Abstract
We exploited the unique ecological niche of oil fly larval guts to isolate a strain of Staphylococcus haemolyticus which may be the most solvent-tolerant gram-positive bacterium yet described. This organism is able to tolerate 100% toluene, benzene, and p-xylene on plate overlays and saturating levels of these solvents in monophasic liquid cultures. A comparison of membrane fatty acids by gas chromatography after growth in liquid media with and without toluene showed that in cells continuously exposed to solvent the proportion of anteiso fatty acids increased from 25.8 to 33.7% while the proportion of 20:0 straight-chain fatty acids decreased from 19.3 to 10.1%. No changes in the membrane phospholipid composition were noted. Thus, S. haemolyticus alters its membrane fluidity via fatty acid composition to become more fluid when it is exposed to solvent. This response is opposite that commonly found in gram-negative bacteria, which change their fatty acids so that the cytoplasmic membrane is less fluid. Extreme solvent tolerance in S. haemolyticus is not accompanied by abnormal resistance to anionic or cationic detergents. Finally, six strains of Staphylococcus aureus and five strains of Staphylococcus epidermidis, which were not obtained by solvent selection, also exhibited exceptional solvent tolerance.
Environments having high concentrations (10 to 50%, vol/vol) of organic solvents are considered extreme (1), and bacteria that are able to tolerate such environments have recently been recognized as a subgroup of the extremophiles (1). Lipophilic hydrocarbons are harmful to bacteria because they accumulate in membrane lipid bilayers, thus affecting the structural and functional properties of the membranes (25), and cytoplasmic membranes are the primary site of cellular damage by both organic solvents (25) and anionic detergents, such as sodium dodecyl sulfate (SDS) (17). Recently, our laboratory has pioneered the study of oil fly larval guts as a source of solvent-tolerant bacteria (12, 13). Larvae of the oil fly, Helaeomyia petrolei, are found exclusively submerged in oil, where they ingest large quantities of oil and asphalt. Thus, any bacteria isolated from oil fly larval guts have been naturally selected for solvent tolerance. Larvae isolated from the asphalt seeps of Rancho LaBrea in Los Angeles, Calif., contained ca. 2 × 105 heterotrophic bacteria per larva (13). Most of these bacteria were identified as Providencia rettgeri and Acinetobacter isolates (13), and most of the Providencia strains were subsequently shown to be naturally resistant to many antibiotics and organic solvents (12).
Significantly, all of the bacteria identified from oil fly larvae at that time were gram-negative bacteria; no sporeformers or gram-positive bacteria were found (13). Presumably, the outer membrane protects the cytoplasmic membrane by reducing the periplasmic concentrations of harmful chemicals to acceptable levels. Our finding that almost all of the bacteria in oil fly larvae were gram negative agreed with the generalization of Aono and Inoue (1) that gram-negative bacteria show higher levels of organic solvent tolerance. It also agreed with previous conclusions from our laboratory that only gram-negative bacteria could tolerate high levels (5 to 25%) of SDS or other anionic detergents (15, 17, 22). We decided to investigate further the importance of the outer membranes in organic solvent tolerance by looking explicitly for anaerobic bacteria in oil fly larval gut contents. In the process we identified the highly solvent-tolerant gram-positive Enterococcus and Staphylococcus strains which are described in this paper.
Organic solvents have many detrimental effects on microbes, and some solvents are more harmful than others. To classify the intrinsic toxicity of a solvent, the logarithm of its partition coefficient in n-octanol and water was measured and termed log Pow. Solvents with a low log Pow (1.5 to 4.0) are considered extremely toxic, while solvents with a higher log Pow are less toxic (7). The reasoning for this toxicity index lies in the fact that solvents with lower log Pow values tend to partition into the cytoplasmic membranes of organisms, compromising the structure and destroying vital functions. In recent years, a growing number of bacterial species able to overcome these toxic effects have been found (1).
Most solvent-tolerant bacterial species are gram negative. Gram-negative bacteria have the advantage of having an additional outer membrane, which allows quick modifications and adaptations in the lipopolysaccharides, efflux pumps, and/or fatty acid composition (23). Due to the inherent disadvantage of lacking an outer membrane, only a few gram-positive organisms have been reported to exhibit solvent tolerance, including species of Bacillus, Rhodococcus, and Enterococcus (8, 18). Mechanisms of solvent tolerance have not been proposed yet for gram-positive species, although it has been suggested that these organisms may use mechanisms similar to those used by gram-negative bacteria (23).
