Abstract
The Rho GTPases play distinctive roles in cytoskeletal reorganization associated with growth and differentiation. The Cdc42/Rac-binding p21-activated kinase (PAK) and Rho-binding kinase (ROK) act as morphological effectors for these GTPases. We have isolated two related novel brain kinases whose p21-binding domains resemble that of PAK whereas the kinase domains resemble that of myotonic dystrophy kinase-related ROK. These ∼190-kDa myotonic dystrophy kinase-related Cdc42-binding kinases (MRCKs) preferentially phosphorylate nonmuscle myosin light chain at serine 19, which is known to be crucial for activating actin-myosin contractility. The p21-binding domain binds GTP-Cdc42 but not GDP-Cdc42. The multidomain structure includes a cysteine-rich motif resembling those of protein kinase C and n-chimaerin and a putative pleckstrin homology domain. MRCKα and Cdc42V12 colocalize, particularly at the cell periphery in transfected HeLa cells. Microinjection of plasmid encoding MRCKα resulted in actin and myosin reorganization. Expression of kinase-dead MRCKα blocked Cdc42V12-dependent formation of focal complexes and peripheral microspikes. This was not due to possible sequestration of the p21, as a kinase-dead MRCKα mutant defective in Cdc42 binding was an equally effective blocker. Coinjection of MRCKα plasmid with Cdc42 plasmid, at concentrations where Cdc42 plasmid by itself elicited no effect, led to the formation of the peripheral structures associated with a Cdc42-induced morphological phenotype. These Cdc42-type effects were not promoted upon coinjection with plasmids of kinase-dead or Cdc42-binding-deficient MRCKα mutants. These results suggest that MRCKα may act as a downstream effector of Cdc42 in cytoskeletal reorganization.
The Ras-related p21 Rho subfamily GTPases are implicated in actin reorganization, although the exact mechanisms involved remain largely obscure (48). In Swiss 3T3 fibroblasts, introduction of Cdc42 into cells resulted in filopodial formation (24, 41), while Rac1 and RhoA give rise to lamellipodia and stress fibers, respectively (43, 44). Apart from cell morphology, the Rho p21s are also involved in processes such as cell growth, cytokinesis, activation of transcription factors, and cell cycle progression (13, 14, 39, 40, 42). An important step toward understanding the biochemical mechanisms by which these p21s exert their diverse cellular effects is to identify and characterize interacting proteins which mediate the actions of a particular p21. To date, a large number of proteins which interact with Rho p21s have been reported. These include regulatory proteins such as GTPase-activating proteins, guanine nucleotide exchange factors, guanine nucleotide dissociation inhibitors and an increasing number of kinases and nonkinases (32, 48). Most of these molecules have multidomain structures (10, 26, 32), suggesting the existence of a wide range of multimolecular complexes in regulating signalling pathways underlying cell morphology and other related cellular activities. Such complexity has been shown in lower organisms such as Saccharomyces cerevisiae in which normal polarized cell growth and cell shape changes are accomplished by the interaction of Cdc42p with several proteins, including Cdc24 (a guanine nucleotide exchange factor), Ste20p kinase, and actin-binding protein (28, 52). In mammalian cells, input from other signalling pathways can also be implicated in the p21 functions; e.g., phosphatidylinositol 3-kinase can mediate signalling from Ras to Rac1 (45).
In searching for potential targets of the p21 Rho family, we and others have identified p21-activated kinases (PAKs) which specifically interact with GTP-Cdc42/Rac1 (36) and the RhoA-binding kinases (ROKs) (19, 30, 31, 38). Interaction of PAK with p21 in vitro results in kinase activation (36, 37). This novel activation process has led us and others to postulate that the yeast homolog of mammalian PAK, Ste20p, may act downstream of Cdc42p in the heterotrimeric G-protein-coupled yeast pheromone Kss1/Fus3 mitogen-activated protein kinase pathway (47, 54). Similarly, mammalian Cdc42 and Rac1 have also been found to have nuclear signalling roles through the JNK/SAPK mitogen-activated protein kinase pathway (13, 39). Several reports have also implicated PAKs in these events (5, 8, 53), suggesting a parallel conservation of components in these signalling events among eukaryotes. In mammalian cells, expression of various constitutively active forms of αPAK results in disassembly of focal complexes and stress fiber dissolution, suggesting that these kinases also have morphological roles (34). ROKs also have effects on morphology, with their overexpression enhancing the formation of stress fibers and focal adhesion complexes (1, 20, 30). This effect of ROKs may be mediated by their inhibition of myosin phosphatase through specific phosphorylation of its myosin-binding subunit, which increases the phosphorylation state of myosin light chain (23). Alternatively, ROKs may also activate myosin through direct phosphorylation of myosin light chain (2). These results suggest that a diverse network of p21 targets, in particular kinases, is involved in both nuclear and cytoskeletal control (32). The use of mutants of Rac1 and Cdc42Hs has also revealed different pathways utilizing distinctive effectors for morphological as well as transcriptional activation (21, 27, 51).
Apart from PAKs, the p21 binding assay has revealed the presence of multiple proteins of 180 to 200 kDa in a variety of rat tissues which bind Cdc42Hs/Rac1 (36). We have purified several ∼180-kDa Cdc42/Rac1-binding proteins from rat brain and liver which turned out to be identical to IQGAP isoforms isolated by others (18, 25). We now report the isolation and characterization of MRCKs, a novel family of ∼190-kDa serine/threonine kinases highly related to the myotonic dystrophy kinase (7, 15) and ROKs (19, 30, 31, 38), which interact strongly with the GTP-bound form of Cdc42. These kinases also contain a cysteine-rich domain capable of binding to phorbol ester and a putative pleckstrin homology (PH) domain. The possible involvement of these kinases as Cdc42 effectors in cytoskeletal reorganization is also presented.
MATERIALS AND METHODS
Screening and expression of MRCKα and -β.
A λgt11 human brain cDNA library (Clontech) was used for expression screening with [γ-32P]GTP–glutathione S-transferase (GST)–Cdc42Hs as previously described (35). The 373-bp positive cDNA clone encoding the 124-amino-acid residues and its deleted and mutated derivatives were subcloned into pGEX vectors for expression and p21 binding analysis. For isolating the full-length clones, a rat brain λZAP cDNA library (Stratagene) was used. The full-length MRCKα was derived from an 8-kb cDNA clone containing the entire coding sequence. The MRCKβ sequence was derived from three overlapping clones. Full-length MRCKα (see below) was subcloned into pBAK-GST vector for expression in the baculovirus system (Clontech). The GST fusion protein was purified through a glutathione-Sepharose column and used for kinase assays with various substrates. For expression in mammalian cells, MRCKα was subcloned from pBluescript SK vector into either plasmid pXJ40-HA or plasmid pXJ40-FLAG (34). A BamHI/PstI-digested PCR product of the 5′ end corresponding to the N-terminal kinase domain was obtained by using a 5′ primer (5′CGGGATCCAACATGTCTGGAGAAGTGCG3′) and a 3′ primer (5′-CTCTGCGAAGCTCCTG-3′) and ligated to the BamHI/PstI-cut pXJ40 vector to generate the kinase domain construct MRCKα1-473. Full-length MRCKα1-1732 was obtained by replacing a BstXI/KpnI fragment of this subclone by a longer 6-kb BstXI/KpnI fragment from the full-length SK vector. For MRCKΔPH, an in-frame deletion of an EcoRV/NheI (blunted) fragment (residues 1117 to 1181) was made. For mutagenesis, a two-step PCR protocol (34) with VENT polymerase (New England Biolabs) was used. The p21-binding-defective mutant (MRCKαH1579A,H1582A) was obtained with primers GTTAAAATTAGTTGGG-3′/T3 and 5′-GCCATAGCAGCATGGGTCCTGGACCTG-3′/T7, and the kinase-dead mutant (MRCKαK106A) was obtained with primers 5′GCCATGGCAAATACTTTATC3′/T7 and 5′GCCATGGCAATTCTGAACAAGTGG3′/T3, by using suitable MRCKα subclones in pBluescript SK vector, and the final constructs were fully sequenced. Expression of the correct-size proteins was confirmed with a TNT in vitro translation kit (Promega) (data not shown) and expression in COS-7 cells (Fig. 3D). All other constructs used have been previously described (30, 34).
