Summary
UFMylation is a ubiquitin-like protein modification of Ubiquitin Fold Modifier 1 (UFM1) applied to substrate proteins and regulates several cellular processes such as protein quality control. Here, we describe the development of an antibody-based enrichment approach to immunoprecipitate remnant UFMylated peptides and identification by mass spectrometry. We used this approach to identify >200 UFMylation sites from various mouse tissues, revealing extensive modification in skeletal muscle. In vivo knockdown of the E2 ligase, UFC1, followed by enrichment and analysis of remnant UFMylated peptides quantified concomitant down-regulation and validation of a subset of modification sites, particularly myosin UFMylation. Furthermore, we show that UFMylation is increased in skeletal muscle biopsies from people living with amyotrophic lateral sclerosis (plwALS). Quantification of UFMylation sites in these biopsies with multiplexed isotopic labeling reveal prominent increases in myosin UFMylation. Our data suggest that in vivo UFMylation is more complex than previously thought.
Keywords: Ubiquitin Fold Modifier 1, UFM1, UFMylation, ubiquitin-like modification
Graphical abstract

Highlights
-
•
Development of a method to site specifically identifies UFMylation sites in vivo
-
•
Generation of anti-VG-ε-K antibody clones to capture “remnant VG” UFMylated sites
-
•
Antibody-based enrichment, isotopic labeling, and LC-MS/MS for UFMylome quantification
-
•
Characterization of myosin UFMylation in mouse muscle and human ALS muscle biopsies
Motivation
Our knowledge of the suite of proteins that are post-translationally modified with Ubiquitin Fold Modifier 1 (UFM1) is limited. Such characterization of the UFMylome would enhance our functional understanding of this elusive modification. Here, we develop an antibody-based enrichment approach to immunoprecipitate remnant UFMylated peptides followed by site-specific identification by mass spectrometry to quantify the UFMylome in vivo.
UFMylation is the post-translational modification of Ubiquitin Fold Modifier1 (UFM1) applied to substrate proteins. Blazev et al. develop an antibody-based enrichment approach followed by LC-MS/MS to identify and quantify the in vivo UFMylome.
Introduction
The Ubiquitin Fold Modifier 1 (UFM1) covalently modifies Lys residues of substrate proteins in a process called UFMylation and has been implicated in various cellular processes and diseases.1,2 UFMylation begins with removal of the C-terminal Ser84Cys85 residues of UFM1 by UFSP1/2 to generate UFM1ΔSC, which is subsequently activated by forming a thioester bond with UBA5, a UFM1-specific E1-activating enzyme. Next, activated-UFM1ΔSC is transferred to the UFM1-specific conjugating E2 enzyme, UFC1. Together with the UFM1-specific ligase E3 complex (UFL1, DDRGK1, and CDK5RAP3), UFM1ΔSC is then transferred to substrate Lys residues.3,4 The Lys residues of conjugated-UFM1 can then undergo further poly-UFMylation.5,6 Currently, <15 UFMylation substrates have been identified primarily via expression of exogenously epitope-tagged UFM1 and enrichment from cell culture models (for a summary see Komatsu et al.2). Here, we describe an enrichment method coupled to liquid chromatography-tandem mass spectrometry (LC-MS/MS) to site specifically identify and quantify endogenous UFMylation sites in vivo. We applied this method to quantify UFMylation sites in human skeletal muscle biopsies from people living with amyotrophic lateral sclerosis (plwALS) and healthy age-matched controls revealing extensive changes in myosin UFMylation.
Results
We generated an antibody-based enrichment strategy coupled to LC-MS/MS to identify in vivo UFMylation sites. The approach is based on the identification of ubiquitination sites using tryptic peptide immunoprecipitation with the “remnant diGly” anti-GG-ε-K antibody.7 Briefly, following the generation of UFM1ΔSC, the sequential action of UFMylation E1-E2-E3 ligases transfer UFM1ΔSC to substrate Lys residues within cells (Figure 1A). Proteins are extracted with denaturing buffers and proteolytically digested with trypsin, which cleaves C-terminal to Lys and Arg residues. Trypsin cleaves after Arg81 on UFM1ΔSC-conjugated substrate proteins and leaves a “remnant” ValGly (VG) attached to the substrate Lys via an isopeptide bond (VG-ε-K isopeptide). The resulting remnant VG-modified peptide is unique to UFMylation, among other ubiquitin-like modifications. We generated three monoclonal pan-anti-VG-ε-K antibody clones to immunoprecipitate remnant VG UFMylated sites independently from the surrounding amino acid context. ELISA showed that the antibody clones had ∼6- to 17-fold enhanced specificity to VG-ε-K-containing peptides compared to GG-ε-K-containing peptides (Figure 1B). To screen these antibody clones, tryptic peptides from mouse gastrocnemius skeletal muscles were immunoprecipitated with each of the three variants or a pooled cocktail followed by two-dimensional (2D)-LC-MS/MS and analysis with two different search algorithms (Sequest+Percolator and MSFragger+PTM-Prophet), each offering distinct statistical models for the interpretation of MS/MS spectra providing orthogonal validation.8 In total, we identified 385 unique VG-modified peptides with either search algorithm, with each clone identifying unique subsets, and the pooled cocktail identifying the greatest number (Figures 1C–1E). The increase in identified peptides using pooled antibody clones is consistent with previous enrichment and identification of lysine acetylated peptides.9 Motif analysis identified no major sequence specificity but a slight upstream acidic amino acid residue preference for all clones, where it was most prominent for clone 2 (Figure 1F). This is interesting as a slight upstream basic amino acid preference has been observed in the diGly-modified proteome7; however, we cannot rule out that our antibodies show sequence bias. A total of 199 unique VG-modified peptides were identified by both algorithms representing the greatest confidence (Figure 1G; Table S1). This includes the previously identified and independently validated UFMylation site of Lys134 on RPL26.6,10 Because a high proportion of the skeletal muscle proteome comprises contractile proteins, we sought to test the capacity of our methods to identify UFMylation of other substrates across other tissue and cell types. Comparing global UFMylation abundance using anti-UFM1 western blot in various mouse and human cells and in various mouse tissues revealed consistent and prominent bands at ∼30 and 60 kDa (Figure 1H). However, the tissue samples exhibited increased immunoreactivity across a range of molecular weights, suggesting more complex UFMylation in vivo, including tissue-specific substrates. We next performed tryptic peptide immunoprecipitations in these mouse tissues using the pooled anti-VG-ε-K antibody cocktail, followed by 2D-LC-MS/MS, and considered only data identified by both search algorithms (Table S1). Figure 1I plots the number of VG-containing peptide spectral matches (PSMs), unique peptides, and sites identified by both search algorithms for each tissue. A total of 250 unique VG-containing peptides from a total of 160 proteins were identified, and enrichment analysis identified the over-representation of proteins associated with the contractile apparatus or localized to extracellular, endoplasmic/sarcoplasmic reticulum (ER/SR) or the mitochondrial Gene Ontology (GO) compartments (Figure 1J). RPL26 UFMylation was identified across multiple tissues, but other previously identified sites on histone H4,11 p53,12 ASC1,5 CYB5R3,13 and DDRGK13 were not identified. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed that UFMylated proteins were associated with central carbon metabolism, amino acid/glucose metabolism, and proteins involved in muscle contraction/cardiomyopathy (Figure 1K). Analysis of UFMylated proteins forming interaction networks in the STRINGdb identified interconnected associations of the contractile apparatus, proteins involved in calcium handling at the ER/SR, glucose metabolism, and translational regulators (Figure 1L). We also identified VG modification of Lys19 and Lys69 on UFM1, providing direct evidence for the presence of di- or poly-UFMylation.5,6
Figure 1.
