Abstract
The relationship between cell inactivation and membrane damage was studied in two gram-positive organisms, Listeria monocytogenes and Bacillus subtilis, and two gram-negative organisms, Yersinia enterocolitica and Escherichia coli, exposed to chlorine in the absence and presence of 150 ppm of organic matter (Trypticase soy broth). L. monocytogenes and B. subtilis were more resistant to chlorine in distilled water. The addition of small amounts of organic matter to the chlorination medium drastically increased the resistance of both types of microorganisms, but this effect was more marked in Y. enterocolitica and E. coli. In addition, the survival curves for these microorganisms in the presence of organic matter had a prolonged shoulder. Sublethal injury was not detected under most experimental conditions, and only gram-positive cells treated in distilled water showed a relevant degree of injury. The exposure of bacterial cells to chlorine in distilled water caused extensive permeabilization of the cytoplasmic membrane, but the concentrations required were much higher than those needed to inactivate cells. Therefore, there was no relationship between the occurrence of membrane permeabilization and cell death. The addition of organic matter to the treatment medium stabilized the cytoplasmic membrane against permeabilization in both the gram-positive and gram-negative bacteria investigated. Exposure of E. coli cells to the outer membrane-permeabilizing agent EDTA increased their sensitivity to chlorine and caused the shoulders in the survival curves to disappear. Based on these observations, we propose that bacterial envelopes could play a role in cell inactivation by modulating the access of chlorine to the key targets within the cell.
Chlorination is one of the most widely used processes for microbial control (5) in both drinking water and wastewater processing (16). Chlorine is a powerful antimicrobial substance due to its potential oxidizing capacity. In addition to drinking water disinfection, there are a number of other commercial uses for chlorine in the food industry, including reduction of microbial populations on the surfaces of raw foods, such as fruits and vegetables, and sanitation of surfaces in food processing environments (5, 7). In general, disinfectants such as chlorine are used at very high concentrations in order to attain a rapid rate of killing. At the concentrations used, it is difficult for microorganisms to survive. However, the use of such high concentrations increases the risk of formation of potentially hazardous by-products or the production of off-tastes and odors, which are the main drawbacks of chlorination (13, 17). Conversely, at low chlorine levels, microorganisms that survive the treatment may be injured rather than inactivated (8). Under suitable conditions injured cells might repair cellular damage and recover (10). An appreciation of the nature of sublethal injury and repair is therefore important in devising chlorination strategies and in developing combination treatments with synergistic actions against the target microorganisms. The mechanisms of action of chlorine on microorganisms have been widely investigated (1, 3, 19). Nevertheless, the mechanism by which chlorine exerts its lethal effect has never been fully elucidated. Moreover, the occurrence of sublethal injury caused by chlorine treatments is scarcely known.
Effective microbial control by chlorine requires appropriate disinfection design criteria to ensure protection of public health and to minimize unwanted effects of the chlorination process. A better understanding of the way that chlorine kills cells would help in defining effective chlorine treatments and in optimizing strategies for chlorination.
The purpose of this study was to investigate the occurrence of sublethal injury and the relationship between membrane damage and loss of viability in two gram-positive and two gram-negative microorganisms after exposure to chlorine in the absence and presence of a chlorine-demanding substrate.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli STCC 471, Yersinia enterocolitica STCC 4315, Listeria monocytogenes ATCC 15313, and Bacillus subtilis NCTC 10073 were used in this study. E. coli, Y. enterocolitica, and L. monocytogenes were grown in tryptic soy broth with 0.6% yeast extract (TSBYE) (Biolife, Milan Italy), and B. subtilis was grown in nutrient broth (Biolife, Milan Italy). A subculture of each strain was obtained by inoculating a test tube containing 5 ml of sterile broth with a single colony and incubating it overnight at 37°C. A flask containing 50 ml of broth was inoculated with the subculture to obtain a concentration of 106 CFU/ml. The bacteria were grown to the stationary phase with agitation at 37°C. Before treatment, microorganisms were centrifuged at 6,000 × g for 5 min at 4°C. The broth was removed, and the cell pellet was rinsed and resuspended in a phosphate buffer solution (0.01 M, pH 7). This washing procedure was repeated several times until the supernatant did not demand chlorine. The chlorine demand was estimated by the N,N-dimethyl-p-phenylenediamine colorimetric method (4). As small variations in the cell concentration could affect chlorine depletion and therefore chlorine efficacy, the microbial concentration was determined by measuring absorbance at 620 nm. Microbial suspensions were diluted in distilled water or in tryptic soy broth (TSB) (1,500 ppm, corresponding to 1,120 ppm of organic load calculated from the formulation) to obtain a concentration of 6 × 108 ± 1 × 108 CFU/ml for inactivation experiments. Before each experiment absorbance measurements were confirmed by plate counting. All experiments were performed in demand-free glassware.
