Abstract
To evaluate the role of the polysaccharide intercellular adhesin as an energy-storage molecule, we investigated the effect of nutrient limitation on S. epidermidis biofilms. The stability of established biofilms depends on σB activity; however, the slow decay of biofilms under conditions of nutrient limitation reveal its use as an energy-storage molecule to be unlikely.
Biofilm formation is the major pathogenic mechanism involved in infections caused by Staphylococcus epidermidis (5) and is associated with an increased antibiotic resistance (14). Cell-to-cell adhesion within S. epidermidis biofilms is mediated by the polysaccharide intercellular adhesin (PIA) (18, 19). PIA is synthesized by the gene products of the icaADBC gene locus (4, 6). PIA synthesis and methicillin resistance are regulated by the gene rsbU via the alternative sigma factor σB (8, 11, 12, 21). The σB activity in staphylococci is regulated by at least three regulatory genes located upstream of sigB (15, 23, 24, 26). The genes rsbU and rsbV encode negative regulators, whereas rsbW encodes a positive regulator of σB activity. Interestingly, in σB-negative mutants PIA synthesis could be restored under ethanol stress (11), as observed in the majority of icaADBC-positive clinical isolates (9). In addition to these regulatory pathways of biofilm formation, additional regulatory gene loci are involved in the regulation of PIA synthesis (2, 13, 17, 20).
Implanted medical devices have only been used in the last few decades, indicating that the role of PIA as a virulence factor plays only a secondary role, whereas the primary physiologic function of PIA synthesis is still poorly understood. The observation of sugar-dependent biofilm formation in staphylococci (3, 10) has led to the hypothesis that PIA could act as an energy storage molecule (7). To further evaluate this possible function of PIA, we investigated the stability of S. epidermidis biofilms under conditions of glucose limitation in the present study. The possibility to restore biofilm formation in mutants with dysfunctional σB by ethanol induction (8, 11) allowed us to characterize the influence of the alternative sigma factor σB on the stability of the established biofilms under different environmental conditions. Mutants of clinical isolates S. epidermidis 1457 and 8400 with inactivation of the regulatory genes rsbU, rsbW, the entire regulatory cascade rsbUVW, the sigB gene, and the entire sigma B operon were investigated (Table 1).
TABLE 1.
Strains used in this study
| S. epidermidis strain | Reference | Comments |
|---|---|---|
| 1457 | 22 | Isolate from infected central venous catheter |
| 1457rsbU | 11 | rsbU::erm derivate from S. epidermidis 1457, generated by allelic gene replacement |
| 1457rsbW | 11 | rsbW::erm derivate from S. epidermidis 1457, generated by allelic gene replacement |
| 1457sigB | 11 | sigB::erm derivate from S. epidermidis 1457, generated by allelic gene replacement |
| 1457rsbUVW | 11 | rsbUVW::erm derivate from S. epidermidis 1457, generated by allelic gene replacement |
| 1457rsbUVWsigB | 11 | rsbUVWsigB::erm derivate from S. epidermidis 1457, generated by allelic gene replacement |
| 8400 | 22 | Isolate from infected central venous catheter |
| 8400rsbU | 11 | rsbU::erm derivate from S. epidermidis 8400, derived by phage transduction |
| 8400rsbW | 11 | rsbW::erm derivate from S. epidermidis 8400, derived by phage transduction |
| 8400sigB | 11 | sigB::erm derivate from S. epidermidis 8400, derived by phage transduction |
| 8400 rsbUVW | 11 | rsbUVW::erm derivate from S. epidermidis 8400, derived by phage transduction |
| 8400rsbUVWsigB | 11 | rsbUVWsigB::erm derivate from S. epidermidis 8400, derived by phage transduction |
Biofilms were grown in 96-well tissue culture plates (Nunc, Roskilde, Denmark), and biofilm formation was measured quantitatively after staining with gentian violet as described previously (1, 16). To induce biofilm formation for the primary cultures Trypticase soy broth (TSB; Becton Dickinson, Cockeysville, Md.) was supplemented with 3% ethanol for all strains. For investigations of biofilm stability, the culture supernatant was replaced daily with TSB, TSB without glucose (TSBwo; 1.7% tryptone, 0.3% soy peptone [Oxoid, Basingstoke, England], 0.5% NaCl, and 0.25% dipotassium phosphate [Merck, Darmstadt, Germany]), or phosphate-buffered saline (PBS). The amount of remaining biofilms was quantified daily.