FIG. 3.
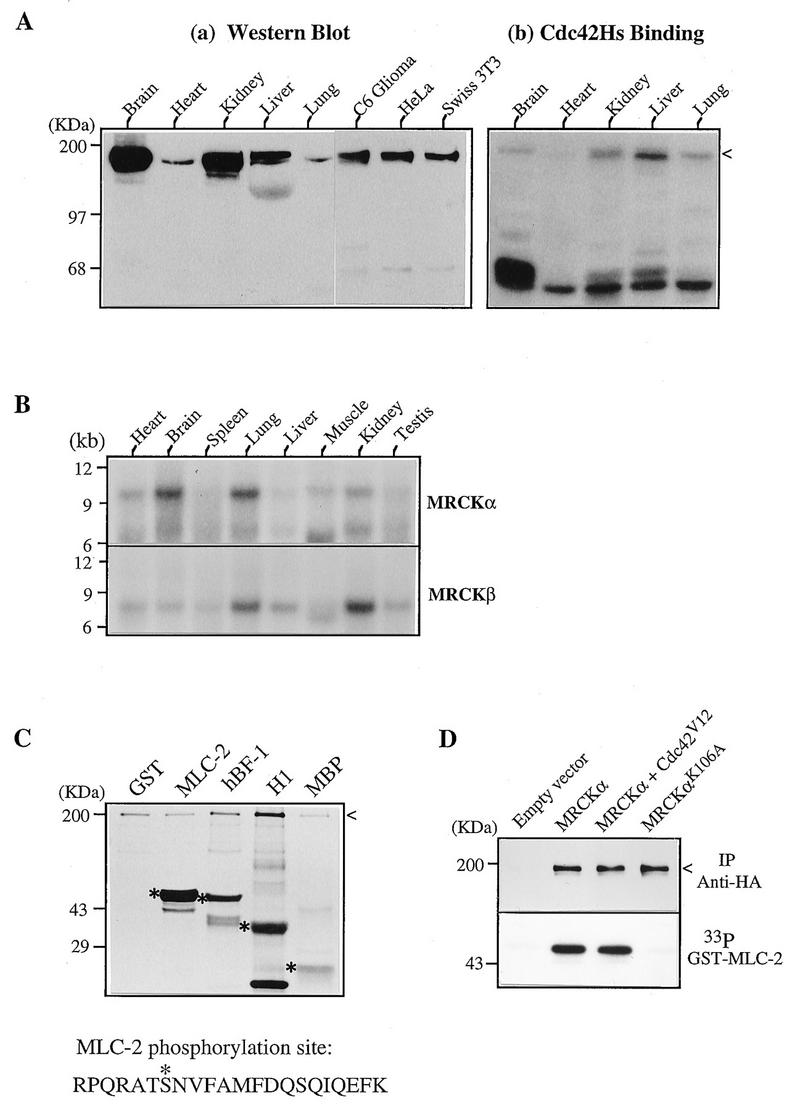
Expression and biochemical characterization of MRCKs. (A) Expression of MRCK in tissues and cultured cells. (a) Soluble protein extracts from various rat tissues and cells were separated by polyacrylamide gel electrophoresis and transferred to nitrocellulose filters for Western analysis using antibodies against the Cdc42-binding domain of human MRCKα (C-terminal 124 residues). (b) A similar blot showing Cdc42 binding. The arrowhead indicates the positions of the immunoreactive and Cdc42-binding regions. (B) Northern (mRNA) blot from rat tissues (Clontech) hybridized to the 32P-labeled MRCKα and MRCKβ cDNA probes. (C) Kinase activity toward different substrates. GST–MLC-2, GST-MRCKα p21-binding domain hBF-1 (residues 1 to 124; Fig. 1), histone H1, and myelin basic protein (MBP) were used as substrates in a kinase assay with purified MRCKα expressed in baculovirus as a GST fusion protein. The 33P-labeled bands corresponding to the Coomassie blue-stained substrate proteins are marked with asterisks, and the autophosphorylated GST-MRCKα band is indicated by an arrowhead. The sequence at the bottom shows the Lys-C peptide with the serine 19 (asterisk) phosphorylation site in MLC-2. (D) Kinase activities in transfected cells. COS-7 cells were transfected with vector pXJ40HA or with a vector containing MRCKα alone, MRCKα in combination with Cdc42V12, or kinase-inactive MRCKαK106A. Tagged proteins were immunoprecipitated (IP) with anti-HA antibody, and kinase activity (lower panel) was assayed with [γ-33P]ATP as described elsewhere (36).
RNA and protein analysis.
Total RNA (20 μg) from rat tissues and cells obtained by a guanidinium thiocyanate method was used to hybridize to either a 2.4-kb EcoRI fragment of MRCKα (nucleotides 3088 to 5474) or a 3-kb StuI fragment of MRCKβ (nucleotides 1966 to 4932). For Western blot analysis and p21 binding, 150 μg of protein from each tissue was used to probe either with the affinity-purified mouse polyclonal antibodies against human MRCKα or with [γ-32P]GTP-Cdc42. Nucleotide-dependent binding was carried out exactly as specified in reference 31, with GST-Cdc42 from a GST-2TK vector. For the kinase assay, myelin basic protein, histone H1, and GST–myosin light chain 2 (MLC-2) were used as substrates. The latter was obtained by PCR of a human brain cDNA preparation by using primers 5′-CAGGATCCATGTCGAGCAAAAGAAC-3′ and 5′-CTGAATTCAGTCATCTTTGTCTTTGG-3′ (16). The PCR product was digested with BamHI-EcoRI and subcloned into pGEX4T3. Phosphorylated protein bands after transfer onto polyvinylidene difluoride filters were subjected to phosphoamino acid analysis (36) or complete Lys-C digestion. A single phosphorylation peak obtained from high-pressure liquid chromatography was analyzed by peptide sequencing with simultaneously radioactive detection of each residue. For detecting kinase activity of MRCKα in overexpressed COS-7 cells, transfected cells were extracted with lysis buffer containing 25 mM HEPES (pH 7.3), 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 20 mM sodium β-glycerophosphate, 1 mM sodium vanadate, 0.5% Triton X-100, 5% glycerol, and freshly added 5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, and 5 μg each of aprotinin and pepstatin per ml. Clarified extracts (0.5 mg) were passed through 40-μl columns of beads preloaded with anti-hemagglutinin (HA) monoclonal antibody (MAb) 12CA5. After washing with lysis buffer and kinase buffer containing 50 mM HEPES (pH 7.3), 50 mM KCl, 10 mM β-glycerophosphate, 10 mM MgCl2, 2 mM MnCl2, and 0.05% Triton X-100, kinase reactions were carried out for 20 min at 30°C with 0.1 mg of GST–MLC-2 per ml and 10 μM [γ-32P]ATP. The reaction was stopped by adding 50 μl of 2× sodium dodecyl sulfate sample buffer. After a brief spin in an Eppendorf tube, the reaction mixture was run on a 10% polyacrylamide gel and transferred onto a nitrocellulose filter for radioactive imaging and subsequent detection of expressed proteins by a rabbit anti-HA antibody (BabCo). 32P phosphorylation of MLC-2 was quantified with a Molecular Dynamics PhosphorImager.
