Abstract
Little is known of the prevalence of Cryptosporidium and Giardia parasites in sheep and the genotypes that they harbor, although potentially sheep may contribute significantly to contamination of watersheds. In the present study, conducted in Western Australia, a total of 1,647 sheep fecal samples were screened for the presence of Cryptosporidium and Giardia spp. using microscopy, and a subset (n = 500) were screened by PCR and genotyped. Analysis revealed that although both parasites were detected in a high proportion of samples by PCR (44% and 26% for Giardia and Cryptosporidium spp., respectively), with the exception of one Cryptosporidium hominis isolate, the majority of isolates genotyped are not commonly found in humans. These results suggest that the public health risk of sheep-derived Cryptosporidium and Giardia spp. in catchment areas and effluent may be overestimated and warrant further investigation.
The protozoan parasite genus Cryptosporidium has been identified as the cause of numerous outbreaks of diarrheal disease in humans and animals worldwide (15). At present, 14 species of Cryptosporidium are regarded as valid on the basis of differences in oocyst morphology, site of infection, vertebrate class specificity, and genetic differences: C. muris in rodents; C. andersoni in cattle; C. parvum in cattle, humans and other mammals; C. suis in pigs; C. hominis in humans; C. meleagridis in birds and humans, C. baileyi and C. galli in birds; C. serpentis and C. saurophilum in snakes and lizards; C. molnari in fish; C. wrairi from guinea pigs; C. felis in cats; and C. canis in dogs (3, 15, 16, 19, 23, 31, 33, 42).
Giardia duodenalis is the most common intestinal parasite of humans and livestock worldwide (37, 38). There are several major genotypes; genotype A is found in humans, other primates, and livestock, and genotype B is found in humans and other primates. The livestock genotype is found in cattle, sheep, and pigs, the dog genotype is found in dogs, and the rodent genotype is found in rats (37-39).
The genotypes of Cryptosporidium and Giardia spp. that are harbored in sheep and other farmed animals have not been widely reported but it has been assumed that for Cryptosporidium spp. at least, oocysts in the size range of 4 to 6 μm are C. parvum (cattle genotype). There is now strong evidence however that there are numerous genetically distinct Cryptosporidium genotypes which are morphologically identical to C. parvum, but which differ in their zoonotic potential and are likely to be cryptic species (23, 41, 42). As sheep may potentially contribute significantly to contamination of watersheds, more information on the transmission dynamics of Cryptosporidium and Giardia spp. is urgently required. The aim of this study was to determine the prevalence of both parasites in sheep and their relationship to diarrhea and to identify which genotypes are present.
MATERIALS AND METHODS
Sampling.
A survey of parasites in sheep sent for slaughter at the Fletcher International abattoir at Narrikup, on the south coast of Western Australia, was conducted from September 2002 to January 2003. Fecal samples were taken each day from six lines of sheep selected at random, except that preference for sampling was given to lines showing evidence of scouring (diarrhea). A line of sheep was defined as a group of 50 or more sheep consigned from an identified source. Lines were classified as either scouring (at least 10 animals showing evidence of active or recent diarrhea) or nonscouring. From all lines, fecal samples were taken from 10 individual nonscouring animals, and in scouring lines, an additional 10 scouring sheep were sampled. Lambs were categorized as less than 12 months of age, and adults as older than 12 months.
Microscopy.
A total of 1,647 sheep fecal samples were screened for the presence of Cryptosporidium and Giardia spp. using microscopy. Due to the large numbers of samples to be analyzed, fecal samples from individual lines were pooled (five samples per pool). If positives were detected, individual samples were analyzed. Microscopy for Cryptosporidium spp. was carried out using malachite green negative staining (12) and saturated salt flotation was used for the detection of Giardia spp. (18).
Statistical analysis.
Chi-square, risk analysis, and nonparametric tests were performed using SPSS 11.0 (Statistical Package for the Social Sciences) for Macintosh OS X (SPSS Inc., Chicago, Ill.). The association between the presence of the protozoa and age categories or the presence of diarrhea was assessed by calculating odds ratios and their 95% confidence intervals.
DNA extraction and PCR amplification.
