Summary
Background
Clinical diagnosis of tuberculosis (TB), referring to diagnosis without bacteriological confirmation, is common and may affect an individuals’ outcomes. We undertook a systematic review to assess the proportion of people with TB who were diagnosed clinically, and mortality compared to those with bacteriologically confirmation in the published literature.
Methods
We searched Medline, Embase, Web of Science and Cochrane Library from January 2010 to December 2024 using terms for ‘TB’ and diagnostic studies. We excluded studies with participants aged <15 years, not reporting clinical and bacteriologically confirmed TB, not conducted in high TB burden settings, and studies that were not trials, cohort or cross-sectional in design. Published summary data was extracted and risk of bias assessed. Summary estimates for proportion of diagnoses that were clinical were calculated overall and by pre-specified subgroups. Risk ratio for mortality of clinical compared to bacteriological diagnosis was evaluated by random effects meta-analysis. This review was prospectively registered (PROSPERO CRD42023404419).
Findings
Our search identified 5693 records, of which 53 datasets were included. 12 studies were rated as low risk of bias. Median proportion of TB diagnosed clinically (n = 85,623) was 40% (95% CI: 31–46%, interquartile range 27%–53%). The proportion of TB diagnosed clinically was higher in people living with HIV and extrapulmonary TB. Clinical diagnosis did not differ by diagnostic modality available or by study year. The pooled risk ratio for mortality (n = 20,523, 10 studies) was 1·5 (95% CI: 1·0–2·2, I2 = 78·7%) indicating higher mortality in people diagnosed clinically.
Interpretation
Clinical diagnosis of TB remains common and was associated with higher mortality risk than bacteriologically confirmed TB, suggesting conditions other than TB that are not being adequately treated. Better understanding of reasons for clinical TB diagnosis and investment in improved diagnostics for TB and non-TB conditions is needed.
Funding
UK National Institute for Health and Care Research and Academy of Medical Sciences; US National Institutes of Health.
Keywords: Tuberculosis, Clinical diagnosis, Empirical treatment, Diagnosis, Mortality
Research in context.
Evidence before this study
Current diagnostic tests for tuberculosis (TB) have suboptimal sensitivity, therefore in countries with a high burden of TB the use of ‘clinical diagnosis’, in the absence of bacteriological confirmation, is commonplace. Programmatic data from the World Health Organization estimates approximately 38% of people with TB had bacteriological confirmation in 2023. However, little is known about how this varies in different settings and populations, and with the availability of different diagnostic tests. Furthermore, outcomes for people started on treatment after clinical diagnosis of TB compared to those with bacteriological confirmed TB is not well described. We searched the Medline database for systematic reviews of clinical diagnosis of TB, and found no such studies.
Added value of this study
This systematic review included data from 54 studies in high TB burden settings, and found a median of 40% of TB diagnoses. We found higher proportions of clinical diagnosis in people living with HIV, in hospital inpatients, and in extrapulmonary TB. We found mortality was higher in those with clinical TB diagnosis than bacteriologically confirmed TB. Clinical trials of diagnostics interventions showed a reduction in clinical diagnosis with more sensitive diagnostics, but this was not seen in sub-group analysis across observational studies.
Implications of all the available evidence
Clinical diagnosis of TB seems to be associated with higher risk of mortality, and is more common in some populations and settings. These data support the need to better understand the reasons for clinical diagnosis of TB, and the need for improved diagnostics.
Introduction
Despite the global commitment to End Tuberculosis (TB) by 2030, there were still an estimated 1·25 million deaths in 2023 attributed to TB worldwide.1 Diagnosis remains the biggest gap in the TB care cascade, and early and accurate diagnosis is key to attain global TB targets of eliminating TB as public health issue.2 It is estimated that in 2023 around 2·7 million people worldwide falling sick with TB did not get diagnosed or treated for TB.1
The bacteriological detection of TB allows for the correct diagnosis of TB, initiation of the most effective treatment, depending on the presence of drug resistance, and can reduce overtreatment resulting from empirical treatment. For decades, diagnosis of TB relied on sputum smear microscopy, which has suboptimal sensitivity, especially in people living with HIV, extrapulmonary disease and earlier in the disease course.3 Therefore, clinical diagnosis (i.e. without a positive bacteriological test for TB) and empirical treatment have been commonplace in practice, and have been important to reduce mortality and morbidity from TB.4 Molecular tests for TB are highly specific and more sensitive than sputum microscopy.5 Since World Health Organization (WHO) endorsement in 2011, rapid, low complexity molecular tests have been scaled-up by national TB programs, gradually replacing sputum smear microscopy. Despite this, there has not been a substantial increase in the proportion of notified TB that was bacteriologically confirmed since 2010 worldwide-only 62% of pulmonary TB was confirmed bacteriologically in 2023.1
Potential reasons for clinical diagnosis of TB include inavailability or delays in bacteriological testing for TB, inability to produce sputum for bacteriological testing, failure to respond to broad-spectrum antibiotics, persistent clinical features compatible with TB despite negative tests, uncertainty about the accuracy of TB tests and a desire to quickly relieve symptoms for patients.6, 7, 8, 9 The extensive use of clinical diagnosis for TB has the potential to undermine the impact of more accurate TB diagnostics and could further contribute to overtreatment.6,10,11 People treated for TB in the absence of bacteriological confirmation may have other conditions missed, and potentially worse outcomes.12,13 However, the drivers of clinical diagnosis, including differential importance between settings and populations are insufficiently understood.
We systematically reviewed and assessed existing evidence for how common clinical diagnosis of TB is, whether this varied by sub-population and setting, and how it affected outcomes compared to outcomes with bacteriologically confirmed TB.
Methods
For this systematic review and meta-analysis, we describe the proportion of people diagnosed clinically among those diagnosed with TB and, secondarily, the clinical outcomes of those people diagnosed clinically compared to those with a bacteriologically confirmed diagnosis. We defined clinical diagnosis as a diagnosis of TB in the absence of any positive bacteriological test (e.g. sputum smear microscopy, mycobacterial culture, molecular diagnostic test or urinary lipoarbinomannan [LAM] antigen test), either because testing was not done or negative. Clinical diagnosis of TB is synonymous with empirical treatment.6,14
Search strategy and selection criteria
We systematically searched Medline, Embase, Web of Science and Cochrane Library databases from 1st January 2010 until 31st December 2024 to identify relevant literature. We included studies if they were conducted in 2010 or later, if they were randomized controlled trials (RCTs), cohort studies or cross-sectional studies, written in English, reporting on clinical diagnosis and bacteriological diagnosis, if the included population was older than 15 years and if they were conducted in a WHO high TB burden or WHO high HIV/TB burden country.15 2010 was chosen as the start date as this is when studies of molecular diagnostics in high burden settings were first conducted. Our search strategy combined broad search terms for TB and diagnostic studies (see Appendix pages 2–4 for the details of the search terms). Studies were excluded if they were case control studies, questionnaire based, cost effectiveness studies, enrolled fewer than ten people, community-based or household contact screening or active case finding studies, reporting on a specific or preselected population (e.g. miners, pregnant women, congregate settings), or were studies on tuberculosis meningitis or drug-resistant TB. We did not exclude studies done exclusively in people living with HIV.
Identified records were screened systematically using Rayyan software.16 A form was used for title, abstract and full text screening, and studies possibly containing data on clinical diagnosis were selected for full text review. Two authors (BF and AS) conducted screening independently. Conflicts were resolved by consensus, involving third reviewer (AGW) if necessary. After blinded double screening and high agreement for the first 10 studies, 50% of records in title and abstract screen and 50% of records in full-text screen were double screened for quality assurance.
Data extraction
Data was extracted into a piloted and standardised data extraction form using Covidence software (Veritas Health Innovation, Australia). First and second reviewer extracted data independently, involving third reviewer (AGW) if necessary. To ensure quality and alignment, first and second reviewer double extracted the first 5% of records. Multiple reports on the same study were summarized in a single extraction form where appropriate. Clinical trials with a diagnostic intervention aiming at improving bacteriologically confirmed TB were extracted into different forms for each intervention arm.
