Abstract
The growth of indoor molds and their resulting products (e.g., spores and mycotoxins) can present health hazards for human beings. The efficacy of chlorine dioxide gas as a fumigation treatment for inactivating sick building syndrome-related fungi and their mycotoxins was evaluated. Filter papers (15 per organism) featuring growth of Stachybotrys chartarum, Chaetomium globosum, Penicillium chrysogenum, and Cladosporium cladosporioides were placed in gas chambers containing chlorine dioxide gas at either 500 or 1,000 ppm for 24 h. C. globosum was exposed to the gas both as colonies and as ascospores without asci and perithecia. After treatment, all organisms were tested for colony growth using an agar plating technique. Colonies of S. chartarum were also tested for toxicity using a yeast toxicity assay with a high specificity for trichothecene mycotoxins. Results showed that chlorine dioxide gas at both concentrations completely inactivated all organisms except for C. globosum colonies which were inactivated an average of 89%. More than 99% of ascospores of C. globosum were nonculturable. For all ascospore counts, mean test readings were lower than the controls (P < 0.001), indicating that some ascospores may also have been destroyed. Colonies of S. chartarum were still toxic after treatment. These data show that chlorine dioxide gas can be effective to a degree as a fumigant for the inactivation of certain fungal colonies, that the perithecia of C. globosum can play a slightly protective role for the ascospores and that S. chartarum, while affected by the fumigation treatment, still remains toxic.
Sick building syndrome (SBS) is a broad term for a range of human health symptoms associated with poor indoor air quality. While poor indoor air quality can be related to a number of issues, the one that has received the most attention in the last 5 to 10 years has been the effects of airborne fungi and their products (2, 6, 12). It has been acknowledged that damp and moldy structures are potentially harmful to the occupants (16). Therefore, from a human health standpoint, it is recommended that the conditions that allowed for fungal proliferation inside human occupied structures be addressed and corrected and that existing fungal growth be removed or treated from any affected substrates. However, a problem is the detection of hidden mold growth sites. Invasive investigations can assist in this regard, but these investigations can be costly to effect and there is also the possibility that some growth sites can be overlooked. It is possible that gaseous fungicides may be able to circumvent this problem because the gas can penetrate into crevices, wall cavities and other hard to access areas, thus treating the inaccessible mold colonies. The contents inside a contaminated structure may also be able to be sterilized (10) using this technique as well. A biocide, chlorine dioxide, has been previously been used in a gaseous form for bacterial decontamination (1, 9). It has been also shown to have a degree of fungicidal activity in solution (15). In this study, we examined the fungicidal effect of chlorine dioxide gas on four species of mold, Stachybotrys chartarum, Cladosporium cladosporioides, Penicillium chrysogenum, and Chaetomium globosum. The first three belong to the class hyphomycetes and the fourth organism to ascomycetes, which are characterized by the presence of an ascus, a membrane-like structure surrounding the ascospores. These organisms have been found in buildings with indoor air quality problems (3, 12, 13). We also looked at the effect of the gas treatment on mycotoxins produced by S. chartarum.
MATERIALS AND METHODS
Experimental design.
The four fungal species were grown separately on sterile Whatman no. 3 filter paper (Whatman, Florham Park, NJ) placed on potato dextrose agar medium (PDA; Difco, Sparks, MD). In addition to growing colonies, the ascospores of one of the organisms, C. globosum was also presented in order to determine if the perithecia and asci present in colonies played a protective role for the ascospores. The filter papers (15 per organism) with fungal colonies and settled ascospores were exposed to either 500 or 1,000 ppm of chlorine dioxide gas for 24 h at room temperature. Control colonies were grown up as for the test colonies but were not exposed to chlorine dioxide gas. After treatment, the filter papers were then placed in 0.15 M phosphate-buffered saline (PBS), pH 7.0 in 50 ml tubes and vortexed resulting in the conidia being in suspension. The resulting conidia suspension was then counted and then serially diluted onto agar plates, with dilution up to 10−4 except Penicillium chrysogenum, which was diluted up to 10−5. All plates were incubated for 7 or more days at 25°C. At this time the plates were removed from the incubator and all colonies were counted. The following data were collected. (i) Total numbers of recovered CFU of tests versus total numbers of CFU of controls were counted. A treatment efficiency (TE) value was calculated using recovered CFU of tests versus controls. (ii) Overall conidia numbers of both tests and controls were counted. (iii) Filter papers with Stachybotrys chartarum colonies were also examined to determine if any posttreatment colonies were still toxic.
