Abstract
Environmental studies of the human-pathogenic bacterium Campylobacter jejuni have focused on linking distributions with potential sources. However, in aquatic ecosystems, the abundance of C. jejuni may also be regulated by predation. We examine the potential for grazing by the freshwater planktonic crustacean Daphnia carinata to reduce the survival of C. jejuni. We use a system for measuring grazing and clearance rates of D. carinata on bacteria and demonstrate that D. carinata can graze C. jejuni cells at a rate of 7% individual−1 h−1 under simulated natural conditions in the presence of an algal food source. We show that passage of C. jejuni through the Daphnia gut and incorporation into fecal material effectively reduces survival of C. jejuni. This is the first evidence to suggest that grazing by planktonic organisms can reduce the abundance of C. jejuni in natural waters. Biomanipulation of planktonic food webs to enhance Daphnia densities offers potential for reducing microbial pathogen densities in drinking water reservoirs and recreational water bodies, thereby reducing the risk of contracting water-borne disease.
Thermophilic bacteria of the genus Campylobacter are the most commonly reported bacterial cause of gastroenteritis in industrialized countries (10), and account for ca. 10% of all diarrhea worldwide (1). Of the gram-negative, spiral campylobacters, Campylobacter jejuni alone can attack otherwise healthy individuals, making it the most common cause of human campylobacteriosis (23, 32, 36). A number of outbreaks of campylobacteriosis have been linked to nine apparently contaminated and insufficiently treated drinking water supplies (20, 34, 39), however, in these instances, C. jejuni has rarely been isolated from the suspected water supplies. Although human sewage and animal fecal matter are the most likely sources of environmental contamination by campylobacters, clear and direct links between these potential sources and campylobacters in drinking or recreational waters have rarely been established, owing partly to the complex behavior of campylobacters once in the aquatic environment.
Campylobacter replication is thought to occur almost exclusively in the intestinal tract of mammalian and avian hosts (2, 16, 31). Once excreted into aquatic environments, campylobacters can undergo physiological change to a viable, nonculturable state (5, 6, 22, 30, 35, 39) and, in both the nonculturable and the culturable states, they can persist in water and be transported from their sources. Thermophilic campylobacters can survive for days (5, 18), weeks (19), and months (8, 30, 37) under various conditions, in a variety of aqueous media. In untreated lake water, survival of C. jejuni has been shown to be reduced compared to filter-sterilized lake water (18, 19).
Skelly and Weinstein (31) propose an ecoenvironmental approach to human campylobacteriosis, which acknowledges “the complexity of campylobacter survival trajectories in terms of environmental constraints and ecological filters.” They suggest that the ingestion of campylobacters by planktonic grazers in aquatic environments influences their survival and contributes to the complexity of modeling their survival. In support of this idea, it is well established that the freshwater crustacean, Daphnia, is a highly efficient grazer of aquatic bacteria (13, 14, 15, 21), having the ability to filter organisms as small as 0.4 to 2 μm (3, 38). Grazing by Daphnia has been shown to affect the biomass, productivity, size structure and species composition of aquatic bacterial communities (13). Daphnia will also effectively graze Escherichia coli (26), however, the ability of Daphnia to control concentrations of human pathogenic bacteria, such as C. jejuni, has never been tested to our knowledge.
The overall aim of our study was to determine the rate at which Daphnia carinata King could remove C. jejuni from aqueous media. We used a model experimental system to examine Daphnia-Campylobacter interactions and determined grazing and clearance rates of D. carinata on C. jejuni under simulated natural conditions and in the presence of an algal food source. We also assessed the effect of gut passage on the survival of C. jejuni by determining the culturability of C. jejuni in the fecal material of D. carinata fed on C. jejuni.
MATERIALS AND METHODS
Experimental organisms.
Campylobacter jejuni subsp. jejuni strain 825/79, biotype 2, was obtained from the New Zealand Reference Culture Collection (ESR, Wellington, New Zealand) and maintained at 4°C on Columbia sheep blood agar (Fort Richard). The liquid medium used was Preston broth which was comprised of nutrient broth no. 2 (CM 67, Oxoid, Global Scientific), 5% defibrinated horse blood, and Campylobacter Growth Supplement (SR84, Oxoid), but not the Campylobacter Selective Supplement (SR 117, Oxoid) or cefoperazone. Single colonies from Columbia agar plates were placed into 12-ml tubes filled with Preston broth and incubated at 37°C for 24 h. The resulting growth was pelleted by centrifugation (10,000 rpm, 10 min, 4°C), and the cell pellet was washed twice and rediluted in 12 ml of 0.1% peptone (Difco, Fort Richard). This procedure consistently provided a concentration of approximately 109 CFU ml−1 in the final suspension.