Transfection and microinjection.
COS-7 cells and HeLa cells maintained in 10% fetal bovine serum were transfected essentially as described previously (30) except that DOSPR (5 μl/ml; Boehringer Mannheim) was used. For immunoprecipitation experiments, cells grown in a 100-mm-diameter dish were transfected and incubated for 16 h before harvest. For immunofluorescence studies, HeLa cells were plated onto glass chamber slides (Nunc), transfected in the presence of 5% serum, and fixed after 16 h of incubation. For microinjection, HeLa cells maintained in minimal essential medium in the presence or absence of 10% fetal bovine serum were used. Subconfluent cells plated on coverslips for 48 h were microinjected with different constructs (50 ng/μl except where indicated otherwise), by using an Eppendorf micromanipulator system. Two to four hours after injection, cells were fixed with 4% paraformaldehyde and incubated in phosphate-buffered saline–0.5% Triton X-100 for 2 h at 25°C with the combination of various primary antibodies at the following dilutions: anti-HA (12CA5) or anti-FLAG (IBI) MAb, 5 μg/ml; antivinculin MAb (hVIN-1; Sigma), 1:300; antipaxillin MAb (Transduction Laboratories), 2 μg/ml; anti-myosin light chain MAb (Sigma), 1:100. Fluorescein isothiocyanate-conjugated second antibodies (1:100; Sigma) and rhodamine-conjugated second antibodies (1:50; Boehringer Mannheim) were incubated for 1 h at 25°C. To visualize polymerized actin, cells were stained with rhodamine-conjugated phalloidin (1 μg/ml; Sigma) for 1 h at room temperature. Stained cells were analyzed with an MRC600 confocal imager adapted to a Zeiss Axioplan microscope. For phase-contrast microscopy, cells after injection were viewed under a Zeiss Axiovert microscope with a temperature-controlled stage, and phase-contrast views were taken at various time intervals.
Nucleotide sequence accession numbers.
The GenBank accession numbers are AF021935 (MRCKα) and AF021936 (MRCKβ).
RESULTS
Identification of a novel Cdc42Hs-binding domain by expression screening.
Using expression screening with [γ-32P]GTP-Cdc42, we obtained a partial cDNA from a human brain cDNA library. This cDNA fragment, a shorter AseI/EcoRI-deleted fragment, and a PCR fragment flanking the putative p21-binding site when expressed as GST fusion proteins all bound GTPγS-Cdc42 but not GDP-Cdc42 (Fig. 1A and B). Double mutation of the conserved histidines led to abolition of binding. Weak binding was also observed to GTP-Rac1 but not to GTP-RhoA (data not shown). The binding domain is conserved in the two rat isoforms isolated from further screening and resembles other p21-binding motifs of this class (9, 36) (Fig. 1C) but not the RhoA-binding sequence of ROK or protein kinase N (PKN) (3, 30, 50).
FIG. 1.
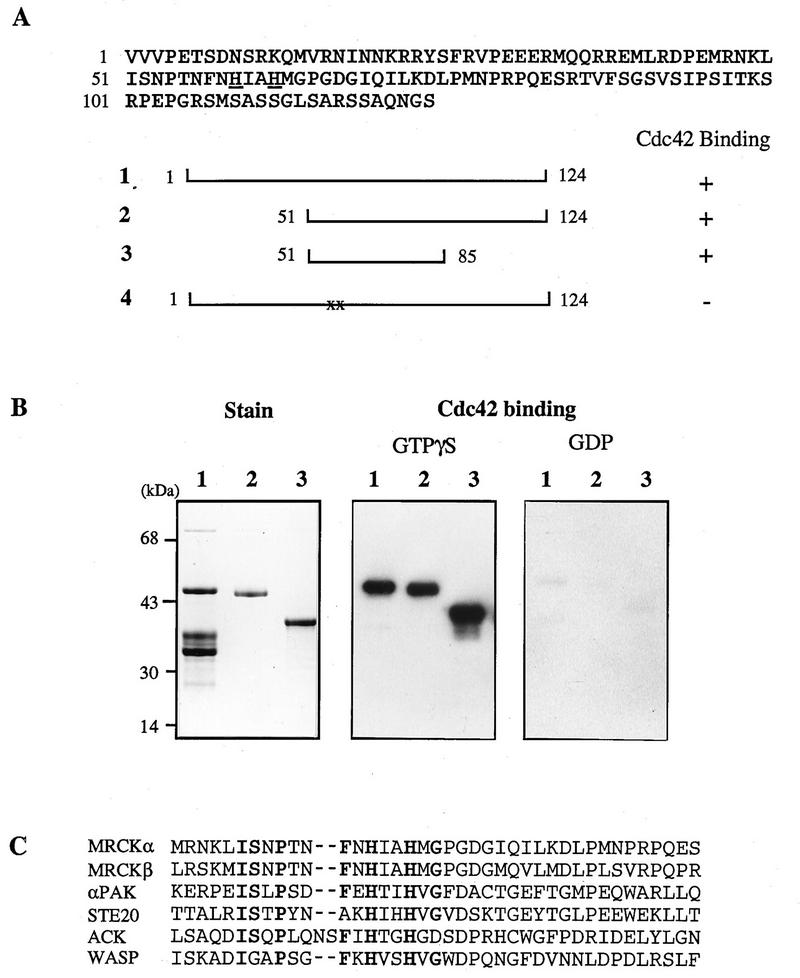
Identification of a family of Cdc42Hs-binding proteins. (A) Deduced amino acid sequence of a human brain partial cDNA clone isolated by expression screening with [γ-32P]GTP-Cdc42. GST fusion proteins were made with wild-type (construct 1), deleted (constructs 2 and 3), or mutated (construct 4) variants. In construct 4, two histidines (underlined) were mutated to alanine. Binding of [γ-32P]GTP-Cdc42 was performed as described previously (35). (B) Nucleotide-dependent binding by two related rat Cdc42-binding proteins. Nitrocellulose filters with 50 ng of GST fusion protein containing the binding domain of human MRCKα (residues 1 to 124; lane 1), rat MRCKβ (residues 1569 to 1690; lane 2), and αPAK (residues 67 to 150; lane 3) were assayed for binding with a 32P-phosphorylated Cdc42Hs (from pGEX-2TK) exchanged with either GTPγS or GDP (31). (C) Consensus sequence of Cdc42-binding motifs of different proteins (9).