A subset of 500 isolates taken at random were screened by PCR for Cryptosporidium and Giardia spp. at the 18S locus. Total DNA was purified from 500 fecal samples using a QiAmp stool kit (QIAGEN, Hilden, Germany). A two-step nested PCR protocol was used to amplify the Giardia 18S rRNA gene and the Cryptosporidium 18S rRNA gene as previously described (18, 32). A subset of Cryptosporidium-positive isolates were also analyzed at the HSP-70 locus as previously described (22).
Sequencing.
PCR products were purified using QIAGEN spin columns (QIAGEN, Hilden, Germany) and sequenced using an ABI Big Dye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Sequences were analyzed using SeqEd v1.0.3. (Applied Biosystems, Foster City, CA).
Phylogenetic analyses.
Nucleotide sequences were aligned using Clustal X (36). Phylogenetic analysis was performed using Treecon version 1.3b (http://www.psb.rug.ac.be/bioinformatics/psb/Userman/treeconw.html), based on evolutionary distances calculated with the Tamura Nei model. In the construction of Cryptosporidium neighbor-joining trees, a sequence of Eimeria bovis (U77084) was used as an outgroup for the 18S rRNA analysis and a sequence of Plasmodium falciparum (M19753) was used for the HSP-70 gene analysis. Giardia ardeae (Z17210) was used as an outgroup for the Giardia neighbor-joining tree. The confidence of grouping was assessed by bootstrapping, using 1,000 replicates.
Nucleotide sequence accession number.
The nucleotide sequence of the novel Cryptosporidium genotype has been deposited in GenBank under accession number AY898790.
RESULTS
Microscopy.
Microscopy detected a total of 43 positives for Cryptosporidium spp. (2.6%) and 144 positives for Giardia spp. (8.7%). Mixed infections were detected in 0.4% of samples by microscopy (Table 1).
TABLE 1.
PCR and genotyping results for Cryptosporidium and Giardia spp.
| Parasite genus | % Positive (no. positive/total)
|
Species/genotype (no.) | |
|---|---|---|---|
| Microscopy | PCR | ||
| Giardia | 8.7 (144/1,647) | 44 (220/500) | Livestock genotype (33) |
| Assemblage A (11) | |||
| Unknown genotype (2) | |||
| Cryptosporidium | 2.6 (43/1,647) | 26.2 (131/500) | Cervid genotype (33) |
| New bovine B genotype (14) | |||
| Pig genotype II (4) | |||
| Marsupial genotype (4) | |||
| C. suis (2) | |||
| C. andersoni (1) | |||
| C. hominis (1) | |||
| Unknown genotype (1) | |||
PCR.
Of the 500 isolates that were screened by PCR for Cryptosporidium and Giardia spp. at the 18S rRNA locus, 131 were positive for Cryptosporidium spp. (a prevalence of 26.2%) and 220 were positive for Giardia spp. (a prevalence of 44%). Mixed infections were detected in 4% of samples by PCR (Table 1).
Statistical analysis.
Lines of lambs were 3.7 times more likely to be positive for Cryptosporidium spp. than adult lines (95% confidence interval: 1.5 to 9.3), and 7.0 times more likely to be positive for Giardia spp. than adult sheep (95% confidence interval: 4.1 to 11.9). Although Cryptosporidium and Giardia spp. were more likely to be detected in lambs, lines of adult sheep positive for Cryptosporidium spp. were 9.7 times more likely to be scouring than negative lines (odds ratio 95% confidence interval: 2.3 to 41.6), and lines positive for Giardia spp. were 3.1 times more likely to be scouring than lines in which Giardia spp. were not detected (95% confidence interval: 1.2 to 8.2). There was no significant relationship between diarrhea and Cryptosporidium or Giardia spp. in lambs.
Genotyping.