Stock chlorine solutions.
Free chlorine solutions were prepared from reagent grade chemicals (10% sodium hypochlorite from Panreac, Barcelona, Spain). A stock solution (100 ppm available chlorine) was prepared using deionized distilled water that did not demand chlorine. Deionized distilled water was also used to prepare all other hypochlorite solutions. The initial chlorine concentrations ranged from 0.3 to 30 ppm. The initial free chlorine (hypochlorous acid plus hypochlorite ion) concentration was determined by the N,N-dimethyl-p-phenylenediamine method (4).
Chlorine decay assays.
Once microorganisms were added to the chlorine solutions, samples were taken after different contact times. Free chlorine concentrations were determined by the method described above. All determinations were done in duplicate, and the results were expressed as the average values.
Microbial inactivation experiments.
Microbial resistance to chlorine was evaluated by adding 0.1 ml of a microbial suspension in distilled water or in 1,500 ppm TSB to 1.5-ml conical flasks containing 0.9 ml of a corresponding chlorine solution at 20°C. Samples were collected after different treatment times ranging from 0.08 to 30 min. After the desired contact time, appropriate serial dilutions were prepared in TSBYE. It was previously determined that TSBYE neutralized the residual chlorine in the corresponding dilutions.
EDTA treatment.
Samples (0.1 ml) of a suspension of E. coli were added to 0.9 ml of distilled water that contained 20 mM of EDTA disodium salt. After 30 min of incubation the EDTA was removed by centrifugation, and the EDTA-treated microorganisms were resuspended in distilled water or in TSB (150 ppm). The chlorine resistance of these EDTA-treated microorganisms was determined as indicated above.
Counting of survivors.
The numbers of survivors of E. coli, Y. enterocolitica, and L. monocytogenes were determined in tryptic soy agar with yeast extract (TSAYE) or in TSAYE with 4, 2, and 5% NaCl, respectively, and the number of survivors of B. subtilis was determined in nutrient agar (NA) or in NA with 6% NaCl. The concentrations used to detect sublethal injury correspond to the highest NaCl concentrations that did not affect the growth of untreated cells. Plates were incubated at 37°C for 24 to 72 h, and after incubation, colonies were counted with an improved image analyzer automatic counter (Protos, Analytical Measuring Systems, Cambridge, United Kingdom) as described elsewhere (2). The number of sublethally injured bacteria corresponded to the difference between the counts obtained in the nonselective medium (TSAYE or NA) and the counts obtained in the selective medium (medium supplemented with NaCl).
Leakage of UV-absorbing substances.
To quantify the intracellular material released from the cells, untreated and treated concentrated samples (6 × 108 ± 1 × 108 CFU/ml) were centrifuged at 6,000 × g for 10 min. The UV absorbance of the supernatant was measured at 260 and 280 nm with a spectrophotometer (UNICAM UV 500 UV-visible spectrometer).
Determination of propidium iodine uptake.
The integrity of the cytoplasmic membranes of chlorine-treated cells was determined using propidium iodide (PI) (Sigma-Aldrich). Untreated and chlorine-treated cells were centrifuged, the supernatants were removed, and the cell pellets were rinsed and resuspended in distilled water to an optical density at 620 nm of approximately 0.4 for E. coli or 1 for L. monocytogenes, corresponding to approximately 108 cells per ml. PI was added to a final concentration of 2 μg/ml. After incubation for 10 min, samples were centrifuged and washed in distilled water to remove the excess dye. Fluorescence was measured with a spectrofluorophotometer (model TECAN; Genius) at an excitation wavelength of 535 nm and an emission wavelength of 625 nm. Fluorescence data for each sample were normalized with the optical density at 620 nm; the values obtained for untreated cells were subtracted from all experimental values.