Daphnia carinata King was collected from the lower pond at the Dunedin Botanical Gardens, Dunedin, New Zealand, and cultured in COMBO culture medium for freshwater algae and zooplankton (17) over a 3-month period. The D. carinata cultures were fed exclusively on a culture of the alga Cryptomonas sp. which was grown at 20°C in modified MBL culture medium (33). Adult D. carinata used in the experiments generally ranged from 2.3 to 3.2 mm in length, from the top of the head to the base of the tail spine.
Impact of D. carinata grazing on C. jejuni survival.
To determine survival of C. jejuni in the presence or absence of D. carinata, and to estimate D. carinata grazing rates, six sterile glass culture vessels were filled with 250 ml of autoclaved, filtered COMBO culture medium. One ml of C. jejuni suspension (109 CFU ml−1) and Cryptomonas sp. (final concentration, 180 ml−1) was added to all vessels. Then, ten D. carinata, which had been rinsed twice in sterile COMBO, were added to three of the six culture vessels. After gentle shaking to mix the contents, the vessels were not agitated during the experiment, which took place in dim light at 13°C, so that Campylobacter survived for the duration of the experiment.
At 0, 24, 48, and 72 h, 1-ml samples were taken from the upper third of each culture vessel (to avoid sampling daphnid fecal material) and diluted 10-fold in 0.1% peptone to yield concentrations of C. jejuni appropriate for using a most probable number method, described as follows. One ml of the diluted samples was added to tubes (12 ml) containing Preston broth, ensuring that there was minimal headspace. The tubes were incubated at 37°C for 4 to 6 h before the addition of selective supplement (SR 117, Oxoid) and cefoperazone (1.25 mg liter−1). Tubes were transferred to 42°C and incubated for a further 24 h. An aliquot from each tube was subsequently streaked onto modified charcoal cefoperazone desoxycholate agar (CCDA; Oxoid, Basingstoke, UK) plates, which were incubated at 42°C in a microaerophilic atmosphere. Confirmation of the presence of C. jejuni was based on the examination of colonial morphology, microscopic examination of Gram-stained samples and the results of oxidase testing. Most probable number methods have been shown to enhance recovery of C. jejuni from water, compared to drop plate methods (7, 25; P. Bremer, unpublished data).
After sampling at 72 h, the D. carinata were removed from the culture vessels, rinsed thoroughly with sterile water, placed in a sterile petri dish and photographed to determine their size. They were then transferred aseptically to vials containing 0.1% peptone (10 ml) and three glass beads (3.5 to 4.5 mm in diameter, BDH) and vortexed for 1 min. The number of C. jejuni in the resulting suspensions was estimated using the most probable number method.
To test whether subsampling the medium was an effective method for estimating total culturable C. jejuni in the vessels, the medium remaining at the end of the experiment, including any fecal material, was filtered through 0.45-μm filters (catalog no. 66278, Pall Gelman Laboratory) and the concentration of culturable C. jejuni on the filter was determined. In addition, to account for potential bacterial adhesion to the vessels, their interior walls were thoroughly wiped with a cotton bud to remove attached bacteria. The filters and cotton buds were placed into 10 ml of 0.1% peptone. The total number of culturable C. jejuni from each vessel was converted to a concentration per ml of medium to compare with the bacterial concentration in subsamples of the medium at 72 h, immediately before filtration.
C. jejuni survival following ingestion and excretion by D. carinata.
Three autoclaved glass culture vessels were filled with 250 ml of autoclaved, filtered COMBO medium. Ten D. carinata, which had been rinsed twice in sterile COMBO, and 1 ml of C. jejuni suspension were added to each vessel. After initial gentle shaking, the vessels were incubated at 13°C in dim light.
