Abstract
The results of this study confirm that adenoviruses are the most resistant enteric viruses to inactivation by UV light and that adenovirus 40 appears to be the most resistant. The effect of freeze-thawing and storage in water may affect the sensitivity of some adenoviruses to inactivation by UV light.
Because of the increasing use of UV light disinfection to control waterborne pathogens in wastewater and drinking water, it is important to gain additional data on the UV light inactivation of the different serotypes of adenoviruses and factors which may influence their inactivation by UV light (1, 3, 7).
The objectives of this study were to determine the effect of UV light on selected serotypes of adenoviruses; the ability of two different cell lines to repair UV light damage; and the influence of freeze-thawing, storage time, and storage temperature on adenoviruses before UV light exposure.
Human adenovirus type 1 (VR-1, strain adenoid 71), type 3 (VR-3), type 4 (VR-4, strain RI-67), type 5 (VR-5), and type 6 (VR-6, strain tonsil 99) were obtained from the American Type Culture Collection (ATTC; Manassas, VA). Propagation of human adenoviruses was performed using the HeLa cell line (CCL-2; ATTC) and purified as described by Gerba et al. (4).
Two different continuous cell lines, PLC/PRF/5 human hepatoma (CRL-8024; ATCC) and HeLa (CCL-2; ATCC), were used for cell culture assays and enumerated by the most-probable-number method (4, 5). The MS-2 bacteriophage was propagated and assayed according to Meng and Gerba, (6).
A collimated-beam apparatus was utilized to conduct the UV disinfection experiments (6). Exposure conditions and dose determination were identical to those of Gerba et al. (4).
Viral stocks were frozen at −80°C and thawed at room temperature for freeze-thaw experiments prior to UV radiation. For the storage temperature experiments, viral stocks were stored at either 4°C or room temperature (25°C) prior to UV radiation.
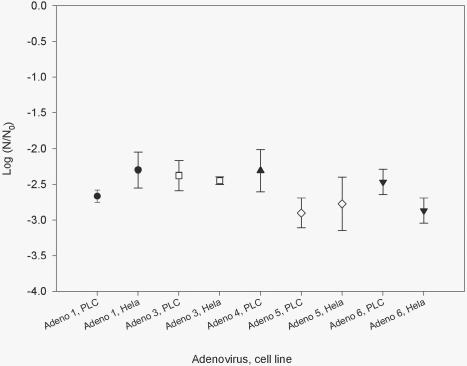
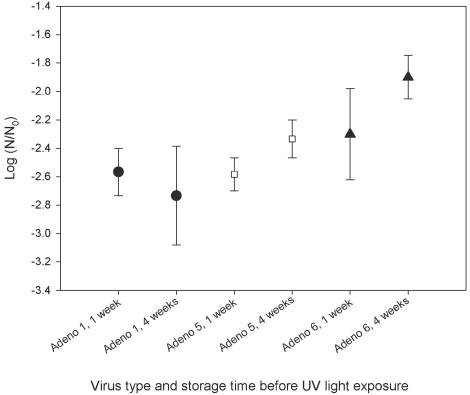
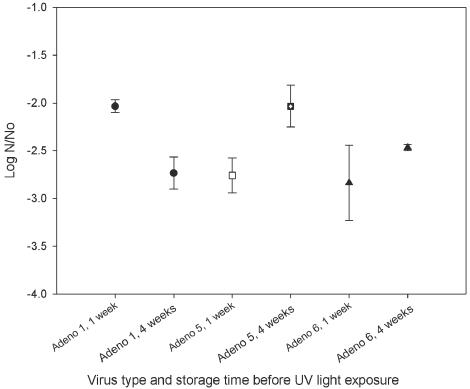
Experiments to determine the effects of host cell reactivation, freeze-thaw, and storage time and temperature were all conducted in 0.01 M phosphate-buffered saline (6) at a UV light dose of 90 mWs/cm2. The inactivation of adenovirus types 1 (AD1), 3 (AD3), 5 (AD5), and 6 (AD6), assayed on PLC and HeLa cell lines, are presented in Table 1 and Fig. 1. AD1, -5, and -6 were used for the freeze-thaw experiments. The log survival for each virus after one to four freeze-thaw events is shown in Table 2. The effect of storage temperature (4 and 25°C) and holding time (1 to 28 days) before UV light exposure was examined for AD1, -5, and -6 (Table 3 and Fig. 2 and 3).
