Abstract
An oleaginous fungus, Mortierella alpina 1S-4, is used commercially for arachidonic acid production. Δ12-Desaturase, which desaturates oleic acid (18:1n-9) to linoleic acid (18:2n-6), is a key enzyme in the arachidonic acid biosynthetic pathway. To determine if RNA interference (RNAi) by double-stranded RNA occurs in M. alpina 1S-4, we silenced the Δ12-desaturase gene. The silenced strains accumulate 18:2n-9, 20:2n-9, and Mead acid (20:3n-9), which are not detected in either the control strain or wild type strain 1S-4. The fatty acid composition of stable transformants was similar to that of Δ12-desaturation-defective mutants previously identified. Thus, RNAi occurs in M. alpina and could be used to alter the types and relative amounts of fatty acids produced by commercial strains of this fungus without mutagenesis or other permanent changes in the genetic background of the producing strains.
An arachidonic acid (AA)-producing fungus, Mortierella alpina 1S-4, is used commercially to produce polyunsaturated fatty acids (PUFAs) such as dihomo-γ-linolenic acid (DGLA) (n-6 PUFA; Δ8, Δ11, Δ14-20:3), AA (n-6 PUFA; Δ5, Δ8, Δ11, Δ14-20:4), and eicosapentaenoic acid (n-3 PUFA; Δ5, Δ8, Δ11, Δ14, Δ17-20:5) (25, 34). PUFAs play important roles as structural components of membrane phospholipids and as precursors of the eicosanoids of signaling molecules, including prostaglandins, thromboxanes, and leukotrienes (16, 26). All mammals synthesize eicosanoids that are involved in inflammatory responses, reproductive function, immune responses, and the regulation of blood pressure (7). Although these fatty acids are useful for both researchers and consumers, their high cost and relative scarcity limit their application, and more efficient production strains are needed (25).
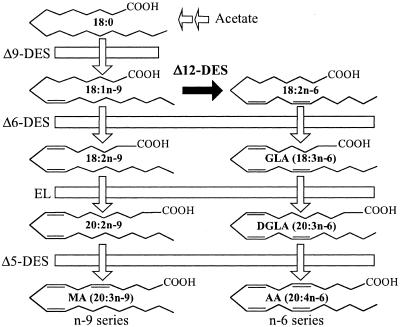
We previously studied the fatty acid metabolism in M. alpina 1S-4 through comparative analyses of fatty acid composition or the accumulation of derivative mutations (9, 11, 12). For example, instead of n-6 fatty acids, Δ12-desaturase-defective mutants accumulate n-9 fatty acids (Fig. 1). We also cloned and sequenced the fatty acid desaturase and elongase genes and analyzed them functionally (21, 22, 23). Wynn et al. (32, 33) identified the rate-limiting step in AA production and the NADPH-producing step responsible for fatty acid synthesis through enzymatic analysis. A rudimentary transformation system is available for M. alpina 1S-4 (27), but this system needs further improvement, and direct gene disruption cannot be performed in this system at this time.
FIG. 1.
Biosynthetic pathway for polyunsaturated fatty aids in Mortierella alpina and its Δ12-desaturase-defective mutants. Mortierella alpina strains produce n-6 fatty acids. Δ12-Desaturase-defective mutants produce n-9 fatty acids by blocking of Δ12-desaturation. DES, desaturase; EL, elongase; 18:0, stearic acid; 18:1n-9, oleic acid; 18:2n-6, linoleic acid; 18:3n-6, γ-linolenic acid; 20:3n-6, DGLA; 20:4n-6, AA; 18:2n-9, 6,9-octadecadienoic acid; 20:2n-9, 8,11-eicosadienoic acid; 20:3n-9, MA (5,8,11-eicosatrienoic acid).