At the start of the experiment, 1-ml subsamples were taken from the upper third of the culture vessels to determine the starting concentration of C. jejuni by the most probable number method. After 2 h, additional subsamples were taken to determine the concentration of C. jejuni in the medium. Subsequently, five of the 10 D. carinata in each vessel were collected, rinsed twice with sterile COMBO, and assayed to determine associated C. jejuni numbers, using the most probable number method. The remaining five D. carinata from each vessel were transferred to fresh, sterile COMBO medium for a further 30 min, before they were removed, rinsed, and assayed to determine C. jejuni numbers. The remaining COMBO, including any fecal material, was filtered and the concentration of C. jejuni determined using the most probable number method.
Clearance rates.
Clearance rates were calculated from the log-linear slopes of relationships between bacterial concentration and time, estimated by least squares linear regression. It was assumed that grazing rates were independent of bacterial densities. The proportion of the population grazed per unit time (g) was calculated as g = 1-10(m1-m2), where m1 and m2 are the log-linear regression slopes of bacterial concentration versus time for Daphnia treatments and controls, respectively. Clearance rates (F, ml animal−1 h−1) were calculated as F = vgn−1 where v is the volume (ml) of the culture and n is the number of animals in the culture.
This method yielded similar but more accurate estimates of clearance rates than the formulae in Marin et al. (24), which used only initial and final bacterial concentrations.
RESULTS
Impact of D. carinata grazing on C. jejuni survival.
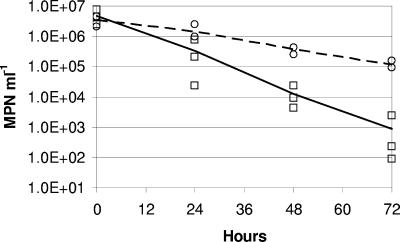
The rate of decline of C. jejuni concentration during the grazing experiment was significantly higher in the presence of D. carinata than in their absence (Fig. 1). After 72 h, D. carinata had reduced C. jejuni survival by ca. 2 orders of magnitude. A grazing rate (corrected for nongrazing mortality in the Daphnia-free culture vessels) of 7% of C. jejuni h−1 by the 10 D. carinata was calculated at a daphnid density of 40 liter−1, a C. jejuni density of between 1.4 × 106 and 1.0 × 103 most probable number ml−1, at 13°C, in the presence of Cryptomonas sp. This grazing rate corresponded to a clearance rate of 1.75 ml ind−1 h−1 (R2 = 0.998, P < 0.001).
FIG. 1.
Daphnia carinata grazing on Campylobacter jejuni as determined by a most probable number method. Points are treatment replicates showing C. jejuni concentrations in COMBO in the presence (squares, solid line) and absence (circles, dashed line) of D. carinata.
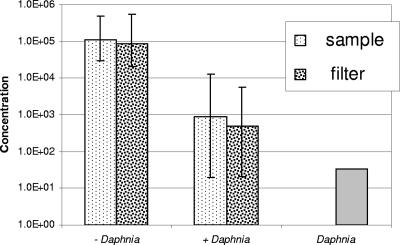
Comparison of the treatments with and without D. carinata revealed that D. carinata effectively rendered the C. jejuni nonculturable (Fig. 2). The bacterial concentration recovered from the filtered medium was not significantly different to that recovered from the unfiltered medium (Fig. 2).
FIG. 2.
Comparison of counts of Campylobacter jejuni in experimental culture vessels after 72 h, as determined by subsampling COMBO medium and by filtering the entire contents of culture vessels (with Daphnia and without Daphnia; most probable number ml−1). Vertical lines indicate the maximum and minimum of three replicates. The count of C. jejuni associated with Daphnia carinata in one treatment replicate after 72 h (Daphnia; most probable number animal−1).
Assays for C. jejuni on or in the D. carinata at the end of the experiment revealed an average of 33 cells of C. jejuni associated with each animal.
C. jejuni survival following ingestion by D. carinata.
In experiments where D. carinata were placed in culture vessels containing C. jejuni at 1.3 × 109 cells vessel−1, an average of 2.3 × 108 cells vessel−1 (or 18% of the cells at t = 0) remained in the medium after 2 h. At this time, the D. carinata in each vessel accounted for 1.2 × 107 C. jejuni, either attached to the Daphnia or in their digestive tracts. After D. carinata were placed in sterile medium for 30 min, the number of bacteria associated with them was 3.2 × 105 cells vessel−1 with a further 7.9 × 105 cells vessel−1 recovered from the total volume of culture medium including fecal material. Therefore, during the 30 min in sterile medium, bacterial numbers initially associated with the transferred D. carinata declined by 91%, indicating that the bacteria were killed by association with D. carinata, presumably during passage through the gut.