Enrichment and site-specific identification of the UFMylome
(A) Overview of UFMylation and schematic for the enrichment of VG-modified peptides and identification by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
(B) Analysis of antibody specificity to VG-ε-K- compared to GG-ε-K-containing peptides by ELISA.
(C) Number of VG-peptide spectral matches (PSMs) and unique VG-peptides/VG-sites identified by each of the antibody clones or the pooled cocktail.
(D and E) Overlap of VG-peptides identified by each antibody clone.
(F) Motif enrichment of the amino acids surrounding the UFMylation sites.
(G) Overlap of VG-peptides identified by each search algorithm.
(H) Anti-UFM1 western blot of various mouse and human cell lines, and various mouse tissues.
(I) Number of VG-PSMs and unique VG-peptides/VG-sites identified in each mouse tissue.
(J and K) (J) Gene Ontology (GO), and (K) Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the UFMylated proteins.
(L) STRINGdb analysis of the UFMylated proteins.
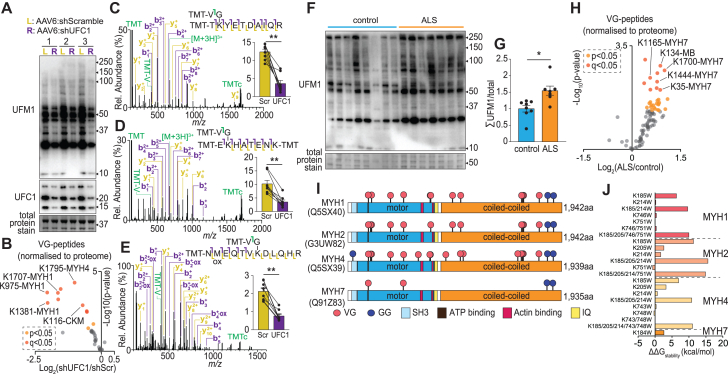
We next sought to validate a subset of skeletal muscle UFMylation sites by knocking down UFC1, which should result in a concomitant decrease in their abundance. Mouse extensor digitorum longus muscles were injected with recombinant adeno-associated virus serotype 6 (rAAV6), with the left leg receiving scramble negative control small hairpin RNA (shRNA) and the right contralateral leg receiving UFC1 shRNAs. Muscles were harvested after 28 days, and western blotting revealed a decrease in UFC1 protein levels, with a concomitant decrease in global UFMylation (Figure 2A). Tryptic peptides were immunoprecipitated using pooled anti-VG-ε-K antibody clones followed by multiplex stable isotope labeling with tandem mass tags (TMTs) and analysis by 2D-LC-MS/MS. A total of 22 unique VG-containing peptides were quantified in every sample, and a general decrease in UFMylation was observed following knockdown of UFC1 (Figure 2B; Table S2). The intensity of VG-containing peptides was normalized to total protein levels obtained via a parallel proteomic analysis without anti-VG-ε-K enrichment. This identified 10 down-regulated VG-containing peptides at a q < 0.05 using Benjamini-Hochberg false discovery rate (FDR), while 12 were down-regulated at a less strict p < 0.05. The MS/MS spectra of the most down-regulated peptides were manually annotated and mapped to multiple myosin isoforms given their shared sequence similarity such as Lys1381/1381/1375/1378/1381 on MYH1/2/3/4, respectively; Lys975/975/969/972 on MYH1/2/3/4, respectively; and 1795/4 on MYH4/8, respectively (Figures 2C–2E). The fewer VG-containing peptides identified in these knockdown experiments compared to the data described above are likely due to the >10-fold less available starting material (100 μg/sample) combined with the reduced identification efficiency of highly charged TMT-labeled peptides.14 Despite this, our data validate that myosin is UFMylated in vivo.
Figure 2.
Site-specific quantification of the UFMylome identifies a differential signature in human skeletal muscle associated with ALS and specific sites of UFMylation on myosin proteins
(A) Anti-UFM1 western blot of mouse extensor digitorum longus (EDL) skeletal muscles treated with recombinant adeno-associated virus serotype 6 (rAAV6) harboring scramble negative control shRNA (L: left leg) or UFC1 shRNA (R: right leg).
(B) Volcano plot of quantified VG-peptides from mouse EDL following knockdown of UFC1.
(C–E) Annotated MS/MS of VG-containing peptides from (C) Lys1381/1381/1375/1378/1381 on MYH1/2/3/4, (D) Lys975/975/969/972 on MYH1/2/3/4, and (E) 1795/4 on MYH4/8, respectively. Inserts show log2(area under the curve) normalized to total myosin levels. ∗∗q < 0.01; paired Student’s t test with Benjamini-Hochberg FDR. Error bars: SEM.
(F) Anti-UFM1 western blot of skeletal muscle biopsies from individuals diagnosed with ALS and age-matched controls.
(G) Immunoreactivity densitometry of the entire lane. ∗p < 0.05; unpaired Student’s t test. Error bars: SEM.
(H) Volcano plot of quantified VG-peptides from human individuals.
(I) VG- and GG-peptides identified on myosin purified from mouse skeletal muscle.
(J) Modeling the effects of Lys>Trp substitutions on myosin stability.
