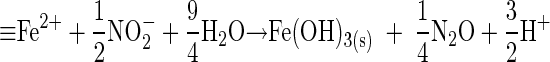 |
(1) |
Sørensen et al. described a similar surface-catalyzed reaction between Fe2+ and NO2− for which reduction of NO2− to N2O was very slow in the absence of a mineral surface but proceeded rapidly in the presence of lepidocrocite (γ-FeOOH) (40). The hypothetical mechanism suggested by Cooper et al. requires the sorption of Fe2+ to the cell surface before reacting with aqueous NO2−. Dissimilatory iron-reducing bacteria are known to sorb Fe2+ to their cell surfaces, and such sorption is believed to passivate the surface and impede further Fe(III) reduction (34, 46, 47). Fe2+ is relatively soluble at neutral pH and, in anoxic, iron-reducing environments, there may also be significant sorption of Fe2+ to cell surfaces of bacteria incapable of iron reduction. NO2−, whether from anthropogenic sources or produced via nitrification or denitrification, will have the opportunity to react with that sorbed Fe2+. The oxidation of surface-bound Fe2+ by NO2− and resulting formation of a Fe (hydr)oxide mineral on the surface of the cell could have a rapid, inhibitory affect on the transport of soluble electron acceptors into the cell. Although recent work has established the ability of some bacteria to catalyze Fe(II)-dependent reduction of NO3− (45, 49), little is known about how abiotic NOx− reduction by cell-sorbed Fe2+ will affect microbial metabolism.
Processes that limit the rate or extent of NOx− reduction may have notable environmental effects. Attempts to bioremediate a NOx−-contaminated site by addition of a suitable substrate, for example, would take longer and be more costly. Since NO3− must often be consumed prior to bioremediation of metals and radionuclides (5, 11), remediation of these compounds may also be negatively affected by processes that limit NOx− removal. The same inhibition mechanism may also affect the reduction of other environmentally relevant electron acceptors, which has broad implications for the understanding of subsurface geochemical cycles. Since N2O, a product of the proposed reaction, is also a potent greenhouse gas also believed to be implicated in stratospheric ozone depletion (7, 10, 48), increased knowledge about possible N2O sources may improve our modeling of global climate change and other environmental stresses.
The inhibitory mechanism proposed by Cooper et al. was supported primarily by nitrogen stable isotope experiments that showed that the increased N2O production was primarily of chemical origin, i.e., produced by the abiotic reduction of NO2− by surface-bound Fe2+ produced during microbial reduction of synthetic goethite. Their proposed mechanism, however, was largely speculative, direct evidence of such Fe-rich cell coatings was not provided, and the potential environmental significance of the reaction was not apparent. In the current work, we demonstrate that NOx− reduction is inhibited during reduction of other Fe(III) minerals, that the utilization of soluble electron acceptors other than NOx− are similarly inhibited, and that NOx− reduction by microorganisms incapable of dissimilatory iron reduction can also be inhibited by sorption of aqueous Fe2+. In addition, we provide direct evidence of an Fe-rich, electron-dense coating formed on bacteria in the presence of NO2− and Fe2+.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. putrefaciens 200 is a gram-negative motile rod with an obligate respiratory metabolism (37), originally isolated from a Canadian oil pipeline by Obuekwe (28). Paracoccus denitrificans (ATCC 13543) is a facultative, denitrifying bacterium incapable of reducing Fe(III) that was used in this study for comparison to S. putrefaciens. The cultures were maintained at −80°C in a 10% glycerol-90% nutrient broth solution. Liquid cultures were grown aerobically to late log phase in a 1-liter flask on a shaker table in Westlake medium (30). Cells were subsequently harvested by centrifugation and resuspended to a target optical density (λ, 600 nm) of approximately 1.2 in low-ionic-strength, artificial groundwater (AGW) medium. The full composition of AGW medium is described elsewhere (5). This medium contains 15 mM lactate as an electron donor, and primary buffering capacity is provided by 10 mM HEPES buffer. Phosphate (0.044 mM) and bicarbonate (0.50 mM) concentrations are relatively low to minimize the potential for precipitation of vivianite or siderite.
Fe(III) and NOx− reduction experiments.
Although the general methodology employed in the Fe(III) and NO3− reduction experiments has been previously described (6), a summary of the procedure is presented here. All experiments were conducted in 150-ml serum bottles, crimp sealed with butyl rubber stoppers under a 95% N2-5% H2 headspace. Except where noted, slurries were established that contained 75 ml AGW medium and the appropriate solid phase. Three solid phases were used in separate experiments as sources of ferric iron: goethite (α-FeOOH,) hematite (Fe2O3), and an iron-containing natural sediment. Goethite was prepared as described by Schwertmann and Cornell (36), hematite was purchased from J.T. Baker/Mallinckrodt Baker Inc., and the natural sediment (MNC-71) was collected from iron-bearing, clayey sediments at Marshall, N.C. (J. Zachara, PNNL, personal communication). The amount of each sediment required for the 50 mM Fe3+ (by citrate dithionate extraction) used in these experiments was approximately 0.34 g goethite, 0.6 g hematite, and 4.9 g MNC-71 in 75 ml of medium. When required, NO3− or NO2− was added to serum bottles from sterile stock solutions of NaNO3 and NaNO2. In experiments requiring a chelator to reduce Fe2+ adsorption to cell surfaces, 1.0 ml sterile anaerobic nitrilotriacetic acid (NTA) stock solution was added to the AGW medium for a final concentration of 5.0 mM.
