Abstract
Animal waste odors arising from products of anaerobic microbial metabolism create community relations problems for livestock producers. We investigated a novel approach to swine waste odor reduction: the addition of FeCl3, a commonly used coagulant in municipal wastewater treatment, to stimulate degradation of odorous compounds by dissimilatory iron-reducing bacteria (DIRB). Two hypotheses were tested: (i) FeCl3 is an effective source of redox-active ferric iron (Fe3+) for dissimilatory reduction by bacteria indigenous to swine manure, and (ii) dissimilatory iron reduction results in significant degradation of odorous compounds within 7 days. Our results demonstrated that Fe3+ from FeCl3 was reduced biologically as well as chemically in laboratory microcosms prepared with prefiltered swine manure slurry and limestone gravel, which provided pH buffering and a substrate for microbial biofilm development. Addition of a 1-g liter−1 equivalent concentration of Fe3+ from FeCl3, but not from presynthesized ferrihydrite, caused initial, rapid solids flocculation, chemical Fe3+ reduction, and Eh increase, followed by a 2-day lag period. Between 2 and 6 days of incubation, increases in Fe2+ concentrations were accompanied by significant reductions in concentrations of volatile fatty acids used as odor indicators. Increases in Fe2+ concentrations between 2 and 6 days did not occur in FeCl3-treated microcosms that were sterilized by gamma irradiation or amended with NaN3, a respiratory inhibitor. DNA sequences obtained from rRNA gene amplicons of bacterial communities in FeCl3-treated microcosms were closely related to Desulfitobacterium spp., which are known representatives of DIRB. Use of iron respiration to abate wastewater odors warrants further investigation.
Accumulated wastes from confined animal feeding operations give rise to anaerobic degradation products that create nuisance odors and community relations problems for livestock producers (10). A prime example is swine manure stored in lagoons or pits, resulting in production of at least 160 identified compounds associated with objectionable odors (3, 19, 21, 31). Although forced aeration assists microbial mineralization of organic wastes to nonodorous carbon dioxide and water (6, 30), intensive aeration is expensive and consumes large amounts of energy, and its effects in stored wastes are usually short-lived due to high chemical oxygen demand. Ozonation is another oxidizing technology with more sustained effects (28), but it has not been adopted extensively for animal waste treatment.
In the absence of O2, diverse microorganisms can obtain energy through dissimilatory respiration by transferring electrons from organic compounds to redox-active elements such as nitrogen, sulfur, or iron in their oxidized states (i.e., as NO3−, SO42−, or Fe3+). Animal wastes contain a vast surfeit of reduced organic compounds, and most forms of nitrogen and sulfur in animal wastes are also reduced, precluding their use as electron acceptors. In this study we investigated the addition to swine wastewater of ferric chloride (FeCl3), a solids coagulant commonly used in municipal wastewater treatment (5). Our objective was to determine whether a one-time addition of FeCl3 could stimulate dissimilatory ferric iron (Fe3+) respiration that could be coupled to the degradation of odorous organic compounds.
The first hypothesis in this study was that FeCl3 serves as a source of redox-active Fe3+ for dissimilatory iron-reducing bacteria (DIRB) indigenous to swine wastewater. Because Fe3+ from dissolved FeCl3 is known to undergo hydrolysis, which results in acid production (24), we proposed to use limestone gravel in laboratory microcosms containing swine waste slurry and FeCl3 to buffer acidity from the following reactions: Fe3+ + H2O → Fe(OH)2+ + H+; Fe(OH)2+ + H2O → Fe(OH)2+ + H+; Fe(OH)2+ + H2O → Fe(OH)3 (solid) + H+. Ferric hydroxides are also known to undergo reductive dissolution through interactions with sulfhydryl groups, organic acids, and reducing ligands (23), all of which are present in excess in swine manure slurries (21, 10). We therefore proposed to incorporate two types of abiotic controls in microcosm experiments to distinguish between the effects of chemical and biological Fe3+ reduction.
Our second hypothesis was that bacterial Fe3+ respiration results in significant reductions in concentrations of odorous swine manure compounds, such as volatile fatty acids (VFAs) and phenols, which DIRB would use as electron donors and carbon sources (15, 16). We therefore measured slurry concentrations of selected VFAs and phenolic compounds that have been identified as major odor contributors in swine wastes (3, 10, 31). The applied goal of our study was to assess the odor reduction potential of the addition of FeCl3 to buffered swine wastewater within a relatively short period of 7 days. We used rRNA intergenic spacer analysis (RISA) to track temporal changes in bacterial populations on gravel biofilms and to obtain DNA sequences of RISA-PCR amplicons to identify putative DIRB.
MATERIALS AND METHODS
Swine manure.
Swine manure slurry was pumped from the underground holding pit of a 400-hog-capacity building at the Swine Research Facility at Penn State University (PSU). The slurry had an average residence time in the tank of approximately 15 days. For these experiments, the raw manure in the underground pit was agitated for 10 min and pumped into a bark filter consisting of a watertight metal container (2.4 by 4.8 m) filled with approximately 5 m3 of wood chips. This prefiltration treatment reduced the amount of total solids (TS) from 40 to 50 g liter−1 to 10 to 15 g liter−1, with volatile solids constituting about 55 to 61% of TS. Filtered manure slurry was then pumped into plastic holding tanks where it was stored at ambient temperatures that ranged from 7 to 38°C. Two batches of slurry were collected in October 2003 and one in March 2004 by pumping from the bottom of the holding tank following agitation for 5 min prior to removing samples. Slurry was stored at 4°C for up to 3 weeks prior to microcosm set-up. Total, suspended, volatile, and dissolved solids of slurry samples were determined by standard methods (7). Solids contents ranged from 11.6 to 12.5 g liter−1, with volatile solids comprising 55 to 60% of total solids.
Effects of iron minerals and limestone gravel in slurry microcosms.