Identification of MRCKs.
Two related full-length cDNAs were isolated upon subsequent screening of a rat brain cDNA library. The N termini of the predicted proteins (Fig. 2A) begin with a kinase domain (Fig. 2B) exhibiting 68% identity to the human myotonic dystrophy kinase. These kinases were designated MRCKα and -β. The kinase domains were followed by an extended α-helix, with coiled-coil features (residues 450 to 950 in MRCKα) and a highly conserved region (residues 810 to 860 in MRCKα) which has some homology to nonmuscle myosin heavy chain and rat nestin (29). Both isoforms of ∼190 kDa contain the p21-binding motif near the C-terminus, with the domain organization of these kinases being quite different from that of ROKα (Fig. 2A). A cysteine-rich domain (Fig. 2D) and a pleckstrin-like domain (Fig. 2C) occur between the kinase domain and the p21-binding motif.
FIG. 2.


Sequence of a family of Ser/Thr kinases containing a Cdc42-binding domain and other functional domains. (A) Deduced amino acid sequences of rat MRCKα and MRCKβ. Regions in boldface represent, in order, kinase, cysteine-rich (CR), PH, and p21 GTPase-binding (GBD) domains. In MRCKα, the region underlined is identical, apart from an initial L→V, to the human sequence shown in Fig. 1A. Domain organization of MRCKs, myotonic dystrophy kinase (DMK), and ROKα, along with percent identities of related domains, is also shown. (B) Kinase domains of MRCKα, MRCKβ, myotonic dystrophy kinase (DMK), and ROKα. (C) PH domains in MRCKα, MRCKβ, ROKα, and pleckstrin N terminus (Pleck N). Amino acids identical to the most commonly occurring consensus sequence in PH domains (in boldface and uppercase) are marked with asterisks. (D) Cysteine-rich domains of MRCKs, PKCα, and n-chimaerin (n-CHIM). Conserved residues (17) are indicated by asterisks.
Biochemical characterization of MRCKs.
The expression of these p190 kinases was examined in protein extracts by Cdc42-GTP binding and Western blot analysis using polyclonal antibodies against the p21-binding domain of human MRCKα. As reported previously, major Cdc42 binding occurs in regions from around 180 to 200 and 62 to 68 kDa; the latter corresponds to PAK isoforms (36). The larger Cdc42-binding proteins probably include MRCKα and -β. Western analysis revealed 180- to 200-kDa proteins, present at higher levels in the brain and kidney (Fig. 3A). High levels of immunoreactivity were detected in lung, in the pellet fraction (data not shown). The Cdc42-binding pattern did not correlate well with p180-200 immunoreactivity in different tissues, possibly because of the presence of other MRCK isoforms or Cdc42-binding proteins such as IQGAPs, which have similar molecular sizes (18, 25). On Northern blot analysis, the 10-kb MRCKα mRNA was highly enriched in the brain and lung and present in lower levels in other tissues (Fig. 3B). MRCKβ mRNA was expressed in all tissues examined and at highest levels in lung and kidney. Both mRNAs were also expressed in epithelial HeLa cells (data not shown).
MRCKα when expressed as a GST fusion protein was found to phosphorylate serine/threonine residues of several substrates, including myelin basic protein, histone H1, and its own binding domain (hBF-1) in vitro, but was especially active toward nonmuscle myosin regulatory light chain (MLC-2), the latter being phosphorylated at serine 19 (Fig. 3C). Immunoprecipitated HA-MRCKα did not exhibit elevated activity when cotransfected with Cdc42V12 (Fig. 3D). Similar results were obtained with the recombinant full-length GST-MRCKα (data not shown), indicating that interaction with Cdc42 was not essential for kinase activation. The mutant MRCKαK106A, with a substitution of the critical lysine in the kinase domain, exhibited no detectable kinase activity.
The cysteine-rich domain in both isoforms resembles those of PKC and chimaerins and was capable of binding to [3H]phorbol myristic acetate in a lipid-dependent manner (data not shown).
Cellular localization of MRCKα and the effect of Cdc42V12.
In transfected HeLa cells, expressed FLAG-MRCKα showed a dispersed punctate cytoplasmic distribution and a more intense staining along the cell periphery, especially at the leading edge and cell-cell junction. Cotransfection with Cdc42V12 led to a typical Cdc42-type morphology, and MRCKα was found to colocalize with Cdc42V12, particularly at the cell-cell junction and periphery, which contained numerous protrusions (Fig. 4, left panels). As PH domains can interact with lipids and the cytoskeleton, we also studied the effects of MRCKΔPH, a construct with the PH domain deleted. Cells transfected with MRCKαΔPH plasmid showed a more even cytoplasmic distribution of the kinase. When cotransfected with Cdc42V12, both MRCKαΔPH and p21 remained largely dispersed within the cytoplasm, and the typical Cdc42-type morphology was not produced (Fig. 4, right panels). These results suggest that the PH domain is important for the correct localization of MRCKα and that MRCKα may be associated with producing a Cdc42 phenotype since the MRCKαΔPH mutant blocked production of this phenotype (possibly acting as a dominant-negative mutant).
FIG. 4.
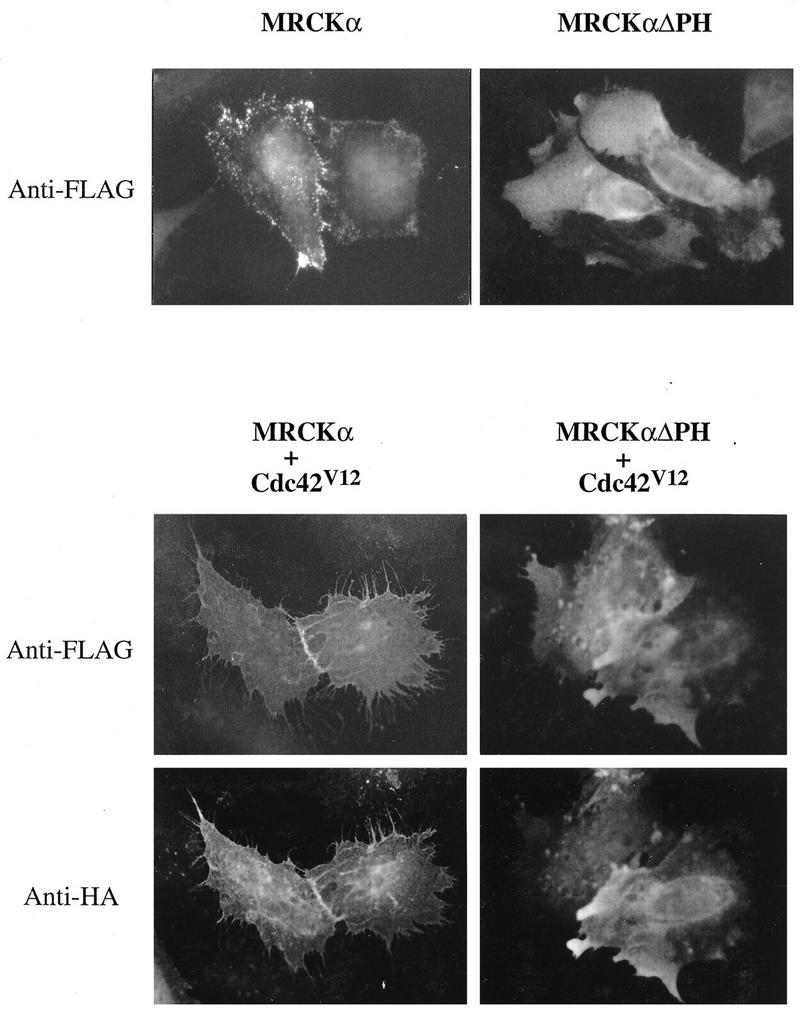
Cellular localization of MRCKα and the effects of Cdc42V12. HeLa cells grown in minimal essential medium with 10% fetal bovine serum were transfected with pXJ40-FLAG plasmids encoding either MRCKα alone or MRCKαΔPH (the latter with the PH domain deleted). Cells were fixed with 4% paraformaldehyde and stained with anti-FLAG antibody after 16 h. For cotransfection experiments, plasmid encoding FLAG-tagged MRCKα or MRCKαΔPH was cotransfected with plasmid encoding HA-tagged Cdc42V12. Cells were fixed and doubly stained with antibodies against FLAG for MRCKα and HA for Cdc42V12.
