Abstract
In the Mexico City metropolitan area (MCMA), 70% of the water for 18 million inhabitants is derived from the Basin of Mexico regional aquifer. To provide an overview of the quality of the groundwater, a longitudinal study was conducted, in which 30 sites were randomly selected from 1,575 registered extraction wells. Samples were taken before and after chlorine disinfection during both the rainy and dry seasons (2000-2001). Microbiological parameters (total coliforms, fecal coliforms, streptococci, and Vibrio spp.), the presence of Helicobacter pylori, and physicochemical parameters, including the amount of trihalomethanes (THMs), were determined. Although microorganisms and inorganic and organic compounds were evident, they did not exceed current permissible limits. Chlorine levels were low, and the bacterial counts were not affected by chlorine disinfection. Eighty-four bacterial species from nine genera normally associated with fecal contamination were identified in water samples. H. pylori was detected in at least 10% of the studied samples. About 40% of the samples surpassed the THM concentration allowed by Mexican and U.S. regulations, with levels of chloroform being high. The quality of the water distributed to the MCMA varied between the rainy and dry seasons, with higher levels of pH, nitrates, chloroform, bromodichloromethane, total organic carbon, and fecal streptococci during the dry season. This study showed that the groundwater distribution system is susceptible to contamination and that there is a need for a strict, year-round disinfection strategy to ensure adequate drinking-water quality. This situation in one of the world's megacities may reflect what is happening in large urban centers in developing countries which rely on a groundwater supply.
The new millennium represented a turning point, with the world population moving from rural to urban communities (17). It is in developing countries that the majority of megacities are found, where over 10 million inhabitants are concentrated. The Mexico City Metropolitan Area (MCMA) is the second largest megacity in the world (27, 47).
These urban agglomerations place an enormous burden on natural resources at a regional level, the most notable of which is water (27). The increasingly difficult problem of supplying adequate water quality is associated with a dispersion of the population, an expansion of the city area, and a diversity of contaminant sources (8, 45).
In most developing cities, population growth precedes the development of an infrastructure capable of handling water and wastewater, which tends to lead to widespread contamination of the groundwater by domestic and industrial effluents. Hence, rapid urbanization has had a detrimental effect on water management systems (11, 16). In some urban areas, minimally treated groundwater for the public water supply is being threatened, and in some cases there is an increased risk due to specific hydrogeological conditions which have been linked to increased health risks (38). This is the case for several megacities that are dependent on groundwater.
The MCMA case study is an example of an area where groundwater is the major source of the public water supply. In terms of environmental conditions, the MCMA is at a critical stage (15, 30), with water being one of the limiting resources that is impacting the sustainability and future development of this urban ecosystem. The aquifer system supplies approximately 70% of the MCMA demand, with the remaining 30% being imported from other watersheds (37). Water availability is a delicate topic, since 18 million people depend on this resource (18). Several studies have been conducted in the MCMA to evaluate the groundwater resources in pilot zones (36) and in specific geological areas important for groundwater recharge (12, 33). These have been based on microbiological parameters, which are primarily comprised of indicator bacteria, in addition to streptococci, Vibrio spp., and Helicobacter pylori, which have also been detected in various aquatic ecosystems within the MCMA (34, 35). There is also concern about the formation of disinfection by-products in drinking water (40), a new issue just being investigated in developing countries.
Although previous studies provided information that points to a fecal contamination problem in groundwater, these have been limited to specific areas of the city. Due to the heterogeneity of the city environment with respect to the geological, hydrological, social, and cultural conditions, a survey using representative samples of the whole urban area is still needed.
Although such information is mainly of local interest, its scope goes beyond the immediate environment, with the results being of benefit to existing and future urban planners who are charged with managing issues such as groundwater dependency for growing populations and urban spread in both the developed and developing world. This may be the case in Lagos, Calcutta, Shanghai, Buenos Aires, Dhaka, Jakarta, Manila, Beijing, Cairo, Paris, Tianjin, and Lima, to name the major megacities in the world at this time (27).
This prospective case study, designed specifically for the MCMA, gives the status of the groundwater in an urban area, which may reflect similar situations in other megacities which rely on groundwater for their water supply.
MATERIALS AND METHODS
Experimental design.
A simple random sampling strategy using random number tables (26) was followed to select representative sites from the 1,575 registered extraction wells in the MCMA. The number and location of selected sites provided a representative sample of the geographical area, allowing the general situation of the groundwater before and after chlorine disinfection to be evaluated and understood. The sample size was calculated under the assumption that none of the samples should culture any bacterial indicator (total and fecal coliforms), according to current Mexican (14) and U.S. (50) standards. Therefore, 30 sites for each season prior to disinfection were selected. A further 20 sites for the dry season and 22 sites for the rainy season were determined in the same locations for the postdisinfection samples. Selection was made with the aim of attaining a 95% confidence interval not greater than 15% whenever a 0% point estimate of positivity for each bacterial indicator in a given set of chlorination-season samples was found (32).
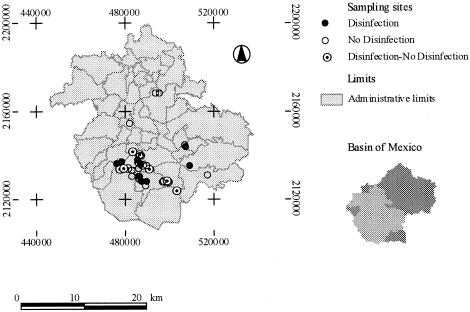
A total of 102 water samples were collected from 30 extraction wells and 22 pumping points, since not all sites have chlorination in situ. Sixty water samples were taken prior to chlorine disinfection (30 in the rainy season and 30 in the dry season), and 42 samples were taken after disinfection (22 in the rainy season and 20 in the dry season). Of the 42 water samples taken after disinfection, 11 in the rainy season and 9 in the dry season were taken from the same wells. Eleven water samples during the rainy season and 11 during the dry season were taken from pumping areas, representing treated water with a different contact time. Note that the chlorination point along the water system varies from 1.5 m to 1.5 km and that the contact time also alters. These variations are due to the infrastructure and water management practices. Well operation is performed by the federal or state (local) water authorities or by companies in the case of privately owned wells. The rainy season ran from June to September 2000, and the dry season was from January to May 2001. The randomly selected water sampling sites in the MCMA are indicated in Fig. 1.
FIG. 1.
Sampling sites in the Mexico City metropolitan area.
Sampling.