RNA interference (RNAi) is a recent technological advance that reduces gene expression at the posttranscriptional level in various organisms, including trypanosomes (17), fruit flies (13), zebra fish (29), frogs (19), mammals (31), plants (30), and fungi (4, 18). RNAi allows researchers to obtain information about gene function relatively quickly and easily. In addition, if the genes involved in PUFA synthesis are analyzed, then the resulting transformants can be used to identify gene function and to produce novel and/or commercially important PUFAs. Creation of strains that can produce PUFAs currently in limited supply (e.g., Mead acid [MA] [20:3n-9], which has an important metabolic role in mammalian cells [3, 6, 8]), should provide new commercial opportunities.
Our objectives in this study were: (i) to determine if RNAi occurs in Mortierella species, (ii) to determine if RNAi can be used to change the types and relative amounts of fatty acids produced by this fungus, and (iii) to determine if RNAi can be used to create strains that produce qualitatively different sets of fatty acids. If genes involved in fatty acid synthesis, e.g., the Δ12-desaturase gene, were silenced, then PUFAs not normally accumulated by the wild-type strain might be obtained. If novel fatty acids are produced, they could have useful pharmacological effects, and if strains can produce higher levels of one or more relatively scarce fatty acids, then they could be used to increase the commercial production of those fatty acids.
MATERIALS AND METHODS
Strains, media, and growth conditions.
The Czapek-Dox and SC media were as previously described (28). SC medium was used as a uracil-free synthetic medium for cultivation of the ura5− strain and the transformants. The ura5− strain was from M. alpina 1S-4, which was maintained on potato dextrose agar (Difco, Detroit, MI) containing 5-fluoroorotic acid (0.5 mg/ml) and uracil (50 μg/ml) and sporulated on Czapek-Dox medium plus 50 μg/ml uracil. GY medium contains 2% (wt/vol) glucose and 1% yeast extract and was used for fatty acid composition analyses. Media were solidified with 1.5% agar and incubated at 28°C after inoculation. All liquid cultures were grown at 28°C with reciprocal shaking (120 strokes/min). Escherichia coli DH5α was used for DNA manipulation and was cultivated at 37°C with vigorous shaking (300 rpm).
Preparation of genomic DNA and construction of a cDNA library.
Genomic DNA from M. alpina was prepared as described previously (14). Purification of mRNA and cDNA synthesis also were conducted as described previously (2, 22).
Gene-silencing constructs and expression vector.
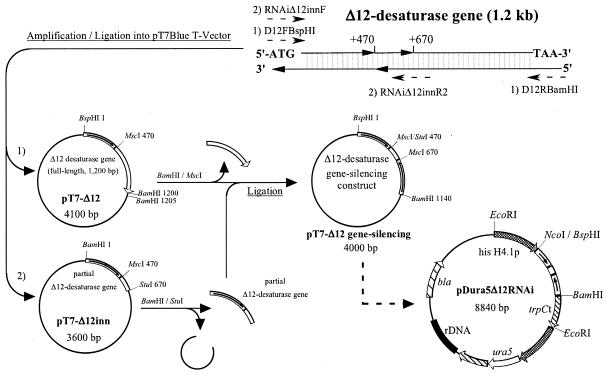
Gene-silencing constructs targeted the Δ12-desaturase gene. The entire Δ12-desaturase gene was amplified with forward primer D12FBspHI (5′-TCATCATGACACCTCCCAACACTA-3′) and reverse primer D12RBamHI (5′-AAAGGATCCTTTACTTCTTGAAAAAGACC-3′), 62°C was the annealing temperature, and 1S-4 genomic DNA was the template. The D12FBspHI primer contains a BspHI restriction site (underlined) and anneals at positions −5 to +19 of the Δ12-desaturase gene (DDBJ accession number AB020033). The D12RBamHI primer contains a BamHI restriction site (underlined) and anneals at positions +1213 to +1185. The ∼1.2-kb PCR product was cloned into the pT7Blue T vector (Novagen, Madison, WI), and designated pT7-Δ12. The BamHI restriction site on primer D12RBamHI is present on the same side as the BamHI restriction site in the multiple cloning sites of the pT7Blue T vector.