Skeletal muscle UFMylation has been shown to increase in the SOD1(G37R) mouse model of ALS.15 To investigate whether this is also observed in humans, we analyzed biopsies of vastus lateralis skeletal muscle from plwALS and age-matched controls and previously showed that these plwALS display disease-associated atrophy.16 Western blotting revealed a conserved increase in the UFMylation of several proteins (Figures 2F and 2G). To identify and quantify these UFMylation sites, tryptic peptides were immunoprecipitated from each of the participants’ biopsies using pooled anti-VG-ε-K antibody clones followed by TMT labeling and analysis by 2D-LC-MS/MS. A total of 56 unique VG-containing peptides were quantified in every biopsy, and a general increase in UFMylation was observed in plwALS (Figure 2H; Table S2). Nine unique VG-containing peptides were up-regulated at a q < 0.05 using Benjamini-Hochberg FDR, while 24 were up-regulated at a less strict p < 0.05. The increase in abundance of these VG-containing peptides was observed independent of changes in their corresponding protein abundance, suggesting site-specific changes in UFMylation. The most significantly up-regulated UFMylation sites were observed on the contractile protein Conventional Myosin-7 (MYH7). Other up-regulated UFMylation sites at the less stringent cutoff were on other contractile and/or structural proteins such as Troponin C1 and Titin. To provide a more detailed analysis of UFMylation and potential interplay with ubiquitinylation, we next purified myosin isoforms from mouse tibialis anterior muscle and analyzed tryptic peptides by LC-MS/MS. Twenty unique VG-containing peptides and four unique GG-containing peptides were identified (Table S2). The majority of these VG-/GG-containing peptides mapped to multiple myosin isoforms given their shared sequence similarity. Mapping VG- and GG-sites onto the primary amino acid sequences of the major isoforms of MYH1, MYH2, MYH4, and MYH7 revealed several interesting clusters (Figure 2I). For example, Lys185 and Lys214 on MYH1/2/4, Lys 204 on MYH2/4, and Lys184 on MYH7 are directly adjacent to the ATP binding site. Other interesting sites include Lys746 on MYH1, Lys743 on MYH4, and Lys751 on MYH1/2, which are between the two actin-binding domains. To investigate potential functional impacts, we used EvoEF, an in silico modeling approach to estimate the effects of single amino acid substitutions on protein stability using physical energy function.17 Here, Lys residues were mutated to Trp either in isolation or in combinations. Mutation of residues adjacent to the ATP binding site dramatically increased ΔΔGStability of >10 kcal/mol, while the sites between the two actin-binding domains had little impact (Figure 2J). For context, the observed ΔΔGStability changes constitute a >10-fold increase in predicted stability compared to myosin phosphorylation.18 Although this in silico approach does not directly assess the functional impact of myosin UFMylation, it highlights that modifications of specific amino acids are highly likely to alter the function of myosin. Our site-specific identification of UFMylation has validated myosin as a target of this modification that is up-regulated during ALS in human patients.
Discussion
To date, knowledge of how UFMylation influences the proteome has been limited by challenges associated with identifying proteins specifically targeted by the UFMylation system. To address this limitation, we developed an approach to identify and quantify the endogenous UFMylome in vivo using tryptic peptide immunoprecipitation with pan-anti-VG-ε-K antibodies coupled with multiplexed stable isotope labeling and LC-MS/MS. This approach provides distinct advantages over traditional approaches to identify UFMylated substrates, which rely on the expression of exogenously epitope-tagged UFM1, followed by protein-level enrichment. First, peptide-level enrichment may increase the ability of the mass spectrometer to sequence the VG-containing peptides to pinpoint the modification sites. Second, samples can be lysed in very harsh denaturing conditions to inhibit all enzyme activity, whereas these lysis buffers may not be compatible with epitope-tagged protein-level enrichment. Additionally, our approach avoids the need for the overexpression of exogenously epitope-tagged UFM1 to unnaturally high concentrations that may result in non-physiological substrate UFMylation. This phenomenon has been observed with high levels of ubiquitin leading to the ubiquitylation of non-physiological substrates.19,20 It is worth noting that several previous studies identifying UFMylated substrates have also combined ectopic expression of tagged-UFM1 with genetic manipulations of the E1-E2-E3 ligase system to further boost cellular UFMylation levels.
Our data have identified >200 UFMylation sites and reveal that this modification is more widespread than previously thought. The UFMylation machinery is enriched at the ER, and CRISPR screens have identified a key role of this modification in various ER stress responses.6,21 A principal mechanism is via the UFMylation of RPL26 and the regulation of ribosome-associated quality control in stalled translocons.10,22,23 In addition to RPL26, we identified other UFMylated proteins enriched at the ER/SR, including CASQ1, RYR1, and ATP2A1/2, which play critical roles in calcium handling. As UFMylation negatively regulates skeletal muscle contractile function,15 and given that we observed UFMylation of both calcium handling proteins and the contractile apparatus, particularly myosin, future experiments could delineate the exact role of this modification upon the excitation-contraction pathway. The extensive modification of myosin also warrants further investigation, particularly regarding sites adjacent to the ATP binding domain, which, given our in silico predictions, suggests large changes in stability following the mutation of these amino acids. Furthermore, whether the activation of UFMylation is causal or protective in diseases such as ALS warrants further investigation.
Limitations of the study
While our approach for identification and quantification of substrate UFMylation offers specific advantages, a potential limitation of our VG-peptide-level enrichment and identification of remnant UFMylation is that the required trypsin cleavage results in the loss of information on site-specific polyUFMylation or linkage patterns. The use of trypsin also has limitations in that complete sequence coverage is not often obtained, and orthogonal proteases may be required for comprehensive coverage. Our study only used male mice because our goal was to simply characterize the developed antibodies; however, future work is warranted to investigate potential sex differences.
Furthermore, UFMylation appears to be very low in abundance and requires a significant amount of protein input, meaning our approach may not be suitable for studies involving limited sample amounts. As such, we envisage that considered application of our techniques will help to expand the understanding of UFMylation across cell types and biological contexts, but ongoing investigation of opportunities to develop complementary methods hold the potential to further expand insight into the full scale of UFMylation complexity.
Resource availability
Lead contact
Requests for further information, resources, and reagents should be directed to and will be fulfilled by the lead contact, Benjamin L. Parker (ben.parker@unimelb.edu.au).
Materials availability
This study generated three monoclonal pan-anti-VG-ε-K antibody clones, which are available from the lead contact upon reasonable request.
Data and code availability
-
•
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE24 partner repository with the dataset identifier PXD051412 and are publicly accessible as of the date of publication.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
We thank Nicholas Williamson, Ching-Seng Ang, Shuai Nie, Swati Varshney, and Michael Leeming for instrument support in the Bio21 Mass Spectrometry and Proteomics Facility. We thank all plwALS and control individuals who participated in this study. This work was funded by an Emerging Leader Investigator grant (APP2009642) from the National Health and Medical Research Council (NHMRC) (Australia) and a University of Melbourne Driving Research Momentum grant to B.L.P., a Motor Neurone Disease Research Australia Charcot grant and NHMRC Ideas grant 1185427 to F.J.S. and S.T.N., the Scott Sullivan MND Research Fellowship (University of Queensland, Royal Brisbane & Women’s Hospital, and the MND and Me Foundation) to S.T.N., and an NHMRC Investigator Grant (APP2017070) to P.G. This work was also supported by a Department of Anatomy and Physiology (The University of Melbourne) ECR Seeding Grant to R.B. Y.-K.N. is the recipient of a School of Biomedical Science Postgraduate Award.