Samples were collected during the annual cycle (2000-2001) in wide-mouth polypropylene sterile flasks. One-liter samples were taken for bacteriological indicator analyses, and 500-ml samples were taken for H. pylori and physicochemical analyses. Separate samples for individual and total trihalomethanes (THMs) were taken in duplicate in 40-ml amber-borosilicate glass vials with screw caps and silicon-Teflon septa, with 160 μl 0.2 M sodium sulfite added as a reducing agent to the samples containing chlorine. Samples were transported and stored refrigerated (4°C) according to standard procedures (1). Operating extraction wells were sampled at points prior to and following chlorine disinfection. The tap was cleaned before the samples were taken, and water was allowed to flow for 5 min before water samples were collected.
Bacteriological analyses.
Water samples were analyzed according to standard membrane filtration procedures for the enumeration of four bacterial types, namely, total coliforms, fecal coliforms, streptococci, and Vibrio spp. Membrane filters (0.45-μm cellulose acetate; Millipore MF type HA) were placed on a pad with M-Endo broth for total coliforms, M-FC broth for fecal coliforms, K-F agar for streptococci, and thiosulfate-citrate-bile salts-sucrose agar for Vibrio spp. (1, 39). Incubation was performed using an incubator (WTB Binder) under conditions described by the American Public Health Association (1) and Murray et al. (39).
Biochemical tests were carried out for the identification of gram-positive and gram-negative bacteria of the Micrococcaceae and Streptococcaceae families, which include Staphylococcus and Enterococcus, as well as for the Enterobacteriaceae family. Final identification was performed using a semiautomatic DADE MicroScan instrument (AutoSCAN-4; DADE International, West Sacramento, CA) (39).
The presence of H. pylori was determined by PCR and confirmed by Southern blot hybridization. Each 500-ml sample was concentrated by centrifugation at 10,000 × g for 30 min at 4°C. Sediments were resuspended in 10 ml of 10 mM Tris-HCl (pH 8.0) and 1 mM EDTA, and aliquots of 1 ml were stored at −70°C and used to extract DNA by the guanidium thiocyanate-EDTA-Sarkosyl-EDTA method (42). The primers and amplification conditions were the same as those used by Mazari-Hiriart et al. (34, 35).
PCR products from the 16S rRNA of H. pylori were purified using a MER plasmid spin kit (BIO101, La Jolla, CA) from a 3% agarose gel stained with ethidium bromide. PCR products of 110 bp were cloned into a pCR2.1 TOPO (Kanr Ampr) plasmid according to the manufacturer's instructions (Invitrogen Life Technologies). A transformation assay was carried out with Escherichia coli XL1-Blue by thermal shock, and cells were grown in Luria medium with X-Gal (5-bromo-4-chloro-3indolyl-β-d-galactopyranoside), IPTG (isopropyl-β-d-thiogalactopyranoside), and kanamycin. Five selected white clones were cultured in 5 ml of a mixture of Luria broth and kanamycin. A pUC18 plasmid was used as a clone control with a pCR21 vector without a DNA fragment insert. Plasmid extraction and purification were conducted by alkaline lysis (44). The PCR extraction products were visualized in a 1% agarose gel with 0.5% Tris-borate-EDTA buffer that was stained with ethidium bromide.
To determine if the plasmid contained a 16S rRNA fragment, a PCR was performed for M13 sequence sites by using M13 reverse and forward primers. PCR was carried out as previously described for 30 cycles. The PCR products were visualized in a 2% agarose gel with 0.5% Tris-borate-EDTA buffer that was stained with ethidium bromide. To confirm the presence of the DNA fragment insert in the clones, the primers Hp1 and Hp2 were used with the PCR cocktail, and similar conditions to those described above for amplification were used. A 110-bp band was visualized in an agarose gel and stained as described above to identify the 16S rRNA nucleotide sequence. The selected clones were sequenced using an ABI PRISM BigDye Terminator cycle sequencing ready reaction kit (PE Applied Biosystems) according to the manufacturer's instructions. One microliter of plasmid DNA was used to sequence the clones in both directions. Electropherograms were obtained by using an ABI PRISM 377 DNA sequencer (PE Applied Biosystems).
H. pylori cagA identification was carried out using the primers cagA1 (93089) (5′-ATACACCAACGCCTCCAAG-3′) and cagA2 (93261) (5′-TTGTTGCCGCTTTTGCTCTC-3′) as described by Castillo-Rojas et al. (9).
For physicochemical analyses, temperature, conductivity, and pH were measured using a portable YSI model 3500 instrument. Residual chlorine was analyzed by using an Orion selective electrode (model 9770BN) calibrated with Orion iodated potassium as a chlorine stock solution, nitrate was analyzed by using a Corning electrode (model 476137) calibrated with standard nitrate solutions prepared from a Corning stock solution, and ammonia was analyzed by using a Corning electrode (model 487234) calibrated with standard solutions prepared from a Corning standard ammonium solution. Samples were analyzed in duplicate. The total organic carbon concentration was determined by using a UIC carbon analyzer (model CM5012) by the coulomb metric methods ASTM D 4129-88 and ASTM D 513-92 (25).
As mentioned previously, samples for individual and total trihalomethanes were taken in duplicate in 40-ml amber-borosilicate glass vials with screw caps and silicon-Teflon septa, with 160 μl 0.2 M sodium sulfite added as a reducing agent to the samples containing chlorine. The concentration was determined by the headspace technique described by Cancho (personal communication) using an HP 7694 headspace autosampler coupled to an HP 5890 Series II gas chromatograph with an electron capture detector and a DB-624 capillary column that was 30 m long, had a 0.25-mm internal diameter, and used 2-μm film. The chromatographic conditions were as follows: injector temperature, 200°C; detector temperature, 250°C; column temperature, 80°C for 1 min, rising by 6°C per minute until 140°C, which was maintained for the final minute. Helium was used as a carrier gas at 1 ml/min, and a special mixture of 5% methane in 95% argon was used for the detector.
Statistical analyses.
In order to maintain a statistically conservative approach, samples from the same site were regarded as independent from each other. Physicochemical data were summarized as medians and interquartile intervals (q1 and q3). All data were stratified according to their chlorination status (yes or no) and season of sample collection (rainy or dry). Three hydrogeologic zones (HGZ) were defined in the MCMA a priori, based on topography and main water flow. HGZ 1, Mexico City, was located in the Northwest, with 13 sampling sites; HGZ 2, Texcoco, was located in the Northeast, with 4 sampling sites; and HGZ 3, Chalco-Xochimilco, was located in the South, with 25 sampling sites. Spearman's rank correlation coefficient was used to appraise the association between the physicochemical variables themselves as well as between these variables and the HGZ within the MCMA or the bacteriological data.
Given that the distribution of the microbiologic data was extremely skewed, it was more appropriate to summarize the data for the number of CFU per 100 ml isolated for each bacterial group by using several indicators, including the arithmetic mean, its standard deviation (SD), the median, and the interquartile intervals (q1 and q3). The proportion of positive samples (i.e., those with at least 1 CFU/100 ml) was calculated, together with its corresponding 95% confidence interval, according to a binomial distribution.