A partial Δ12-desaturase gene was amplified with forward primer RNAiΔ12innF (5′-TCAGGATCCCACCTCCCAACACTAT-3′) and reverse primer RNAiΔ12innR2 (5′-CAGAGGCCTTCATAATAAGGTACGCA-3′), 62°C was the annealing temperature, and 1S-4 genomic DNA was the template. The RNAiΔ12innF primer contains a BamHI restriction site (underlined) and anneals at positions −5 to +20 of the Δ12-desaturase gene. The RNAiΔ12innR2 primer contains an StuI restriction site (underlined) and anneals at positions +642 to +667. The ∼700-bp PCR product was cloned into the pT7Blue T vector and designated pT7-Δ12inn. The partial Δ12-desaturase gene obtained by digesting pT7-Δ12 with BamHI and StuI was ligated to pT7-Δ12 treated with BamHI and MscI. The MscI recognition site exists at positions +465 to +470 of the Δ12-desaturase gene. The gene constructs were under the control of the homologous histone H4 promoter, giving rise to a transformation vector for gene silencing designated pDura5Δ12RNAi (Fig. 2).
FIG. 2.
Construction of transformation vector for M. alpina 1S-4 ura5− mutants. Two primers, D12FBspHI/D12RBamHI and RNAiΔ12innF/RNAiΔ12innR2, were used to amplify the partial and whole sequences of the Δ12-desaturase gene, respectively. his H4.1p, Mortierella alpina histone H4.1 promoter; ura5, Mortierella alpina 1S-4 ura5 gene; trpCt, Aspergillus nidulans N-(5′-phosphoribosyl)anthranilate isomerase (trpC) transcription terminator; rDNA, M. alpina 1S-4 rRNA gene fragment; bla, ampicillin resistance gene.
Transformation of M. alpina 1S-4 ura5−, screening of stable transformants, and confirmation of transformation.
M. alpina 1S-4 ura5− was transformed as described previously (27). A PDS-1000/He particle delivery system (Bio-Rad Laboratories Inc., Hercules, CA) was used for the transformation. Tungsten particles (1.1 μm in diameter) coated with pDura5Δ12RNAi were used according to the manufacture's instructions. Intact spores were harvested from the surface of Czapek-Dox agar medium containing uracil (4.0 × 108 spores/300 cm2). Each plate was bombarded twice. After the bombardment, plates were incubated statically at 28°C for 2 to 5 days. Mycelia appearing on the plates were regarded as putative transformants. Transformants were transferred to SC agar medium and maintained for further analyses.
Screening of stable transformants and confirmation of transformation were performed as previously described (27). A vector-specific forward primer, RDNA1 (5′-ACAGGTACACTTGTTTAGAG-3′), which anneals just upstream of the trpC terminator region, and reverse primer RDNA2 (5′-CGCTGCGTTCTTCATCGATG-3′), which anneals to the 5.8S rRNA gene located downstream of the 18S rRNA gene region but not in the vector, were used.
Fatty acid analysis.
Fatty acid analysis was performed as previously described (21, 22, 23). The mycelia of five M. alpina 1S-4-derived strains transformed with pDura5Δ12RNAi were inoculated into 500-ml Erlenmeyer flasks containing 100 ml of GY medium and then cultivated at 28°C with reciprocal shaking (120 strokes/min) for 4 days. Fungal mycelia were harvested by suction filtration, washed with distilled water, and then dried at 120°C for 3 h. The dried cells were directly transmethylated with 10% methanolic HCl at 50°C for 3 h, and the resultant fatty acid methyl esters were extracted with n-hexane, concentrated, and then analyzed by gas-liquid chromatography.
RESULTS
Transformation of M. alpina 1S-4 ura5− with pDura5Δ12RNAi.
The Δ12-desaturase gene was the target for RNAi biosynthesis. Δ12-Desaturase-defective mutants are available (10, 24), and their existence suggests that it is possible to obtain transformants in which the Δ12-desaturase activity has been at least temporarily silenced.