RESULTS
Microbial chlorine resistance in distilled water and TSB (150 ppm).
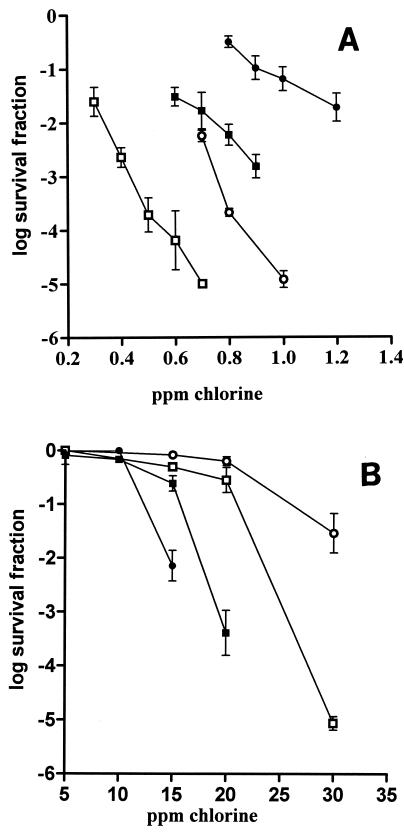
In order to study chlorine resistance, the initial free chlorine concentrations were varied depending on the intrinsic resistance of the microorganism tested. In distilled water, the chlorine sensitivities of the gram-negative microorganisms E. coli and Y. enterocolitica were investigated by using concentrations of 0.3 to 0.7 ppm and 0.8 to 1 ppm, respectively, while the chlorine sensitivities of the gram-positive microorganisms B. subtilis and L. monocytogenes were investigated by using concentrations of 0.8 to 1.2 ppm and 0.6 to 0.9 ppm, respectively. When cells were exposed to the ranges of chlorine concentrations indicated above for 2 min, the gram-positive microorganisms showed greater resistance (Fig. 1A). E. coli was more sensitive than Y. enterocolitica, and L. monocytogenes was more sensitive than B. subtilis.
FIG. 1.
Microbial inactivation by treatment with different initial chlorine concentrations for 2 min in the absence of TSB (A) and in the presence of TSB (150 ppm) (B) at 20°C. Symbols: •, B. subtilis; ▪, L. monocytogenes; ○, Y. enterocolitica; □, E. coli.
The presence of organic matter in the treatment medium drastically increased the chlorine resistance of the four microorganisms investigated. In the presence of organic matter the free chlorine concentrations assayed ranged from 10 to 30 ppm. The increase in resistance was especially marked for the gram-negative microorganisms, which were even more chlorine resistant than the gram-positive microorganisms (Fig. 1B). After contact for 2 min, cell death began at initial chlorine concentrations of more than 20 and 10 ppm for gram-negative and gram-positive microorganisms, respectively. As observed in distilled water, Y. enterocolitica was more resistant than E. coli; however, B. subtilis was more resistant than L. monocytogenes.
Inactivation kinetics and occurrence of sublethal injury in distilled water and TSB (150 ppm).
Examples of survival curves showing the inactivation of the four microorganisms in distilled water are shown in Fig. 2A and B. In order to show whether chlorine caused sublethal injury, survival curves for the treated microorganisms recovered in a selective medium are also included. As shown in Fig. 2, the survival curves for gram-negative microorganisms showed that initially there was very fast linear inactivation, which was followed by zero-slope tailing (Fig. 2A), whereas the survival curves for gram-positive microorganisms were concave upward (Fig. 2B).
FIG. 2.