TABLE 1.
Effect of cell line on adenovirus reactivation after UV light exposurea
| Adenovirus type | Log (N/N0) for cell line:
|
|
|---|---|---|
| HeLa | PLC | |
| 1 | −2.3 | −2.7 |
| 3 | −2.5 | −2.3 |
| 5 | −2.5 | −2.8 |
| 6 | −2.8 | −2.4 |
Dose, 90 ± 2 mWs/cm2. N0, initial concentration of virus; N, concentration of virus after exposure to UV light.
FIG. 1.
UV light inactivation of adenoviruses at a dose of 90 ± 2 mWs/cm2, as assayed on HeLa and PLC cell lines.
TABLE 2.
Effect of freeze-thaw on UV light inactivation of adenovirusesa
| Adenovirus type | Log (N/N0) for indicated no. of freeze-thaws
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 1 | −2.3 | −1.7 | −2.6 | −1.6 |
| 5 | −2.5 | −2.3 | −2.3 | −2.8 |
| 6 | −2.8 | −2.4 | −2.6 | −3.5 |
Dose, 90 ± 2 mWs/cm2. N0, initial concentration of virus; N, concentration of virus after exposure to UV light.
TABLE 3.
Effect of storage temperature on UV light inactivation of adenovirusesa
| Adenovirus type | Log (N/N0) for:
|
||||
|---|---|---|---|---|---|
| No storage | Storage at:
|
||||
| 4°C for:
|
25°C for:
|
||||
| 1 wk | 4 wk | 1 wk | 4 wk | ||
| 1 | −2.3 | −2.5 | −2.5 | −2.0 | −2.7 |
| 5 | −2.5 | −2.6 | −2.3 | −2.7 | −1.9 |
| 6 | −2.8 | −2.1 | −1.9 | −2.6 | −2.3 |
Dose, 90 ± 2 mWs/cm2. N0, initial concentration of virus; N, concentration of virus after exposure to UV light.
FIG. 2.
UV inactivation of AD1, AD5, and AD6 at 90 ± 2 mWs/cm2 after 1 week and 4 weeks of storage at 4°C.
FIG. 3.
Inactivation of AD1, AD5, and AD6 at 90 mWs/cm2 after storage at 25°C.
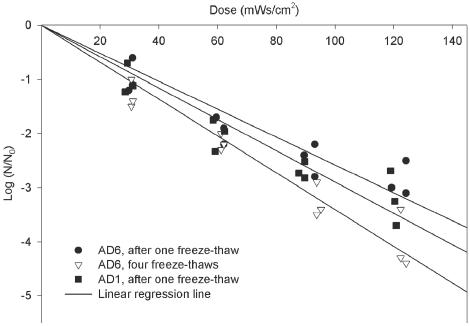
The effects of UV exposure for AD1 after the first freeze-thaw and for AD6 after the first and fourth freeze-thaws are presented in Tables 4 and 5 and Fig. 4. Both viruses exhibited typical first-order inactivation kinetics. Associated R2 values and inactivation constants are listed in Table 6. The predicted doses calculated for 1 to 4 logs of inactivation for Ad1 and -6 are shown in Table 7. In addition, the predicted values for adenovirus 40 and poliovirus 1 are also presented in the same table for comparison.
TABLE 4.
Comparison of observed log (N/N0) of adenovirus types 1 and 6 at different UV light doses after one freeze-thawa
| Dose (mWs/cm2) | Log (N/N0) for adenovirus type:
|
|
|---|---|---|
| 1 | 6 | |
| 30 | −1.0 | −0.9 |
| 60 | −2.0 | −1.9 |
| 90 | −2.7 | −2.4 |
| 120 | −3.0 | −2.8 |
N0, initial concentration of virus; N, concentration of virus after exposure to UV light.
TABLE 5.