Experiments were initiated by inoculating slurries with 1 ml of washed S. putrefaciens suspension under anoxic conditions (ca. 2 ×106 cells ml−1). Serum bottle reactors were wrapped in foil and incubated horizontally on a shaker table at room temperature. Initial samples were taken prior to inoculation, and subsequent samples were taken at regular intervals thereafter. During sampling, bottles were transferred to the anaerobic chamber, shaken vigorously, and immediately sampled with a 3-ml syringe (23-gauge needle). A 1.5-ml aliquot of slurry was transferred to a microcentrifuge tube, and solids were separated by centrifugation. The supernatant was immediately sampled for pH, NO3−, and NO2−. To determine if the observed inhibition of NOx− reduction was due to unknown goethite toxicity, a different bacterium (Paracoccus denitrificans) was substituted for S. putrefaciens. P. denitrificans is able to utilize NO3− as a terminal electron acceptor but is unable to reduce Fe(III). The same growth and experimental procedures described above for goethite and NO3− reduction were followed, using P. denitrificans in place of S. putrefaciens.
Reaction of NO2− with sorbed Fe2+.
Experiments were done to measure N2O production from the abiotic reduction of NO2− by aqueous Fe2+ or Fe2+ sorbed to a goethite surface. Previously deoxygenated AGW medium lacking vitamins and lactate was dispensed into replicate (n = 3) sets of serum bottle batch reactors in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI). The first set contained 3 mM Fe2+ added from a stock solution of FeCl2 that had been prepared anoxically, adjusted to pH 7, and filtered (0.45 μm) to remove fine particulates. This filtration step was required to ensure that only aqueous Fe2+ was utilized and Fe(III) oxides present as contaminants on the FeCl2 crystals or formed in trace amounts during stock solution preparation were not inadvertently added. The second and third sets of replicates contained 50 mM goethite and 50 mM goethite plus 3 mM Fe2+, respectively. After 24 h, all systems received an amendment of NO2− as NaNO2 to a final concentration of 3 mM. N2O production was measured in all three systems over time as described below.
Fe2+ was sorbed to the surface of S. putrefaciens and P. denitrificans (4, 38) to compare the inhibition of NOx− reduction by bacteria able and unable to reduce Fe(III). S. putrefaciens and P. denitrificans were grown and harvested as described above and then resuspended in anaerobic AGW medium containing 50 mM FeCl2 (pH, ∼7) in the anaerobic chamber. The resuspension was gently mixed for 15 min to allow adsorption of Fe2+ onto the cell surfaces. The cells were then centrifuged again in tightly sealed centrifuge tubes to prevent Fe2+ oxidation and washed with anaerobic, Fe-free AGW before being used to inoculate replicate reactors containing 3 mM NO3− or NO2−. Control reactors were set up identically except they were initially resuspended in Fe-free AGW medium.
Additional experiments were similarly done (only with S. putrefaciens) to determine if sorption of Fe2+ and subsequent exposure to NO2− would impede the utilization of soluble electron acceptors other than NO3− or NO2−. In these experiments, Fe2+ was sorbed to cells and suspensions were harvested and washed as described above. The washed cells were then incubated in anaerobic AGW medium containing 5 mM NO2− which was allowed to react for 1 h with the Fe2+ sorbed to cells. Cells were washed and subsequently incubated in AGW amended either with 5 mM fumarate or ∼8.6 mg liter−1 O2. Consumption of the electron acceptor over time was compared with identical suspensions that had not undergone the Fe2+ sorption step. Where reported, initial and final cell numbers were determined by acridine orange direct counting (15).
Electron microscopy and electron dispersion spectroscopy.
Cells of S. putrefaciens for examination using transmission and scanning electron microscopy (TEM and SEM) were prepared by sequential incubation with Fe2+ and NO2− as described above to abiotically simulate the formation of the Fe(III) (hydr)oxide coating believed to form in NOx−- reducing incubations containing goethite. Control cells were incubated solely with either Fe2+ or NO2− for comparison. All preparation of samples for TEM and SEM analyses was performed in an anaerobic chamber to prevent oxidation of Fe2+. For TEM analysis, prepared cells were placed on parlodian support films on 300 mesh copper grids and allowed to dry in the anaerobic chamber. Dried grids with cells were placed in an anaerobic container (BBL GasPak system) for transport to the TEM facility and were exposed to the air for less than 1 minute while being transferred to the TEM vacuum chamber. Treated and control cells were observed at 100 kV on a JEOL JEM-1010 transmission electron microscope, and images were taken at a magnification of ×12,000. For SEM analysis, prepared cells were filtered onto a 0.45-μm membrane filter, dehydrated using graded alcohol-water washes, and critical point dried in hexamethyldisilazane. Following gold coating, treated and control cells were observed at 20.0 kV on a FEI Quanta 400F scanning electron microscope at a magnifications between ×23,000 and ×78,000. Surface point elemental analysis was performed while the samples were being viewed in the SEM using a Princeton Gamma-Tech energy-dispersive spectrometer.
Analytical methods.
Samples for NO3− and NO2− analyses were diluted twofold in Milli-Q water, frozen, and stored for later analysis by ion chromatography as previously described (6). NH4+ was quantified in some experiments using the colorimetric phenate method (15). N2O production was quantified by removing 25.0 μl of headspace gas from the batch reactors using a gas-tight syringe and analyzed via gas chromatography as previously described (6).
