Abstract
Pulsed-field gel electrophoresis and PCR were applied for the first time to the molecular characterization of Clostridium tetani. Among five strains tested, one (CN1339) turned out to contain a mixture of two genetically different clones and two (D11 and G761) to contain bacteria differing by the presence or absence of the 74-kb plasmid harboring the tetX gene.
Clostridium tetani, an obligatory anaerobic spore-forming bacillus, is the causative agent of tetanus, whose clinical effects result from the action of a powerful exotoxin, tetanus toxin (TetX). The genetic factor controlling the production of tetanus toxin is associated with an extrachromosomal plasmid (4). Genetic information available for C. tetani is mainly restricted to the nucleotide sequences of the gene encoding the tetanus toxin (tetX) and that encoding its direct transcriptional activator, TetR, both of which are on a 74-kb megaplasmid (2, 3, 9, 10, 11). While the genome of one strain (E88) is now available (1), to date no tools for the typing of C. tetani strains have been published. Consequently, the presence of the plasmid leading to the toxigenic potential has not been studied at the clonal level. Pulsed-field gel electrophoresis (PFGE) is a highly discriminatory technique that has been successfully applied not only to the study of food poisoning cases but also for the identification of clinical isolates of Clostridium perfringens (12, 14), Clostridium difficile (7, 8, 13, 16), and Clostridium botulinum (6, 17). In this context, it was of interest to develop a reliable PFGE protocol for assessing C. tetani strains at the clonal level. In combination with a PCR specific for tetX, this method was used for the molecular characterization of five C. tetani strains.
C. tetani strain A, derived from reference strain Harvard, was kindly provided to the Institut Mérieux in 1955 by the Rijks Instituut voor Volksgezondheid at Bilthoven, The Netherlands. Strain IM1116 was obtained by clinical diagnostic sampling in 1964. Strain D11, obtained in 1966, was derived from the Massachusetts reference strain, like strain E88, which was used for genome sequencing (1). Strain CN1339 was obtained in 1964 from the Wellcome Research Laboratories, and strain G761 was obtained from the Institut Pasteur, Toulouse France, in 1969.
Freeze-dried seeds were inoculated into 10 ml thioglycolate broth (TYG; bioMérieux, Marcy l'Etoile, France) and grown for 24 h. Isolated colonies were obtained by plating serial dilutions of the preculture on Columbia blood agar plates (bioMérieux). All cultures were incubated at 35°C in an atmosphere containing 5% H2, 85% N2, and 10% CO2 using an anaerobic cabinet (Concept Plus; Jouan) for either 24 h for preculture or for 48 h for solid cultures. For PCR assays, isolated colonies were harvested from plates in 50 μl TE buffer (10 mM Tris-HCl [pH 7.4], 0.1 mM Na2EDTA) and boiled for 30 min at 60°C and then for 10 min at 95°C to extract genomic DNA. The sequences of the oligonucleotide primers for PCR amplification of a 1,354-bp fragment of the tetX gene were designed using the GeneJockey II software (Biosoft, Cambridge, United Kingdom) to allow the amplification of a 1,354-bp fragment: Primer sequences were as follows: 5′-CTG GAT TGT TGG GTT GAT AAT G-3′ and 5′-ATT TGT CCA TCC TTC ATC TGT AGG-3′. Five microliters of lysed cells was added to a PCR containing 25 pmol of each primer and 2 U of Taq polymerase (Bio Gene). PCR was performed using a T3 PCR apparatus (Biometra). Cycling conditions used were as follows: an initial denaturation of 95°C for 10 min, followed by 25 cycles each consisting of 1 min at 94°C, 1 min at 52°C, and 1.5 min at 72°C. PCR products were analyzed by electrophoresis on a 1.5% agarose gel. For PFGE and Southern blot analysis, bacteria from solid cultures were collected, adjusted to 2 × 108 CFU/ml, and then mixed 1:1 with 2% agarose (SeaPlaque GTG agarose; FMC