The data extracted included study characteristics and design, study population, TB diagnostic tests, and our outcomes of interest (see Appendix page 5). The primary outcome was the proportion of people whose diagnosis of TB was clinical among all people diagnosed with TB. Secondary outcomes were the risk ratio (RR) of mortality between the group of clinically diagnosed compared to bacteriologically diagnosed. Missing Information was not imputed.
Assessment of bias
We assessed risk of bias using a bespoke tool based on the JBI risk of bias tool for prevalence studies and diagnostic accuracy studies (Appendix Table S1).17,18 Each domain was rated as high risk, low risk or unclear if inadequate information was available. The eight domains were then summarized to a score value (0–8) indicating overall risk for each study. Studies with a score value of 7–8 were rated as low risk studies, studies with a value of 5–6 as high risk staudies and studies with a value ≤4 as very high risk studies. Risk of Bias was assessed independently by first and second reviewer, and conflicts resolved by a third reviewer. Publication bias was assessed by visual inspection of a funnel plot.
Data analysis and synthesis
Studies meeting our eligibility criteria and reporting data for at least our main objective were used for quantitative data synthesis. Data extracted on absolute numbers of people with clinically diagnosed TB and bacteriologically confirmed TB were used to calculate proportions. Where there was a high degree of heterogeneity between studies on visual assessment of forest plots and using I2, overall summary estimates for the proportion of clinically diagnosed TB are presented as bootstrapped median with 95% confidence interval, supplemented by interquartile ranges.19
Heterogeneity was explored by pre-specified subgroup analyses, both within studies (where disaggregated data was available) or at study level. Subgroups included HIV status, pulmonary or extrapulmonary TB, healthcare setting and level (primary i.e. community health centers or outpatients, and secondary i.e. hospitals, inpatients), the main diagnostic methods used (e.g. smear or molecular testing), and if all people were offered bacteriological tests. To investigate the strength of evidence for differences between subgroups, we used random effects meta-analysis, with two-sided alpha at 0·05. To investigate differences over time, we conducted meta-regression of proportion with clinical diagnosis by mean year of study recruitment.
To assess clinical outcomes, disaggregated absolute numbers of people with clinical and bacteriological diagnosis with data on mortality were collected, and RR for mortality calculated. Meta-analysis of binary outcome data was conducted when at least four papers reported on the outcome. We used a random effects model and inverse variance method DerSimonian Laird, combined with Hartung-Knapp adjustment as we expected high degree of heterogeneity.19 Meta-regression of the mean year of study recruitment was conducted to further investigate difference over time.
In post-hoc analysis, we calculated the absolute proportion reduction in clinical TB diagnosis between trial arms in controlled trials that included a diagnostic intervention aiming at improving bacteriological TB diagnosis. Absolute proportion reduction between the study arms of each clinical trial and standard error were calculated, and data were pooled using a generic inverse variance random effects meta-analysis with Hartung-Knapp adjustment.
Data analysis was conducted using RStudio (version: 2024·04·0 + 73) using meta, metaprop and forestploter packages. Results of individual studies are presented by forest plot. For each meta-analysis conducted, 95% confidence interval as well as prediction interval are reported and I2 statistic used to assess heterogeneity.
We follow the PRISMA 2020 guideline for reporting systematic reviews and meta-analyses.20 The protocol for the systematic review was prospectively registered on PROSPERO database (CRD42023404419).
Role of funding source
No funders had any role in the study design, conduct or analysis.
Results
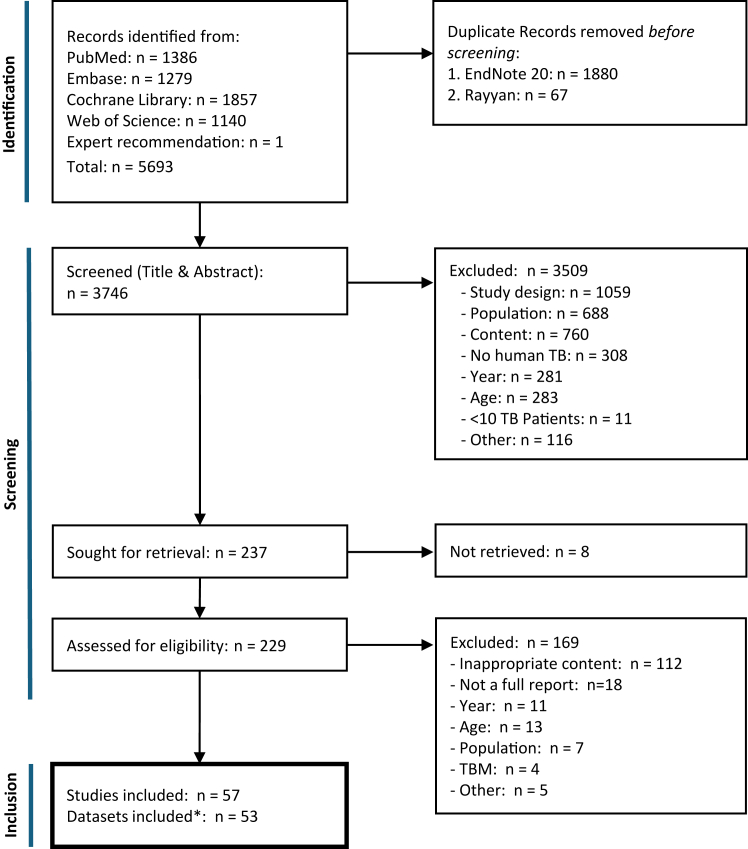
We identified 5693 articles from database searches, which reduced to 3746 after removing duplicates, and to 237 after screening abstract and titles. After full-text review and summarising of multiple articles reporting data on the same cohort, 53 datasets from 57 studies were included in the systematic review (Fig. 1).
Fig. 1.
PRISMA flow diagram. Flow diagram on study selection process according to PRISMA 2020 guidelines20 with list of exclusion reasons. TBM, tuberculosis meningitis, ∗four studies reported the same patient cohort as another included study.
The eligible studies included data from 253,829 participants assessed for TB, of whom 85,623 were diagnosed with TB disease and had data on the basis of diagnosis (bacteriologically confirmed or clinically diagnosed). Characteristics are presented in Table 1 and Table S2. The median number of people diagnosed with TB in each study was 420 (range from 38 to 24,265), median age was 37 years (range 30–54 years), and a median of 55% were male (range 31–78%). Most studies (62%) were from the WHO African region, and 17% were from South-East Asia. 53% reported on participants with both extrapulmonary and pulmonary TB, with 30% being limited to pulmonary TB only. The majority of studies were in people with symptoms of TB, although 6 studies included PLHIV irrespective of symptoms. 24% of studies only used sputum smear microscopy as the main diagnostic method for TB, 55% of studies used molecular diagnostics, and 47% included mycobacterial culture. 24% of studies were in a primary healthcare setting, 40% in secondary healthcare. 15% of studies included only inpatients and 24% only outpatients. 38% were cross sectional studies, 34% controlled clinical trials and 23% cohort studies. Overall only 23% of studies had low risk of bias, 77% had high or very high risk of bias (Appendix Table S3).
Table 1.