The two slurry batches from October were used to carry out replicated microcosm experiments in sterile, 50-ml polypropylene conical tubes containing 25-ml amounts of slurry. One experiment was conducted to test the bioavailability of Fe3+ from ferric chloride (FeCl3.6H2O) and magnetite (Fe3O4), both of which were obtained from Sigma-Aldrich (Milwaukee, WI), and from ferrihydrite (amorphous ferric oxyhydroxide), which was freshly synthesized according to the method of Schwertmann and Taylor (20). Microcosms were prepared with equivalent total concentrations of 1,000 and 2,500 mg of Fe3+ liter−1 from the three minerals.
Another experiment assessed the effect of limestone gravel on slurry pH and Fe3+ reduction. Duplicate series of microcosms were prepared in 50-ml conical tubes containing 25 ml of slurry amended with FeCl3 to obtain concentrations of 10, 20, 40, and 50 mM Fe3+ (corresponding to 500, 1,000, 2,000, and 2,500 mg of Fe3+ liter−1, respectively). Limestone gravel (18 g) was added to one series of tubes (2:1 volume ratio of slurry:gravel), while the other series received no gravel. Diameters of the washed gravel pieces (size 1-B) ranged from 0.25 to 0.5 cm. Duplicate tubes with and without gravel but no FeCl3 served as negative controls. All microcosms were incubated at 25°C in the dark. Statistically significant differences between microcosm treatments for mean measurements of pH and HCl-soluble Fe2+ were determined for each sampling day at P values of ≤0.05) using PROC TTEST (SAS Institute Inc., Cary, NC).
Since nonsterile gravel was used in preliminary experiments, we conducted tests to rule out Fe2+ reduction by microorganisms introduced with the gravel. In slurry microcosms containing 1,000 mg of Fe3+ liter−1, we compared the effects of autoclaved and nonautoclaved gravel on measured Fe2+ concentrations and found no significant differences (P < 0.05) in mean Fe2+ concentrations after 0, 2, or 6 days. Nonsterile gravel was used in all subsequent experiments.
Measurements of pH, Fe2+ concentrations, and oxidation-reduction potentials.
Changes in pH were measured with a Ross pH electrode and Thermo Orion 230A pH meter. Fe2+ concentrations were measured as acid-soluble Fe with the Ferrozine iron reagent, or 50 mM HEPES and 2 mM Ferrozine (J.T. Baker, Phillipsburg, NJ), as described by Lovley and Phillips (14). Slurry samples (300 μl) were removed from the upper liquid fractions of microcosms without agitation and transferred to test tubes containing 2.5 ml of 0.5 M HCl. Acidified samples were incubated at room temperature for ∼12 h before 20-μl aliquots were added to 2.5 ml of Ferrozine reagent. After a 15-min incubation, the amounts of Fe2+-Ferrozine complex were determined from absorbance readings at 562 nm (Perkin Elmer Lambda 40). Fe2+ concentrations were calculated from absorbance values using an equation derived from a standard curve based on ferrous ammonium sulfate (Fisher Scientific). Changes in oxidation-reduction potential (Eh) were measured with a Pt/Ag/AgCl reference electrode and Thermo Orion 250A+ meter.
Abiotic control microcosms.
To confirm the biological nature of Fe3+ reduction from FeCl3, experiments were conducted with two types of abiotic controls. The first controls were sterilized by gamma irradiation with 4 megarads from a cobalt-60 source (Gamma Cell 220; Penn State University Breazeale Nuclear Reactor). Duplicate irradiated and nonirradiated microcosms, prepared from the same batch of slurry sampled in October, consisted of 2:1 mixtures of slurry:gravel in 50-ml conical tubes amended with 500 mg of Fe3+ liter−1 from FeCl3. The second controls employed the respiratory inhibitor sodium azide, NaN3 (J. T. Baker, Phillipsburg, NJ). An effective concentration of NaN3 was determined first by adding 0.05% to 2% NaN3 to slurry microcosms in 250-ml Wheaton bottles containing 80 g of limestone gravel and 100 ml of slurry (volume ratio of 2:1; mass ratio of approximately 1.3:1, or 105 g of slurry added to 80 g of gravel). After incubation for 6 days, slurries were checked for growth by transferring 0.1-ml aliquots to tubes containing 9 ml of brain heart infusion broth (Difco Laboratories, Detroit, MI). Separate tubes were incubated aerobically and anoxically at 25°C and checked for growth after 5 days. Growth in brain heart infusion broth was observed for slurries treated with 0.05% to 1% NaN3 but not for slurries treated with 2% NaN3 or gamma irradiation. The 2% NaN3 concentration was therefore used.
Odor indicator analysis using abiotic controls.
Concomitant measurements were made of odor indicators and Fe2+ in liquid fractions of microcosms prepared with slurries sampled in October and March. For each trial, duplicate microcosms were prepared for each of the following treatments: no Fe3+ (unamended), 1,000 mg liter−1 Fe3+ (added as 5.4 g liter−1 FeCl3 · 6H2O), 1,000 mg liter−1 Fe3+ with 2% NaN3, and 2.6 g liter−1 AlCl3 (EM Science, Darmstadt, Germany). The AlCl3-amended microcosms were included to serve as controls for the effect of flocculation alone. These microcosms contained equimolar amounts of Al3+, which flocculates solids but is not redox active.
Seven VFAs (propionic, butyric, isobutyric, valeric, isovaleric, caproic, and isocaproic acids), three phenolic compounds (p-cresol, p-ethylphenol, and phenol), and two indoles (skatole and indole) were measured as odor indicators (21). Manure slurry samples (10 ml) were removed for extraction of volatile organic compounds and acidified with 2.0 ml of 1.0 M HCl. Mixtures were overlaid with 2.5 ml of diethyl ether (Sigma-Aldrich, St. Louis, MO) and incubated at 4°C for 4 h. Concentrations of odor indicators were measured in aliquots of ether extracts injected into a gas chromatograph (Hewlett-Packard 5890 Series II with HP G1030A ChemStation Controller) after separation in a capillary column (RESTEK Rtx-1; length, 30 m; internal diameter, 0.32 mm; film width, 4.0 μm) and quantification with a flame ionization detector. Standard solutions of each compound (Sigma-Aldrich) were used to establish calibration curves. For measurements of individual VFAs and phenols, initial concentrations (day 0) were expressed as 100%, with measurements after 6 days expressed as percentages of initial concentrations.