In their review, Sardessai and Bhosle (24) paid particular attention to the limited reports of solvent tolerance among gram-positive bacteria. These authors concluded that (i) there is a large void in the available data on solvent tolerance mechanisms in gram-positive bacteria; (ii) there should be studies to determine whether molecular mechanisms of solvent tolerance elucidated in gram-negative bacteria are also conserved in gram-positive bacteria; and (iii) new habitats need to be explored to isolate other species displaying such tolerance (24). In the present paper we describe our use of oil fly larvae as a novel source of solvent-tolerant gram-positive bacteria. We then examined the fatty acid and phospholipid compositions of one isolate (Staphylococcus haemolyticus) grown in the presence and absence of saturating levels of toluene. We isolated an S. haemolyticus strain, as identified by MIDI and 16S RNA sequence analyses, which is able to tolerate levels of cyclohexane, toluene, benzene, and p-xylene on plate overlays that are greater than the levels tolerated by any other previously isolated gram-positive bacterium. We also examined these bacteria when they were grown in monophasic (no phase separation) liquid cultures. Below we describe changes in fatty acid composition as a possible mechanism of solvent tolerance for S. haemolyticus. We postulate that when grown in toluene-saturated media, S. haemolyticus is similar to its gram-negative counterparts in that it alters its fatty acid membrane composition but is dissimilar in that the membrane becomes more fluid rather than less fluid. A survey of 13 strains of Staphylococcus aureus and Staphylococcus epidermidis, which had not previously been exposed to solvent selection, revealed solvent tolerance similar to that of S. haemolyticus.
MATERIALS AND METHODS
Isolation of solvent-tolerant anaerobes from H. petrolei larvae.
All steps were performed under strictly anaerobic conditions in a Coy (Grass Lake, Mich.) anaerobic chamber containing a mixture of 5% hydrogen and 95% nitrogen. Four H. petrolei larvae were surface sterilized as previously described (13). The larvae were placed in a sterilized hand-held Potter-Elvehjem homogenizer containing 2 ml of Clostridium isolation broth (CIB) (2). The larvae were homogenized for 5 min, after which 0.1 ml was used to inoculate four tubes containing 9.9 ml of CIB. Growth and spore formation were monitored daily by phase-contrast microscopy. Each of the growing cultures was subcultured in CIB containing 5% (vol/vol) acetone or butanol and incubated anaerobically at 28°C for 48 h. For the cultures exhibiting growth, we used a series of dilution tubes and plating on CIB agar to isolate single colonies. Two isolated colonies were subcultured in CIB, and their purity and morphology were assessed by Gram staining and phase-contrast microscopy. They were sent to MIDI Laboratories (Newark, DE) for identification by 16S rRNA sequence and fatty acid analyses. A gram-positive coccus was identified as Enterococcus faecalis, and a gram-positive rod was identified as Clostridium sporogenes. Only the E. faecalis isolate was used for further study.
Other strains used.
S. haemolyticus was isolated from oil fly larval guts by selection on an LB agar plate which was overlaid with 15% benzene/85% cyclohexane. The cells were gram-positive spheres. They were coagulase negative. The organism was identified as S. haemolyticus on the basis of its fatty acid composition (MIDI Inc., Newark, DE) and 16S RNA sequence. The 16S RNA sequence (accession no. MAFF 911476) differed at only one base from the sequence of another known S. haemolyticus strain (ATCC 29970T). Other Staphylococcus strains used were provided by Greg Somerville and Eugene Martin (Table 1).
TABLE 1.
