Abstract
A traditional Chinese medicine (TCM) monomer is a bioactive compound extracted from Chinese herbal medicines possessing determined biological activity and pharmacological effects, and has gained much attention for treating neuronal diseases. However, the application of TCM monomers is limited by their low solubility and poor ability to cross the blood-brain barrier (BBB). Exosomes are small extracellular vesicles (EVs) ranging in size from 30 to 150 nm in diameter and can be used as drug delivery carriers that directly target cells or tissues with unique advantages, including low toxicity, low immunogenicity, high stability in blood, and the ability to cross the BBB. This review discusses the biogenesis, components, stability, surface modification, isolation technology, advantages, and disadvantages of exosomes as drug carriers and compares exosomes and other similar drug delivery systems. Furthermore, exosome-encapsulated TCM monomers exert neuroprotective roles, such as anti-inflammation, anti-apoptosis, anti-mitophagy, and anti-oxidation, in various neuronal diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS), and cerebral ischemia and reperfusion (CI/R) injury, as well as anti-drug resistance, anti-tumorigenesis, anti-angiogenesis, and promotion of apoptosis in brain tumors, providing more inspiration to promote the development of an exosome-based delivery tool in targeted therapy for neuronal diseases.
Keywords: Traditional Chinese medicine monomer, Exosome, Drug delivery, Neuronal disease
Graphical abstract
Highlights
-
•
Exosomes can be used as TCM monomer delivery carriers.
-
•
Exosomes encapsulated TCM monomers exerted neuroprotective roles in AD, PD, MS, and CI/R.
-
•
Exosomes encapsulated TCM monomers exerted anti-tumor effects in glioma.
1. Introduction
Traditional Chinese medicine (TCM) is a medical system with a specific theoretical style that is gradually built on medical and life practices and experiences based on long-term accumulation and continuous summarization [1]. It is characterized by indigenous medicine that contributes to the maintenance of health to hinder, diagnose, and intervene in physical and mental illnesses differently from modern Western medicine founded on theories, beliefs, and experiences. The oldest classic of TCM originated in Huangdi Neijing, also known as the Inner Canon of Huangdi or the Yellow Emperor's Medicine Classic, which dates back to antiquity, with a history spanning more than 4,000 years [2]. TCM is deeply rooted in the Chinese philosophy of Yin-Yang and the Five Elements (Wu Xing), including a mixture of ancient philosophy, clinical experiences, primitive knowledge of medicine, regional cultures, and religious beliefs. It is worth noting that TCM is a holistic system and emphasizes harmony with the universe [1,3]. Therefore, TCM exerts multifaceted therapeutic effects through a comprehensive approach that combines multiple methods, including Chinese herbal medicine, herbal enemas, acupuncture, Chinese massage (Tui Na), mind/body exercises, and dietary therapy [4]. Treatment is characterized by a definite curative effect, relatively few toxic and side effects, and wide indications; however, the properties of matrix complexity, component diversity, and low levels of active components pose challenges for the study of TCM [5]. TCMs mainly include natural medicines such as plant, animal, and mineral medicines, as well as preparations in which these natural medicines are formulated into various dosage forms [6]. The composition of TCMs is complex and generally comprise a large number of active ingredients with different chemical structures, physicochemical properties, concentrations, and pharmaceutical and/or toxic activities, such as flavonoids, alkaloids, lignins, phenols, terpenoids, amino acid derivatives, organic acids, polyketides, steroids, and sugars [7].
A TCM monomer extracted from a single TCM or TCM prescription, is considered as an active component with a determinate molecular formula and spatial structure in TCM (Table 1) [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]], and has shown biological activities and pharmacological effects, such as anti-inflammatory, antibacterial, anti-diabetic, and anti-tumor effects [26]. TCM monomers have unique and novel pharmacological advantages that allow them to achieve therapeutic outcomes similar to those of conventional therapies with reduced toxicity and side effects. The study of TCM monomers improves the efficacy of TCM and provides a scientific basis for its application in modern medicine [27].
Table 1.
The list of chemical structure of traditional Chinese medicine (TCM) monomers introduced in this article [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]].
| TCM monomer | Chemical structure | Refs. |
|---|---|---|
| Pal | 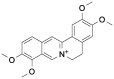 |
[8] |
| Ber | 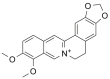 |
[8,9] |
| Cory-B | 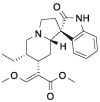 |
[10] |
| Hed | 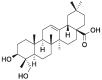 |
[11] |
| Nef | 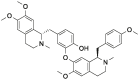 |
[11] |
| Bai | 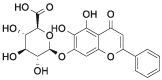 |
[11,12] |
| Cur | 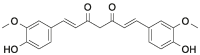 |
[[13], [14], [15], [16], [17], [18], [19], [20]] |
| ECG | 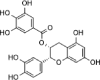 |
[21] |
| RSV | 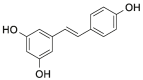 |
[22] |
| Que | 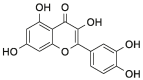 |
[23] |
| DHT | 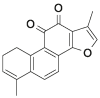 |
[24] |
| SAB |  |
[25] |
| CPT | 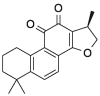 |
[25] |
Pal: palmatine; Ber: berberine; Cory-B: corynoxine-B; Hed: hederagenin; Nef: neferine; Bai: baicalin; Cur: curcumin; ECG: epicatechin gallate; RSV: resveratrol; Que: quercetin; DHT: dihydrotanshinone; SAB: salvianolic acid B; CPT: cryptotanshinone.
TCM monomers have also gained attention due to their potential to treat neuronal diseases. However, the use of TCM monomers to treat neuronal disorders presents some challenges. Among them, the characteristics of poor solubility and bioavailability in aqueous conditions, high degradation rate, poor targeting, and low delivery efficiency, especially the poor ability to cross the blood-brain barrier (BBB) to target the central nervous system (CNS) of TCM, have restricted their clinical efficacy [28]. To address these issues, researchers have begun to explore novel drug delivery systems, such as exosomes. This review systematically introduces the mechanisms by which TCM monomers are loaded into exosomes to prevent and treat various neuronal diseases.
2. The introduction of extraction of TCM active component
TCM monomers can be divided into alkaloids, flavonoids, terpenoids, saponins, phenols, quinones, and other substances, and are characterized by low concentrations in TCM, complicated structures, various types, and unstable properties. These special characteristics of TCM monomers pose a significant challenge for the isolation and purification of a single pure active component from complex TCM mixtures. The hot water isolation method, which is the earliest leaching method, is a traditional and popular approach. Enzyme-assisted extraction (EAE) facilitates water isolation with high impurity removal and purification efficiency. Physical methods such as ultrasound and microwave-associated extraction (MAE) are also common approaches for TCM extraction. Recently, with the development of technology, new separation methods, including semi-bionic-, supercritical fluid-, and molecular imprinting technology (MIT)-associated extraction, have also been used for TCM extraction.
2.1. Hot water extraction (HWE)
Water is an ideal polar solvent for the extraction of polar and hydrophilic molecules. High temperatures promote the dissolution of polar and hydrophilic macromolecules from the cell wall, making them more easily dissolved in water [29]. The HWE process is simple, with a single extraction solvent, a simple device, and low cost, especially without solvent pollution and environmental protection [30]. However, the long duration and high temperatures make it difficult to control the temperature, resulting in the degradation of the compound and reduction of its biological activity [31]. Furthermore, this traditional isolation technology has a large number of water-soluble impurities, and it is difficult to isolate the target product from the mixtures, leading to a low extraction efficiency [32]. In other words, HWE is the most common and convenient method of extracting a target product.
2.2. EAE
The EAE technique uses the catalytic power of enzymes to break down complex biological structures, facilitating the release of intracellular active components from cells [32]. This approach is highly valued for its mild reaction conditions, which make it environmentally friendly, efficient, easy to operate, and cost-effective in terms of both investment and energy requirements [33]. The specificity and selectivity of enzymes are key characteristics that can influence the extraction process. Enzymes such as proteases, cellulases, amylases, glucanases, and endoproteases are used in EAE to catalyze cell wall degradation. However, because a single enzyme may not be sufficient to meet the extraction objectives, a cocktail of enzymes with a broad spectrum of activities is often used to disrupt the cell wall more effectively [33,34]. EAE can improve the level of hydrolysis as a result of the synergistic effects between different enzymes. Optimizing conditions for enzymatic activity is crucial for EAE. Each enzyme has an optimal set of reaction conditions, including enzyme concentration, temperature, time, and pH, which collectively affect the extraction efficiency [35]. The optimal conditions for a mixture of enzymes may differ from those for a single enzyme. Therefore, it is important to consider the synergistic effects, types of substrates, and presence of enzyme inhibitors. EAE is not typically used in isolation but is often combined with other extraction methods to increase the yield of chemical components. EAE not only serves as an auxiliary process for water extraction, but also aids in impurity removal and purification, thus enhancing the extraction activity.
2.3. Ultrasound-associated extraction (UAE)
UAE is a modern technique that utilizes the physical effects of ultrasound waves to enhance the release of active components from plant materials. This technology operates based on the principle of cavitation, in which ultrasound waves generate microbubbles in a liquid medium. These bubbles grow and collapse violently, producing shock waves and microstreams that disrupt cell walls and release their contents [36]. Compared to traditional extraction methods such as HWE, UAE operates at lower temperatures, has a higher yield and efficiency, shorter extraction time, is more versatile and more suitable for various plant materials and target compounds [37]. However, the application of UAE has some challenges. The extraction efficiency is affected by the limited conversion rate of sound energy to mechanical energy, insufficient ultrasonic power can lead to low extraction rates, and prolonged sonication can cause thermal effects that can degrade heat-sensitive ingredients [38]. The yield and purity of the target compound can be affected by extraction processes that involve several factors, such as ultrasonic frequency and power, number of ultrasound cycles, ratio of plant material to solvent, and time [39]. In summary, UAE is a promising technique for the extraction of medicinal compounds and offers advantages such as speed, efficiency, and preservation of heat-sensitive ingredients. However, careful consideration of operational parameters and equipment characteristics is necessary to optimize the extraction process and achieve optimal results.
2.4. MAE
MAE is a separation method to extract bioactive compounds from natural sources. It is based on the thermal effect of microwaves, which causes polar substances, particularly water molecules in the cells of the material, to absorb microwave energy and produce heat. This rapid increase in intracellular temperature leads to the vaporization of water, which in turn generates pressure that breaks cell walls and membranes, creates pores, and facilitates the release of active ingredients into the solvent [40]. MAE is widely used to isolate compounds such as polysaccharides, volatile oils, flavonoids, and alkaloids. MAE offers advantages, such as a high extraction rate, short extraction time, and reduced solvent consumption. However, it is not applicable to active ingredients with poor water absorption and heat-sensitive components, such as proteins and peptides [32]. In other words, the MAE is considered as an auxiliary tool that is limited by the transformation rate of heat power.
2.5. Semi-bionic extraction (SBE)
SBE technology simulates the transport process of oral medicine in the gastrointestinal tract using pH-optimized artificial gastric and intestinal juices for successive elution and isolation, based on the principle of a biopharmacological perspective, combining holistic drug research with molecular drug research [41]. SBE can improve extraction efficiency, shorten the production cycle, and reduce costs. However, it introduces invalid constituents and is adversely concentrated and refined [38]. SBE is widely applied in pharmacological research in the laboratory; however, its practical large-scale applications have not yet been realized.
2.6. Supercritical fluid extraction (SFE)
SFE is a sophisticated green separation technique that controls the critical temperature and pressure of a component using a supercritical fluid as an extracting agent to selectively isolate and purify components according to their molecular mass, boiling point, and polarity [42]. SFE integrates the properties of distillation and liquid-liquid extraction, and uses various solvents such as CO2, which have low toxicity, non-flammability, cost-effectiveness, and high purity [43]. Moreover, the low temperature of supercritical fluids can protect heat-sensitive substances and is suitable for isolating bioactive components from TCM. However, long-term processing can cause solvent waste and degradation of bioactive substances. Furthermore, the problems of large equipment size, high equipment costs, and high operational demand also make the application of SFE a challenge [38]. SFE is an advancement in extraction technology that provides a sequence of benefits that can strengthen the efficiency and quality of natural product research. However, the challenges involved in its implementation must be considered, and further optimization and technological advancements may be necessary to overcome these limitations.
2.7. MIT
MIT is a molecular recognition technology based on the principle of selective recognition and binding to specific target molecules that mimic the recognition properties of biological antibodies or receptors [44]. Molecularly imprinted polymers (MIPs) are prepared in three steps: i) imprinting, where functional monomers are arranged around a template molecule to form complexes through covalent or non-covalent interactions; ii) polymerization, where the complexes are cross-linked to form a stable network, which is then solidified; and iii) template removal, where the template is extracted, leaving behind specific binding sites that can selectively recognize the target molecule [45]. The prepared MIPs have three main characteristics: structure predictability, identification specificity, and application extensiveness [46]. MIPs are easy to synthesize and can be produced in large quantities with strong mechanical and chemical stabilities, high selectivity, and high affinity [38]. However, some problems are also associated with MIPs, such as inefficient adsorption and desorption, complex preparation processes, poor batch-to-batch reproducibility, and potential contamination. Despite these challenges, the potential applications of MIPs are universal and they are used in many fields, such as sample pre-concentration for the extraction and concentration of analytes from complex samples, as stationary phases in chromatography, as bioassays and biosensors, and as enzyme and receptor mimics [47]. With the development of technology, new methods will be developed to address the current limitations of MIPs, such as improving their reproducibility and reducing the complexity of their preparation, further expanding the range of applications of MIT technology in various scientific and industrial fields.
3. Exosomes
Small extracellular vesicles (EVs) produced in the endosomes of eukaryotic cells are called exosomes, and their diameter is usually 30−150 nm [48]. All cell types secrete exosomes, which are found in blood, urine, gastric acid, and other components [49]. Exosomes play crucial roles in cell communication by transferring various proteins, metabolites, and nucleic acids to recipient cells, thus affecting many life activities and are also associated with the occurrence of many diseases [50]. Furthermore, exosomes can be used as drug delivery carriers to treat various diseases, particularly neuronal disorders. Exosome-mediated drug delivery has the advantages of low toxicity, low immunogenicity, and high engineering capabilities, which can improve drug targeting and delivery efficiency. By encapsulating drugs in exosomes, drugs containing TCM monomers can be directed at cells more efficiently, improving therapeutic efficacy and reducing side effects [51].