Characteristics of studies included in systematic review.
| Study ID | RoB/8a | Design | Country (-ies) | Healthcare level | Diagnostics available | Population descriptionb | Total TBc | HIV prevalenced |
|---|---|---|---|---|---|---|---|---|
| Burke 202421 | 7 | RCT | Malawi | Secondary | CU, XP, LAM, CXR, CAD | ≥18 y, PLHIV admitted to hospital, irrespective of TB symptoms, no TB treatment 6 months before enrolment | 415 | 100% |
| Kibirige 202322 | 6 | DAS | Uganda | Secondary | SM | ≥18 y, either TB treatment naïve or initiated on TB treatment | 232 | 38·6% |
| Sachdeva 201523 | 6 | CHS | India | Primary | SM, XP, CXR | TB symptoms and producing sputum | 17,587 | – |
| Adewole 201524 | 5 | RCT | Nigeria | Secondary | SM | ≥15 y, not on TB treatment, no relevant coexisting medical conditions | 150 | 6·7% |
| Balcha 201425 | 6 | CHS | Ethiopia | Primary | CU >50%, SM, XP | Adult PLHIV, not on ART, irrespective of TB symptoms, producing sputum | 158 | 100% |
| Durovni 201426 | 4 | CRT | Brazil | Mixed | SM, CU, XP, CXR | Producing sputum, PTB only | 4660 | 8·9% |
| McCarthy 201827,28 | 5 | CRT | South Africa | Primary | SM, CU, XP, CXR | ≥18 y, started on TB treatment, negative initial XTEND study sputum results, clinical indication for sputum investigation | 541 | 62% |
| Bekele 201829 | 4 | RCT | Ethiopia | Mixed | CU >50%, SM, IGRA | Adults, newly diagnosed PTB, no relevant coexisting medical conditions | 348 | 0% |
| Eneogu 202430 | 3 | CSS | Nigeria | – | – | People diagnosed with TB, data from laboratory register | 4823 | – |
| Cox 201431 | 7 | RCT | South Africa | Primary | SM, CU, XP | Adults, TB symptoms, no TB Treatment for >3 d before enrolment | 506 | 58·8% |
| Majella 202132 | 6 | RCT | India | Secondary | SM | New diagnosis of TB, possession of mobile phone | 310 | 5·8% |
| DeCastro 202133 | 4 | RCT | Brazil, Côte d’Ivoire, +3 | – | SM, CU, XP, LAM, CXR | ≥18 y, ART naive, Rifampicin containing TB Treatment initiated within 2 months, no relevant coexisting medical conditions | 457 | 100% |
| Rima 202434 | 7 | CSS | Ethiopia | Mixed | SM, XP, CXR | ≥15 y, PTB, on directly observed therapy, mainly rural, not critically ill | 393 | – |
| Dave 201335 | 7 | CSS | India | Mixed | SM | Adults and children with TB diagnosis | 556 | 4·3% |
| Gupta-Wright 201836 | 7 | RCT | Malawi, South Africa | Secondary | SM, CU, XP, LAM, CXR | ≥18 y, hospitalized, urban and rural, irrespective of TB symptoms, not on TB treatment for 12 months before enrolment | 474 | 100% |
| Hanifa 201637 | 4 | CHS | South Africa | – | SM, CU, XP, LAM, CXR | ≥18 y, CD4 count <200 cells/μl, in HIV care, irrespective of TB symptoms, no TB treatment within 3 months before enrolment | 56 | 100% |
| Getahun 201638 | 6 | CSS | Ethiopia | – | SM | Adults, new TB cases, on directly observed therapy at least for 1 month | 576 | – |
| Atekem 201839 | 6 | CSS | Cameroon | Primary | SM | – | 895 | 47·7% |
| Ereso 202440 | 4 | CSS | Ethiopia | Mixed | – | ≥15 y, initiated TB treatment | 755 | 3·3% |
| Weber 201841 | 6 | CHS | India | Secondary | SM, CU, CXR | ≥16 y, TB symptoms | 285 | 14% |
| Cattamanchi 202142 | 6 | RCT | Uganda | Primary | SM, XP | Adults, PTB, [we included people started on treatment only] | 824 | 43·8% |
| Prudhivi 201943 | 7 | CHS | India | Secondary | SM | New and retreatment PTB, data on treatment outcome available | 1113 | 23% |
| Åhsberg 202344 | 7 | CRT | Ghana | Secondary | CU, XP, LAM | Adult PLHIV, TB symptoms, severe illness or advanced HIV, not receiving TB treatment in the preceding 60 days | 105 | 100% |
| Padda 201545 | 6 | CSS | India | – | SM | TB treatment directly observed therapy started | 2571 | – |
| Manosuthi 201246 | 5 | RCT | Thailand | Secondary | SM, CU, CXR | 18–65 y, CD4 count <350 cells/μl, ART naive, no relevant coexisting medical conditions | 156 | 100% |
| Bock 201847 | 5 | CHS | South Africa | Primary | CU, XP, CXR | ≥18 y, started ART | 97 | 100% |
| Theron 201448,49 | 8 | RCT | South Africa, Zimbabwe, +2 | Primary | CU >50%, SM, XP, CXR | ≥18 y, periurban, TB symptoms, no treatment in previous 60 d, producing sputum | 645 | 59·6% |
| Bjerrum 201550 | 6 | CSS | Ghana | Secondary | SM, CU, XP, CXR, LAM | ≥18 y, CD4 count ≤350 cells/μl, irrespective of TB symptoms, producing sputum, no TB treatment 3months before enrolment | 100 | 100% |
| Shivalingaiah 202451 | 5 | CHS | India | – | – | All TB cases ≥18 y, registered for treatment at study site | 516 | 1·7% |
| Hanrahan 201352 | 7 | CHS | South Africa | Primary | CU >50%, SM, XP | TB symptoms, informal settlement communities | 116 | 69·1% |
| Zerihun 202353 | 7 | CSS | Ethiopia | Primary | SM | ≥18 y, PTB, newly started on TB treatment, mainly urban | 636 | 16·7% |
| Shrivastava 201354 | 6 | CHS | India | Secondary | SM | TB symptoms | 61 | 10·2% |
| Bezerra 202055 | 4 | CHS | Brazil | Secondary | – | Adults, started on TB Treatment | 148 | 37·2% |
| Songkhla 201956 | 6 | CHS | Thailand | Secondary | CU >50%, SM, LAM, CXR | Adults, CD4 cell count ≤200/μl, symptomatic, irrespective of sputum production, no TB Treatment within 3 months before enrolment | 137 | 100% |
| Jiang 202357 | 5 | CSS | China | – | SM, CU, XP | Not on TB treatment yet, not recorded as “dead”, “treatment failure”, or who were “not evaluated” | 24,265 | – |
| Hemalatha 202358 | 3 | CSS | India | Secondary | – | PTB and EPTB | 45 | – |
| Abdullahi 202112 | 7 | CSS | Kenya | Mixed | SM, XP, CXR | Adults, started on TB treatment | 12,856 | 29% |
| Kaku 202459 | 4 | CSS | Indonesia | – | XP | – | 158 | 1·2% |
| Jin 202060 | 5 | DAS | China | Secondary | CU >50%, XP | People with different diseases (infectious and non-infectious) | 125 | – |
| Ncube 201961 | 5 | CSS | Zimbabwe | Primary | – | ≥15 y, newly registered, densely populated poor urban suburb resident | 1617 | 67·5% |
| Peter 201662,63 | 6 | RCT | South Africa, Tanzania, +2 | Secondary | CU >50%, SM, XP, LAM, CXR | ≥18 y, symptomatic, severely ill, no TB treatment within 60 d before testing | 1246 | 100% |
| Gebreegziabher 201664 | 6 | CSS | Ethiopia | Mixed | SM | ≥15 y, newly diagnosed PTB, no TB retreatment cases | 706 | 11·6% |
| Mupfumi 201465 | 6 | RCT | Zimbabwe | Secondary | SM, XP, CXR | ≥18 y, ART naive initiating ART, not on TB treatment, urban resident, irresp. of TB symptoms | 88 | 100% |
| Mohammed 202066 | 4 | CSS | Ethiopia | Mixed | SM, XP, CXR | TB symptoms | 2483 | 12·5% |
| Qiu 201567 | 6 | RCT | China | Secondary | CU >50%, SM, CXR, IGRA | TB symptoms | 597 | 0% |
| Seid 201868 | 5 | CSS | Ethiopia | Mixed | SM, XP, CXR | ≥18 y, newly diagnosed TB, <15 d of TB treatment, not critically ill | 382 | 20·4% |
| Kebede 202169 | 7 | CSS | Ethiopia | Secondary | SM | ≥15 y, started on TB treatment | 465 | 27·4% |
| Getiye 202470 | 6 | CSS | Ethiopia | Mixed | – | Adults, newly diagnosed PTB attending TB clinics in public health facilities, mainly rural | 420 | 2% |
| Auld 201671 | 7 | CHS | Cambodia | Mixed | SM, CU, XP, CXR | PLHIV, rural, symptomatic | 234 | 100% |
| O'Connor 201772 | 6 | CRT | Lesotho | Mixed | SM, CU, XP, CXR | ≥18 y, newly registered for TB treatment | 1233 | 100% |
| Mishra 202373 | 5 | CSS | India | Secondary | SM | Adult, on DOTS, new cases of PTB, on treatment for <10 days, no relevant coexisting medical conditions | 341 | 0% |
| Humphrey 202074 | 5 | CHS | Kenya, Uganda, +10 | Secondary | SM, CU, XP, LAM, CXR | ≥15 y, started on TB treatment | 2091 | 100% |
| Beckwith 202175,76 | 6 | RCT | South Africa | Secondary | SM, CU, XP, CXR | ≥18 y, CD4 count ≤150 cells/μl, no relevant coexisting medical conditions, newly diagnosed TB | 95 | 100% |
CSS, cross sectional; RCT, randomised controlled trial; DAS, diagnostic accuracy study; CRT, cluster randomized trial; SM, smear microscopy; CU, culture; XP, X-pert; CXR, chest X-ray; IGRA, interferon gamma release assay; LAM, lipoarabinomannan on urine; CAD, digital chest X-ray with computer-aided diagnosis; PLHIV, people living with HIV; PHC, primary health care; ART, antiretroviral therapy; DOTS, direct observed therapy.