When applicable, aqueous and cell-bound Fe2+ was analyzed via a modification of the ferrozine method (35, 42). Surface areas of the various iron oxides used were determined by multipoint Brunauer-Emmett-Teller (BET) N2 adsorption on a NOVA 1000 surface area analyzer. Oxygen utilization rate was determined using a YSI biological oxygen monitor. Fumarate concentrations were quantified on a Waters high-performance liquid chromatography apparatus using an absorbance detector at a wavelength of 238 nm.
RESULTS AND DISCUSSION
Fe(III) reduction and inhibition of NOx− reduction.
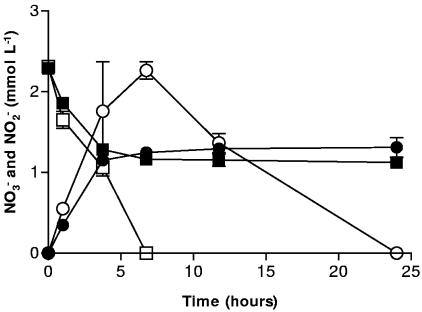
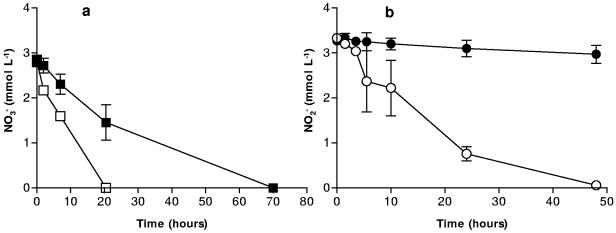
Our hypothetical mechanism of inhibition relies on a surface-catalyzed reaction between Fe2+ and NO2− and the subsequent formation of an Fe (hydr)oxide coating on the surface of cells. Fe2+ produced from the microbial reduction of Fe(III) sorbs to the surface of the cell and then is reoxidized by NO2−. Goethite was the only Fe (hydr)oxide used in previous experiments which resulted in the inhibition of NOx− (6). When hematite was used as the Fe(III) oxide, NO3− reduction and NO2− production were severely inhibited after 4 h (Fig. 1). In controls without hematite, NO3− reduction was complete after 7 h, and NO2− reduction was complete in less than 25 h. In incubations with hematite and NO3−, inhibition of NO3− reduction was not apparent for about 4 h, which may be related to the reduced Fe(III) reduction rates (not shown) and lower surface area of the hematite used in our experiments. The surface area of this hematite (∼9 m2 g−1) is much less than that of the goethite (∼72 m2 g−1) used in previous experiments where inhibition of NO3− reduction occurred almost immediately (6). The microbially accessible surface area of an iron oxide has a significant effect on the rate and extent of iron oxide reduction (34, 35). Less Fe(III) reduced to Fe2+ would in turn affect the rate of the hypothesized surface reaction between Fe2+ and NO2−.
FIG. 1.
NO3− reduction by S. putrefaciens in the presence of 50 mmol liter−1 hematite (▪) and without hematite (□) and NO2− production/reduction in the presence of 50 mmol liter−1 hematite (•) and without hematite (○). Error bars indicate the standard deviations of the means (n = 3).
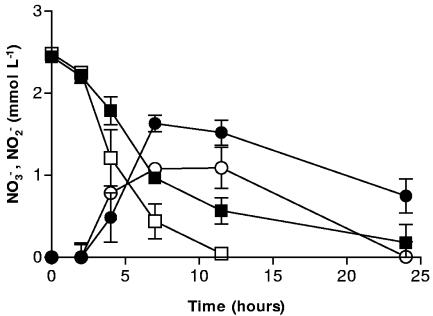
Nitrate reduction in the presence of sediment MNC-71 also progressed more slowly than in sediment-free systems. After approximately 12 h, more than 10 times as much NO3− remained in systems containing MNC-71 (Fig. 2). NO3− took at least twice as long (>24 h) to be completely reduced in the systems containing MNC-71. The inhibition of NOx− reduction in the MNC-71 systems was less pronounced than in pure iron oxide systems. This may be due to the presence of clay and sand particles which make up a significant portion of the natural sediment (A. Coby, unpublished results). The negatively charged surfaces of clays in particular could compete with the bacterial surfaces for sorption of Fe2+. Indeed, clays have been used as solid-phase complexants to adsorb microbially produced Fe2+ and thereby increase the rate and extent of Fe(III) reduction that would otherwise be limited by Fe2+ adsorption to cells and cause cell surface passivation (47). Still, a significant lag in NOx− reduction resulted, which is in agreement with results using goethite or hematite (6). This suggests that the hypothetical surface reaction between Fe2+ and NO2− and the putative cell coating are not restricted to pure iron oxides and may be significant in a variety of iron-containing natural sediments and soils.
FIG. 2.
NO3− reduction by S. putrefaciens in the presence of the natural sediment MNC-71 (▪) and without MNC-71 (□) and NO2− production/reduction in the presence of MNC-71 (•) and without MNC-71 (○). Error bars indicate the standard deviations of the means (n = 3).