3.1. Biogenesis of exosomes
Fusion of nuclear endosomes and plasma membranes is the main method of exosome production [48]. The limiting membrane of early endosomes sprouts inward to form exosomes [49]. Subsequently, bioactive substances begin to accumulate in the early sorting endosomes. Early sorting endosomes become late sorting endosomes under the combined action of other related proteins required for transport and endocytic sorting complexes [52]. Double invaginations of the plasma membranes then form multivesicular bodies (MVBs). After completion of this process, MVBs fuse with the plasma membrane to secrete exosomes [53].
3.2. Components of exosomes
Exosomes contain many bioactive cargo originating from the cell, including nucleic acids, lipids, metabolites, and cytosolic and cell-surface proteins, which can be delivered to target cells to execute biological functions [54]. The exosome incorporates many compounds of late endosomes, such as tetraspanins (CD63, CD9, and CD81), membrane transport and fusion proteins (Rab, GTPases, and flotillin); moreover, it also contains various heat shock proteins (e.g, heat shock protein 70 (HSP70) and HSP90) and MVB biogenesis proteins (e.g., alg-2 interacting protein-x (Alix) and tumor susceptibility gene 101 (TSG101)), and all of these proteins can be considered as markers of exosomes [55]. Furthermore, certain proteins that exist in specific exosomes, determined by their maternal cells, can reflect the functional states of parental cells under pathological or physiological conditions [56].
3.3. Stability
Stability is strongly associated with resistance to aggregation, structure maintenance, and avoidance of protein degradation, which are crucial for exosomes to act as drug delivery carriers. Methods for detecting exosomal stability include quantifying surface markers, such as CD63 or CD81, to check protein degradation; characterizing colloidal stability through measurements of zeta potential, size distribution, and concentration using nanoparticle tracking analysis (NTA); and visualizing morphology by electron microscopy [57]. Exosome stability is significantly affected by storage parameters, including temperature, time, and freeze-thaw cycles; osmotic pressure and pH are also important for exosomal stability [58]. For example, the levels of protein and RNA inclusions of exosomes decreased at 10 days at room temperature compared to storage at −70 and 4 °C. Exosomes are stable at 4 °C for the short term (within 7 days) and below −70 °C for the long term, with the storage temperature being the most favorable condition for long-term preservation of fresh exosomes [59].
3.4. Surface modification of exosomes
Recently, exosomes have been explored as nanoscale carriers for drug delivery in biomedicine. Several studies have shown that natural exosomes spread in the extracellular spaces and biofluids by free diffusion and are randomly uptaken by recipient cells. For example, exosomes labeled with fluorescence dye/probe can be detected in the liver, spleen, kidney, pancreas, and other organs by intravenous, intraperitoneal, or subcutaneous injection, indicating the uncontrolled location of exosomes in vivo [60]. Ensuring the targeting capacity of natural exosomes is required to deliver the desired cargo to specific tissues or cells. Several technologies have been developed for exosome surface modification: 1) genetic engineering, which is a common approach for producing surface-modified exosomes, in which exosome-originating cells use plasmid vectors that encode targeting ligands fused with transmembrane proteins such as tetraspanins, lysosome-associated membrane protein (Lamp), and glycosylphosphatidylinositol (GPI) [61]; 2) the click chemistry technology covalent modification, which involves attaching an alkyne group to the exosome surface via a condensation reaction with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide-N-hydroxysuccinimide (EDC-NHS). This alkyne group can then be covalently linked to an azido group on a target moiety in the presence of copper, a reaction known as “click” due to its efficiency and reliability [20]. Genetic engineering and click chemistry are widely used approaches to produce surface-modified exosomes. In addition, some non-covalent modification methods are also exploited for yielding targeted exosomes: 1) multivalent electrostatic interactions, which strengthen the efficiency of exosome targeting to negatively charged biological membranes by coating the exosome membrane with a positive charge imparting moiety [62]; 2) the ligand-receptor interaction, which is the correlation between exosome surface natural receptors and targeting ligands [63]; 3) hydrophobic interaction/membrane engineering, where the exosome-liposome hybrid membrane acquires targeting capacity through the fusion of exosomal membranes with functionalized liposomal membranes [64]; 4) aptamer-based surface modification, where the aptamer technique that fuses with nucleotide sequences is used for exosomal surface modification [65]; and 5) modification by anchoring the CP05 peptide, in which the CP05 peptide has a strong affinity for the second extracellular loop of CD63, a protein that is frequently found on the surface of exosomes. This makes it an effective linker between therapeutic agents and the exosome surface [66].
3.5. Separation of exosomes
Standardized isolation strategies are crucial for the study and clinical application of exosomes. Different exosome separation technologies have been explored based on their size, shape, density, and surface proteins, including ultracentrifugation (UC), polymer precipitation (PP), immunoaffinity capture (IC), ultrafiltration (UF), size exclusion chromatography (SEC), and microfluidics-based techniques [67].
3.5.1. UC
UC is the most common exosomal separation technology and is considered as the gold standard for exosome isolation. UC is based on divergence in the density and size of the exosomes and impurities in the sample. First, the samples were centrifuged at 300, 2000, and 10,000 g to remove dead cells and cell debris, which could be replaced by filtration, and the supernatant was centrifuged at high speed (∼100,000 g) to pellet exosomes. Typically, an additional high-speed centrifugation step is implemented to wash the exosome pellet in phosphate-buffered saline (PBS) to reduce protein contaminants [68]. As a mature method, UC is suitable for isolating most samples at low operating costs. However, the separation process is time-consuming (>4 h), with poor repeatability, impurity potential, and risk of exosome damage that affects biological activity [69].
3.5.2. IC
The IC method for exosome isolation uses specific interactions between antigens and antibodies to selectively capture and purify exosomes from biological samples [70]. Exosomes contain specific proteins, lipids, and polysaccharides on their surfaces that can be targeted by antibodies. By immobilizing these antibodies on a solid surface, such as magnetic beads, exosomes with matching antigens can be specifically bound and separated [71]. The IC method can isolate specific subpopulations of exosomes based on target antigens, which is crucial to studying the functions of particular exosome types, yielding highly pure exosome preparations that are beneficial for downstream applications such as proteomics or functional assays. Various exosome subpopulations can be isolated by selecting different antibodies [72]. However, this method is time-consuming and requires expensive and specific antibodies, which ultimately result in a low yield of exosomes due to their incomplete elution of beads [73]. This separation method is particularly useful for research on specific diseases in which disease-specific exosome markers can be detected and studied.
3.5.3. PP
PP technology alters the solubility of exosomes in solution. Polymers, such as polyethylene glycol (PEG), are added to the sample, which interacts with water molecules and reduces the solubility of exosomes, causing them to precipitate from the solution [72]. After low-speed centrifugation to remove cells and debris, the supernatant was mixed with a polymer solution, incubated and centrifuged again at low speed to pellet exosomes [74]. This method can be used for large sample volumes, yield a high quantity of exosomes, and preserve the integrity of exosomes without complex equipment or lengthy procedures [75]. However, the use of polymers may introduce impurities, such as protein aggregates and lipoproteins, into exosome preparations, potentially affecting their biological activity and interfering with subsequent analyses, such as proteomics or mass spectrometry [76]. To overcome this challenge, the precipitation method can be used together with other separation techniques, such as the immunoaffinity method, to improve the purity and specificity of the isolation.
3.5.4. UF
UF is a membrane-based separation technique that is gaining popularity in the field of exosome isolation because of its simplicity and efficiency. The principle of UC is based on its size using a membrane with a specific pore diameter or molecular weight cutoff (MWCO), which retains larger impurities while passing through smaller particles, such as exosomes [77]. This technology can be used in combination with low-speed centrifugation to concentrate samples and remove large impurities. UF can be divided into dead-end filtration (DEF; liquid flow is in the same direction as filtration, leading to rapid filter clogging) and tangential flow filtration (TFF; liquid flow is perpendicular to filtration, reducing clogging and improving membrane life and equipment stability) depending on the pressure [78]. UF is one of the simplest methods for exosome separation, requiring minimal equipment and expertise, allowing rapid purification of large sample volumes in a short amount of time, and it does not require expensive special equipment or harmful chemical reagents. However, this method has some disadvantages, including membrane clogging, exosome deformation, loss of exosomes, and the risk of contamination with fluid components smaller than the filter pore size [79]. UF is a valuable tool for exosome isolation due to its ease of use and efficiency.
3.5.5. SEC
SEC, also known as gel filtration chromatography, is a technique used to separate particles according to their size and is particularly useful for the isolation of exosomes from biological fluids. The principle of UC is based on differences in size, using a porous stationary phase (e.g., Sephadex, Sepharose, Sephacryl, and BioGel P) in a column through which a mobile phase (biological fluid) is passed and the larger particles elute first because they cannot enter the pores and thus take a shorter path, and the smaller particles, including exosomes, penetrate the pores and elute later [80,81]. SEC has many advantages, including the high purity of isolated exosomes by minimizing protein contamination, maintaining the biological activity and structure of exosomes, minimizing sample loss compared to UF, requiring less time to complete in 15 min, and isolating specific subsets of EVs using materials with different pore sizes [82]. However, SEC cannot effectively separate contaminants of sizes similar to those of exosomes, resulting in a low yield of exosomes [82]. In summary, SEC is a valuable method for exosome isolation that offers high purity, preservation of biological activity, and scalability.
3.5.6. Microfluidics-based techniques
The microfluidics-based method is a cutting-edge technology that has emerged as a powerful tool for the isolation and analysis of exosomes due to its ability to integrate multiple processes into a single chip [83]. The principle of microfluidic-isolated exosomes is based on physical properties (size, density, surface antigens, etc.), immunoaffinity, and contact-free [82]. This method could be applied to point-of-care diagnostics, liquid biopsy, and personalized medicine in the future [84]. However, it also meets certain requirements, including the need for further validation and large-scale testing of microfluidics-based methodologies, improving the sensitivity and specificity of exosome detection and isolation, and combining other detection and analysis methods for comprehensive exosome research. In summary, microfluidics offers a versatile and efficient approach to exosome research with the potential to transform diagnostics and therapeutics.
3.6. The advantages and disadvantages of exosomes as drug carriers
3.6.1. The advantages of exosomes as drug carriers for targeting the brain
Exosomes have been identified as promising natural carriers for drug loading and delivery for the following reasons (Fig. 1). The first is its ability to cross the BBB. The BBB is a semi-permeable barrier composed of glial cells (astrocytes and microglia), pericytes, specialized brain microvascular endothelial cells (BMECs) connected by tight junctions, and a basement membrane [85]. This barrier governs homeostasis, which can limit the transportation of ions and micro- and macro-molecules between blood and neural tissues [85]. The BBB is one of the main problems faced by traditional drug delivery to the CNS because almost all large-molecule drugs and more than 98% of small-molecule drugs have difficulty penetrating the CNS. Exosomes can freely cross the vascular wall and the extracellular matrix benefits from its endogenous characteristics and small size [86]. Furthermore, several studies have suggested mechanisms underlying the interaction between exosomes and the BBB. For example, Yuan et al. [87] found that macrophage-derived exosomes can bypass the BBB, depending on the interaction of integrin lymphocyte function-associated antigen 1 (LFA-1) with intercellular adhesion molecule 1 (ICAM-1) of brain microvessel endothelial cells. Furthermore, Chen et al. [88] found that HEK293T-derived exosomes can overcome the BBB through activation of BMECs endocytosis induced by the inflammatory cytokine tumor necrosis factor-α (TNF-α). TCM monomers can cross the BBB after being loaded into exosomes to treat neuronal diseases (Fig. 2). Second, it has a better targeting capability. Studies have found that exosomes have lower immunogenicity and stronger specific targeting abilities than traditional drug carriers when used as drug delivery vehicles. Compared to liposomes, their homing ability to tumor tissues is ten times greater, which also confirms the good targeting ability of exosomes [89]. Exosomes contain various homing molecules (ligands, magnetic materials, and pH-responsive motifs) on their lipid bilayer membranes, which contribute to the identification of specific cell types and enable the acquisition of targeting features for drug delivery in vivo or in vitro. For example, exosomes containing Tspan8 are prone to combine with CD11b and CD54-positive cells [90]. Third, it improves security. The surfaces of exosomes are wrapped in the membranes of various cells, improving their biocompatibility and safety [91]. Traditional drug carriers can cause toxic immune reactions in vivo, resulting in greatly reduced therapeutic effects, while exosomes are not prone to immune reactions due to their improved endogenous biocompatibility [92]. Based on the good biocompatibility of exosomes, the stability and effectiveness of drugs contained in exosomes can be effectively improved [93]. Various proteins contained in the exosomal membrane also facilitate their use as drug delivery vehicles. Four transmembrane proteins, CD9 and CD81 (on the exosome membrane promote fusion between exosomes and cells), CD55, and CD59, enhance the stability of exosomes in circulation. CD47 improves macrophage resistance to exosomes [93]. Fourth, it is easier to administer (Table 2) [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]]. Exosomes can be delivered via various routes, including intravenous, subcutaneous, intraperitoneal, intratumoral, nasal, and oral administration, which are conducive to target tissues in different disease models [94]. The diversity of drug administration ways also indicates the high flexibility and compatibility of exosome-based drug delivery. Finally, the TCM monomers were encapsulated (Table 3) [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]]. Monomeric active ingredients of TCMs can be loaded into exosomes due to their low toxicity and side effects. Several loading methods have been applied using physical treatments, including sonication, electroporation, extrusion, freeze-thawing, surfactant treatment, and dialysis, to achieve various therapeutic effects [94].