Comparison of observed log (N/N0) of adenovirus type 6 after one and four freeze-thaws at different UV light dosesa
| Dose (mWs/cm2) | Log (N/N0) after:
|
|
|---|---|---|
| 1 freeze-thaw | 4 freeze-thaws | |
| 30 | −0.9 | −1.3 |
| 60 | −1.9 | −2.2 |
| 90 | −2.4 | −3.2 |
| 120 | −2.8 | −3.8 |
N0, initial concentration of virus; N, concentration of virus after exposure to UV light.
FIG. 4.
UV light inactivation curve of AD6 (after one freeze-thaw and four freeze-thaws) and AD1 (after one freeze-thaw).
TABLE 6.
Inactivation constants, standard errors, and R2 values obtained by regression analysis for UV disinfection of AD1 and AD6a
| Virus, no. of thaws | K (coefficient) | SE | R2 |
|---|---|---|---|
| AD6, 1 | 0.026 | 0.0023 | 0.89 |
| AD6, 4 | 0.034 | 0.0022 | 0.95 |
| AD1, 1 | 0.029 | 0.0024 | 0.91 |
| MS-2, 0 | 0.034 | 0.0023 | 0.97 |
For each virus, three experiments were performed. K, inactivation constant.
TABLE 7.
Predicted dose requirements for log10 inactivation using UV light
There was virtually no statistical difference in log survival of adenoviruses stored at two dissimilar temperatures for up to 4 weeks before UV light exposure, as determined by analyses of variance (ANOVA) (Table 8). The exception was AD1, which had a greater rate of inactivation after 4 weeks of storage at 25°C (P = 0.02). There was not a significant difference between inactivation of AD1 and AD6 after one freeze-thaw (Table 9). The slopes of linear regression for inactivation of AD6, after one freeze-thaw and four freeze-thaws, demonstrated a significant difference (Table 9). This indicated that AD6 was more susceptible to UV light after four freeze-thaws at a dose of 120 mWs/cm2.
TABLE 8.
ANOVA test results of log (N/N0) at 90 mWs/cm2a
| Source of variation | Virus/condition | Degrees of freedom | Sum of squares | F ratio | P |
|---|---|---|---|---|---|
| Virus type | AD1, -3, -5, -6/ HeLa cell line | 3 | 0.64 | 1.2 | 0.37 |
| Virus type | AD1, -3, -4, -5, -6/ PLC cell line | 4 | 1.12 | 1.2 | 0.37 |
| No. of freeze-thaws | AD1 | 3 | 2.0 | 2.11 | 0.18 |
| No. of freeze-thaws | AD5 | 3 | 8.8 | 1.5 | 0.28 |
| No. of freeze-thaws | AD6 | 3 | 0.96 | 1.2 | 0.4 |
| Storage time | AD1/25°C | 1 | 0.7 | 15.2 | 0.02 |
| Storage time | AD5/25°C | 1 | 0.8 | 6.4 | 0.06 |
| Storage time | AD6/25°C | 1 | 0.2 | 0.86 | 0.4 |
| Storage time | AD1/4°C | 1 | 0.04 | 0.18 | 0.7 |
| Storage time | AD5/4°C | 1 | 0.09 | 1.9 | 0.23 |
| Storage time | AD6/4°C | 1 | 0.24 | 1.3 | 0.32 |
Boldface indicates significance with more than 95% confidence level (P < 0.05). N0, initial concentration of virus; N, concentration of virus after exposure to UV light.
TABLE 9.