In the studies by Cooper et al. using goethite (6), the inhibition of NOx− was incomplete, i.e., both NO3− and NO2− were eventually exhausted after approximately 50 and 800 h, respectively. With the hematite incubations shown in Fig. 1, although NO3− and NO2− were both still present when the experiment was stopped at 24 h, it is possible that they would have been slowly utilized and eventually exhausted. In incubations with sediment MNC-71 (Fig. 2), NO3− was almost exhausted at the conclusion of the experiment and NO2− was clearly being consumed. This lack of complete inhibition may be due to incomplete formation of cell coatings on some cells or the ability of cells to eventually “clear” themselves of such coatings. During studies of microbial Fe(III) reduction by S. putrefaciens CN32, Liu et al. used TEM and energy-dispersive spectroscopy to show that cells released small (100-nm) membrane vesicles that apparently helped clear them of Fe precipitates that formed on the cells and limited Fe(III) reduction rates (17). Although the nature of the precipitates formed in our NOx−-containing systems is likely different from those observed by Liu et al., it is possible that release of Fe (hydr)oxide-coated membrane blebs could eventually relieve inhibition caused by mineral coatings.
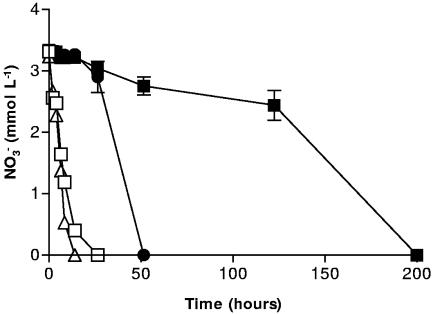
In order to test whether the reaction between NO2− and Fe2+ was indeed surface catalyzed, the chelator NTA was used to complex with Fe2+ produced during the reduction of goethite. The formation of an NTA-Fe2+ complex would reduce Fe2+ sorption and, if the reaction with NO2− was indeed surface catalyzed, we expected that this would minimize the formation of the hypothetical Fe (hydr)oxide coating on the cell and allow NO3− reduction to proceed normally. Previous studies under similar conditions have reported no detectible Fe(III)aq in NTA-amended (5 mM) systems containing 50 mM goethite and inoculated with Shewanella alga (47). The NTA amendments were therefore not expected to significantly contribute to the dissolution of Fe(III) from goethite. Figure 3 summarizes the results, which support the surface reaction hypothesis. Without NTA, NO3− reduction was inhibited by up to 200 h in systems containing both goethite and NO3−. When NTA was present at 5 mM in goethite-containing systems, NO3− reduction was complete within 50 h. In goethite-free controls (with and without NTA), NO3− reduction was complete by 30 h. The temporary inhibition seen in the system containing goethite and NTA may be due to Fe2+ sorbing to the cell surface before having a chance to complex with NTA. The rate and extent of Fe(III) mineral reduction is increased in the presence of NTA (2, 47), and it is reasonable to postulate that a portion of the Fe2+ formed at the mineral-microbe interface may be temporarily sorbed to the cell before removal by the chelators.
FIG. 3.
NO3− reduction by S. putrefaciens with 5 mmol liter−1 NTA (Δ), without NTA (□), with 50 mmol liter−1 goethite (▪), and with 5 mmol liter−1 NTA plus 50 mmol liter−1 goethite (•). Error bars are not shown for cultures lacking goethite, for the sake of clarity.
The possibility exists that the observed inhibition of NOx− reduction in the presence of goethite (6) was a result of an unknown toxic effect of goethite on the cells in the sediment slurries. Paracoccus denitrificans, a gram-negative, denitrifying organism unable to reduce Fe(III), was chosen as a surrogate for S. putrefaciens in additional experiments. The rate of NO3− reduction, in systems with or without goethite, was nearly identical when using P. denitrificans (data not shown). This suggests that there is no toxic effect of goethite in the absence of Fe(III) reduction and supports our hypothesis that a small amount of Fe(III) needs to be reduced to Fe2+ for the inhibition of NOx− reduction to occur.
Sorption of Fe2+ and reaction with NO2.
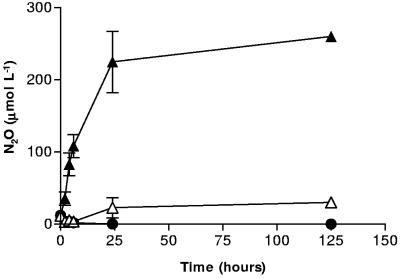
In the absence of an iron (hydr)oxide, S. putrefaciens 200 primarily reduces NO3− and NO2− to ammonia and typically produces only trace amounts of N2O (6), presumably as an enzymatic side reaction during dissimilatory reduction of nitrate to ammonia (3, 43). In systems containing small amounts of biogenic Fe2+, goethite, and S. putrefaciens, though, Cooper et al. found N2O production in amounts 10-fold greater than when goethite was absent (6). Additional experiments were done to clearly determine if the enhanced N2O production observed in microbial systems containing NOx− and goethite could be explained solely by the abiotic reduction by surface-bound Fe2+. The first set of abiotic, replicate batch reactors contained 3 mM aqueous Fe2+ and 3 mM NO2−; the second set contained 50 mM of goethite and 3 mM NO2−; the third contained all three components, Fe2+, NO2−, and goethite. As can be seen in Fig. 4, bottles containing only aqueous Fe2+ and NO2− generated only 30 μM N2O after 125 h. In contrast, bottles containing Fe2+, NO2−, and goethite had generated 225 μM N2O after 30 h and, eventually, 260 μM after 125 h. Controls containing only NO2− and goethite amendments did not produce any N2O over the course of the experiment. These results are in agreement with studies by Sørensen et al., in which very little N2O was produced by reaction of aqueous Fe2+ and NOx− in systems lacking a surface catalyst (40). The results also confirm that, under our experimental conditions, Fe2+ will sorb to a surface such as goethite and abiotically reduce NO2− to N2O. Although the mechanistic details of the surface reaction remain unknown, it is possible that surface sorption of Fe2+ increases the local electron density over that in aqueous solution, thereby increasing the possibility of reduction reactions involving multiple electrons. It is possible that a similar reaction could occur on cell surfaces which have adsorbed Fe2+.
