Abstract
The presence of iron, used both as a nutrient and as an electron acceptor, was demonstrated to give an advantage to Escherichia coli bacteria in drinking water. Slight additions of ferrous sulfate to water with initial low iron concentrations led to a significant increase in the number of E. coli bacteria. The presence of ferric oxide in water under anaerobic conditions increased bacterial cultivability.
The presence of iron corrosion products is reported to favor bacterial activity in drinking water networks, resulting in increases of both suspended bacteria and biofilm-associated bacteria (2, 8, 13, 22) or in increased occurrence and/or cultivability of coliform bacteria, usually used as the indicator of water bacteriological quality (17, 18, 26). The large accumulations of bacterial cells observed on environmental ferric mineral (9) and on corroded metallic structures (19) may be partially attributed to the surface properties of iron oxides in that their high surface area and their surface charge may promote attachment and colonization by microorganisms (1, 24).
Iron may also be considered a nutrient necessary for bacterial growth, like other elements, such as carbon, nitrogen, and phosphorus. It is, for example, a constituent of all heme enzymes, which include cytochromes and hydroperoxidase. Iron has been shown to limit microbial growth in aquatic environments (6, 7, 14, 16), where bacteria must display particular mechanisms, such as production of strong iron chelators called siderophores, when faced with extreme iron deficiency (10, 11).
Iron is also an electron acceptor for bacteria that can couple organic matter oxidation to Fe(III) reduction in the absence of oxygen (20). Iron-reducing bacteria are thus able to gain energy from the reduction of soluble (12) or solid iron species (15, 23).
The present study was conducted to observe the effect of iron, considered both as nutrient and as electron acceptor, on the growth and cultivability of Escherichia coli strain SH 702 in drinking water.
To investigate the role of iron as a limiting nutrient, drinking water sampled after treatment chain but before distribution was sterilized by heat (121°C, 2 atm, 20 min) and 0.22-μm filtration. Concentrations of dissolved constituents were the following (mg liter−1): Ca, 23.2; Mg, 6.4; K, 2.2; Na, 16.5; SO4, 51.1; Cl, 16.4; NO3, 4.4; PO4, <0.05; organic carbon, 1.56; Fe, <0.002. The water was then placed in 500-ml glass flasks and supplemented with ferrous sulfate as a soluble mineral iron source, at concentrations close to those described as the minimum necessary to support bacterial growth (21). Three flasks were then prepared for each iron concentration (no added iron and 2, 5, 10, 15, and 20 μg liter−1), and final concentrations were confirmed by inductively coupled plasma mass spectrometry analysis. E. coli bacteria previously isolated from a distribution system and characterized as strain SH 702 were grown in Luria-Bertani broth media (Difco 0446-17-3) at 25°C. After 48 h, the cell cultures were washed three times by centrifugation (5 min; 10,000 × g) and suspended in sterile (0.22-μm filtration) commercial mineral water with an organic carbon concentration of about 0.2 mg liter−1. To simulate the type of oligotrophic conditions found in drinking water, the cells were starved by incubation in the mineral water at 20°C for 24 h before being subjected to another washing cycle. E. coli bacteria were then harvested and added to drinking water in glass flasks, at a final concentration of 1.5 × 103 cells ml−1. This concentration was supposed to be low enough to allow bacterial growth in such poor-nutrient media. Flasks were incubated at 25°C, in the dark, with agitation at 350 rpm. Bacterial growth was monitored by epifluorescence microscopy counting after cells had been stained with DAPI (4′,6′-diamidino-2-phenylindole) (2). Cultivability was monitored by cultivation on agar (plate count agar; Difco 247940) at 30°C for 24 h, as is used for drinking water quality control.
To investigate iron as an electron acceptor, 12 flasks were prepared with the same water as previously described. Six were supplemented with a 50 mM concentration of a solid iron oxide (lepidocrocite; γ-FeOOH) simulating iron corrosion products. For three flasks with iron oxide and three without, anaerobic conditions were generated by purging with nitrogen for 30 min and closing the flasks with butyl stoppers. In the remaining flasks, aerobic conditions were maintained by agitating and simply closing flasks with sterile cotton. E. coli bacteria were prepared as described above before being added to flasks, at a final concentration of 5 × 106 cells ml−1. This concentration was deliberately set high, in order to detect cultivable E. coli for a long time (several days) and hence observe a potential effect of iron oxide or anaerobic conditions on cultivability. Total and cultivable bacteria were enumerated as described above. To observe iron reduction, ferrous iron measurements were performed by the o-phenanthroline spectrophotometric method (12) in flasks containing iron oxide at the beginnings and ends of experiments. Abiotic controls were also analyzed.