Microinjection of MRCKα affects cellular structures.
To investigate whether MRCKα had a direct effect on morphology, we microinjected HeLa cells with plasmids encoding MRCKα and various derivatives. Expression of wild-type MRCKα enhanced the formation of stress fibers, some of which exhibited a crisscross pattern (Fig. 5a and b). The kinase domain alone (which is constitutively active) elicited gross changes in actin- and myosin-containing structures involving marked actin condensation (Fig. 5e and f). Some increase in focal complexes were seen (Fig. 5g), but microtubules were unaffected (Fig. 5h). The action of MRCKα in promoting formation of stress fibers was reminiscent of the action of the related ROKα. However, MRCKα notably differed from ROKα in that its kinase-dead mutant (MRCKαK106A) did not promote dissolution of existing stress fibers (Fig. 5c and d). The MRCKα promotion of stress fibers was also not affected by the dominant-negative ROKαK112A (not shown, being very similar to Fig. 5a and b), indicating that MRCKα did not act via ROKα. These results show that although overexpressed MRCKα can mimic some effects of ROK through the presence of a kinase domain which is highly homologous among a family of diverse proteins, MRCKα appears to have a role different from that of the Rho-binding ROK.
FIG. 5.
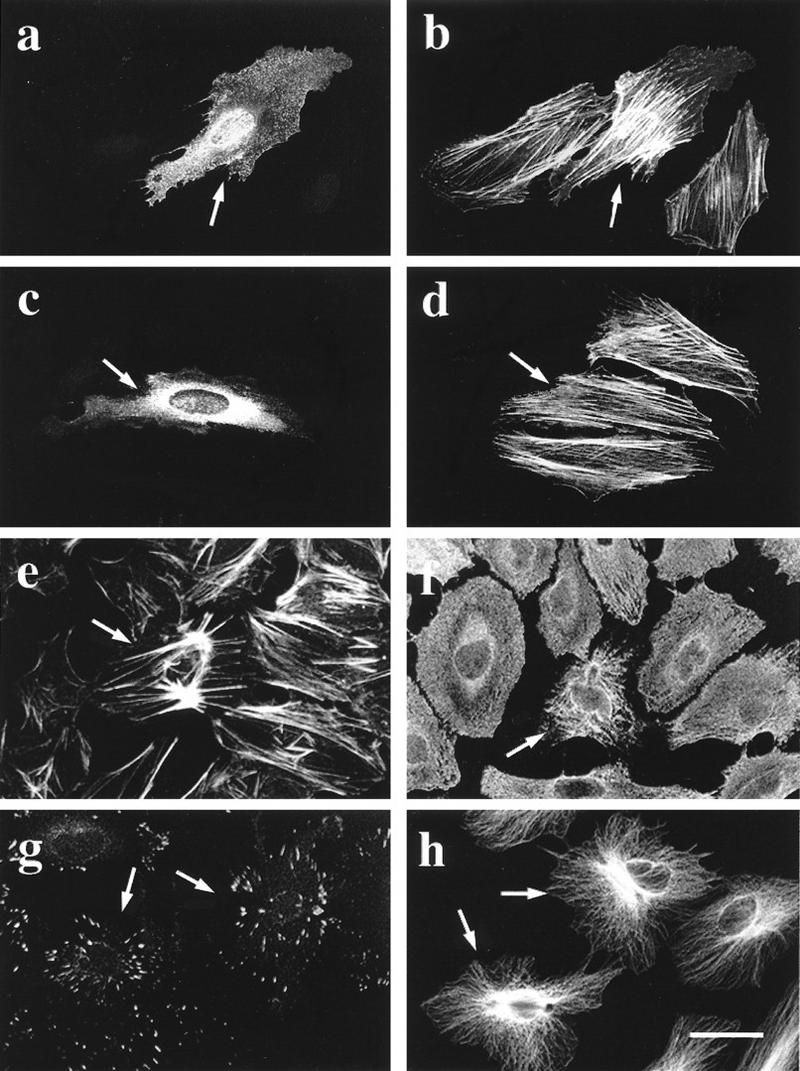
MRCKα affects the organization of cellular structures. HeLa cells grown on coverslips were microinjected with a plasmid encoding HA-tagged wild-type MRCKα (a and b), kinase-dead MRCKαK106A (c and d), or kinase domain alone (e to h). Two hours after incubation, cells were fixed and stained with anti-HA antibody (a and c) or doubly stained with phalloidin (b, d, and e) or antibodies against myosin light chain (f), vinculin (g), or tubulin (h). Arrows indicate the injected cells located by HA staining (not shown in panels e to h). Bar = 10 μm.
MRCKα modulates Cdc42-dependent morphology.
We then examined whether MRCKα could have a role in the morphological effects promoted by Cdc42. In HeLa cells, these morphological effects include microspike formation and production of stellate peripheral focal complexes readily observed 2 h after injection of Cdc42V12 plasmid (50 ng/μl) (Fig. 6A, panels c and d). When these cells were first injected with plasmid encoding kinase-dead MRCKαK106A (using this as a putative dominant-negative mutant) 3 h before the injection of Cdc42V12, these morphological effects were not seen. This blocking effect of the kinase-dead MRCKαK106A mutant was not due to its possible sequestration of Cdc42V12, since prior expression of the kinase-dead and Cdc42-binding-deficient MRCKαK106A,H1579,H1582A mutant was as effective in inhibiting the morphological action of Cdc42V12 (Fig. 6A, panels a and b). This MRCKα mutant had no effect on RacV12-induced focal complexes or cell spreading (Fig. 6A, panels e to h), showing that it specifically affected Cdc42 actions.
FIG. 6.