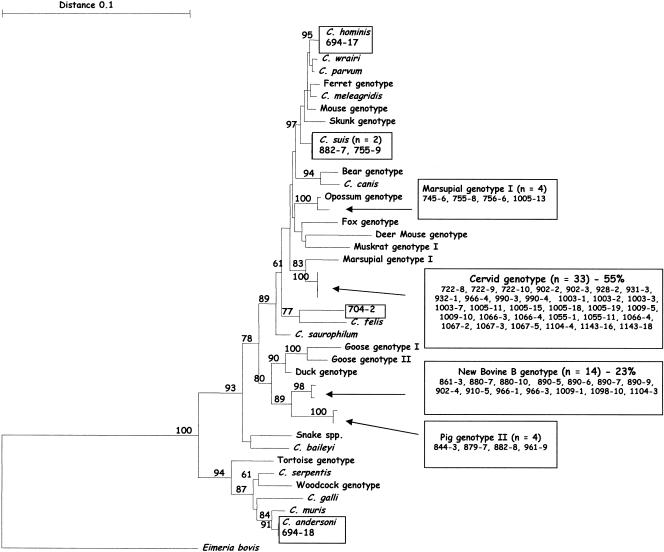
Sequence analysis of 60 Cryptosporidium-positive isolates identified eight distinct genotypes/species of Cryptosporidium spp. at the 18S rRNA locus; C. hominis (one isolate), C. andersoni (one isolate), the cervid genotype (33 isolates), the bovine B genotype (14 isolates), the marsupial genotype (four isolates), the pig genotype II (four isolates), C. suis (two isolates ), and a novel unidentified genotype (one isolate) (Fig. 1).
FIG. 1.
Phylogenetic relationships of Cryptosporidium isolates inferred by neighbor joining analysis of Tamura Nei distances calculated from pairwise comparisons of the 18S rRNA sequences. Percent bootstrap support (>70%) from 1,000 replicate samples is indicated at the left of the supported node.
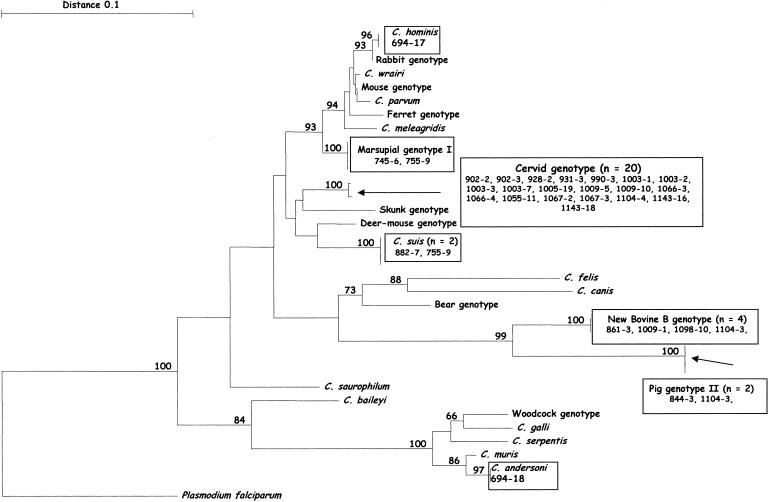
Sequence analysis of a subset of these isolates (32 of 60) at the HSP-70 locus identified seven distinct genotypes/species of Cryptosporidium; the cervid genotype (20 isolates), the bovine B genotype (four isolates), the marsupial genotype (two isolates), C. suis (two isolates) the pig genotype II (two isolates), C. hominis (one isolate) and C. andersoni (one isolate). The novel genotype failed to amplify at the HSP-70 locus (Fig. 2).
FIG. 2.
Phylogenetic relationships of Cryptosporidium isolates inferred by neighbor joining analysis of Tamura Nei distances calculated from pairwise comparisons of the HSP-70 DNA sequences. Percent bootstrap support (>70%) from 1,000 replicate samples is indicated at the left of the supported node.
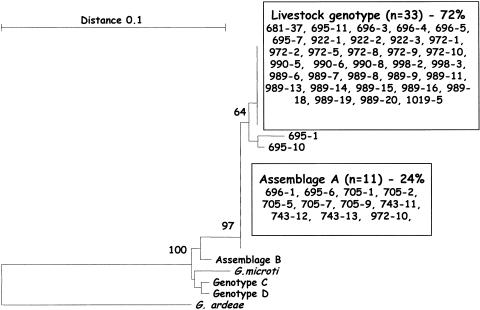
Sequence analysis of 46 Giardia isolates at the 18S rRNA locus identified the majority of genotypes as belonging to the livestock genotype (33 isolates) and assemblage A (11 isolates) and with two isolates (695-1 and 695-10) which exhibited some genetic differences but grouped most closely with the livestock genotype (Fig. 3).
