Abstract
BACKGROUND
Mild behavioral impairment (MBI) refers to the neurobehavioral symptoms observed in older adults that may be potential risk factors for neurodegenerative diseases. While a significant number studies have explored the association between cerebrospinal fluid and MBI, only a few have examined the connection between plasma biomarkers and MBI.
AIM
To examine the prevalence of MBI in healthy older adults (HOAs) and individuals with mild cognitive impairment (MCI), as well as the association between MBI and plasma biomarkers of Alzheimer’s disease (AD).
METHODS
We enrolled a total of 241 subjects, which included 136 HOAs and 105 MCIs, from the Yuhua District of Shijiazhuang City, Hebei Province, China. The MBI symptom checklist (MBI-C) was utilized for the assessment and diagnosis of MBI, and a score of MBI-C ≥ 6.5 was considered indicative of the condition. Fasting venous blood samples were collected from 70 patients, 32 HOAs and 38 MCIs, and levels of amyloid β-protein (Aβ) 40, Aβ42, and hyperphosphorylated tau (p-Tau217) in these samples were measured using an enzyme-linked immunosorbent assay.
RESULTS
The prevalence of MBI in the HOAs and MCI groups was 4.4% and 15.3%, respectively (χ2 = 7.262, P = 0.007), with particularly notable decreases in motivation and increases in impulse dyscontrol (the highest detection rate) and social inappropriateness (P < 0.05). The total MBI score correlated with Aβ42 and p-Tau217 (r = -0.385, P = 0.019; r = -0.330, P = 0.041), but not with Aβ40 or the Aβ42/40 ratio. Among the subdomains, impulse dyscontrol was correlated with Aβ42 (r = -0.401, P = 0.025).
CONCLUSION
Both MCI and HOAs have exhibited a higher prevalence of MBI, with changes in impulse control behavior being the most common. MBI not only presents as an independent risk factor for cognitive decline but is also linked with AD-related peripheral biomarkers.
Keywords: Mild cognitive impairment, Mild behavioral impairment, Alzheimer’s disease, Neuropsychiatric symptom, Amyloid β-protein 42, P-tau
Core Tip: Both mild cognitive impairment and healthy older adults have shown a higher prevalence of mild behavioral impairment (MBI), with changes in impulse control behavior being the most common. MBI not only stands as an independent risk factor for cognitive decline but is also associated with Alzheimer’s disease-related peripheral biomarkers.
INTRODUCTION
Mild cognitive impairment (MCI)[1] and dementia are not solely characterized not just by cognitive dysfunction, but also by decreased motivation, anxiety, depression, and uninhibited behavior. These non-cognitive symptoms are referred to neuropsychiatric symptoms (NPS). These symptoms can span the entire course of cognitive disorders and in some cases, may even present as psychiatric and behavioral anomalies before the emergence of cognitive deficits become apparent[2]. In recent years, the concept of mild behavioral impairment (MBI), a concept first defined by Taragano and Allegri at the Eleventh Congress of the International Psychogeriatric Association, has increasingly drawn the attention of researchers. MBI encapsulates neurobehavioral symptoms observable in older adults for at least 6 months, without meeting diagnostic criteria for any other psychiatric syndromes. MBI can occasionally precede cognitive impairment and might indicate early signs neurodegeneration. Both longitudinal and cross-sectional studies consistently suggest an association between MBI and cognitive decline, as well as a faster progression toward dementia[3,4]. This suggests that MBI could potentially be a risk factor for neurodegenerative diseases[5-8].
The Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment working group proposed the MBI research diagnostic criteria in 2016[9]. The MBI-checklist (MBI-C)[10] was developed to assess the severity of behavioral symptoms across various domains. Currently, the comprehensive and subdomain validity of the MBI-C for clinical diagnosis and prognosis is being established. Xu et al[11] examined the reliability and validity of the Chinese version of MBI-C, concluding that it maintains consistent internal validity and is effectively used to measure patients’ psychological and behavioral changes. Although MBI symbolizes non-cognitive symptoms and is seen as an early sign of Alzheimer’s disease (AD), its association with AD’s preclinical pathophysiology remains unclear. Preliminary investigations into the etiology and pathology of MBI have revealed links between MBI and biological markers of AD, including amyloid β-protein (Aβ)-positron emission tomography (PET)[12], tau-PET[13], cerebrospinal fluid (CSF)-tau, plasma neurofilament light[14], and AD risk gene loci[15,16]. From an imaging perspective, MBI patients display brain changes similar to those with AD. In a cross-sectional study by Shu et al[17] documented atrophy in the left frontal cortex and right thalamus of MBI patients associated with NPS in dementia, suggesting MBI may be an early warning sign of cognitive decline and dementia.
Furthermore, these plasma biomarkers display a link with the potential development of AD. However, most of these studies are based on Western populations. Given that race and ethnicity could influence the evaluation of these markers, it’s critical to validate these findings in the context of the Chinese population. While numerous studies have explored the relationship between CSF and MBI, only a few have examined the connection between plasma biomarkers and MBI. Our study in the Chinese population reinforces previous European and American findings that plasma biomarkers are indeed altered during the MBI phase, based on evidence from the Chinese population.
The primary aim of this study is to examine the prevalence of MBI in healthy older adults (HOAs) and MCI populations. Furthermore, the investigation will focus on the correlation between MBI and AD biomarkers in MCI populations, specifically Aβ40, Aβ42, and hyperphosphorylated tau (p-tau217) levels.
MATERIALS AND METHODS
Study population
Sample size calculation: Prior literature[6,18] has reported an incidence of MBI in HOAs and MCI at 6%-9% and 14%, respectively, with a standard deviation of 6.41. Using the PASS15.0.5 software for tests examining the difference between two Poisson rates, the total sample size is estimated to be 212 cases. This calculation assumes a degree of assurance 1-β = 0.90 and the test level α = 0.05 were set. The sample sizes for both the HOA group and the MCI group were equivalent, with each group containing 106 cases.
This research was designed as a cross-sectional study, conducted from April to October 2021. The participant pool consisted of older adults from the Yuhua District of Shijiazhuang City, in Hebei Province, China. The inclusion criteria were as follows: Individuals aged 55 years and above, who were willing to participate and complete both the questionnaire survey and cognitive function assessment, and consent to provide blood samples.
The study population comprised 241 participants, ranging in age from 55 to 93 years, with an average age of 74.2 years. Out of these, 85 were males and 161 were females. The average educational attainment within the cohort was 10.5 years.
The exclusion criteria included: Credible alternative causes of cognitive decline such as a definitive history of stroke (either cerebral infarction or cerebral hemorrhage), brain tumors, Parkinson’s disease, epilepsy, head or nose injuries; psychiatric disorders namely schizophrenia, bipolar disorder, and depression; incapacity to voluntarily participate in neurological and psychiatric examinations; and severe physical ailments or notable visual or auditory impairments that could hinder participation in cognitive functionality tests. Each participant provided signed informed consent, and the study gained approval from the Ethics Committee of the First Hospital of Hebei Medical University (ethical approval No. 20190416). The population was divided into two groups: HOAs and MCI.
Diagnostic criteria
HOAs were defined as follows: (1) No self-reported cognitive decline; (2) Objective evidence of normal cognitive function, with mini-mental state examination (MMSE) scores of ≥ 19 for illiterate individuals, ≥ 22 for individuals with primary school-education, and ≥ 24 for those with junior high school education or higher[19]. For Montreal Cognitive Assessment (MoCA) scores, the boundaries were ≥ 13 for illiterate individuals, ≥ 19 for those with primary school education, and ≥ 24 for those with junior high school education or higher education[20]; (3) A clinical dementia rating (CDR) of 0[21]; (4) Normal daily functioning, as indicated by an activity of daily living (ADL) scale[22] score of ≤ 26; and (5) Minimal anxiety or depression, as indicated by a Hamilton anxiety scale score of < 7 and a Hamilton depression scale score of < 7.