Risk of Bias Score out of 8, higher scores indicate lowest risk of bias.
Population description as described in the study manuscript. TB symptoms refer to cough, fever, weight loss or night sweats.
Total amount of people diagnosed with TB.
HIV prevalence in the study population.
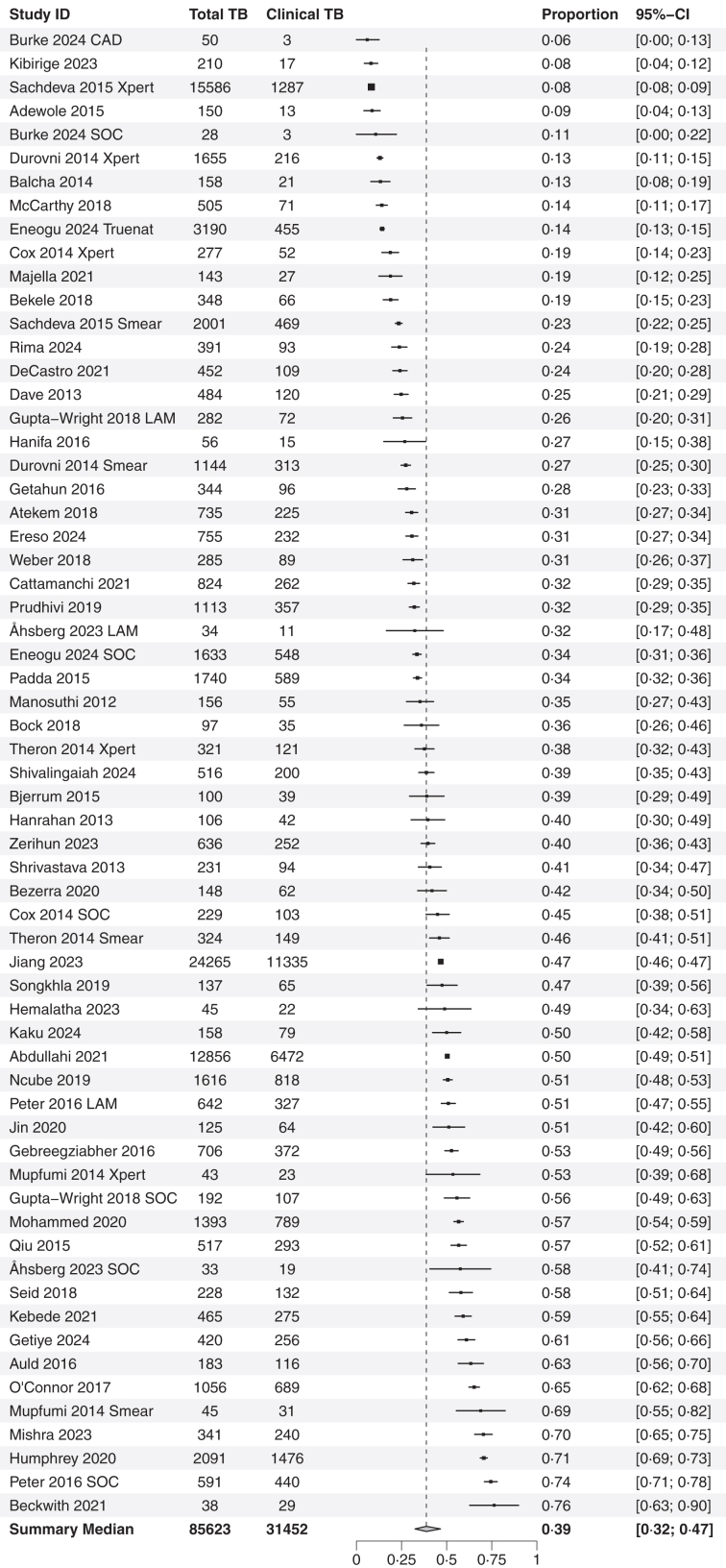
The median overall proportion of study participants with a clinical diagnosis of TB was 40% (95% CI: 31–46%, IQR: 27%–53%, n = 63, Fig. 2), with high levels of heterogeneity between studies (I2 = 99%, Figure S1). The proportion of clinical diagnosis was substantially higher for extrapulmonary TB (78%, 95% CI: 48–93%, n = 6, Figure S2), compared to only pulmonary TB (34%, 95% CI: 28–40%, n = 35, p = 0·001). Similarly, clinical diagnosis was more common in people living with HIV (45%, 95% CI: 38–52%, n = 35, Figure S4), compared to those not living with HIV (33%, 95% CI: 25–42%, n = 15, p = 0·026 Figure S5). TB was also diagnosed clinically more often in secondary care compared to primary care (47% versus 34%, p = 0·01, Figure S6), and in inpatients compared to outpatients (51% versus 40%, p = 0·29, Figure S7).
Fig. 2.
Proportion of clinical TB. Forest Plot on the proportion of clinically diagnosed TB people in all people diagnosed as TB with data on confirmation status available. Overall proportion with 95% CI below is the bootstrapped median of data above. Absolute numbers of people diagnosed with TB (Total TB) and clinically diagnosed TB (Clinical TB) are on the left, and proportion and 95% CI on the right. Where clinical trial arms have been presented separately, the description of the arm is presented after the citation: SOC Standard of Care, Smear is sputum smear microscopy, Xpert is Xpert MTB/RIF or Xpert MTB/RIF Ultra arm, LAM is Urine lipoarabinaomannan (LAM) testing, CAD is digital chest X-ray with computer-aided diagnosis and Truenat is Truenat real time PCR device.
Clinical diagnosis was more common in studies where not all participants received bacteriological testing compared to studies where all participants had at least one bacteriological test (60% versus 35%, p < 0·001, Figure S8). The proportion of clinical TB did not differ by whether studies used sputum smear microscopy or molecular tests as the main diagnostic test for TB (Figure S9), or by risk of bias score (Figure S10). Meta-regression by year of study found no changes over time (Figure S11), and there was no evidence of publication bias (Figure S12).