FIG. 3.
Phylogenetic relationships of Giardia isolates inferred by neighbor joining analysis of Tamura Nei distances calculated from pairwise comparisons of the 18S rRNA sequences. Percent bootstrap support (>70%) from 1,000 replicate samples is indicated at the left of the supported node.
DISCUSSION
The prevalence and pathogenic significance and production effects of protozoan infections in sheep have received comparatively little attention in Australia and other nations. Previous studies that have been conducted on the prevalence of Cryptosporidium and Giardia spp. in sheep based on microscopy have reported prevalences ranging from 6.2% to 68.6% for Giardia spp. (6, 10, 25, 35) and 10.1% to 68.3% for Cryptosporidium spp. (1, 7, 15, 20, 21, 26).
In the present study, PCR detection was much more sensitive than microscopy; Giardia was detected in 8.7% (144 of 1,647) of samples by microscopy versus 45.5% (67 of 147) by PCR and Cryptosporidium was detected in 2.6% (43 of 1,647) of samples by microscopy versus 26.25% (63 of 240) by PCR. The disparity between the microscopy and PCR results could have been affected by the fact that samples were pooled for microscopy thereby reducing the sensitivity. However, prior to screening, fecal samples were spiked with known numbers of oocysts/cysts and then pooled and examined. Results revealed that pooling did not appear to significantly alter the sensitivity (data not shown). Another reason for the disparity could also be that shedding of Giardia and Cryptosporidium spp. is often sporadic and in low numbers, which can make microscopy difficult (12).
Cryptosporidium parvum and C. hominis are responsible for most human infections (23) and it has been assumed that the majority of Cryptosporidium infections in farmed animals that had oocysts in the size range of 4 to 6 μm were due to C. parvum (cattle genotype) and that farm animals represent an important zoonotic reservoir for human cryptosporidiosis. The present study suggests that this may not always be the case, as the zoonotic C. parvum (cattle genotype), which has previously been assumed to be the most common species in sheep (15), was not detected in any of the 60 isolates sequenced. The most common Cryptosporidium genotypes identified were the cervid genotype (33 isolates) and the novel bovine B genotype (14 isolates). Both genotypes are genetically very distinct and the cervid genotype appears to have a wide host range, including one report in a human and another in a primate (9, 27, 32, 41). The novel bovine B genotype was first identified in cattle in the USA in 2002 (34, 41), little is known of its prevalence or distribution. This is the first report of this genotype in sheep.
Cryptosporidium andersoni was identified in one sample (694-18). This is the first report of C. andersoni in Australia and also the first report of this species in sheep. Cryptosporidium andersoni is not known to be zoonotic but as C. andersoni is associated with long-term chronic infections and reduced weight gain (19), its finding in Australian sheep is significant and warrants further investigation.
An unknown genotype was identified in one sample (704-2). This genotype is genetically distinct and may represent a new species of Cryptosporidium. Further studies are required to confirm this. Recent research in the United Kingdom has also identified a novel genotype of Cryptosporidium in sheep (8). PCR-restriction fragment length polymorphism analysis of the Cryptosporidium oocyst wall protein (COWP) gene identified the novel genotype in 26 of 60 (43%) isolates sampled (8). Sequence analysis of the COWP gene showed the novel isolate to differ from other Cryptosporidium species and C. parvum isolates by 7 to 21%. The sheep in which the novel isolate was identified were healthy and showed no symptoms of cryptosporidiosis (8). Unfortunately, it is not possible to compare the sequences found in the United Kingdom study with those found in the present study as different genetic loci were sequenced.
Cryptosporidium hominis was detected in one isolate (694-17). This is the first report, to our knowledge, of a natural C. hominis infection in sheep, although recently, several groups have shown that lambs can be experimentally infected with C. hominis (2, 11, 17). Cryptosporidium hominis is the only Cryptosporidium species/genotype detected in the present study that is known to regularly infect humans (42) and as only one isolate was identified, this indicates that the public health risk from contamination of catchment areas, effluent and also abattoirs is probably low.
