Abstract
Lipids are the major form of carbon storage in arbuscular-mycorrhizal fungi. We studied fatty acid synthesis by Glomus intraradices and Gigaspora rosea. [14C]Acetate and [14C]sucrose were incorporated into a synthetic culture medium to test fatty acid synthetic ability in germinating spores (G. intraradices and G. rosea), mycorrhized carrot roots, and extraradical fungal mycelium (G. intraradices). Germinating spores and extraradical hyphae could not synthesize 16-carbon fatty acids but could elongate and desaturate fatty acids already present. The growth stimulation of germinating spores by root exudates did not stimulate fatty acid synthesis. 16-Carbon fatty acids (16:0 and 16:1) were synthesized only by the fungi in the mycorrhized roots. Our data strongly suggest that the fatty acid synthase activity of arbuscular-mycorrhizal fungi is expressed exclusively in the intraradical mycelium and indicate that fatty acid metabolism may play a major role in the obligate biotrophism of arbuscular-mycorrhizal fungi.
Arbuscular-mycorrhizal (AM) fungi form obligate symbioses with more than 80% of plant species in all terrestrial habitats (30). These fungi are highly beneficial for plants, but their obligate biotrophic nature makes them difficult to study and to exploit for plant crop improvement. Identifying the mechanisms involved in this obligate biotrophism is therefore of both fundamental and applied interest. Although it is not yet clear which metabolic pathway is involved in the obligate symbiosis of AM fungi, a better understanding of the AM symbiosis, particularly of the obligate endosymbiotic nature of AM fungi, requires an understanding of the biosynthesis of the major carbon metabolites in the fungus (2).
In exchange for mineral nutrients, e.g., phosphorus, the plant supplies the fungal partner with reduced carbon (23, 29, 31). This carbon is stored primarily as lipids (5, 28), with smaller amounts of carbohydrates stored as glycogen (3, 12) and trehalose (1, 6). For example, up to 70% of the dry weight of Glomus caledonius is composed of lipids, predominantly triglycerides (9). The major fatty acid in Glomus intraradices, and in many other mycorrhizal fungi, is 16:1 ω5 (10, 18, 21). This unique molecule has an unusual saturation position that seems to be specific to certain AM fungi and bacteria (24) and has been used as a specific biochemical marker to estimate the biomass of AM mycelia in soil (25, 26).
Previous work (4) has shown that germinating spores of G. intraradices cannot synthesize fatty acids. This metabolic deficiency also is found in the extraradical hyphae of the fungus (27), where quantitative fungal fatty acid synthesis was detected only in mycorrhized roots. These studies used nuclear magnetic resonance spectroscopic investigations, which are not very sensitive, and did not provide detailed analysis of fatty acids, relying on 16:1 ω5 as the marker fatty acid. However, this work led to the important hypothesis that AM fungi rely on a host-created environment to induce lipid synthesis (2). In contrast, other work with [14C]acetate as the source for fungal fatty acid synthesis suggested that germinating spores and the extraradical hyphae of AM fungi could synthesize their own fatty acids (8, 17). However, since individual fatty acids were not assayed for 14C incorporation, it is not clear if the observed incorporation was due to acetate use as the initial synthetic precursor for fatty acid synthesis or if existing fatty acids incorporated 14C through the fatty acid elongation pathway (synthesis of long-chain fatty acids).
Fatty acid synthesis in fungi is catalyzed by a fatty acid synthase (FAS) complex (34). FAS is a cytosolic complex of two nonidentical subunits, α (Mr, 213,000), which has three enzymatic activities, and β (Mr, 203,000), which has five different activities. The active enzyme is an α6β6 complex that contains six palmitate-synthesizing units. FAS uses acetyl-coenzyme A (CoA) and malonyl-CoA for the initial condensation and malonyl-CoA for further condensations until the acyl chain reaches 16 carbons. Successive elongations and desaturations do not require the FAS. Elongation occurs in the endoplasmic reticulum as a result of elongase enzyme activity to obtain 18-, 20-, or 22-carbon fatty acids. Further desaturations (by desaturase) yield all the unsaturated fatty acids. Regulation of fatty acid synthesis is principally controlled by acetyl-CoA carboxylase, which converts acetyl-CoA to malonyl-CoA, the building block for fatty acids. Activity of acetyl-CoA carboxylase can be controlled by transcriptional regulation (14), by phosphorylation (22), or by allosteric conversion (34). Expression of fatty acid synthase genes is regulated at the level of transcription (14), and for the moment, no posttranscriptional regulation has been clearly demonstrated.
The objective of our study was to resolve contradictions concerning the ability (or inability) of AM fungi to synthesize fatty acids. We hypothesize that the root apoplast is a unique environment in which AM fungi become competent for fatty acid synthesis and that this environment provides plant signals that are required to directly or indirectly induce FAS genes and/or enzymatic activity. Identifying the mechanisms involved in fatty acid synthesis could highlight the role of FAS in the obligate symbiosis between AM fungi and their plant hosts.
MATERIALS AND METHODS
Growth and labeling of spores and roots.
Approximately 2.5 × 104 spores of G. intraradices Smith & Schenck (strain DAOM 197198) produced from the in vitro culture of root-inducing transferred-DNA (Ri T-DNA)-transformed carrot (Daucus carota L.) roots (Premier Tech, Rivière-du-Loup, Canada) were suspended in 2 ml of liquid M medium (7). The spores were pregerminated at 28°C for 4 days before adding 2 μCi (0.38 μmol; 1 Ci = 37 GBq) of sodium [1-14C]acetate (5.3 mCi/mmol) (ICN Biomed, Aurora, OH). After an additional 4 days of incubation, the spores were washed with distilled water and harvested by filtration through a 40-μm nylon filter (Nitex; Sefar Canada Inc., Montréal, Québec, Canada). The experiment was repeated twice.