Author contributions
R.B., B.M.Z., H.P., Y.-K.N., C.T.A.L., C.Z., J.W.M., C.A.G., and B.L.P acquired and analyzed the data. F.J.S. and S.T.N. generated the human samples. J.O., P.G., M.P.S., and B.L.P. supervised the work. All authors approved the manuscript.
Declaration of interests
B.M.Z., H.P., and M.P.S. are employees of Cell Signaling Technology. C.T.A.L. is an employee of Novo Nordisk A/S.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| UFM1 | abcam | Abcam Cat# ab109305, RRID:AB_10864675 |
| UFC1 | abcam | ab189251 [EPR15014] |
| UFSP2 | abcam | ab192597 [EP13424-49] |
| VG-ε-K | This paper | N/A |
| Bacterial and virus strains | ||
| Recombinant adeno-associated virus serotype 6 – scramble shRNA (5′ GATCGAATGTGTACTTCGA) | This paper | N/A |
| Recombinant adeno-associated virus serotype 6 – mouse UFC1 shRNA (5′ CTGGAGACAACCTGTC AATAAA; 5′ GCAAGGTCACAAAGTTTCTAAA; 5′ TCCACGATTTCCTCAAATATGA) |
This paper | N/A |
| Biological Samples | ||
| Human muscle biopsies from people living with ALS and healthy controls | Wong et al.25 | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Dulbecco’s Modified Eagle Medium | GIBCO by Life Technologies | 11995065 |
| fetal bovine serum (FBS) | GIBCO by Life Technologies | 26140079 |
| GlutaMAX | GIBCO by Life Technologies | 35050061 |
| Sodium pyruvate | GIBCO by Life Technologies | 11360070 |
| Guanidine hydrochloride | Sigma-Aldrich | G4505 |
| Tris(2-carboxyethyl)phosphine | Sigma-Aldrich | 75259 |
| 2-Chloroacetamide | Sigma-Aldrich | 22790 |
| Trifluoroethanol | Sigma-Aldrich | T63002 |
| HEPES | Sigma-Aldrich | H3375 |
| Tris hydrochloride | Sigma-Aldrich | 9310 |
| Tween | Sigma-Aldrich | P1379 |
| BCA Protein Assay | Thermo Fisher Scientific | 23325 |
| Laemelli Buffer | BioRad | 1610747 |
| MOPS SDS Running Buffer | Thermo Fisher Scientific | NP0001 |
| Sequencing grade trypsin | Sigma-Aldrich | T6567 |
| LysC | Wako | 129–02541 |
| Western Chemiluminescent HRP Substrate | Millipore | WBKLS0500 |
| BLOt-FastStain | GBiosciences | 786–34 |
| Trifluoroacetic acid | Thermo Fisher Scientific | FSBA116 |
| Tandem Mass Tags 16-plex | Thermo Fisher Scientific | A44520 |
| MOPS | Sigma-Aldrich | M5162 |
| Sodium phosphate monobasic | Sigma-Aldrich | S0751 |
| Sodium Chloride | Sigma-Aldrich | S9888 |
| LC-MS water | Thermo Fisher Scientific | 047146 |
| LC-MS acetonitrile | Thermo Fisher Scientific | A9551 |
| LC-MS isopropanol | Thermo Fisher Scientific | A4614 |
| Acetone | Sigma-Aldrich | 270725 |
| Ammonium hydroxide | Sigma-Aldrich | 5.33003 |
| Formic acid | Thermo Fisher Scientific | 28905 |
| Ammonium formate | Sigma-Aldrich | 70221 |
| Potassium chloride | Sigma-Aldrich | P9333 |
| Sodium pyrophosphate decahydrate | Sigma-Aldrich | 221368 |
| Magnesium chloride | Sigma-Aldrich | M8266 |
| Imidazole | Sigma-Aldrich | I202 |
| Dithiothreitol | Sigma-Aldrich | 43815 |
| Protease inhibitor cocktail | Sigma-Aldrich | P8340 |
| EGTA | Sigma-Aldrich | E4378 |
| Critical commercial assays | ||
| DC protein assay kit | Biorad | 5000111 |
| Deposited data | ||
| Mass spectrometry proteomics | This paper | ProteomeXchange Dataset Identifier: PXD051412 |
| Experimental models: Cell lines | ||
| HEK-293 | ATCC | CRL-1573 |
| HeLa | ATCC | CCL-2 |
| C2C12 | ATCC | CRL-1772 |
| Experimental models: Organisms/strains | ||
| C57BL/6J mice | Animal Resource Center (Australia) | JAX 000664 |
| New Zealand White rabbits | Cell Signaling Technology | N/A |
| Software and algorithms | ||
| MS Fragger | Kong et al.26 | https://msfragger.nesvilab.org/ |
| Perseus | Tyanova et al.27 | https://maxquant.net/perseus/ |
| EvoEF | Huang et al.28 | https://zhanggroup.org/EvoEF/ |
Experimental model and study participant details
Human ALS participants and experimental procedures
Eight participants with ALS who met the revised El-Escorial criteria for ALS were enrolled from the Royal Brisbane and Women’s Hospital (RBWH) motor neuron disease clinic for the collection of skeletal muscle biopsies along with eight healthy control participants who were the spouses, friends or family members of ALS participants. The study was approved by the RBWH and University of Queensland human research ethics committees. Patient characteristics and ethics approval is provided in a previous publication.26 Biopsy samples were collected, and lysates were generated as described previously.26
Cell culture
C2C12, HEK293T and HeLa cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) (GIBCO by Life Technologies; # 11995065), supplemented with 10% fetal bovine serum (FBS) (Life Technologies; #26140079), pyruvate and GlutaMAX (GIBCO by Life Technologies). Cells were kept at 37°C and 5% CO2 in a humidified incubator Direct Heat CO2 Incubator featuring Oxygen Control (In Vitro Technologies).
Mouse housing and rAAV6 intramuscular injection
All mouse experiments were approved by The University of Melbourne Animal Ethics Committee (#26527), and adhere to the guidelines set out by the National Health and Medical Research Council of Australia for the care and use of animals for scientific purposes. Male C57BL/6J mice were housed at 22°C (±1°C) in groups of five/cage and maintained on a Standard Chow diet (Specialty Feeds, Glen Forest, WA, Australia, #SF15-049) with a 12-h light/dark cycle and ad libitum access to food and water. rAAV6 production, purification and shRNA sequences are previously described.15 For intramuscular injections of rAAV6, mice were anesthetized with isoflurane (4% in oxygen at 1L/min) and then transferred to a dissecting microscope stage with heat pad and isoflurane inhalation nose piece (2% in oxygen at 1 L/min). Unconsciousness was assessed via the lack of leg and optical reflexes for at least 1 min to ensure head position does not affect normal breathing. Mice received subcutaneous analgesic injection of meloxicam between the shoulder blades (5 mg/kg) and the surface of the hindlimbs were sterilized with 80% ethanol. The EDL muscles were injected with 2 x 1010 vector genomes/30 μL of rAAV6 using a 32G needle. Mice were returned to cages and body weights monitored daily for the first 3 days and then weekly prior to cull at 28 days post-injection.