Sequence analysis of 46 Giardia isolates at the 18S rRNA locus identified the majority of genotypes as belonging to the livestock genotype (33 isolates) and assemblage A (11 isolates) and with two isolates (695-1 and 695-10) which exhibited some genetic differences but grouped most closely with the livestock genotype. The livestock genotype is commonly found in cattle and other hoofed animals including sheep (5, 14, 24) and is not considered zoonotic (37, 38). Assemblage A is geographically the most widespread genotype and as it has been identified previously in both livestock (including sheep) and humans, it is thought to be zoonotic (13, 38). PCR-restriction fragment length polymorphism analysis of trophozoite variant surface proteins has also identified a novel genotype in sheep (13).
The results also indicated that both parasites may play a potential role as pathogens in sheep as there was a significant association between lines of adult sheep that were positive for Cryptosporidium or Giardia spp. and diarrhea. It was not possible to determine if the diarrhea could be attributed entirely to Cryptosporidium or Giardia spp., as the presence of viruses or bacteria were not tested for. However, a study in Canada reported that Giardia infection in specific-pathogen-free sheep was associated with a decrease in rate of weight gain and impairment in feed efficiency. In addition, time to reach slaughter weight was extended in infected lambs, and the carcass weight of Giardia-infected lambs was lower than that of control lambs (25). The authors concluded that “giardiasis in domestic ruminants is an economically important disease, thus necessitating control or elimination of the infection.” In Australia there has been little previous indication of a major problem, but both subclinical effects and unrecognized disease outbreaks are likely in some circumstances. Whether these infections have an unrecognized but occasionally costly sheep production impact in Australia has yet to be determined.
In conclusion, the majority of Cryptosporidium (∼98%) and Giardia (∼76%) isolates genotyped as part of this study are not known to commonly infect humans. This result is surprising, as sheep have often been assumed to be an important reservoir for human infection via both direct transmission and contamination of water catchment areas, effluent, and possibly abattoirs. These results highlight the importance of genotyping analysis as there are significant differences in the zoonotic risk between genotypes and therefore hazard analysis critical control point analysis based on data from microscopy alone could significantly overestimate the risk of human cryptosporidiosis and giardiasis from sheep-derived protozoan contamination of catchments, effluents, and abattoirs.
This is further supported by recent studies in the United States and Australia which reported that mammals and marsupials in watersheds excreted host-adapted Cryptosporidium oocysts not known to be of significant public health importance and that the potential role of wildlife in the transmission of human Cryptosporidium infection had been overestimated (30, 43). This is also in agreement with the previous finding of only wildlife Cryptosporidium genotypes in runoff (storm water) in feral areas in the United States (40). Another study which examined 971 fecal specimens from 15 dairy farms in seven states on the east coast of the United States revealed that C. parvum (cattle genotype) constituted 85% of the Cryptosporidium infections in preweaned calves but only 1% of the Cryptosporidium infections in postweaned calves (the bovine B genotype and a novel deer-like genotype constituted 86% and the remaining 13% were C. andersoni) (34).
In addition, the detection of C. parvum in humans does not always indicate a zoonotic source, as the results of recent subtyping studies have shown the presence of a human-adapted C. parvum subtype which has been widely found in humans in South Africa, Portugal, the United States, and Peru, but which has never been found in animals (4, 28, 29, 42). A limitation of the present study is that preweaned lambs were not included and it is possible, as with the recent study conducted in cattle in the United States, that C. parvum is the most prevalent species in preweaned lambs. Further studies are required to determine the prevalence of Cryptosporidium and Giardia genotypes in all age groups of sheep and lambs, the zoonotic risk they pose, and the extent of economic loss associated with these genotypes.
Acknowledgments
This work was supported by funds from Meat and Livestock Australia (MLA).
We thank Fletchers International Abattoir for their assistance in this study.