Spores of Gigaspora rosea Nicol & Schenck (strain DAOM 194757) were produced on potted leek plants (Allium ampeloprasum L.), isolated by wet sieving, sterilized twice for 10 min in 2% chloramine T, and stored overnight in a streptomycin (100 mg liter−1)/gentamicin (50 mg liter−1) solution at 4°C. To reduce the risk of contamination, enzyme-linked immunosorbent assay (ELISA) multiwell plates were used. Each well contained 95 μl of M medium with 0.1 μCi (19 nmol) sodium [1-14C]acetate. Five spores were transferred to each well. Uncontaminated germinating spores and hyphae were collected following 14 days of incubation. The experiment was repeated twice.
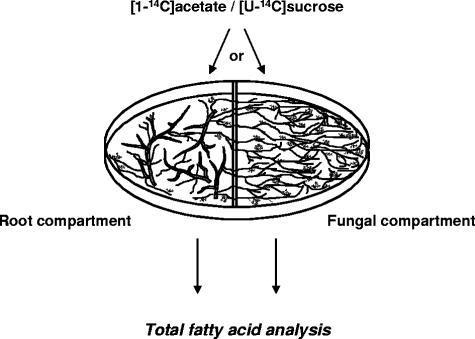
Ri T-DNA-transformed carrot roots colonized by G. intraradices were grown in a two-compartment petri dish system (32) with complete M medium in both compartments. Mycorrhized roots were inoculated in one compartment, i.e., the root compartment. The fungus was allowed to grow in both compartments and to proliferate in the second compartment, i.e., the fungal compartment. Root colonization by the fungus was 20 to 30% before proceeding. Two radioactive solutions were prepared by adding 5 μCi (0.38 μmol) sodium [1-14C]acetate or 5 μCi (9.1 nmol) [U-14C]sucrose (550 mCi/mmol; PerkinElmer, Boston, MA) in 5 ml sterile distilled water. Two ml of these solutions was added to either compartment of 5-week-old cultures in petri dishes (Fig. 1). The petri dishes were incubated for 1 week at 28°C. Roots, spores, and hyphae were harvested by solubilizing the gel in 10 mM sodium citrate buffer at pH 6.0 (15) under agitation on a magnetic stirring plate for 15 min at room temperature. The tissues were collected by passing the suspension through a 40-μm filter and rinsing them with distilled water. The experiment was repeated three times.
FIG. 1.
Experimental system for labeling mycorrhized roots or extraradical hyphae with [1-14C]acetate or [U-14C]sucrose. Mycorrhized roots are grown in one compartment (root compartment), while the majority of the extraradical fungal mycelium grows in the second compartment (fungal compartment).
Production of stimulatory root exudates.
Lipophilic carrot root exudates were prepared as previously described (13). This lipophilic root exudate stimulates the growth and hyphal branching of germinating spores of AM fungi (13) as well as fungal mitochondria biogenesis and respiration (33).
Effect of root exudates on the fatty acid synthesis of germinating spores.
After germination for 2 to 3 days, 5 μl of concentrated root exudate solution was added to each well containing G. rosea. Five μl of 50% methanol-water served as the control. Spores and hyphae were collected following 14 days of incubation in the presence of sodium [1-14C]acetate for fatty acid analysis. The experiment was repeated twice.
G. intraradices spores (6 × 104) were germinated for 4 days in 950 μl of liquid M medium. Following the germination period, 50 μl of the concentrated root exudate solution was added to the medium. Fifty μl of 50% methanol-water served as the control. Spores and hyphae were collected after 12 days of incubation in the presence of sodium [1-14C]acetate for fatty acid analysis. The experiment was repeated twice.
Extraction and derivatization of total fatty acids.
Samples were ground in a mortar with a pestle and transferred to an 8-ml screw-cap tube with 2.5 ml of 95% ethanol. Total fatty acids were saponified by adding 0.4 ml KOH 3.3 g liter−1 and heating the samples at 90°C for 90 min. The saponification mixture was washed with 3 ml of hexane to remove nonsaponified components, and the hexane fraction was discarded. After reacidification with 1 ml of 6 N HCl, fatty acids were extracted with 3 ml of hexane, transferred to a fresh screw-cap tube, evaporated under a stream of nitrogen, and resuspended in 1 ml of hexane. The saponified extract was divided in two, and each half was used for the preparation of fatty acid methyl esters (FAME) or fatty acid phenacylesters (FAPE).
FAME were prepared by adding 2.5 ml of a 14% solution of boron trifluoride-methanol (Sigma-Aldrich, Mississauga, Ontario, Canada) in an 8-ml screw-cap tube containing dry saponified extract. Methylation of total fatty acids was performed under reflux at 90°C for 1 h. After cooling on ice, 2 ml of water and 2 ml of hexane were added, followed by 2 min of vigorous shaking and 10 min of centrifugation (300 × g) at room temperature. The upper phase was recovered, the lower aqueous phase was washed twice more with 1 ml hexane, and the three hexane fractions were pooled. The pooled hexane fraction was dried under a stream of nitrogen, resuspended in 1 ml hexane, and subjected to gas chromatography analysis. Preparation of FAME was also performed directly on nonsaponified samples using the same boron trifluoride-methanol method and gave similar results.