MCI was diagnosed based on the consensus of the Chinese expert group on MCI diagnosis from 2006[23]. This included: (1) Self-perceived or family-reported significant memory impairment; (2) Relatively intact or mildly impaired cognitive functions other than memory, with MMSE scores akin to those of the HOAs group but MoCA scores of < 13 for illiterate individuals, < 19 for those with primary school education, and < 24 for those with junior high school education or higher; (3) Essentially normal daily functions, as indicated by an ADL score of ≤ 26; (4) A CDR of 0.5; and (5) Exclusion of the possibility of dementia, that is, not yet meeting the diagnostic criteria for dementia.
Data collection procedures
In this study, the researchers undertook formal training to ensure the unified management of the questionnaires and provided standardized guidance for the subjects. The investigators followed a one-on-one approach to conduct neuropsychological assessments and fill out questionnaires for each subject. Each survey took approximately 45 minutes per subject to finish.
A general information survey, created in-house, was used to collect basic information. This included age, gender, education level, marital status, as well as smoking and drinking habits. The survey also inquired about past medical history, specifically focusing on conditions such as diabetes, coronary heart disease, hypertension, and hyperlipidemia.
Neuropsychological testing
The MMSE[19] and MoCA[20] were utilized to assess overall cognitive function. The MMSE is presently the most favored scale, it covers orientation, memory, calculation, language, visual-spatial ability, application, and attention, totaling 30 points. The MoCA has demonstrated a superior screening sensitivity compared to the MMSE[24]. The clock drawing task[25] was employed to measure visual-spatial ability. The Boston naming test[26] was utilized to evaluate language naming proficiency. The digit span test[27] gauged immediate memory function, attention, and information processing speed. The functional activities questionnaire (FAQ) measured difficulties in daily living activities[28], with higher scores indicating increased difficulty.
The behavioral assessment was conducted using the MBI-C 10 tool, comprised of 34 items divided into five domains: (1) Decreased motivation (covering six items that evaluate cognitive, behavioral, and emotional apathy); (2) Emotional dysregulation (6 items evaluating feeling of depression, anhedonia, despair, guilt, anxiety, and panic); (3) Impulse dyscontrol (12 items probing into agitation, aggression, impulsiveness, recklessness, and so forth); (4) Social inappropriateness (5 items that assess sensitivity, empathy, and disinhibited behavior); and (5) Abnormal perception or thought content (5 items evaluating suspicion, exaggeration, auditory hallucinations, and visual hallucinations). Participants were required to respond with either “yes” or “no” for each item. Any item with a “yes” response must be in line with behavior for at least 6 months, therefore indicating a significant deviation from baseline behavior. Here, the severity level could range from 1 (mild), 2 (moderate), to 3 (severe). Scores for each MBI-C subdomain could be computed by summing the severity scores for each respective category. Classification into MBI positive (MBI +) or MBI negative (MBI -) was assessed using the MBI-C validation study for MCI patients, with a cut-off point of 6.5 points[29] for diagnosing MBI. Consequently, MBI-C scores equal to or exceeding 6.5 were classified as MBI +, while those below 6.5 were considered MBI -.
Blood sample collection and biochemical detection
Fasting blood samples were drawn from 70 cases into coagulation tubes at a specific time (8:00 to 9:00). The drawn blood samples were then allowed to sit at room temperature for 2 h before being centrifugation at 3000 rpm for 10 minutes duration. The serum collected post-centrifugation was aliquoted and stored at -80 °C for subsequent analyses.
The human Aβ40, Aβ42, and p-tau217 enzyme-linked immunosorbent assay kits were acquired from Shanghai Zhuocai Biotechnology Co., Ltd. Before use, the kit and samples were allowed to reach room temperature (18 °C-25 °C). Each kit contained six standards with known concentrations (10, 20, 40, 80, 160, and 320 pg/mL), which were used to plot a standard curve with these six points.