Established biofilms were stable over at least 6 days in all mutants when TSB was used to replace the culture supernatants, indicating that σB activity is not required to maintain established S. epidermidis biofilms under energy-rich conditions in these strains (Fig. 1). In TSBwo and PBS used for replacement of the culture supernatants, biofilms of strains with deletions of rsbU, as well as of sigB and the entire σB operon, in S. epidermidis 8400 disintegrated within 2 to 3 days, whereas in S. epidermidis 1457 the respective mutants displayed even in PBS, after an initially rapid decrease, still substantial biofilm. In contrast, biofilms of strains with mutations of the negative regulator RsbW or the entire regulatory cascade rsbUVW displayed a high stability over at least 6 days in TSBwo. In wild-type S. epidermidis 8400 a slight decay of the primarily established biofilm was observed. However, even after this time period the strains would be classified as strongly biofilm positive (9). The biofilm of S. epidermidis 1457 remained stable under these conditions.
FIG. 1.
Stability of biofilms under nutrient limitation. The wild-type strains S. epidermidis 1457 (A) and 8400 (B), as well as their respective mutants with inactivation of rsbU, rsbW, sigB, the regulatory cascade rsbUVW, and the entire σB operon, were investigated. After primary biofilm formation in TSB supplemented with 3% ethanol culture, the supernatants were replaced daily with fresh TSB, TSB lacking glucose (TSBwo), or phosphate-buffered saline for 6 days. All experiments were performed three times in quadruplicate. The displayed bars represent mean values of all results obtained in three experiments. Error bars represent the standard error.
In PBS the rsbW and rsbUVW mutants, as well as both wild-type strains, displayed a slow decay of primary biofilms over 6 days; however, no complete disintegration of biofilms was observed, and the remaining levels of biofilms were higher compared to the mutants with dysfunctional σB activity. Similar phenotypic results were obtained for wild-type strains and mutants with inactivation of rsbW or rsbUVW if TSB without ethanol was used for primary biofilm formation (data not shown), indicating that ethanol induction has no influence on biofilm stability under the investigated conditions.
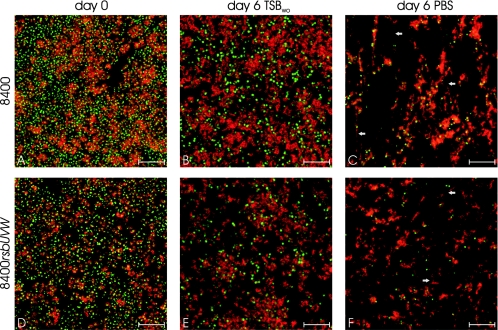
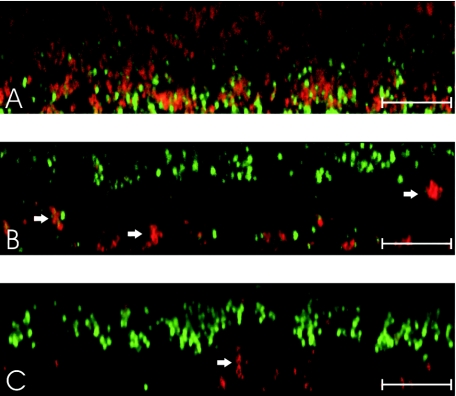
The relation of biofilm stability with PIA stability was investigated for representative mutants of strain 8400 by confocal microscopy using SYTO9 to stain cells and TRITC (tetramethyl rhodamine isothiocyanate)-labeled wheat germ agglutinin (Molecular Probes, Eugene, OR), a lectin which binds to PIA (25), to stain the extracellular polysaccharide matrix. Even after 6 days of energy limitation, similar amounts of PIA could be detected in strains still displaying a strong biofilm-positive phenotype (Fig. 2) indicating that the stability of PIA is responsible for the observed stability of biofilms in mutants with intact σB activity. Interestingly, only a few intact cells could be detected after 6 days, whereas large masses of PIA with significantly altered structure were still detectable (Fig. 2). For mutants with dysfunctional σB activity, thin biofilms could be detected at day 4 in TSBwo which also displayed a significantly altered structure (Fig. 3). A thin layer with almost no detectable PIA was located at the top with ca. 5 to 10 μm distance to the basal layer of attached cells at the bottom of the chambers only fixed by a few PIA-containing clusters (Fig. 3).
FIG. 2.