Organisms.
Four fungal species, Penicillium chrysogenum (ATCC 9179), Chaetomium globosum (ATCC 16021), a nontoxic strain of Stachybotrys chartarum (ATCC 9633), a toxic strain of Stachybotrys chartarum, and Cladosporium cladosporioides were used. C. cladosporioides and the toxic strain of S. chartarum were collected from building materials and identified in the Center for Indoor Air Research Texas Tech University Health Science Center using reference texts (4, 17). Toxicity of the environmental isolate of S. chartarum was determined by a yeast toxicity assay (5). All organisms were identified and verified using reference texts (4, 17).
Fungal colony preparation.
For each species, conidia were collected from mature cultures on PDA and suspended in PBS. The concentration of conidia was determined by averaging at least four counts on hemocytometer and multiplying the dilution factor.
A total of 1 × 105 conidia were then inoculated onto a 2- by 2-cm sterile Whatman no. 3 filter paper that had been placed in the center of 35- by 10-mm PDA medium plates. The plates were sealed and then incubated at 25°C and 40 to 60% relative humidity until fungal colonies were both sporulating and established over the filter papers.
Ascospore preparation.
For ascospore collection, only PDA plates with mature colonies were used. It was ascertained microscopically that the majority of the perithecia had erupted, freeing the ascospores. Following that, 10 to 15 ml of PBS was added to the plate and a sterile glass rod was used to gently remove the spores from the colonies. The spore suspension was then centrifuged and the pellets of the spores were resuspended to have a concentration of 200,000 spores per microliter. The spore concentration of suspension was determined as described above. No parts of perithecia or hyphae were seen microscopically in the spore suspension. A total of 2 × 106 conidia were then inoculated onto a 2- by 2-cm sterile Whatman filter paper that had been placed in the center of a 35- by 10-mm plate and dried inside a biohazard cabinet.
Chlorine dioxide treatment.
Each gas treatment consisted of three separate runs with five replicates of each fungal species per run. The filter papers with fungal colonies were then placed separately on Petri dish lids inside a 20.8-liter gas chamber which was created by modifying a commercially available pressure cooker. Chlorine dioxide gas at either 500 ppm or 1,000 ppm was created according to the instructions of the manufacturer in the gas chamber through the use of 6 g Aseptrol S10-Tab tablets (Engelhard Corporation, Iselin, New Jersey) dissolved in distilled water. The gas chamber was then sealed for 24 h at room temperature under a fume hood. Control samples, that is, filter papers with identical fungal growth on them were placed in an identical gas chamber without ClO2 gas. As an additional check on the production of ClO2 gas in the chambers, one day old Escherichia coli cultures (ATCC DH5-α) on sheep blood agar plates provided by Clinical Microbiology Department of Texas Tech University Health Sciences Center (Becton Dickinson and Company, Sparks, MD) were placed inside the test and control gas chambers at room temperature. After the treatments, the E. coli colonies were streaked on to blood agar plates and checked for the next 2 days for bacterial growth at 37°C. This organism has been shown to be sensitive to low ClO2 gas concentrations (8). The actual measurement of the concentration of ClO2 gas was conducted as a separate run of experiments.
CFU determination.
After treatment, the filter papers with fungal colonies were removed from the gas chamber and placed into a 10-ml sterile tube containing 5 ml of PBS. After 3 h of occasional vortexing, the suspension was diluted up to four times in 10-fold serial dilutions. For each of five replicates, a total of 100 μl of each dilution was spread plated on PDA media in 150- by 10-mm petri dishes. The plates were sealed and then incubated at 25°C for 5 to 7 days at which time they were examined for fungal growth, which was recorded as CFU. A treatment efficiency (TE) value was calculated for comparison purposes between test and controls. The value was derived by subtracting the recovered test CFU (T) from the recovered control CFU (C) and expressing the difference as a percentage, e.g., %TE = [(C−T)/C)]×100.
During the first step of CFU determination, an aliquot was removed from the suspension and placed in a hemocytometer where the fungal conidia were counted. Total conidia numbers were then compared to the number of recovered CFU for tests and control. The ratio of recovered CFU to total conidia was also determined, resulting in a percent culturable spores value.