The double-stranded RNA (dsRNA) of Δ12-desaturase from the gene-silencing construct in pDura5Δ12RNAi consists of a 467-bp stem and a 194-bp loop (Fig. 2). Sixty transformants were recovered following transformation with pDura5Δ12RNAi. The morphology and growth rate of the transformants were the same as those of the parent. Five stable transformants, i.e., no. 11, 30, 47, 52, and 56, retained the heterologous DNA for at least three transfers on GY medium (data not shown) and were used for further experiments.
Genomic DNA was prepared following growth in uracil-free SC medium and the transformation confirmed by PCR. A vector-specific primer, RDNA1, and a genomic DNA-specific primer, RDNA2 were used to confirm the transformation. The fragment was amplified by PCR with the two primers and genomic DNA as the template. The heterologous DNA in all of these transformants integrated into the rRNA gene locus (data not shown).
Fatty acid analysis of the transformants.
Stable transformants were cultured in 100 ml of GY medium at 28°C and their fatty acid compositions determined (Table 1). Mut48 strain is a Δ12-desaturase-defective mutant derived from wild type strain 1S-4 (10). The pDura5 transformant was used as a control strain and carries the homologous ura5 gene (27).
TABLE 1.
Comparison of the fatty acid compositions of M. alpina transformantsa
| Strain | Total fatty acid (mg/g of dry cell weight) | Fatty acid composition (mol%)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1n-9 | 18:2n-9 | 20:2n-9 | MA | 18:2n-6 | 18:3n-6 | DGLA | AA | Other | ||
| Wild-type 1S-4 | 160 ± 15 | 19 ± 0.24 | 9.8 ± 0.23 | 24 ± 0.13 | NDb | ND | ND | 7.3 ± 0.01 | 5.5 ± 0.11 | 5.7 ± 0.02 | 21 ± 0.34 | 7.6 ± 0.07 |
| Mut48c | 66 ± 0.32 | 7.8 ± 0.16 | 3.9 ± 0.03 | 63 ± 0.25 | 14 ± 0.06 | 0.63 ± 0.03 | 2.1 ± 0.08 | ND | ND | ND | ND | 7.9 ± 0.03 |
| pDura5 transformantd | 52 ± 7.0 | 12 ± 1.3 | 3.7 ± 0.40 | 23 ± 1.6 | ND | ND | ND | 14 ± 0.74 | 16 ± 1.6 | 4.1 ± 0.07 | 19 ± 0.22 | 8.5 ± 0.63 |
| pDura5Δ12RNAi transformants | ||||||||||||
| 11 | 88 ± 4.1 | 8.1 ± 0.40 | 2.6 ± 0.05 | 57 ± 0.98 | 14 ± 0.66 | 0.50 ± 0.16 | 1.7 ± 0.13 | 0.68 ± 0.21 | 4.2 ± 0.79 | 0.88 ± 0.15 | 5.7 ± 0.71 | 4.3 ± 0.30 |
| 30 | 142 ± 16 | 6.4 ± 0.06 | 4.8 ± 0.02 | 56 ± 0.98 | 14 ± 0.11 | 0.60 ± 0.02 | 1.9 ± 0.03 | 0.17 ± 0.03 | 1.9 ± 0.09 | 0.30 ± 0.04 | 3.4 ± 0.24 | 11 ± 0.57 |
| 47 | 75 ± 1.0 | 7.0 ± 0.06 | 3.8 ± 0.08 | 57 ± 0.11 | 15 ± 0.21 | 0.68 ± 0.01 | 1.9 ± 0.03 | 0.14 ± 0.06 | 1.4 ± 0.04 | 0.27 ± 0.10 | 2.8 ± 0.01 | 10 ± 0.44 |
| 52 | 71 ± 0.71 | 7.3 ± 0.20 | 3.5 ± 1.1 | 54 ± 0.38 | 10 ± 0.26 | 0.54 ± 0.03 | 1.3 ± 0.02 | 0.56 ± 0.02 | 3.3 ± 0.08 | 0.78 ± 0.11 | 6.2 ± 0.08 | 12 ± 0.36 |
| 56 | 41 ± 22 | 5.5 ± 0.05 | 2.3 ± 0.04 | 62 ± 0.07 | 18 ± 0.21 | 0.64 ± 0.0 | 2.3 ± 0.0 | 0.10 ± 0.10 | 1.9 ± 0.05 | 0.60 ± 0.10 | 2.9 ± 0.05 | 3.4 ± 0.04 |
Fungal cultures were grown at 28°C for 4 days in 100 ml of GY medium with vigorous shaking (120 strokes/min).Values are means and standard deviations from duplicate experiments.