ANOVA test results of slopes (coefficients) of linear regression line
| Source of variation | Virus or condition | Degrees of freedom | Sum of squares | F ratio | P |
|---|---|---|---|---|---|
| No. of freeze-thaws | AD6 | 1 | 0.00011 | 13.4 | 0.021 |
| Virus type (AD6, AD1) | After 1 freeze-thaw | 1 | 0.000015 | 3.4 | 0.14 |
Battigelli et al. (2) and Thurston-Enriquez et al. (9) reported values of 29.3 and 87.8 mWs/cm2 for 1- and 3-log inactivation of MS-2, respectively, which are very similar to the values obtained in this study (Table 7). The presence of host cell repair enzymes affects the ability of the DNA viruses to repair the damage caused by UV light. Two different continuous cell lines, PLC/PRF/5 and HeLa, were used to determine if the assay of UV light-exposed adenoviruses would provide different results; however, no statistical difference was found (Table 8). Multiple freeze-thawing of virus stocks has been speculated to damage the viral capsids and as a result make them more susceptible to UV radiation (4). No significant difference between inactivation rate of AD1 and AD6 after one freeze-thaw was observed (Table 9). However, the slopes of linear regression lines of AD6 after one freeze-thaw and four freeze-thaws showed a significant difference (Table 9). This indicates that AD6 was more susceptible to UV light after four freeze-thaws at a dose of 120 mWs/cm2.
There was no statistically significant difference in log reduction of AD5 and -6 stored at two dissimilar temperatures for up to 4 weeks before UV light exposure (Table 8). However, AD1 demonstrated a higher inactivation rate after 4 weeks of storage at 25°C (P = 0.02).
Gerba et al. (4) found that doses required to achieve 2- to 4-log inactivation of AD2 were very similar to the ones required for AD6 in this study and slightly higher than doses for AD1 (Table 7). Thurston-Enriquez et al. (9) found that enteric AD40 was significantly more resistant to UV light than any of the respiratory adenoviruses used in this study. Meng and Gerba (6) reported 30 and 124 mWs/cm2 for 1- and 4-log inactivation of AD40, which are very similar to those for AD6 after four freeze-thaws (29 and 117.6 mWs/cm2). Also, the reported 3.3-log reduction of AD40 at 90 mWs/cm2 was almost identical to AD6 (Table 7).
The results of this study confirm that adenoviruses are the most resistant enteric viruses to inactivation by UV light and that adenovirus 40 appears to be the most resistant. The effect of freeze-thawing and storage in water may affect the sensitivity of some adenoviruses to inactivation by UV light.
Acknowledgments
This work was supported by the Office of Water, Office of Science and Technology, U.S. Environmental Protection Agency, under contract 68-C-99232.
The opinions expressed here are those of the authors and not necessarily those of the U.S. Environmental Protection Agency. Mention of equipment used in this study does not constitute an endorsement by the authors or the U.S. Environmental Protection Agency.
REFERENCES
- 1.Ballester, N. A., and V. P. Malley. 2004. Sequential disinfection of adenovirus type 2 with UV-chlorine-chloramine. J. Am. Water Works Assoc. 96:97-103. [Google Scholar]
- 2.Battigelli, D. A., M. D. Sobsey, and D. C. Lobe. 1993. The inactivation of hepatitis A virus and other model viruses by UV irradiation. Water Sci. Technol. 27:339-342. [Google Scholar]
- 3.Gerba, C. P., N. Nwachuku, and K. R. Riley. 2003. Disinfection resistance of waterborne pathogens on the United States Environmental Protection Agency's contaminant candidate list (CCL). J. Water Supply-Aqua. 52:81-94. [Google Scholar]
- 4.Gerba, C. P., D. M. Gramos, and N. Nwachuku. 2002. Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Appl. Environ. Microbiol. 68:5167-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurley, M. A., and M. E. Roscoe. 1983. Automated statistical analysis of microbial enumeration by dilution series. J. Appl. Bacteriol. 55:159-164. [Google Scholar]
- 6.Meng, Q. S., and C. P. Gerba. 1996. Comparative inactivation of enteric adenoviruses, poliovirus and coliphages by ultraviolet irradiation. Water Res. 30:2665-2668. [Google Scholar]
- 7.Nwachuku, N., and C. P. Gerba. 2004. Emerging waterborne pathogens: can we kill them all? Curr. Opin. Biotehnol. 15:175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roessler, P. F., and B. F. Severin. 1996. Ultraviolet light disinfection of water and wastewater, p. 313-368. In C. J. Hurst (ed.), Modeling disease transmission and its prevention by disinfection. Cambridge University Press, Cambridge, United Kingdom.
- 9.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, K. Riley, and C. P. Gerba. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]