Differences among combinations of data, such as the chlorination status and season of sample collection as well as the HGZ, were tested using the Kruskal-Wallis and the Wilcoxon-Mann-Whitney procedures. Proportions were compared by the chi-square test and the Fisher-Freeman-Halton test.
In order to identify multivariate associations, multiple linear regression and multiple logistic regression models were built and tested. The dependent variable in the first case was the total count of CFU/100 ml for each bacterial category, and in the second case, the variable was the positivity of each category. Independent variables were physicochemical data, chlorination status, season, and HGZ.
The alpha value was set at 0.05. Analyses were performed with Stata statistical software (Intercooled Stata 7.0 for Windows 98/95/NT).
Nucleotide sequence accession numbers.
The nucleotide sequences of the 16S rRNA gene fragments were deposited in GenBank under accession numbers AY961375 and AY960213 (pA16S4-2 and pA16S5-3, respectively).
RESULTS
The results of microbiological analyses of groundwater extracted from wells in the MCMA by season and chlorination effect are shown in Table 1. The presence of total coliforms, fecal coliforms, streptococci, and Vibrio spp. was identified throughout the whole study. According to Mexican water quality standards, which specify 0 CFU/100 ml as the limit for total and fecal coliforms, the arithmetic means for samples from this study surpass the levels recommended in the regulations for water that is intended for human use and consumption. For total and fecal coliforms only, 19% of the samples (10/52) during the rainy season surpassed the regulations and 16% of samples (8/50) for the dry season surpassed the agreed standard. Taking all samples into account, 18% of the water samples (18/102) throughout the year did not comply with the regulations. These samples do not comply with the U.S. EPA Groundwater and Drinking Water regulations, either, for which total coliforms, including fecal coliforms and E. coli, should be zero. Any sample containing total coliforms must be analyzed for fecal coliforms and E. coli (48, 50).
TABLE 1.
Counts of indicator bacteria in water samples from MCMA
| Parametera | Value (CFU/100 ml)
|
Pb | |||
|---|---|---|---|---|---|
| Rainy season
|
Dry season
|
||||
| Cl (n = 22) | No Cl (n = 30) | Cl (n = 20) | No Cl (n = 30) | ||
| Total coliforms | |||||
| Mean (SD) | 0.23 (0.53) | 0.73 (3.92) | 2.33 (9.61) | 1.62 (7.66) | |
| Md (q1, q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | NS |
| Positivity fraction (95% CI) | 0.182 (0.052-0.403) | 0.067 (0.008-0.221) | 0.1 (0.012-0.317) | 0.167 (0.056-0.347) | |
| Fecal coliforms | |||||
| Mean (SD) | 0.27 (0.97) | 0.03 (0.13) | 0.23 (1.01) | 2.35 (11.96) | |
| Md (q1, q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | NS |
| Positivity fraction (95% CI) | 0.136 (0.029-0.349) | 0.067 (0.008-0.221) | 0.05 (0.012-0.249) | 0.067 (0.008-0.221) | |
| Streptococci | |||||
| Mean (SD) | 0.14 (0.44) | 0.08 (0.19) | 8.25 (19.61) | 21.05 (58.10) | |
| Md (q1, q3) | 0 (0, 0) | 0 (0, 0) | 0.25 (0, 9.5) | 0.5 (0, 5) | 0.001 |
| Positivity fraction (95% CI) | 0.136 (0.029-0.349) | 0.167 (0.056-0.347) | 0.55 (0.315-0.769) | 0.5 (0.313-0.687) | |
| Vibrio spp. | |||||
| Mean (SD) | 0.07 (0.23) | 16.77 (91.27) | 0.28 (.83) | 0.32 (1.03) | |
| Md (q1, q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | NS |
| Positivity fraction (95% CI) | 0.091 (0.011-0.292) | 0.133 (0.038-0.307) | 0.15 (0.032-0.379) | 0.2 (0.077-0.386) | |
Md, median; q1 and q3, first and third quartiles, respectively; CI, confidence interval.
From Kruskal-Wallis test. NS, not significant.
Although the presence and levels of fecal streptococci and Vibrio spp. are not considered in the Mexican and U.S. EPA water standards, these bacteria were targeted since they have been detected continuously in the Mexico City area water. In the case of fecal streptococci, there was a significant difference according to the season and chlorine presence, with a substantial increase in the dry season from 15% to 52% positive samples.
The relevant results for physicochemical parameters during the annual cycle are shown in Table 2. Within the physicochemical data, the changes in nitrate concentration (NO3) stand out, with chlorinated samples during the rainy season showing the lowest level (2.9 mg/liter) and nonchlorinated samples in both the rainy and dry seasons showing the highest levels (P = 0.0001). The figures for total organic carbon show accentuated changes according to the season (a maximum median value of nearly 43 mg/liter during the rainy season versus a minimum median value between 4 and 6 mg/liter in the dry season [P = 0.0001]). As expected, there were higher concentrations of residual chlorine in the chlorinated samples (0.06 mg/liter) than in the nonchlorinated samples (<0.02 mg/liter) (P = 0.018). Both values are extremely low, reflecting the inefficient chlorination process adopted in the MCMA, which may also be the case in other developing countries. The differences in ammonia were also significant (a minimum of 0.9 mg/liter in chlorinated samples in the rainy season versus a maximum of 5.1 mg/liter in chlorinated samples during the dry season [P = 0.034]). The total THM concentration showed a variation from 40.2 μg/liter for nonchlorinated samples in the dry season to 103.2 μg/liter for chlorinated samples in the same season (P = 0.042). This relatively high concentration of THM is mainly due to the presence of chloroform. Splitting the results according to the HGZ and dividing the samples showed a difference in terms of temperature, with a minimum of 16.9°C for HGZ 3 (Chalco-Xochimilco) and a maximum of 25.4°C for HGZ 2 (Texcoco) (P = 0.001). In terms of pH, there was a minimum value of 6.68 for HGZ 1 (Mexico City) and a maximum of 7.06 for HGZ 2 (P = 0.047).
TABLE 2.