Iron as a nutrient.
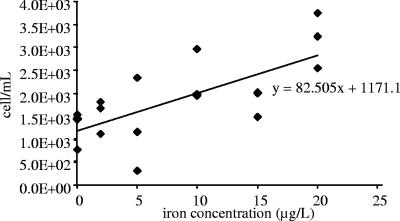
The number of total E. coli bacteria (epifluorescence counts) increased with time and reached steady state after 16 days. At this point, a clear relationship was observed between total bacteria and iron concentration (r = 0.87, P < 0.001; analysis of variance, F = 4.101, P = 0.021), and the number of total cells was on average three times higher in flasks with 20 μg Fe liter−1 than in flasks without added iron, where almost no growth was observed (Fig. 1) (the Tukey test was significant for 20 versus 0 μg Fe liter−1, and for 20 versus 2 μg Fe liter−1: P = 0.015 and P = 0.042, respectively). Unlike total bacteria, the number of cultivable E. coli bacteria rapidly decreased from the initial concentration of 1.3 × 103 CFU ml−1 at the beginning of the experiment to values from 3.3 × 10−1 to 4.0 × 101 CFU ml−1 for flasks containing 2 and 10 μg Fe liter−1, respectively, after 16 days. No simple relationship between the iron concentration and the cultivability of E. coli was observed at any time of the experiment. No organic carbon consumption was displayed by measurements performed at the beginnings and ends of experiments.
FIG. 1.
Number of total E. coli bacteria (epifluorescence counts) in drinking water flasks after 16 days, according to iron concentration. Each data point represents the result from a single flask.
The increase in total cells observed here must be due to iron utilization, because all assays were carried out under the same conditions and with similar initial organic carbon and inorganic nutrient concentrations. Bacterial growth is not attributable to prior nutrient storage in cells during their growth, as no increase in bacterial number was observed without added iron. The apparent absence of organic carbon consumption during the assays is not surprising, due to the very small amount of nutrients necessary to produce 2 × 103 cells ml−1, as observed in flasks with 20 μg Fe liter−1. According to an earlier hypothesis based on previous observations (2), bacterial production in such conditions requires 1 × 10−12 g of organic carbon per cell. On this basis, the highest organic carbon consumption needed to support bacterial growth in the present experiments would be only 0.2 μg liter−1 (in flasks supplemented with 20 μg Fe liter−1), which is lower than the sensitivity of the apparatus used here. In this experiment, the benefit provided by the presence of iron to bacterial growth is unlikely to be due to the electron acceptor character of iron, because iron was initially provided as Fe(II), and experiments were conducted in the presence of oxygen, which is preferentially reduced by bacteria.
Iron as an electron acceptor.
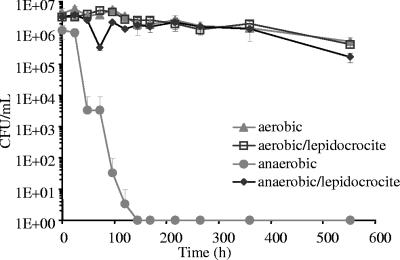
In the assays performed under aerobic conditions, E. coli remained cultivable during the 23-day experiment, with only a slight decrease over time, and the presence of iron oxide had no effect on cultivable bacteria in such conditions. In the anaerobic flasks without iron oxide, cultivable E. coli decreased 6 orders of magnitude after only 6 days and was undetectable thereafter. When iron oxide was included under anaerobic conditions, the cultivability of E. coli was comparable to that in the assays performed under aerobic conditions (Fig. 2). Epifluorescence counts after 23 days did not reflect the trend in CFU and were 1.6 (±0.1) × 106 and 4.0 (±0.5) × 106 cells/ml in the anaerobic assays (with and without iron oxide, respectively), and 1.4 (±0.1) × 106 and 5.0 (±0.2) × 106 in the aerobic assays (with and without iron oxide, respectively). Cell concentrations reported for assays with iron oxide are probably slightly underestimated, owing to the difficulty of enumerating bacteria attached to oxide particles. Fe(II) measurements revealed that iron oxide had been partially reduced during the experiments: the Fe(II) concentration increased from 0.11 ± 0.02 mM under initial conditions in assays with lepidocrocite to 1.06 ± 0.09 mM and 0.82 ± 0.05 mM after 23 days under aerobic and anaerobic conditions, respectively. No reduction was observed in similar assays performed under abiotic conditions. A repetition of this experiment performed with the same E. coli in the same type of water led to similar results.
FIG. 2.