Biofilm sampling and processing.
Biofilm coverage on gravel surfaces was compared for manure slurry microcosms with and without 1,000 mg of Fe3+ liter−1. Two duplicate microcosms for each treatment were prepared from one batch of manure slurry sampled in October. After incubation for 6 days, two pieces of gravel were randomly picked from each microcosm (n = 4) and placed in 10 mM phosphate buffer (pH 7.0) with BacLite stain (Molecular Probes, Eugene, OR) for direct examination with an Olympus Fluoview 300 confocal scanning laser microscope (CSLM) at a magnification of ×400. Digital images were captured along the z axis at 1-μm intervals and analyzed with the COMSTAT program to estimate total volume of viable cells in biofilms (11). Biofilm dimensions were calculated from stacked 8-bit CSLM images, and statistical significance (P < 0.05) was determined with a PROC TTEST.
For biofilm community DNA analyses, two sets of microcosm treatments were prepared with different batches of manure slurry sampled in October. One microcosm was selected from treatment duplicates in each set for community DNA analysis. Ten pieces of gravel were removed from each microcosm and transferred to a clean tube for gentle rinsing in 10 mM phosphate buffer (pH 7.2) to remove unattached cells and organic matter. The buffer was decanted, and 10 ml of fresh buffer was added for vigorous shaking on a vortex mixer at maximum speed for 2 min. After vortexing, 1.9 ml of the supernatant was transferred to a 2.0-ml Eppendorf tube and centrifuged at 10,000 rpm for 2 min. This procedure was repeated three times to obtain a cell pellet from a total volume of 5.7 ml of wash buffer. Pellets were frozen at −80 C and thawed in a water bath (80°C) to help disrupt the bacterial cells prior to DNA extraction. All processing steps were carried out on the bench top. No attempts were made to maintain anaerobiosis of samples.
DNA extraction and RISA fingerprinting.
DNA was extracted from the biofilm pellets using a procedure modified from Berthelet et al. (2). Cell lysis was accomplished by two freeze-thaw cycles, followed by bead beating for 1 min at the highest setting (BioSpec, Bartlesville, OK). DNA yield and quality were assessed by measuring absorbance from 230 to 300 nm using a Perkin Elmer spectrophotometer (4). The 16S-23S rRNA intergenic spacer region with the flanking 150 bp from the 3′ terminus of the 16S rRNA gene was amplified with “universal” bacterial primers described by Lane (13): 16S-1406f (5′-TGTACACACCGCCCGT-3′) and 23S-115r (5′-GGGTBCCCCATTCGG-3′). PCRs were prepared in 25-μl reaction volumes as follows: 2.5 μl of 10× Hotmaster PCR buffer with 25 mM MgCl2; 0.25 mM concentrations of dATP, dGTP, dCTP, and dTTP; 6 pmol of each primer; 12.5 μg of bovine serum albumin; 2 U of Hotmaster Taq polymerase (Eppendorf AG, Hamburg, Germany); and 10 ng of template DNA. PCRs were performed in triplicate in a 2400 Perkin Elmer thermocycler using an initial denaturing step at 95°C for 5 min, followed by 25 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. After individual reaction mixtures were checked in ethidium bromide-stained 1.0% agarose gels, PCR products from three reactions were pooled for fingerprint analysis to reduce possible bias due to random events within individual PCRs (26).
RISA-PCR amplicons were separated in 6% Tris-borate-EDTA polyacrylamide gels at 200 V for 6 h and stained with 0.01% SYBR green. RISA gels were photographed with EpiChemi II equipment (UVP Inc., Upland, CA), and digital images were loaded into the GelCompar 2.5 program in BioNumerics (Applied Maths, Sint-Martens-Latem, Belgium) and normalized using a 200-bp molecular weight ladder (Promega Biosciences, Inc., San Luis Obispo, CA) as an external reference marker. The positions of amplicon bands were determined manually using side-by-side comparisons of the fingerprint image and the densitometric curve for each lane. All fingerprint comparisons were made using the same tolerance setting in BioNumerics (0.2% of the entire gel length). Community similarity values were obtained with the BioNumerics program using a similarity matrix calculated from pairwise comparisons of RISA fingerprints (presence/absence of amplicon bands) using the Dice coefficient, and dendrograms were generated using the unweighted-pair group method using average linkages algorithm.
DNA sequencing and GenBank accession numbers.
Select RISA-amplicon bands were excised from gels for DNA purification and cloning. Sequences from the 3′ ends of 16S rRNA genes (ca. 150 bp) were analyzed with BLAST (1) to determine the closest relatives of populations that gave rise to the amplicons in gel bands. To check DNA uniformity, sequences were obtained from at least two independent clones of DNA from each excised gel sample. All duplicate sequences were > 99.99% identical in the 16S rRNA and spacer regions. GenBank accession numbers were AY714587 through AY714591.
RESULTS
Effect of limestone gravel on pH of manure slurries.