For FAPE analysis, fatty acids were derivatized by adding 200 μl acetonitrile, 100 μl phenacyl-8 (Pierce Biotechnology, Rockford, IL), and 75 mg K2CO3 (16) to the dry saponified extract in an 8-ml screw-cap tube. The mixture was heated at 80°C for 1 h with stirring every 10 min. FAPE were filtered through a 0.45-μm syringe filter prior to high-performance liquid chromatography (HPLC) analysis.
Separation of fatty acids by using a gas chromatography-flame ionization detector.
FAME were analyzed by using a gas chromatograph (Hewlett Packard model 5890) coupled to a flame ionization detector, using a 30-m DB225 column (J&W Scientific, Folsom, CA). Injector and detector temperatures were 200°C and 250°C, respectively. The oven temperature program was as follows: 100°C for 2 min, 40°C/min to 195°C, holding for 7 min, 40°C/min to 220°C, and holding for 18 min.
FAME were identified by comparing retention times and by spiking with individual standard fatty acids that were derivatized as previously described. All individual standards were purchased from Sigma-Aldrich, with the exception of 16:1 ω5, which was obtained from Matreya Inc. (Pleasant Gap, PA). Individual FAME were quantified with calibration curves by using a mixture of 37 authentic methylated standards (F.A.M.E. C4 through C24; Supelco, Bellefonte, PA). 18:1 ω5, 18:1 ω7, and 16:1 ω5 were added to the standard mixture prior to analysis. Methylheptadecanoate (C17) was used as an internal standard for comparison of relative retention times and was not present in any of the samples.
Separation of fatty acids by HPLC and determination of radioactivity.
FAPE were separated on a Waters HPLC system (Pump Model 996) (Waters Limited, Mississauga, Canada) using a 250- by 4.6-mm, 5-μm-thick HyPURITY Elite C18 column at a flow of 1 ml/min with the following program: linear gradient elution of 80 to 100% acetonitrile for 15 min, 5 min of isocratic elution of 100% acetonitrile, and linear gradient elution for 5 min of 100 to 80% acetonitrile. FAPE were detected with a photodiode array detector (Waters model 600) at 242 nm. Fatty acids standards were derivatized as described above and used to identify fatty acid peaks by spiking the individual samples. Samples were collected every 8 s by using an automatic fraction collector. One ml of liquid scintillation cocktail (Ecolite; ICN Biomed, Aurora, OH) was added to each fraction, and radioactivity was measured with a Wallac 1409 liquid scintillation counter (PerkinElmer Life Sciences, Boston, MA). All of the labeling experiments were done in duplicate or triplicate as indicated in the figures.
Specific radioactivity was calculated for individual fatty acids by dividing a fatty acid's radioactivity by its molar percentage. The specific 18:2/16:1 radioactivity ratio was determined by dividing the specific radioactivity of 18:2 (principally of plant origin) by that of 16:1 (fungal origin).
RESULTS
Fatty acid profiles of G. intraradices, G. rosea, and carrot roots.
Over 20 different fatty acids were identified in germinating spores and the extraradical mycelium of G. intraradices and G. rosea and in colonized carrot roots (Table 1). 16:1 was the predominant fatty acid in G. intraradices. Combined, both 16 carbon fatty acids (16:0 and 16:1) account for over 85% of all fatty acids present in G. intraradices. Long-chain fatty acids also were present, in particular, 20:3, 20:4, 20:5, and 24:1. Few differences in fatty acid profiles were observed before and after germination of the spores.
TABLE 1.
Molar percentage (total, 100) of fatty acids in germinating spores of G. intraradices and G. rosea and in carrot roots colonized by G. intraradices expressed as means ± standard errors
| Fatty acid | Carrot rootsa | Carrot roots colonized by G. intraradicesa |
G. intraradices spores
|
G. rosea spores
|
||
|---|---|---|---|---|---|---|
| Before germinationb | After germinationb | Before germinationb | After germinationb | |||
| 12:0 | 0.13 ± 0.01 | 0.04 ± 0.01 | NDc | 0.05 ± 0.01 | ND | ND |