Survival curves corresponding to microbial inactivation by chlorine in distilled water at 20°C. (A) Symbols: •, Y. enterocolitica, 0.7 ppm chlorine, enumeration in TSAYE; ○, Y. enterocolitica, 0.7 ppm chlorine, enumeration in TSAYE containing 2% NaCl; ▪, E. coli, 0.5 ppm chlorine, enumeration in TSAYE; □, E. coli, 0.5 ppm chlorine, enumeration in TSAYE containing 4% NaCl. (B) Symbols: •, L. monocytogenes, 0.7 ppm chlorine, enumeration in TSAYE; ○, L. monocytogenes, 0.7 ppm chlorine, enumeration in TSAYE containing 5% NaCl; ▪, B. subtilis, 1 ppm chlorine, enumeration in NA; □, B. subtilis, 1 ppm chlorine, enumeration in NA containing 6% NaCl.
For both gram-negative bacteria there was no difference between the counts in nonselective plating medium and the counts in selective plating medium (Fig. 2A). However, it was found that chlorine caused sublethal injury in gram-positive microorganisms since a greater reduction in viability was observed when cells were recovered in the selective medium. After 1 min of treatment more than 99% of the B. subtilis and L. monocytogenes survivors were injured and therefore unable to grow in the presence of sodium chloride. Similar results were observed for various initial chlorine concentrations (data not shown).
In all cases investigated the levels of chlorine consumption for the populations of the four microorganisms were found to be similar. Microbial suspensions in distilled water exerted a high chlorine demand, and the free chlorine was almost completely consumed by the bacterial cell suspensions during the first moments of the treatment (data not shown).
Examples of survival curves in TSB (150 ppm) corresponding to inactivation of the four microorganisms investigated recovered in nonselective and selective plating media are shown in Fig. 3. The presence of organic matter in the treatment medium modified the shape of the survival curves for the gram-negative microorganisms, which had a shoulder followed by a linear inactivation. Shoulders in survival curves have been attributed to accumulation of repairable damage that becomes irreversible only beyond a critical level. However, for both gram-negative and gram-positive microorganisms there were no differences between the counts in nonselective medium and the counts in selective plating medium. Therefore, in the presence of organic matter, chlorine did not injure cells before they were completely inactivated. Similar behavior was observed in experiments performed with different chlorine concentrations.
FIG. 3.
Survival curves corresponding to microbial inactivation by chlorine in the presence of TSB (150 ppm) at 20°C. (A) Symbols: •, Y. enterocolitica, 20 ppm chlorine, enumeration in TSAYE; ○, Y. enterocolitica, 20 ppm chlorine, enumeration in TSAYE containing 2% NaCl; ▪, E. coli, 20 ppm chlorine, enumeration in TSAYE; □, E. coli, 20 ppm chlorine, enumeration in TSAYE containing 4% NaCl. (B) Symbols: •, L. monocytogenes, 15 ppm chlorine, enumeration in TSAYE; ○, L. monocytogenes, 15 ppm chlorine, enumeration in TSAYE containing 5% NaCl; ▪, B. subtilis, 150 ppm chlorine, enumeration in NA; □, B. subtilis, 150 ppm chlorine, enumeration in NA containing 6% NaCl.
TSB (150 ppm) in solutions containing bacterial suspensions was found to exert a rapid chlorine demand in the first 1 min of treatment, followed by a slow demand with a longer contact time. For the four microorganisms, the levels of residual free chlorine during the treatment were always higher than 3 ppm.
Leakage of UV-absorbing substances caused by chlorine treatment.