REFERENCES
- 1.Abd-El-Wahed, M. M. 1999. Cryptosporidium infection among sheep in Qalubia Governorate, Egypt. J. Egypt Soc. Parasitol. 29:113-118. [PubMed] [Google Scholar]
- 2.Akiyoshi, D. E., X. Feng, M. A. Buckholt, G. Widmer, and S. Tzipori. 2002. Genetic analysis of a Cryptosporidium parvum human genotype 1 isolate passaged through different host species. Infect. Immun. 70:5670-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Pellitero, P., and A. Sitja-Bobadilla. 2002. Cryptosporidium molnari n. sp. (Apicomplexa: Cryptosporidiidae) infecting two marine fish species, Sparus aurata L. and Dicentrarchus labrax L. Int. J. Parasitol. 32:1007-1021. [DOI] [PubMed] [Google Scholar]
- 4.Alves, M., L. Xiao, I. Sulaiman, O. Matos, and F. Antunes. 2003. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 41:2744-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appelbee, A. J., L. M. Frederick, T. L. Heitman, and M. E. Olson. 2003. Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Vet. Parasitol. 25:112:289-294. [DOI] [PubMed] [Google Scholar]
- 6.Buret, A., N. denHollander, P. M. Wallis, D. Befus, and M. E. Olson. 1990. Zoonotic potential of giardiasis in domestic ruminants. J. Infect. Dis. 162:231-237. [DOI] [PubMed] [Google Scholar]
- 7.Causape, A. C., J. Quilez, C. Sanchez-Acedo, E. del Cacho, and F. Lopez-Bernad. 2002. Prevalence and analysis of potential risk factors for Cryptosporidium parvum infection in lambs in Zaragoza (northeastern Spain). Vet. Parasitol. 104:287-298. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers, R. M., K. Elwin, W. J. Reilly, H. Irvine, A. L. Thomas, and P. R. Hunter. 2002. Cryptosporidium in farmed animals: the detection of a novel isolate in sheep. Int. J. Parasitol. 32:21-26. [DOI] [PubMed] [Google Scholar]
- 9.da Silva, A. J., S. Cacciò, C. Williams, K. Y. Won, E. K. Nace, C. Whittier, N. J. Pieniazek, and M. J. Eberhard. 2003. Molecular and morphologic characterization of a Cryptosporidium genotype identified in lemurs. Vet. Parasitol. 111:297-307. [DOI] [PubMed] [Google Scholar]
- 10.Diaz, V., M. Campos, J. Lozano, I. Manas, and J. Gonzalez. 1996. Aspects of animal giardiasis in Granada province (southern Spain). Vet. Parasitol. 64:171-176. [DOI] [PubMed] [Google Scholar]
- 11.Ebeid, M., A. Mathis, A. Pospischil, and P. Deplazes. 2003. Infectivity of Cryptosporidium parvum genotype I in conventionally reared piglets and lambs. Parasitol. Res. 90:232-235. [DOI] [PubMed] [Google Scholar]
- 12.Elliot, A., U. M. Morgan, and R. C. A. Thompson. 1999. Improved staining method for detecting Cryptosporidium oocysts in stools using malachite green. J. Gen. Appl. Microbiol. 45:139-142. [DOI] [PubMed] [Google Scholar]
- 13.Ey, P. L., T. Bruderer, C. Wehrli, and P. Kohler. 1996. Comparison of genetic groups determined by molecular and immunological analyses of Giardia isolated from animals and humans in Switzerland and Australia. Parasitol. Res. 82:52-60. [DOI] [PubMed] [Google Scholar]
- 14.Ey, P. L., M. Mansouri, J. Kulda, E. Nohynkova, P. T. Monis, R. H. Andrews, and G. Mayrhofer. 1997. Genetic analysis of Giardia from hoofed farm animals reveals artiodactyl-specific and potentially zoonotic genotypes. J. Eukaryot. Microbiol. 44:626-635. [DOI] [PubMed] [Google Scholar]
- 15.Fayer, R., U. M. Morgan, and S. J. Upton. 2000. Cryptosporidium as a parasitic zoonotic. Int. J. Parasitol. Special Issue 30:1305-1321. [DOI] [PubMed] [Google Scholar]