| 14:0 | 0.37 ± 0.03 | 0.14 ± 0.03 | 0.05 ± 0.02 | 0.08 ± 0.02 | 0.43 ± 0.08 | 1.4 ± 0.6 |
| 14:1 | ND | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.04 ± 0.02 | ND | ND |
| 15:0 | 0.43 ± 0.05 | 0.18 ± 0.03 | 0.16 ± 0.10 | 0.08 ± 0.00 | 0.18 ± 0.03 | 1.3 ± 0.5 |
| 15:1 | ND | 0.06 ± 0.00 | TRd | 0.11 ± 0.01 | ND | ND |
| 16:0 | 18 ± 1 | 17 ± 1 | 10 ± 1 | 9.8 ± 0.7 | 22 ± 1 | 21 ± 1 |
| 16:1 ω5 | ND | 47 ± 5 | 78 ± 2 | 78 ± 1 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| 16:1 ω7 | 0.41 ± 0.13 | 0.53 ± 0.05 | 0.57 ± 0.08 | 0.50 ± 0.09 | 0.49 ± 0.04 | 1.1 ± 0.2 |
| 18:0 | 2.4 ± 0.3 | 1.0 ± 0.2 | 0.26 ± 0.18 | 0.24 ± 0.11 | 0.38 ± 0.16 | 5.0 ± 1.2 |
| 18:1 ω5 | ND | 0.68 ± 0.01 | 0.93 ± 0.08 | 1.1 ± 0.2 | 0.53 ± 0.02 | 0.23 ± 0.04 |
| 18:1 ω7 | 0.67 ± 0.02 | 2.1 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.1 | 5.7 ± 0.1 | 4.2 ± 0.2 |
| 18:1 ω9 | 5.9 ± 0.2 | 2.3 ± 0.9 | 1.2 ± 0.1 | 1.3 ± 0.1 | 35 ± 1 | 26 ± 1 |
| 18:2 | 52 ± 1 | 18 ± 4 | 0.65 ± 0.01 | 0.73 ± 0.10 | 2.2 ± 0.1 | 1.6 ± 0.2 |
| 18:3 | 5.2 ± 0.3 | 4.4 ± 1.0 | 0.78 ± 0.14 | 0.77 ± 0.13 | 1.1 ± 0.1 | 3.1 ± 1.1 |
| 20:0 | 3.1 ± 0.1 | 0.97 ± 0.13 | TR | TR | ND | ND |
| 20:1 | 0.28 ± 0.04 | 0.15 ± 0.03 | 0.06 ± 0.02 | 0.08 ± 0.03 | 14 ± 1 | 13 ± 1 |
| 20:2 | 0.32 ± 0.04 | 0.12 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.01 | 6.5 ± 0.1 | 6.0 ± 0.4 |
| 20:3 | 0.38 ± 0.05 | 1.5 ± 0.1 | 2.2 ± 0.1 | 2.4 ± 0.1 | 2.2 ± 0.1 | 3.0 ± 0.6 |
| 20:4 | ND | 0.23 ± 0.05 | 0.83 ± 0.03 | 0.89 ± 0.03 | 2.1 ± 0.1 | 2.6 ± 0.7 |
| 20:5 | ND | 0.56 ± 0.02 | 1.2 ± 0.3 | 1.3 ± 0.4 | 3.9 ± 0.1 | 4.0 ± 1.4 |
| 22:0 | 6.7 ± 0.8 | 1.5 ± 0.1 | ND | ND | ND | ND |
| 22:1 | 0.98 ± 0.27 | 0.19 ± 0.02 | 0.25 ± 0.09 | 0.19 ± 0.01 | 2.4 ± 0.1 | 2.8 ± 0.1 |
| 24:0 | 2.5 ± 0.8 | 0.49 ± 0.08 | ND | ND | ND | ND |
| 24:1 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.36 ± 0.08 | 0.40 ± 0.09 | 0.25 ± 0.01 | ND |
| % Weighte | 3.3 ± 0.2 | 10 ± 3 | 42 ± 3 | 40 ± 2 | - | - |
n = 3.
n = 2.
ND, not detected.
TR, trace.
Percentage of the fatty acids (wt/wt) in dry biomass.
The fatty acid profile of G. rosea was very different from that of G. intraradices. In germinating spores of G. rosea, the most abundant fatty acids were 18:1 ω9 and 16:0. 16:1 was present but at a lower concentration than in G. intraradices, and 20:1 was present but at a higher concentration than in G. intraradices. In general, G. rosea has fatty acids with longer acyl chains than G. intraradices, i.e., 76% of fatty acids were longer than 16 carbons in G. rosea, but only 11% of the fatty acids were over 16 carbons long in G. intraradices. Molar percentages of fatty acids differed before and after the germination of G. rosea spores. Stearic acid (18:0) increased from 0.38% (before germination) to 5% (after germination), and 18:3 increased from 0.45% to 3.3%. The most abundant G. rosea fatty acid (18:1 ω9) decreased from 35% to 26%.
Fatty acid dry mass of carrot root was very low relative to that of G. intraradices. Major root fatty acids were 18:2 and 16:0. 22:0 and 24:0 fatty acids were specific to carrot roots and were not found in the two fungi, and 16:1 ω5, 20:4, and 20:5 were specific to the fungi but were not found in the carrot root.
[14C]Acetate labeling of fatty acids from germinating G. intraradices and G. rosea spores.
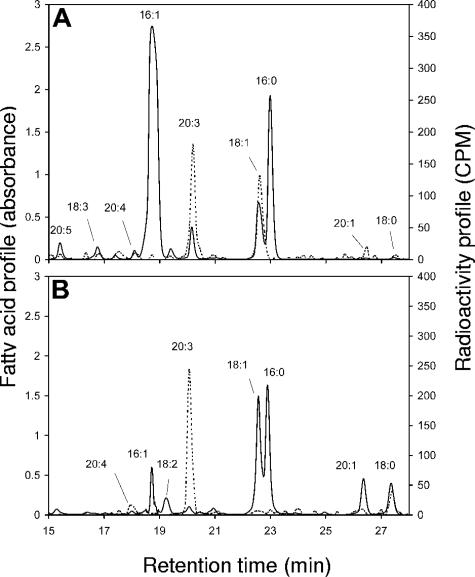
The HPLC chromatogram and radioactivity of fatty acids ([1-14C]acetate labeling) in germinating spores of G. intraradices showed strong labeling of 18:1 and 20:3, while little labeling was observed for 18:0, 18:3, 20:1, and 20:4 (Fig. 2A). For G. rosea, strong labeling was observed in 20:3 and 18:0, but not in 18:1, contrary to that in G. intraradices. No radioactivity was detected in the 16:0 and 16:1 fatty acids of G. intraradices or G. rosea even though 16:0 is common in both fungi and 16:1 is the most common fatty acid for G. intraradices (Fig. 2). For both fungal species, labeling was detected only in fatty acids with carbon chains longer than 16 atoms.