Physicochemical parameters for water samples from MCMA
| Parameter | Value (median [1st and 3rd quartiles]) for indicated samples
|
Pa | Permissible level
|
||||
|---|---|---|---|---|---|---|---|
| Rainy season
|
Dry season
|
U.S. EPAb | DOFb | ||||
| Cl (n = 22) | No Cl (n = 30) | Cl (n = 20) | No Cl (n = 30) | ||||
| Temperature (°C) | 18.1 (16, 20.2) | 18.3 (15, 20.2) | 19.3 (15.8, 20.9) | 17.5 (15.1, 20) | NS | ||
| Conductivity (μS/cm) | 429 (376, 616) | 491.5 (325, 769) | 445.5 (317.5, 655) | 456.5 (333, 774) | NS | ||
| pH | 7.03 (6.55, 7.35) | 6.89 (6.69, 7.15) | 6.61 (6.37, 6.95) | 6.70 (6.49, 7.05) | NS | 6.5-8.5 | 6.5-8.5 |
| Dissolved oxygen (mg/liter) | 3.4 (3, 4.2) | 4.1 (3.2, 6.6) | 4.2 (3.4, 6.00) | 4.8 (3.4, 6) | NS | ||
| Nitrates (mg/liter) | 2.9 (1.4, 3.7) | 8.8 (3.8, 18.4) | 5.6 (2.2, 13.8) | 8.3 (4.7, 29.4) | 0.0001 | 10 | 10 |
| Ammonia (mg/liter) | 0.9 (0.5, 2.0) | 2.5 (0.8, 12.1) | 5.1 (0.8, 24.3) | 4.0 (1.0, 14.0) | 0.034 | 0.50 | |
| Residual chlorine (mg/liter) | 0.06 (0.00, 0.23) | 0.014 (0.006, 0.022) | 0.06 (0.02, 0.92) | 0.02 (0.01, 0.04) | 0.018 | 0.2-1.5 | |
| Chloroform (μg/liter) | 48.8 (40.0, 71.8) | 43.9 (36.6, 55.3) | 103.2 (46.7, 197.7) | 39.0 (14.4, 123.7) | 0.019 | ||
| Bromodichloromethane (μg/liter) | 14.6 (6.1, 34.7) | <8 (0, 11.8) | <8 (0, 33.7) | <8 (0, 0) | 0.0001 | ||
| Dibromochloromethane (μg/liter) | <18 (0, 14.0) | <18 (0, 0) | <18 (0, 0) | <18 (0, 0) | NS | ||
| Bromoform (μg/liter) | <22 (0, 0) | <22 (0, 0) | <22 (0, 0) | <22 (0, 0) | NS | ||
| Total trihalomethanes (μg/ liter) | 77.3 (49.9, 109) | 48.5 (41.0, 94.9) | 103.2 (46.7, 233.0) | 40.2 (14.4, 186.0) | 0.042 | 100 | 200 |
| Total organic carbon (mg/ liter) | 41.7 (22.2, 66) | 42.8 (24.5, 56.3) | 3.8 (0, 26.8) | 5.7 (0.2, 16.6) | 0.0001 | ||
Stratification by season-chlorination showed significant variations between HGZs for the following parameters: total THMs in chlorinated samples from the rainy season (P = 0.05), temperature in nonchlorinated samples from both seasons (P = 0.009 and 0.011), dissolved oxygen in chlorinated samples from the dry season (P = 0.04), and total organic carbon in nonchlorinated samples from the dry season (P = 0.04).
An analysis of the physicochemical markers showed a positive correlation between conductivity and pH (rs = 0.54; P = 0.0000) and between conductivity and temperature (rs = 0.47; P = 0.0000). In other analyses, pH and temperature showed a positive correlation of 0.43 (P = 0.0000), and there was a positive correlation of 0.34 (P = 0.0005) between pH and total organic carbon. Negative correlations were found between dissolved oxygen and conductivity (rs = −0.34; P = 0.0005) and between dissolved oxygen and pH (rs = −0.33; P = 0.0007). Regarding the microbiological data, a moderate negative correlation was found between the residual chlorine level and fecal coliform counts (rs = −0.32; P = 0.0011).
The majority of the samples were free of microorganisms, exception for streptococci, for which there was a statistically significant increase from the rainy to the dry season independent of chlorine disinfection (P = 0.0002 for the total count of CFU/100 ml and P = 0.001 for the proportion of positive samples). The frequency of at least 1 CFU being detected varied from 5% of the chlorinated samples from the dry season for fecal coliforms to a maximum of 55% of the chlorinated samples from the dry season for fecal streptococci. This suggests a selective effect of the season-chlorination status on the bacteriological spectrum studied. There were no samples that developed more than 1 × 103 CFU (range, 0 to 500), which produced the upper limits of the 95% confidence intervals for the 0% point estimate at 16.8% for 20 samples, 15.6% for 22 samples, and 11.6% for 30 samples.
Residual chlorine levels do not show a positive impact on reducing bacterial counts, except in the case of fecal coliforms. Bacterial isolates from water samples throughout the annual cycle are listed in Table 3. A total of 84 species from nine genera were identified in water samples. Bacteria identified as total coliform and fecal coliform groups were found throughout the year, with E. coli being the most frequent organism. With regard to streptococci, the most frequent species were Enterococcus sp. and viridans group streptococci. There was a higher prevalence during the dry season, although the prevalence remained stable with chlorination.
TABLE 3.
Microorganisms in water samples from MCMA
| Microorganism | No. of samples with microorganisma
|
||||
|---|---|---|---|---|---|
| Rainy season
|
Dry season
|
Total | |||
| Cl (n = 22) | No Cl (n = 30) | Cl (n = 20) | No Cl (n = 30) | ||
| Total coliforms | |||||
| Escherichia coli | 3 | 1 | 1 | 2 | 7 |
| Klebsiella pneumoniae | 1 | 1 | 1 | 3 | |
| Enterobacter cloacae | 1 | 1 | 1 | 3 | |
| Enterobacter agglomerans | 1 | 1 | |||
| Fecal coliforms | |||||
| Escherichia coli | 2 | 2 | 4 | ||
| Klebsiella pneumoniae | 1 | 1 | |||
| Catalase-positive, gram-positive cocci | |||||
| Micrococcus sp. | 2 | 1 | 2 | 5 | |
| Streptococci | |||||
| Enterococcus sp. | 3 | 5 | 6 | 12 | 26 |
| Viridans group streptococci | 1 | 9 | 7 | 17 | |
| Klebsiella, Enterobacter, Citrobacter, Serratia, Plesiomonas, and others | |||||
| Enterobacteriaceae | |||||
| Serratia odorifera | 1 | 1 | |||
| Vibrio spp. | |||||
| Vibrio fluvialis | 1 | 1 | |||
| Vibrio parahaemolyticus | 1 | 1 | |||
| Pseudomonas spp. | |||||
| Pseudomonas aeruginosa | 1 | 1 | 1 | 1 | 4 |
| Pseudomonas stutzeri | 1 | 1 | |||
| Pseudomonas sp. | 1 | 1 | |||
| Nonfermentative, gram-negative bacilli | 4 | 1 | 3 | 8 | |
| Total | 12 | 16 | 23 | 33 | 84 |
Cl, with chlorine disinfection; no Cl, without chlorine disinfection; n, total number of samples.