Solvent tolerance of 14 Staphylococcus strains
| Straina | Growth withb:
|
|||
|---|---|---|---|---|
| Cyclohexane (log Pow, 3.3) | p-Xylene (log Pow, 3.1) | Toluene (log Pow, 2.6) | Benzene (log Pow, 2.0) | |
| S. aureus strains | ||||
| MSSA 476 | ++ | ++ | ++ | ? |
| RN6390 SigB− | ++ | -- | -- | -- |
| SH1000 SigB+ | ++ | ++ | ++ | ++ |
| MRSA 252 | ++ | ++ | + | + |
| MW2 | ++ | ++ | ++ | + |
| RF122 | ++ | -- | -- | -- |
| 25923 | ++ | ++ | ++ | ++ |
| 213 | ++ | ++ | ++ | ++ |
| S. epidermidis strains | ||||
| RP62A | ++ | ++ | ? | -- |
| SE229 | ++ | ++ | + | -- |
| SE220 | ++ | + | + | -- |
| ATCC 14990 | ++ | ++ | ++ | + |
| ATCC 12228 | ++ | ++ | ++ | -- |
| S. haemolyticus | ++ | ++ | ++ | ++ |
| E. faecalis | ++ | +c | +c | +c |
S. haemolyticus and E. faecalis were isolated as part of our oil fly screening, and S. aureus strains 213 and 25923 were obtained from Eugene Martin. All other strains were obtained from Greg Somerville (23).
Log Pow is a measure of the intrinsic solvent toxicity depending on the partition ratios of the solvent into E. coli membranes. Growth was scored as follows: ++, confluent growth; +, some growth; −, no growth; ?, variable results. All data were based on at least three independent replicates.
E. faecalis did not tolerate 100% p-xylene, toluene, or benzene; the maximum levels tolerated were 50, 35, and 30%, respectively.
Measurement of solvent tolerance.
Plate overlay solvent tolerance was determined by spot inoculating 20 μl (106 cells) of S. haemolyticus from an overnight culture grown in LB broth at 37°C onto glass petri plates containing LB agar. The spots were then allowed to dry for 20 to 30 min at 37°C. The plates were transferred to a properly ventilated room temperature area, and solvent was directly poured on top of the agar plate surface to a depth of 5 mm. After 8 h the solvent was pipetted off, and the plates were inverted and incubated at 37°C for another 24 h. The solvents tested included cyclohexane, toluene, and benzene. Reagent grade toluene was obtained from Matheson, Coleman, and Bell Inc., Norwood, Ohio. All other chemicals were obtained from Sigma-Aldrich, St. Louis, Mo.
Monophasic solvent medium.
A 100-μl aliquot of an overnight culture was transferred to 20 ml of 37°C prewarmed LB medium which contained either cyclohexane, toluene, or benzene. Monophasic (saturated) medium contained either 0.95 mM cyclohexane, 6.2 mM toluene, or 21 mM benzene (i.e., 2.08, 13.4, and 37.6 μl per 20 ml, respectively). To ensure that there was no loss of solvent due to volatilization, flasks were capped with aluminum foil-covered rubber stoppers. Cells were grown at 37°C in 250-ml sidearm nephaloflasks (Bellco Glass, Vineland, N.J.) with rotary shaking at 200 rpm. Optical density readings were taken every 2 h for 30 h using a Klett-Summerson colorimeter (New York, NY) with a red filter. This arrangement permitted us to measure cell density without opening the flasks.
Measurement of detergent resistance.
Cultures were grown overnight in LB medium and inoculated into nephaloflasks containing LB medium and detergent. The detergents used were SDS and cetyltrimethylammonium bromide (CTAB). Each detergent was initially diluted in water, filter sterilized, and then added to the medium to the appropriate final concentration. Growth was measured every hour for 8 h with a Klett-Summerson colorimeter.
Growth conditions for lipid extraction.
S. haemolyticus was grown in LB broth (200 ml in l-liter flasks) at 37°C either with or without toluene. For the cells grown with solvent 50 μl of toluene was added to the LB broth every 3 h to maintain continuous exposure to the solvent. All treatments were replicated four times. Cells were harvested for lipid analysis when they reached an optical density at 600 nm of 0.60.
Lipid extraction.
Lipids were extracted using a modification of the Bligh-Dyer method (3). Two hundred milliliters of cells was grown as described above. The cells were centrifuged, and the lipids were extracted twice using 2 ml of methanol:chloroform (1:1). Extracts were combined, phase separated, and centrifuged, and the methanol layer was discarded. The chloroform layer was filtered (Whatman no. 40, 5.5 cm) and evaporated under nitrogen. Lipids were redissolved in 0.500 ml chloroform stabilized with amylenes for both phospholipid analysis and fatty acid methyl ester derivatization.
Phospholipid analysis.