FIG. 2.
HPLC chromatograms (A242) and [1-14C]acetate-labeled radioactivity measurements (CPM) of fatty acids from germinating spores. G. intraradices (A) and G. rosea (B) are shown. Solid lines denote the fatty acid profile, and dotted lines denote the radioactivity profile. The experiment was repeated twice and gave similar results.
[14C]Acetate labeling of fatty acids from mycorrhized roots and G. intraradices extraradical mycelium.
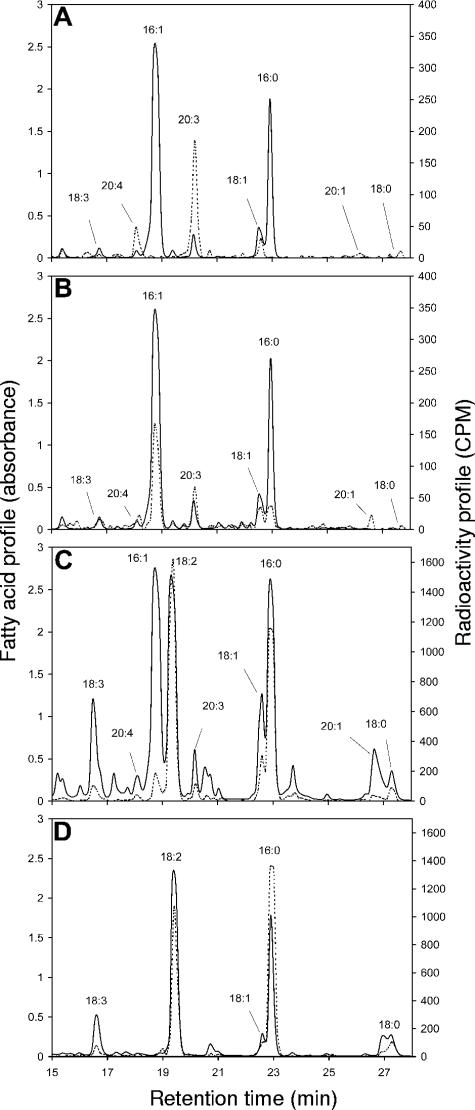
Fatty acids of hyphae and spores fed [14C]acetate in the fungal compartment (Fig. 3A) had labeling patterns very similar to those of germinating spores (Fig. 2). 18:1 and 20:3 were highly labeled, while 16:0 and 16:1 were not labeled. No labeling was detected in the root compartment when the extraradical mycelium of the fungus was fed with [14C]acetate in the fungal compartment (data not shown). When mycorrhized roots in the root compartment were fed [14C]acetate, all of the fungal fatty acids in the fungal compartment were radioactive (Fig. 3B). In the root compartment of the same petri dish, all of the fatty acids were strongly labeled (Fig. 3C). With [14C]acetate labeling, the 18:2/16:1 specific radioactivity ratio was very high at 9.8. By comparison, the fatty acid profile of noncolonized Ri T-DNA-transformed carrot roots (Fig. 3D) contained very little 16:1 and no 20:3. The major fatty acid, 18:2, was strongly labeled.
FIG. 3.
HPLC chromatograms (A242) and [1-14C]acetate-labeled radioactivity measurements (CPM) of fatty acids in compartmented petri dishes. (A) Fatty acid analysis of the fungal compartment (labeling in the fungal compartment). (B) Fatty acid analysis of the fungal compartment (labeling in root compartment). (C) Fatty acid analysis of the root compartment (labeling in the root compartment). (D) Fatty acid analysis of noncolonized roots (labeling in root compartment). Solid lines denote the fatty acid profile, and dotted lines denote the radioactivity profile. The experiment was repeated three times and gave similar results.
[14C]Sucrose labeling of fatty acids from mycorrhized roots or the G. intraradices extraradical mycelium.
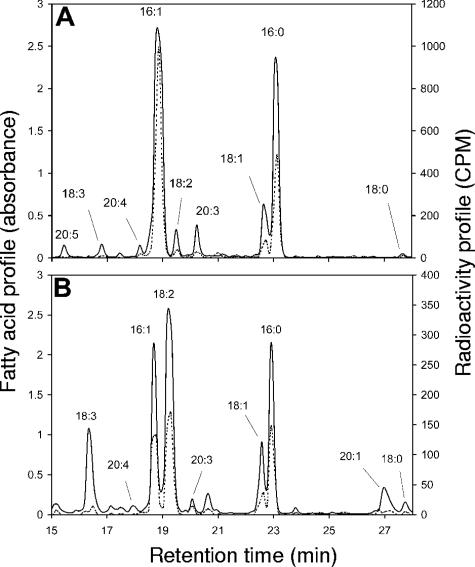
When [14C]sucrose was added to the root compartment, all fatty acids were labeled in both compartments (Fig. 4). In the root compartment, 18:2, 16:0, 16:1, and 18:1 were strongly labeled (Fig. 4B) and some minor fatty acids (18:2, 20:3) also were labeled. The 18:2/16:1 specific radioactivity ratio was relatively low at 0.76. In the fungal compartment, the major labeled fatty acid was 16:1 (Fig. 4A). Strong labeling also was seen for 16:0 and 18:1, while low labeling was observed for 20:3 and 18:2. In contrast, when [14C]sucrose was added to the fungal compartment, no radioactivity was detected in any of the fatty acids, in either compartment.
FIG. 4.