Cultivability of E. coli in drinking water flasks, determined under aerobic and anaerobic conditions with or without iron oxide supplementation.
Despite the presence of oxygen in the bulk media of aerobic flasks, ferrous iron is likely to be stabilized by adsorption on iron oxide particles, the presence of organic or microbial chelators, and local microanaerobic conditions occurring at water-mineral-bacterium interfaces.
Fe(III) reduction has been observed under such conditions with E. coli and in comparable amounts under anaerobic and aerobic conditions (4, 27). According to different authors, Fe(III) reduction by E. coli would either be a respiration process (25), meaning a dissimilatory reduction of iron, or a constitutive process involving transport within cells before its utilization as functional constituent (3, 5, 27), meaning an assimilatory mechanism. In the present assays, if increased cultivability was due to iron as a nutrient only, no difference should occur between aerobic and anaerobic conditions. The differing behavior of cultivable E. coli between anaerobic assays with and without iron hydroxide must be due to more than the use of iron as a nutrient. Another observation suggesting that iron reduction was not due to bacterial assimilation is that the amount of ferrous iron produced in the assays was close to 1 mM (55.8 × 10−3 g liter−1) in both aerobic and anaerobic assays. According to the E. coli cell concentration in the medium (about 2 × 106 cells ml−1), and to the hypothesis of a cell mass of 0.2 × 10−12 g cell−1, the biomass present would be around 4 × 10−5 g of bacterial cells liter−1. With 3 orders of magnitude between biomass and ferrous iron, the reduced iron is hence definitely unlikely to have been only assimilated by bacteria and stored in cells, as described by Briat (3). Rather, the main iron reduction pathway must be dissimilation.
This study demonstrates that iron in drinking water may promote both growth and cultivability of E. coli. Results suggest that iron may be considered a potentially limiting nutrient in drinking water and that increasing iron concentration may thus lead to an increase in E. coli growth. We also demonstrate that while anaerobic conditions may have a negative effect on E. coli cultivability, this can be cancelled by the presence of iron oxide. These findings confirm and partially explain numerous observations made on distribution systems in which corroded pipe material has been related to bacterial growth and highlight the fact that the presence of iron oxides in drinking water distribution systems may lead to actual degradation in microbiological water quality.
Acknowledgments
This work was carried out as part of a larger research program entitled “Biofilm” and coordinated by the Centre International de l'Eau de Nancy (NanCIE, France). It was funded by Compagnie Générale des Eaux (CGE, France), Agence de l'Eau Seine-Normandie (AESN, France), Anjou-Recherche (VEOLIA, France), Syndicat des Eaux d'Ile de France (SEDIF, France), and the Communauté Urbaine du Grand Nancy (CUGN).
REFERENCES
- 1.Appenzeller, B. M. R., Y. B. Duval, F. Thomas, and J.-C. Block. 2002. Influence of phosphate on bacterial adhesion onto iron oxyhydroxide in drinking water. Environ. Sci. Technol. 36:646-652. [DOI] [PubMed] [Google Scholar]
- 2.Appenzeller, B. M. R., M. Batté, L. Mathieu, J.-C. Block, V. Lahoussine, J. Cavard, and D. Gatel. 2001. Effect of adding phosphate to drinking water on bacterial growth in slightly and highly corroded pipes. Water Res. 35:1100-1105. [DOI] [PubMed] [Google Scholar]
- 3.Briat, J.-F. 1992. Iron assimilation and storage in prokaryotes. J. Gen. Microbiol. 138:2475-2483. [DOI] [PubMed] [Google Scholar]
- 4.Bromfield, S. M. 1954. The reduction of iron oxide by bacteria. J. Soil Sci. 5:129-139. [Google Scholar]
- 5.Brons, H. J., W. R. Hagen, and A. J. B. Zehnder. 1991. Ferrous iron dependent nitric oxide production in nitrate reducing cultures of Escherichia coli. Arch. Microbiol. 155:341-347. [DOI] [PubMed] [Google Scholar]
- 6.Church, M. J., D. A. Hutchins, and H. W. Ducklow. 2000. Limitation of bacterial growth by dissolved organic matter and iron in the Southern Ocean. Appl. Environ. Microbiol. 66:455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochlan, W. P. 2001. The heterotrophic bacterial response during a mesoscale iron enrichment experiment (IronEx II) in the eastern equatorial Pacific Ocean. Limnol. Oceanogr. 46:428-435. [Google Scholar]