Mortality data stratified by clinical or bacteriologically confirmed TB was available for ten studies including 20,523 participants, of whom 48% were diagnosed clinically and 52% bacteriologically. The RR for mortality varied from 0·6 to 3·1, with three studies reporting lower mortality risk in people with clinical TB diagnosis. The pooled RR was 1·5 (95% CI: 1·1–2·2, I2 = 78, 7%, Fig. 3), indicating a higher mortality risk in those with a clinical diagnosis. For studies with a low risk of bias, pooled RR was higher than in studies with high risk of bias (RR 1·9, 95% CI: 1·4–2·7, n = 6 compared to 1·0, 95% CI: 0·4–2·4, n = 4 respectively, p = 0·04, Figure S13). Meta-regression by year found a positive correlation, with a higher risk ratio for mortality in more recent years (p = 0·004, Figure S14), with more recent studies having a two-fold higher RR.
Fig. 3.
Mortality of clinical TB. Forest plot depicting random effects inverse variance meta-analysis on mortality risk ratio of clinically diagnosed TB people compared to bacteriologically confirmed TB people with data on confirmation status available. Absolute numbers on the left are deaths and total number of people (cases) with clinical or bacteriological diagnosis. RR per study with 95% CIs and weight on the right. Pooled RR with 95% CI displayed below as well as heterogeneity assessment. Median duration of follow-up is 6·0 months (IQR 4·5 months).
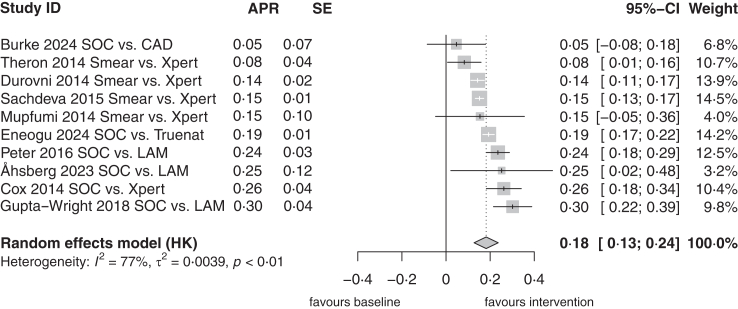
We identified ten clinical trials of diagnostic interventions aiming to improve bacteriological diagnosis, including trials of implementation of Xpert MTB/RIF and/or urine lipoarabinomannan assays (Appendix Table S4). All studies demonstrated a decrease in proportion of clinically diagnosed TB in the intervention arms compared to standard of care, which varied from 5% to 30%, with a pooled absolute reduction of 18% (95% CI: 13–24%, Fig. 4).
Fig. 4.
Reduction of porportion of clinical TB in clinical trials with diagnostic intervention. Forest plot depicting inverse variance random effects meta-analysis on absolute proportion reduction (APR) between the two arms of clinical trials aiming on the reduction of the proportion of clinical TB in their diagnostic intervention. Pooled APR indicates, that interventions of clinical trials reduced the proportion of clinical TB. The APR and standard error (SE) on the left. APR with 95% CI and weight on the right. Below the pooled estimate with 95% CI as well as heterogeneity assessment. SOC is Standard of Care, Smear is sputum smear microscopy, Xpert is Xpert MTB/RIF or Xpert MTB/RIF Ultra arm, and LAM is Urine lipoarabinaomannan (LAM) testing. CAD is digital chest X-ray with computer-aided diagnosis and Truenat is Truenat real time PCR device.
Discussion
We report, to our knowledge, the first systematic review to assess clinical diagnosis of TB and associated mortality in high burden settings. Our main findings are that clinical diagnosis of TB remains common, occurring in a median of 40% of people diagnosed with TB, and has not decreased over time or by availability of molecular diagnostic tests. There was high variability between studies, but clinical diagnosis was more common in people living with HIV, in secondary care and inpatients, and in extrapulmonary TB. Mortality risk was approximately 50% higher in those with clinical diagnosis compared to those with bacteriological confirmation.
Our findings concur with programmatic data reported in the WHO Global TB report, with the most recent global data reporting only 62% of pulmonary TB was bacteriologically confirmed in 2023.1 The implementation of more sensitive molecular diagnostics should, theoretically, lead to a decrease in the need for clinical diagnosis and empirical treatment compared to only having sputum smear microscopy available. However, based on both our results, and programmatic data collated by WHO, this is yet to be seen. While the impact might present only slowly over time, we did not observe a trend in our data. In contrast, we found clinical trials comparing more sensitive TB diagnostic tests (predominantly Xpert MTB/RIF and urine lipoarabinomannan antigen tests) to standard demonstrated a median absolute reduction in clinical TB diagnosis of 19%. This discrepancy between clinical trials, and non-interventional study and programmatic data might partially be explained by a study (Hawthorne) effect,77 where healthcare workers in trials are aware that decisions about TB diagnosis are being observed.
Whilst the introduction of more sensitive tests should decrease the proportion of people treated for TB based on clinical diagnosis, public health interventions to reduce TB incidence may have the opposite effect. Data from Blantyre, Malawi, found clinically diagnosed TB did not fall to the same degree as bacteriologically confirmed TB in the context of reducing TB incidence between 2011 and 2019, suggesting significant overtreatment.78 The decision to start TB treatment in the absence of a positive bacteriological test is complex, and will be affected by poorly quantifiable factors including national and local guidelines and epidemiology, access to other diagnostics (e.g. radiology, microbiology), and individual health worker factors (e.g. training and clinical experience). Clinicians may have a high index of suspicion for diagnosing TB and thus a low threshold for starting TB treatment. Improving healthcare workers’ knowledge on pretest probability of TB, including the impact of more sensitive diagnostics and changing incidence, may reduce overtreatment through clinical diagnosis.79 However, if people are diagnosed earlier in the disease course with more paucibacillary disease, this may also reduce the sensitivity of diagnostic tests for TB.
We found higher proportions of clinical diagnosis amongst people living with HIV and extrapulmonary TB. Sputum based diagnostics are known to be less sensitive in people living with HIV, although the difference in sensitivity is less for newer molecular assays such as Xpert MTB/RIF Ultra (Cepheid, USA) compared with sputum smear microscopy.80 This, coupled with high mortality associated with missed TB in people living with HIV, is likely driving more clinical diagnoses and empirical treatment.4,13 In studies not able to test all those with presumptive TB using bacteriological tests, the proportion of clinical diagnoses was also higher than in studies testing everyone. This may be due to challenges in obtaining sputum samples for TB testing.81 However, even when everyone underwent bacteriological testing, one-third of diagnoses were clinical. Together, these findings strongly support the need for TB diagnostics that (1) do not solely rely on sputum, (2) are easily implementable at peripheral levels of healthcare, and (3) can accurately diagnose extrapulmonary and HIV-associated TB.82
Our results found people diagnosed clinically with TB had worse outcomes, including a 50% higher relative risk of mortality. One possible explanation is other undiagnosed conditions that are not treated, leading to morbidity and mortality.83, 84, 85, 86 This was even more marked in people living with HIV, where other opportunistic infection can mimic TB clinically. As the incidence of TB decreases, there is a need for improved access to non-TB diagnostics for people presenting to healthcare facilities in high TB burden settings with presumptive TB, especially at peripheral healthcare levels. This could help reduce morbidity and mortality associated with clinic diagnosis of TB. Alternative mechanisms for the association with mortality is that more critically unwell individuals are more likely to have difficulty in producting sputum for testing, and therefore are more likely to be treated for TB based on clinical diagnosis. It is not clear why mortality risk was higher in more recent studies, it maybe that as diagnostics have improved a lower proportion of those with clinically diagnosed TB actually have TB.