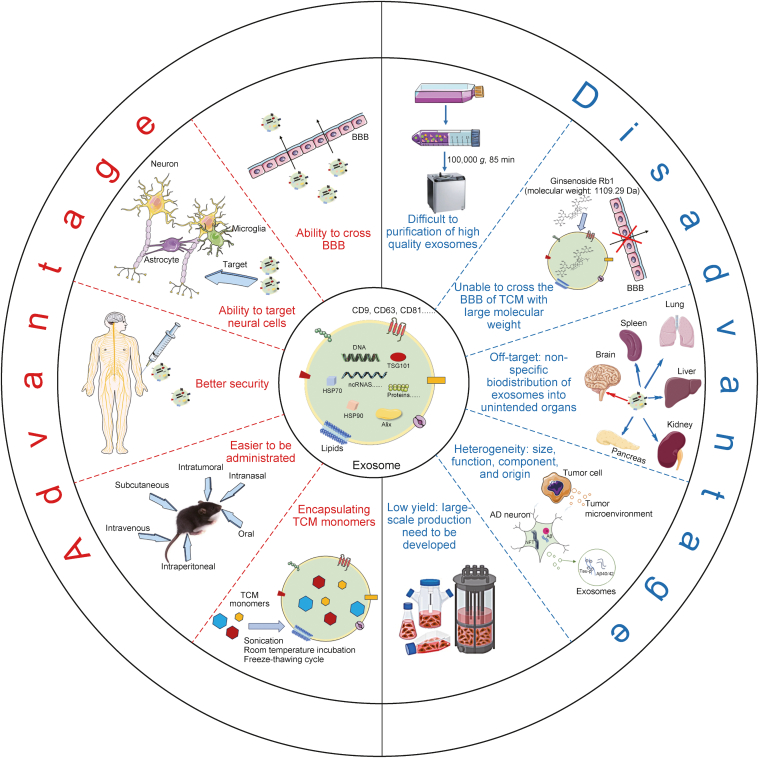
Fig. 1.
The description of advantages and disadvantages of exosomes as a drug carrier for treatment of neuronal diseases. As a drug delivery tool, exosome have the ability to cross blood-brain barrier (BBB), to target neural cells, better security, various ways of administration in vivo, and effectively encapsulating traditional Chinese medicine (TCM) monomers. However, the properties of difficult to separation of exosome, poor ability to cross the BBB of TCM monomers with large molecular weight, off-target, heterogeneity, and low yield have restricted exosome to be TCM monomers delivery system in clinical application. AD: Alzheimer's disease; TSG101: tumor susceptibility gene 101; ncRNAs: non-coding RNAs; HSP: heat shock protein; Alix: alg-2 interacting protein-x.
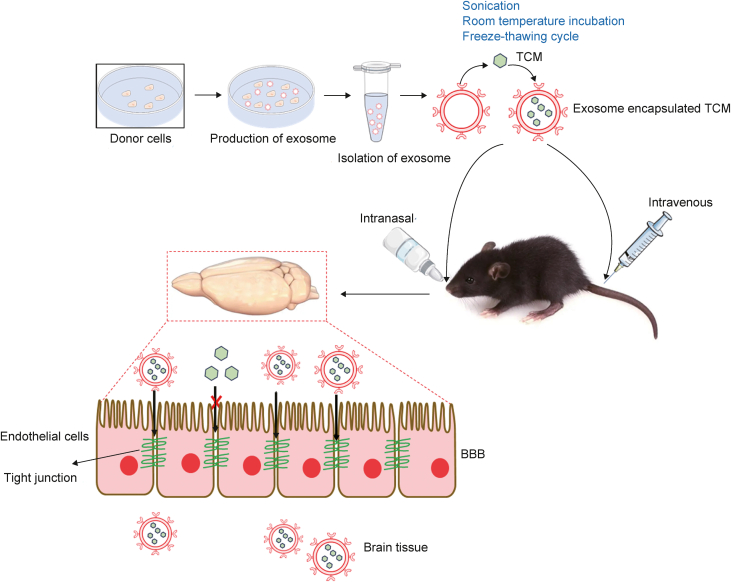
Fig. 2.
Traditional Chinese medicine (TCM) monomers can cross the blood-brain barrier (BBB) after being loaded into exosomes. After separation from the supernatant of cell's culture medium, exosome can load with TCM monomers using sonication, room temperature incubation, and freeze-thawing cycle methods. Exosomes encapsulated TCM monomers were injected into rats or mice major through intranasally, intravenously delivery pathway, then exosome-TCMs monomers can cross BBB to target diseased brain region.
Table 2.
The therapeutic outcome and pros/cons of exosome encapsulated traditional Chinese medicine (TCM) monomer in various neuronal disease model [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]].
| Naïve or modified exosome | TCM monomer | Animal/cell model | Administration way | Therapeutic outcome | Pros/cons | Refs. |
|---|---|---|---|---|---|---|
| Naïve exosomes | Ber/Pal | APP/PS1 mice | Intravenously: tail vein injection at the same time every two days for a total of 21 days | Anti-inflammatory effects both in vitro and in vivo; increasing drug accumulation in the hippocampus, cortex, and striatum; strengthening cognitive impairment and nerve injury in vivo by accelerating Aβ elimination and anti-inflammatory cytokine secretion | Pros: the Ber/Pal combined therapy exhibits better and more comprehensive therapeutic effects than single-drug treatment Cons: low yield of exosomes, lack of organic targeting |
[8] |
| Naïve exosomes | Ber | SCI mice | Intravenously: tail vein injection | Anti-inflammatory and anti-apoptotic effect, alleviation moto function | Pros: exosome-Ber can cross BBB and blood spinal barrier, reach the spinal cord injury site through the cerebrospinal fluid circulation; exosomes provide a drug platform and have synergistic effect with drugs | [9] |
| Fe65-exosomes | Cory-B | 5× FAD mice | Intraperitoneally or intravenously: alternate days for 45 days | Inducing neuronal cells autophagy and ameliorating cognitive function of AD mice | Prons: Fe65-exosomes can bypass BBB and target APP overexpressed neuronal cells | [10] |
| Naïve exosomes | Bai, Hed, and Nef | APP/PS1 mice | Intravenously: tail vein injection at a dose of 100 μg/kg | Reducing the level of huntingtin74, p301L tau, A53T α-synuclein, aggregation of Aβ (1−42), enhancing autophagy, and ameliorating the learning and memory ability in vivo | Pros: high bio-availability, crossing BBB with the molecular weight lower than 1109 Da of monomer Cons: off-target effects; not applicable to large-scale |
[11] |
| Naïve exosomes | Bai | tMCAO and pMCAO rats | Intravenously: tail vein injection | Reducing cell apoptosis by attenuating ROS production in vitro and depressing the infarct area neurological scores while the integrity of neuronal structure in vivo | Pros: exosome-Bai easily cross the BBB, allowing more Bai to target brain tissues; the migratory and brain targeting abilities features of exosome-Bai was acquired from its origin cells (macrophages), to promote the accumulation of Bai in the brain ischemic region following pMCAO | [12] |
| Naïve exosomes | Cur | Injection of OA on one side of hippocampal area of mice | Intravenously: a single dose of cur at 0.4 mg/kg | Inhibiting tau phosphorylation through the Akt/GSK-3β pathway, ameliorating of learning and memory deficiencies in OA-induced AD mice | Pros: Cur-treated macrophage derived exosomes can enhance the penetration of cur across the BBB into the brain through interaction between LFA-1 and ICAM-1 | [13] |
| Naïve exosomes | Cur | 6-OHDA induced PD | Nasally: at a dose of 10 mg/kg body weight | Decreased the brain inflammation, aggregated of α-synuclein, and cell apoptosis in the dopaminergic TH positive neuron, and improved the impaired learning and memory ability in PD mice | Pros: hEnSCs-exosomes can delivery and transfer safe and effective payload in long-term, and sustain release manner into neuronal cells through intranasal injection way | [14] |
| PR-exosome | Cur | MPTP-induced PD model mice | Intranasally injection | Eliminating α-synuclein aggregation and promotion of the growth of neurite length and branches in vitro and the improvement of movement behavior and coordination ability in PD mice | Pros: three-Pronged Synergistic Treatment of PR-exosome/PP@Cur | [15] |
| Naïve exosomes | Cur | MOG peptide induced EAE mice | Intranasally: exosome-Cur was administered intranasally daily for 31 days using the protocol described above and was initiated on day 4 after immunization with the MOG peptide | Anti-inflammatory effects: reduction in the number of microglial cells | Pros: no side effects with nasally injection; exosomes are taken up by microglial cells (∼60%)/nonmicroglial cells (∼40%) | [16] |
| Naïve exosomes | Cur | MCAO rats | Intravenously: tail vein injection, exosomes-cur (10 μg/mL cur) was administered to MCAO rats after 2 h of occlusion | Decreasing of inflammatory cytokines expression level, ROS generation, Cyt c release, and infarct area; rescuing the loss of tight junction proteins and improving neurological performance in MCAO rats | Prons: exosomes protected Cur from degradation in plasma; accumulation of exosome-Cur in ischemic regions were driven by the inflammation-mediated targeting ability of exosomes | [17] |
| Naïve exosomes | Cur | Ischemia reperfusion-injured mic | Intranasally: alternate nostrils (2 μL × 5 times) started within an hour of I/R and sham surgery and continued till seven days. | Improvement in neurological scores, lessening in lesion volume, brain water content, and inflammation effect; normalization astrocytes and neuronal expression, decreasing in ICAM and improvement in VE-cadherin levels in brain vessels; and alleviating tight junction proteins loss | Pros: more solubility and stability of Cur in exosomes and the integrity of exosomes-Cur was preserved. Cons: the treatment of I/R-injured mice was started within an hour of injury which does not clinically implicate the conditions of the stroke patients who sometimes reach to the hospitals after hours of ischemic insult; the lack of additional mice groups treated with Cur and embryonic stem cells alone to compare the combined MESC-exosome-Cur effects; and time points analysis of available Cur concentrations in the blood and mice brain tissues |
[18] |
| c(RGDyK)-exosomes | Cur | MACO mice | Intravenously: vein tail injection 12 h or 24 h after reperfusion | Decreasing pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) mediated by NF-κB and inhibiting expression level of caspase-3 | Pros: the exosomes surface can be modified by bio-orthogonal chemistry easily and rapidly; cRGD-exosome can preserve intact exosomes shape and the bioactivity of their cargo, and can be internalized into brain cells to exert its neuroprotective roles Cons: whether the modified exosomes maintain the basic characteristics of natural exosomes like low immunogenicity should be evaluated in the future | [19] |
| RGE-exosomes | Cur | Orthotopic glioma-bearing nude mouse model | Intravenously: tail vein injection, every other day for total seven times | RGE-exosome-SPION/Cur had capability for targeted imaging of glioma and inhibiting the growth of tumor | Prons: the technology combination of exosome-SPION/Cur with MRI can early precise diagnosis and response evaluation of glioma Cons: the precise mechanism of exosome interaction with cells, exosome crossing BBB, the long-term effects of exosome, the optimal dose, treatment time, temperature control, and monitoring of magnetic flow hyperthermia for the synergistic effect of SPIONs need to be further explored | [20] |
| Naïve exosomes | ECG | Rotenone-induced SHSY5Y cells | – | Enhancing cell viability and antioxidative effects and inhibiting autophagy and cell apoptosis | [21] | |
| Naïve exosomes | RSV | EAE mice | Intranasally: daily doses of RSV and exosome (3 mg/kg RSV, 30 μL) | Reducing of the expressions of pro-inflammatory cytokine (TGF-β, INF-γ, IL-6, and IL-17) | Pros: macrophages derived exosomes modified by click chemistry methods had little effect on the natural properties of exosomes, and fit for treatment of neurodegenerative diseases | [22] |
| mAb GAP43-exosomes | Que | MCAO/R model | Intravenously: tail vein injection | Inhibiting ROS production via activation of the Nrf2/HO-1 pathway in vitro and in vivo | Pros: the stability and solubility of exosomal Que were significantly enhanced; mAb GAP43 conjugated to the surface of exosomes can be internalized into I/R injury neuronal cells dependent on the interaction between exosome and GAP43 expressed on neurons in the ischemic region; and naïve plasma exosome exerted a synergistic neuroprotective effect against I/R injury | [23] |
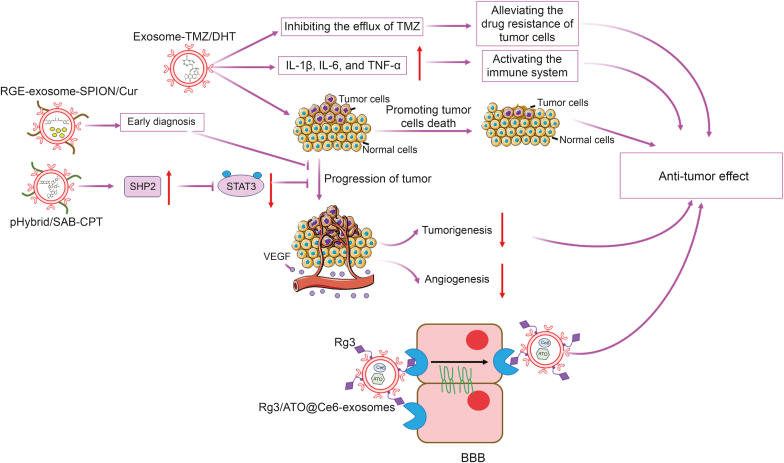
| R-exosomes | TMZ/DHT | Tumor-bearing mice | Intravenously: tail vein every day, a total of six times. | R-exosome- TMZ/DHT could accurately target the tumor site and exerted good antitumor roles via activating immune system in vivo and in vitro, and reduction TMZ resistance in gliomas | Pros: R-exosome-TMZ/DHT have no toxicity or visible organ impairment in vivo | [24] |
| pHybrid-exosomes-Lips nanovesicles | SAB/CPT | orthotopic BALB/c nude mice bearing U87-Luc xenograft tumors |
Intravenously: tail vein injection | The synergistic efficiency of pHybrid-SAB-CPT is major dependent on its cytotoxicity on cancer cells and anti-angiogenesis via depriving the nutrients supplied by new blood vessels in tumor, which are associated with SHP-2 upregulation induced STAT3 signal pathway suppression and anti-angiogenesis caused by VEGF inhibition | Prons: pHybrid/SAB-CPT can target tumor and accumulated in deep tumor regions and pHybrid nanovesicles have features in drug delivery, cell targeting, and tissue penetration | [25] |
Ber: berberine; Pal: palmatine; APP: amyloid precursor protein; PS1: presenilin 1 ; SCI: spinal cord injury; BBB: blood-brain barrier; Cory-B: corynoxine-B; FAD: family Alzheimer's disease (AD); Bai: baicalin; Hed: hederagenin; Nef: neferine; tMCAO: transient middle cerebral artery occlusion; pMCAO: permanent middle cerebral artery occlusion/reperfusion; ROS: reactive oxygen species; Cur: curcumin; Akt: protein kinase B; GSK-3β: glycogen synthase kinase-3β; OA: okadaic acid; LFA-1: lymphocyte funciton-associated antigen 1; ICAM-1: endothelial intercellular adhesion molecule 1; 6-OHDA: 6-hydroxydopamine hydrochloride; PD: Parkinson's disease; TH: tyrosine hydroxylase; hEnSCs: human endometrial stem cells; PR: penetratin (P) and rabies virus glycoprotein (RVG29) peptides; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PP: ROS-responsive amphiphilic polymer poly(propylene sulfide)-polyethylene glycol (PPS-PEG); MOG: myelin oligodendrocyte glycoprotein; EAE: refractory experimental autoimmune encephalitis; I/R: ischemia and reperfusion injury; VE: vascular endothelial; MESC: mouse embryonic stem cell; c(RGDyK): cyclo(Arg-Gly-Asp-D-Tyr-Lys) peptide; TNF-α: Tumor necrosis factor-α; IL-1β: interleukin-1β; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; cRGD: cyclo(Arg-Gly-Asp); RGE: RGERPPR; SPION: superparamagnetic iron oxide nanoparticles; MRI: magnetic resonance imaging; ECG: epicatechin gallate; RSV: resveratrol; TGF-β: transforming growth factor-β; IFN-γ: interferon-γ; mAb: monoclonal antibody; GAP43: growth-associated protein-43; MCAO/R: middle cerebral artery occlusion/reperfusion ; Nrf2: nuclear factor erythroid-2-related factor 2; HO-1: heme oxygenase-1; R: reassembly; TMZ: temozolomide; DHT: dihydrotanshinone; pHybrid: BBB penetrated exosomes-liposome hybrid nanovesicles; Lips: lipidosomes; SAB: salvianolic acid B; CPT: cryptotanshinone; SHP-2: Src homology-2 domain-containing protein tyrosine phosphatase 2; STAT3: signal transducer and activator of transcription 3.