FIG. 4.
N2O production in systems containing aqueous Fe2+ (2 mmol liter−1) and NO2− (3 mmol liter−1) (Δ), aqueous Fe2+, NO2−, and 50 mmol liter−1 goethite (▴), and NO2− and 50 mmol liter−1 goethite (•). Error bars indicate the standard deviations of the means (n = 3).
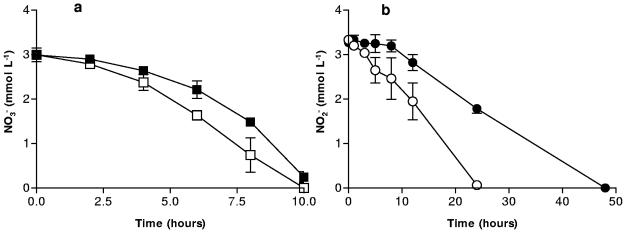
To test the hypothesis that Fe2+ sorbed to cell surfaces will react with NO2−, producing an Fe (hydr)oxide coating on the cell and an inhibition of NOx− reduction, Fe2+ was sorbed to cells by equilibration of aqueous Fe2+ with a concentrated bacterial culture. Those cells were then incubated with a medium containing NO3− or NO2− as the sole electron acceptor. After 20 h, all the NO3− had been reduced in systems not treated with Fe2+, while approximately 1.5 mM NO3− remained in systems containing treated cells (Fig. 5a). Inhibition of NO2− reduction by prior sorption of Fe2+ was even more dramatic. Reduction of 3.25 mM of NO2− by untreated cells was complete within 50 h, whereas treated cells only reduced 1/10 of the original NO2− after 50 h (Fig. 5b). After 5 days of incubation in NO2−-reducing systems, we observed only 5.3 ± 2.9 μM N2O in systems containing untreated cells, whereas 14.1 ± 2.6 μM N2O was produced in systems containing cells treated with Fe2+. These results all support the hypothesis that Fe2+ sorbs to the cell surface and reacts with NO2− to form an Fe (hydr)oxide coating that may be responsible for the observed inhibition of NOx− reduction.
FIG. 5.
(a) NO3− reduction by S. putrefaciens treated with Fe2+ (▪) and without treatment (□). (b) NO2− reduction by S. putrefaciens treated with Fe2+ and NO2− (•) and without treatment (○). Error bars indicate the standard deviations of the means (n = 3).
The above experiment was repeated using P. denitrificans to see if NO3− and NO2− reduction by a non-Fe(III)-reducing organism could be inhibited after treatment with Fe2+ and NO2−. In these experiments the inhibition of NOx− reduction was less pronounced, but still significant. In Fig. 6a, after 6 h, 1.7 times more NO3− had been reduced in untreated systems versus those treated with Fe2+. This is in close agreement with the analogous experiment inoculated with S. putrefaciens after 7 h (Fig. 5a). In Fig. 6b, almost 2 mM of NO2− still remained after 24 h in the systems treated with Fe2+, while untreated cells had reduced nearly all the NO2− at the same time point. These results are particularly significant because they suggest that, in environments where Fe(II) is being produced, the reduction of NOx− or other soluble electron acceptors could be inhibited across a wide range of microorganisms. These results show that the inhibition of NOx− reduction is not due to the putative iron (hydr)oxide coating serving as an alternate electron acceptor and competitively inhibiting NOx− reduction, since P. denitrificans is unable to utilize Fe(III) as an electron acceptor. The less-pronounced inhibition of NO3− reduction with P. denitrificans compared to S. putrefaciens may be due to interspecies differences in the nature of the cell surface that might affect the extent of Fe2+ sorption. Alternately, P. denitrificans may be able to recover more quickly by clearing coatings from its surface.
FIG. 6.
(a) NO3− reduction by P. denitrificans treated with Fe2+ and NO2− (▪) and without treatment (□). (b) NO2− reduction by P. denitrificans treated with Fe2+ and NO2− (•) and without treatment (○). Error bars indicate the standard deviations of the means (n = 3).
If reduction of NOx− is inhibited as a result of formation of mineral coatings on the cell, the utilization of other soluble, terminal electron acceptors should also be inhibited. A greater environmental significance of the Fe (hydr)oxide coating could be argued if it were seen to have an effect on utilization of other respiratory substrates. Following incubation of Fe2+-treated cells with 5 mM NO2−, we measured O2 utilization rates and fumarate reduction rates. The O2 utilization rate for cells that were incubated only with 5 mM NO2− (0.41 mg liter−1 min−1) was about 2.2 times faster than for cells that had been incubated with Fe2+ prior to NO2− (0.19 mg liter−1 min−1). This experiment was repeated using twice the cell number (2 ×108 cells ml−1) with comparable results.