DISCUSSION
Previous studies of C. jejuni in aqueous media have shown that the period of survival in water is generally inversely proportional to temperature of the medium, within the range of ca. 4 to 40°C (5, 18, 19, 27, 30, 36, 37). Different strains, isolated from different sources, show considerable variability in survival parameters (8). Other environmental factors such as UVB light level, the level of oxygenation, water source and the presence of other microorganisms have been reported to influence C. jejuni survival (5, 7, 27, 36). Korhonen and Martikainen (18, 19) showed that survival of C. jejuni was significantly higher in filter-sterilised (0.2-μm-filtered) lake water than in untreated lake water or 5.0-μm-filtered lake water. The authors suggested that predation by protozoans may have reduced survival of C. jejuni in unsterilised lake waters, as has been shown to influence E. coli survival in previous studies (references 18 and 19 and references therein). Indeed, once in the aquatic environment, C. jejuni becomes a component of the aquatic microbial food web and its survival could potentially be affected by predation.
We used a model system for studying the interactions between D. carinata and C. jejuni in the presence of a Cryptomonas sp. as a supplementary algal food source for Daphnia. We found that the presence of D. carinata strongly reduced C. jejuni numbers in COMBO culture medium (Fig. 1 and 2). Furthermore, it appears that the association of C. jejuni with D. carinata results in death of the C. jejuni rather than sequestration. We did not determine the nature of this association but suggest that the death of C. jejuni cells was most likely due to their ingestion by D. carinata.
Daphnia are effective grazers of bacteria in lakes and ponds, with typical bacterial clearance rates ranging from 0.1 to 2.8 ml ind−1 h−1 (13, 29, 38). We have demonstrated that D. carinata can clear water of C. jejuni under experimental conditions at a rate of 1.75 ml ind−1 h−1. Given that C. jejuni cannot reproduce in waterways and is typically larger than most of the bacteria present in surface waters (0.1 to 1 μm) (11, 13), it is likely that the grazing activity of D. carinata and other daphnids could reduce the abundance of C. jejuni more effectively than that of native aquatic bacteria in aquatic ecosystems.
The calculated clearance rate of C. jejuni of 1.75 ml ind−1 h−1 falls within the range of clearance rates of Daphnia grazing on aquatic bacterial assemblages (13) and is similar to that for D. carinata grazing on E. coli at 18°C in a preliminary experiment (P. Bremer, unpublished data). Daphnia commonly occur at densities >30 ind liter−1 and can exceed 100 ind liter−1 (4). At a clearance rate of 1.75 ml ind−1 h−1, Daphnia at a density of 25 ind liter−1 could theoretically clear a water body of nondividing Campylobacter cells in <24 h. Our clearance rate is calculated for a range of Campylobacter concentrations that is typical of wastewaters (12, 31); therefore, further study is required to confirm similar clearance rates at lower concentrations of C. jejuni, more typical of surface waters (9, 31).
Grazing by the protozoan Cyclidium glaucoma has recently been shown to reduce the survival of another pathogenic bacterium, Vibrio cholerae, in brackish waters (28). Our study appears to be the first to show that crustacean zooplankton can reduce the concentration of human pathogens under simulated natural conditions. We confirmed that our model system could be used to explore the role of microbial food webs in regulating bacterial pathogen abundance in aquatic ecosystems. The results of our study suggest that the use of food web biomanipulation in recreational water bodies and drinking water reservoirs could enhance Daphnia densities and reduce pathogen density and the risk of contracting waterborne diseases.
Acknowledgments
We thank the University of Otago for funding this study.
REFERENCES
- 1.Benenson, A. 1995. Control of communicable diseases in man. American Public Health Association, Washington, D.C.
- 2.Blaser, M. J., Taylor, D., N., and R. A. Feldman. 1984. Epidemiology of Campylobacter infections, p. 143-161. In J. P. Butzler (ed.), Campylobacter infection in man and animals. CRC Press, Boca Raton, Fla.