Table 3.
The list of loading method, encapsulation efficiency (EE)/loading capacity (LC), and exosome origin in various neuronal diseases [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]].
| TCM monomer | Exosome origin | Loading method | EE(%)/LC (%) | Disease | Refs. |
|---|---|---|---|---|---|
| Ber/Pal | Microglial cells | Sonication | LC: 15.43 ± 2.19 (Ber) and14.05 ± 2.90 (Pal) | AD | [8] |
| Ber | M2-type primary peritoneal macrophages | Sonication | LC: 17.13 ± 1.64 | SCI | [9] |
| Cory-B | HT22 cells | Sonication | LC: 28% | AD | [10] |
| Bai, Hed, and Nef | Neuro-2a and differentiated PC-12 cells | Room temperature incubation | – | AD | [11] |
| Bai | RAW264.7 cells | Sonication | EE: 45.7 and LC: 11.4 | I/R | [12] |
| Cur | Macrophage RAW264.7 | Cur pre-treated cells | EE: 84.8 and LC: 15.1 | AD | [13] |
| Cur | hEnSCs | Room temperature incubation | EE: 31 | PD | [14] |
| Cur | MSCs | – | LC: 75.53 | PD | [15] |
| Cur | EL-4 cells | Incubation | – | MS | [16] |
| Cur | RAW264.7 cells | Incubation | EE: 82.7 and LC: 14.5 | I/R | [17] |
| Cur | MESC | Freeze-thawing cycle | – | I/R | [18] |
| Cur | Whole blood | Sonication | EE: 34.0 and LC: 13.6 | I/R | [19] |
| Cur | RAW264.7 cells | Electroporation | – | Glioma | [20] |
| ECG | Fresh bovine milk | Sonication | LC: 25.96 ± 0.45 | PD | [21] |
| RSV | RAW264.7 cells | Sonication | LC: 19.34 ± 0.37 | MS | [22] |
| Que | Whole blood of SD rats | Sonication | EE: 34.0 and LC:13.6 | I/R | [23] |
| TMZ/DHT | GL261 | Sonication | EE: 83.99 ± 2.54 (DHT) | Glioma | [24] |
| SAB/CPT | Serum of rats | Electroporation | EE: 89.7 ± 2.57 (SAB) and 84.7 ± 9.35 (CPT) | Glioma | [25] |
EE% = (weight of exosomal TCM monomer/weight of initially added TCM monomer) × 100%; LC% = (Weight of exosomal TCM monomer/Weight of exosomal TCM monomer) × 100%. Ber: berberine; Pal: palmatine; AD: Alzheimer's disease; SCI: spinal cord injury; Cory-B: corynoxine-B; Bai: baicalin; Hed: hederageni; Nef: neferine; I/R: ischemia and reperfusion injury; Cur: curcumin; hEnSCs: human endometrial stem cells; MSCs: mesenchymal stem cell; PD: Parkinson’s disease; MS: multiple sclerosis; MESC: mouse embryonic stem cell; ECG: epicatechin gallate; RSV: resveratrol; Que: quercetin; SD: Sprague-Dawley; TMZ: temozolomide; DHT: dihydrotanshinone; SAB: salvianolic acid B; CPT: cryptotanshinone.
3.6.2. The challenges of exosomes as drug carriers to target the brain
Although exosomes have broad prospects as drug carriers, their applications face some challenges (Fig. 1). First, it is very difficult to isolate and extract exosomes due to the similarity in physical and chemical properties between the EV subtypes. Mainstream exosome extraction methods have advantages and disadvantages. As discussed above, UC can yield high-purity exosomes, but equipment is expensive and can easily damage exosomes during the separation process. Although UF is simple and can achieve high-throughput separation, the purity of isolated exosomes is low. The chemical precipitation method is simple and high-throughput; however, it cannot isolate high-purity exosomes. Exosomes isolated by SEC have high purity and are suitable for all types of samples; however, the protein is easily contaminated during operation, the instrument is expensive, and the operation procedure is complex [48]. Second, exosomes have a good capability to cross the BBB; however, the relationship between the molecular weight of TCM and exosomes needs to be further explored. Tang et al. [11] found that TCM such as ginsenoside Rb1 (molecular weight: 1109.29 Da) with a molecular weight greater than 1109.29 Da cannot cross the BBB even after encapsulating into exosomes, implying that while exosomes facilitate TCM by bypassing the BBB. Exosomes also depend on the molecular weight of the cargo compounds, highlighting a key parameter for the future pharmacological development of exosomes as drug carriers. Third, exosomes are considered as suitable candidates to carry various cargoes, including TCM, RNAs, and proteins. However, it should be noted that exosomes can still have off-target effects with non-specific biodistribution to unintended organs (liver, spleen, lungs, kidneys, and pancreas). For example, exosome-berberine/palmatine (Ber/Pal) can penetrate the heart and kidney besides the target brain [8]. Interestingly, the surfaces of exosomes can be modified or engineered for diseased cell- or tissue-specific targeting. For example, engineered exosomes were produced by hippocampal neuronal cells by transfection of the Fe65 overexpressing plasmid, followed by exosomes expressing Fe65 on their surface to interact with amyloid precursor protein (APP) in Alzheimer's disease (AD) cells, strengthening the ability of exosomes to internalize by AD diseased cells [10]. Moreover, the attendant problem is whether engineered exosomes that preserve the basic features of natural exosomes, such as low immunogenicity, should be explored in the future. Fourth, exosomes derived from different cell sources have different characteristics, such as the transmission of Aβ as well as hyperphosphorylated tau cleavage products contained in exosomes secreted by AD neuronal cells AD [95]; tumor cell-derived exosomes can spread various components, including gene, protein, and cytokines to affect other types of cells [96]. In addition to encapsulated compounds, their characteristics can affect diseases. Therefore, the selection of exosomes from appropriate cell sources plays a crucial role in their use as drug carriers. Fifth, even given the recent developments in exosome technology, large-scale production of exosomes is still not employed, and the low yield of cell-derived exosomes and the long preparation time limit their use in clinical treatment. For example, every 2.13 × 108 cells produced 1 mg of exosomes [8]. Furthermore, the composition of exosomes exhibits high heterogeneity, due to differences in cell sources and physiological and pathological states. Therefore, it is essential to develop technologies that can increase exosome productivity and reduce costs. Interestingly, some studies have shown that the yield of exosomes can be dramatically increased dozens of times through low-level electrical treatment (0.34 mA/cm2) and production boosters, revealing a promising exosome extraction method [97]. Some studies have suggested that conditioned media from the stem cell industry could be a stable source and that immortalized mesenchymal stem cell (MSC) cell lines could be used to replace primary cells to improve the yield, quality, and homogeneity of exosomes [98].
3.7. Comparisons of exosomes and other synthetic nanoparticles like liposomes
Recently, exosomes have gained considerable attention as a novel type of bionanoparticle for drug delivery compared to synthetic nanoparticles, such as synthetic polymeric and lipidic nanoparticles (liposomes), which are associated with high production costs, reproducibility issues, toxicity, and high immunogenicity. Similar drug-delivery systems have been extensively explored as nanocarriers for drug delivery. Liposomes are composed of lipids, mainly phospholipids, which have a hydrophilic head and hydrophobic tail to form a bilayer structure that encloses an aqueous space [99]. Exosomes offer several advantages over liposomes in drug delivery. First, as natural endogenous nanocarriers, exosomes exhibit excellent biocompatibility and bioavailability, reducing the risk of immune rejection and toxicity compared to liposomes, which can be immunogenic due to their synthetic nature [100]. Second, exosomes possess targeting specificity due to the presence of transmembrane and membrane-anchored proteins that facilitate endocytosis and interaction with specific cell types, thus improving the delivery of their cargo to target cells [101]. Third, unlike liposomes, which are manually equipped with cargo, exosomes inherit bioactive materials from their parent cells that can have therapeutic benefits [102]. Fourth, exosomes functionality can be improved through preconditioning (cytokines, hypoxia/hypoxia, drugs, and physical interventions) or engineering, further enhancing their therapeutic potential [103].
However, exosomes also have several disadvantages compared to liposomes in drug delivery. First, the yield of exosomes is much lower than that of liposomes. Exosome production is limited by the capacity of parent cells to secrete them, making large-scale production challenging and costly [104]. Second, exosomes are naturally packed with proteins and nucleic acids, making it difficult to load them with the desired therapeutic cargo, resulting in lower cargo-loading efficiency compared to liposomes [101]. Third, exosomes can be heterogeneous in size and composition, affecting their stability, biodistribution, and therapeutic efficacy. The quality control of exosomes is more complex than that of liposomes due to their endogenous origin and variability in composition and properties [105]. Fourth, although exosomes can be engineered to improve their functionality, the process is complex and requires a deep understanding of the molecular mechanisms involved in exosome biogenesis and function [94]. Fifth, the regulatory landscape of exosome-based therapeutics is still evolving, and there may be challenges in defining standards for their production, characterization, and clinical use.
4. The potential effect of an exosome loaded with TCM monomers in neuronal diseases
4.1. Exosome-encapsulated TCM monomer in AD
AD is a highly influential neurodegenerative disease characterized by plaques, tau phosphorylation, and neurodegeneration [106]. Individuals with AD experience severe memory deficits and cognitive impairments, with gradual deterioration in all aspects of brain function. Currently, no fully effective medications have been approved for clinical treatment due to the complexity of AD, which is caused by multiple factors [107]. Mainstream treatments for AD involve the use of cholinesterase inhibitors, such as donepezil, galantamine, and N-methyl-d-aspartate-receptor antagonists, as well as glutamate receptor antagonists, such as rivastigmine and memantine [108]. However, these drugs provide only temporary relief from symptoms and cause serious adverse reactions. Therefore, there is an urgent need to identify new and effective drugs for the treatment of AD. In view of the complexity of AD, TCM monomers often have low toxicity and rare adverse effects, providing safe and effective options for drug development; however, their application is limited by the BBB [109]. Moreover, exosomes, as natural delivery vectors, have better biocompatibility than other synthetic nanocarriers and can cross the BBB by carrying TCM monomers to target the brain. This property makes exosomes a promising research direction for drug delivery, particularly for the treatment of AD [110].