Toxicity determination.
Kluyveromyces marxianus (ATCC 8554), a yeast demonstrated to be particularly sensitive to trichothecene mycotoxins (5), was maintained in YPG-50 (1% yeast extract, 1% bacteriological peptone, and 50 mM glucose) (5) at −80°C in a 1-ml aliquot. Cultures for inoculation of the bioassay were prepared by adding 1 ml aliqout to 50 ml of YPG-50 in a culture flask. The flask was incubated in a rotary incubator for approximately 16 h at 37°C. For the bioassay procedure, YPG-50 was supplemented from a stock solution of polymixin B sulfate (PMBS; ICN Biomedicals, Seoul, Korea) to give a final bioassay PMBS concentration of 15 mg/ml (5). A total of 136 μl of PMBS supplemented YPG-50 medium was added to the wells of a 96-well polystyrene microtiter plate in triplicate. A total of 8 μl of test sample or control was added to each well, followed by 16 μl of yeast inoculum to yield an initial cell density of approximately 1 × 108 cells/ml. Blank wells contained 152 μl of medium and 8 μl of sterile distilled water. Negative control wells consisted of 144 μl of medium and 16 μl of yeast inoculum. Positive control wells were also included which contained the medium, yeast inoculum and one microgram of roridin A. The plates were sealed and incubated on a plate shaker at 37°C for 8 h. Turbidity was measured every two hours by measuring the absorbance at 550 nm in a microtiter plate reader. Toxicity of the sample was based on these absorbances. Yeast growth resulted in high absorbance, indicating that the sample was not toxic. Conversely, the lower the absorbance, the more toxic the sample was to the yeast.
Statistical analysis.
To determine if the treatments may have destroyed conidia rather than compromised them, the mean number of C. globosum ascospores for the two test treatments were compared to the control mean (N = 30 control and 15 test). This analysis was not performed on the hyphomycetes organisms because the pre-treatment conidia numbers could not be determined and an analysis on the final numbers therefore could not be made. A one-way analysis of variance (ANOVA) was performed on the ascospore test and control means followed by a Tukey's post hoc test (SigmaStat software, version 2.0; SPSS Inc., Chicago, IL). Conditions of normality and equal variance were met for the analysis.
RESULTS
No culturable E. coli was recovered after chlorine dioxide gas treatment indicating that the gas was present in the gas chambers. For both the 500 and 1,000 ppm levels of ClO2 gas treatment, P. chrysogenum, S. chartarum, and C. cladosporioides were not able to be cultured, indicating that the treatments were successful in completely inhibiting the culturability of these organisms. Table 1 shows the results for the trial for the hyphomycetes organisms. With regard to C. globosum colonies the treatment of ClO2 at 500 ppm resulted in a TE of 91%. At 1,000 ppm, the TE was 87%. The C. globosum ascospores were virtually inactivated 100% by both concentrations. The mean number of ascospores of the control was significantly higher than that for both treatments (P < 0.001). Table 2 shows the results for colonies and ascospores of C. globosum.
TABLE 1.
Means and standard errors for results of a trial that examined the efficacy of CLO2 gas over 24 h on the culturability and overall conidia numbers of three hyphomycetes mold species
| Organisms and parameters | Treatment with ClO2a
|
||
|---|---|---|---|
| 0 ppm | 500 ppm | 1,000 ppm | |
| S. chartarum | |||
| Recovered CFU | 2.6 × 106 (0.19 × 106) | 0 (0) | 0 (0) |
| Recovered conidia | 1.071 × 107 (2.26 × 106) | 8.13 × 106 (1.95 × 106) | 9.5 × 106 (2.1 × 106) |
| % Culturable spores | 48.0 (6.70) | 0 (0) | 0 (0) |
| Treatment efficiency (%) | 100.00 | 100.00 | |
| C. cladosporioides | |||
| Recovered CFU | 1.8 × 106 (0.18 × 106) | 0 (0) | 0 (0) |
| Recovered conidia | 2.8 × 106 (0.33 × 106) | 2.3 × 106 (0.32 × 106) | 3.6 × 106 (0.31 × 106) |
| % Culturable spores | 69.0 (7.80) | 0 (0) | 0 (0) |
| Treatment efficiency (%) | 100.00 | 100.00 | |
| P. chrysogenum | |||
| Recovered CFU | 7.3 × 106 (0.05 × 106) | 0 (0) | 0 (0) |
| Recovered conidia | 1.76 × 107 (0.15 × 106) | 1.67 × 107 (0.84 × 106) | 1.45 × 107 (0.16 × 106) |
| % Culturable spores | 45.0 (3.6) | 0 (0) | 0 (0) |
| Treatment efficiency (%) | 100.00 | 100.00 | |
Values in parentheses represent standard errors.