Among other bacteria, Pseudomonas aeruginosa was the most prevalent. With regards to the gram-positive, catalase-positive coccus group, Micrococcus spp. were also identified throughout the year. The following fermentative bacteria were also found: Enterobacter spp., Escherichia coli, Klebsiella spp., Serratia spp., and Vibrio spp. The nonfermentative bacteria isolated were Bacillus spp. and Pseudomonas spp.
H. pylori detection rates were not affected by season or chlorination. The data for H. pylori and the cytotoxin-associated gene (cagA) are shown in Table 4. The presence of H. pylori was detected in 10% to 20% of the samples, with no significant differences between seasons and chlorination statuses. cagA was detected in one of eight samples during the rainy season and in five of seven samples during the dry season. However, in relation to the chlorination status, cagA was detected in four of seven chlorinated samples and in two of eight nonchlorinated samples, showing that disinfection did not lead to a significant difference in the expression of cagA.
TABLE 4.
Presence of H. pylori in water samples from MCMA
| Helicobacter pylori parameter | Valuea
|
|||
|---|---|---|---|---|
| Rainy season
|
Dry season
|
|||
| Cl (n = 22) | No Cl (n = 30) | Cl (n = 20) | No Cl (n = 30) | |
| No. (%) of 16S rRNA-positive samples | 3 (15) | 5 (16.7) | 4 (20) | 3 (10) |
| 95% Confidence interval | 0.032-0.379 | 0.056-0.347 | 0.057-0.437 | 0.021-0.265 |
| No. of cagA-positive samples/no. of positive samples | 1/3 | 0/5 | 3/4 | 2/3 |
Cl, with chlorine disinfection; no Cl, without chlorine disinfection; n, number of samples.
Genome sequencing was performed as part of this study. The 16S rRNA nucleotide sequence results are presented in Table 5, which shows a high homology between the human and environmental H. pylori 16S rRNA genes.
TABLE 5.
H. pylori 16S rRNA gene sequence
The physicochemical parameters (Table 2) that did not show any significant difference by season or by chlorination status were temperature, conductivity, dissolved oxygen, dibromochloromethane, and bromoform. No significant differences were found for residual chlorine between chlorinated and nonchlorinated samples. Parameters that showed significant differences by both season and disinfection status were nitrates, chloroform, and bromodichloromethane. The parameters affected by season were pH, ammonia, and total organic carbon, while those affected by chlorination were total trihalomethanes and pH, which complied with the Mexican regulations. Nitrate concentrations were relatively high, but these did not exceed the levels stipulated by the Mexican and U.S. regulations. Total organic carbon is not considered in these water standards.
There was no association between changes in bacteriological indicators (including H. pylori 16S rRNA) and HGZs, even when data were stratified by season and chlorination status.
A logistic-regression multivariate analysis showed a predictive role for the season (odds ratio [OR] = 5.17 for the dry season; P = 0.009) and for streptococci and total organic carbon, with an OR of 1.02 (P = 0.038) for H. pylori 16S rRNA. The bacterial counts showed an independent association between residual chlorine and total coliforms (b = −1.17; P = 0.018) and between nitrates and fecal coliforms (b = 0.092; P = 0.025) and nitrates and fecal streptococci (b = 0.530; P = 0.016). Finally, the total THMs were discretely associated with the bacterial aggregate represented by the sum of all of the subgroups (b = 0.0026; P = 0.042).
Subdivision according to the HGZ, including physicochemical, chlorination, and seasonal analyses, did not produce significant differences, which supports the capacity of the model. The areas selected for study were geographically similar to all other areas within the MCMA, thereby showing that the samples can be considered as truly representative of the MCMA as a whole.
DISCUSSION
The results of this prospective study of the MCMA showed that the groundwater distribution network is susceptible to contamination, which might be the case in other developing nations.
From the 102 water samples, 84 microorganisms from nine genera were identified, most of which are considered to be a normal part of the gut floras of humans and animals. This strongly suggests that these microorganisms found their way into the groundwater via human and animal feces.
Some of these bacteria represent potential threats to human health, including bacteria causing common diseases such as acute gastroenteritis, urinary tract infections, and nosocomial infections. The effects arising from contact with such water vary depending on the volume of water ingested by an individual and the individual's immune status, with children and the elderly being the most susceptible (2, 3, 6, 13, 23, 41).
Despite chlorination being detected in 90% of water samples, and considering the Mexican and American standards for water intended for human use and consumption based on the absence of total and fecal coliforms and E. coli (14, 50), the study shows that 18% of the samples (18/102) did not meet these standards. In terms of the presence of streptococci (Enterococcus and Streptococcus) and Vibrio spp., 37% of the water samples (19/52) from the rainy season and 58% (29/50) of the samples from the dry season were positive, giving an average of 47% (48/102) for the total period. In other words, almost half of the water samples studied showed some bacterial presence.
The results showed that chlorine concentrations in both chlorinated and nonchlorinated samples were extremely low. Chlorine is added to the water at low concentrations which are lower than those stipulated by the Mexican regulations (0.2 to 1.5 mg/liter). This is mostly carried out manually, and there is often not enough residual chlorine to react.
Some of the water samples were positive for more than one bacterial indicator group. Of the four groups of bacteria analyzed (total coliforms, fecal coliforms, fecal streptococci, and Vibrio spp.), the most frequent groups in both seasons were fecal streptococci and Vibrionaceae, while fecal coliforms and total coliforms were found in a lower percentage of the samples. Since the objective of using bacterial indicators is to achieve good water quality, the data from this study should be considered to improve the current monitoring strategy and to increase the use of more adequate indicators.
For more than 100 years, routine water quality assessments have relied on methods that detect or count indicator bacteria, but the reliability of these indicators is controversial (4, 5, 24, 41, 43). Coliforms include organisms such as Escherichia, Citrobacter, Klebsiella, Enterobacter, and Serratia that can survive and grow in water, and hence they are not useful as an index for fecal pathogens (52). However, they have been used as indicators of treatment effectiveness and to assess the integrity of the distribution system (20). The intestinal enterococcus group has been suggested as a possible indicator, since most species do not multiply in water environments, tend to survive longer in aquatic systems than E. coli, and are more resistant to drying and chlorination (52). For this study, several groups of bacteria were used in order to assess the quality of the MCMA groundwater supply in an attempt to expand the partial vision that is generated by using coliforms only, but even so, the assessment is still incomplete.
The resistance of coliforms to disinfection is lower than that of many pathogenic microorganisms. Viruses and parasites are generally more resistant to water treatment than coliform bacteria, and water that is apparently free of pathogens due to the absence of coliforms might contain other bacteria such as Helicobacter or Legionella, disease-causing viruses such as hepatitis A or E, rotavirus, coxsackievirus, adenoviruses, and Norwalk viruses, and parasites such as Giardia and Cryptosporidium (19, 41, 43, 46).