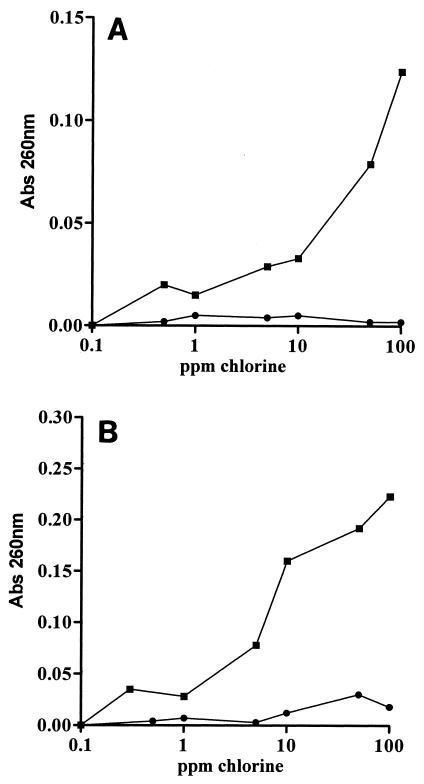
The values for absorbance at 260 nm for supernatants of E. coli and L. monocytogenes suspensions treated in distilled water and TSB (150 ppm) are shown in Fig. 4A and B. The results obtained at 280 nm were similar (data not shown). Increasing the chlorine concentration resulted in increased levels of UV-absorbing substances for both microorganisms when they were treated in distilled water. However, leakage of UV-absorbing substances began to be remarkable at chlorine concentrations higher than the concentration necessary to inactivate more than 99.9% of the populations of both microorganisms.
FIG. 4.
Leakage of UV-absorbing substances from E. coli and L. monocytogenes treated with chlorine for 2 min. The graphs show representative results. (A) Symbols: ▪, E. coli treated in distilled water; •, E. coli treated in TSB (150 ppm). (B) Symbols: ▪, L. monocytogenes treated in distilled water; •, L. monocytogenes treated in TSB (150 ppm).
When TSB was added to the treatment medium, no leakage of UV-absorbing substances occurred even at chlorine concentrations as high as 100 ppm for the two microorganisms tested.
Uptake of PI after chlorine treatment.
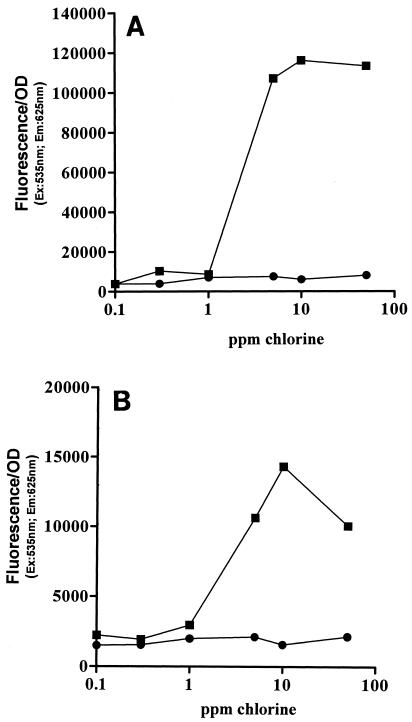
Propidium iodide is commonly used as an indicator of cytoplasmic membrane damage. PI is able to enter cells only if the membrane is permeabilized. Once inside the cytoplasm, it binds to single- and double-stranded nucleic acids, yielding fluorescence in the red wavelength region. Data for the uptake of PI by chlorine-treated cells of E. coli and L. monocytogenes when they were treated in distilled water and TSB (150 ppm) are shown in Fig. 5A and B, respectively. The uptake of propidium iodide exhibited the same pattern as the leakage of UV-absorbing material shown in Fig. 4A and B. When treated in distilled water, both microorganisms took up the dye at chlorine concentrations above 1 ppm. Since chlorine concentrations below 1 ppm for 2 min inactivated more than 99.9% of the cells of both microorganisms, the onset of cell staining occurred once the cells were dead. No uptake was observed in the two microorganisms when they were treated in TSB (150 ppm), even at high chlorine concentrations.
FIG. 5.
Permeability of chlorine-treated cells for 2 min to propidium iodine. The graphs show representative results. (A) Symbols: ▪, E. coli treated in distilled water; •, E. coli treated in TSB (150 ppm). (B) Symbols: ▪, L. monocytogenes treated in distilled water; •, L. monocytogenes treated in TSB (150 ppm). OD, optical density; Ex, excitation; Em, emission.
Chlorine resistance of EDTA-treated E. coli cells.