- 8.Donlan, R. M., W. O. Pipes, and, T. L. Yohe. 1994. Biofilm formation on cast iron substrata in water distribution systems. Water Res. 28:1497-1503. [Google Scholar]
- 9.Emerson, D., J. V. Weiss, and J. P. Megonigal. 1999. Iron-oxidizing bacteria are associated with ferric hydroxide precipitates (Fe-plaque) on the roots of wetland plants. Appl. Environ. Microbiol. 65:2758-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan, L. L., K. Kanoh, and K. Kamino. 2001. Effect of exogenous siderophores on iron uptake activity of marine bacteria under iron-limited conditions. Appl. Environ. Microbiol. 67:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hersman, L. E., A. Huang, P. A. Maurice, and J. H. Forsythe. 2000. Siderophore production and iron reduction by Pseudomonas mendocina in response to iron deprivation. Geomicrobiol. J. 17:261-273. [Google Scholar]
- 12.Jorand, F., B. Appenzeller, M. Abdelmoula, P. Refait, J.-C. Block, and J. M. R. Génin. 2001. Assessment of vivianite formation in Shewanella putrefaciens culture. Environ. Technol. 21:1001-1005. [Google Scholar]
- 13.Kerr, C. J., K. S. Osborn, G. D. Robson, and P. S. Handley. 1999. The relationship between pipe material and biofilm formation in a laboratory model system. J. Appl. Microbiol. Symp. Suppl. 85:29S-38S. [DOI] [PubMed] [Google Scholar]
- 14.Kirchman, D. L., B. Meon, M. T. Cottrell, D. A. Hutchins, D. Weeks, and K. W. Bruland. 2000. Carbon versus iron limitation of bacterial growth in the California upwelling regime. Limnol. Oceanogr. 45:1681-1688. [Google Scholar]
- 15.Kotska, J. E., and K. H. Nealson. 1995. Dissolution and reduction of magnetite by bacteria. Environ. Sci. Technol. 29:2535-2540. [DOI] [PubMed] [Google Scholar]
- 16.Lancelot, C., E. Hannon, S. Becquevort, C. Veth, and H. J. W. De Baar. 2000. Modeling phytoplankton blooms and carbon export production in the Southern Ocean: dominant controls by light and iron in the Atlantic sector in austral spring 1992. Deep-Sea Res. Pt. I 47:1621-1662. [Google Scholar]
- 17.LeChevallier, M. W. 1990. Coliform regrowth in drinking water: a review. J. AWWA 82(11):74-86. [Google Scholar]
- 18.LeChevallier, M. W., N. J. Welch, and D. B. Smith. 1996. Full-scale studies of factors related to coliform regrowth in drinking water. Appl. Environ. Microbiol. 62:2201-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little, B. J., R. I. Ray, P. A. Wagner, J. Jones-Meehan, C. Lee, and F. Mansfeld. 1999. Spatial relationships between marine bacteria and localised corrosion on polymer coated steel. Biofouling 13:301-321. [Google Scholar]
- 20.Lovley, D. R., and E. J. Phillips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neilands, J. B., and K. Nakamura. 1991. Detection, determination, isolation, characterization, and regulation of microbial iron chelates, p. 1-14. In G. Winkelmann (ed.), Handbook of microbial iron chelates. CRC Press, Inc., Boca Raton, Fla.
- 22.Niquette, P., P. Servais, and R. Savoir. 2000. Impacts of pipe materials on densities of fixed bacterial biomass in a drinking water distribution system. Water Res. 34:1952-1956. [DOI] [PubMed] [Google Scholar]
- 23.Ona-Nguema, G., M. Abdelmoula, F. Jorand, O. Benali, A. Géhin, J.-C. Block, and J. M. R. Génin. 2002. Iron(II, III) hydroxycarbonate green rust formation and stabilization from the lepidocrocite bioreduction. Environ. Sci. Technol. 36:16-20. [DOI] [PubMed] [Google Scholar]
- 24.Roden, E., and J. M. Zachara. 1996. Microbial reduction of crystalline iron(III) oxides: influence of oxide surface area and potential for cell growth. Environ. Sci. Technol. 30:1618-1628. [Google Scholar]
- 25.Short, K. A., and R. P. Blakemore. 1986. Iron respiration-driven proton translocation in aerobic bacteria. J. Bacteriol. 167:729-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Victoreen, H. T. 1984. The role of rust in coliform regrowth, p. 253-264. In Proceedings of the AWWA Water Quality Technology Conference. American Water Works Research Foundation, Denver, Colo.
- 27.Williams, H. D., and R. K. Poole. 1987. Reduction of iron(III) by Escherichia coli K12: lack of involvement of the respiratory chains. Curr. Microbiol. 15:319-324. [Google Scholar]