HPLC chromatograms (A242) and radioactivity measurements (CPM) of fatty acids labeled with [U-14C]sucrose in the root compartment. (A) Fungal compartment (G. intraradices); (B) root compartment. Solid lines denote the fatty acid profile, and dotted lines denote the radioactivity profile. The experiment was repeated three times and gave similar results.
[14C]Acetate labeling in germinating spores of G. intraradices and G. rosea in the presence of root exudates.
The presence of root exudates rapidly stimulated the growth and hyphal branching of germinating spores. This branching is easy to observe in germinating spores of G. rosea but not in G. intraradices, which naturally branches without root exudates. However, neither G. rosea nor G. intraradices showed any difference in the [14C]acetate labeling patterns of fatty acids in the presence of root exudates compared to the germinating spore controls (data not shown). Again, there was no labeling of 16-carbon fatty acids.
DISCUSSION
The end product of fungal FAS is palmitic acid (16:0), which can then be elongated and desaturated to produce all of the other fatty acids (34). In germinating spores and the extraradical hyphae of G. intraradices supplied with [14C]acetate, only fatty acids longer than 16 carbons were labeled, which suggests that no de novo fatty acid synthesis by FAS was occurring in these two fungal cell domains. Previous work (27) also found no labeling of 16:1ω5 when extraradical hyphae were supplied with 13C-acetate. In the present study, germinating spores and the extraradical mycelium of G. intraradices could elongate pre-existing 16-carbon fatty acids (16:0 or 16:1) with labeled acetate to form 18- and 20-carbon fatty acids (Fig. 2A and 3A). An equivalent result was obtained with G. rosea, demonstrating that this fungus also lacks FAS activity in germinating spores. That these two phylogenetically distant AM fungi share the same lack of enzymatic activity suggests that the lack of FAS synthase might be a general feature of the early development of AM fungi.
16-Carbon fatty acids of G. intraradices were labeled only when [14C]acetate or [14C]sucrose was added to colonized roots (Fig. 3B and 4A). In the root compartment, plant fatty acids were highly labeled and mixed with the fungal fatty acids. Carrot roots contain only minute levels of 16:1, so this strongly labeled fatty acid was almost exclusively of fungal origin. Both organisms synthesize the other fatty acids, and their origins cannot be distinguished. When radioactive precursors were included in the root compartment, all fungal compartment fatty acids also became highly labeled, especially the most abundant one: 16:1.
A likely hypothesis is that the fungus, which lacks FAS activity, acquires palmitic acid from its plant host and uses it to synthesize 16:1 and longer fatty acids. The large amount of fatty acids typically found in AM fungi could be the result of a very efficient lipid transfer mechanism between the two organisms. The lipid transfer proteins (20) normally associated with plant defense reactions could be produced during hyphal penetration (11) and involved in the transfer mechanism. However, the differential results obtained when labeling the root compartment with [14C]acetate (Fig. 3C) or [14C]sucrose (Fig. 4B) invalidates this hypothesis of massive lipid transfer from the plant root to the fungus as the specific radioactivity ratio between 18:2 (principally of plant origin) and 16:1 (fungal origin) was very high (9.8) when using [14C]acetate. This indicates that the root, much more than the fungus, is using acetate to synthesize fatty acids. This ratio is not surprising since the roots have greater biomass and a larger contact surface with the medium and since acetate enters the cell by simple diffusion. A much lower 18:2/16:1 specific radioactivity ratio (0.76) was obtained when [14C]sucrose was provided. If fungal fatty acids, including 16:1, were synthesized exclusively from the 16:0 plant precursor, a ratio similar to that of [14C]acetate should have been obtained regardless of the radioactive precursor, since the end product used for fatty acid synthesis is [14C]acetyl-CoA. Instead, the sucrose probably is rapidly converted to glucose and fructose in the medium and then taken up by the fungus and used for its own 16-carbon fatty acid synthesis. The difference in the two ratios suggests that the fungus has a hexose transport system that is more efficient than the roots and can overcome the lower fungal biomass and smaller contact surface disadvantages. A similar conclusion regarding hexose transport and efficiency of utilization was drawn in 13C labeling experiments with mycorrhized leek roots and Glomus etunicatum (29) as well as in radiorespirometry experiments with Gigaspora margarita (31).
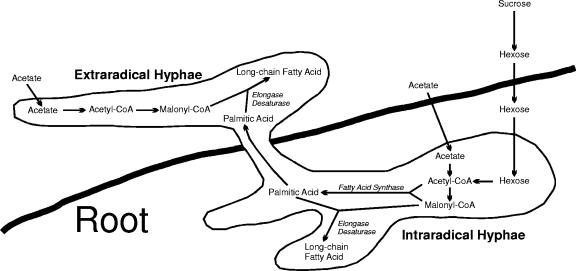
Overall, our data suggest that G. intraradices synthesizes 16-carbon fatty acids exclusively in planta in its intraradical hyphae (Fig. 5). Some AM genes, e.g., the high-affinity phosphate transporters of Glomus versiforme, are expressed only in extraradical hyphae (19), and we suggest that the fatty acid synthase genes of G. intraradices are expressed only in intraradical hyphae. This regulation may be one of the reasons for the obligate symbiosis between AM fungi and their plant hosts. Following the synthesis of the fungal fatty acids, or at least their palmitic acid precursor, by the intraradical mycelium of the fungus, the fatty acids would then be transferred to the extraradical hyphae and spores. Bago et al. (5) had previously observed the movement of lipid bodies along the hyphae in vivo, which is consistent with this hypothesis.