Standard and sample wells were prepared. Each standard well-received 50 μL of various concentration standards, while the enzyme-labeled plate was given 40 μL of sample diluent. Subsequently, 10 μL of the sample was added to each sample well, resulting in a final dilution factor of 5. The sample was administered by adding it at the bottom of the enzyme-labeled well, with care taken to avoid touching the well walls, followed by gentle shaking for mixing. Next, 100 μL of enzyme-labeled reagent was dispensed into each well, except the blank. The plate was sealed with a film and incubated at 37 °C for 60 minutes. The 20 × concentrated washing solution was diluted by a factor of 20 with distilled water and set aside. The sealing film was meticulously removed, the liquid was discarded, and the plate was dried by tapping. The washing solution was introduced to each well, and left for 30 seconds, before being discarded. This process was repeated five times, after which the plate was tapped dry again. Afterward, 50 μL of color developer A and 50 μL of color developer B were added to each well, which were gently mixed before being kept in the dark at 37 °C for 15 minutes. The reaction was quenched by adding 50 μL of stop solution to each well, causing the color switch from blue to yellow. The absorbance (optical density value) of each well was measured sequentially at 450 nm wavelength, using the blank well for zero adjustment, and the measurement was conducted within 15 minutes of adding the stop solution.
Statistical analysis
Statistical analysis was conducted using SPSS 24.0. Independent sample t-tests enabled comparisons among roughly normally distributed quantitative data between two groups, with means and SD for description. For severely distorted continuous variables, medians (lower quartile, upper quartile) were used for description, while Mann-Whitney U non-parametric tests were used for comparison. Frequencies and percentages were derived for categorical variables, with χ2 tests being utilized for contingency tables. Due to the low prevalence of abnormal perception or thought content symptoms in this sample, these were not included in the analysis. The partial correlation analysis method was used to investigate the relationship between MBI-C scores, their subdomains, and AD biomarkers, controlling for age, gender, years of education, and cognitive factors incorporating them into the covariate list. The correlation coefficient (r) indicated the correlation between MBI and biomarker levels. To explore the influences of various factors on Aβ42 levels, multivariable linear regression was executed. In the multiple linear regression analysis, demographic variables such as age, gender, and education level were forcibly included in the basic model. For screening of candidate indicators: (1) Univariate analysis: Initial screening of variables significantly correlated with the dependent variable (P < 0.10); (2) Collinearity diagnosis: Exclusion of variables with multicollinearity variables through variance inflation factor (< 5); and (3) Stepwise regression validation: Use of backward stepwise method (P-exclusion > 0.10, P-inclusion < 0.05) to verify variable were undertaken. GraphPad Prism 9 served as the graphing software. P < 0.05 was deemed statistically significant.
RESULTS
Demographic, cognitive, and behavioral characteristics of HOAs and MCI populations
The study’s flow diagram, including with the specific number of participants, is illustrated in Figure 1. Our sample included 136 individuals with HOAs (45 males/91 females) and 105 patients with MCI (38 males/67 females). All the subjects were sorted into two groups: Non-MBI (n = 89) and MBI (n = 16), based on an MBI-C score of ≥ 6.5.
Figure 1.
Flow diagram of the trial. HOA: Healthy old adults; MCI: Mild cognitive impairment; MBI: Mild behavioral impairment; M: Male; F: Female.
As depicted in Table 1, significant differences were observed between the two groups in terms of age (t = 5.160, P < 0.000), diabetes (χ2 = 5.275, P = 0.022), cognitive assessment, and FAQ score (P < 0.05). However, we found no significant differences in gender, years of education, lifestyle habits, and medical history (P > 0.05).
Table 1.