Strengths of this systematic review include the large number of studies identified for the main analysis, representing a wide range of settings, and allowing subgroup analyses and meta regression. A further strength is our analysis of mortality outcomes. Our main limitation is the high degree of heterogeneity, likely resulting from differing practice, availability of TB diagnostics (e.g. smear versus molecular diagnsotics, the use of urinary LAM antigen tests) procedures and TB prevalence across settings and studies. The reasons for clinical diagnosis, tests performed as part of diagnostic algorithm and criteria for initiation of TB treatment could not be investigated in more detail. We did not undertake subgroup analysis by region as the majority of studies were from the African region. The nature of many studies indicated high risk of bias, although our findings did not change significantly when limiting to low risk of bias studies (Appendix Figure S10). We were unable to report any outcomes other than mortality due to data availability, and follow-up time varied between studies.
In conclusion, we found clinical diagnosis of TB to be commonplace despite implementation of more sensitive molecular diagnostics. Clinical diagnosis was also associated with higher mortality. There is a need to better understand the reasons for clinical TB treatment in high burden settings, and investment in improved diagnostics for TB and non-TB conditions.
Contributors
AGW and CD developed the idea and initiated the article. BF and AGW drafted study design and protocol with input from AS, MGaeddart and CD. The search was designed and conducted by BF, MGrilli and AGW. Screening, data extraction and risk of bias assessment was done by BF, AS and AGW. BF and AGW undertook the data analysis. BF, AGW, MGaeddart, OA, SW and CD interpreted results, BF and AGW wrote the first draft of the manuscript. All authors contributed to the manuscript by edits and providing critical feedback. All authors had full access to the data and had final responsibility for submission.
Data sharing statement
This manuscript is based on secondary data which is published and publicaly available. Data extracted for the analysis is presented within the results section and Supplementary Appendix.
All data reported in this study derives from published studies cited in the reference section. Our data set will not be made available public. The code used for the analyses is available upon request.
Declaration of interests
There are no conflicts of interest to declare.
Acknowledgements
CD and AGW are funded by the R2D2 TB Network (National Institute of Allergy and Infectious Diseases of the US National Institutes of Health under award number U01AI152087). AGW is supported by the UK National Institute for Health and Care Research (NIHR305136) and Academy of Medical Sciences (SGCL0251043). SW is recipient of Clinical Leave Stipend for unrelated project by Deutsches Zentrum für Infektionsforschung (DZIF). No funders had any role in the study design, conduct or analysis.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2025.103251.
Appendix A. Supplementary data
References
- 1.WHO . World Health Organisation; Geneva: 2024. Global tuberculosis report 2024. [Google Scholar]
- 2.Subbaraman R., Jhaveri T., Nathavitharana R.R. Closing gaps in the tuberculosis care cascade: an action-oriented research agenda. J Clin Tuberc Other Mycobact Dis. 2020;19 doi: 10.1016/j.jctube.2020.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steingart K.R., Schiller I., Horne D.J., Pai M., Boehme C.C., Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;2014(1):Cd009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katagira W., Walter N.D., Den Boon S., et al. Empiric TB treatment of severely ill patients with HIV and presumed pulmonary TB improves survival. J Acquir Immune Defic Syndr. 2016;72(3):297–303. doi: 10.1097/QAI.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horne D.J., Kohli M., Zifodya J.S., et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2019;6(6):Cd009593. doi: 10.1002/14651858.CD009593.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theron G., Peter J., Dowdy D., Langley I., Squire S.B., Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014;14(6):527–532. doi: 10.1016/S1473-3099(13)70360-8. [DOI] [PubMed] [Google Scholar]
- 7.Basile F.W., Sweeney S., Singh M.P., et al. Uncertainty in tuberculosis clinical decision-making: an umbrella review with systematic methods and thematic analysis. PLOS Glob Public Health. 2024;4(7) doi: 10.1371/journal.pgph.0003429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Divala T.H., Fielding K.L., Kandulu C., et al. Utility of broad-spectrum antibiotics for diagnosing pulmonary tuberculosis in adults: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20(9):1089–1098. doi: 10.1016/S1473-3099(20)30143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDowell A., Pai M. Treatment as diagnosis and diagnosis as treatment: empirical management of presumptive tuberculosis in India. Int J Tuberc Lung Dis. 2016;20(4):536–543. doi: 10.5588/ijtld.15.0562. [DOI] [PubMed] [Google Scholar]
- 10.Haraka F., Kakolwa M., Schumacher S.G., et al. Impact of the diagnostic test Xpert MTB/RIF on patient outcomes for tuberculosis. Cochrane Database Syst Rev. 2021;5(5):Cd012972. doi: 10.1002/14651858.CD012972.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kebede W., Abebe G., Gudina E.K., De Vos E., Riviere E., Van Rie A. Role of empiric treatment in hospitalized patients with Xpert MTB/RIF-negative presumptive pulmonary tuberculosis: a prospective cohort study. Int J Infect Dis. 2020;97:30–37. doi: 10.1016/j.ijid.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Abdullahi O., Moses N., Sanga D., Annie W. The effect of empirical and laboratory-confirmed tuberculosis on treatment outcomes. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-94153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bresges C., Wilson D., Fielding K., et al. Early empirical tuberculosis treatment in HIV-positive patients admitted to hospital in South Africa: an observational cohort study. Open Forum Infect Dis. 2021;8(7) doi: 10.1093/ofid/ofab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . World Health Organisation; Geneva: 2013. Definitions and reporting framework for tuberculosis – 2013 revision. [Google Scholar]
- 15.WHO . World Health Organisation; Geneva: 2021. WHO global lists of high burden countries for TB, multidrug/rifampicin-resistant TB (MDR/RR-TB) and TB/HIV, 2021–2025. [Google Scholar]
- 16.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 18.Campbell J.M., Klugar M., Ding S., et al. Diagnostic test accuracy: methods for systematic review and meta-analysis. Int J Evid Based Healthc. 2015;13(3):154–162. doi: 10.1097/XEB.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P.T.T.J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. 2023. Cochrane Handbook for Systematic Reviews of Interventions Cochrane. [Google Scholar]
- 20.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke R.M., Nyirenda S.K., Mtenga T., et al. Enhanced tuberculosis diagnosis with computer-aided chest X-ray and urine lipoarabinomannan in adults with human immunodeficiency virus admitted to hospital (CASTLE Study): a cluster randomized trial. Clin Infect Dis. 2024 doi: 10.1093/cid/ciae273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kibirige D. Diagnostic accuracy of two confirmatory tests for diabetes mellitus in adult Ugandans with recently diagnosed tuberculosis. Ther Adv Infect Dis. 2023;10 doi: 10.1177/20499361231216799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sachdeva K.S., Raizada N., Sreenivas A., et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adewole O.O., Oladele T., Osunkoya A.H., et al. A randomized controlled study comparing community based with health facility based direct observation of treatment models on patients' satisfaction and TB treatment outcome in Nigeria. Trans R Soc Trop Med Hyg. 2015;109(12):783–792. doi: 10.1093/trstmh/trv091. [DOI] [PubMed] [Google Scholar]