The phospholipid compositions were analyzed and compared by using the methods of Nahaie et al. (16). Briefly, lipid extracts from cells grown with and without toluene were spotted onto a Silica Gel 60 F254 aluminum-backed thin-layer chromatography (TLC) plate (6.6 by 6.6 cm; Alltech, Deerfield, IL) that was developed in chloroform:methanol:water (70:30:4). Phospholipids were detected by dipping the plate into 10% phosphomolybdic acid in ethanol (Sigma). Glycolipids were detected by spraying the plate with α-naphthol-sulfuric acid reagent and charring it for 15 min at 110°C (9). Phospholipid and glycolipid classes were determined by using standards and by comparison to previously reported TLC results for other known Staphylococcus species (16). Diphosphatidylglycerol, phosphatidylglycerol (PG), and lysylphosphatidylglycerol (LPG) standards were used (Sigma, St. Louis, Mo.).
Fatty acid derivatization and analysis.
A 100-μl aliquot of chloroform-suspended lipids (prepared as described above) was evaporated under nitrogen and resuspended in 1 ml toluene:methanol (1:1). Lipids were hydrolyzed using freshly prepared 0.2 M potassium hydroxide in methanol for 30 min at 37°C and neutralized with acetic acid, and the resulting fatty acid methyl esters were partitioned into hexane:chloroform (4:1). After centrifugation, the hexane:chloroform layer was transferred to a new tube, and the hydrolystate was reextracted twice more with hexane:chloroform. The combined extracts were evaporated to dryness under nitrogen and resuspended in 50 μl hexane with 0.05 mg/ml of C19:0 as an internal standard. Fatty acid methyl esters were separated by capillary gas chromatography with a Hewlett-Packard 5890 Series II gas chromatograph using helium as the carrier gas. This instrument contained an Ultra 2 HP column (length, 50 m; inside diameter, 0.2 mm; film thickness, 0.33 μm) and was run in split mode (44:1) with a 0.75-min purge time. The injector and flame ionization detectors were maintained at 280 and 300°C, respectively, and the oven temperature was ramped from 50 to 160°C at a rate of 40°C min−1, held for 2 min, and then ramped at a rate of 3°C min−1 to 300°C and held for 30 min. Individual retention times were compared with the retention times of known fatty acid methyl ester standards. Fatty acid methyl esters were quantified by normalizing the peak area to the peak areas of the internal standards.
RESULTS
Solvent tolerance.
Strains of S. haemolyticus and E. faecalis were isolated with solvent selection, and, as expected, they exhibited exceptional solvent tolerance (Table 1). In plate overlay assays (20 μl, ca. 106 cells), S. haemolyticus tolerated 100% cyclohexane, p-xylene, toluene, or benzene for 8 h (Table 1). After removal of the solvent and subsequent incubation at 37°C, confluent cell growth was seen after 24 h. Subsequently, eight strains of S. aureus and five strains of S. epidermidis were also examined to determine their solvent tolerance (Table 1). None of these strains had been previously exposed to solvent via solvent selection. All 13 of these Staphylococcus strains tolerated cyclohexane (log Pow, 3.3) overlays, and all of the strains except S. aureus strains RN6390 and RF122 tolerated p-xylene (log Pow,3.1). Toluene ( log Pow, 2.6) had effects similar to those of p-xylene, while benzene (log Pow, 2.0) was the most detrimental of the solvents tested (Table 1).
Growth under solvent overlays.
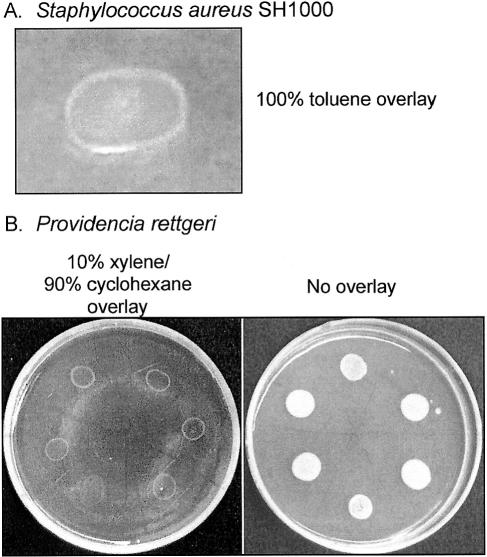
Of the solvent-tolerant bacteria described in Table 1, only S. aureus SH1000 showed visible growth under all of the solvents tested. The other strains merely tolerated the solvents. Visual evidence for growth under solvent was provided (Fig. 1A) by the expanding-ring appearance of S. aureus SH1000 under a 100% toluene overlay. The same expanding-ring appearance was noted in a study (11) of the gram-negative organism P. rettgeri growing under 10% xylene/90% cyclohexane (Fig. 1B). One explanation for the ring-like growth seen under a solvent is that solvent tolerance is an energy-dependent phenomenon and the ring consists of metabolically active cells generating sufficient energy to maintain solvent tolerance. This model is adapted from the well-known energy dependence of SDS resistance in enteric bacteria (22).