In developing countries such as Mexico, current microbial standards are based on total and fecal coliforms. These do not ensure safe drinking water, since they do not offer any indication of a diversity of bacterial, viral, and parasitic presences in the water. Tackling water quality by using traditional microbiological means is not the optimal approach (20). A reevaluation of microbial water quality tools for detection and risk assessment has been proposed (21, 43), and these ideas need to be applied in developing countries. The sampling frequency also needs to be considered in terms of a routine monitoring program, as utilized in the United States, where the sampling effort (number and frequency of samples) is based on the population being supplied (49, 50).
The detection of H. pylori in 20% of the groundwater samples was interesting, as this contrasts with a previous study in which 68% of groundwater samples from a specific area in southern Mexico City tested positive for H. pylori (34). This difference may be due to more intensive sampling in a smaller geographical area than that of the previous study.
The cagA gene was detected in only six samples in total, showing that the prevalence of this gene appears to be low. A positivity rate of 40% (6/15) is congruent with the frequency of this indicator in other studies, considering that the 95% confidence intervals range from 16.3% to 67.7%. In the water samples analyzed, there may have been a discretely higher prevalence of strains without this pathogenicity factor. For isolates from the Mexican population, the cagA gene was detected in approximately 93% of samples, which seems very high considering that approximately 60% of H. pylori isolates in Western countries are cagA positive.
In the current study, two 16S rRNA nucleotide sequences amplified by PCR from water samples and compared with existing DNA bank sequences showed great homology (GenBank sequences J99 [AE001556] and Hp 26695 [AE000620]) with H. pylori ATCC 43504 (U01330), suggesting that the DNA detected in the water samples came from H. pylori, whose only host is humans. Although H. pylori DNA was detected by PCR, this does not suggest directly that viable (and potentially infectious) H. pylori cells were present in the water samples studied. Independent studies concerning the prevalence and seroprevalence of H. pylori have suggested that drinking water might play some role in its transmission (28, 29, 31, 34, 35).
In developing countries, infection by H. pylori occurs early in life, with a reported prevalence above 50%, while in developed countries the prevalence of infection in children is usually below 10% (22). In Mexico, as well as other developing countries, the prevalence of H. pylori infection begins in children before they are 10 years of age, and by the age of 40, >90% of the population is seropositive for H. pylori (10), indicating the importance of assessing the potential transmission of this bacterium via water.
The quality of the water distributed in the MCMA differs between the dry and rainy seasons; higher pHs and higher levels of nitrates, chloroform, bromodichloromethane, total organic carbon, and fecal streptococci are observed during the dry season. The contaminant sources are evident throughout the year, but in the dry season the concentrations of some parameters increase.
It is known that high levels of certain compounds are associated with health problems, although in this case study, a direct link cannot be established with the discrete to moderate concentrations found. It would be too adventurous to link contamination with the effects arising from the consumption of water. One limitation is that the water supply cannot be specifically associated with a water source due to an intricate system that has developed over a very long period of time.
The potential formation of THMs as a result of total organic carbon reacting with residual chlorine also represents a health risk, and this is just being explored. As an initial screening method, THMs in this study were measured using the headspace technique by gas chromatography. In 4% of the samples (2/52) from the rainy season and 29% (14/48) of the samples from the dry season, the concentration of trihalomethanes exceeded the Mexican water quality standard of 200 μg/liter total trihalomethanes. Compared with the 60-μg/liter maximum level recommended by U.S. standards, 46% of the samples (24/52) exceeded the maximum concentration level during the rainy season and 42% (20/48) exceeded it during the dry season. These levels should be monitored over the long term, considering that the risk for bladder, colon, and rectal cancer, as well as neural tube defects, rises with increases in the THM concentration.
These types of organic compounds are rarely measured in developing countries due to the lack of equipment and trained personnel to perform the analysis, although in developed countries they are considered priority pollutants due to the potential long-term health problems associated with them.
Two events have changed the habits of MCMA inhabitants with respect to drinking water. Firstly, the 1985 earthquake broke water pipelines and sewers, causing cross-contamination; and secondly, the cholera pandemic reached Mexico in 1991 (7), with cholera cases registered from 1991 to 1998 (7, 51). People generally filter and/or boil their drinking water or use bottled water or sodas.
A previous groundwater study in the southern and western zones of Mexico City (12, 33) that focused on water quality and looked at household characteristics, including sanitation and the risk of enteric diseases, showed that one-half of the 1,250 households visited on a random basis often bought commercially bottled water, and the other half said they regularly use drinking water disinfectant.
Despite the highly complex water supply system for a large urban area like Mexico City, it should be emphasized that random probability sampling within a careful census of all water wells provides strong support for the findings of this study in terms of estimating the corresponding parameters for microbiological and physicochemical measurements of these water sources.
The only reliable way to link water quality and health problems in urban areas where a large population is exposed would be to perform an epidemiological study in which water would be sampled at the point of delivery. It would also be necessary to consider socioeconomic factors, which have a direct influence on water use and management. This would include nutrition status, vaccination, and practices for cleaning fruits and vegetables, since agricultural land in developing countries is sometimes irrigated with residual waters.
Water quality continues to be a basic public health issue in Mexico City and other cities of the world, with the presence of microorganisms and the deficiencies in control systems leading to the recurrence of gastrointestinal diseases. In addition, little is known about the effects of organic compounds, including trihalomethanes and other disinfection by-products, on health due to a lack of systematic monitoring. A better planning strategy needs to be implemented in order to incorporate risk analysis for synthetic chemicals and also to balance the presence and number of different microorganisms with the formation of disinfection by-products in water.
Due to the seasonal variations in bacteriological and physicochemical parameters, a different strategy could be implemented during the dry season and general improvements could be made to the efficiency of disinfection systems throughout the year. Regarding the deficient chlorination practices in cities like the MCMA, the selection of reliable and efficient compounds with no secondary effects needs to be performed, in addition to obtaining inexpensive, automatic, and easily maintained water system equipment, in order to supply potable water. Other options should be considered, such as UV light, chloramines, and ozone, although economic restrictions will dictate the way and speed with which such technologies will be incorporated into improved systems in developing countries.
One of the main problems with the distribution systems of developing countries, including some areas of the MCMA, is intermittent water distribution. This causes temporary negative pressure in the pipelines, which increases the chance of back-siphonage and back pressure of nonpotable water, which may carry microorganisms, bacteria, and viruses as well as contaminants present in the soil and groundwater, into the distribution system.