Rabbit housing
New Zealand White rabbits were housed at 22°C (±1°C) in groups with a 12-h light/dark cycle and ad libitum access to food and water. All procedures involving rabbits were overseen by the Institutional Animal Care and Use Committees (IACUC), ensuring compliance with ethical standards throughout the study.
Method details
Anti-VG-ε-K antibody production and bead production
New Zealand White male and female rabbits 3–6 months old were immunized with a KLH-conjugated degenerate peptide library containing a VG-remnant modified lysine (n = 6; 3/sex). Serum samples were screened for PTM specificity by ELISA. Monoclonal antibodies were then generated using proprietary methods developed at Cell Signaling Technology, Danvers, MA. Each IP was performed with 1:10 antibody:protein ratio e.g., 1mg of antibody:10mg of digested mouse protein lysate or 100 μg of antibody:1 mg of digested human muscle biopsy protein lysate. Antibodies were incubated on Protein-A agarose beads at a ratio of 100 μg of antibody/30 μL of beads overnight at 4°C with rotation. The beads were washed four times with 1 mL of PBS and used immediately for the enrichment. The pooled cocktail mix was generated by conjugating each antibody clone and then pooling the beads. For comparison of antibody clones vs. the pooled antibody cocktail, the amount of antibody on the beads was constant e.g., 1 mg for individual clones vs. 3 × 333 μg of individual clones mixed together.
Lysate preparation
Tissue was lysed by tip-probe sonication in 6M guanidine HCl (Sigma-Aldrich, St. Louis, MO, USA, #G4505), 100mM Tris pH 8.5 containing 10mM Tris(2-carboxyethyl)phosphine (Sigma-Aldrich, #75259) and 40mM 2-Chloroacetamide (Sigma-Aldrich, #22790). The lysate was then heated at 95°C for 5 min and centrifuged at 18,000 g for 30 min at 4°C. The supernatant was diluted 1:1 with LC-MS/MS water and precipitated overnight in a final concentration of 80% acetone at −30°C. The lysate was centrifuged at 4,500 g for 5 min at 4°C and the supernatant was disregarded. The protein pellet was washed with 80% ice-cold acetone and centrifuged at 4,500 g for 5 min at 4°C, then resuspended in digestion buffer (10% trifluoroethanol (Sigma-Aldrich; T63002) in 100 mM HEPES pH 7.5). Protein was quantified using a BCA protein assay (Thermo Fisher Scientific, #23325) and normalized to 2μg/μL containing Laemelli buffer (BioRad, Hercules, CA, USA, #1610747) for western blotting. For comparison of the 3 antibody clones and pooled cocktail, 4 × 10 mg protein aliquots of the identical mouse skeletal muscle was normalized to 1 mL each. For enrichment of VG-peptides from mouse tissues, 10mg of protein was normalized to 1 mL of digestion buffer, while for enrichment from human skeletal muscle biopsies, 1mg of protein was normalized to 200 μL. Protein lysates were digested in sequencing grade trypsin (Sigma-Aldrich, #T6567) and LysC (Wako, Chuo-Ku, Osaka, Japan, #129–02541) (1:100 enzyme:substrate ratio) overnight at 37°C with shaking at 2000 rpm.
Western blotting
Proteins were separated on NuPAGE 4–12% Bis-Tris protein gels (Thermoscientific) in MOPS SDS running buffer at 145V for 60 min at room temperature. Proteins were then transferred to PVDF membranes (Millipore, Burlington, MA, USA, #IPFL00010) in NuPAGE transfer buffer at 20V for 60 min at room temperature. Membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for at least 60 min at room temperature with gentle shaking. Membranes were then incubated overnight at 4°C with gentle shaking in anti-UFM1 antibody (1:1000) (Abcam; ab109305) containing 5% BSA, 0.02% NaN3 in TBST. Membranes were incubated with HRP-secondary antibody in 5% skim milk in TBST for at least 60 min at room temperature with gentle shaking. Immunoreactivity was visualized with Immobilon Western Chemiluminescent HRP Substrate (Millipore, #WBKLS0500) and imaged on a ChemiDoc (BioRad). Membranes were stained with BLOt-FastStain (GBiosciences; 786-34) for protein visualization. Densitometry was performed in ImageJ.27
Peptide purification and anti-VG-ε-K enrichment
Peptides were acidified by Trifluoroacetic acid (TFA) (Thermo Fisher Scientific, #FSBA116) to a final concentration of 1% TFA and centrifuged at 16,000 g for 10 min at room temperature. Peptides were desalted using reversed-phase chromatography with HLB-SPE columns (Waters, Milford, MA, USA) and washed with 0.1% TFA, then eluted with 80% acetonitrile, containing 0.1% TFA. Peptides were vacuum-dried at 45°C. Peptides were resuspended in 500 μL of Immunoprecipitation buffer (50mM MOPS, 10mM NaH2PO4, 50mM NaCl, pH 7.5), and adjusted to a pH of 7.5 with 5M NaOH, then centrifuged at 16,000 g for 5 min at 4°C. The supernatant containing peptides were rotated with antibody:Protein-G bead complex overnight at 4°C. The beads were washed three times with 1mL of IP buffer and then twice with 1 mL of LC-MS/MS water. Peptides were eluted twice with 75 μL of 0.2% TFA for 5 min at 10°C with shaking at 1200 rpm, and beads filtered using in-house packed C8 tips (3M, Saint Paul, MN, USA, #11913614). Enriched peptides from mouse tissues were purified through in-house packed SDB-RPS columns as described below. Enriched peptides from human skeletal muscle lysates were dried by vacuum centrifugation and resuspended in 10 μL 200mM HEPES, pH 7.4, for 5 min with shaking at 2000 RPM, and then 80 μg/10 μL of TMTpro-16plex in 100% acetontrile was added, followed by 1 h incubation at room temperature (Thermo Fisher Scientific, #A44520). The labeling scheme and sample identification has been uploaded to the PRIDE Proteomics repository as described below. The reaction was deacylated to a final concentration of 0.3% hydroxylamine then quenched with 1% TFA. All 16 samples were pooled together and purified through in-house packed SDB-RPS (Sigma-Aldrich, #66886-U) tip, and washed with 99% isopropanol, 1% TFA followed by 5% acetonitrile, 0.2% TFA, then eluted with 80% acetonitrile, 5% NH4OH. Sample was resuspended in 2% acetonitrile, 0.1% TFA and stored at −80°C, then fractionated using a HpH C18 column into 12 concatenated fractions as previously described.28 Briefly, fractionation was performed on a Dionex 3500 equipped with a UV detector. Enriched peptides were injected onto a C18BEH 0.3 mm ∗ 150 mm column containing 1.7 μm particles (Waters) at a flow rate of 5 μL/min. Separation was achieved using a 50-min gradient of 5–40% Buffer B (Buffer A = 10 mM ammonium formate pH 7.9; Buffer B = 90% acetonitrile). A total of 48 fractions were collected and automatically concatenated into 12 fractions in a looping fashion directly into a 96-well plate which was dried by vacuum centrifugation. Peptides were resuspended in 2% acetonitrile containing 0.1% TFA and store at −80 prior to analysis.