FIG. 1.
Ring-like growth of bacteria under organic solvent overlays. (A) S. aureus SH1000 under 100% toluene. (B) P. rettgeri under 10% xylene/90% cyclohexane. Both cultures (15 μl) were spotted onto LB agar plus 2% glucose, allowed to dry for 10 to 15 min, overlaid with solvent to a depth of 2 mm, and incubated at 30°C for 6 to 8 h prior to photography.
Monophasic liquid culture.
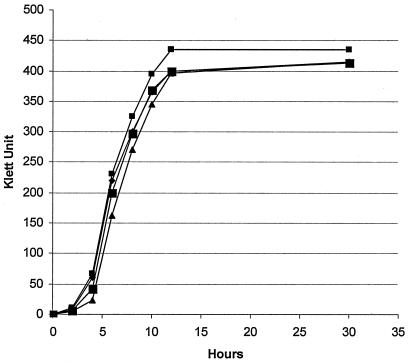
S. haemolyticus also exhibited exceptional solvent tolerance during liquid monophasic growth. In LB medium containing saturating levels of solvent (21 mM benzene, 6.2 mM toluene, and 0.95 mM cyclohexane), S. haemolyticus exhibited no significant differences in growth rate or cell yield compared to cells grown without solvent (Fig. 2). Also, cell lysis was not observed during the stationary phase (Fig. 2). S. haemolyticus was chosen for further investigation of adaptations in the lipid portion of its cytoplasmic membrane during growth in solvent-saturated liquid media.
FIG. 2.
S. haemolyticus growth curves in the presence of monophasic, saturated solvent. Samples were grown in liquid LB medium at 37°C. Symbols: ▪, cyclohexane (0.95 mM); ▪, toluene (6.2 mM); ▴, benzene (21 mM); ⧫, no solvent.
Phospholipid analysis.
The phospholipids that occur most commonly in Staphylococcus species are diphosphatidylglycerol (cardiolipin), phosphatidylglycerol, lysylphosphatidylglycerol, and another unidentified phospholipid (16). Characterization of the phospholipids in S. haemolyticus showed that the major classes of lipids were phosphatidylglycerol and another unknown glycolipid. These findings are consistent with phospholipids found in S. haemolyticus cells according to Nahaie et.al (16). Following exposure to toluene, small shifts in spots were noted. However, no changes were evident in the abundance or distribution of any phospholipid compared to non-toluene-exposed cells, as determined by the visual size and shape of the TLC spot. In particular, no spots due to lysylphosphatidylglycerol were observed for S. haemolyticus cells grown with and without toluene. All 10 strains of S. aureus and S. epidermidis tested (Table 1) had phospholipid compositions consistent with those described by Nahaie et al. (16) in that they contained both phosphatidylglycerol and diphosphatidylglycerol (data not shown).
Fatty acid analysis.
The same crude lipid extracts used for phospholipid classification of S. haemolyticus were converted to fatty acid methyl ester derivatives and analyzed by gas chromatography. Fatty acid profiles for both solvent-exposed cells and non-solvent-exposed cells are shown in Table 2. The cells not exposed to toluene contained roughly 46% straight-chain fatty acids, 26% anteiso-branched fatty acids, and 28% iso-branched fatty acids, whereas the cells grown in the continued presence of toluene contained 37% straight-chain fatty acids, 34% anteiso-branched fatty acids, and 29% iso-branched fatty acids. Overall, in the toluene-grown cells there was a 9% decrease in straight-chain fatty acids that was balanced by an 8% increase in anteiso-branched fatty acids and a 1% increase in iso-branched fatty acids. The largest differences were in the 20-carbon straight-chain, 15-carbon anteiso, and 17-carbon anteiso fatty acids (Table 2). The largest percentage change was a decrease in the 20:0 straight-chain fatty acids, from 19.4% in cells not exposed to toluene to 10.1% in toluene-grown cells. There was a marked increase in the percentage of the 15:0 anteiso fatty acid, which comprised 20.6% of the total fatty acids in the non-solvent-exposed cells and 25.9% of the total fatty acids in the toluene-grown cells (Table 2).