TABLE 2.
Means and standard errors for results of a trial that examined the efficacy of ClO2 gas over 24 h on the culturability and overall conidia numbers of the ascomycete C. globosum
| Organisms and parameters | Treatment with ClO2a
|
||
|---|---|---|---|
| 0 ppm | 500 ppm | 1,000 ppm | |
| C. globosum colonies | |||
| Recovered CFU | 1.5 × 106 (0.09 × 106) | 0.19 × 106 (0.03 × 106) | 0.14 × 106 (0.02 × 106) |
| Recovered conidia | 3.0 × 106 (0.02 × 106) | 3.3 × 106 (0.05 × 106) | 3.6 × 106 (0.04 × 106) |
| % Culturable spores | 65 (7.1) | 7 (1.5) | 3 (0.6) |
| Treatment efficiency (%) | 90.76 | 87.35 | |
| C. globosum ascospores | |||
| Recovered CFU | 8.3 × 105 (0.40 × 105) | 76 (40.0) | 30 (15.5) |
| Recovered conidia | 1.01 × 106 (0.39 × 105)A | 6.6 × 105 (0.35 × 105)B | 7.7 × 105 (0.67 × 105)B |
| % Culturable spores | 82 (2.5) | 0.01 (0.007) | 0.003 (0.001) |
| Treatment efficiency (%) | 99.99 | 99.99 | |
Different superscripts on the rows of recovered conidia indicate a significant difference at the P = 0.001 level. Values in parentheses represent standard errors.
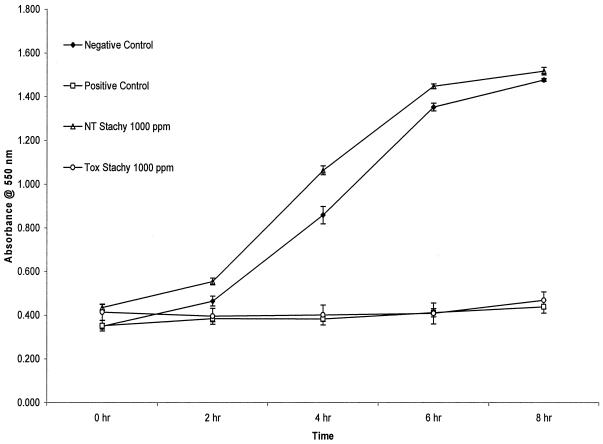
The yeast toxicity assay results showed that the ClO2 gas treatment was unsuccessful in detoxifying the colonies of the toxic strain of S. chartarum. Figure 1 and Table 3 show the results for this assay. These results show that the toxicity of S. chartarum was due to the properties of the organism rather than to a combination of ClO2 and organism.
FIG. 1.
Graph showing the representative result from a trial that examined the efficacy of ClO2 gas over 24 h on the toxicity of S. chartarum (Stachy) colonies using a yeast toxicity assay. High absorbance readings indicate nontoxicity. NT, nontoxic strain; Tox, toxic strain.
TABLE 3.
Means and standard errors for results from a yeast toxicity assay showing mean optical densities at a wavelength of 550 nm taken at 8 h of incubation for toxic and nontoxic Stachybotrys chartarum compared to positive and negative controls
| ClO2 concn (ppm) | Absorbance at OD550a
|
|||
|---|---|---|---|---|
| Toxic | Nontoxic | Positive control | Negative control | |
| 500 | 0.567 (0.038) | 1.490 (0.016) | 0.296 (0.073) | 1.398 (0.062) |
| 1,000 | 0.679 (0.055) | 1.474 (0.008) | 0.296 (0.073) | 1.398 (0.062) |
OD500, optical density at 550 nm. Low mean absorbancy indicates toxicity. Values in parentheses represent standard errors.