The contents loaded into exosomes were confirmed to possess enhanced bioavailability and can be applied at a lower, non-toxic concentration than those of the compounds alone for therapeutic effects. The TCM monomers baicalin (Bai), hederagenin (Hed), and neferine (Nef) were isolated from Scutellaria baicalensis Georgi, Hedera nepalensis K. Koch var. sinensis, and Nelumbo nucifera Gaertn, respectively. They were loaded separately into exosomes and then can dramatically reduce the level of neurodegenerative disease proteins, including huntingtin 74 (HTT74), P301L tau, and A53T α-synuclein and increase autophagy and facilitate autophagic degradation of mutant proteins compared to monomers alone. Furthermore, exosome-encapsulated Nef (10 mg/kg) could significantly eliminate Aβ (1−42) aggregates, prevent their formation in vitro, and improve the learning and memory ability of APP/PS1 double transgenic mice in vivo [11]. Zhao et al. [8] explored the roles of exosomes encapsulated Ber and Pal, which are isoquinoline alkaloids and the main extracts of Rhizoma Coptidis, and proved to be more effective than the free Ber/Pal group in the treatment of AD. Microglia-derived exosomes were isolated and purified using SEC and then coloaded with Ber and Pal into exosomes (exosomes-Ber/Pal, 10 mg/kg; Ber, 1.22 mg/kg; and Pal, 1.5 mg/kg) using ultrasonication combination with the freeze-thaw cycle method. Microglia-derived exosomes coloaded with the Ber/Pal delivery vehicle improved targeting and penetrating of drugs into the brain and strengthened the therapeutic role of Ber/Pal, including inhibiting expression levels of inflammatory cytokine-nitric oxide (NO), TNF-α, interleukin-1β (IL-1β), IL-4, and IL-1, protecting neurons, reducing synaptic damage, and ameliorating Aβ pathological symptoms. The inflammatory response of microglia was suppressed by Pal/Ber by prohibiting the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) by blocking the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) and mitogen-activated protein kinase (MAPK) signaling pathway; meanwhile, activating the adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) pathway [8]. Furthermore, curcumin (Cur) was isolated from Curcuma longa rhizomes and has been identified as a natural polyphenol with the ability to regulate tau protein phosphorylation [111]. To address the challenges in the solubility and bioavailability of Cur, researchers have treated mouse macrophages with Cur and produced Cur-containing exosomes (Exosome-Cur) from these cells [112]. This strategy significantly improved the solubility and stability of Cur, while improving its bioavailability in tissues [13]. The therapeutic efficacy of Exosome-Cur was evaluated in the okadaic acid (OA)-induced AD animal model. Exosome-Cur (100 μg/mL of Cur for seven consecutive days of injection) can activate the neuronal survival signaling pathway-Akt/glycogen synthase kinase-3β (GSK-3β) and further inhibit tau phosphorylation, thus preventing neuronal death both inside and outside the cell and alleviating the symptoms associated with AD [13]. Engineered exosomes have been constructed to increase the ability of the brain to target and cross the BBB. Iyaswamy et al. [10] designed Fe65 overexpressing exosomes on their surface derived from hippocampal neurons (Fe65-Exosome), which can target APP overexpressed-neuron cells under AD conditions in vivo or in vitro. Fe65-Exosome encapsulated corynoxine-B (Cory-B) (Fe65-Exosome-Cory-B; 20 mg/kg), a natural autophagy inducer isolated from Uncaria rhynchophylla, allowed APP-targeted delivery of Cory-B through APP receptor-dependent endocytosis and induced autophagy through beclin-1 (BECN1), autophagy-related 5 (Atg5), and autophagy-related 7 (Atg7) of APP overexpression neuronal cells by restraining the natural interaction between Fe65 and APP, ultimately decreased AP pathology and improved cognitive and locomotor behavior in AD mice [10]. The design of these novel drug delivery systems not only improves the bioavailability of the TCM monomer, but also opens up new possibilities for the precise treatment of AD (Fig. 3).
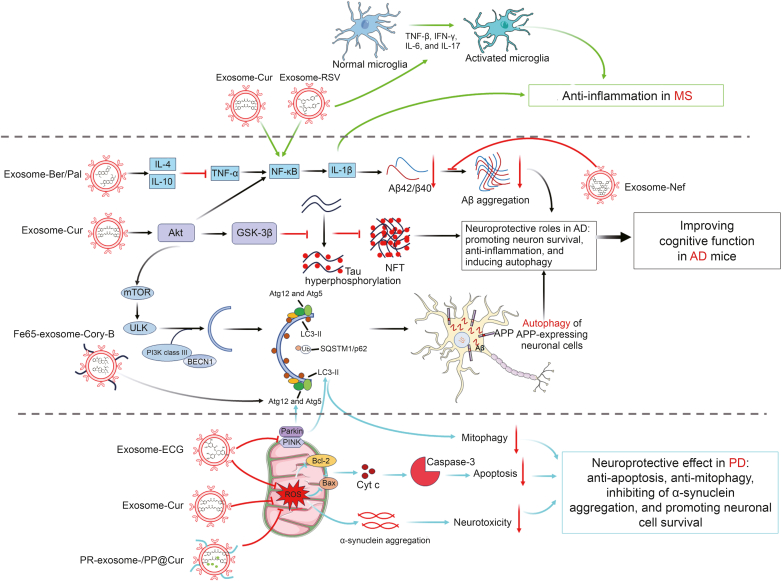
Fig. 3.
Integrative figure of molecular mechanism of traditional Chinese medicine (TCM) monomers-loaded exosomes for the treatment of neurodegenerative disease. (A) Curcumin (Cur) and resveratrol (RSV) encapsulated into exosomes exerted their anti-inflammation functions via weakening the expressions of tumor necrosis factor-β (TNF-β), interferon-γ (IFN-γ), interleukin-6 (IL-6), IL-17, which are associated with the activation of microglia in multiple sclerosis (MS). (B) Berberine (Ber)/palmatine (Pal), Cur, corynoxine-B (Cory-B), and neferine (Nef) encapsulated into naïve exosome or engineered exosomes played neuroprotective roles in Alzheimer's diseases (AD), including anti-inflammation, pro-survival, and pro-autophagy through inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and activating protein kinase B (Akt) signaling pathway. (C) Cur or epicatechin gallate (ECG) encapsulated into naïve exosome or engineered exosomes played neuroprotective roles in Parkinson's disease (PD), including anti-apoptosis, anti-mitophagy, and pro-survival via inhibiting reactive oxygen species (ROS) induced apoptosis and putative kinase 1 (PINK)/Parkin mediated autophagy, ultimately depressed α-synuclein aggregation and neurotoxicity in PD conditions. GSK-3β: glycogen synthase kinase-3β; NFT: neurofibrillary tangles; mTOR: mammalian target of rapamycin; ULK: Unc-51 like autophagy activating kinase 1; PI3K: phosphatidylinositol 3-kinase; BECN1: beclin-1; Atg12: autophagy-related 12; LC3-II: microtubule-associated protein-1 light chain 3-II; Ub: ubiquitin; SQSTM1/P62: sequestosome-1; APP: amyloid precursor protein; Bcl-2: B-cell lymphoma-2; Bax: Bcl-2-associated X protein; Cyt c: cytochrome c.
4.2. Exosome-encapsulated TCM monomer in Parkinson's disease (PD)
PD is the second most common neurodegenerative disorder. The neuropathological hallmarks of PD are that severe impairment of dopaminergic projection neurons in the substantia nigra (SN) causes deficiency of dopaminergic innervation in the striatum and is accompanied by accumulation of intracellular inclusions containing α-synuclein (called Lewy bodies) in other brain tissues, ultimately resulting in deterioration of motor functions [113]. The most common treatment strategy for PD is based on the pharmacological substitution of striatal dopamine with enzyme inhibitors and levodopa and involves deep brain stimulation through surgery [114]. However, long-term drug use generally reduces efficacy and produces side effects in patients with PD. Currently, there is no effective treatment to prevent or terminate the pathological processes of PD [115]. One of the greatest challenges is the development of novel drugs with good therapeutic effects and fewer adverse effects.
The enhancement of glial cell activation, oxidative stress, and inflammation is closely associated with the pathological process of PD. Various studies have shown that Cur has anti-inflammatory and antioxidant effects in neurodegenerative diseases [116]. Cur was loaded into exosomes derived from human endometrial stem cells (hEnSCs) (exosome-Cur), whose roles were detected in a 6-hydroxydopamine hydrochloride (6-OHDA)-induced PD mice model by intranasal injection. hEnSCs exosome-Cur (10 mg/kg) can decrease brain inflammation, α-synuclein aggregation, and cell apoptosis in the dopaminergic tyrosine hydroxylase (TH)-positive neuron, as well as improve the impaired learning and memory ability in PD mice [14]. Additionally, epicatechin gallate (ECG), one of the most effective catechins present in various plants and foods, was incorporated into fresh bovine milk-derived exosomes (exosome-ECG). Exosome-ECG showed more effective anti-apoptosis protective functions, including reducing the apoptosis rate and the expression of caspase-3, B-cell lymphoma-2 (Bcl-2)-associated X protein (Bax), and anti-mitophagy, including downregulated expression of parkin/phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1) and Atg5, compared to free ECG in the rotenone-induced PD cell model [21]. Furthermore, Peng et al. [15] constructed a self-oriented nanocarrier with a three-pronged synergistic treatment for PD. The micellar core was self-assembled by reactive oxygen species (ROS)-responsive amphiphilic polymer poly (propylene sulfide) (PPS)-PEG loading Cur and superparamagnetic iron oxide nanoparticles (SPIONs) (PP@Cur), and the outer core was MSC-derived exosomes modified with octadecyl chains-penetratin (P) (SA-p) and -rabies virus glycoprotein (SA-RVG29) peptides (PR-exosome). The PR-exosome/PP@Cur nanocarrier could cross the BBB and target the SN. This was facilitated by the MSC-derived exosome shell that promoted drug migration to the lesion, and the Cur concentration in the SN of PD mice administered PR-exosome/PP@Cur was 67.26 μg/g, which was higher than that of wild-type mice. Furthermore, Cur was accurately delivered to dopaminergic neurons by PR-exosome/PP@Cur nanocarriers and played a neuroprotective role by improving nerve axon growth and decreasing the α-synuclein aggregation-induced neurotoxicity, ultimately improving movement behavior and coordination ability of PD mice [15]. Therefore, the drug delivery and curative effects of TCM monomers in PD improved greatly when loaded into exosomes compared to free drugs (Fig. 3).
4.3. Exosome-encapsulated TCM monomer in multiple sclerosis (MS)
As the most common inflammatory disease of the CNS, MS is generally characterized by demyelination and neuronal damage and loss, which can eventually lead to neurological dysfunction [117]. It is caused by autoimmune reactions to self-antigens. Clinical symptoms vary depending on the location of nervous system lesions. Generally, it is related to BBB erosion by inflammatory cells [118].
Their limited ability to cross the BBB is one of the obstacles to traditional cell therapy and drug therapy in the treatment of MS, while exosomes can freely cross the BBB and diffuse into the blood. Furthermore, its non-immunogenicity endows exosomes with good stability and long-lasting systemic circulation and does not cause cytotoxicity, with greater safety [119]. Cur loaded in exosomes (exosomes-Cur, 1.5 nmol in 10 μL PBS) can significantly reduce neuroinflammation by targeting activated microglia and reducing the number of microglial cells by nasal administration in lipopolysaccharide (LPS) induced mice and myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis mice [16]. Although exosomes encapsulated in TCM drugs have good therapeutic effects on brain inflammation, their distribution in the CNS requires further exploration. Zheng et al. [22] first designed and synthesized five N-acyl side chain sialic acid analogs of different lengths and then screened the best metabolic precursors for exosome labeling through biorthogonal click chemistry. Then, they found that macrophage exosomes had obvious fluorescence signals and long residence times in the brain and spinal cord and were strongly colocalized with microglia. Based on this finding, microglia-derived exosome-encapsulated resveratrol (RSV&exosome) was further developed for the treatment of MS, and intranasal administration of RSV&exosome (3 mg/kg RSV, 30 μL) significantly decreased the expression of inflammatory factors, including transforming growth factor-β (TGF-β), interferon-γ (IFN-γ), IL-1β, IL-6, and IL-17, further inhibited the inflammatory response mediated by activated microglia and NF-κB, ultimately suppressed weight loss and improved behavior of EAE mice [22]. Furthermore, spinal cord injury (SCI) caused by irreversible motor neurons is associated with immune activation of resident macrophages/microglia. Ber loaded into M2-type primary peritoneal macrophage-derived exosomes (exosomes-Ber) using an ultrasonic method plays a crucial role in mediating its anti-inflammatory effects. Exosomes-Ber can reduce the M1 protein marker inducible NO synthase (iNOS) and apoptotic cytokines (TNF-α, IL-1β, IL-6, caspase-9, and caspase-8) and improve the M2 protein marker mannose receptor (CD206) in vitro. Exosome-Ber plays an important the anti-inflammatory and anti-apoptotic effect by inducing macrophages/microglia from the M1 phenotype to M2 phenotype polarization by reducing the expressions of the M1 protein marker iNOS and apoptotic cytokines (TNF-α, IL-1β, IL-6, caspase-9, and caspase-8) but enhancing the expression of the M2 protein marker CD206 in vitro, ultimately alleviated motor function in SCI mice model with exosomes-Ber injection (5 mg/kg) [9]. Therefore, the development of endogenous immunocytes as delivery vehicles offers new opportunities for inflammatory brain disease treatment, providing experimental evidence for the development of novel exosome-based diagnostics and therapeutics in the future (Fig. 3).
4.4. Exosome-encapsulated TCM monomer in cerebral ischemia and reperfusion (CI/R) injury
Ischemic stroke is an acute cerebrovascular disease characterized by sudden onset and focal neurological deficits, accounting for 85% of all cerebral strokes [120]. It is one of the leading causes of death worldwide, with high rates of disability, morbidity, and recurrence. Approximately 6 million people die from stroke each year, and the lifetime risk of stroke is estimated to range from 8% to 10% [120]. The main cause of ischemic stroke is CI/R injury, a pathological condition characterized by recovery of blood supply to the brain after restriction; however, the concomitant reoxygenation process can cause further damage [27]. Thrombolysis therapy is currently a common clinical method for restoring blood and oxygen supply to the damaged brain; however, sudden recanalization of an occluded artery can produce ROS, trigger an inflammatory response, and lead to tissue cytotoxicity and CI/R injury [121]. The recombinant tissue plasminogen activator (rtPA) is the only drug approved by the U.S. Food and Drug Administration (FDA) to treat acute cerebral infarction. However, many patients are unable to benefit from this treatment due to its narrow treatment time window and association with hemorrhagic complications [122].