TABLE 2.
Fatty acid composition of S. haemolyticus grown with and without toluene
| Fatty acid | %a
|
P value | |
|---|---|---|---|
| No solvent | Toluene | ||
| C14:0 iso | 2.41 ± 0.93 | 1.62 ± 0.12 | 0.143 |
| C14:0 | 0.51 ± 0.12 | 0.43 ± 0.02 | 0.220 |
| C15:0 iso | 13.56 ± 1.94 | 13.15 ± 0.81 | 0.708 |
| C15:0 anteiso | 20.59 ± 3.61 | 25.65 ± 2.00 | 0.049 |
| C16:0 iso | 1.95 ± 0.08 | 1.74 ± 0.17 | 0.058 |
| C16:0 | 3.53 ± 0.34 | 4.72 ± 1.07 | 0.077 |
| C17:0 iso | 5.84 ± 0.158 | 6.8 ± 0.11 | 0.000 |
| C17:0 anteiso | 4.36 ± 0.341 | 6.94 ± 0.827 | 0.001 |
| C18:0 iso | 1.73 ± 0.189 | 2.06 ± 0.331 | 0.072 |
| C18:0 | 22.4 ± 2.83 | 22.79 ± 1.85 | 0.824 |
| C19:0 iso | 2.56 ± 0.154 | 2.12 ± 0.116 | 0.004 |
| C19:0 anteiso | 0.86 ± 0.10 | 1.12 ± 0.25 | 0.098 |
| Unknown | 0.35 ± 0.114 | 0.62 ± 0.07 | 0.011 |
| C20:0 | 19.35 ± 3.92 | 10.14 ± 0.582 | 0.004 |
| Total | 100 | 99.9 | |
| Sum of anteiso | 25.81 | 33.71 | |
S. haemolyticus cells were grown in liquid LB medium at 37°C with or without toluene. The values are the means ± standard deviations of four independent measurements. P values were obtained by the Student t test. They indicate whether the fatty acid differences observed between cells grown with and without toluene were statistically significant (P < 0.05).
Detergent resistance.
S. haemolyticus, E. faecalis, and S. aureus strains 213 and 25923 were tested for resistance to both anionic (SDS) and cationic (CTAB) detergents. None of the strains grew in the presence of ≥0.1% SDS or ≥0.04% CTAB. Thus, in gram-positive bacteria we observed no correspondence between organic solvent tolerance and SDS resistance.
DISCUSSION
We exploited the unique ecological niche of oil fly larval guts to identify highly solvent-tolerant gram-positive bacteria. Clearly, an outer membrane is not needed for bacteria to tolerate organic solvents. The gram-positive bacteria studied were E. faecalis, S. haemolyticus, eight strains of S. aureus, and five strains of S. epidermidis. The first two species were isolated by positive solvent selection, whereas none of the S. aureus and S. epidermidis strains had ever previously been exposed to solvents. In 1991, S. aureus strain IFO was reported to be growth limited by solvents, with log Pow values less than 4.8, as measured by plate overlays (7). We found that all 14 of the Staphylococcus strains examined tolerated harsher solvents (Table 1) and that S. haemolyticus was able to grow in the presence of cyclohexane, benzene, and toluene, both in monophasic liquid cultures and in organic solvent plate overlays. Thus, S. haemolyticus can tolerate organic solvents with log Pow values as low as 2.0 (benzene). Given the ubiquitous nature of the bacteria identified, it seems likely that solvent tolerance is merely a previously unrecognized resistance characteristic and the designation extremophile is not warranted. However, the prevalence of extreme solvent tolerance among the staphylococci is relevant to the efficacy of many chemical antiseptics in clinical use.