- 25.Balcha T.T., Skogmar S., Sturegård E., et al. A clinical scoring algorithm for determination of the risk of tuberculosis in HIV-infected adults: a cohort study performed at Ethiopian health centers. Open Forum Infect Dis. 2014;1(3) doi: 10.1093/ofid/ofu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durovni B., Saraceni V., van den Hof S., et al. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in Brazil: a stepped-wedge cluster-randomized trial. PLoS Med. 2014;11(12) doi: 10.1371/journal.pmed.1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy K., Fielding K., Churchyard G.J., Grant A.D. Empiric tuberculosis treatment in South African primary health care facilities - for whom, where, when and why: implications for the development of tuberculosis diagnostic tests. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0191608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Churchyard G.J., Stevens W.S., Mametja L.D., et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3(8):e450–e457. doi: 10.1016/S2214-109X(15)00100-X. [DOI] [PubMed] [Google Scholar]
- 29.Bekele A., Gebreselassie N., Ashenafi S., et al. Daily adjunctive therapy with vitamin D(3) and phenylbutyrate supports clinical recovery from pulmonary tuberculosis: a randomized controlled trial in Ethiopia. J Intern Med. 2018;284(3):292–306. doi: 10.1111/joim.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eneogu R., Olabamiji J., Ihesie A., et al. Impact of Truenat on TB diagnosis in Nigeria. Public Health Action. 2024;14(3):124–128. doi: 10.5588/pha.24.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox H.S., Mbhele S., Mohess N., et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLoS Med. 2014;11(11) doi: 10.1371/journal.pmed.1001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majella M.G., Thekkur P., Kumar A.M., Chinnakali P., Saka V.K., Roy G. Effect of mobile voice calls on treatment initiation among patients diagnosed with tuberculosis in a tertiary care hospital of Puducherry: a randomized controlled trial. J Postgrad Med. 2021;67(4):205–212. doi: 10.4103/jpgm.JPGM_1105_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Castro N., Marcy O., Chazallon C., et al. Standard dose raltegravir or efavirenz-based antiretroviral treatment for patients co-infected with HIV and tuberculosis (ANRS 12 300 Reflate TB 2): an open-label, non-inferiority, randomised, phase 3 trial. Lancet Infect Dis. 2021;21(6):813–822. doi: 10.1016/S1473-3099(20)30869-0. [DOI] [PubMed] [Google Scholar]
- 34.Rima D.D., Legese D., Woldesemayat E.M. Tuberculosis treatment delay and associated factors among pulmonary tuberculosis patients at public health facilities in Dale District and Yirgalem Town administration, Sidama Region, South Ethiopia. BMC Infect Dis. 2024;24(1):517. doi: 10.1186/s12879-024-09397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dave P., Shah A., Chauhan M., et al. Screening patients with tuberculosis for diabetes mellitus in Gujarat, India. Public Health Action. 2013;3:S29–S33. doi: 10.5588/pha.13.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta-Wright A., Corbett E.L., van Oosterhout J.J., et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet. 2018;392(10144):292–301. doi: 10.1016/S0140-6736(18)31267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanifa Y., Fielding K.L., Chihota V.N., Adonis L., Charalambous S., Karstaedt A. Diagnostic accuracy of lateral flow urine LAM assay for TB screening of adults with advanced immunosuppression attending routine HIV care in South Africa. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Getahun B., Wubie M., Dejenu G., Manyazewal T. Tuberculosis care strategies and their economic consequences for patients: the missing link to end tuberculosis. Infect Dis Poverty. 2016;5(1):93. doi: 10.1186/s40249-016-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atekem K.A., Tanih N.F., Ndip R.N., Ndip L.M. Evaluation of the tuberculosis control program in South West Cameroon: factors affecting treatment outcomes. Int J Mycobacteriol. 2018;7(2):137–142. doi: 10.4103/ijmy.ijmy_20_18. [DOI] [PubMed] [Google Scholar]
- 40.Ereso B.M., Sagbakken M., Gradmann C., Yimer S.A. Determinants of an unfavorable treatment outcome among tuberculosis patients in the Jimma Zone, Southwest Ethiopia. Sci Rep. 2024;14(1) doi: 10.1038/s41598-024-78084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber S.F., Saravu K., Heller T., et al. Point-of-Care ultrasound for extrapulmonary tuberculosis in India: a prospective cohort study in hiv-positive and hiv-negative presumptive tuberculosis patients. Am J Trop Med Hyg. 2018;98(1):266–273. doi: 10.4269/ajtmh.17-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cattamanchi A., Reza T.F., Nalugwa T., et al. Multicomponent strategy with decentralized molecular testing for tuberculosis. N Engl J Med. 2021;385(26):2441–2450. doi: 10.1056/NEJMoa2105470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prudhivi R., Challa S.R., Basaveswara Rao M.V., Veena G., Bhaskara Rao N., Narne H.M. Assessment of success rate of directly observed treatment short-course (DOTS) in tuberculosis patients of South India. J Young Pharm. 2019;11(1):67–72. [Google Scholar]
- 44.Åhsberg J., Puplampu P., Kwashie A., et al. Point-of-Care urine lipoarabinomannan testing to guide tuberculosis treatment among Severely Ill inpatients with human immunodeficiency virus in real-world practice: a multicenter stepped wedge cluster-randomized trial from Ghana. Clin Infect Dis. 2023;77(8):1185–1193. doi: 10.1093/cid/ciad316. [DOI] [PubMed] [Google Scholar]
- 45.Padda P., Gupta V., Devgan S., Chaudhary S., Singh G. Treatment outcome of TB patients in a district of north India: a three year study. Nepal J Epidemiol. 2015;5(1):457–461. [Google Scholar]
- 46.Manosuthi W., Mankatitham W., Lueangniyomkul A., et al. Time to initiate antiretroviral therapy between 4 weeks and 12 weeks of tuberculosis treatment in HIV-infected patients: results from the TIME study. J Acquir Immune Defic Syndr. 2012;60(4):377–383. doi: 10.1097/QAI.0b013e31825b5e06. [DOI] [PubMed] [Google Scholar]
- 47.Bock P., Jennings K., Vermaak R., et al. Incidence of tuberculosis among HIV-positive individuals initiating antiretroviral treatment at higher CD4 counts in the HPTN 071 (PopART) trial in South Africa. J Acquir Immune Defic Syndr. 2018;77(1):93–101. doi: 10.1097/QAI.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theron G., Zijenah L., Chanda D., et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383(9915):424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 49.Peter J., Theron G., Chanda D., et al. Test characteristics and potential impact of the urine LAM lateral flow assay in HIV-infected outpatients under investigation for TB and able to self-expectorate sputum for diagnostic testing. BMC Infect Dis. 2015;15(1):262. doi: 10.1186/s12879-015-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bjerrum S., Kenu E., Lartey M., Newman M.J., Addo K.K., Andersen A.B. Diagnostic accuracy of the rapid urine lipoarabinomannan test for pulmonary tuberculosis among HIV-infected adults in Ghana- findings from the DETECT HIV-TB study. BMC Infect Dis. 2015;15:407. doi: 10.1186/s12879-015-1151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shivalingaiah A.H., Ayyappan P., Kiruffi D.V., Muralidhar M. Impact of COVID-19 pandemic on tuberculosis treatment outcomes at a tuberculosis unit in southern India: a retrospective observational study. J Clin Diagn Res. 2024;18(2):LC12–LC14. [Google Scholar]
- 52.Hanrahan Colleen F., Selibas K., Deery Christopher B., Dansey H., Clouse K., Bassett J. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zerihun M., Mekonnen H., Gebretensaye T.G. Treatment outcome and associated factors among adult patients with pulmonary tuberculosis in selected health centers in Addis Ababa Ethiopia. PLoS One. 2023;18(10) doi: 10.1371/journal.pone.0292218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shrivastava S.R., Shrivastava P.S. HIV-tuberculosis interface: a comparison of collateral prevalence of HIV and tuberculosis in an urban health centre. Ann Trop Med Publ Health. 2013;6(3):290–296. [Google Scholar]