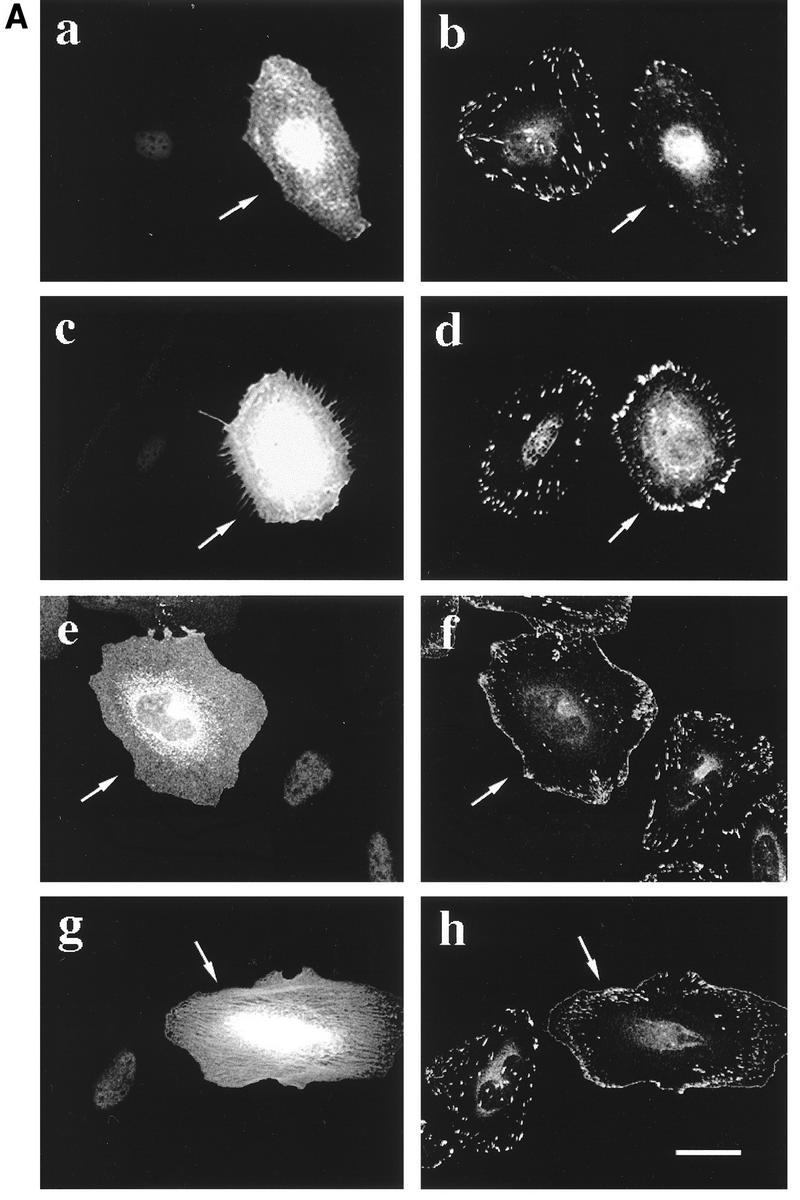
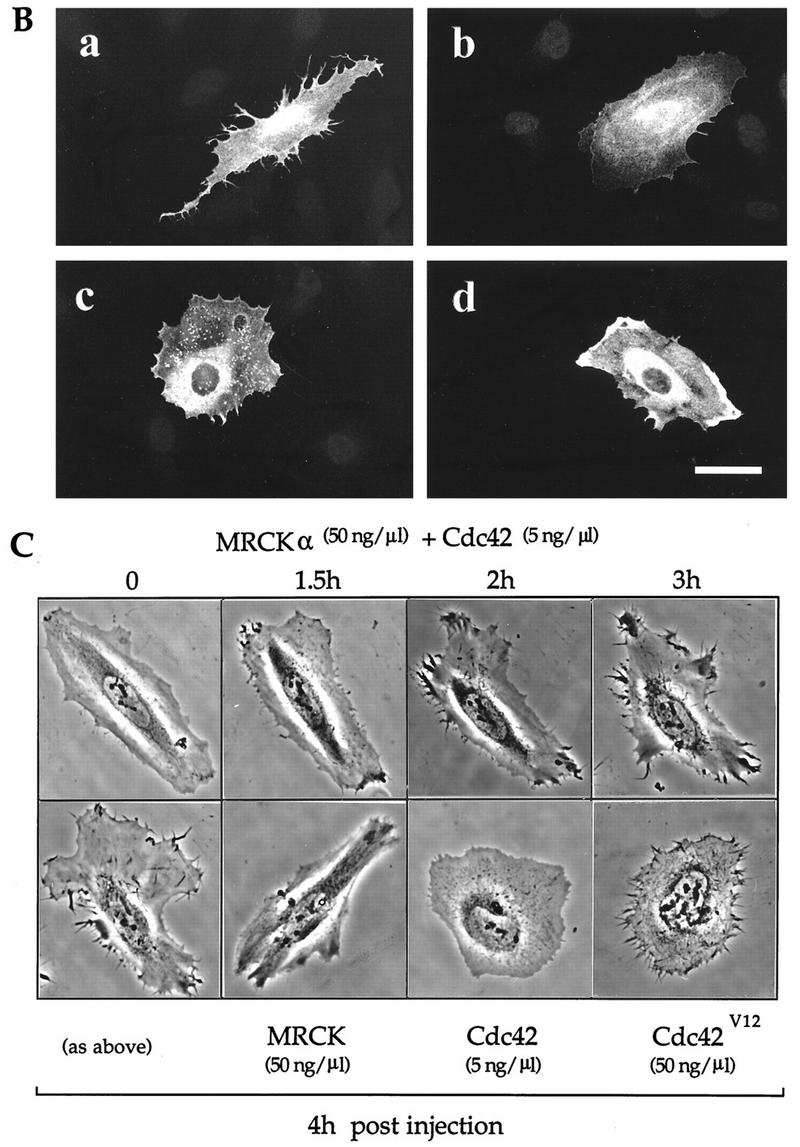
MRCKα potentiates the effects of Cdc42 on microspike formation. (A) Kinase-dead MRCKα blocks Cdc42-mediated effects on focal complexes and morphology. Serum-starved HeLa cells were injected with plasmid encoding FLAG-tagged kinase-dead/p21-binding-deficient mutant MRCKαK106A,H1579A,H1581A (50 ng/μl); 3 h later, these preinjected cells (a, b, e, and f) and uninjected control cells (c, d, g, and h) were injected with plasmid pXJ40-HA (50 ng/μl) encoding Cdc42V12 (a to d) or Rac1V12 (e to h). Cells were fixed and stained with antibodies against FLAG (a and e), HA (c and g), or paxillin (b, d, f, and h) after incubating for 2 h. Essentially similar results were obtained with kinase-dead MRCKαK106A. (B) Morphological effect of expression of MRCKα and limiting amounts of Cdc42. HeLa cells grown in serum-containing medium were injected with plasmids encoding FLAG-tagged Cdc42 (5 ng/μl) together with plasmid encoding either HA-tagged wild-type MRCKα (a), MRCKαK106A (b), p21-binding-defective MRCKαH1579A,H1582A (c), or ROKα (d) at 50 ng/μl. Cells incubated for 2 h were fixed and stained with anti-HA antibody. Bar = 10 μm. (C) Time-lapse phase-contrast microscopy of HeLa cells coinjected with plasmids encoding MRCKα and Cdc42 as in panel B. Morphological changes in a typical coinjected cell are shown up to 4 h after the coinjection. Cells 4 h after injection with plasmids encoding MRCKα (50 ng/μl) alone, Cdc42 (5 ng/μl) alone, or Cdc42V12 (50 ng/μl) are included for comparison.