Several mechanisms of organic solvent tolerance have been found in gram-negative bacteria (23). Among these mechanisms is alteration of the membrane fatty acid composition and membrane fluidity. These mechanisms have been studied best in E. coli and Pseudomonas putida. They include a short-term response to solvents in which the unsaturated fatty acids are changed from cis to trans utilizing a cis-to-trans isomerase (5, 10). As longer-term responses to solvent exposure, in some gram-negative cells the saturated fatty acid content of the phospholipids increases, while in other cells the ratio of long-chain fatty acids to short-chain fatty acids increases (21). All of these responses, both short and long term, result in a less fluid cellular membrane. Regarding phospholipids, the gram-negative organisms P. putida and E. coli have been shown to respond to the presence of toluene by decreasing the membrane content of phosphatidylethanolamine and increasing the content of phosphatidylglycerol and diphosphatidylglycerol, also known as cardiolipin (4, 20). After toluene addition, 90% of the de novo phospholipid synthesis is diphosphatidylglycerol synthesis (23). The elevated diphosphatidylglycerol level decreases membrane fluidity, again stabilizing the cellular membrane in the presence of solvent (23). However, these fatty acid and phospholipid shifts have been studied mostly in gram-negative bacteria; few gram-positive bacteria have been studied for mechanisms of solvent tolerance. In trying to identify the mechanisms of solvent tolerance in S. haemolyticus, we expected to find mechanisms similar to those of the gram-negative bacteria operating via decreased membrane fluidity. We did not.
For phospholipids, we found only a small shift in the phospholipid spots for S. haemolyticus grown with and without toluene. This suggests that phospholipid alteration is not a mechanism of solvent tolerance for S. haemolyticus. We believe that the small shifts observed, as discussed by Touchstone et al (27), are due to changes in the attached fatty acid composition of the phospholipids. Also, the lack of involvement of lysylphosphatidylglycerol is not surprising. The formation of LPG converts a negatively charged phospholipid into a positively charged phospholipid. This conversion is essential for the resistance of S. aureus to positively charged, antibacterial peptides, such as the human defensins (19). Presumably, the PG-to-LPG conversion protects S. aureus from antibacterial peptides via electrostatic repulsion (19). Thus, a PG-to-LPG conversion should not be needed for solvent tolerance.
While S. haemolyticus does alter its membrane fatty acid composition, it does so in a manner opposite that observed in gram-negative bacteria; that is, it increases its membrane fluidity by increasing its level of anteiso fatty acids while decreasing its level of straight-chain fatty acids. A higher percentage of anteiso fatty acids results in a more fluid membrane because the melting points of the anteiso fatty acids are 25 to 35°C lower that those of the normal fatty acids (14). Therefore, S. haemolyticus seems to have developed a different response to solvents than its gram-negative counterparts. This “opposite effect” on membrane fluidity may be confined to S. haemolyticus, or it may be a more universal effect among the gram-positive bacteria. A conclusive statement cannot be made at this time due to a lack of data for other gram-positive solvent-tolerant organisms. Also, we have investigated the membrane lipid composition of only toluene-grown S. haemolyticus. Gram-positive bacteria likely employ some of the other solvent tolerance mechanisms characteristic of the gram-negative bacteria, such as membrane efflux pumps (23, 24).
One target for future investigation should be the role of σB in the solvent stress tolerance of S. aureus (6). The toluene-sensitive organism strain RN6390 differs from the toluene-resistant organism strain SH1000 only in that it contains an 11-bp deletion in rsbU, a positive regulator of σB function in S. aureus, which causes a strong defect in σB activity (6, 26). Similarly, the toluene-sensitive organism S. aureus RF122 is also suspected of being a sigB mutant (Greg A. Somerville, personal communication).
Acknowledgments
We thank John J. McGraw for determining the 16S RNA sequence of S. haemolyticus.
This work was supported by the Undergraduate Creative Activity and Research Experience (UCARE) Program, University of Nebraska, and by a grant from the Nebraska Corn Board.
REFERENCES
- 1.Aono, R., and A. Inoue. 1998. Organic solvent tolerance in microorganisms, p. 287-310. In K. Horikoshi and W. D. Grant (ed.), Extremophiles: microbial life in extreme environments. Wiley-Liss, New York, N.Y.
- 2.Atlas, R. M. 1993. Handbook of microbiological media. CRC Press, Boca Raton, Fla.