Myosin heavy chain purification
Myofibrillar protein purification was performed based on the method of.25 Tibialis anterior (TA) muscles from 10 weeks old male C57Bl/6 mice were homogenised for 2 × 20 s with an Omni Homogeniser (TH-02, Omni International, Kennesaw, GA, USA) in 2mL of buffer containing: 300 mM KCl, 10 mM Na4P2O7, 1.0 mM MgCl2, 150 mM Imidazole, 1.0 mM DTT, Protease inhibitor cocktail (P8340, Sigma, St Louis, MO, USA), pH 6.8. Samples were rotated for 90 min at 4°C and then centrifuged at 178,000 x g for 90 min at 4°C. The resulting supernatant was added to 32 mL of ice-cold H2O containing 1.0 mM DTT and protease inhibitor and incubated on ice for 60 min. After centrifugation at 20,000 x g at 4°C, the supernatant was carefully removed and the remaining pellet washed with cold H2O and then resuspended in 200 μl of storage buffer containing: 300 mM KCl, 1.0 mM EGTA, 4.0 mM MgCl2, 25mM Imidazole, 1.0 mM DTT and protease inhibitor cocktail, pH 7.3. Protein concentration was quantified using a DC protein assay kit (Bio-Rad, Hercules, CA). Proteins (6ug total) were then separated by SDS-PAE on a 4–20% gradient tris/glycine gel (Bio-Rad, Hercules, CA), and protein bands were visualized after Coomassie blue staining and destaining.
LC-MS/MS data acquisition
For analysis of mouse tissues, enriched and fractionated peptides were analyzed on a Dionex 3500 nanoHPLC coupled to an Orbitrap Exploris 480 mass spectrometer (Thermo Fisher Scientific) via electrospray ionisation in positive mode with 1.9 kV at 275°C and RF set to 40%. Separation was achieved on a 50 cm × 75 μm column packed with C18AQ (Dr Maisch, Ammerbuch, Germany, 1.9 μm) (PepSep, Marslev, Denmark) over 54 min at a flow rate of 300 nL/min. The peptides were eluted over a linear gradient of 3–25% Buffer B (Buffer A: 0.1% formic acid [FA]; Buffer B: 90% v/v acetonitrile, 0.1% v/v FA) and the column was maintained at 50°C. The instrument was operated in data-dependent acquisition (DDA) mode with an MS1 spectrum acquired over the mass range 350–1,550 m/z (120,000 resolution, 300% automatic gain control (AGC) and 25 ms maximum injection time) followed by MS/MS analysis via HCD fragmentation mode and detection in the orbitrap (15,000 resolution, 1.2 m/z isolation, 200% AGC, 55 ms maximum injection time; 30% normalized collision energy). Fractionated TMT labeled enriched peptides from human skeletal muscle biopsies were analyzed on the identical chromatography conditions as described above except acquisition performed on an Orbitrap Ascend mass spectrometer (Thermo Fisher Scientific) via electrospray ionisation in positive mode with 2.0 kV at 275°C and RF set to 30%. The instrument was operated in DDA mode with an MS1 spectrum acquired over the mass range 350–1,550 m/z (120,000 resolution, 250% AGC and 25 ms maximum injection time) followed by MS/MS analysis via HCD fragmentation mode and detection in the orbitrap (30,000 resolution, 1.2 m/z isolation, 200% AGC, 59 ms maximum injection time; 35% normalized collision energy, Enhanced Resolution Mode for TMTpro reagents).
LC-MS/MS data processing
Analysis of enriched peptides from various mouse tissues with SequestHT and Percolator29 was performed in Proteome Discoverer (v2.5.0.4) against the UniProt mouse database (March 2022; UP000000589_10090 including isoforms with 63,592 entries). Data were filtered to 1% FDR at the peptide spectral match, peptide and protein level using QVALITY in the Protein FDR Validator node.30 All data were searched with oxidation of methionine (+15.994915), N-terminal protein acetylation (+42.010565), and VG of lysine (+156.08988) set as the variable modification. Carbamidomethylation of cysteine (+57.021464) was set as fixed modification. The MS1 mass tolerance was set to 10 ppm, while the mass tolerance for MS/MS fragments was set to 0.02 Da with maximum of 2 missed cleavage sites. Localisation of VG-sites was performed with PhosphoRS.31 Analysis of enriched peptides from various mouse tissues with MSFragger and PTMprophet was performed in FragPipe (v18.0)32 against the identical database and same modifications described above. MSBooster33 was enabled and data were filtered to 1% FDR at the peptide spectral match, peptide and protein level using ProteinProhet.34 Enriched and TMT-labelled peptides from mouse skeletal muscle treated with rAAV6 or human skeletal muscle biopsies were analyzed with SequestHT and Percolator in Proteome Discoverer (v2.5.0.4) against the UniProt mouse database (March 2022; UP000000589_10090 including isoforms with 63,592 entries) or UniProt human database (March 2023; UP000005640_9606 including isoforms with 103,484 sequences), respectively. Data were searched with the parameters and modifications described above but also included TMTpro-VG of lysine (+460.297026) as a variable modification, and TMTpro of peptide N-terminus (+304.207146) as a fixed modification. Quantification was performed with the reporter ion quantification node for TMT quantification in Proteome Discoverer. TMT precision was set to 20 ppm and corrected for isotopic impurities. Only spectra with <50% co-isolation interference were used for quantification.
EvoEF protein stability simulations
EvoEF Protein Stability Simulations EvoEF (version 1) was used to calculate the stability change upon mutation, in terms of ΔΔG. To this end, we first used "EvoEF --command = RepairStructure" to repair clashes and torsional angles of the wild type structure. "EvoEF --command = BuildMutant" is then used to mutate the repaired wild type structures into the mutant by changing the side chain amino acid type followed by a local side chain repacking. "EvoEF --command = ComputeStability" is then applied to both the repair wild type and the mutant to calculate their respective stabilities (ΔGWT and ΔGmutant). The stability change upon mutation can then be derived by ΔΔG = ΔGmutant-ΔGWT. A ΔΔG below zero means that the mutation causes destabilization; otherwise, it induces stabilization.17,35 The sequence of the MYH2 coiled-coil backbone was used for EvoEF stability calculations due to size limitations in the software when using the entire MYH2 protein sequence.