Horizontal sections of biofilms in representative strains displaying a stable biofilm-positive phenotype under conditions of energy limitation. Strains 8400 (A, B, and C) and 8400rsbUVW (D, E, and F) were grown in coverglass cell culture chambers (Nunc, Roskilde, Denmark) in TSB containing 3% ethanol (A and D), and culture supernatants were replaced daily with TSBwo (B and E) or PBS (C and F) for 6 days. Chambers were washed four times with PBS, and remaining biofilms were stained with SYTO9 and TRITC labeled wheat germ agglutinin to stain cells (green) and PIA (red), respectively. Compared to day 0 (A and D), both strains displayed a similar structure with slight reduction of cells in TSBwo and a similar amount of PIA at day 6 (B and E). In PBS both strains displayed a significantly altered structure of the biofilm. Almost no intact cells could be detected, and PIA-containing fibers (arrows) were observed connecting the remaining PIA clusters. For strains with dysfunctional σB activity, no remaining biofilms could be detected at day 6 (data not shown). Displayed scales indicate 10 μm.
FIG. 3.
Vertical sections of biofilms. Strains 8400 (A), 8400rsbU (B), and 8400rsbUVWsigB (C) were grown in coverglass cell culture chambers in TSB containing 3% ethanol, and culture supernatants were replaced daily with TSBwo for 4 days. Chambers were washed four times with PBS, and remaining biofilms were stained with SYTO9- and TRITC-labeled wheat germ agglutinin to stain cells (green) and PIA (red), respectively. Strain 8400 displayed a compact cell mass with single cells enclosed by PIA (A), as it was observed for all strains at day 0 and for strains with a strong biofilm-positive phenotype in TSBwo after 6 days (data not shown). Due to the high density of the biofilm cells of the upper layers are insufficiently illuminated. In contrast, strains 8400rsbU and 8400rsbUVWsigB displayed a thin cell layer with almost no detectable PIA (B and C) that was located at the top with about 5 to 10 μm distance to the basal layer of attached cells at the bottom of the chambers only fixed by a few PIA-containing clusters (arrows). In contrast to the biofilm observed for strain 8400, the cells embedded in the thin layer displayed a more elastic cell-to-cell contact (see movies in the supplemental material) as observed for a protein dependent biofilm (J. K.-M. Knobloch and H. Rohde, unpublished data). Displayed scales indicate 20 μm.
The data presented here suggest that the stability of S. epidermidis biofilms under nutrient limitation depends on σB activity. The slightly different observations in two investigated S. epidermidis isolates indicate that additional factors could influence biofilm stability under conditions of energy limitation. However, the slow reduction of wild-type strain biofilms and the stability of biofilms of the rsbW and rsbUVW mutants in TSB lacking glucose indicates that it is unlikely that PIA is used as an energy storage molecule. The regulation of biofilm formation by σB, which is a major inductor of genes required to cope with environmental stress, suggests that the primary role of PIA synthesis could be interpreted as a kind of sporulation equivalent that is protecting biofilm-embedded cells from environmental stresses.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Kn560/7-1 to J.K.-M.K. and D.M). S.J. is the recipient of a fellowship of the Werner Otto-Stiftung Hamburg.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conlon, K. M., H. Humphreys, and J. P. O'Gara. 2004. Inactivations of rsbU and sarA by IS256 represent novel mechanisms of biofilm phenotypic variation in Staphylococcus epidermidis. J. Bacteriol. 186:6208-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobinsky, S., K. Kiel, H. Rohde, K. Bartscht, J. K. M. Knobloch, M. A. Horstkotte, and D. Mack. 2003. Glucose-related dissociation between icaADBC transcription and biofilm expression by Staphylococcus epidermidis: evidence for an additional factor required for polysaccharide intercellular adhesin synthesis. J. Bacteriol. 185:2879-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerke, C., A. Kraft, R. Süssmuth, O. Schweitzer, and F. Götz. 1998. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J. Biol. Chem. 273:18586-18593. [DOI] [PubMed] [Google Scholar]