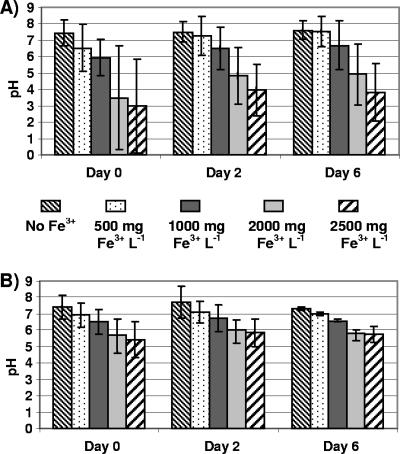
The addition of FeCl3 to manure slurries without gravel induced immediate flocculation and pH decreases. Initial mean pH values decreased as Fe3+ increased from 500 to 2,500 mg liter−1 (Fig. 1A). The presence of limestone gravel did not cause measurable changes in the pH of slurries that contained no FeCl3 (Fig. 1B), nor did it appear to affect the degree of solids flocculation when FeCl3 was added. The initial rapid declines in pH resulting from FeCl3 additions tended to be less in gravel-containing slurry microcosms (0.4 to 2.0 pH units) than they were in microcosms without gravel (0.4 to 4.5 pH units), although means (n = 4) were not significantly different at P < 0.05. After a 6-day incubation, mean pH values of slurries in gravel-based microcosms containing 500 and 2,500 mg of Fe3+ liter−1 were 7.4 and 5.8, respectively (Fig. 1B), whereas mean pH values without gravel were 7.6 and 3.8 (Fig. 1A). Thus, limestone gravel resulted in more neutral mean pH values and less pH variability overall.
FIG. 1.
Manure slurry pH after 0, 2, and 6 days in microcosms prepared without gravel (A) and with gravel (B). Microcosms were amended with FeCl3 to obtain initial concentrations of 0, 500, 1,000, 2,000, and 2,500 mg of Fe3+ liter−1. Column heights represent means of four measurements; error bars represent standard deviations from the means.
Effect of limestone gravel on Fe3+ reduction in manure slurries.
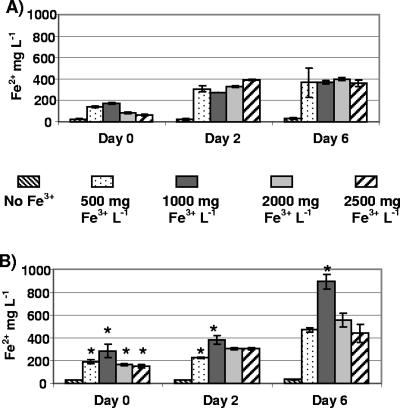
Within minutes of adding 500 and 1,000 mg of Fe3+ liter−1 to slurries without gravel, mean Fe2+ concentrations were 140 and 180 mg liter−1, or 28% and 18% of added Fe3+, respectively (Fig. 2A). Such rapid Fe3+ reduction was attributed to abiotic interactions with slurry solids, although higher amendments of 2,000 and 2,500 mg of Fe3+ liter−1 resulted in rapid reduction of only 4% and 2% of added Fe3+, respectively (Fig. 2A). After a 2-day incubation, mean Fe2+ concentrations in slurries amended with 1,000, 2,000, and 2,500 mg of Fe3+ liter−1 increased to 28%, 17%, and 16% of added Fe3+, respectively. After 6 days, these percentages were 37%, 20%, and 14%. The Fe2+ increases observed in slurries after 2 and 6 days could have resulted from biological Fe3+ reduction, and they were greater in slurries amended with 500 and 1,000 mg of Fe3+ liter−1 than in slurries with 2,000 and 2,500 mg of Fe3+ liter−1 (Fig. 2A).
FIG. 2.
HCl-soluble Fe2+ concentrations in manure slurries after 0, 2, and 6 days in microcosms prepared without gravel (A) and with gravel (B). Microcosms were amended with FeCl3 to obtain initial concentrations of 0, 500, 1,000, 2,000, and 2,500 mg of Fe3+ liter−1. Column heights represent means of four measurements; error bars represent standard deviations from the means. Stars above columns indicate significantly higher mean Fe2+ concentrations (P < 0.05) based on paired t tests with means from microcosms without gravel and amended with the same amount of FeCl3.
When slurry microcosms were prepared with limestone gravel, significantly more Fe3+ was rapidly reduced at all amendment levels than in slurries without gravel (Fig. 2B). Addition of 1,000, 2,000, and 2,500 mg of Fe3+ liter−1 with gravel resulted in the rapid reduction of 30%, 8%, and 6% of added Fe3+, respectively. The presence of gravel did not appear to cause measurable changes in Fe2+ concentrations of unamended slurries, which averaged 30 mg of Fe2+ liter−1 throughout the 6-day incubation (Fig. 2A and B). After 2 days, mean Fe2+ concentrations in manure slurries with gravel were significantly higher than in slurries without gravel, but only at amendment levels of 500 and 1,000 mg of Fe3+ liter−1 (Fig. 2B). After 6 days, mean Fe2+ concentrations in microcosms with gravel were highest (900 mg of Fe2+ liter−1) after adding 1,000 mg of Fe3+ liter−1. This was the only amendment level that showed significantly higher mean Fe2+ relative to microcosms amended with the same amount of Fe3+ but without gravel (Fig. 2B). The addition of 1,000 mg of Fe3+ liter−1 to gravel-based microcosms, therefore, was determined to provide the best conditions for the presumed biological Fe3+ reduction occurring in the microcosms during incubation.
Solids flocculation in manure slurry microcosms with limestone gravel.
All filtered swine manure slurries used in this study had TS contents of 1.2 to 1.4%. Prior to adding FeCl3 to gravel-based slurry microcosms, total dissolved solids and total suspended solids comprised 70% and 30%, respectively, of slurry solids on average. In the flocculation process which occurred after adding 1,000 mg of Fe3+ liter−1 (as FeCl3), most of the suspended solids became complexed in the floc so that total suspended solids comprised only 4% of total solids in liquid slurry following flocculation. During flocculation the gaseous foam produced from the chemical reactions between the solids and soluble Fe3+ caused the floc to rise and localize at the surface of the slurry. The flocculated material remained in the upper portion of the microcosms for 2 days, after which it settled below the surface of the liquid. Floc settling appeared to be associated with the greater increases in Fe3+ reduction observed after 2 days (Fig. 2B).
Slurry microcosm trials to confirm biological Fe3+ reduction.