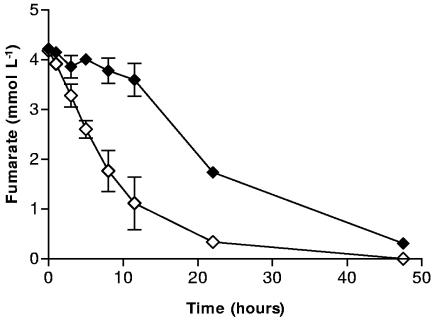
Fumarate reduction by cells treated with Fe2+ and NO2− was also severely inhibited compared to cells exposed to just NO2− (Fig. 7). After 12 h, about 3.5 times more fumarate remained in systems containing cells exposed to both Fe2+ and NO2− than in those systems containing cells previously exposed to NO2− alone. Fumarate reduction in treated systems was still not complete after 48 h. Both the O2 and fumarate reduction results suggest that the inhibitory effect of the Fe (hydr)oxide coating on the reduction of soluble electron acceptors is not restricted to NO3− and NO2 but may have broad environmental implications concerning the utilization of environmentally significant electron acceptors.
FIG. 7.
Fumarate reduction by S. putrefaciens treated with Fe2+ and NO2− (⧫) and without treatment (⋄). Error bars indicate the standard deviations of the means (n = 3).
Electron microscopy and energy-dispersive spectroscopy.
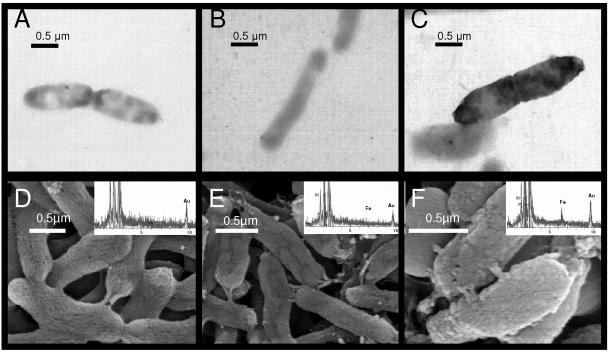
The six panels shown in Fig. 8 show the effect of NO2− reacting with Fe2+ which had been previously sorbed to the surface of the cells. Panels A and D are TEM images of S. putrefaciens incubated in AGW medium containing 5 mM NO2− without previous Fe2+ sorption to the cell surface. Panels B and E are of cells which were treated with 50 mM aqueous Fe2+ for 30 min but without subsequent NO2− incubation. The cells and surrounding medium in the TEM image (Fig. 8B) are slightly darker, indicating perhaps the presence of Fe2+ both sorbed to the cell surface and in solution. Panels C and F are of cells that have been treated with 50 mM aqueous Fe2+ and then allowed to react with NO2− (5 mM) for 3 hours. This is the same treatment used on cells in the previous experiments examining the reduction of soluble electron acceptors. The much darker quality and thickened appearance of the cells in panel C suggest an electron-dense coating on the cells, likely a Fe (hydr)oxide precipitate resulting from the surface-catalyzed reaction between sorbed Fe2+ and aqueous NO2−. This is supported by the presence of a rough coating on the surface in the SEM images shown in panel F. The EDS data for each of the SEM cell treatments indicate a clear enrichment of Fe on the surface of the cells treated with both Fe2 and NO2− (Fig. 8F). Taken together, these data clearly show that electron-dense, Fe-enriched coatings on cells exposed to NO2− are formed only following sorption of Fe2+. Sorption of Fe2+ alone is also insufficient to form such coatings.
FIG. 8.
Transmission and scanning electron micrographs of S. putrefaciens under three treatments. (A and D) Cells treated with NO2−; (B and E) cells treated with Fe2+; (C and F) cells treated with both Fe2+ and NO2−. The electron-dense coating on the cells in panel C and the rough surface on the cells in panel F represent an Fe (oxy)hydroxide coating formed from the reaction between surface-bound Fe2+ and NO2−. The inset on each SEM image is an EDS point scan that corresponds to the cells in the image.
The identity of the Fe (hydr)oxide formed on the surface of S. putrefaciens in our systems is not known, but it does not appear that the cells are able to rapidly utilize this precipitate as an electron acceptor. Some forms of Fe minerals, such as magnetite [Fe2+(Fe3+)2O4] or green rusts [Fe2+(1 − x)Fe3+(x)(OH)2]x+ are resistant to microbial reduction (25). Experiments by Cooper et al. have demonstrated the formation of magnetite following the reduction of lepidocrocite by S. putrefaciens 200 (5), and Sørensen et al. reported the formation of magnetite as a product of the reaction of Fe2+ with NO2− on the surface of lepidocrocite (40). Although our studies did not utilize lepidocrocite, it is clear that the Fe(III) (hydr)oxide coatings formed in our experiments were not readily or completely reducible by S. putrefaciens over the duration of our experiments.
Conclusions.
Support for the proposed inhibition mechanism involving the reaction of NO2− and surface-bound Fe2+ is evident in the results demonstrating that the presence of Fe2+ on the surface of cells leads to the production of N2O and an inhibition of NOx− reduction even at small concentrations of Fe2+. This reaction occurs using a variety of Fe oxides, including an iron-bearing natural sediment, can occur with bacterial species incapable of Fe(III) reduction if they are in environments containing aqueous Fe2+, and affects several soluble terminal electron acceptors. The exact composition of the Fe (hydr)oxide coating is not yet known. The extent and significance of N2O formation via this reaction in the environment is not known but could be significant in iron-bearing soils during microbial remediation of NOx− contamination. The potential increase of both Fe2+ and NO2− production during bioremediation efforts could significantly increase the formation of N2O as a by-product, and the implications of this reaction for metal bioremediation efforts, geochemical cycling, and greenhouse gas production make it an important subject for further investigation.
Acknowledgments
We thank the School of Public and Environmental Affairs for its financial support to this research.