- 5.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 6.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 7.Jefferson, K. K., D. B. Pier, D. A. Goldmann, and G. B. Pier. 2004. The teicoplanin-associated locus regulator (TcaR) and the intercellular adhesin locus regulator (IcaR) are transcriptional inhibitors of the ica locus in Staphylococcus aureus. J. Bacteriol. 186:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knobloch, J. K. M., K. Bartscht, A. Sabottke, H. Rohde, H. H. Feucht, and D. Mack. 2001. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183:2624-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knobloch, J. K. M., M. A. Horstkotte, H. Rohde, P. M. Kaulfers, and D. Mack. 2002. Alcoholic ingredients in skin disinfectants increase biofilm expression of Staphylococcus epidermidis. J. Antimicrob. Chemother. 49:683-687. [DOI] [PubMed] [Google Scholar]
- 10.Knobloch, J. K. M., M. A. Horstkotte, H. Rohde, and D. Mack. 2002. Evaluation of different detection methods of biofilm formation in Staphylococcus aureus. Med. Microbiol. Immunol. 191:101-106. [DOI] [PubMed] [Google Scholar]
- 11.Knobloch, J. K. M., S. Jäger, M. A. Horstkotte, H. Rohde, S. Dobinsky, and D. Mack. 2004. RsbU-dependent regulation of Staphylococcus epidermidis biofilm formation is mediated via the alternative sigma factor σB by repression of the negative regulator gene icaR. Infect. Immun. 72:3838-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knobloch, J. K. M., S. Jäger, J. Huck, M. A. Horstkotte, and D. Mack. 2005. mecA is not involved in the σB-dependent switch of the expression phenotype of methicillin resistance in Staphylococcus epidermidis. Antimicrob. Agents Chemother. 49:1216-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knobloch, J. K. M., M. Nedelmann, K. Kiel, K. Bartscht, M. A. Horstkotte, S. Dobinsky, H. Rohde, and D. Mack. 2003. Establishment of an arbitrary PCR for rapid identification of Tn917 insertion sites in Staphylococcus epidermidis: characterization of biofilm-negative and nonmucoid mutants. Appl. Environ. Microbiol. 69:5812-5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knobloch, J. K. M., H. von Osten, M. A. Horstkotte, H. Rohde, and D. Mack. 2002. Minimal attachment killing (MAK): a versatile method for susceptibility testing of attached biofilm-positive and -negative Staphylococcus epidermidis. Med. Microbiol. Immunol. 191:107-114. [DOI] [PubMed] [Google Scholar]
- 15.Kullik, I., and P. Giachino. 1997. The alternative sigma factor σB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch. Microbiol. 167:151-159. [DOI] [PubMed] [Google Scholar]
- 16.Mack, D., K. Bartscht, C. Fischer, H. Rohde, C. de Grahl, S. Dobinsky, M. A. Horstkotte, K. Kiel, and J. K. M. Knobloch. 2001. Genetic and biochemical analysis of Staphylococcus epidermidis biofilm accumulation. Methods Enzymol. 336:215-239. [DOI] [PubMed] [Google Scholar]
- 17.Mack, D., P. Becker, I. Chatterjee, S. Dobinsky, J. K. M. Knobloch, G. Peters, H. Rohde, and M. Herrmann. 2004. Mechanisms of biofilm formation in Staphylococcus epidermidis and Staphylococcus aureus: functional molecules, regulatory circuits, and adaptive responses. Int. J. Med. Microbiol. 294:203-212. [DOI] [PubMed] [Google Scholar]
- 18.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack, D., M. Haeder, N. Siemssen, and R. Laufs. 1996. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J. Infect. Dis. 174:881-884. [DOI] [PubMed] [Google Scholar]
- 20.Mack, D., H. Rohde, S. Dobinsky, J. Riedewald, M. Nedelmann, J. K. M. Knobloch, H. A. Elsner, and H. H. Feucht. 2000. Identification of three essential regulatory gene loci governing expression of Staphylococcus epidermidis polysaccharide intercellular adhesin and biofilm formation. Infect. Immun. 68:3799-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mack, D., A. Sabottke, S. Dobinsky, H. Rohde, M. A. Horstkotte, and J. K. M. Knobloch. 2002. Differential expression of methicillin resistance by different biofilm-negative Staphylococcus epidermidis transposon mutant classes. Antimicrob. Agents Chemother. 46:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack, D., N. Siemssen, and R. Laufs. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki, E., J. M. Chen, C. Ko, and W. R. Bishai. 1999. The Staphylococcus aureus rsbW (orf159) gene encodes an anti-sigma factor of SigB. J. Bacteriol. 181:2846-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palma, M., and A. L. Cheung. 2001. σB activity in Staphylococcus aureus is controlled by RsbU and an additional factor(s) during bacterial growth. Infect. Immun. 69:7858-7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas, V. L., B. A. Sanford, R. Moreno, and M. A. Ramsay. 1997. Enzyme-linked lectinsorbent assay measures N-acetyl-d-glucosamine in matrix of biofilm produced by Staphylococcus epidermidis. Curr. Microbiol. 35:249-254. [DOI] [PubMed] [Google Scholar]
- 26.Wu, S., H. de Lencastre, and A. Tomasz. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J. Bacteriol. 178:6036-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.