Demographic, cognitive, and behavioral characteristics of healthy older adult and mild cognitive impairment populations, n (%)
|
Characteristic
|
HOA (n = 136)
|
MCI (n = 105)
|
t/χ2/K-W
|
P value
|
| Age (years, mean ± SD) | 71.8 ± 8.1 | 77.1 ± 7.9 | 5.160 | < 0.001 |
| Male | 45 (33.1) | 38 (36.2) | 0.253 | 0.615 |
| Years of education median (P25, P75) | 12 (9, 14) | 10 (7, 13.5) | 1.305 | 0.192 |
| Bereavement | 29 (21.3) | 25 (23.8) | 0.211 | 0.646 |
| Smoking history | 6 (4.4) | 7 (6.7) | 0.606 | 0.738 |
| Drinking history | 16 (11.8) | 15 (14.3) | 1.987 | 0.370 |
| Hypertension | 48 (35.3) | 41 (39.0) | 0.358 | 0.549 |
| Coronary heart disease | 26 (19.1) | 31 (29.5) | 3.553 | 0.059 |
| Diabetes | 18 (13.2) | 26 (24.8) | 5.275 | 0.022 |
| Hyperlipidemia | 40 (29.4) | 29 (27.6) | 0.093 | 0.760 |
| SCD (yes, cases) | 109 (80.1) | 89 (84.8) | 0.861 | 0.354 |
| MMSE median (P25, P75) | 28 (28, 29) | 25 (24, 27) | 11.048 | < 0.001 |
| MoCA median (P25, P75) | 27 (26, 28) | 23 (19, 24) | 11.862 | < 0.001 |
| CDT median (P25, P75) | 4 (4, 4) | 3 (2, 4) | 5.780 | < 0.001 |
| BNT median (P25, P75) | 26 (23, 27) | 24 (21, 26) | 4.129 | < 0.001 |
| DST median (P25, P75) | 10 (8, 11) | 8 (6, 10) | 4.271 | < 0.001 |
| MBI total score median (P25, P75) | 0 (0, 2) | 0 (0, 3) | 2.223 | 0.026 |
| MBI > 1 | 50 (36.8) | 50 (47.6) | 2.876 | 0.090 |
| MBI > 6.5 | 6 (4.4) | 16 (15.3) | 7.262 | 0.007 |
| Decreased motivation > 0 | 14 (10.3) | 21 (20.2) | 4.635 | 0.031 |
| Emotional dysregulation | 17 (12.5) | 20 (19.2) | 2.047 | 0.152 |
| Impulse dyscontrol | 39 (28.7) | 51 (49.4) | 5.063 | 0.025 |
| Social inappropriateness | 1 (0.7) | 5 (4.8) | 4.010 | 0.045 |
| Abnormal perception or thought content | ||||
| FAQ median (P25, P75) | 0 (0, 0) | 0 (0, 2) | 3.372 | 0.001 |
Independent sample t-test for variable of normal distribution, Mann-Whitney U for variable of non-normal distribution, χ2 test for categorical variables. HOA: Healthy old adults; MCI: Mild cognitive impairment; SCD: Subjective cognitive impairment; MMSE: Mini-mental state examination; MoCA: Montreal cognitive assessment; CDT: Clock drawing test; BNT: Boston naming test; DST: Digit span test; MBI: Mild behavioral impairment; P25: Lower quartile; P75: Upper quartile; FAQ: Functional activities questionnaire.
The prevalence of MBI-C > 1 was 36.8% in the HOAs group and 47.6% in the MCI group. Meanwhile, the prevalence of MBI + (MBI-C ≥ 6.5) was 4.4% and 15.3%, respectively. This difference was statistically significant (χ2 = 7.262, P = 0.007). A subdomain analysis of MBI-C showed significant differences between the two groups in decreased motivation (χ2 = 4.635, P = 0.031), impulse dyscontrol (χ2 = 5.063, P = 0.025), and emotional dysregulation (χ2 = 4.010, P = 0.045). However, there were no notable differences in emotional dysregulation or abnormal perception/thought content between the groups.
In the HOAs group, the most common MBI symptoms were impulse dyscontrol (28.7%) and emotional dysregulation (12.5%). Less common symptoms in this group included emotional dysregulation (0.7%). Among the MCI group, the most common MBI symptoms were impulse dyscontrol (33.8%) and decreased motivation (20.2%), while the less common symptoms in the MCI group included emotional dysregulation (4.8%). Neither group met the criteria for abnormal perception or thought content.