BioProducts) to prepare agarose plugs. The embedded cells were lysed in buffer I (6 mM Tris-HCl [pH 7.4], 1 M NaCl, 10 mM Na2EDTA, 0.5% Brij 58, 0.2% Na-deoxycholate, 0.2% Na-lauroylsarcosine) supplemented with 5-mg/ml lysozyme (Sigma) and 1-μg/ml RNase (Roche diagnostic) for 5 h at 37°C. Following this first lysis step, the plugs were incubated overnight at 50°C in 1 ml of buffer II (10 mM Tris-HCl [pH 7.4], 1 M Na2EDTA, 1% sodium lauroylsarcosine, 1 mg of proteinase K per ml). In order to inactivate the proteinase K, the plugs were thoroughly washed with 4 ml of TE buffer (10 mM Tris-HCl [pH 7.4], 0.1 mM Na2EDTA). Agarose plugs were digested overnight at 25°C with 20 U of ApaI or SmaI (Gibco BRL). Electrophoresis was performed on 1% PFGE grade agarose (Bio-Rad) using a CHEF Mapper XA system (Bio-Rad Laboratories). Running conditions were 0.5 to 20 s for both enzymes, using switch times of 0.5 to 20 s for 27 h at 6.0 V/cm and 14°C in 0.5× TBE (44.5 mM Tris-borate, 12.5 mM EDTA [pH 8.0]). Gels were either stained with ethidium bromide and analyzed using a Gel-Doc 1000 system (Bio-Rad Laboratories) or used to prepare Southern blots, which were subsequently probed with a 1,354-bp digoxigenin (DIG)-labeled, double-stranded, tetX-specific DNA fragment. This probe was prepared by PCR using the DIG Chem-Link labeling and detection set kit as recommended by the manufacturer (Diagnostics Roche). PFGE-separated DNA fragments were transferred to a positively charged nylon membrane (Amersham) and UV fixed at 312 nm for 2 min. The blots were then hybridized against the DIG-labeled tetX probe as described in the Genius System User Guide for Filter Hybridization (Roche). Detection of the hybridized tetX probe was performed using a DIG chemiluminescence detection system with chloroplastic drought stress protein (CDSP) as the substrate (Roche).
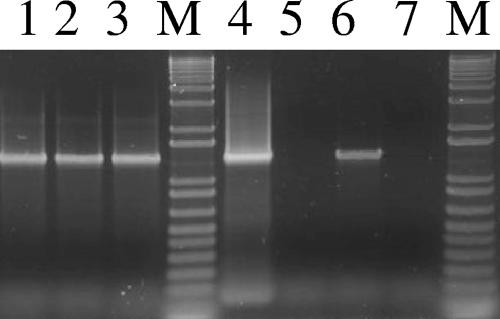
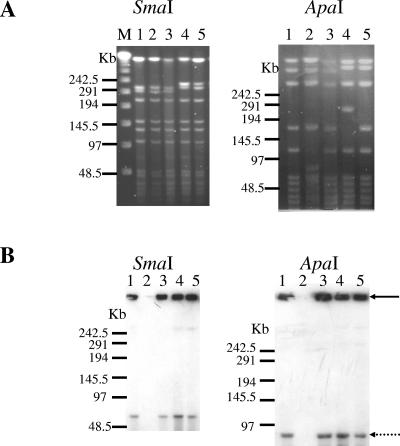
By PCR assay, the five strains studied were found to be positive for tetX, thus confirming their potential to produce the tetanus toxin due to the presence of the 74-kb megaplasmid. However, for two of the five strains (strains G761 and D11), colonies lacking the tetX gene were also detected (Fig. 1, lanes 5 and 7). The high frequency of colonies lacking the tetX gene in strain G761 was found to correlate with its low toxin production (data not shown). The study of Laird and colleagues (9) demonstrated that loss of the 74-kb megaplasmid correlated with the loss of toxinogenicity. However, the issue of whether the same clones were analyzed or whether this result might be explained by the presence of a mixture of a heterogeneous population of bacteria has not been investigated. To address this question and further characterize C. tetani strains, macrorestriction profiling by PFGE was performed. As the G+C content of the chromosome of C. tetani E88 is 28.6% and its plasmid pE88 has a remarkably low G+C content of 24.5% (1), restriction enzymes that cut within GC-rich sites are rare. Therefore, the SmaI and ApaI enzymes were selected for use in PFGE analysis as they were found to yield a suitable number of fragments. Among the five strains tested, two different SmaI and four different ApaI profiles were observed (Fig. 2A). Strains A and D11 displayed the same PFGE profiles with both enzymes. To verify the results obtained by PCR, strains were subsequently subjected to PFGE and Southern blot analysis using the 1,534-bp tetX PCR fragment as a probe. As ApaI and SmaI are located exclusively on the C. tetani chromosome, digestion of DNA samples with each of these two enzymes should produce chromosomal DNA fragments that are able to enter pulsed-field gels but should not affect the migration of the plasmid DNA. The 74-kb plasmid was detected in all strains tested except strain G761 (Fig. 2B, lane 2). This result correlated with tetX PCR colony results.