ND, not detected.
Mut48 is a Δ12-desaturase-defective mutant derived from the wild-type IS-4 strain (10).
The pDura5 transformant is a control strain. This strain was created by complementation with only the ura5 gene homologous to the host cell (27).
One apparent difference was that all of the pDura5Δ12RNAi transformants produced MA (20:3n-9). The levels of n-9 fatty acids, e.g., 18:1n-9 and 18:2n-9, increased relative to those in the control strain. The amount of n-6 fatty acids, e.g., 18:2n-6, 18:3n-6, DGLA, and AA, decreased. In particular, 18:1n-9, a substrate for Δ12-desaturase, accumulated to up to 60% of the total fatty acid. MA was synthesized through Δ6-desaturation, elongation, and Δ5-desaturation from the accumulated 18:1n-9. Transformant 56 had the lowest Δ12-desaturase activity and the highest MA and lowest AA contents. Thus, Δ12-desaturase gene silencing occurred in these transformants, as a result of RNAi.
DISCUSSION
Previously, studies of RNAi in yeasts or filamentous fungi generally have had basic science objectives (15, 18, 20). However, RNAi also could enable biosynthesis of previously rare and economically valuable compounds. For example, by silencing the structural gene for an enzyme in the PUFA biosynthetic pathway, an unusual and commercially unavailable compound could be synthesized.
We improved the fatty acid content of M. alpina through RNAi. Dietary supplementation of MA inhibits leukotriene B4 synthesis in rats, probably through the inhibition of leukotriene A hydrolase (8). The human neutrophil leukotriene B4 synthesis was reduced by MA more than by eicosapentaenoic acid, which has a known anti-inflammatory effect (3). Previous, Δ12-desaturase-defective mutants, which accumulate MA, were obtained from wild-type strain 1S-4 by chemical mutagenesis with N-methyl-N′-nitro-N-nitrosoguanidine (10). There were no differences between the mutants and the wild type except in fatty acid composition and a slightly lower growth rate (Table 1). Instead of n-6 fatty acids, the Δ12-desaurase-defective mutants accumulated n-9 fatty acids, such as 18:2n-9, 20:2n-9, and MA (20:3n-9), that are not detected in the wild-type strain. The transformants we obtained could produce n-9 and n-6 fatty acids (Table 1). The simultaneous production of two different series of PUFAs is not thought to be commercially advantageous. The productivity of n-9 fatty acids must be reduced by carbon flow into the n-6 fatty acid synthetic pathway. The MA productivity of the RNAi transformants was comparable to that of the Δ12-desaturase-defective mutant. Sakuradani et al. (24) reported that increased production of MA could result from an increase in the Δ6- and Δ5-desaturase activities in a Δ12-desaturse-deficient mutant. Lipid accumulation by the ura5− recipient strain was lower than that by the wild-type strain even if the ura5 gene was complemented (Table 1). These differences in lipid accumulation may be due to mutations at several loci other than ura5. Therefore, the additional changes, e.g., increased PUFA biosynthetic enzyme activity or the creation of more desirable host cells that perform higher lipid accumulation, is needed before RNAi can be used in a commercial production strain.