Neither magnetite nor ferrihydrite caused solids flocculation, pH decreases, or Fe2+ increases in unbuffered manure slurries within 6 days of incubation (data not shown). Therefore, FeCl3 amendments were used in all gravel-based microcosms as the source of redox-active Fe3+. In the test to assess Fe3+ reduction in slurries presterilized by gamma irradiation, similar Fe2+ concentrations were observed in both nonirradiated (210 ± 8 mg liter−1) and gamma-irradiated (255 ± 36 mg liter−1) slurry microcosms immediately after the addition of 500 mg of Fe3+ liter−1. Thus, Fe2+ increases in both microcosm treatments were attributable to chemical rather than biological reactions. After 4 days of incubation, little change was observed in the Fe2+ concentrations of either type of slurry microcosm. After 9 days, however, mean Fe2+ concentrations were significantly higher in the nonirradiated slurry microcosms (360 ± 53 mg liter−1) than in the irradiated slurry microcosms (110 ± 5 mg liter−1). Presterilization of slurry thus appeared to prevent the rise in Fe2+ concentrations observed in nonirradiated slurry microcosms between 4 and 9 days.
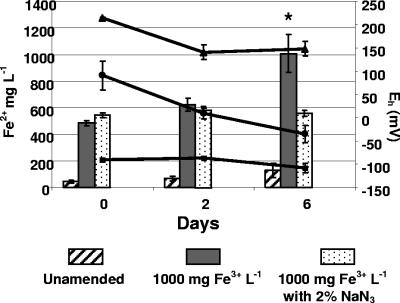
In the test for biological Fe3+ reduction involving a bacterial respiratory inhibitor, slurries treated with FeCl3 and FeCl3+NaN3 showed an immediate increase of Fe2+, which approximated 50% of the total soluble Fe3+ added (Fig. 3). These concentrations were not changed after 2 days of incubation. After 6 days, Fe2+ concentrations in the FeCl3-treated slurries were significantly higher than concentrations in FeCl3+NaN3-treated slurries, reflecting nearly complete reduction of the initial 1,000 mg liter−1 Fe2+ added. Unamended and AlCl3-treated slurry microcosms were also included in these tests and were found to contain low concentrations of Fe2+ (30 mg liter−1), which remained unchanged through 6 days of incubation (Fig. 3). Initial Eh values of slurry microcosms after the addition of either FeCl3, FeCl3+NaN3, or AlCl3 were positive (+70 to + 170 mV), whereas the Eh values of unamended microcosms ranged from −89 to −109 mV (Fig. 3). On further incubation, unamended slurries exhibited no change in Eh, while slurries treated with FeCl3+NaN3 showed a slight reduction in Eh after 2 days but no further reduction after 6 days. The Eh values of slurries treated with FeCl3, on the other hand, exhibited a continuous decline and were negative on day 6 (Fig. 3).
FIG. 3.
Effect of 2% NaN3 on HCl-soluble Fe2+ concentrations (left axis) and Eh (right axis) of gravel-containing manure slurry microcosms after 0, 2, and 6 days. Bar heights and error bars represent means and standard deviations of Fe2+ concentrations in microcosms with no FeCl3 added (Unamended), with FeCl3 to obtain 1,000 mg Fe3+ liter−1, and with FeCl3 to obtain 1,000 mg of Fe3+ liter−1 plus 2% NaN3. Lines show Eh for microcosms with no FeCl3 added ▪,with FeCl3 •, and FeCl3+2% NaN3 ▴. Means and standard deviations in both data sets are from three independent experiments. The star above the column for day 6, no inhibitor, indicates a significantly higher mean Fe2+ concentration (P < 0.05) based on a paired t test with the mean from microcosms with NaN3.
Gas chromatography measurements of odor indicators.
On day 0, summed concentrations of the seven measured VFAs averaged 14,000 ppm, with a standard deviation of 7,200 ppm. Propionic and n-butyric acids each constituted 39 to 44% of the total, valeric and isovaleric acids were each about 6%, isobutyric acid was 5%, caproic acid was <1%, and isocaproic acid was <0.1%. Summed concentrations of three phenolic odor indicators averaged 176 ppm with a standard deviation of 78 ppm. Indoles and skatoles were not detected at all in most samples.
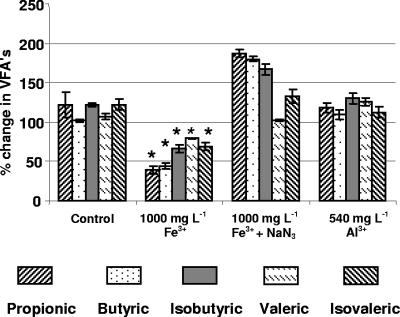
Since most biological Fe3+ reduction appeared to take place in FeCl3-amended microcosms between 2 and 6 days of incubation, experiments were conducted to determine whether changes in odor indicator concentrations also occurred during this period in amended microcosms but not in abiotic controls. Changes in the concentrations of five individual VFAs between 0 and 6 days are shown as percentages of initial (day 0) concentrations (Fig. 4). The remaining two VFA indicators, caproic and isocaproic acids, were not consistently detected, and these data are not shown. Significant reductions (P < 0.05) in all VFAs were observed between 0 and 6 days in the FeCl3-amended microcosms but not in the unamended, FeCl3+NaN3, or AlCl3 microcosms (Fig. 4). After 6 days, concentrations of three phenolic odor indicators were not significantly different from day 0 concentrations in any of the microcosms (data not shown). In general, concentrations of phenolic indicators in slurries varied to a greater degree than the VFA concentrations.
FIG. 4.
Percentages of initial VFA concentrations of swine manure slurries after 6 days of incubation in microcosms prepared with no amendments, 1,000 mg of Fe3+ liter−1, 1,000 mg of Fe3+ liter−1 plus NaN3, and 540 mg of Al3+ liter−1. Relative concentration changes are shown for propionic, butyric, isobutyric, valeric, and isovaleric acids. Stars above columns indicate significant differences between mean VFA concentrations after 0 and 6 days.