- 55.Bezerra W.S.P., Lemos E.F., Do Prado T.N., et al. Risk stratification and factors associated with abandonment of tuberculosis treatment in a secondary referral unit. Patient Prefer Adherence. 2020;14:2389–2397. doi: 10.2147/PPA.S266475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Songkhla M.N., Tantipong H., Tongsai S., Angkasekwinai N. Lateral flow urine lipoarabinomannan assay for diagnosis of active tuberculosis in adults with human immunodeficiency virus infection: a prospective cohort study. Open Forum Infect Dis. 2019;6(4):ofz132. doi: 10.1093/ofid/ofz132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang Y., Chen J., Ying M., et al. Factors associated with loss to follow-up before and after treatment initiation among patients with tuberculosis: a 5-year observation in China. Front Med. 2023;10 doi: 10.3389/fmed.2023.1136094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hemalatha V.S., Thaliath Z.X., Somson H.T. Comparison of pulmonary tuberculosis and extra pulmonary tuberculosis in a tertiary care setting. Int J Acad Med Pharmacy. 2023;5(1):225–228. [Google Scholar]
- 59.Kaku J.S., Ahmad R.A., Main S., et al. Tuberculosis case finding in Kulon Progo district, Yogyakarta, Indonesia: passive versus active case finding using mobile chest X-ray. Trop Med Infect Dis. 2024;9(4):75. doi: 10.3390/tropicalmed9040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin W., Pan J., Miao Q., et al. Diagnostic accuracy of metagenomic next-generation sequencing for active tuberculosis in clinical practice at a tertiary general hospital. Ann Transl Med. 2020;8(17):1065. doi: 10.21037/atm-20-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ncube R.T., Dube S.A., Machekera S.M., et al. Feasibility and yield of screening for diabetes mellitus among tuberculosis patients in Harare, Zimbabwe. Public Health Action. 2019;9(2):72–77. doi: 10.5588/pha.18.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peter J.G., Zijenah L.S., Chanda D., et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet. 2016;387(10024):1187–1197. doi: 10.1016/S0140-6736(15)01092-2. [DOI] [PubMed] [Google Scholar]
- 63.Zijenah L.S., Kadzirange G., ason T., et al. Comparative performance characteristics of the urine lipoarabinomannan strip test and sputum smear microscopy in hospitalized HIV-infected patients with suspected tuberculosis in Harare, Zimbabwe. BMC Infect Dis. 2016;16:20. doi: 10.1186/s12879-016-1339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gebreegziabher S.B., Bjune G.A., Yimer S.A. Patients' and health system's delays in the diagnosis and treatment of new pulmonary tuberculosis patients in west Gojjam zone, Northwest Ethiopia: a cross-sectional study. BMC Infect Dis. 2016;16(1):673. doi: 10.1186/s12879-016-1995-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mupfumi L., Makamure B., Chirehwa M., et al. Impact of Xpert MTB/RIF on antiretroviral therapy-associated tuberculosis and mortality: a pragmatic randomized controlled trial. Open Forum Infect Dis. 2014;1(1) doi: 10.1093/ofid/ofu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohammed H., Oljira L., Roba K.T., et al. Burden of tuberculosis and challenges related to screening and diagnosis in Ethiopia. J Clin Tuberc Other Mycobact Dis. 2020;19:100158. doi: 10.1016/j.jctube.2020.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu Y., Wang Y.S., Lin N., et al. Multicenter clinical evaluation of three commercial reagent kits based on the interferon-gamma release assay for the rapid diagnosis of tuberculosis in China. Int J Infect Dis. 2015;40:108–112. doi: 10.1016/j.ijid.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Seid A., Metaferia Y. Factors associated with treatment delay among newly diagnosed tuberculosis patients in Dessie city and surroundings, Northern Central Ethiopia: a cross-sectional study. BMC Public Health. 2018;18(1):931. doi: 10.1186/s12889-018-5823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kebede W., Gudina E.K., Balay G., Abebe G. Diagnostic implications and inpatient mortality related to tuberculosis at Jimma medical center, southwest Ethiopia. J Clin Tuberc Other Mycobact Dis. 2021;23:100220. doi: 10.1016/j.jctube.2021.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Getiye A., Zakaria H.F., Deressa A., et al. Magnitude and factors associated with delay in treatment-seeking among new pulmonary tuberculosis patients in public health facilities in Habro district, eastern Ethiopia. Health Serv Insights. 2024;17 doi: 10.1177/11786329241232532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Auld S.C., Moore B.K., Kyle R.P., et al. Mixed impact of xpert® MTB/RIF on tuberculosis diagnosis in Cambodia. Public Health Action. 2016;6(2):129–135. doi: 10.5588/pha.16.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Connor D.E., Frederix K., Saito S., et al. Pulmonary tuberculosis diagnostic practices among people living with the human immunodeficiency virus in Lesotho. Int J Tuberc Lung Dis. 2017;21(10):1133–1138. doi: 10.5588/ijtld.17.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mishra P., Bhargava S., Vihan S. Handgrip strength deficit and time lag between symptom onset and starting of chemotherapy in pulmonary tuberculosis: a cross-sectional study in North India. Indian J Physiol Pharmacol. 2023;67(3):205–211. [Google Scholar]
- 74.Humphrey J.M., Mpofu P., Pettit A.C., et al. Mortality among people with HIV treated for tuberculosis based on positive, negative, or no bacteriologic test results for tuberculosis: the IEDEA consortium. Open Forum Infect Dis. 2020;7(1) doi: 10.1093/ofid/ofaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beckwith P.G., Tlali M., Charalambous S., et al. Causes and outcomes of admission and investigation of tuberculosis in adults with advanced HIV in South African hospitals: data from the TB fast track trial. Am J Trop Med Hyg. 2021;105(6):1662–1671. doi: 10.4269/ajtmh.21-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grant A.D., Charalambous S., Tlali M., et al. Algorithm-guided empirical tuberculosis treatment for people with advanced HIV (TB Fast Track): an open-label, cluster-randomised trial. Lancet HIV. 2020;7(1):e27–e37. doi: 10.1016/S2352-3018(19)30266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lawn S.D., Nicol M.P., Corbett E.L. Effect of empirical treatment on outcomes of clinical trials of diagnostic assays for tuberculosis. Lancet Infect Dis. 2015;15(1):17–18. doi: 10.1016/S1473-3099(14)71049-7. [DOI] [PubMed] [Google Scholar]
- 78.Burke R.M., Nliwasa M., Dodd P.J., et al. Impact of community-wide tuberculosis active case finding and human immunodeficiency virus testing on tuberculosis trends in Malawi. Clin Infect Dis. 2023;77(1):94–100. doi: 10.1093/cid/ciad238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boyles T.H., Kengne A.P. A priori estimation of diagnostic thresholds could improve the reporting of diagnostic studies of tuberculosis. Int J Tuberc Lung Dis. 2016;20(2):147–149. doi: 10.5588/ijtld.15.0832. [DOI] [PubMed] [Google Scholar]
- 80.Zifodya J.S., Kreniske J.S., Schiller I., et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev. 2021;2:Cd009593. doi: 10.1002/14651858.CD009593.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peter J.G., Theron G., Singh N., Singh A., Dheda K. Sputum induction to aid diagnosis of smear-negative or sputum-scarce tuberculosis in adults in HIV-endemic settings. Eur Respir J. 2014;43(1):185–194. doi: 10.1183/09031936.00198012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.WHO . World Health Organisation; Geneva: 2024. Target product profiles for tuberculosis diagnosis and detection of drug resistance. [Google Scholar]
- 83.Jayasooriya S., Dimambro-Denson F., Beecroft C., et al. Patients with presumed tuberculosis in sub-Saharan Africa that are not diagnosed with tuberculosis: a systematic review and meta-analysis. Thorax. 2023;78(1):50–60. doi: 10.1136/thoraxjnl-2021-217663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chaaba E., Bwembya J., Nyambe E., et al. Mortality among persons receiving tuberculosis treatment in Itezhi-Tezhi District of Zambia: a retrospective cohort study. PLOS Glob Public Health. 2023;3(2) doi: 10.1371/journal.pgph.0001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Afriyie-Mensah J.S., Aryee R., Zigah F., Amaning-Kwarteng E., Séraphin M.N. The burden of bacteriologically negative TB diagnosis: a four-year review of tuberculosis cases at a tertiary facility. Tuberc Res Treat. 2023;2023 doi: 10.1155/2023/6648137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adamu A.L., Gadanya M.A., Abubakar I.S., et al. High mortality among tuberculosis patients on treatment in Nigeria: a retrospective cohort study. BMC Infect Dis. 2017;17(1):170. doi: 10.1186/s12879-017-2249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.