DISCUSSION
The results show that the two dosages of the ClO2 gas treatment were successful in rendering the all conidia completely nonculturable of hyphomycetes organisms Penicillium chrysogenum, Stachybotrys chartarum, Cladosporium cladosporioides, and the majority of ascospores of ascomycete Chaetomium globosum growing on the filter paper. With regard to culturable ascospore numbers, they were significantly reduced (P < 0.001) after treatment and this in turn indicates that some destruction of ascospores may have occurred. It is also possible that the ascospores and conidia were compromised. ClO2 is a strong oxidant (14) and previous work with bacterial spores has shown that it can affect the integrity of cell membranes and germination inhibition (20).
It is not clear if the nonculturable spores themselves will be a health hazard in the same way that culturable spores are. However, nonculturable spores will prevent further growth of colonies and inhibition of fungal growth will prevent further mycotoxin production.
In this experiment, filter paper is used to represent the actual building materials in terms of food source and physical support. Further research is needed to show the effectiveness of the treatment of mold damaged buildings with high concentration ClO2 gas.
Chaetomium globosum (Chaetomiaceae, Sordariales, Sordariomycetidae, Ascomycetes) is an ascomycete that produces conidia known as ascospores which are characterized by a thick multilayered spore coat. Numerous ascospores are situated within an ascus which is a sac-like structure. The asci in turn are situated inside another enclosure called perithecia (11). Nothing is known of the function of the ascus and the perithecia with relation to fungicidal agents. The results of this trial indicate that these two additional layers played a slightly protective role for the Chaetomium ascospores against the oxidative effect of ClO2. This may possibly be due to the ascus and perithecia providing a physical protective barrier against the gas. The treatment of C. globosum with ClO2 at 500 ppm resulted in a TE of 91%, while the 1,000 ppm treatment resulted in a TE of 87%. This indicates that a ClO2 concentration of 500 ppm is high enough to inhibit fungal growth.
The treatment was not successful in detoxifying the nonculturable toxic strain of S. chartarum. Chlorine was chosen as the active agent in this study because previous studies have shown that sodium hypochlorite can inactivate trichothecene mycotoxins (18). It has also been effective in inactivating aflatoxins (19). We have also shown that ClO2 is effective against the trichothecene mycotoxins roridin A and verrucarin A placed in water (data not shown). Satratoxin G is the main trichothecene mycotoxin produced by. S. chartarum and it is found predominantly on S. chartarum conidia, both on the outer membrane and the inner layer of the conidia (7) and it is possible that because of their location the gas was not able to inactivate the trichothecenes.
While the gas treatment is successful in inactivating mold and bacterial colonies, the treatment comes with a caveat in that chlorine dioxide gas has corrosive properties and care has to be taken that the treatment does not result in the degradation of the contents as well as the components of the structure. It has been used in the past, as the disinfection of the offices of the Hart Senate Office Building by the Environmental Protection Agency in 2002 was achieved with the help of ClO2 gas. This treatment can be considered where buildings are emptied of sensitive items and the construction of the building is such that there are inaccessible mold contaminated areas.
In conclusion, this study has shown that chlorine dioxide gas can be effective to a degree as a fumigant for the inactivation of certain fungal colonies, that the perithecia/asci of C. globosum can play a protective role for the ascospores and that S. chartarum, while inactivated by the fumigation treatment, still remains toxic at the tested dosages.
Acknowledgments
We thank Assured Indoor Air Quality Ltd., Dallas, Texas, and Texas Tech University Health Sciences Center (TTUHSC) for their financial support.
S. C. Wilson and D. C. Straus were supported by a Center of Excellence grant from TTUHSC. D. C. Straus and T. L. Brasel were supported by a grant from the Texas Higher Education Coordinating Board.
REFERENCES
- 1.Buttner, M. P., P. Cruz, L. D. Stetzenbach, A. K. Clima-Komba, V. L. Stevens, and T. D. Cronin. 2004. Determination of the efficacy of two building decontamination strategies by surface sampling with culture and quantitative PCR analysis. Appl. Environ. Microbiol. 70:4740-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman, J. A. 2003. Stachybotrys chartarum (chartarum = atra = alternans) and other problems caused by allergenic fungi. Allergy Asthma Proc. 24:1-7. [PubMed] [Google Scholar]
- 3.Cooley, J. D., W. C. Wong, C. A. Jumper, and D. C. Straus. 1998. Correlation between the prevalence of certain fungi and sick building syndrome. Occup. Environ. Med. 55:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domsch, K. H., W. Gams, and T. Andersen. 1993. Compendium of soil fungi, vol. 1. IHW-Verlag, Eching, Germany.