Numerous studies have indicated that ROS rapidly accumulates after ischemic stroke. These ROS further exacerbate brain damage by inducing mitochondrial-mediated apoptosis and BBB impairment. The timely clearance of ROS can help alleviate these injuries and promote recovery of brain function [17]. Therefore, strategies to reduce ROS production are generally adopted to promote recovery after ischemic stroke. Quercetin (Que), a natural flavonoid polyphenol widely found in various foods, exerts antioxidant effects by removing free radicals. Que incorporated into monoclonal antibody (mAb) growth-associated protein-43 (GAP43) coupled exosomes (mAb GAP43 exosome-Que) can improve neuronal survival by specifically targeting damaged neurons and interacting with GAP43, which is expressed in damaged neurons via a monoclonal antibody against GAP43 (mAb GAP43). mAb GAP43 exosome-Que (3.4 mg/mL injection) activated the nuclear factor erythroid-2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway to inhibit ROS production, reduced infarct volume, and resulted in more significant neurological recovery compared to free Que or Que carrying exosomes treatment in the middle cerebral artery occlusion/reperfusion (MCAO/R) induced rat [23]. Moreover, Bai incorporated into macrophage-derived exosomes (exosome-Bai, containing Bai at a concentration of 1.6 mg/mL injection) also exerted its therapeutic role by reducing ROS production and inhibiting neuronal cell apoptosis through upregulation of the Nrf2/HO-1 pathway in MCAO rats [12]. The anti-inflammatory effect of Cur was further strengthened by its incorporation into embryonic stem cell-derived exosomes (mouse embryonic stem cell (MESC)-exosome-Cur) or macrophage-derived exosomes (exosomes-Cur), which can target the ischemic region driven by inflammation. MESC-exosome-Cur (total 10 μL injection, Cur:Exos = 1:4) or Exos-Cur (Cur: 10 μg/mL) can dramatically reduce ROS accumulation in lesions and inhibit BBB damage by increasing the expression of the tight junction protein-claudin-5/occludin and mitochondrial-mediated neuronal apoptosis, which is confirmed by the downregulated expressions of Bax, cleaved caspase-3, and release of cytochrome c (Cyt c), and normalize astrocytes and neuronal expression in MCAO animal model [17,18].
To improve the targeting ability of exosomes for clinical applications, engineered cyclo(Arg-Gly-Asp-D-Tyr-Lys) peptide (c(RGDyK))-conjugated exosomes (cRGD-exosomes) were constructed using click chemistry. cRGD-exosome can specifically target the ischemic brain depending on the high affinity of c(RGDyK) for the integrin αvβ3 in reactive cerebral vascular endothelial cells after ischemia. Cur packaged in cRGD-exosome (cRGD-exosome-Cur, 100 or 300 μg for different experiments) has a strong inhibition of the inflammatory response and cellular apoptosis in the lesion region in MCAO mice, which was confirmed by the downregulated expressions of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and pro-apoptotic protein-cleaved caspase-3 [19]. Luo et al. [123] successfully constructed cRGD-exosome-encapsulated ginsenoside Rg1 (G-Rg1) (cRGD-exosome-Rg1, 1 mg/mL injection), which could also effectively and specifically enter the ischemic brain of rats and exert neuroprotective effects by promoting angiogenesis and neurogenesis by activating the PI3K/Akt pathway [123]. Therefore, TCM monomers loaded onto exosomes can significantly decrease ROS generation, inhibit inflammation, and eventually suppress neuronal cell apoptosis induced by CI/R injury compared to drugs alone (Fig. 4).
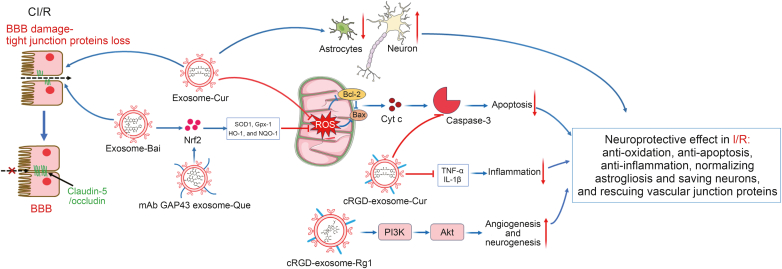
Fig. 4.
Integrative figure of molecular mechanism of traditional Chinese medicine (TCM) monomers-loaded exosomes for the treatment of cerebral ischemia and reperfusion (CI/R). Curcumin (Cur), baicalin (Bai), quercetin (Que), and ginsenoside Rg1 (Rg1) encapsulated into naïve or engineered exosomes played neuroprotective roles in CI/R injury, including anti-oxidation, anti-apoptosis, pro-survival of neurons, enhancing the structure integrity of blood-brain barrier (BBB), and anti-inflammation via inhibiting reactive oxygen species (ROS) induced neurons apoptosis, decreasing tumor necrosis factor-α (TNF-α)/interleukin-1β (IL-1β), activating phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway. Nrf2: nuclear factor erythroid-2-related factor 2; SOD1: superoxide dismutase 1; Gpx-1: glutathione peroxidase 1; HO-1: heme oxygenase-1; NQO1: NAD(P)H quinone dehydrogenase 1; mAb: monoclonal antibody; Bcl-2: B-cell lymphoma-2; Bax: Bcl-2-associated X protein; Cyt c: Cytochrome c; cRGD: cyclo(Arg-Gly-Asp).
4.5. Exosome-encapsulated TCM monomer in glioma
Brain cancer, especially glioma, has a low survival rate due to its aggressiveness and difficulty in treatment [124]. Currently, surgery, chemotherapy, radiotherapy, and immunotherapy, which are the main cancer treatment methods, face certain challenges and side effects when used alone or in combination. For example, brain surgery requires extreme precision to avoid damaging sensitive brain tissues, while the use of chemotherapy drugs is often limited by their low specificity, high toxicity, unstable efficacy, and drug resistance [125]. Brain cancer is extremely challenging to treat, mainly because the BBB not only helps prevent common bacterial infections, but also limits drug access to the brain [126]. Therefore, there is an urgent need to develop novel drugs and delivery tools that can cross the BBB to target brain tumor cells.
First-line chemotherapeutic agents, temozolomide (TMZ) and dihydrotanshinone (DHT), loaded into homologous glioma cell-derived exosomes (R-exosomes-TMZ/DHT), have been shown to reverse drug resistance and enhance lesion-targeted drug delivery. Moreover, R-exosomes-TMZ/DHT (the doses of TMZ and DHT were 1 mg/kg and 35 μg/kg) has synergistic anti-tumor effects, including activation of the immune system for immunotherapy, inhibition of glioma proliferation, and enhancement of glioma cell death with the combined treatment of TMZ and DHT in the orthotopic glioblastoma (GBM)-bearing mice model [24]. Furthermore, Cur and SPIONs encapsulated in neuropilin-1-targeted peptide (RGERPPR, RGE)-modified exosomes (RGE-exosome-SPION/Cur) have good glioma-targeting ability, mediated by the interaction between RGE and glioma cells overexpressing protein, neuronilin-1 (NRP-1), and good anti-tumor therapy efficiency with reduced side effects [20]. RGE-exosome-SPION/Cur (Cur concentration of 15 mg/mL) had a strong role in targeted imaging of glioma mediated by SPION and significantly reduced tumor size when treated with Cur, which showed a good synergistic effect of early diagnosis and precise evaluation of glioma [20]. Recently, synthetically bioengineered exosome-liposome hybrid nanovesicles have provided a novel strategy for drug delivery and brain diseases. These hybrid nanovesicles possess the advantages of exosomes, including their ability to cross biological barriers and the positive role of liposomes in improving the drug-loading capacity and stability of nanovesicles. A novel biomimetic nanovesicle, BBB penetrated exosomes-liposome hybrid nanovesicles (pHybrid), was designed for drug delivery to the brain via membrane fusion between blood exosomes and CGNKRTR peptide (tLyp-1) peptide-modified liposomes. pHybrid nanovesicles coloaded with salvianolic acid B (SAB) and cryptotanshinone (CPT) (the doses of SAB and CPT were 851.2 and 248.1 μg/kg) showed synergistic anti-tumor effects of the two drugs, including directly killing cancer cells and inhibiting the growth of new blood vessels in the tumor, providing a new approach for the treatment of glioma [25]. Interestingly, Rg3, the main active ingredient of ginseng, can be concurrently developed as an active ligand for BBB crossing and GBM targeting through interactions with the glucose transporter 1 (GLUT1) overexpressed in the BBB, depending on its glucosyl residues. Li et al. exploited a Rg3/arsenic trioxide (ATO)@chlorin e6 (Ce6) modified exosome (Rg3/ATO@Ce6-exosomes), which could actively cross the BBB and precisely target GBM in vivo, repress in-situ growth of tumor cells, improve the polarization of tumor-associated macrophages and M1/M2, strengthen infiltration of cytotoxic T lymphocytes, and significantly improve survival benefits. This nanoformulation improves the synergistic effect of chemotherapy and photoimmunotherapy, providing a new strategy for the precise treatment of gliomas using active TCM ingredients [127]. This technology has demonstrated the ability to use exosomes to encapsulate TCM and other substances for synergistic immunotherapy to overcome challenges such as low drug enrichment, poor lesion targeting, and immunosuppressive microenvironment barriers in the treatment of gliomas (Fig. 5).
Fig. 5.
Integrative figure of molecular mechanism of traditional Chinese medicine (TCM) monomer-loaded exosomes for the treatment of glioma. TCM monomers including dihydrotanshinone (DHT), curcumin (Cur), salvianolic acid B (SAB)/cryptotanshinone (CPT), and ginsenoside Rg3 (Rg3) coloaded into engineered exosomes with other components exerted synergistic anti-tumor roles, including anti-drug resistance, anti-tumorigenesis, anti-angiogenesis, and promoting apoptosis in glioma. TMZ: Temozolomide; IL-1β: Interleukin-1β; TNF-α: Tumor necrosis factor-α; RGE: RGERPPR; SPION: superparamagnetic iron oxide nanoparticle; SHP2: Src homology-2 domain-containing protein tyrosine phosphatase 2; STAT3: Signal transducer and activator of transcription 3; VEGF: Vascular endothelial growth factor; ATO: arsenic trioxide; Ce6: chlorin e6; BBB: blood-brain barrier.
5. Conclusions and perspective
In general, the capacities of TCMs, including bypassing the BBB, advanced accumulation in the lesion region, and increased bioactivity and bioavailability, were strengthened by loading them into exosomes compared to free TCMs (Fig. 2). Exosome encapsulated TCM monomers were injected into rats or mice through an intranasal or intravenous delivery pathway, which can cross the BBB to target diseased brain regions and exert anti-inflammation, anti-apoptosis, anti-mitophagy, and anti-oxidation effects in AD, PD, MS, and I/R, as well as anti-drug resistance, anti-tumorigenesis, anti-angiogenesis, and apoptosis promotion in GBM (Fig. 3, Fig. 4, Fig. 5). TCMs, including Ber/Pal, Cur, and Cory-B exerted their protective roles in reducing Aβ pathological symptoms by enhancing clearance of Aβ plaques, diminishing the formation of neurofibrillary tangles (NFT) by inhibiting tau hyperphosphorylation and eliminating APP overexpression neurons major, inhibiting TNF-α/NF-κB signaling pathway, activating AKT/GSK-3β signaling pathway, and promoting PI3K class III/Atg5,7/microtubule-associated protein-1 light chain 3-II (LC3-II) mediated autophagy, which ultimately improved cognitive function of AD. Interestingly, exosome-ECG inhibited autophagy to protect neuronal cells under PD conditions by significantly inhibiting the PINK/Parkin/Atg5 mitophagy pathway. Additionally, exosome-Cur and exosome-ECG can weaken neurotoxicity by removing aggregating of α-synuclein, depressing neuroinflammation and promoting neuronal cell survival by prohibiting ROS and its mediated Cyt c/caspase-3 apoptosis signaling pathways, ultimately improving the ability of movement behavior and coordination in PD mice. Exosome-RSV and exosome-Cur decreased inflammatory responses by targeting NF-κB, TGF-β, IFN-γ, IL-1β, IL-6, and IL-17 in EAE mice. Moreover, exosome-Que, Exo-Bai, and exosome-Cur exerted neuroprotective effects in I/R, including anti-oxidation, anti-apoptosis, normalizing astrogliosis, saving neurons, and rescuing vascular junction proteins, mainly through activation of Nrf2/superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (Gpx-1), HO-1, and NAD(P)H quinone dehydrogenase 1 (NQO1) to remove ROS, thus increasing the expression levels of tight junction proteins-claudin-5/occludin. Furthermore, exosome-TMZ/DHT, RGE-exosome-SPION/Cur, and pHybrid/SAB-CPT have an anti-tumor effect in GBM by inhibiting drug resistance, activating the immune system, promoting tumor cell death, and restraining the development of tumor by enhancing IL-1β, IL-6, and TNF-α, upregulation of the Src homology-2 domain-containing protein tyrosine phosphatase 2 (SHP-2) with following inhibition on phosphorylation of signal transducer and activator of transcription 3 (STAT3) signal pathway.
Undoubtedly, the most commonly TCM monomer encapsulated into exosome is Cur, which exerts the functions of anti-inflammation, anti-oxidization, and anti-tumor through Akt, NF-κB, and ROS mediated apoptosis in AD, PD, MS, CI/R, and glioma. Its neuroprotective role is dramatically enhanced when loaded into naïve or engineered exosomes. Moreover, one beneficial part of the encapsulation in exosomes can be loaded with two monomers or other compounds, which is beneficial for synergistic therapeutic effects. For example, RGE-TMZ/DHT can activate the immune system to suppress the development of tumors while alleviating the first-line chemotherapeutic agent-TMZ resistance in glioma cells, and RGE-exosome-SPION/Cur has a strong ability for targeted imaging of glioma, which can be used for early diagnosis due to SPION for magnetic resonance imaging (MRI) and anti-tumor effects mediated by Cur. Furthermore, exosomes can be modified or engineered to enhance their ability to cross the BBB and target the brain. For example, PR-Exosome/PP@Cur possessed a three-pronged synergistic treatment for PD; the outer core was MSC-derived modified exosomes to promote drug migration to the lesion, and the inner core of SPION was applied as MRI for guiding therapy and Cur for clearing α-synuclein aggregates.