- 16.Fayer, R., J. M. Trout, L. Xiao, U. M. Morgan, A. A. Lal, and J. P. Dubey. 2001. Cryptosporidium canis n. sp from domestic dogs. J. Parasitol. 87:1415-1422. [DOI] [PubMed] [Google Scholar]
- 17.Giles, M., K. A. Webster, J. A. Marshall, J. Catchpole, and T. M. Goddard. 2001. Experimental infection of a lamb with Cryptosporidium parvum genotype 1. Vet. Rec. 149:523-525. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins, R. M., B. P. Meloni, D. M. Groth, J. D. Wetherall, J. A. Reynoldson, and R. C. A. Thompson. 1997. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. J. Parasitol. 83:44-51. [PubMed] [Google Scholar]
- 19.Lindsay, D. S., S. J. Upton, D. S. Owens, U. M. Morgan, J. R. Mead, and B. L. Blagburn. 2000. Cryptosporidium andersoni n. sp. (Apicomplexa: Cryptosporiidae) from cattle, Bos taurus. J. Eukaryot. Microbiol. 47:91-95. [DOI] [PubMed] [Google Scholar]
- 20.Mahdi, N. K., and N. H. Ali. 2002. Cryptosporidiosis among animal handlers and their livestock in Basrah, Iraq. East Afr. Med. J. 79:550-553. [DOI] [PubMed] [Google Scholar]
- 21.Majewska, A. C., A. Werner, P. Sulima, and T. Luty. 2000. Prevalence of Cryptosporidium in sheep and goats bred on five farms in west-central region of Poland. Vet. Parasitol. 89:269-275. [DOI] [PubMed] [Google Scholar]
- 22.Morgan, U. M., P. Monis, L. Xiao, J. Limor, S. Raidal, P. O'Donoghue, R. Gasser, A. Murray, R. Fayer, B. Blagburn, A. A. Lal, and R. C. A. Thompson. 2001. Molecular and phylogenetic characterisation of Cryptosporidium from birds. Int. J. Parasitol. 31:289-296. [DOI] [PubMed] [Google Scholar]
- 23.Morgan-Ryan, U. M., A. Fall, L. A. Ward, N. Hijjawi, I. Sulaiman, R. Fayer, R. C. A. Thompson, M. Olson, A. Lal, and L. Xiao. 2002. Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae). J. Eukaryot. Microbiol. 49:433-440. [DOI] [PubMed] [Google Scholar]
- 24.O'Handley, R. M., M. E. Olson, D. Fraser, P. Adams, and R. C. A. Thompson. 2000. Prevalence and genotypic characterisation of Giardia in dairy calves from Western Australia and Western Canada. Vet. Parasitol. 90:193-200. [DOI] [PubMed] [Google Scholar]
- 25.Olson, M. E., T. A. McAllister, L. Deselliers, D. W. Morck, K. J. Cheng, K.J. A. G. Buret, and H. Ceri. 1995. Effects of giardiasis on production in a domestic ruminant (lamb) model. Am. J. Vet. Res. 56:1470-1474. [PubMed] [Google Scholar]
- 26.Olson, M. E., C. L. Thorlakson, L. Deselliers, D. W. Morck, and T. A. McAllister. 1997. Giardia and Cryptosporidium in Canadian farm animals. Vet. Parasitol. 68:375-381. [DOI] [PubMed] [Google Scholar]
- 27.Ong, C. S., D. L. Eisler, A. Alikhani, V. W. Fung, J. Tomblin, J., W. R. Bowie, and J. L. Isaac-Renton. 2002. Novel Cryptosporidium genotypes in sporadic cryptosporidiosis cases: first report of human infections with a cervine genotype. Emerg. Infect. Dis. 8:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng, M. M., S. R. Meshnick, N. A. Cunliffe, B. D. Thindwa, C. A. Hart R. L. Broadhead, and L. Xiao. 2003. Molecular epidemiology of cryptosporidiosis in children in Malawi. J. Eukaryot. Microbiol. 50(Suppl.):557-559. [DOI] [PubMed] [Google Scholar]
- 29.Peng, M. M., M. L. Wilson, R. E. Holland, S. R. Meshnick, A. A. Lal, and L. Xiao. 2003. Genetic diversity of Cryptosporidium spp. in cattle in Michigan: implications for understanding the transmission dynamics. Parasitol. Res. 90:175-180. [DOI] [PubMed] [Google Scholar]