FIG. 5.
Proposed pathway for the synthesis of fatty acids by the extraradical and intraradical mycelium of arbuscular-mycorrhizal fungi when supplied with acetate or sucrose. Fatty acid synthase activity never occurs in the extraradical hyphae.
Our data also indicate that hyphae and spores in the fungal compartment could elongate and desaturate palmitic acids. These data could explain the observation of Fontaine et al. (17), who found in contrast with other work (4, 27) that the fungus could spontaneously synthesize its own fatty acids. However, quantitatively, our data are in agreement with those of Pfeffer et al. (27) who showed, in a similar experimental system, that most fungal lipid synthesis occurs in the root compartment.
We hypothesize that the root apoplast is a unique environment in which the fungus becomes competent for 16-C fatty acid synthesis. This environment might provide some plant signals required to directly or indirectly induce FAS genes and/or enzymatic activity. An active root exudate fraction, presumably containing specific plant signals, is involved in the presymbiotic development of AM fungi (13). This root exudate fraction induces several fungal genes and biochemical activity, e.g., fungal respiration (33). Since fatty acid synthesis was not stimulated in either G. intraradices or G. rosea by this root exudate fraction, we conclude that the root-exuded signals that function during presymbiosis and the putative signal involved in symbiosis are different and that the lack of fatty acid synthesis in germinating spores is not due to insufficient respiration and energy.
Hexose uptake also seems to be particularly active in the intraradical hyphae (27, 29, 31). Whether the induction of fatty acid synthesis and hexose transport is independent or coupled remains to be tested. That both processes are under some type of plant control, however, could provide further clues for understanding the obligate biotrophic nature of AM fungi.
In conclusion, our data clarify and consolidate some contradictory published results concerning the ability (or inability) of mycorrhizal fungi to synthesize their own fatty acids. We found that germinating spores of AM fungi do not have FAS activity but do have the ability to elongate and desaturate pre-existing 16-carbon fatty acids. This enzymatic profile was observed both in G. intraradices (Glomaceae) and in G. rosea (Gigasporaceae), two phylogenetically distant AM fungi. We also found that FAS activity is absent in extraradical hyphae. Our results strongly suggest that fungal FAS activity occurs exclusively in the intraradical mycelia and that its induction probably requires some kind of plant signal. The regulation of fatty acid biosynethesis may be reflective of the obligate symbiosis between AM fungi and their plant hosts.
Acknowledgments
We thank Rénald Boulanger, Caroline Labbé, and Michel Rossignol for technical assistance with HPLC, Marc Béland, Anne-Marie Cantin, Julie Gingras, Anick Viel, and Horst Vierheilig for providing biological material (spore cultivation), and Sébastien Roy for the production of root exudates.
This research was supported by F.C.A.R. and N.S.E.R.C. grants.
REFERENCES
- 1.Amijee, F., and P. Stribley. 1987. Soluble carbohydrates of vesicular-arbuscular mycorrhizal fungi. Mycologist 21:20-21. [Google Scholar]
- 2.Bago, B., and G. Bécard. 2002. Bases of the obligate biotrophy of arbuscular mycorhizal fungi, p. 33-48. In Gianinazzi, S. H. Schüepp, J. M. Barea, and K. Haselwandter (ed.), Mycorrhizal technology in agriculture. Birkhäuser Verlag, Basel, Switzerland.
- 3.Bago, B., P. E. Pfeffer, J. Abubaker, J. Jun, J. W. Allen, J. Brouillette, D. D. Douds, P. J. Lammers, and Y. Shachar-Hill. 2003. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol. 131:1496-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bago, B., P. E. Pfeffer, D. D. Douds, Jr., J. Brouillette, G. Bécard, and Y. Shachar-Hill. 1999. Carbon metabolism in spores of the arbuscular mycorrhizal fungus Glomus intraradices as revealed by nuclear magnetic resonance spectroscopy. Plant Physiol. 121:263-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bago, B., W. Zipfel, R. M. Williams, J. Jun, R. Arreola, P. J. Lammers, P. E. Pfeffer, and Y. Shachar-Hill. 2002. Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiol. 128:108-124. [PMC free article] [PubMed] [Google Scholar]
- 6.Bécard, G., L. W. Doner, D. B. Rolin, D. D. Douds, and P. E. Pfeffer. 1991. Identification and quantification of trehalose in vesicular-arbuscular mycorrhizal fungi by in vitro 13C NMR and HPLC analyses. New Phytol. 118:547-552. [Google Scholar]
- 7.Bécard, G., and J. A. Fortin. 1988. Early events of vesicular-arbuscular mycorrhiza formation on Ri-T-DNA transformed roots. New Phytol. 108:547-552. [DOI] [PubMed] [Google Scholar]
- 8.Beilby, J. P. 1983. Effects of inhibitors on early protein, RNA, and lipid synthesis in germinating vesicular-arbuscular mycorrhizal fungal spores of Glomus caledonium. Can. J. Bot. 29:596-601. [DOI] [PubMed] [Google Scholar]
- 9.Beilby, J. P., and D. K. Kidby. 1980. Biochemistry of ungerminated and germinated spores of the vesicular-arbuscular mycorrhizal fungus, Glomus caledonius: changes in neutral and polar lipids. J. Lipid Res. 21:739-750. [PubMed] [Google Scholar]