We also thank Craig Cooper for valuable discussions and mentorship, Barry Stein for guidance using the TEM, Juergen Schieber for patience and persistence with the SEM/EDS analyses, and Ai Nguyen for assistance in the some of the experiments.
REFERENCES
- 1.Achtnich, C. A., F. Bak, and R. Conrad. 1995. Competition for electron donors among nitrate reducers, ferric iron reducers, sulfate reducers, and methanogens in anoxic paddy soil. Biol. Fertil. Soils 19:65-72. [Google Scholar]
- 2.Arnold, R. G., T. J. DiChristina, and M. R. Hoffmann. 1988. Reductive dissolution of Fe(III) oxides by Pseudomonas sp. 200. Biotechnol. Bioeng. 32:1081-1096. [DOI] [PubMed] [Google Scholar]
- 3.Averill, B. A., and J. M. Tiedje. 1982. The chemical mechanism of microbial denitrification. FEBS Lett. 138:8-12. [DOI] [PubMed] [Google Scholar]
- 4.Claessens, J., T. Behrends, and P. Van Cappellen. 2004. What do acid-base titrations of live bacteria tell us? A preliminary assessment. Aquat. Sci. 66:19-26. [Google Scholar]
- 5.Cooper, D. C., F. Picardal, J. Rivera, and C. Talbot. 2000. Zinc immobilization and magnetite formation via ferric oxide reduction by Shewanella putrefaciens 200. Environ. Sci. Technol. 34:100-106. [Google Scholar]
- 6.Cooper, D. C., F. W. Picardal, A. Schimmelmann, and A. J. Coby. 2003. Chemical and biological interactions during nitrate and goethite reduction by Shewanella putrefaciens 200. Appl. Environ. Microbiol. 69:3517-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crutzen, P. J. 1970. The influence of nitrogen oxides on the atmospheric ozone content. Q. J. R. Meteorol. Soc. 96:320-325. [Google Scholar]
- 8.Cummings, D. E., F. Caccavo, S. Fendorf, and R. F. Rosenzweig. 1999. Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ. Sci. Technol. 33:723-729. [Google Scholar]
- 9.DiChristina, T. J. 1992. Effects of nitrate and nitrite on dissimilatory iron reduction by Shewanella putrefaciens 200. J. Bacteriol. 174:1891-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Environmental Protection Agency. 2002. Report 430-R-02-003, inventory of U.S. greenhouse gas emissions and sinks: 1990-2000. Environmental Protection Agency, Office of Atmospheric Programs, Washington, D.C.
- 11.Finneran, K. T., M. E. Housewright, and D. R. Lovley. 2002. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ. Microbiol. 4:510-516. [DOI] [PubMed] [Google Scholar]
- 12.Fredrickson, J. K., J. M. Zachara, D. L. Balkwill, D. W. Kennedy, W. L. Shu-mei, H. M. Kostandarithes, M. J. Daly, M. F. Romine, and F. J. Brockman. 2004. Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the Hanford site, Washington State. Appl. Environ. Microbiol. 70:4230-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, M. C. Duff, Y. A. Gorby, S. Li, and K. M. Krupka. 2000. Reduction of U(VI) in goethite (α-FeOOH) suspensions by a dissimilatory metal-reducing bacterium. Geochim. Cosmochim. Acta 64:3085-3098. [Google Scholar]
- 14.Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, R. K. Kukkadapu, J. P. McKinley, S. M. Heald, C. X. Liu, and A. E. Plymale. 2004. Reduction of TcO4− by sediment-associated biogenic Fe(II). Geochim. Cosmochim. Acta 68:3171-3187. [Google Scholar]
- 15.Greenberg, A. E., L. S. Clesceri, and A. E. Eaton (ed.). 1992. Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, Washington, D.C.
- 16.Kim, S., and F. W. Picardal. 1999. Enhanced anaerobic biodegradation of carbon tetrachloride in the presence of reduced iron oxides. Environ. Toxicol. Chem. 18:2142-2150. [DOI] [PubMed] [Google Scholar]
- 17.Liu, C., J. M. Zachara, Y. A. Gorby, J. E. Szecsody, and C. F. Brown. 2001. Microbial reduction of Fe(III) and sorption/precipitation of Fe(II) on Shewanella putrefaciens strain CN32. Environ. Sci. Technol. 35:1385-1393. [DOI] [PubMed] [Google Scholar]
- 18.Liu, C. X., Y. A. Gorby, J. M. Zachara, J. K. Fredrickson, and C. F. Brown. 2002. Reduction kinetics of Fe(III), Co(III), U(VI) Cr(VI) and Tc(VII) in cultures of dissimilatory metal-reducing bacteria. Biotechnol. Bioeng. 80:637-649. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd, J. R. 2003. Microbial reduction of metals and radionuclides. Microbiol. Rev. 27:411-425. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd, J. R., P. Yong, and L. E. Macaskie. 2000. Biological reduction and removal of Np(V) by two microorganisms. Environ. Sci. Technol. 34:1297-1301. [Google Scholar]
- 21.Lovley, D. R. 1991. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 55:259-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovley, D. R. 2000. Fe(III) and Mn(IV) reduction, p. 3-30. In D. R. Lovley (ed.), Environmental microbe-metal interactions. ASM Press, Washington, D.C.