- 30.Power, M. L., M. B. Slade, N. C. Sangster, and D. A. Veal. 2004. Genetic characterisation of Cryptosporidium from a wild population of eastern grey kangaroos Macropus giganteus inhabiting a water catchment. Infect. Genet. Evol. 4:59-67. [DOI] [PubMed] [Google Scholar]
- 31.Ryan, U. M., P. Monis, H. L. Enemark, I. Sulaiman, B. Samarasinghe, C. Read, R. Buddle, I. Robertson, L. Zhou, R. C. A. Thompson, and L. Xiao. 2004. Cryptosporidium suis. n. spp. (Apicomplexa: Cryptosporidiidae) in pigs (Sus scrofa). J. Parasitol. 90:769-773. [DOI] [PubMed] [Google Scholar]
- 32.Ryan, U. M., L. Xiao, C. Read, L. Zhou, A. A. Lal, and I. Pavlasek, I. 2003. Identification of novel Cryptosporidium genotypes from the Czech Republic. Appl. Environ. Microbiol. 69:4302-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan, U. M., L. Xiao, C. Read, I. M. Sulaiman, P. Monis, A. A. Lal, R. Fayer, and I. Pavlasek. 2003. A redescription of Cryptosporidium galli Pavlasek 1999, 2001 (Apicomplexa: Cryptospodiidae) from birds. J. Parasitol. 89:809-813. [DOI] [PubMed] [Google Scholar]
- 34.Santin, M. J. M. Trout, L. Xiao, L. Zhou, E. Greiner, and R. Fayer, R. 2004. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 122:103-117. [DOI] [PubMed] [Google Scholar]
- 35.Taylor, M. A., J. Catchpole, R. N. Marshall, and J. Green. 1993. Giardiasis in lambs at pasture. Vet. Rec. 133:131-133. [DOI] [PubMed] [Google Scholar]
- 36.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, F., and D. G. Higgins. 1997. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson, R. C., R. M. Hopkins, and W. L. Homan. 2000. Nomenclature and genetic groupings of Giardia infecting mammals. Parasitol. Today 16:210-213. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, R. C. A., U. M. Morgan, R. M. Hopkins, and L. J. Pallant. 2000. Enteric protozoan infections, p. 194-209. In R. C. A. Thompson (ed.), Molecular epidemiology of infectious diseases. Arnold, London, United Kingdom.
- 39.van Keulen, H., P. T. Macechko, S. Wade, S. Schaaf, P. M. Wallis, and S. L. Erlandsen. 2002. Presence of human Giardia in domestic, farm and wild animals, and environmental samples suggests a zoonotic potential for giardiasis. Vet. Parasitol. 108:97-107. [DOI] [PubMed] [Google Scholar]
- 40.Xiao, L., K. Alderisio, J. Limor, M. Royer, and A. A. Lal. 2000. Identification of species and sources of Cryptosporidium oocysts in storm waters with a small-subunit rRNA-based diagnostic and genotyping tool. Appl. Environ. Microbiol. 66:5492-5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao, L., I. Sulaiman, U. Ryan, L. Zhou, E. R. Atwill, M. L. Tischler, X. Zhang, R. Fayer, and A. A. Lal. 2002. Host adaptation and host-parasite co-evolution in Cryptosporidium. Int. J. Parasitol. 32:1773-1785. [DOI] [PubMed] [Google Scholar]
- 42.Xiao, L., R. Fayer, U. Ryan, and S. J. Upton. 2004. Cryptosporidium taxonomy: recent advances and implications for public health. Clin. Microbiol. Rev. 17:72-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou, L., R. Fayer, J. M. Trout, U. M. Ryan, F. W. Schaefer III, and L. Xiao. 2004. Genotypes of Cryptosporidium infecting fur-bearing mammals differ from those infecting humans. Appl. Environ. Microbiol. 70:7574-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]