- 10.Bentivenga, S. P., and J. B. Morton. 1996. Congruence of fatty acid methyl ester profiles and morphological characters of arbuscular mycorrhizal fungi in Gigasporaceae. Proc. Natl. Acad. Sci. USA 93:5659-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blilou, I., J. A. Ocampo, and J. M. Garcia-Garrído. 2000. Induction of LTP (lipid transfer protein) and PAL (phenylalanine ammonia-lyase) gene expression in rice roots colonized by the arbuscular mycorrhizal fungus Glomus mosseae. J. Exp. Bot. 51:1969-1977. [DOI] [PubMed] [Google Scholar]
- 12.Bonfante, P., and S. Perotto. 1995. Tansley review no. 82: strategies of arbuscular mycorrhizal fungi when infecting host plants. New Phytol. 130:3-21. [Google Scholar]
- 13.Buée, M., M. Rossignol, A. Jauneau, R. Ranjeva, and G. Bécard. 2000. The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol. Plant-Microbe Interact. 13:693-698. [DOI] [PubMed] [Google Scholar]
- 14.Chirala, S. S. 1992. Coordinated regulation and inositol-mediated and fatty acid-mediated repression of fatty acid synthase genes in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 89:10232-10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doner, L. W., and G. Bécard. 1991. Solubilization of gellan gels by chelation of cations. BioTechniques 5:25-29. [Google Scholar]
- 16.Durst, H. D., M. Milano, E. J. Kikta, Jr., S. A. Connelly, and E. Grushka. 1975. Phenacyl esters of fatty acids via crown ether catalysts for enhanced ultraviolet detection in liquid chromatography. Anal. Chem. 47:797-1801. [DOI] [PubMed] [Google Scholar]
- 17.Fontaine, J., A. Grandmougin-Ferjani, and M. Sancholle. 2001. Métabolisme lipidique du champignon endomycorhizien: Glomus intraradices. C. R. Acad. Sci. III 324:847-853. [DOI] [PubMed] [Google Scholar]
- 18.Graham, J. H., N. C. Hodge, and J. B. Morton. 1995. Fatty acid methyl ester profiles for characterization of glomalean fungi and their endomycorrhizae. Appl. Environ. Microbiol. 61:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison, M. J., and M. L. Van Buuren. 1995. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 378:626-629. [DOI] [PubMed] [Google Scholar]
- 20.Kader, J.-C. 1996. Lipid-transfer proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47:627-654. [DOI] [PubMed] [Google Scholar]
- 21.Madan, R., C. Pankhurst, B. Hawke, and S. Smith. 2002. Use of fatty acids for identification of AM fungi and estimation of the biomass of AM spores in soil. Soil Biol. Biochem. 34:125-128. [Google Scholar]
- 22.Munday, M. R. 2002. Regulation of mammalian acetyl-CoA carboxylase. Biochem. Soc. Trans. 30:1059-1064. [DOI] [PubMed] [Google Scholar]
- 23.Newsham, K. K., A. H. Fitter, and A. R. Watkinson. 1995. Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol. Evol. 10:407-411. [DOI] [PubMed] [Google Scholar]
- 24.Nichols, P. D., B. K. Stulp, J. G. Jones, and D. C. White. 1986. Comparison of fatty acid content and DNA homology of the filamentous gliding bacteria Vitreoscilla, Flexibacter, Filibacter. Arch. Microbiol. 146:1-6. [Google Scholar]
- 25.Olsson, P. A., E. Bååth, and I. Jakobsen. 1997. Phosphorus effects on the mycelium and storage structures of an arbuscular mycorhizal fungus as studied in the soil and roots by analysis of fatty acid signatures. Appl. Environ. Microbiol. 63:3531-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsson, P. A., E. Bååth, I. Jakobsen, and B. Söderström. 1995. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol. Res. 99:623-629. [Google Scholar]
- 27.Pfeffer, P. E., D. D. Douds Jr., G. Bécard, and Y. Shachar-Hill. 1999. Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol. 120:587-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sancholle, M., Y. Dalpé, and A. Grandmougin-Ferjani. 2001. Lipids of mycorrhizae, p. 63-92. In B. Hock (ed.), The mycota, vol. 9: fungal association. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 29.Shachar-Hill, Y., P. E. Pfeffer, D. Douds, S. F. Osman, L. W. Doner, and R. G. Ratcliffe. 1995. Partitioning of intermediary carbon metabolism in vesicular-arbuscular mycorrhizal leek. Plant Physiol. 108:7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, S. E., and D. J. Read. 1996. Mycorrhizal symbiosis. Academic Press, London, United Kingdom.
- 31.Solaiman, M. Z., and M. Saito. 1997. Use of sugars by intraradical hyphae of arbuscular mycorrhizal fungi revealed by radiorespirometry. New Phytol. 136:533-538. [DOI] [PubMed] [Google Scholar]
- 32.St-Arnaud, M., C. Hamel, B. Vimard, M. Caron, and J. A. Fortin. 1996. Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycol. Res. 100:328-332. [Google Scholar]
- 33.Tamasloukht, B., N. Séjalon-Delmas, A. Kluever, A. Jauneau, C. Roux, G. Bécard, and P. Franken. 2003. Root factors induce mitochondrial-related-gene expression and fungal respiration during the developmental switch from asymbiosis to presymbiosis in the arbuscular mycorrhizal fungus Gigaspora rosea. Plant Physiol. 131:1469-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakil, S. J., J. K. Stoops, and V. C. Joshi. 1983. Fatty acid synthesis and its regulation. Annu. Rev. Biochem. 52:537-579. [DOI] [PubMed] [Google Scholar]