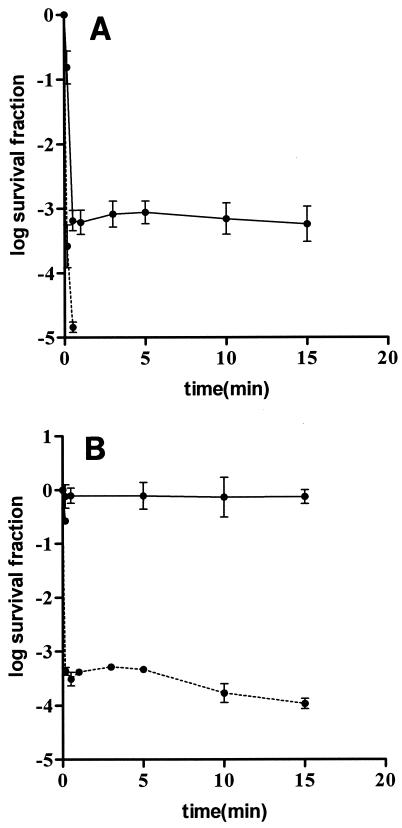
It is well known that the outer membrane of gram-negative bacteria has a clear role in modulating the accessibility of low-molecular-weight substances to the cytoplasm. We studied whether previous treatment of E. coli cells with an outer membrane-permeabilizing agent, such as EDTA, could sensitize cells against chlorine. Figure 6 shows that preincubation with EDTA drastically decreased the chlorine resistance of E. coli in both distilled water and TSB (150 ppm). Sublethal injury was not detected in any case (data not shown).
FIG. 6.
Influence of preincubation with EDTA (20 mM) for 30 min on E. coli resistance to chlorine. (A) Solid line, native cells treated in distilled water, 0.4 ppm chlorine; dashed line, cells preincubated with EDTA and treated in distilled water, 0.4 ppm chlorine. (B) Solid line, native cells treated in TSB (150 ppm), 15 ppm chlorine; dashed line, cells preincubated with EDTA and treated in TSB (150 ppm), 15 ppm chlorine.
DISCUSSION
Results obtained in this investigation showed that there were large differences in chlorine resistance depending on the microorganism investigated and the composition of the treatment medium. In distilled water gram-negative microorganisms (E. coli and Y. enterocolitica) were more chlorine sensitive than gram-positive microorganisms (B. subtilis and L. monocytogenes). The microbial resistance drastically increased when TSB was present in the treatment medium. This effect was more marked for the two gram-negative microorganisms, which exhibited different inactivation kinetics and became even more chlorine resistant than the two gram-positive microorganisms.
The protective effect of organic matter against chlorine has been described previously (6). This effect has been attributed to the higher chlorine demand of organic compounds, which results in a rapid decline in the available free chlorine (5, 9, 15). However, in experiments conducted in this investigation the residual chlorine concentration in the presence of organic matter was much higher than the chlorine concentration necessary to completely inactivate the microbial population in distilled water. Therefore, the protective effect cannot be attributed solely to the chlorine demand of TSB but may be due to effective stabilization of some cellular structures.
Another aspect observed in this study was the different inactivation kinetics depending on the microorganism and the treatment medium. In distilled water most inactivation of gram-negative microorganisms occurred during the first 1 min of treatment, and the counts remained unchanged when the treatment was extended. However, in TSB (150 ppm) the survival curves had a shoulder, followed by linear inactivation. The occurrence of a shoulder in survival curves with different lethal agents has been attributed to accumulation of damage that becomes irreparable only beyond a critical level. The occurrence of sublethal injury to the membrane was studied by the selective plating technique using sodium chloride as the inhibitory compound. In this investigation it was found that chlorine did not cause sublethal injury in gram-negative microorganisms when they were suspended both in distilled water and in TSB (150 ppm). Therefore, we concluded that the occurrence of a shoulder in inactivation curves for gram-negative bacteria in the presence of organic matter does not seem to be due to cellular injury.
Chlorine is generally considered to be a nonselective oxidant which reacts avidly with a variety of cellular components and affects metabolic processes (14). The cytoplasmic membrane has been proposed to be a possible key target involved in bacterial inactivation by chlorine (19), since alterations in its permeability after chlorination have frequently been described. Sensitivity to salt in the recovery medium is recognized as a symptom of damage to the cytoplasmic membrane (10, 19). In this investigation no sublethally injured cells were detected in most cases, and only gram-positive cells treated in distilled water showed a significant degree of sublethal injury. Therefore, only in this case did sublethal damage to the membrane precede cell death. In other words, cells were able to repair their membrane when they were recovered in an adequate plating medium (TSAYE). In contrast, for gram-negative cells and for cells treated in the presence of organic matter, damage to the membrane was either irreparable or nonexistent.