If the strains were cultivated on GY medium without selective pressure to maintain the ura+ allele, then unstable transformants lost the plasmid and became uracil auxotrophs like the host strain within a one-time cultivation. The fatty acid composition of the unstable transformants was always similar to that of the original ura5− recipient strain (data not shown). Transformation of M. alpina usually results in the integration of multiple copies of the transforming vector. In our case, the vector was constructed so as to be integrated in tandem arrays in the rRNA gene coding region. Some transformants lost the integrated vector by homologous recombination of the sequences (1, 35), but stable transformants never lost the transforming DNA, even if sequentially subcultured on GY medium more than three times (27). Thus, production of n-6 fatty acids by the RNAi transformants was not a result of the excision of the RNAi vector.
Based on the production of n-6 fatty acids, Δ12-desaturase gene expression was not completely suppressed in the RNAi transformants. Δ12-desaturase is present as only a single-copy gene in this fungus, so the gene silencing is presumed to be incomplete. There are several possible explanations for these results. First, our gene-silencing construct, which results in a dsRNA of ∼500 bp in length, may be too short. In other organisms (5, 17), a longer dsRNA is more effective for gene silencing. Thus, an analysis to determine the minimum effective dsRNA length required for complete gene silencing is needed. The regions of the Δ12-desaturase gene that are most effective as a dsRNA for gene silencing also need to be determined. Finally, the number of Δ12-desaturase gene transcripts might be larger than the number of dsRNA molecules, which means there might not be enough dsRNA molecules to interact with all of the Δ12-desaturase transcripts.
We have previously cloned and characterized genes involved in fatty acid synthesis and expressed them heterologously in Aspergillus oryzae (21, 22, 23). These experiments are difficult because insoluble PUFAs must be added to the cultures for incorporation into the transformed strains. In addition, a heterologous protein need not have the correct activity in the host cells, e.g., because a suitable electron donor is not present or the localization of the protein was inappropriate. RNA silencing is a considerable improvement over heterologous expression and allows the function of a protein encoded by an unidentified gene to be determined in situ. We think that gene silencing can be applied to strains used for PUFA production and to functional analysis of genes that encode proteins with unknown function in M. alpina 1S-4. For commercial purposes, the PUFA biosynthetic pathway may be modified by simultaneously silencing various genes in the pathway.
Acknowledgments
We thank David B. Archer (School of Life and Environmental Sciences, University of Nottingham, United Kingdom) for providing the Mortierella transformation vector.
This work was supported in part by the New Energy and Industrial Technology Development Organization (NEDO) and a Grant-in-Aid for Scientific Research (no. 15658024 to S.S.) from the Ministry of Education, Science, Sports, and Culture, Japan.
REFERENCES
- 1.Burmester, A., A. Wostemeyer, and J. Wostemeyer. 1990. Integrative transformation of a zygomycete, Absidia glauca, with vectors containing repetitive DNA. Curr. Genet. 17:155-161. [Google Scholar]
- 2.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 3.Cleland, L. G., M. J. James, S. M. Proudman, M. A. Neumann, and R. A. Gibson. 1994. Inhibition of human neutrophil leukotiriene B4 synthesis in essential fatty acid deficiency: role of leukotriene A hydrolase. Lipids 29:151-155. [DOI] [PubMed] [Google Scholar]