- 5.Engler, K. H., R. D. Coker, and I. H. Evans. 1999. A colorimetric technique for detecting trichothecenes and assessing relative potencies. Appl. Environ. Microbiol. 65:1854-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flannigan, B., and J. D. Miller. 1994. Health implications of fungi in indoor environments: an overview, p. 3-28. In R. A. Samson, B. Flannigan, A. P. Verhoeff, O. C. G Adan, and E. S. Hoekstra (ed.), Health implications of fungi in indoor environments. Elsevier, New York, N.Y.
- 7.Gregory, L., J. J. Pestka, D. G. Dearborn, and T. G. Rand. 2004. Localization of satratoxin-G in Stachybotrys chartarum spores and spore-impacted mouse lung using immunocytochemistry. Toxicol. Pathol. 32:26-34. [DOI] [PubMed] [Google Scholar]
- 8.Han, Y., J. D. Floros, R. H. Linton, S. S. Nielsen, and P. E. Nelson. 2001. Response surface modeling for the inactivation of Escherichia coli O157:H7 on green peppers (Capsicum annuum L.) by chlorine dioxide gas treatments. J. Food Prot. 64:1128-1133. [DOI] [PubMed] [Google Scholar]
- 9.Han, Y., B. Applegate, R. H. Linton, and P. E. Nelson. 2003. Decontamination of Bacillus thuringiensis spores on selected surfaces by chlorine dioxide gas. J. Environ Health. 66:16-21. [PubMed] [Google Scholar]
- 10.Jeng, D. K., and A. G. Woodworth. 1990. Chlorine dioxide gas sterilization of oxygenators in an industrial scale sterilizer: a successful model. Artif. Organs 14:361-368. [DOI] [PubMed] [Google Scholar]
- 11.Kirk, P. M., P. E. Cannon, J. C. David, and J. A. Stalpers. 2001. Ainsworth and Bisby's dictionary of the fungi, 9th ed., p. 625. CABI Bioscience, New York, N.Y.
- 12.Lee, T. G., 2003. Health symptoms caused by molds in a courthouse. Arch. Environ. Health 58:442-446. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoudi, M., and M. E. Gershwin. 2000. Sick building syndrome. 111. Stachybotrys chartarum. J. Asthma 37:191-198. [DOI] [PubMed] [Google Scholar]
- 14.Moore, G. S., E. J. Calabrese, S. R. DiNardi, and R. W. Tuthill. 1978. Potential health effects of chlorine dioxide as a disinfectant in potable water supplies. Med. Hypotheses 4:481-496. [DOI] [PubMed] [Google Scholar]
- 15.Price, D. L., and D. G. Ahearn. 1999. Sanitation of wallboard colonized with Stachybotrys chartarum. Curr. Microbiol. 39:21-26. [DOI] [PubMed] [Google Scholar]
- 16.Redd, S. C. 2002. State of the science on molds and human health. Statement for the record before the Subcommittees on Oversight and Investigations and Housing and Community Opportunity, Committee on Financial Services, United States House of Representatives. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, Ga.
- 17.St. Germain, G., and R. Summerbell. 1996. Identifying filamentous fungi: a clinical laboratory handbook, p. 263. Star, Belmont, Calif.
- 18.Wannemacher, R. W., and S. L. Wiener. 1997. Trichothecene mycotoxins, p. 660. In F. R. Sidell, E. T. Takafuji, and D. R. Franz (ed.), Medical aspects of chemical and biological warfare. Office of the Surgeon General, U.S. Department of the Army, Washington, D.C.
- 19.Yang, C. 1972. Comparative studies on the detoxification of aflatoxins by sodium hypochlorite and commercial bleaches. Appl. Microbiol. 24:885-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young, S. B., and P. Setlow. 2003. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J. Appl. Microbiol. 95:54-67. [DOI] [PubMed] [Google Scholar]