FIG. 1.
tetX PCR amplification products of DNA extracted from isolated colonies of the C. tetani strains. Lane 1, strain A; lane 2, strain IM1116; lane 3, strain CN1339; lane 4, strain D11, plasmid-positive colony; lane 5, strain D11, plasmid-negative colony; lane 6, strain G761, plasmid-positive colony; lane 7, strain G761, plasmid-negative colony; lanes M, 1-kb plus DNA ladder PCR marker.
FIG. 2.
PFGE and Southern blot analysis of the six tested strains.(A) PFGE of genomic DNA after digestion with SmaI or ApaI. (B) Southern blotting of PFGE-separated fragment digested with SmaI or ApaI using a tetX-specific probe. Strains A (lane 1), G761 (lane 2), D11 (lane 3), IM1116 (lane 4), and CN1339 (lane 5) are shown. Lane M, DNA ladder PFGE marker. The black arrow indicates the position of the gel well, and the dotted arrow indicates the position of the plasmid (74 kb). Results are representative of three independent experiments.
In the case of strain G761, the majority of the colonies were found to have lost the plasmid harboring tetX. However, a tetX-specific fragment was successfully amplified by PCR from 1% of the colonies. Each type of colony (with or without plasmid) was analyzed by PFGE. For both types of colony, the SmaI (Fig. 3A, lanes 3 and 4) and ApaI profiles (data not shown) were identical, demonstrating that the genetic background of colonies selected from strain G761 is the same, irrespective of the presence or absence of the plasmid in the tested colonies (Fig. 3B, lane 3 and 4). Among the five strains studied, strain CN1339 displayed two types of colony, as distinguished by their diffusion and hemolytic characteristics. Based on these visual criteria, two subcultures were prepared from either a small and highly hemolytic colony (clone CN1339-A) or a diffuse and nonhemolytic colony (clone CN13339-B). In order to characterize these clones, PFGE profiles were performed using the SmaI and ApaI enzymes. Two different profiles were observed for the two enzymes (Fig. 3A, lanes 1 and 2), demonstrating that strain CN1339 was in fact a mixture of at least two genetically different clones. The presence of the plasmid was confirmed by Southern analysis following digestion with each of the two enzymes (Fig. 3B, lanes 1 and 2).
FIG. 3.
PFGE and Southern blot analysis of the selected colony types from strains CN1339 and G761. (A) PFGE of genomic DNA after SmaI or ApaI digestion. (B) Southern blotting following PFGE of genomic DNA fragments digested with SmaI or ApaI using a tetX-specific probe. Lane 1, clone CN1339-A; lane 2, clone CN1339-B; lane 3, plasmid-negative clone of the G761 strain; lane 4, plasmid-positive of the G761 strain; lanes M, DNA ladder PFGE marker. The black arrow indicates the position of the gel well, and the dotted arrow indicates the position of the plasmid (74 kb). Results are representative of three independent experiments.
In this study, we have demonstrated for the first time the feasibility of using PFGE for the analysis of C. tetani. Furthermore, we have shown that heterogeneity may be found even within a limited panel of strains and have devised a protocol that allows us to obtain reproducible results for the genotyping of C. tetani. Despite the fact that the genome of C. tetani is available (1), only macroscopic descriptions of C. tetani strains are present in the literature (5, 18, 19). It may be that lack of publications on the genotyping of this species is mainly due to difficulties in obtaining high-quality DNA for PFGE and other genotyping methods. This difficulty is often the result of DNA degradation occurring during the process of DNA isolation due to a high level of endonuclease activity, a feature well described for many species of clostridia (6, 15). As molecular typing methods are generally regarded as superior to phenotypic methods in terms of marker expression, stability, and providing improved levels of typeability, this and future typing studies of C. tetani are likely to facilitate attempts to increase our understanding of the global epidemiology of this pathogen and its associated disease.
Acknowledgments
We are grateful to E. Trannoy and Sharmila Bakshi for critical reading of the manuscript.