Differences in biofilms and bacterial RISA fingerprints.
CSLM images of biofilms on gravel pieces from 6-day microcosms were used to obtain means and standard deviations for total cell volumes and biofilm thicknesses. Biofilms from FeCl3-amended microcosms had significantly higher total cell volumes (11.1 ± 6.7 μ3 per μ2 gravel surface) than did biofilms from unamended microcosms (3.0 ± 0.8 μ3 per μ2 gravel surface). On the other hand, biofilm thicknesses based on detection of cells as a function of distance from the gravel surface were highly variable and not significantly different between FeCl3-amended (11.4 ± 11.2 μ) and unamended (7.6 ± 1.7 μ) microcosms. Thus, biofilms in FeCl3-amended microcosms appeared to have higher cell densities but greater spatial heterogeneity than biofilms in unamended microcosms.
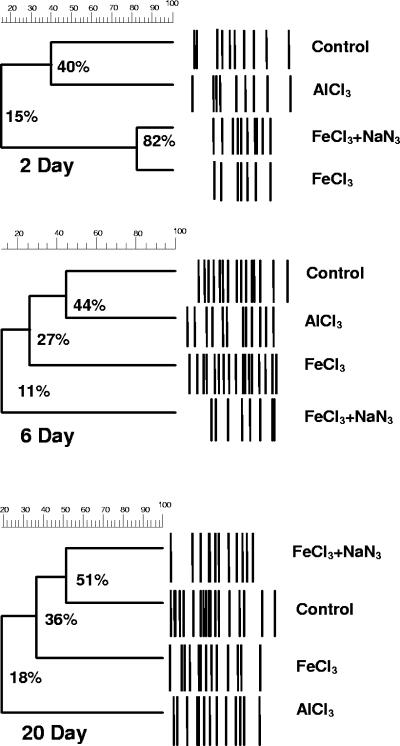
Similarities of RISA fingerprints after 2, 6, and 20 days of incubation were compared among control microcosms (no amendments) and microcosms with 1,000 mg liter−1 Fe3+, 1,000 mg liter−1 Fe3+ and NaN3, and AlCl3 (Fig. 5). Temporal changes in RISA fingerprints of biofilm communities were similar for microcosms receiving the same treatments but prepared with different slurry batches (data not shown). A strong effect of FeCl3 addition was observed after 2 days of incubation, when RISA fingerprints from both types of FeCl3-amended microcosms (with and without NaN3) were 82% similar to each other but only 15% similar to fingerprints from the control and AlCl3-amended microcosms (Fig. 5). After 6 days, RISA patterns from the two types of FeCl3-amended microcosms had diverged (11% similarity). A comparatively large biofilm community shift occurred in the FeCl3-amended microcosms between 2 and 6 days and was characterized by the appearance of 10 new amplicon bands. This community shift was not observed in the microcosms with NaN3 inhibitor (Fig. 5). After 20 days, RISA fingerprints from the FeCl3-amended microcosms became more similar (36%) to those from the NaN3-containing and control microcosms, while fingerprints from the AlCl3-amended microcosms were least similar (18%) to the others (Fig. 5). Among all comparisons of RISA fingerprints in these experiments, therefore, community shifts with the greatest magnitude occurred in FeCl3-only treatments between 2 and 6 days.
FIG. 5.
Dendrograms showing similarities among RISA bacterial fingerprints of biofilm communities after 2, 6, and 20 days of incubation of gravel-based microcosms: control (no amendments), FeCl3 (1,000 mg liter−1 Fe3+), FeCl3+NaN3 (1,000 mg liter−1 Fe3+ with 2% NaN3), and AlCl3 (2,500 mg liter−1 Al3+). Each dendrogram is based on amplicon band patterns from one RISA gel.
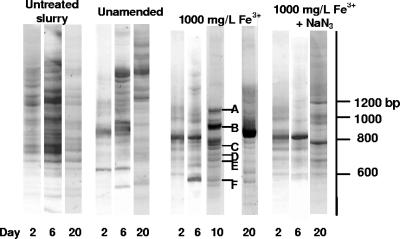
RISA amplicon bands unique to the FeCl3 treatment appeared after 6 and 10 days and yielded sequences closely related to known DIRB, Desulfitobacterium frappieri (97 to 99%) and Desulfitobacterium metallireducens (Fig. 6). Other populations detected after 10 days were most closely related to Arcobacter spp., Alcaligenes faecalis, Bacteroides spp., and Kurthia spp., some of which have been cultured previously from swine manure (3, 27). Most bands in 10-day RISA fingerprints from the FeCl3-amended microcosms were not observed after 20 days, suggesting population turnover after Fe3+ depletion. RISA fingerprints from unamended microcosms were similar at 2 and 6 days but diverged after 20 days. RISA fingerprints from communities in untreated manure slurries varied little throughout the 20-day incubation period (Fig. 6).
FIG. 6.
RISA fingerprints from biofilms extracted from untreated manure, unamended microcosms, and FeCl3 and FeCl3+NaN3 microcosms. Each lane corresponds to a community fingerprint at different incubation times (2, 6, and 20 days). Selected bands unique to FeCl3-grown communities at day 10 were sequenced, and flanking portions of the 16S rRNA genes (150 bp) were used in BLAST analyses to determine closest relatives. A, Arcobacter sp. AY314754 (95%); B, Alcaligenes faecalis AF155147 (99%); C, Bacteroides thetaiotamicron AY773156 (94%); D, D. metallireducens AF297871 (100%); E, D. frappieri U40078 (97 to 99%); F, Kurthia sibirica AY773151 (98%).