Quantification and statistical analysis
Statistics of western-blot were performed in Graph-Pad PRISM using unpaired t-test and significance determined using p < 0.05. For comparison of the 3 antibody clones and pooled cocktail, experiments were performed with one biological replicate. For comparison of mouse tissues, experiments were performed with one biological replicate. For comparison of mouse skeletal muscle treated with rAAV6:shScramble or rAAV6:shUFC1, experiments were performed with eight biological replicates each. For comparison of skeletal muscle from plwALS and age-matched controls, experiments were performed with eight biological replicates each. Data were processed with Perseus36 with Log2-transformation and first normalized by subtracting the median of all the quantified non-VG-modified peptides of each sample to account of subtle differences in the amount of peptide enriched and labeled. The data were further normalized by subtracting the abundance of the protein to account of possible differences in the abundance of the VG-peptide vs. protein levels. For comparison of mouse skeletal muscle treated with rAAV6:shScramble or rAAV6:shUFC1, statistical analysis was performed using two-way paired student’s t-tests and p-values correcting for multiple hypothesis testing using Benjamini Hochberg to obtain q-values. For comparison of skeletal muscle from plwALS and age-matched controls, statistical analysis was performed using two-way unpaired student’s t-tests and p-values correcting for multiple hypothesis testing using Benjamini Hochberg to obtain q-values. Significance thresholds are indicated in Figure Legends with p-value <0.05 or q-value <0.05.
Published: May 9, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.crmeth.2025.101048.
Supplemental information
Tab 1: VG-modified peptide spectral matches identified from mouse skeletal muscle using each of the three antibody clones or a pooled cocktail and identified with both Sequest+Percolator and FragPipe+PTMprophet.
Tab 2: VG-modified peptide spectral matches identified from various mouse tissues using a pooled anitbody cocktail and identified with Sequest+Percolator and MSFragger+PTMProphet.
Tab 1: VG-modified peptide spectral matches from mouse skeletal muscle following rAAV6 treatment with either scramble shRNA or UFC1 shRNA and identified with Sequest+Percolator.
Tab 2: VG-modified peptide spectral matches from human skeletal muscle biopsies obtained from people living with ALS and age-matched controls identified with Sequest+Percolator. Tab 3: VG-modified peptide spectral matches from Myosin purified from mouse skeletal muscle identified with Sequest+Percolator.
References
- 1.Komatsu M., Chiba T., Tatsumi K., Iemura S.i., Tanida I., Okazaki N., Ueno T., Kominami E., Natsume T., Tanaka K. A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO J. 2004;23:1977–1986. doi: 10.1038/sj.emboj.7600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komatsu M., Inada T., Noda N.N. The UFM1 system: Working principles, cellular functions, and pathophysiology. Mol. Cell. 2024;84:156–169. doi: 10.1016/j.molcel.2023.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Tatsumi K., Sou Y.S., Tada N., Nakamura E., Iemura S.i., Natsume T., Kang S.H., Chung C.H., Kasahara M., Kominami E., et al. A novel type of E3 ligase for the Ufm1 conjugation system. J. Biol. Chem. 2010;285:5417–5427. doi: 10.1074/jbc.M109.036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peter J.J., Magnussen H.M., DaRosa P.A., Millrine D., Matthews S.P., Lamoliatte F., Sundaramoorthy R., Kopito R.R., Kulathu Y. A non-canonical scaffold-type E3 ligase complex mediates protein UFMylation. EMBO J. 2022;41 doi: 10.15252/embj.2022111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo H.M., Kang S.H., Kim J.Y., Lee J.E., Seong M.W., Lee S.W., Ka S.H., Sou Y.S., Komatsu M., Tanaka K., et al. Modification of ASC1 by UFM1 is crucial for ERalpha transactivation and breast cancer development. Mol. Cell. 2014;56:261–274. doi: 10.1016/j.molcel.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Walczak C.P., Leto D.E., Zhang L., Riepe C., Muller R.Y., DaRosa P.A., Ingolia N.T., Elias J.E., Kopito R.R. Ribosomal protein RPL26 is the principal target of UFMylation. Proc. Natl. Acad. Sci. USA. 2019;116:1299–1308. doi: 10.1073/pnas.1816202116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim W., Bennett E.J., Huttlin E.L., Guo A., Li J., Possemato A., Sowa M.E., Rad R., Rush J., Comb M.J., et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He T., Liu Y., Zhou Y., Li L., Wang H., Chen S., Gao J., Jiang W., Yu Y., Ge W., et al. Comparative Evaluation of Proteome Discoverer and FragPipe for the TMT-Based Proteome Quantification. J. Proteome Res. 2022;21:3007–3015. doi: 10.1021/acs.jproteome.2c00390. [DOI] [PubMed] [Google Scholar]
- 9.Svinkina T., Gu H., Silva J.C., Mertins P., Qiao J., Fereshetian S., Jaffe J.D., Kuhn E., Udeshi N.D., Carr S.A. Deep, Quantitative Coverage of the Lysine Acetylome Using Novel Anti-acetyl-lysine Antibodies and an Optimized Proteomic Workflow. Mol. Cell. Proteomics. 2015;14:2429–2440. doi: 10.1074/mcp.O114.047555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Xu Y., Rogers H., Saidi L., Noguchi C.T., Li H., Yewdell J.W., Guydosh N.R., Ye Y. UFMylation of RPL26 links translocation-associated quality control to endoplasmic reticulum protein homeostasis. Cell Res. 2020;30:5–20. doi: 10.1038/s41422-019-0236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin B., Yu J., Nowsheen S., Wang M., Tu X., Liu T., Li H., Wang L., Lou Z. UFL1 promotes histone H4 ufmylation and ATM activation. Nat. Commun. 2019;10:1242. doi: 10.1038/s41467-019-09175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Guan D., Dong M., Yang J., Wei H., Liang Q., Song L., Xu L., Bai J., Liu C., et al. UFMylation maintains tumour suppressor p53 stability by antagonizing its ubiquitination. Nat. Cell Biol. 2020;22:1056–1063. doi: 10.1038/s41556-020-0559-z. [DOI] [PubMed] [Google Scholar]
- 13.Ishimura R., El-Gowily A.H., Noshiro D., Komatsu-Hirota S., Ono Y., Shindo M., Hatta T., Abe M., Uemura T., Lee-Okada H.C., et al. The UFM1 system regulates ER-phagy through the ufmylation of CYB5R3. Nat. Commun. 2022;13:7857. doi: 10.1038/s41467-022-35501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thingholm T.E., Palmisano G., Kjeldsen F., Larsen M.R. Undesirable charge-enhancement of isobaric tagged phosphopeptides leads to reduced identification efficiency. J. Proteome Res. 2010;9:4045–4052. doi: 10.1021/pr100230q. [DOI] [PubMed] [Google Scholar]