To further study the nature of the membrane damage, the leakage of intracellular substances in the gram-positive organism L. monocytogenes and the gram-negative organism E. coli was investigated. We observed extensive leakage of UV-absorbing material after chlorine treatment in water. However, addition of small amounts of organic matter to the treatment medium completely prevented the leakage of UV-absorbing material, suggesting that there was protection of the cell walls for both the gram-positive and gram-negative microorganisms investigated.
Since the molecular size of RNA and proteins or peptides, which are the main molecules detected by the UV absorbance measurements, is quite large, experiments with propidium iodide were also carried out. Propidium iodide is a low-molecular-mass dye (668.4 Da) which emits strong red fluorescence when it binds to single- and double-stranded nucleic acids. Its entrance into the bacterial cell is prevented by an intact cytoplasmic membrane. The results of experiments performed with propidium iodide had the same profile as the results described above for the leakage of UV-absorbing material, confirming that there was permeabilization of the cytoplasmic membrane when cells were treated in distilled water. However, membrane permeabilization was observed when bacteria were exposed to chlorine concentrations as high as 50 ppm, which is severalfold higher than the concentration required for cell killing. This indicates that extensive cytoplasmic membrane damage is not a key event leading to cell death due to chlorine.
However, we cannot disregard the possibility that the cell wall plays a role in the inactivation of cells by chlorine since the presence of organic matter in the treatment medium protected cell membranes against permeabilization and simultaneously increased the free chlorine concentration needed to attain cell killing. For bactericides to be effective, they must be able to penetrate the cell envelope and attain a concentration at the target site high enough to exert their antimicrobial action. Results obtained in this investigation suggest that the presence of organic matter could stabilize the envelopes and thus slow the penetration of chlorine into the cell. Whereas the envelopes of gram-positive bacteria consist of the cytoplasmic membrane surrounded by a thick peptidoglycan wall, the envelopes of gram-negative bacteria posses an external layer, the outer membrane, which provides an extra barrier against antimicrobial compounds. The fact that the protective effect of organic matter was greater for gram-negative microorganisms than for gram-positive microorganisms also suggested that there was stabilization of the outer membrane. This hypothesis was also supported by the occurrence of shoulders in survival curves for gram-negative bacteria treated in the presence of organic matter. Stabilization of the outer membrane by small amounts of organic matter has been reported previously for Salmonella (11). To explore the role of the outer membrane in gram-negative bacteria, E. coli was preincubated with the outer membrane-permeabilizing agent EDTA. EDTA provokes destabilization of the outer membrane through chelation of divalent cations, such as calcium and magnesium (18). Divalent cations play an important role in stabilizing the external leaflet of the outer membrane by neutralizing the electrostatic repulsion between neighboring lipopolysaccharide molecules. If the divalent cations are removed from their positions in the outer membrane, the stability of the lipopolysaccharide layer is disturbed and the permeability to several compounds is increased (12). Preincubation with EDTA drastically increased E. coli sensitivity to chlorine in both distilled water and TSB. Furthermore, this preincubation treatment also resulted in the disappearance of the shoulders in survival curves in TSB (150 ppm), confirming that the outer membrane is implicated in the protective effect of organic matter in E. coli.
Results obtained in this investigation support that extensive membrane damage is not a key event in the inactivation of bacteria by chlorine and confirm the observations of other authors that suggest that more subtle events, such as uncoupling of the electron chain or enzyme inactivation either in the membrane or in the cell interior, are involved in the bactericidal mechanism of chlorine (1, 19). However, the envelopes play an important role in the bacterial resistance to chlorine in the presence of organic matter, which is probably related to the accessibility of chlorine to targets within the cell. Further investigations are required to fully elucidate these aspects and to better exploit chlorination technology.