- 4.Cogoni, C., J. T. Irelan, M. Schumacher, T. J. Schmidhauser, E. U. Selker, and G. Macino. 1996. Transgene silencing of the al-1 gene in vegetative cells of Neurospora is mediated by a cytoplasmic effector and does not depend on DNA-DNA interactions or DNA methylation. EMBO J. 15:3153-3163. [PMC free article] [PubMed] [Google Scholar]
- 5.Elbashir, S. M., W. Lendeckel, and T. Tuschl. 2001. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15:188-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eynard, A. R., W. G. Jiang, and R. E. Mansel. 1998. Eicosatrienoic acid (20:3n-9) inhibits the expression of E-cadherin and desmoglein in human squamous cell carcinoma in vitro. Prostaglandins Leukot. Essent. Fatty Acids 59:371-377. [DOI] [PubMed] [Google Scholar]
- 7.Horrobin, D. F. 1992. Nutritional and medical importance of γ-linolenic acid. Prog. Lipid Res. 31:163-194. [DOI] [PubMed] [Google Scholar]
- 8.James, M. J., R. A. Gibson, M. A. Neumann, and L. G. Cleland. 1993. Effect of dietary supplementation with n-9 eicosatrienoic acid on leukotriene B4 synthesis in rats: a novel approach to inhibition of eicosanoid synthesis. J. Exp. Med. 178:2261-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jareonkitmongkol, S., H. Kawashima, N. Shirasaka, S. Shimizu, and H. Yamada. 1992. Production of dihimo-γ-linolenic acid by a Δ5-desaturase-defective mutant of Mortierella alpina 1S-4. Appl. Environ. Microbiol. 58:2196-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jareonkitmongkol, S., S. Shimizu, and H. Yamada. 1992. Fatty acid desaturation-defective mutants of an arachidonic-acid-producing fungus, Mortierella alpina 1S-4. J. Gen. Microbiol. 138:997-1002. [Google Scholar]
- 11.Jareonkitmongkol, S., S. Shimizu, and H. Yamada. 1993. Occurrence of two nonmethylene-interrupted Δ5 polyunsaturated fatty acids in a Δ6-desaturase-defective mutant of the fungus Mortierella alpina 1S-4. Biochim. Biophys. Acta 1167:137-141. [DOI] [PubMed] [Google Scholar]
- 12.Jareonkitmongkol, S., S. Shimizu, and H. Yamada. 1993. Production of an eicosapentaenoic acid-containing oil by a Δ12 desaturase-defective mutant of Mortierella alpina 1S-4. J. Am. Oil Chem. Soc. 70:119-123. [Google Scholar]
- 13.Kennerdell, J. R., and R. W. Carthew. 1998. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 95:1017-1026. [DOI] [PubMed] [Google Scholar]
- 14.Malardier, L., M. J. Daboussi, J. Julien, F. Roussel, C. Scazzocchio, and Y. Brygoo. 1989. Cloning of the nitrate reductase gene (niaD) of Aspergillus nidulans and its use for transformation of Fusarium oxysporum. Gene 78:147-156. [DOI] [PubMed] [Google Scholar]
- 15.Mouyna, I., C. Henry, T. L. Doering, and J. P. Latge. 2004. Gene silencing with RNA interference in the human pathogenic fungus Aspergillus fumigatus. FEMS Microbiol. Lett. 237:317-324. [DOI] [PubMed] [Google Scholar]
- 16.Needleman, P., J. Turk, B. A. Jakschik, A. R. Morrison, and J. B. Lefkowith. 1986. Arachidonic acid metabolism. Annu. Rev. Biochem. 55:69-102. [DOI] [PubMed] [Google Scholar]
- 17.Ngo, H., C. Tschudi, K. Gull, and E. Ullu. 1998. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 95:14687-14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolas, F. E., S. Torres-Martines, and R. M. Ruiz-Vazquez. 2003. Two classes of small antisense RNAs in fungal RNA silencing triggered by non-integrative transgenes. EMBO J. 22:3983-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oelgeschlager, M., J. Larrain, D. Geissert, and E. M. De Robertis. 2000. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature 405:757-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raponi, M., and G. M. Arndt. 2003. Double-stranded RNA-mediated gene silencing in fission yeast. Nucleic Acids Res. 31:4481-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakuradani, E., M. Kobayashi, and S. Shimizu. 1999. Δ6-Fatty acid desaturase from an arachidonic acid-producing Mortierella fungus. Gene cloning and its heterologous expression in a fungus, Aspergillus. Gene 238:445-453. [DOI] [PubMed] [Google Scholar]