REFERENCES
- 1.Brüggemann, H., S. Baumer, W. F. Fricke, A. Wiezer, H. Liesegang, I. Decker, C. Herzberg, R. Martinez-Arias, R. Merkl, A. Henne, and G. Gottschalk 2003. The genome sequence of Clostridium tetani, the causative agent of tetanus disease. Proc. Natl. Acad. Sci. USA 100:1316-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisel, U., W. Jarausch, K. Goretzki, A. Henschen, J. Engels, U. Weller, M. Hudel, E. Habermann, and H. Niemann. 1986. Tetanus toxin: primary structure, expression in E. coli, and homology with botulinum toxins. EMBO J. 5:2495-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn, C. W., R. P. Silver, W. H. Habig, and M. C. Hardegree. 1984. The structural gene for tetanus neurotoxin is on a plasmid. Science 224:881-884. [DOI] [PubMed] [Google Scholar]
- 4.Hara, T., M. Matsuda, and M. Yoneda. 1977. Isolation and some properties of nontoxigenic derivatives of a strain of Clostridium tetani. Biken J. 20:105-115. [PubMed] [Google Scholar]
- 5.Henrichsen, J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36:478-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hielm, S., J. Bjorkroth, E. Hyytia, and H. Korkeala. 1998. Genomic analysis of Clostridium botulinum group II by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 64:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato, H., H. Kita, T. Karasawa, T. Maegawa, Y. Koino, H. Takakuwa, T. Saikai, K. Kobayashi, T. Yamagishi, and S. Nakamura. 2001. Colonization and transmission of Clostridium difficile in healthy individuals examined by PCR ribotyping and pulsed-field gel electrophoresis. J. Med. Microbiol. 50:720-727. [DOI] [PubMed] [Google Scholar]
- 8.Klaassen, C. H., H. A. van Haren, and A. M. Horrevorts. 2002. Molecular fingerprinting of Clostridium difficile isolates: pulsed-field gel electrophoresis versus amplified fragment length polymorphism. J. Clin. Microbiol. 40:101-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laird, W. J., W. Aaronson, R. P. Silver, W. H. Habig, and M. C. Hardegnee. 1980. Plasmid associated toxigenicity in Clostridium tetani. J. Infect. Dis. 142:623. [DOI] [PubMed] [Google Scholar]
- 10.Marvaud, J. C., U. Eisel, T. Binz, H. Niemann, and M. R. Popoff. 1998. TetR is a positive regulator of the tetanus toxin gene in Clostridium tetani and is homologous to BotR. Infect. Immun. 66:5698-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marvaud, J. C., S. Raffestin, M. Gibert, and M. R. Popoff. 2000. Regulation of the toxinogenesis in Clostridium botulinum and Clostridium tetani. Biol. Cell 92:455-457. [DOI] [PubMed] [Google Scholar]
- 12.Maslanka, S. E., J. G. Kerr, G. Williams, J. M. Barbaree, L. A. Carson, J. M. Miller, and B. Swaminathan. 1999. Molecular subtyping of Clostridium perfringens by pulsed-field gel electrophoresis to facilitate food-borne-disease outbreak investigations. J. Clin. Microbiol. 37:2209-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rupnik, M., V. Avesani, M. Janc, C. von Eichel-Streiber, and M. Delmee. 1998. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J. Clin. Microbiol. 36:2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schalch, B., B. Sperner, H. Eisgruber, and A. Stolle. 1999. Molecular methods for the analysis of Clostridium perfringens relevant to food hygiene. FEMS Immunol. Med. Microbiol. 24:281-286. [DOI] [PubMed] [Google Scholar]
- 15.Stolle, A., B. Schalch, and H. Eisgruber. 2001. Comparison of protocols for pulsed-field gel electrophoresis of clostridia. Electrophoresis 22:1585-1589. [DOI] [PubMed] [Google Scholar]
- 16.Talon, D., P. Bailly, M. Delmee, M. Thouverez, B. Mulin, M. Iehl-Robert, V. Cailleaux, and Y. Michel-Briand. 1995. Use of pulsed-field gel electrophoresis for investigation of an outbreak of Clostridium difficile infection among geriatric patients. Eur. J. Clin. Microbiol. Infect. Dis. 14:987-993. [DOI] [PubMed] [Google Scholar]
- 17.Wang, X., T. Maegawa, T. Karasawa, S. Kozaki, K. Tsukamoto, Y. Gyobu, K. Yamakawa, K. Oguma, Y. Sakaguchi, and S. Nakamura. 2000. Genetic analysis of type E botulinum toxin-producing Clostridium butyricum strains. Appl. Environ. Microbiol. 66:4992-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams, K., and A. T. Willis. 1970. A method of performing surface viable counts with Clostridium tetani. J. Med. Microbiol. 3:639-642. [DOI] [PubMed] [Google Scholar]
- 19.Willis, A. T., and K. Williams. 1970. Some cultural reactions of Clostridium tetani. J. Med. Microbiol. 3:291-301. [DOI] [PubMed] [Google Scholar]