The exosomes introduced in this review for the treatment of various neurological diseases were derived from various cell types, including tumor cells (neuron-2a, PC-12, EL-4, and GL261), microglial cells (RAW264.7), stem cells (hEnSCs and MESCs), blood, and fresh milk (Table 3) [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]]. TCMs encapsulated in exosomes have better biocompatibility and bioavailability and lower toxicity and immunogenicity than free TCMs treatment. However, exosomes have different properties because they inherit bioactive ingredients from their donor cells, which implies that some exosomes possess other functions in neuronal diseases in addition to being TCM delivery carriers. Exosomes can facilitate the crossing of the BBB, such as naïve macrophage exosomes, which promote TCM to bypass the BBB through the interaction between LFA-1 and endothelial ICAM-1 with brain microvessel endothelial cells, which comprise the BBB [13]. Exosomes can possess neuroprotective functions that are equally beneficial as the originating cells, such as embryonic stem cell-derived exosomes loaded with abundant paracrine factors of stem cells, which play crucial roles in improving functional recovery, neurovascular plasticity, neurodegeneration, and regulation of peripheral post-stroke immune responses [18]. In addition, macrophage-derived exosomes can target ischemic regions depending on the migration ability of inflammation-driven exosomes [17]. Tumor-derived exosomes have the characteristic of tumor-homing accumulation, as they contain the same adhesion proteins as tumor cells. In contrast, exosomes can spread detrimental factors, such as exosomes derived from neuron-2a and PC-12 cells were found to play a neuroprotective role on Aβ degradation or synaptic pruning stimulation, respectively; however, a recent study suggested exosomal prion release in neuron-2a cells [11]. In summary, exosomes derived from different cell types exhibit distinct properties.
Traditional drug therapies for brain diseases have many limitations, including the high selectivity of the BBB. It strictly controls the molecules entering and leaving the brain tissue, resulting in 98% of small-molecule drugs. Almost all macromolecular drugs cannot cross the BBB, and the content of drugs in the brain is only 0.01%−0.1% of that in the plasma [128]. Exosomes can only alleviate the pain associated with traditional treatment. Due to their small size and endogenous characteristics, exosomes can avoid capture and clearance by the reticuloendothelial system to achieve the purpose of crossing the blood-brain or placental barrier and achieving a targeted effect. As mentioned in this article, the homing ability of exosomes in tumor tissues is nearly ten times higher than that of traditional liposomes as drug carriers [89]. Exosomes play an important role in cellular communication dependent on various adhesive proteins on their surface, making it possible for exosomes to target cells and deliver various drugs encapsulated within them. In addition, EVs can be modified to construct targeted fragments on their surface, better fuse with receptor target cells, and release contents or drugs into the interior of the cell [129]. In addition to the unique advantage of shuttling in vivo, exosomes are suitable for drug encapsulation. Exosomes have a stable lipid bilayer structure with the same topology as cells (inside-in and outside-out). The microphase of the hydrophobic region in the middle of the lipid layer can encapsulate hydrophobic drugs and the internal cavity can carry a large number of water-soluble drugs (insight into the exosomal membrane: from viewpoints of membrane fluidity and polarity) [130]. This special structure can protect its contents from degradation or dilution in harsh extracellular environments for a long time.
As mentioned above, the scope of clinical applications has expanded with technological developments. In addition to the brain diseases described in this article, exosomes have unique advantages in terms of anti-aging, anti-cancer, and other aspects. In the near future, exosomes may become a new way for people to fight against diseases.
CRediT authorship contribution statement
Chen Pang: Writing – review & editing, Investigation. Jie Zhang: Writing – original draft, Investigation. Yujin Gu: Writing – original draft, Investigation. Qili Zhang: Writing – review & editing. Yanfang Zhao: Writing – review & editing, Writing – original draft.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (Grant No: 31900694).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Qili Zhang, Email: qili_0223@sdut.edu.cn.
Yanfang Zhao, Email: zhyf@sdut.edu.cn.
References
- 1.Wang Y.Y., Zhang H.M. Philosophical basis of original thinking in Chinese medicine. Zhonghua Yi Shi Za Zhi. 2017;47:259–261. doi: 10.3760/cma.j.issn.0255-7053.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 2.P U. Unschuld. Ii. the meaning of the title Huang di Nei Jing su Wen. Huang Di Nei Jing Su Wen. University of California Press; 2019. p. 21. [Google Scholar]
- 3.Xu J., Yang Y. Traditional Chinese medicine in the Chinese health care system. Health Pol. 2009;90:133–139. doi: 10.1016/j.healthpol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Hao Y., Yuan L., et al. Ferroptosis: A new mechanism of traditional Chinese medicine for treating ulcerative colitis. Front. Pharmacol. 2024;15 doi: 10.3389/fphar.2024.1379058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong X., Sucher N.J. Stroke therapy in traditional Chinese medicine (TCM): Prospects for drug discovery and development. Mov. Disord. Clin. Pract. 1999;20:191–196. doi: 10.1016/s0165-6147(98)01276-0. [DOI] [PubMed] [Google Scholar]
- 6.Jaiswal Y., Liang Z., Zhao Z. Botanical drugs in ayurveda and traditional Chinese medicine. J. Ethnopharmacol. 2016;194:245–259. doi: 10.1016/j.jep.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 7.Jin W., Zhou T., Li G. Recent advances of modern sample preparation techniques for traditional Chinese medicines. J. Chromatogr. A. 2019;1606 doi: 10.1016/j.chroma.2019.460377. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X., Ge P., Lei S., et al. An exosome-based therapeutic strategy targeting neuroinflammation in Alzheimer's disease with berberine and palmatine. Drug Des. Dev. Ther. 2023;17:2401–2420. doi: 10.2147/DDDT.S417465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Z., Zhang C., Xia N., et al. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021;126:211–223. doi: 10.1016/j.actbio.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Iyaswamy A., Thakur A., Guan X.J., et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer's disease. Signal Transduct. Targeted Ther. 2023;8 doi: 10.1038/s41392-023-01657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang B., Zeng W., Song L.L., et al. Extracellular vesicle delivery of neferine for the attenuation of neurodegenerative disease proteins and motor deficit in an Alzheimer's disease mouse model. Pharmaceuticals (Basel) 2022;15 doi: 10.3390/ph15010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z., Guo L., Huang L., et al. Baicalin-loaded macrophage-derived exosomes ameliorate ischemic brain injury via the antioxidative pathway. Mater. Sci. Eng., C. 2021;126 doi: 10.1016/j.msec.2021.112123. [DOI] [PubMed] [Google Scholar]
- 13.Wang H., Sui H., Zheng Y., et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale. 2019;11:7481–7496. doi: 10.1039/c9nr01255a. [DOI] [PubMed] [Google Scholar]
- 14.Mobahat M., Sadroddiny E., Nooshabadi V.T., et al. Curcumin-loaded human endometrial stem cells derived exosomes as an effective carrier to suppress alpha-synuclein aggregates in 6OHDA-induced Parkinson's disease mouse model. Cell Tissue Bank. 2023;24:75–91. doi: 10.1007/s10561-022-10008-6. [DOI] [PubMed] [Google Scholar]
- 15.Peng H., Li Y., Ji W., et al. Intranasal administration of self-oriented nanocarriers based on therapeutic exosomes for synergistic treatment of Parkinson's disease. ACS Nano. 2022;16:869–884. doi: 10.1021/acsnano.1c08473. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang X., Xiang X., Grizzle W., et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He R., Jiang Y., Shi Y., et al. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater. Sci. Eng., C. 2020;117 doi: 10.1016/j.msec.2020.111314. [DOI] [PubMed] [Google Scholar]
- 18.Kalani A., Chaturvedi P., Kamat P.K., et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 2016;79:360–369. doi: 10.1016/j.biocel.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian T., Zhang H., He C., et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Jia G., Han Y., An Y., et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. doi: 10.1016/j.biomaterials.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Luo S., Sun X., Huang M., et al. Enhanced neuroprotective effects of epicatechin gallate encapsulated by bovine milk-derived exosomes against Parkinson's disease through antiapoptosis and antimitophagy. J. Agric. Food Chem. 2021;69:5134–5143. doi: 10.1021/acs.jafc.0c07658. [DOI] [PubMed] [Google Scholar]
- 22.Zheng X., Sun K., Liu Y., et al. Resveratrol-loaded macrophage exosomes alleviate multiple sclerosis through targeting microglia. J. Contr. Release. 2023;353:675–684. doi: 10.1016/j.jconrel.2022.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Guo L., Huang Z., Huang L., et al. Surface-modified engineered exosomes attenuated cerebral ischemia/reperfusion injury by targeting the delivery of quercetin towards impaired neurons. J. Nanobiotechnol. 2021;19 doi: 10.1186/s12951-021-00879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R., Liang Q., Zhang X., et al. Tumor-derived exosomes reversing TMZ resistance by synergistic drug delivery for glioma-targeting treatment. Colloids Surf. B Biointerfaces. 2022;215 doi: 10.1016/j.colsurfb.2022.112505. [DOI] [PubMed] [Google Scholar]
- 25.Wang R., Wang X., Zhao H., et al. Targeted delivery of hybrid nanovesicles for enhanced brain penetration to achieve synergistic therapy of glioma. J. Contr. Release. 2024;365:331–347. doi: 10.1016/j.jconrel.2023.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y., Ouyang Z., Du H., et al. New opportunities and challenges of natural products research: When target identification meets single-cell multiomics. Acta Pharm. Sin. B. 2022;12:4011–4039. doi: 10.1016/j.apsb.2022.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng T., Jiang T., Huang Z., et al. Role of traditional Chinese medicine monomers in cerebral ischemia/reperfusion injury: A review of the mechanism. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1220862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L. Pharmacokinetics and drug delivery systems for puerarin, a bioactive flavone from traditional Chinese medicine. Drug Deliv. 2019;26:860–869. doi: 10.1080/10717544.2019.1660732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nie S., Zhang H., Li W., et al. Current development of polysaccharides from Ganoderma: Isolation, structure and bioactivities. Bioact. Carbohydr. Diet. Fibre. 2013;1:10–20. [Google Scholar]
- 30.Cheng S., He F., Fu L., et al. Polysaccharide from rubescens: Extraction, optimization, characterization and antioxidant activities. RSC Adv. 2021;11:18974–18983. doi: 10.1039/d1ra01365c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma T., Sun X., Tian C., et al. Polysaccharide extraction from Sphallerocarpus gracilis roots by response surface methodology. Int. J. Biol. Macromol. 2016;88:162–170. doi: 10.1016/j.ijbiomac.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Xiong X., Huang G. Ultrasound-assisted extraction and analysis of maidenhairtree polysaccharides. Ultrason. Sonochem. 2023;95 doi: 10.1016/j.ultsonch.2023.106395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Y., Li Q., Mao G., et al. Optimization of enzyme-assisted extraction and characterization of polysaccharides from Hericium erinaceus. Carbohydr. Polym. 2014;101:606–613. doi: 10.1016/j.carbpol.2013.09.099. [DOI] [PubMed] [Google Scholar]
- 34.Thomas L., Parameswaran B., Pandey A. Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp. for bioethanol production. Renew. Energy. 2016;98:9–15. [Google Scholar]
- 35.You Q., Yin X., Zhao Y. Enzyme assisted extraction of polysaccharides from the fruit of Cornus officinalis. Carbohydr. Polym. 2013;98:607–610. doi: 10.1016/j.carbpol.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 36.Zhang D., Wan Y., Xu J., et al. Ultrasound extraction of polysaccharides from mulberry leaves and their effect on enhancing antioxidant activity. Carbohydr. Polym. 2016;137:473–479. doi: 10.1016/j.carbpol.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J. Applications and prospects of ultrasound-assisted extraction in Chinese herbal medicine. Open Access J. Biomed. Sci. 2019;1 (5–15) [Google Scholar]
- 38.Li G., Chen D. Comparison of different extraction methods of active ingredients of Chinese medicine and natural products. J. Separ. Sci. 2024;47 doi: 10.1002/jssc.202300712. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y., Bi J., Zhang L., et al. Ultrasound-assisted extraction of three bufadienolides from Chinese medicine ChanSu. Ultrason. Sonochem. 2012;19:1150–1154. doi: 10.1016/j.ultsonch.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Huang H., Huang G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem. Biol. Drug Des. 2020;96:1209–1222. doi: 10.1111/cbdd.13794. [DOI] [PubMed] [Google Scholar]
- 41.Wang R., Wu G., Du L., et al. Semi-bionic extraction of compound turmeric protects against dextran sulfate sodium-induced acute enteritis in rats. J. Ethnopharmacol. 2016;190:288–300. doi: 10.1016/j.jep.2016.05.054. [DOI] [PubMed] [Google Scholar]
- 42.Wu W., Song L., Wang H., et al. Supercritical CO2 fluid extract from Stellariae Radix ameliorates 2, 4-dinitrochlorobenzene-induced atopic dermatitis by inhibit M1 macrophages polarization via AMPK activation. Environ. Toxicol. 2024;39:3188–3197. doi: 10.1002/tox.24145. [DOI] [PubMed] [Google Scholar]
- 43.Uwineza P.A., Waśkiewicz A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules. 2020;25 doi: 10.3390/molecules25173847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L., Wang X., Lu W., et al. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016;45:2137–2211. doi: 10.1039/c6cs00061d. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Zhao G., Han K., et al. Applications of molecular imprinting technology in the study of traditional Chinese medicine. Molecules. 2022;28 doi: 10.3390/molecules28010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X., Li J., Chen L. Advanced preparation technologies and strategies for molecularly imprinted materials. Chin. Sci. Bull. 2019;64:1352–1367. [Google Scholar]
- 47.Liu S., Yi L., Liang Y. Traditional Chinese medicine and separation science. J. Separ. Sci. 2008;31:2113–2137. doi: 10.1002/jssc.200800134. [DOI] [PubMed] [Google Scholar]