DISCUSSION
In these experiments it was critical to include controls that confirmed the biological nature of Fe3+ reduction. As much as 30 to 40% of added Fe3+ appeared to be reduced by strictly chemical processes, such as reductive dissolution reactions (e.g., with organic acids or catechols) (15) and solids coagulation involving interactions between ferric hydroxides and acidic, sulfhydryl, and other functional groups during formation of organic complexes (23). It also appeared that the presence of limestone gravel resulted in significantly higher amounts of Fe3+ being reduced immediately (Fig. 2A and B), indicating that rapid Fe3+ reduction in these high-organic-load slurries was sensitive to pH and possibly to specific interactions with limestone carbonates (24). The more gradual increases in Fe2+ concentrations upon further incubation, however, appeared to be due to biological Fe3+ reduction (Fig. 2B), because these increases did not occur in microcosms prepared with NaN3 or presterilized slurries. The most Fe3+ reduction occurred in microcosms containing 1,000 mg of Fe3+ liter−1 between 2 and 6 days, during which significant decreases in VFA concentrations were also measured (Fig. 3 and 4). Thus, multiple lines of evidence supported our hypotheses that DIRB activity in swine manure slurries was stimulated by FeCl3 addition and that biological activity was accompanied by reductions in concentrations of odor-causing compounds.
Because of the wide variety and variability of odor-causing compounds in animal wastes, the odor reduction results observed in our study may not be applicable to manure from other types of livestock or to different slurry storage and handling conditions. It should be emphasized that the manure slurries in this study had been prefiltered to reduce total solids content by 70 to 75%. While 1,000 mg of Fe3+ liter−1 appeared to be the most favorable concentration for stimulating DIRB activity in prefiltered slurries, higher amounts of Fe3+ are needed to produce similar results with manure having a higher solids content (unpublished results). It should also be emphasized that VFA and phenol concentrations increased between 6 and 20 days in FeCl3-amended, prefiltered slurry microcosms, despite informal observations by laboratory personnel that odors from these microcosms were inoffensive. Increased concentrations of odor indicators were likely due to depletion of added Fe3+ electron acceptor and continued activity by microbial populations other than DIRB. Thus, Fe3+ may need to be added in stages to sustain DIRB activity for odor control.
We used RISA to assess qualitative changes in biofilm communities following FeCl3 addition to slurry microcosms. Because RISA is based on standard rather than quantitative PCR, amplicon band data should be interpreted with caution due to the potential for bias from inefficient DNA extraction, preferential PCR amplification, and the possibility of multiple DNA bands from a single population (9). Nevertheless, comparative patterns of amplicon bands from community DNA provided a direct means to track temporal changes in bacterial populations, as has been demonstrated for diverse environmental samples by other laboratories (12, 25, 29). In our study, RISA amplicon bands unique to the FeCl3 treatment appeared after 6 and 10 days and yielded sequences closely related to known DIRB, D. frappieri (97 to 99%) and D. metallireducens (8). Use of organic acids by putative DIRB in swine manure slurry is a plausible explanation for the observed reductions in VFAs. DIRB such as D. frappieri have been reported to use lactate, formate, butyrate, and ethanol as carbon and electron sources when using metals and metalloids as terminal electron acceptors (18).
As expected, we found that the type of mineral added to swine waste slurries was an important determinant of subsequent abiotic and biotic Fe3+ reduction. The lack of Fe3+ reduction from presynthesized ferrihydrite within 20 days was unexpected because it is commonly used as a selective agent for DIRB isolation (17). The high-organic loading of manure slurries or unfavorable Eh may have contributed to the different responses observed with Fe3+ from FeCl3 and presynthesized ferrihydrite (22). In actuality, ferrihydrite was likely to be one, if not the major, constituent of the products formed during rapid solids flocculation upon FeCl3 addition (23). One important difference between adding presynthesized ferrihydrite to slurry and producing it in situ would be the potential in the latter case to entrain bacterial cells in the iron-containing floc, thus enhancing physical contact and probability of electron transfer between cells and Fe3+.
Although FeCl3 is widely used as a flocculating agent in municipal wastewater treatment, its potential role as a source of electron acceptors for anaerobic respiration has not been recognized as a means to promote odor abatement. FeCl3 is also used extensively to remove phosphorus during secondary municipal wastewater treatment. Thus, FeCl3 treatment of swine wastes would generate two fractions—a clarified liquid that could be applied to land as a nitrogen source and phosphorus-enriched flocculated solids that could be composted or recycled as inoculants. The enhancement of iron respiration by limestone gravel may also have applications for chemical oxygen demand removal by other trickling filter systems. Further work will be required to evaluate treatments on a larger scale and to address the challenges of waste heterogeneity.
Acknowledgments
Research was supported by the Pennsylvania Department of Agriculture, contract no. ME 443246, and the Penn State Institutes of the Environment. H.A.C.-G. acknowledges the financial support of the Consejo Nacional de Ciencia y Tecnologia (CONACYT) of Mexico and the Fulbright Fellowship Program for Graduate Studies.
The authors acknowledge the support of Eileen Wheeler and Robert Graves of the PSU Department of Agricultural and Biological Engineering, helpful discussions with Carmen Enid Martinez of the PSU Department of Crop and Soil Sciences, and excellent technical assistance by Erica Petre, Jon Channel, Patrick Topper, Jenn Zajakowski, Jean Voigt, and Masami Tonegawa.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Berthelet, M., L. G. White, and C. W. Greer. 1996. Rapid, direct extraction of DNA from soils for PCR analysis using polyvinylpolypyrrolidone spin columns. FEMS Microbiol. Lett. 138:17-22. [DOI] [PubMed] [Google Scholar]
- 3.Bourque, D., J. G. Bisaillon, R. Beaudet, M. Sylvestre, M. Ishaque, and A. Morin. 1987. Microbiological degradation of malodorous substances of swine waste under aerobic conditions. Appl. Environ. Microbiol. 53:137-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruns, M. A., and D. H. Buckley. 2002. Isolation and purification of microbial community nucleic acids from environmental samples, p. 564-572. In C. J. Hurst et al. (ed.), Manual of environmental microbiology, 2nd ed., ASM Press, Washington, DC.