We next investigated the functional relationship of MRCKα to its p21 partner Cdc42, adopting an approach similar to one recently used to study the effects of POR1, a Rac1-binding protein, on cytoskeletal reorganization (49). We first established that injection of low concentrations (5 ng/μl) of wild-type Cdc42 plasmid was phenotypically ineffective in inducing morphological changes in HeLa cells and subsequently coinjected MRCKα and Cdc42 plasmids. This led to an enhanced formation of cellular extensions and microspikes with a marked redistribution of MRCKα to cortical regions, especially at the tip of the former structures (Fig. 6B, panel a). Coinjection of Cdc42 plasmid with plasmids encoding kinase-dead MRCKαK106A, Cdc42-binding-deficient MRCKαH1579A/H1582A, and ROKα (Fig. 6B, panels b to d) led to no such enhanced formation of peripheral structures. These results indicate that both kinase and Cdc42-binding domains of MRCKα are required for its effects on Cdc42 functions.
Injected cells were then subjected to time-lapse analysis. When injected with a low concentration (5 ng/μl) of Cdc42 plasmid, cells showed very little change even after 4 h. Higher concentrations (50 ng/μl) of activated Cdc42V12 plasmid led to the appearance of short microspikes (Fig. 6C, bottom row). When MRCKα plasmid was coinjected with low concentrations of Cdc42 plasmid (by itself ineffective), cellular protrusions including microspikes appeared within 90 min after the coinjection, with the peripheral regions undergoing continual retraction and extension over several hours (Fig. 6C, top row). These dynamic and protracted changes resulted in cells displaying extended cytoplasmic tracts 4 h after injection (Fig. 6C, left lower panel). The morphology contrasts sharply with that of control cells injected with plasmids encoding either MRCKα or Cdc42 on their own examined at this time interval.
DISCUSSION
We and others have recently reported the isolation of Rho-binding serine/threonine kinases (ROKs) which act downstream of Rho (1, 2, 19, 20, 23, 30, 31). The isolation of another family of ROK-related kinases with Cdc42 and weak Rac1 binding (MRCKs) strengthens the notion that functionally related members of these kinases are adapted to different switches for diverse biological activities. These multidomain kinases show some similarity in domain organization, with coiled-coil α-helix, cysteine-rich, and PH domains, although the exact arrangements differ. They also share substrates. Like ROKα, MRCKα readily phosphorylates MLC-2 predominantly at serine 19. Phosphorylation of this residue has been reported to be essential for the activation of myosin in vitro (6, 22) and its subsequent effect on the actin-myosin contractile apparatus which has been suggested to underlie the formation of stress fibers and focal adhesion complexes (12). With MRCKα, the exact relationship of its kinase activity to its morphological action remains to be established. It is plausible that phosphorylation of myosin(s) is a common feature of these different kinases and that the site of action will determine the appropriate morphological activity, with selectivity being imparted by specific p21-binding domains.
MRCKα has a characteristic cellular localization which is different from that of ROKα. In general, ROKα is distributed evenly in the cytoplasm and concentrated in the cell periphery only upon translocation by transfected RhoA (31). MRCKα is stained in punctate structures in the cytoplasm, with more intense staining at the periphery of transfected cells, particularly at the leading edge and cell-cell junction. This localization may in part be due to nonkinase regulatory domains such as the PH domain, since its deletion resulted in a more even cellular distribution. The PH domain of the Ras exchange factor Sos had been shown to play a role in targeting the protein to the cell periphery and leading edge of motile cells, in response to serum stimulation (11). Similarly, the N-terminal PH domain of pleckstrin is required for its membrane localization and induction of membrane projections which is regulated by its phosphorylation (33). Although the families of ROKs and MRCKs contain PH domains, these are not identical, and it would be interesting to determine whether and how the different PH domains influence membrane localization.
Not unexpectedly given the similarity in the kinase domain, overexpression of ROKs and MRCKs can result in overlapping morphological activities under certain experimental conditions. Introduction of plasmids for either ROKα or MRCKα led to enhanced formation of stress fibers and focal complexes, which require their kinase activity (Fig. 5 and references 1, 20, and 30). However, while the kinase-dead (dominant-negative) ROKα mutant effected dissolution of stress fibers and focal adhesion complexes, in keeping with ROK’s role downstream of Rho, kinase-dead MRCKα did not affect these Rho-dependent structures. This finding strongly indicates that the functional role of MRCKs is different from that of ROKs. Several lines of evidence from the work presented here suggest that MRCKα is associated with Cdc42 functions. First, Cdc42 colocalizes with MRCKα on cotransfection. Second, introduction of the kinase-dead MRCKα blocks the morphological effects of Cdc42V12. Third, coexpression of MRCKα with limiting concentrations of wild-type Cdc42 (which elicits no effect on its own) promoted the formation of dynamic peripheral structures including microspikes and filopodia. The formation of these structures require Cdc42 mediation (24, 41).
The relationship of MRCK to the other Cdc42-binding kinase PAK clearly warrants further investigation. αPAK disassembles stress fibers and focal adhesion complexes in HeLa cells when activated (34). It has been suggested that this disassembly may facilitate (or be necessary for) the formation of the Cdc42-dependent peripheral structures perhaps because of shared components or cytomechanical needs, reflecting opposing roles of ROK and PAK (32). In cells injected with activated PAK, dissolution of the Rho-mediated structures is followed eventually by massive cell contraction, with long retraction fibers being visible 90 to 120 min after injection (34). In the present study, coexpression of limiting concentrations of Cdc42 with MRCKα led to pronounced microspike activity and restructuring of peripheral portions of the cell, involving continual retraction and protrusion over an extended time (4 h). Rather than the final overall cell contraction observed with PAK, this restructuring resulted in marked expansion of some parts of the cytoplasm. This finding suggests that MRCK and PAK activities may need to be coordinated in normal cells displaying Cdc42-mediated effects. (It is possible that the Cdc42-binding nonkinases such as the Wiskott-Aldrich syndrome protein [4, 46] are also involved.) MRCK, unlike PAK (34), does not appear to be involved in Rac-induced activities (Fig. 6) which can occur subsequent to Cdc42 activation (24, 41). Certainly, the occurrence of different kinase domains with related p21-binding domains within MRCK and PAK, and conversely of similar kinase domains with different p21-binding domains within MRCK and ROK, may well serve to allow the Rho family effectors to engage in cross talk essential for integration of the wide repertoire of cellular activities mediated by these p21s.
ACKNOWLEDGMENTS
We thank Teo Hsiang Ling for expert technical assistance, Robin Philps for phosphopeptide microsequencing, and Francis Leong for photographic reproduction.
This work was supported by the Glaxo Singapore Research Fund.