- 15.Molendijk J., Blazev R., Mills R.J., Ng Y.K., Watt K.I., Chau D., Gregorevic P., Crouch P.J., Hilton J.B.W., Lisowski L., et al. Proteome-wide systems genetics identifies UFMylation as a regulator of skeletal muscle function. Elife. 2022;11 doi: 10.7554/eLife.82951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong J.P.H., Blazev R., Ng Y.K., Goodman C.A., Montgomery M.K., Watt K.I., Carl C.S., Watt M.J., Voldstedlund C.T., Richter E.A., et al. Characterization of the skeletal muscle arginine methylome in health and disease reveals remodeling in Amyotrophic Lateral Sclerosis. bioRxiv. 2024 doi: 10.1101/2024.01.08.574551. Preprint at. [DOI] [PubMed] [Google Scholar]
- 17.Pearce R., Huang X., Setiawan D., Zhang Y. EvoDesign: Designing Protein-Protein Binding Interactions Using Evolutionary Interface Profiles in Conjunction with an Optimized Physical Energy Function. J. Mol. Biol. 2019;431:2467–2476. doi: 10.1016/j.jmb.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis C.T.A., Melhedegaard E.G., Ognjanovic M.M., Olsen M.S., Laitila J., Seaborne R.A.E., Gronset M.N., Zhang C., Iwamoto H., Hessel A.L., et al. Remodelling of Skeletal Muscle Myosin Metabolic States in Hibernating Mammals. bioRxiv. 2024;888:888. doi: 10.1101/2023.11.14.566992. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoeller D., Hecker C.M., Wagner S., Rogov V., Dötsch V., Dikic I. E3-independent monoubiquitination of ubiquitin-binding proteins. Mol. Cell. 2007;26:891–898. doi: 10.1016/j.molcel.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Hjerpe R., Thomas Y., Kurz T. NEDD8 overexpression results in neddylation of ubiquitin substrates by the ubiquitin pathway. J. Mol. Biol. 2012;421:27–29. doi: 10.1016/j.jmb.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Liang J.R., Lingeman E., Luong T., Ahmed S., Muhar M., Nguyen T., Olzmann J.A., Corn J.E. A Genome-wide ER-phagy Screen Highlights Key Roles of Mitochondrial Metabolism and ER-Resident UFMylation. Cell. 2020;180:1160–1177.e20. doi: 10.1016/j.cell.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scavone F., Gumbin S.C., Da Rosa P.A., Kopito R.R. RPL26/uL24 UFMylation is essential for ribosome-associated quality control at the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2220340120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishimura R., Ito S., Mao G., Komatsu-Hirota S., Inada T., Noda N.N., Komatsu M. Mechanistic insights into the roles of the UFM1 E3 ligase complex in ufmylation and ribosome-associated protein quality control. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adh3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Riverol Y., Bai J., Bandla C., García-Seisdedos D., Hewapathirana S., Kamatchinathan S., Kundu D.J., Prakash A., Frericks-Zipper A., Eisenacher M., et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–D552. doi: 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNamara J.W., Singh R.R., Sadayappan S. Cardiac myosin binding protein-C phosphorylation regulates the super-relaxed state of myosin. Proc. Natl. Acad. Sci. USA. 2019;116:11731–11736. doi: 10.1073/pnas.1821660116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong J.P.H., Blazev R., Ng Y.K., Goodman C.A., Montgomery M.K., Watt K.I., Carl C.S., Watt M.J., Voldstedlund C.T., Richter E.A., et al. Characterization of the skeletal muscle arginine methylome in health and disease reveals remodeling in amyotrophic lateral sclerosis. FASEB J. 2024;38 doi: 10.1096/fj.202400045R. [DOI] [PubMed] [Google Scholar]
- 27.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blazev R., Carl C.S., Ng Y.K., Molendijk J., Voldstedlund C.T., Zhao Y., Xiao D., Kueh A.J., Miotto P.M., Haynes V.R., et al. Phosphoproteomics of three exercise modalities identifies canonical signaling and C18ORF25 as an AMPK substrate regulating skeletal muscle function. Cell Metab. 2022;34:1561–1577.e9. doi: 10.1016/j.cmet.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Kall L., Canterbury J.D., Weston J., Noble W.S., MacCoss M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods. 2007;4:923–925. doi: 10.1038/nmeth1113. [DOI] [PubMed] [Google Scholar]
- 30.Kall L., Storey J.D., Noble W.S. QVALITY: non-parametric estimation of q-values and posterior error probabilities. Bioinformatics. 2009;25:964–966. doi: 10.1093/bioinformatics/btp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taus T., Köcher T., Pichler P., Paschke C., Schmidt A., Henrich C., Mechtler K. Universal and confident phosphorylation site localization using phosphoRS. J. Proteome Res. 2011;10:5354–5362. doi: 10.1021/pr200611n. [DOI] [PubMed] [Google Scholar]
- 32.Kong A.T., Leprevost F.V., Avtonomov D.M., Mellacheruvu D., Nesvizhskii A.I. MSFragger: ultrafast and comprehensive peptide identification in mass spectrometry-based proteomics. Nat. Methods. 2017;14:513–520. doi: 10.1038/nmeth.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang K.L., Yu F., Teo G.C., Li K., Demichev V., Ralser M., Nesvizhskii A.I. MSBooster: improving peptide identification rates using deep learning-based features. Nat. Commun. 2023;14:4539. doi: 10.1038/s41467-023-40129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nesvizhskii A.I., Keller A., Kolker E., Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 35.Huang X., Pearce R., Zhang Y. EvoEF2: accurate and fast energy function for computational protein design. Bioinformatics. 2020;36:1135–1142. doi: 10.1093/bioinformatics/btz740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyanova S., Temu T., Sinitcyn P., Carlson A., Hein M.Y., Geiger T., Mann M., Cox J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods. 2016;13:731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab 1: VG-modified peptide spectral matches identified from mouse skeletal muscle using each of the three antibody clones or a pooled cocktail and identified with both Sequest+Percolator and FragPipe+PTMprophet.
Tab 2: VG-modified peptide spectral matches identified from various mouse tissues using a pooled anitbody cocktail and identified with Sequest+Percolator and MSFragger+PTMProphet.
Tab 1: VG-modified peptide spectral matches from mouse skeletal muscle following rAAV6 treatment with either scramble shRNA or UFC1 shRNA and identified with Sequest+Percolator.
Tab 2: VG-modified peptide spectral matches from human skeletal muscle biopsies obtained from people living with ALS and age-matched controls identified with Sequest+Percolator. Tab 3: VG-modified peptide spectral matches from Myosin purified from mouse skeletal muscle identified with Sequest+Percolator.
Data Availability Statement
-
•
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE24 partner repository with the dataset identifier PXD051412 and are publicly accessible as of the date of publication.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.