- 22.Sakuradani, E., M. Kobayashi, and S. Shimizu. 1999. Δ9-Fatty acid desaturase from arachidonic acid-producing fungus. Unique gene sequence and its heterologous expression in a fungus, Aspergillus. Eur. J. Biochem. 260:208-216. [DOI] [PubMed] [Google Scholar]
- 23.Sakuradani, E., M. Kobayashi, T. Ashikari, and S. Shimizu. 1999. Identification of Δ12-fatty acid desaturase from arachidonic acid-producing Mortierella fungus by heterologous expression in the yeast Saccharomyces cerevisiae and the fungus Aspergillus oryzae. Eur. J. Biochem. 261:812-820. [DOI] [PubMed] [Google Scholar]
- 24.Sakuradani, E., N. Kamada, Y. Hirano, M. Nishihara, H. Kawashima, K. Akimoto, K. Higashiyama, J. Ogawa, and S. Shimizu. 2002. Production of 5,8,11-eicosatrienoic acid by a Δ5 and Δ6 desaturation activity-enhanced mutant derived from a Δ12 desaturation activity-defective mutant of Mortierella alpina 1S-4. Appl. Microbiol. Biotechnol. 60:281-287. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu, S., J. Ogawa, M. Kataoka, and M. Kobayashi. 1997. Screening of novel microbial enzymes for the production of biologically and chemically useful compounds, p. 45-87. In T. Schepter (ed.), Advances in biochemical engineering/biotechnology, vol. 58. Springer-Verlag, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 26.Smith, W. L., and P. Borgeat. 1985. The eicosanoids: prostaglandins, thromboxanes, leukotrienes, and hydroxy-eicosaenoic acids, p. 325-360. In D. E. Vance and J. E. Vance (ed.), Biochemistry of lipids and membranes. Benjamin/Cummings, Menlo Park, Calif.
- 27.Takeno, S., E. Sakuradani, S. Murata, M. Inohara-Ochiai, H. Kawashima, T. Ashikari, and S. Shimizu. 2004. Establishment of an overall transformation system for an oil-producing filamentous fungus, Mortierella alpina 1S-4. Appl. Microbiol. Biotechnol. 65:419-425. [DOI] [PubMed] [Google Scholar]
- 28.Takeno, S., E. Sakuradani, S. Murata, M. Inohara-Ochiai, H. Kawashima, T. Ashikari, and S. Shimizu. 2004. Cloning and sequencing of the ura3 and ura5 genes, and isolation and characterization of uracil auxotrophs of the fungus Mortierella alpina 1S-4. Biosci. Biotechnol. Biochem. 68:277-285. [DOI] [PubMed] [Google Scholar]
- 29.Wargelius, A., S. Ellingsen, and A. Fjose. 1999. Double-stranded RNA induces specific developmental defects in zebrafish embryos. Biochem. Biophys. Res. Commun. 263:156-161. [DOI] [PubMed] [Google Scholar]
- 30.Waterhouse, P. M., M. W. Graham, and M. B. Wang. 1998. Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc. Natl. Acad. Sci. USA 95:13959-13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wianny, F., and M. Zernicka-Goetz. 2000. Specific interference with gene function by double-stranded RNA in early mouse development. Nat. Cell Biol. 2:70-75. [DOI] [PubMed] [Google Scholar]
- 32.Wynn, J. P., A. A. Hamid, and C. Ratledge. 1999. The role of malic enzyme in the regulation of lipid accumulation in filamentous fungi. Microbiology 145:1911-1917. [DOI] [PubMed] [Google Scholar]
- 33.Wynn, J. P., and C. Ratledge. 2000. Evidence that the rate-limiting step for the biosynthesis of arachidonic acid in Mortierella alpina is at the level of the 18:3 to 20:3 elongase. Microbiology 146:2325-2331. [DOI] [PubMed] [Google Scholar]
- 34.Yamada, H., S. Shimizu, and Y. Shinmen. 1987. Production of arachidonic acid by Mortierella elongata 1S-5. Agric. Biol. Chem. 51:785-790. [Google Scholar]
- 35.Yanai, K., H. Horiuchi, M. Takagi, and K. Yano. 1990. Preparation of protoplasts of Rhizopus niveus and their transformation with plasmid DNA. Agric. Biol. Chem. 54:2689-2696. [PubMed] [Google Scholar]