- 3.Brendelberger, H. 1985. Filter mesh size and retention efficiency for small particles: comparative studies with Cladocera. Arch. Hydrobiol. Beih. Ergebn. Limnol. 21:135-146. [Google Scholar]
- 4.Burns, C. W. 2000. Crowding-induced changes in growth, reproduction and morphology of Daphnia. Freshwater Biol. 43:19-29. [Google Scholar]
- 5.Buswell, C. M., Y. M. Herlihy, L. M. Lawrence, J. T. M. McGuiggan, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1998. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and rRNA staining. Appl. Environ. Microbiol. 64:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaveerach, P., A. A. H. M. ter Huurne, L. J. A. Lipman, and F. van Knapen. 2003. Survival and resuscitation of ten strains of Campylobacter jejuni and Campylobacter coli under acid conditions. Appl. Environ. Microbiol. 69:711-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chynoweth, R. W., J. A. Hudson, and K. Thom. 1998. Aerobic growth and survival of Campylobacter jejuni in food and stream water. Lett. Appl. Microbiol. 27:341-344. [DOI] [PubMed] [Google Scholar]
- 8.Cools, I., M. Uyttendaele, C. Caro, E. D'Haese, H. D. Neils, and J. Debevere. 2003. Survival of Campylobacter jejuni strains of different origin in drinking water. J. Appl. Microbiol. 94:886-892. [DOI] [PubMed] [Google Scholar]
- 9.Eyles, R., D. Niyogi, C. Townsend, G. Benwell, and P. Weinstein. 2003. Spatial and temporal patterns of Campylobacter contamination underlying public health risk in the Taieri River, New Zealand. J. Environ. Qual. 32:1820-1828. [DOI] [PubMed] [Google Scholar]
- 10.Friedman, C. R., J. Neimann, H. C. Wegener, and others. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 3-26. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd edition. American Society for Microbiology, Washington, D.C.
- 11.Hobbie, J. E. 1979. Activity and bacterial biomass. Arch. Hydrobiol. Beih. Ergebn. Limnol. 12:59-63. [Google Scholar]
- 12.Jones, K., M. Betaieb, and D. R. Telford. 1990. Correlation between environmental monitoring of thermophilic campylobacters in sewage effluent and the incidence of Campylobacter infection in the community. J. Appl. Bacteriol. 69:235-240. [DOI] [PubMed] [Google Scholar]
- 13.Jürgens, K. 1994. Impact of Daphnia on planktonic microbial food webs-a review. Mar. Microb. Food Webs 8:295-324. [Google Scholar]
- 14.Kamjunke, N., A. Benndorf, C. Wilbert, M. Opitz, J. Kranich, M. Bollenbach, and J. Benndorf. 1999. Bacteria ingestion by Daphnia galeata in a biomanipulated reservoir: a mechanism stabilizing biomanipulation? Hydrobiologia 403:109-121. [Google Scholar]
- 15.Kandel, A., K. Christoffersen, and O. Nybroe. 1993. Filtration rates of Daphnia cucullata on Alcaligenes eutrophus JMP134 estimated by a fluorescent antibody method. FEMS Microbiol. Ecol. 12:1-8. [Google Scholar]
- 16.Ketley, J. M. 1997. Pathogenesis of enteric infection by Campylobacter. Microbiology 143:5-21. [DOI] [PubMed] [Google Scholar]
- 17.Kilham, S. S., D. A. Kreeger, S. G. Lynn, C. E. Goulden, and L. Herrera. 1998. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377:147-159. [Google Scholar]
- 18.Korhonen, L. K., and P. J. Martikainen. 1991a. Comparison of the survival of Campylobacter jejuni and Campylobacter coli in culturable form in surface water. Can. J. Microbiol. 37:530-533. [DOI] [PubMed] [Google Scholar]
- 19.Korhonen, L. K., and P. J. Martikainen. 1991b. Survival of Escherichia coli and Campylobacter jejuni in untreated and filtered lake water. J. Appl. Bacteriol. 71:379-382. [DOI] [PubMed] [Google Scholar]
- 20.Kuusi, M., P. Klemets, I. Miettinen, I. Laaksonene, H. Sarkkinen, M. L. Hänninen, H. Rautelin, E. Kela, and J. P. Nuarti. 2004. An outbreak of gastroenteritis from a non-chlorinated community water supply. J. Epidemiol. Comm. Health 58:273-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lampert, W. 1987. Feeding and nutrition in Daphnia, p. 143-192. In R. H. Peters and R. de Bernardi (ed.) Daphnia. Mem. Ist. Ital. Idrobiol., 45 Dott. Marco De Marchi, Pallanza, Italy.