- 5.Chen, W. P. 2002. Comparison of hydrolysis/coagulation behavior of polymeric and monomeric iron coagulants in humic acid solution. Chemosphere 47:963-969. [DOI] [PubMed] [Google Scholar]
- 6.Doyle, Y., and J. Noue. 1987. Aerobic treatment of swine manure: physico-chemical aspects. Biol. Wastes 19:187-208. [Google Scholar]
- 7.Eaton, A. D., L. S. Clesceri, and A. E. Greenberg (ed.). 1995. Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association and American Water Works Association, Washington, D.C.
- 8.Finneran, K. T., H. M. Forbush, C. V. G. Van Praagh, and D. R. Lovely. 2002. Desulfitobacterium metallireducens sp. nov., and anaerobic bacterium that couples growth to the reduction of metals and humic acids as well as chlorinated compounds. Int. J. Syst. Evol. Microbiol. 52:1929-1935. [DOI] [PubMed] [Google Scholar]
- 9.Gürtler, V., and V. A. Stanisich. 1996. New approaches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology 142:3-16. [DOI] [PubMed] [Google Scholar]
- 10.Hatfield, J. L., Y. S. Do, A. A. DiSpirito, D. A. Laird, and R. L. Pfeiffer. 1997. Characterization of volatile organic emissions and wastes from a swine production facility. J. Environ. Qual. 26:1687-1696. [Google Scholar]
- 11.Heydorn, A., A. Toftgaard Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersbøll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 12.Ibekwe, A. M., C. M. Grieve, and S. R. Lyon. 2003. Characterization of microbial communities and composition in constructed dairy wetland wastewater effluent. Appl. Environ. Microbiol. 69:5060-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane, D. J.1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, New York, N.Y.
- 14.Lovley, D. R., and E. J. P. Phillips. 1986. Organic matter mineralization of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovley, D. R., E. J. P. Phillips, and D. J. Lonergan. 1991. Enzymatic versus nonenzymatic mechanisms for Fe(III) reduction in aquatic sediments. Environ. Sci. Technol. 25:1062-1067. [Google Scholar]
- 16.Nealson, K. H., and C. R. Myers. 1992. Microbial reduction of manganese and iron: new approaches to carbon cycling. Appl. Environ. Microbiol. 58:439-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen, J. L., S. Juretschko, M. Wagner, P. H. Nielsen. 2002. Abundance and phylogenetic affiliation of iron reducers in activated sludge as assessed by fluorescence in situ hybridization and microautoradiography. Appl. Environ. Microbiol. 68:4629-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niggemyer, A., S. Spring, E. Stackebrandt, and R. F. Rosenzweig. 2001. Isolation and characterization of a novel As(V)-reducing bacterium: implications for arsenic mobilization and the genus Desulfitobacterium. Appl. Environ. Microbiol. 67:5568-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Neill, D. H., and V. R. Phillips. 1992. A review of the control of odour nuisance from livestock buildings. Part 3: properties of the odorous substances which have been identified in livestock wastes or in the air around them. J. Agric. Eng. Res. 53:23-50. [Google Scholar]
- 20.Schwertman, U., and R. M. Taylor. 1989. Iron oxides, p. 379-438. In J. B. Dixon and S. B. Weed (ed.), Minerals in soil environments, 2nd ed. Soil Science Society of America, Madison, Wis.
- 21.Spoelstra, S. F. 1983. Origin of objectionable odorous components in piggery wastes and the possibility of applying indicator components for studying odor development. Agric. Environ. 5:241-260. [Google Scholar]
- 22.Straub, K. L., and B. Schink. 2004. Ferrihydrite reduction by Geobacter species is stimulated by secondary bacteria. Arch. Microbiol. 182:175-181. [DOI] [PubMed] [Google Scholar]
- 23.Stumm, W. 1992. Chemistry of the solid-water interface: processes at the mineral-water and particle-water interface in natural systems. Wiley-Interscience, Inc., New York, N.Y.
- 24.Stumm, W., and J. J. Morgan. 1996. Aquatic chemistry. Wiley-Interscience, Inc., New York, N.Y.
- 25.von Canstein, L., Ying, J. Leonhauser, E. Haase, A. Felske, W-D. Deckwer, and I. Wagner-Dobler. 2002. Spatially oscillating activity and microbial succession of mercury-reducing biofilms in a technical-scale bioremediation system. Appl. Environ. Microbiol. 68:1938-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner, M., N. Blackstone, P. Cartwright, M. Dick, B. Misof, P. Snow, G. P. Wagner, J. Bartels, M. Murtha, and J. Pendleton. 1994. Surveys of gene families using polymerase chain reaction: PCR selection and PCR drift. Syst. Biol. 43:250-264. [Google Scholar]
- 27.Whitehead, T. R., and M. A. Cotta. 2001. Characterization and comparison of microbial populations in swine faeces and manure storage pits by 16S rDNA gene sequence analyses. Anaerobe 7:181-187. [Google Scholar]
- 28.Wu, J. J., S. Park, S. M. Hengemuehle, M. T. Yokoyama, H. L. Person, J. B. Gerrish, and S. J. Masten. 1999. The use of ozone to reduce the concentration of malodorous metabolites in swine manure slurry. J. Agric. Res. 72:317-327. [Google Scholar]
- 29.Yu, Z., and W. W. Mohn. 2001. Bacterial diversity and community structure in an aerated lagoon revealed by ribosomal intergenic spacer analyses and 16S ribosomal DNA sequencing. Appl. Environ. Microbiol. 67:1565-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, R. H., P. N. Dugba, and D. S. Bundy. 1997. Laboratory study of surface aeration of anaerobic lagoons for odor control of swine manure. Trans. ASAE 40:185-190. [Google Scholar]
- 31.Zhu, J., D. S. Bundy, X. Li, and N. Rashid. 1997. Controlling odor and volatile substances in liquid hog manure by amendment. J. Environ. Qual. 26:740-743. [Google Scholar]