Acknowledgments
R.V. gratefully acknowledges the financial support for her doctoral studies from the Departamento de Educación y Ciencia del Gobierno de Navarra.
REFERENCES
- 1.Barrette, W. C., Jr., J. K. Hurst, B. R. Michel, and H. Rosen. 1991. Hypochlorous acid and myeloperoxidase-catalysed oxidation of iron-sulfur clusters in bacterial respiratory dehydrogenases. Eur. J. Biochem. 202:1275-1282. [DOI] [PubMed] [Google Scholar]
- 2.Condón, S., A. Palop, J. Raso, and F. J. Sala. 1996. Influence of the incubation temperature after heat treatment upon the estimated heat resistance values of spores of Bacillus subtilis. Lett. Appl. Microbiol. 22:149-152. [Google Scholar]
- 3.Dukan, S., and D. Touati. 1996. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J. Bacteriol. 178:6145-6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg, A. E. 1995. Standard methods for the examination of water and waterwaster, 19th ed. American Public Health Association, Washington, D.C.
- 5.Kotula, I. K., A. W. Kotula, B. E. Rose, C. J. Pierson, and M. Camp. 1997. Reduction of aqueous chlorine by organic material. J. Food Prot. 60:276-282. [DOI] [PubMed] [Google Scholar]
- 6.LeChevallier, M. W., T. M. Evans, and R. J. Seidler. 1981. Effect of turbidity on chlorination efficiency and bacterial persistence in drinking water. Appl. Environ. Microbiol. 42:159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeChevallier, M. W., C. D. Cawthon, and R. G. Lee. 1988. Inactivation of biofilm bacteria. Appl. Environ. Microbiol. 54:2492-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisle, J. T., Broadaway, A. M., B. H. Pyle, C. Fricker, and G. A. McFeters. 1998. Effects of starvation on physiological activity and chlorine disinfection resistance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 64:4658-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyndon, L., and R. Gordon. 1998. Modeling water treatment chemical disinfection kinetics. J. Environ. Eng. 124:783-793. [Google Scholar]
- 10.Mackey, B. M. 2000. Injured bacteria, p. 315-341. In B. M. Lund, T. C. Baird-Parker, and G. W. Gould (ed.), The microbiological safety and quality of foods. Aspen Publishers, Gaithersburg, Md.
- 11.Mañas, P., R. Pagán, F. J. Sala, and S. Condón. 2001. Low molecular weight milk whey components protect Salmonella senftenberg 775W against heat by a mechanism involving divalent cations. J. Appl. Microbiol. 91:871-877. [DOI] [PubMed] [Google Scholar]
- 12.Nikaido, H. 1996. Outer membrane, p. 29-47. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 13.Richardson, S. D., A. D. Thruston, J. R., T. V. Caughran, T. W. Collette, K. S Patterson, and W. W. Lykins, Jr. 1998. Chemical by-products of chlorine and alternative disinfectants. Food Technol. 52:58-61. [Google Scholar]
- 14.Shang, C., and E. R. Blatchley III. 1999. Differentiation and quantification of free chlorine and inorganic chloramines in aqueous solution by MIMS. Environ. Sci. Technol. 33:2218-2223. [Google Scholar]
- 15.Shang, C., and E. R. Blatchley III. 2001. Chlorination of pure bacterial cultures in aqueous solution. Water Res. 35:244-254. [DOI] [PubMed] [Google Scholar]
- 16.Sisti, M., A. Albano, and G. Brandi. 1998. Bactericidal effect of chlorine on motile Aeromonas spp. in drinking water supplies and influence of temperature on disinfection efficacy. Lett. Appl. Microbiol. 26:347-351. [DOI] [PubMed] [Google Scholar]
- 17.Thomas, E. L., M. M. Jefferson, J. J. Bennett, and D. B. Learn. 1987. Mutagenic activity of chloramines. Mutant Res. 188:35-43. [DOI] [PubMed] [Google Scholar]
- 18.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkobachar, C., L. Iyengar, and A. V. S. P. Rao. 1997. Mechanism of disinfection: effect of chlorine on cell membrane functions. Water Res. 11:727-729. [Google Scholar]