- 23.Lovley, D. R., and E. J. P. Phillips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormick, M. L., E. J. Bouwer, and P. Adriaens. 2002. Carbon tetrachloride transformation in a model iron-reducing culture: relative kinetics of biotic and abiotic reactions. Environ. Sci. Technol. 36:403-410. [DOI] [PubMed] [Google Scholar]
- 25.Neal, A. L., K. M. Rosso, G. G. Geesey, Y. A. Gorby, and B. J. Little. 2003. Surface structure effects on direct reduction of iron oxides by Shewanella oneidensis. Geochim. Cosmochim. Acta 67:4489-4503. [Google Scholar]
- 26.Nealson, K. H., and D. Saffarini. 1994. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 48:311-343. [DOI] [PubMed] [Google Scholar]
- 27.Nuttall, H. E., L. Deng, A. Abdelouas, and W. Lutze. 1999. In situ denitrification: a field demonstration, p. 59-64. The Fifth International In Situ and On-Site Bioremediation Symposium, vol. 5, no. 4. Battelle Press, San Diego, Calif. [Google Scholar]
- 28.Obuekwe, C. O. 1980. Microbial corrosion of a crude oil pipeline. Ph.D. dissertation. University of Alberta, Edmonton, Alberta, Canada.
- 29.Obuekwe, C. O., and D. W. S. Westlake. 1981. Effect of nitrate on reduction of ferric iron by a bacterium isolated from crude oil. Can. J. Microbiol. 27:692-697. [DOI] [PubMed] [Google Scholar]
- 30.Obuekwe, C. O., and D. W. S. Westlake. 1982. Effect of reducible compounds (potential electron acceptors) on reduction of ferric iron by a Pseudomonas species. Microbiol. Lett. 19:57-62. [Google Scholar]
- 31.Riley, R. G., J. M. Zachara, and F. J. Wobber. 1992. Chemical contaminants on DOE lands and selection of contaminant mixtures for subsurface science research. DOE/ER-0547T. U.S. Department of Energy, Washington, D.C.
- 32.Roden, E. E. 2004. Analysis of long-term bacterial vs. chemical Fe(III) oxide reduction kinetics. Geochim. Cosmochim. Acta 68:3205-3216. [Google Scholar]
- 33.Roden, E. E., M. R. Leonardo, and F. G. Ferris. 2002. Immobilization of strontium during iron biomineralization coupled to dissimilatory hydrous ferric oxide reduction. Geochim. Cosmochim. Acta 66:2823-2839. [Google Scholar]
- 34.Roden, E. E., and M. M. Urrutia. 1999. Ferrous iron removal promotes microbial reduction of crystalline iron(III) oxides. Environ. Sci. Technol. 33:1847-1853. [Google Scholar]
- 35.Roden, E. E., and J. M. Zachara. 1996. Microbial reduction of crystalline iron(III) oxides: influence of oxide surface area and potential for cell growth. Environ. Sci. Technol. 30:1618-1628. [Google Scholar]
- 36.Schwertmann, U., and R. M. Cornell. 1991. Iron oxides in the laboratory: preparation and characterization. VCH Publishers, Inc., New York, N.Y.
- 37.Semple, K. M., and D. W. S. Westlake. 1987. Characterization of iron-reducing Alteromonas putrefaciens strains from oil-field fluids. Can. J. Microbiol. 33:366-371. [Google Scholar]
- 38.Sokolov, I., D. S. Smith, G. S. Henderson, Y. A. Gorby, and F. G. Ferris. 2001. Cell surface electrochemical heterogeneity of the Fe(III)-reducing bacteria Shewanella putrefaciens. Environ. Sci. Technol. 35:341-347. [DOI] [PubMed] [Google Scholar]
- 39.Sørensen, J. 1982. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl. Environ. Microbiol. 43:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sørensen, J., and L. Thorling. 1991. Stimulation by lepidocrocite (γ-FeOOH) of Fe(II)-dependent nitrite reduction. Geochim. Cosmochim. Acta 55:1289-1294. [Google Scholar]
- 41.Spalding, R. F., and M. E. Exner 1993. Occurence of nitrate in groundwater—a review. J. Environ. Qual. 22:392-402. [Google Scholar]
- 42.Stookey, L. L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 43.Stouthamer, A. H. 1988. Dissimilatory reduction of oxidized nitrogen compounds, p. 245-303. In A. J. B. Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley and Sons, New York, N.Y.
- 44.Straub, K. L., M. Benz, and B. Schink. 2001. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 34:181-186. [DOI] [PubMed] [Google Scholar]
- 45.Straub, K. L., M. Benz, B. Schink, and F. Widdel. 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ Microbiol. 62:1458-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urrutia, M. M., E. E. Roden, J. K. Fredrickson, and J. M. Zachara. 1998. Microbial and surface chemistry controls on reduction of synthetic Fe(III) oxide minerals by the dissimilatory iron-reducting bacterium Shewanella alga. Geomicrobiology 15:269-291. [Google Scholar]
- 47.Urrutia, M. M., E. E. Roden, and J. M. Zachara. 1999. Influence of aqueous and solid-phase Fe(II) complexants on microbial reduction of cystalline iron(III) oxides. Environ. Sci. Technol. 33:4022-4028. [Google Scholar]
- 48.Wang, W. C., Y. L. Yung, A. A. Lacis, J. Mo, and J. E. Hansen. 1976. Greenhouse effects due to man-made perturbations of trace gases. Science 194:685-690. [DOI] [PubMed] [Google Scholar]
- 49.Weber, K. A., F. W. Picardal, and E. E. Roden. 2001. Microbially catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(II) compounds. Environ. Sci. Technol. 35:1644-1650. [DOI] [PubMed] [Google Scholar]