- 22.Lázaro, B., J. Cárcamo, A. Audícana, I. Perales, and A. Fernández-Astorga. 1999. Viability and DNA maintenance in nonculturable spiral Campylobacter jejuni cells after long-term exposure to low temperatures. Appl. Environ. Microbiol. 65:4677-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandal, B. K., P. De Mol, and J. P. Butzler. 1986. Clinical aspects of Campylobacter infections in humans, p. 21-31. In J. P. Butzler (ed.) Campylobacter infection in man and animals. CRC Press, Boca Raton, Fla.
- 24.Marin, V., M. E. Huntley, and B. Frost. 1986. Measuring feeding rates of pelagic herbivores: analysis of experimental design and methods. Mar. Biol. 93:49-58. [Google Scholar]
- 25.Mason, M. J., T. J. Humphrey, and K. W. Martin. 1996. Isolation of sublethally injured campylobacters from water, p. 129-133. In Newell et al. (ed.), Campylobacters, helicobacters, and related organisms. Plenum Press, New York, NY.
- 26.McMahon, J. W., and F. H. Rigler. 1965. Feeding rate of Daphnia magna Straus in different foods labeled with radioactive phosphorus. Limnol. Oceanogr. 10:105-113. [Google Scholar]
- 27.Obiri-Danso, K., N. Paul, and K. Jones. 2001. The effect of UVB and temperature on the survival of natural populations and pure cultures of Campylobacter jejuni, Camp. coli, Camp. lari and urease-positive thermophilic campylobacters (UPTC) in surface waters. J. Appl. Microbiol. 90:256-267. [DOI] [PubMed] [Google Scholar]
- 28.Pérez, M. E. M., M. Macek, and M. T. C. Galván. 2004. Do protozoa control the elimination of Vibrio cholerae in brackish water? Int. Rev. Hydrobiol. 89:215-227. [DOI] [PubMed] [Google Scholar]
- 29.Porter, K. G., Y. S. Feig, and E. F. Vetter. 1983. Morphology, flow regimes, and filtering rates of Daphnia, Ceriodaphnia and Bosmina fed natural bacteria. Oecologia 58:156-163. [DOI] [PubMed] [Google Scholar]
- 30.Rollins, D. M., and R. R. Colwell. 1986. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 52:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skelly, C., and P. Weinstein. 2003. Pathogen survival trajectories: an eco-environmental approach to the modeling of human campylobacteriosis ecology. Environ. Health Persp. 111:19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skirrow, M. B., and M. J. Blaser. 1992. Clinical epidemiologic considerations, p. 3-8. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, D.C.
- 33.Stemberger, R. S. 1981. A general approach to the culture of planktonic rotifers. Can. J. Fish. Aquat. Sci. 38:721-724. [Google Scholar]
- 34.Taylor, D. N., K. T. McDermott, J. R. Little, J. G. Wells, and M. J. Blaser. 1983. Campylobacter enteritis from untreated water in the Rocky Mountains. Ann. Intern. Med. 99:38-40. [DOI] [PubMed] [Google Scholar]
- 35.Tholozan, J. L., J. M. Cappelier, J. P. Tissier, G. Delattre, and M. Federighi. 1999. Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl. Environ. Microbiol. 65:1110-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas, C., H. Gibson, D. J. Hill, and M. Mabey. 1999. Campylobacter epidemiology: an aquatic perspective. J. Appl. Microbiol. Symp. Suppl. 85:168S-177S. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, C., D. J. Hill, and M. Mabey. 2002. Culturability, injury and morphological dynamics of thermophilic Campylobacter spp. within a laboratory-based aquatic model system. J. Appl. Microbiol. 92:433-442. [DOI] [PubMed] [Google Scholar]
- 38.Tóth, L. G., K. Kato, and D. Abe. 2001. Grazing of Daphnia galeata (Crustacea, Cladocera) on epi- and hypolimnetic bacteria in the mesotrophic Lake Kizaki, Japan. Verh. Internat. Verein. Limnol. 27:3712-3717. [Google Scholar]
- 39.Vogt, R. L., et al. 1982. Campylobacter enteritis associated with contaminated water. Ann. Intern. Med. 96:292-296. [DOI] [PubMed] [Google Scholar]