Major effort needs to be given to sprawling metropolitan areas in order to improve and guarantee the quality of water being supplied to the population. The MCMA study may be considered a typical example of what is happening in other cities in the developing world and may act as a catalyst for analyses of and hopefully improvements in the quality of water worldwide.
Acknowledgments
We acknowledge the support of the Consejo Nacional de Ciencia y Tecnología (CONACYT) to the project “Calidad microbiológica y química del agua en sistemas acuáticos de la Ciudad de México” (32505-T) from 1999 to 2001.
We thank Pilar Islas, Rosa Isabel Amieva, Josué Sandoval, Xóchitl Vega, Elia Velázquez, Brianda Barrios, and Adriana Vega for their invaluable help in the field and with laboratory work. We thank Mauricio Rodríguez for his assistance with manuscript preparation and Laura Luna-González for the figure.
REFERENCES
- 1.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- 2.Araujo, R. M., R. M. Arribas, F. Lucena, and R. Pares. 1989. Relation between Aeromonas and faecal coliforms in fresh waters. J. Appl. Bacteriol. 67:213-217. [DOI] [PubMed] [Google Scholar]
- 3.Aronson, T., A. Holtzman, N. Glover, M. Boian, S. Froman, O. G. Berlin, H. Hill, and G. Stelma, Jr. 1999. Comparison of large restriction fragments of Mycobacterium avium isolates recovered from AIDS and non-AIDS patients with those of isolates from potable water. J. Clin. Microbiol. 37:1008-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashbolt, N. J., W. O. Grabow, and M. Snozzi. 2001. Indicators of microbial water quality, p. 289-315. In L. Fewtrell and J. Bartram (ed.), Water quality: guidelines, standards and health. Assessment of risk and risk management for water-related infectious diseases. IWA Publishing-World Health Organization, Cornwall, United Kingdom.
- 5.Baggi, F., A. Demarta, and R. Peduzzi. 2001. Persistence of viral pathogens and bacteriophages during sewage treatment: lack of correlation with indicator bacteria. Res. Microbiol. 152:743-751. [DOI] [PubMed] [Google Scholar]
- 6.Basu, A., P. Garg, S. Datta, S. Chakraborty, T. Bhattacharya, A. Khan, S. Ramamurthy, S. K. Bhattacharya, S. Yamasaki, Y. Takeda, and G. B. Nair. 2000. Vibrio cholerae O139 in Calcutta, 1992-1998: incidence, antibiograms, and genotypes. Emerg. Infect. Dis. 6:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borroto, R. J., and R. Martínez-Piedra. 2000. Geographical patterns of cholera in Mexico, 1991-1996. Int. J. Epidemiol. 29:764-772. [DOI] [PubMed] [Google Scholar]
- 8.Bugliarello, G. 1999. Technology and the city, p. 131-146. In R. J. Fuchs, E. Brennan, J. Chamie, F. C. Lo, and J. I. Uitto (ed.), Mega-city growth and the future. United Nations University Press, Tokyo, Japan.
- 9.Castillo-Rojas, G., M. A. Ballesteros, S. Ponce de León, R. Morales-Espinosa, A. Cravioto, and Y. López-Vidal. 2002. Bleeding peptic ulcers and presence of Helicobacter pylori by various tests: a case-control study. Eur. J. Gastroenterol. Hepatol. 14:1113-1118. [DOI] [PubMed] [Google Scholar]
- 10.Castillo-Rojas, G., M. Mazari-Hiriart, and Y. López-Vidal. 2004. Helicobacter pylori: focus on CagA and VacA major virulence factors. Salud Publica Mex. 46:538-548. [DOI] [PubMed] [Google Scholar]
- 11.Cheema, G. S. 1999. Priority urban management issues in developing countries: the research agenda for the 1990s, p. 412-428. In R. J. Fuchs, E. Brennan, J. Chamie, F. C. Lo, and J. I. Uitto (ed.), Mega-city growth and the future. United Nations University Press, Tokyo, Japan.
- 12.Cifuentes, E., M. Mazari-Hiriart, F. Carneiro, F. Bianchi, and D. González. 2002. The risk of enteric diseases in young children and environmental indicators in sentinel areas of Mexico City. Int. J. Environ. Health Res. 12:53-62. [DOI] [PubMed] [Google Scholar]
- 13.Craun, G. F. 1992. Waterborne disease outbreaks in the United States of America: causes and prevention. World Health Stat. Q. 45:192-199. [PubMed] [Google Scholar]
- 14.Diario Oficial de la Federación. 2000. Modificación a la norma oficial Mexicana NOM-127-SSA1-1994. Salud ambiental, agua para uso y consumo humano. Límites permisibles de calidad y tratamientos a que debe someterse el agua para su potabilización. Diario Oficial de la Federación, Mexico City, Mexico.
- 15.Ezcurra, E., M. Mazari-Hiriart, I. Pisanty, and A. G. Aguilar. 1999. The Basin of Mexico: critical environmental issues and sustainability. Clark University-United Nations University Press, Tokyo, Japan.
- 16.Foster, S., A. Lawrence, and B. Morris. 1998. Groundwater in urban development. Assessing management needs and formulating policy strategies. The World Bank, Washington, D.C.
- 17.Fuchs, R. J. 1999. Introduction, p. 1-13. In R. J. Fuchs, E. Brennan, J. Chamie, F. C. Lo, and J. I. Uitto (ed.), Mega-city growth and the future. United Nations University Press, Tokyo, Japan.
- 18.Garza, G. 2000. Ámbitos de expansión territorial, p. 237-246. In G. Garza (ed.), La Ciudad de México en el fin del segundo milenio. Gobierno del Distrito Federal-El Colegio de México, Mexico City, Mexico.
- 19.Gerba, C. H., and J. B. Rose. 1990. Virus in sources and drinking water, p. 380-396. In G. A. McFeters (ed.), Drinking water microbiology. Progress and recent developments. Springer-Verlag, New York, N.Y.
- 20.Gerba, C. P. 2000. Indicator microorganisms, p. 491-503. In R. M. Maier, I. L. Pepper, and C. P. Gerba (ed.), Environmental microbiology. Academic Press, San Diego, Calif.
- 21.Grabow, W. O., M. B. Taylor, and J. C. de Villiers. 2001. New methods for the detection of viruses: call for review of drinking water quality guidelines. Water Sci. Technol. 43:1-8. [PubMed] [Google Scholar]
- 22.Graham, D. Y., H. M. Malaty, D. G. Evans, D. J. Evans, Jr., P. D. Klein, and E. Adam. 1991. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology 100:1495-1501. [DOI] [PubMed] [Google Scholar]
- 23.Guerrero, L., J. J. Calva, A. L. Morrow, F. R. Velazquez, F. Tuz-Dzib, Y. López-Vidal, H. Ortega, H. Arroyo, T. G. Cleary, L. K. Pickering, and G. M. Ruíz. 1994. Asymptomatic Shigella infections in a cohort of Mexican children younger than two years of age. Pediatr. Infect. Dis. J. 13:597-602. [DOI] [PubMed] [Google Scholar]
- 24.Haavelar, A., U. R. Blumenthal, M. Strauss, D. Kay, and J. Bartram. 2001. Guidelines: the current position, p. 17-22. In L. Fewtrell and J. Bartram (ed.), Water quality: guidelines, standards and health. Assessment of risk and risk management for water-related infectious diseases. IWA Publishing-World Health Organization, Cornwall, United Kingdom.