REFERENCES
- 1.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura N, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 2.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 3.Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science. 1996;271:648–650. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- 4.Aspenstrom P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol. 1995;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 5.Bragodia S, Derijard B, Davis R J, Cerione R A. Cdc42 and PAK-mediated signalling leads to JNK and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 6.Bresnick A R, Wolff-Long V L, Baumann O, Pollard T D. Phosphorylation on threonine-18 of the regulatory light chain dissociates the ATPase and motor properties of smooth muscle myosin II. Biochemistry. 1995;34:12576–12583. doi: 10.1021/bi00039a012. [DOI] [PubMed] [Google Scholar]
- 7.Brook J D, McCurrach M E, Harley H G, Buckler A J, Church D, Aburatani H, Hunter K, Stanton V P, Thirion J-P, Hudson T, Sohn R, Zemelman B, Snell R G, Rundle S A, Crow S, Davies J, Shelbourne P, Buxton J, Jones C, Juvonen V, Johnson K, Harper P S, Shaw D J, Housman D E. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 8.Brown J L, Stowers L, Baer M, Trejo J A, Coughlin S, Chant J. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr Biol. 1996;6:598–605. doi: 10.1016/s0960-9822(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 9.Burbelo P D, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- 10.Cerione R A, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- 11.Chen R H, Corbalan-Garcia S, Bar-Sagi D. The role of the PH domain in the signal-dependent membrane targeting of Sos. EMBO J. 1997;16:1351–1359. doi: 10.1093/emboj/16.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coso O A, Chiariello M, Yu J-C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signalling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 14.Drechsel D N, Hyman A A, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y-H, Pizzuti A, Fenwick R G, Jr, King J, Rajnarayan S, Dunne P W, Dubel J, Nasser G A, Ashizawa T, de Jong P, Wieringa B, Korneluk R, Perryman M B, Epstein H F, Caskey C T. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- 16.Grant J W, Zhong R Q, McEwen O M, Church S L. Human nonsarcomeric 20,000 Da myosin regulatory light chain cDNA. Nucleic Acids Res. 1990;18:5892. doi: 10.1093/nar/18.19.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall C, Monfries C, Smith P, Lim H H, Kozma R, Ahmed S, Vanniasingham V, Leung T, Lim L. Novel human brain cDNA encoding a 34,000 Mr protein, n-chimaerin, related to both the regulatory domain of protein kinase C and BCR, the product of the breakpoint cluster region gene. J Mol Biol. 1990;211:11–16. doi: 10.1016/0022-2836(90)90006-8. [DOI] [PubMed] [Google Scholar]
- 18.Hart M J, Callow M G, Souza B, Polakis P. IQGAP1, a calmodulin binding protein with a rasGAP-related domain, is a potential effector for Cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 20.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 21.Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. Rac regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- 22.Kamisoyama H, Araki Y, Ikebe M. Mutagenesis of the phosphorylation site (serine 19) of smooth muscle myosin regulatory light chain and its effects on the properties of myosin. Biochemistry. 1994;33:840–847. doi: 10.1021/bi00169a027. [DOI] [PubMed] [Google Scholar]
- 23.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 24.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases Cdc42 and Rac1. J Biol Chem. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- 26.Lamarche N, Hall A. GAPs for rho-related GTPases. Trends Genet. 1994;10:436–440. doi: 10.1016/0168-9525(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 27.Lamarche N, Tapon N, Stowers L, Burbelo P D, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 28.Leeuw T, Fourest-Lieuvin A, Wu C, Chenevert J, Clark K, Whiteway M, Thomas D Y, Leberer E. Pheromone response in yeast: association of Bem1p with proteins of the MAP kinase cascade and actin. Science. 1995;270:1210–1213. doi: 10.1126/science.270.5239.1210. [DOI] [PubMed] [Google Scholar]
- 29.Lendahl U, Zimmerman L B, McKay R D. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 30.Leung T, Chen X-Q, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- 32.Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases: the p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways. Eur J Biochem. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- 33.Ma A D, Brass L F, Abrams C S. Pleckstrin associates with plasma membranes and induces the formation of membrane projections: requirements for phosphorylation and the NH2-terminal PH domain. J Cell Biol. 1997;136:1071–1079. doi: 10.1083/jcb.136.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manser E, Huang H-Y, Loo T-H, Chen X-Q, Dong J-M, Leung T, Lim L. Expression of constitutively active αPAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manser E, Leung T, Salihuddin H, Tan L, Lim L. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature (London) 1993;363:364–367. doi: 10.1038/363364a0. [DOI] [PubMed] [Google Scholar]
- 36.Manser E, Leung T, Salihuddin H, Zhao Z-S, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature (London) 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 37.Martin G A, Bollag G, McCormick F, Abo A. A novel serine kinase activated by Rac1/Cdc42Hs-dependent autophosphorylation is related to PAK65 and Ste20. EMBO J. 1995;14:1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine threonine kinase, as a putative target for the small GTP-binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 39.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signalling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 40.Molnar A, Theodoras A M, Zon L I, Kyriakis J M. Cdc42Hs, but not Rac1, inhibits serum-stimulated cell cycle progression at G1/S through a mechanism requiring p38/RK. J Biol Chem. 1997;272:13229–13235. doi: 10.1074/jbc.272.20.13229. [DOI] [PubMed] [Google Scholar]
- 41.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibres, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 42.Qiu R-G, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–3458. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridley A J, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibres in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 44.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 46.Symons M, Derry J M J, Karlak B, Jiang S, Lemahieu V, McCormick F, Francke U, Abo A. Wiskott-Aldrich Syndrome protein, a novel effector for the GTPase Cdc42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 47.Symons M, Derry J M J, Souza B, Pringle J R, Abo A, Reed S I. Role for the Rho-family GTPase Cdc42 in yeast mating pheromone signal pathway. Nature (London) 1995;376:702–705. doi: 10.1038/376702a0. [DOI] [PubMed] [Google Scholar]
- 48.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 49.Van Aelst L, Joneson T, Bar-Sagi D. Identification of a novel Rac1-interacting protein involved in membrane ruffling. EMBO J. 1996;15:3778–3786. [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe G, Saito Y, Madaule P, Ishizake T, Fujisawa K, Morri N, Mukai H, Ono Y, Kakizuka A, Narumiya S. Protein kinase N (PKN) and PKN-related protein Rhophilin as targets of the small GTPase Rho. Science. 1996;271:645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- 51.Westwick J K, Lambert Q T, Clark G J, Symons M, Van Aelst L, Pestell R G, Der C J. Rac regulation of transformation, gene expression, and actin polymerization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wittenberg C, Reed S I. Plugging it in: signalling circuits and the yeast cell cycle. Curr Opin Cell Biol. 1996;8:223–230. doi: 10.1016/s0955-0674(96)80069-x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Han J, Sells M A, Chernoff J, Knaus U G, Ulevitch R J, Bokoch G M. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 54.Zhao Z-S, Leung T, Manser E, Lim L. Pheromone signalling in Saccharomyces cerevisiae requires the small GTP-binding protein Cdc42p and its activator CDC24. Mol Cell Biol. 1995;15:5246–5257. doi: 10.1128/mcb.15.10.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]