- 3.Bligh, E. G., and W. T. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 39:911-917. [DOI] [PubMed] [Google Scholar]
- 4.Clark, D. P., and J. P. Beard. 1979. Altered phospholipid composition in mutants of Escherichia coli sensitive or resistant to organic solvents. J. Gen. Microbiol. 113:267-274. [DOI] [PubMed] [Google Scholar]
- 5.Heipieper, H. J., R. Diefenbach, and H. Keweloh. 1992. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl. Environ. Microbiol. 58:1847-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue, A., and K. Horikoshi. 1991. Estimation of Solvent-tolerance of bacteria by the solvent parameter log P. J. Ferment. Bioeng. 71:194-196. [Google Scholar]
- 8.Isken, S., and J. A. M. de Bont. 1998. Bacteria tolerant to organic solvents. Extremophiles 2:229-238. [DOI] [PubMed] [Google Scholar]
- 9.Jacin, H., and A. R. Mishkin. 1965. Separation of carbohydrates on borate-impregnated silica gel G plates. J. Chromatogr. 18:170-173. [DOI] [PubMed] [Google Scholar]
- 10.Junker, F., and J. L. Ramos. 1999. Involvement of the cis-trans isomerase CtiT1 in solvent resistance in Pseudomonas putida DOTT1. J. Bacteriol. 181:5693-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadavy, D. R. 2001. Characterization of microorganisms associated with Helaeomyia petrolei (oil fly) larvae. Ph.D. thesis. University of Nebraska, Lincoln.
- 12.Kadavy, D. R., J. M. Hornby, T. Haverkost, and K. W. Nickerson. 2000. Natural antibiotic resistance of bacteria isolated from larvae of the oil fly, Helaeomyia petrolei. Appl. Environ. Microbiol. 66:4615-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadavy, D. R., B. Plantz, C. A. Shaw, J. Myatt, T. A. Kokjohn, and K. W. Nickerson,. 1999. Microbiology of the oil fly, Helaeomyia petrolei. Appl. Environ. Microbiol. 65:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneda, T. 1977. Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol. Rev. 41:391-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer, V. C., K. W. Nickerson, N. V. Hamlett, and C. O'Hara. 1984. Prevalence of extreme detergent resistance among the Enterobacteriaceae. Can. J. Microbiol. 30:711-713. [DOI] [PubMed] [Google Scholar]
- 16.Nahaie, M. R., M Goodfellow, D. E. Minnikin, and V. Hajek. 1984. Polar lipid and isoprenoid quinone composition in the classification of Staphylococcus. J. Gen. Microbiol. 130:2427-2437. [DOI] [PubMed] [Google Scholar]
- 17.Nickerson, K. W., and A. Aspedon. 1992. Detergent-shock response in enteric bacteria. Mol. Microbiol. 6:957-961. [DOI] [PubMed] [Google Scholar]
- 18.Paje, M. L. F., B. A. Nielan, and I. Couperwhite. 1997. A Rhodococcus species that thrives on medium saturated with liquid benzene. Microbiology 143:2975-2981. [DOI] [PubMed] [Google Scholar]
- 19.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. P. M. van Kessel, and J. A. G. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinkart, H. C., and D. C. White. 1997. Phospholipid biosynthesis and solvent tolerance in Pseudomonas putida strains. J. Bacteriol. 179:4219-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinkart. H. C., J. W. Wolfram, R. Rogers, and D. C. White. 1996. Cell envelope changes in solvent-tolerant and solvent-sensitive Pseudomonas putida strains following exposure to o-xylene. Appl. Environ. Microbiol. 62:1129-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajagopal, S., N. Sudarsan, and K. W. Nickerson. 2002. Sodium dodecyl sulfate hypersensitivity of clpP and clpB mutants of Escherichia coli. Appl. Environ. Microbiol. 68:4117-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-Gonzalez, A. Rojas, W. Teran, and A. Segura. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56:743-768. [DOI] [PubMed] [Google Scholar]
- 24.Sardessai, Y., and S. Bhosle. 2002. Tolerance of bacteria to organic solvents. Res. Microbiol. 153:263-268. [DOI] [PubMed] [Google Scholar]
- 25.Sikkema, J., J. A. M. deBont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somerville, G. A., S.-S. Battouli, J. M. Wickman, S. J. Raffel, B. N. Kreiswirth, and J. M. Musser. 2003. Correlation of acetate catabolism and growth yield in Staphylococcus aureus: implications for host-pathogen interactions. Infect. Immun. 71:4724-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Touchstone, J. C., S. S. Levin, M. F. Dobbins, and P. J. Carter. 1981. Differentiation of saturated and unsaturated phospholipid on thin layer chromograms. J. High Resolut. Chromatogr. Chromatogr. Commun. 4:423-424. [Google Scholar]