- 48.Lai J.J., Chau Z.L., Chen S.Y., et al. Exosome processing and characterization approaches for research and technology development. Adv Sci Weinh. 2022;9 doi: 10.1002/advs.202103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doyle L.M., Wang M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8 doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gurung S., Perocheau D., Touramanidou L., et al. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021;19 doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Y., Duan L., Lu J., et al. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Bi J., Huang J., et al. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai X., Ye Y., He F. Emerging innovations on exosome-based onco-therapeutics. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.865245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song Y., Kim Y., Ha S., et al. The emerging role of exosomes as novel therapeutics: Biology, technologies, clinical applications, and the next. Am. J. Reprod. Immunol. 2021;85 doi: 10.1111/aji.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kilchert C., Wittmann S., Vasiljeva L. The regulation and functions of the nuclear RNA exosome complex. Nat. Rev. Mol. Cell Biol. 2016;17:227–239. doi: 10.1038/nrm.2015.15. [DOI] [PubMed] [Google Scholar]
- 57.Théry C., Witwer K.W., Aikawa E., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J., Chen D., Ho E.A. Challenges in the development and establishment of exosome-based drug delivery systems. J. Contr. Release. 2021;329:894–906. doi: 10.1016/j.jconrel.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Lee M., Ban J.J., Im W., et al. Influence of storage condition on exosome recovery. Biotechnol. Bioproc. Eng. 2016;21:299–304. [Google Scholar]
- 60.Wiklander O.P., Nordin J.Z., O'Loughlin A., et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles. 2015;4 doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mentkowski K.I., Snitzer J.D., Rusnak S., et al. Therapeutic potential of engineered extracellular vesicles. AAPS J. 2018;20 doi: 10.1208/s12248-018-0211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armstrong J.P.K., Holme M.N., Stevens M.M. re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J., Li W., Zhang L., et al. Chemically edited exosomes with dual ligand purified by microfluidic device for active targeted drug delivery to tumor cells. ACS Appl. Mater. Interfaces. 2017;9:27441–27452. doi: 10.1021/acsami.7b06464. [DOI] [PubMed] [Google Scholar]
- 64.Zheng L., Hu B., Zhao D., et al. Recent progresses of exosome–liposome fusions in drug delivery. Chin. Chem. Lett. 2024;35 [Google Scholar]
- 65.Wan S., Zhang L., Wang S., et al. Molecular recognition-based DNA nanoassemblies on the surfaces of nanosized exosomes. J. Am. Chem. Soc. 2017;139:5289–5292. doi: 10.1021/jacs.7b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kooijmans S.A., Aleza C.G., Roffler S.R., et al. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles. 2016;5 doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang Z., Zhang X., Huang H., et al. Exosome based miRNA delivery strategy for disease treatment. Chin. Chem. Lett. 2022;33:1693–1704. [Google Scholar]
- 68.Théry C., Amigorena S., Raposo G., et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Chapter. 2006;3 doi: 10.1002/0471143030.cb0322s30. Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 69.Zhang M., Jin K., Gao L., et al. Methods and technologies for exosome isolation and characterization. Small Methods. 2018;2 [Google Scholar]
- 70.Simpson R.J., Lim J.W., Moritz R.L., et al. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 71.Ruivo C.F., Adem B., Silva M., et al. The biology of cancer exosomes: Insights and new perspectives. Cancer Res. 2017;77:6480–6488. doi: 10.1158/0008-5472.CAN-17-0994. [DOI] [PubMed] [Google Scholar]
- 72.Chen J., Li P., Zhang T., et al. Review on strategies and technologies for exosome isolation and purification. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.811971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rupp A.K., Rupp C., Keller S., et al. Loss of EpCAM expression in breast cancer derived serum exosomes: Role of proteolytic cleavage. Gynecol. Oncol. 2011;122:437–446. doi: 10.1016/j.ygyno.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 74.Martins T.S., Vaz M., Henriques A.G. A review on comparative studies addressing exosome isolation methods from body fluids. Anal. Bioanal. Chem. 2023;415:1239–1263. doi: 10.1007/s00216-022-04174-5. [DOI] [PubMed] [Google Scholar]
- 75.Patel G.K., Khan M.A., Zubair H., et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-41800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryu K.J., Lee J.Y., Park C., et al. Isolation of small extracellular vesicles from human serum using a combination of ultracentrifugation with polymer-based precipitation. Ann. Lab. Med. 2020;40:253–258. doi: 10.3343/alm.2020.40.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu R., Simpson R.J., Greening D.W. A protocol for isolation and proteomic characterization of distinct extracellular vesicle subtypes by sequential centrifugal ultrafiltration. Methods Mol. Biol. 2017;1545:91–116. doi: 10.1007/978-1-4939-6728-5_7. [DOI] [PubMed] [Google Scholar]
- 78.Busatto S., Vilanilam G., Ticer T., et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells. 2018;7 doi: 10.3390/cells7120273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yakubovich E.I., Polischouk A.G., Evtushenko V.I. Principles and problems of exosome isolation from biological fluids. Biochem. (Mosc) Suppl. Ser. A Membr. Cell Biol. 2022;16:115–126. doi: 10.1134/S1990747822030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gámez-Valero A., Monguió-Tortajada M., Carreras-Planella L., et al. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles' characteristics compared to precipitating agents. Sci. Rep. 2016;6 doi: 10.1038/srep33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baranyai T., Herczeg K., Onódi Z., et al. Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu W., Ma Z., Kang X. Current status and outlook of advances in exosome isolation. Anal. Bioanal. Chem. 2022;414:7123–7141. doi: 10.1007/s00216-022-04253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greening D.W., Xu R., Ji H., et al. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 84.Contreras-Naranjo J.C., Wu H.J., Ugaz V.M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17:3558–3577. doi: 10.1039/c7lc00592j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie J., Shen Z., Anraku Y., et al. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224 doi: 10.1016/j.biomaterials.2019.119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salarpour S., Barani M., Pardakhty A., et al. The application of exosomes and Exosome-nanoparticle in treating brain disorders. J. Mol. Liq. 2022;350 [Google Scholar]
- 87.Yuan D., Zhao Y., Banks W.A., et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen C.C., Liu L., Ma F., et al. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell. Mol. Bioeng. 2016;9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dad H.A., Gu T.W., Zhu A.Q., et al. Plant exosome-like nanovesicles: Emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 2021;29:13–31. doi: 10.1016/j.ymthe.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rana S., Yue S., Stadel D., et al. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012;44:1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 91.Li D., Yang M., Xu J., et al. Extracellular vesicles: The next generation theranostic nanomedicine for inflammatory bowel disease. Int. J. Nanomed. 2022;17:3893–3911. doi: 10.2147/IJN.S370784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meng W., He C., Hao Y., et al. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li M., Liu L., Dong M. Progress on pivotal role and application of exosome in lung cancer carcinogenesis, diagnosis, therapy and prognosis. Mol. Cancer. 2021;20 doi: 10.1186/s12943-021-01312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu S., Wang Y., Xia X., et al. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact. 2020;20 [Google Scholar]
- 95.Zhao Y., Gu Y., Zhang Q., et al. The potential roles of exosomes carrying APP and tau cleavage products in Alzheimer's disease. J. Clin. Med. 2023;12 doi: 10.3390/jcm12051883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.T W., Y F., S S., et al. Exosome-based drug delivery systems in cancer therapy. Chin. Chem. Lett. 2023;34:89–96. [Google Scholar]
- 97.Zhao Z., Qu L., Shuang T., et al. Low-intensity ultrasound radiation increases exosome yield for efficient drug delivery. J. Drug Deliv. Sci. Technol. 2020;57 [Google Scholar]
- 98.Yeo R.W., Lai R.C., Zhang B., et al. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Mendes B.B., Conniot J., Avital A., et al. Nanodelivery of nucleic acids. Nat. Rev. Meth. Primers. 2022;2 doi: 10.1038/s43586-022-00104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X., Liu J., Yu B., et al. Effects of mesenchymal stem cells and their exosomes on the healing of large and refractory macular holes. Graefes Arch. Clin. Exp. Ophthalmol. 2018;256:2041–2052. doi: 10.1007/s00417-018-4097-3. [DOI] [PubMed] [Google Scholar]
- 101.Das C.K., Jena B.C., Banerjee I., et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol. Pharm. 2019;16:24–40. doi: 10.1021/acs.molpharmaceut.8b00901. [DOI] [PubMed] [Google Scholar]
- 102.Ma Y., Li C., Huang Y., et al. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun. Signal. 2019;17 doi: 10.1186/s12964-019-0418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mathew B., Ravindran S., Liu X., et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019;197:146–160. doi: 10.1016/j.biomaterials.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Soltani F., Parhiz H., Mokhtarzadeh A., et al. Synthetic and biological vesicular nano-carriers designed for gene delivery. Curr. Pharmaceut. Des. 2015;21:6214–6235. doi: 10.2174/1381612821666151027153410. [DOI] [PubMed] [Google Scholar]
- 105.Pegtel D.M., Gould S.J. Exosomes. Annu. Rev. Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 106.Spina S., Joie R.L., Petersen C., et al. Comorbid neuropathological diagnoses in early versus late-onset Alzheimer's disease. Brain. 2021;144:2186–2198. doi: 10.1093/brain/awab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hausner L., Frölich L. Antidementia drug therapy of Alzheimer's dementia: Status 2018 and outlook. Dtsch. Med. Wochenschr. 2019;144:156–160. doi: 10.1055/a-0658-6720. [DOI] [PubMed] [Google Scholar]
- 108.Bachurin S.O., Bovina E.V., Ustyugov A.A. Drugs in clinical trials for Alzheimer's disease: The major trends. Med. Res. Rev. 2017;37:1186–1225. doi: 10.1002/med.21434. [DOI] [PubMed] [Google Scholar]
- 109.Ahmad A., Tandon S., Xuan T.D., et al. A review on phytoconstituents and biological activities of cuscuta species. Biomed. Pharmacother. 2017;92:772–795. doi: 10.1016/j.biopha.2017.05.124. [DOI] [PubMed] [Google Scholar]
- 110.Zhang T., Ma S., Lv J., et al. The emerging role of exosomes in Alzheimer's disease. Ageing Res. Rev. 2021;68 doi: 10.1016/j.arr.2021.101321. [DOI] [PubMed] [Google Scholar]
- 111.Sun J., Zhang X., Wang C., et al. Curcumin decreases hyperphosphorylation of tau by down-regulating caveolin-1/GSK-3β in N2a/APP695swe cells and APP/PS1 double transgenic Alzheimer's disease mice. Am. J. Chin. Med. 2017;45:1667–1682. doi: 10.1142/S0192415X17500902. [DOI] [PubMed] [Google Scholar]
- 112.Yavarpour-Bali H., Ghasemi-Kasman M., Pirzadeh M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019;14:4449–4460. doi: 10.2147/IJN.S208332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poewe W., Seppi K., Tanner C.M., et al. Parkinson disease. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 114.Fabbri M., Rosa M.M., Ferreira J.J. Adjunctive therapies in Parkinson's disease: How to choose the best treatment strategy approach. Drugs Aging. 2018;35:1041–1054. doi: 10.1007/s40266-018-0599-2. [DOI] [PubMed] [Google Scholar]
- 115.Odekerken V.J., van Laar T., Staal M.J., et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): A randomised controlled trial. Lancet Neurol. 2013;12:37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- 116.Cui C., Han Y., Li H., et al. Curcumin-driven reprogramming of the gut microbiota and metabolome ameliorates motor deficits and neuroinflammation in a mouse model of Parkinson's disease. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.887407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shen Z., Huang W., Liu J., et al. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.749192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Axisa P.P., Hafler D.A. Multiple sclerosis: Genetics, biomarkers, treatments. Curr. Opin. Neurol. 2016;29:345–353. doi: 10.1097/WCO.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ojeda-Hernández D.D., Hernández-Sapiéns M.A., Reza-Zaldívar E.E., et al. Exosomes and biomaterials: In search of a new therapeutic strategy for multiple sclerosis. Life (Basel) 2022;12 doi: 10.3390/life12091417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Woodruff T.M., Thundyil J., Tang S.C., et al. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011;6 doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang J., Zhang W., Lv C., et al. A novel biscoumarin compound ameliorates cerebral ischemia reperfusion-induced mitochondrial oxidative injury via Nrf2/Keap1/ARE signaling. Neuropharmacology. 2020;167 doi: 10.1016/j.neuropharm.2019.107918. [DOI] [PubMed] [Google Scholar]
- 122.Li Y., Zhong W., Jiang Z., et al. New progress in the approaches for blood-brain barrier protection in acute ischemic stroke. Brain Res. Bull. 2019;144:46–57. doi: 10.1016/j.brainresbull.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 123.Luo H., Huang Z., Shi Y. Functionalized exosome-loaded ginsenoside Rg1 for the treatment of ischemic stroke. Sheng Wu Gong Cheng Xue Bao. 2023;39:275–285. doi: 10.13345/j.cjb.220253. [DOI] [PubMed] [Google Scholar]
- 124.Romano E., Netti P.A., Torino E. Exosomes in gliomas: Biogenesis, isolation, and preliminary applications in nanomedicine. Pharmaceuticals (Basel) 2020;13 doi: 10.3390/ph13100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oliveira F.D., Castanho M.A.R.B., Neves V. Exosomes and brain metastases: A review on their role and potential applications. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim J., Zhu Y., Chen S., et al. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J. Nanobiotechnol. 2023;21 doi: 10.1186/s12951-023-02006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li J., Wang X., Guo Y., et al. Ginsenoside Rg3-engineered exosomes as effective delivery platform for potentiated chemotherapy and photoimmunotherapy of glioblastoma. Chem. Eng. J. 2023;471 [Google Scholar]
- 128.Khatami S.H., Karami N., Taheri-Anganeh M., et al. Exosomes: Promising delivery tools for overcoming blood-brain barrier and glioblastoma therapy. Mol. Neurobiol. 2023;60:4659–4678. doi: 10.1007/s12035-023-03365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Contr. Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suga K., Matsui D., Watanabe N., et al. Insight into the exosomal membrane: From viewpoints of membrane fluidity and polarity. Langmuir. 2021;37:11195–11202. doi: 10.1021/acs.langmuir.1c00687. [DOI] [PubMed] [Google Scholar]