- 25.Harris, C. D. 1992. Análisis químico cuantitativo. Grupo Editorial Iberoamérica, Mexico City, Mexico.
- 26.Hill, B. 1971. Principles of medical statistics, 9th ed. The Lancet, London, United Kingdom.
- 27.Howard, K. W. F., and K. K. Gelo. 2003. Intensive use in urban areas: the case of megacities, p. 35-58. In R. Llamas and E. Custodio (ed.), Intensive use of groundwater. Challenges and opportunities. A. A. Balkema, Rotterdam, The Netherlands.
- 28.Hulten, K., H. Enroth, T. Nystrom, and L. Engstrand. 1998. Presence of Helicobacter species DNA in Swedish water. J. Appl. Microbiol. 85:282-286. [DOI] [PubMed] [Google Scholar]
- 29.Hulten, K., S. W. Han, H. Enroth, P. D. Klein, A. R. Opekun, R. H. Gilman, D. G. Evans, L. Engstrand, D. Y. Graham, and F. A. El-Zaatari. 1996. Helicobacter pylori in the drinking water in Peru. Gastroenterology 110:1031-1035. [DOI] [PubMed] [Google Scholar]
- 30.Kasperson, J. X., E. Kasperson, and B. L. Turner II (ed.). 1995. Regions at risk: comparisons of threatened environments. United Nations University Press, Tokyo, Japan.
- 31.Klein, P. D., D. Y. Graham, A. Gaillour, A. R. Opekun, and E. O. Smith. 1991. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Gastrointestinal Physiology Working Group. Lancet 337:1503-1506. [DOI] [PubMed] [Google Scholar]
- 32.Machin, D., and M. J. Campbell. 1987. Statistical tables for the design of clinical trials. Blackwell Scientific Publications, Oxford, Great Britain.
- 33.Mazari-Hiriart, M., E. Cifuentes, E. Velázquez, and J. J. Calva. 2000. Microbiological groundwater quality and health indicators in Mexico City. Urban Ecosys. 4:91-103. [Google Scholar]
- 34.Mazari-Hiriart, M., Y. López-Vidal, and J. J. Calva. 2001. Helicobacter pylori in water systems for human use in Mexico City. Water Sci. Technol. 43:93-98. [PubMed] [Google Scholar]
- 35.Mazari-Hiriart, M., Y. López-Vidal, G. Castillo-Rojas, S. Ponce de León, and A. Cravioto. 2001. Presence of Helicobacter pylori and other enteric bacteria in freshwater environments. Arch. Med. Res. 32:1-10. [DOI] [PubMed] [Google Scholar]
- 36.Mazari-Hiriart, M., B. Torres-Beristain, E. Velazquez, J. J. Calva, and S. D. Pillai. 1999. Bacterial and viral indicators of fecal pollution in Mexico City's southern aquifer. J. Environ. Sci. Health A 34:1715-1735. [Google Scholar]
- 37.Merino, H. 2000. Sistema hidráulico, p. 344-351. In J. Garza (ed.), La Ciudad de México en el fin del segundo milenio. Gobierno del Distrito Federal-El Colegio de México, Mexico City, Mexico.
- 38.Morris, B. L., A. R. Lawrence, and S. D. Foster. 1997. Sustainable groundwater management for fast-growing cities: mission achievable or mission impossible?, p. 55-66. In J. Chilton (ed.), Groundwater in urban environment: problems, process and management. Proceedings of the XXVII IAH Congress on Groundwater in the Urban Environment, Nottingham, United Kingdom. A. A. Balkema, Rotterdam, The Netherlands.
- 39.Murray, P. R., E. J. Baron, M.-A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.). 2003. Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 40.Patel-Coleman, K. 2003. Exposure assessment and disinfection management implications of trihalomethane and coliform levels in a Mexico City drinking water supply. D.Env. dissertation. University of California, Los Angeles.
- 41.Payment, P., A. Berte, M. Prevost, B. Menard, and B. Barbeau. 2000. Occurrence of pathogenic microorganisms in the Saint Lawrence River (Canada) and comparison of health risks for populations using it as their source of drinking water. Can. J. Microbiol. 46:565-576. [PubMed] [Google Scholar]
- 42.Pitcher, D. G., N. A. Saunders, and R. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 43.Rose, J. B., and D. J. Grimes. 2001. Reevaluation of microbial water quality: powerful new tools for detection and risk assessment. American Academy of Microbiology, Washington, D.C. [PubMed]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., p. 1.21-1.52. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Soto Galera, E., M. Mazari-Hiriart, and L. A. Bojórquez Tapia. 2000. Entidades de la zona metropolitana de la Ciudad de México propensas a la contaminación de agua subterránea. Investig. Geográf. 43:60-75. [Google Scholar]
- 46.Szewzyk, U., R. Szewzyk, W. Manz, and K.-H. Scheifer. 2000. Microbiological safety of drinking water. Annu. Rev. Microbiol. 54:81-127. [DOI] [PubMed] [Google Scholar]
- 47.United Nations. 2002. World urbanization prospects. The 2001 revision. Department of Economic and Social Affairs, Population Division. ST/ESA/SER.A/216. United Nations, New York, N.Y.
- 48.U.S. Environmental Protection Agency. 2002. List of drinking water contaminants and MCLs. EPA 816-F-02-013. [Online.] http://www.epa.gov/safewater/mcl.html.
- 49.U.S. Environmental Protection Agency. 2003. List of drinking water contaminants and MCLs. EPA 826-F-02-016. [Online.] http://www.epa.gov/safewater/mcl.html.
- 50.U.S. Environmental Protection Agency. 2003. Water on tap, what you need to know. EPA 816-K-03-007. [Online.] http://www.epa.gov/safewater.
- 51.Vilchis-Guizar, A. E., S. Uribe-Márquez, and P. L. Pérez-Sánchez. 1999. Características clínico-epidemiológicas de pacientes con cólera en la Ciudad de México. Salud Publica Mex. 41:487-491. [PubMed] [Google Scholar]
- 52.World Health Organization. 2004. Guidelines for drinking-water quality. Volume 1 recommendations. World Health Organization, Geneva, Switzerland.