Comparison of MBI-C scores between HOAs and MCI groups with MBI symptoms
The median total scores for MBI-C for the HOAs and MCI groups were 7 and 9.5, respectively, displaying a statistically significant difference between the two groups (Z = 2.459, P < 0.05). However, no statistical significance was observed in the MBI-C subdomain scores (P > 0.05, Supplementary Table 1).
Comparison of MBI-C and subdomain scores between different genders
There was no significant difference in the total score of MBI-C and its subdomain scores between genders (P > 0.05, Supplementary Table 2).
Demographic, cognitive, and behavioral characteristics of non-MBI and MBI groups in MCI
The average age of patients in the MCI group was 77.1 ± 7.9 years, of whom 67 were females (63.8%). The study did not find any significant differences in the baseline demographic characteristics and cognitive assessments between the MBI and non-MBI groups (all P > 0.05, Table 2). The prevalence of hypertension in the non-MBI and MBI groups was 33.7% and 68.8%, respectively, a difference which was statistically significant difference (χ2 = 6.997, P < 0.05). However, no statistical differences were found for diabetes, coronary heart disease, and hyperlipidemia. A statistically significant difference in MBI-C total scores was found between the non-MBI and MBI groups (Z = 6.322, P < 0.001), with median scores of 0 and 9.5 for the non-MBI and MBI groups, respectively. A difference was also found in the FAQ scores between the non-MBI and MBI groups (Z = 2.042, P < 0.05), indicating a worse social function in the MBI group than the non-MBI group.
Table 2.
Demographic characteristics and cognitive and behavioral features of non-mild behavioral impairment and mild behavioral impairment groups in mild cognitive impairment, mean ± SD/n (%)
| Characteristic |
MCI (n = 105)
|
t/χ2/K-W
|
P value
|
|
|
MBI- (n = 89)
|
MBI+ (n = 16)
|
|||
| Age (years) | 77.5 ± 8.1 | 75.3 ± 6.1 | 0.997 | 0.321 |
| Male | 31 (34.8) | 7 (43.8) | 0.467 | 0.494 |
| Education years median (P25, P75) | 10 (6, 14) | 12 (9.5, 12) | 0.901 | 0.367 |
| Bereavement | 22 (24.7) | 3 (13.8) | 0.266 | 0.606 |
| Current smoking | 4 (4.5) | 3 (18.8) | 5.328 | 0.058 |
| Current drinking | 11 (12.4) | 4 (25.0) | 3.413 | 0.171 |
| Hypertension | 30 (33.7) | 11 (68.8) | 6.997 | 0.008 |
| Coronary heart disease | 24 (27.0) | 7 (43.8) | 1.836 | 0.175 |
| Diabetes | 20 (22.5) | 6 (37.5) | 1.644 | 0.200 |
| Hyperlipidemia | 23 (25.8) | 6 (37.5) | 0.922 | 0.337 |
| SCD | 73 (82.0) | 16 (100) | 3.394 | 0.065 |
| MMSE median (P25, P75) | 25 (24, 27) | 25 (23.3, 27) | 0.250 | 0.803 |
| MoCA median (P25, P75) | 22 (19, 24) | 23 (19.5, 24) | 0.405 | 0.686 |
| CDT median (P25, P75) | 3 (2, 4) | 3.5 (2, 4) | 0.195 | 0.845 |
| BNT (points) | 22.8 ± 4.5 | 23.8 ± 3.2 | 0.839 | 0.403 |
| DST (points) | 8.5 ± 2.7 | 8.3 ± 2.0 | 0.227 | 0.821 |
| MBI total score median (P25, P75) | 0 (0, 2) | 9.5 (7.5, 12.8) | 6.322 | < 0.001 |
| FAQ median (P25, P75) | 0 (0, 2) | 1 (0, 4.8) | 2.042 | 0.041 |
Independent sample t-test for variable of normal distribution, Mann-Whitney U for variable of non-normal distribution, χ2 test for categorical variables. MBI: Mild behavioral impairment; MCI: Mild cognitive impairment; SCD: Subjective cognitive impairment; MMSE: Mini-mental state examination; MoCA: Montreal cognitive assessment; CDT: Clock drawing test; BNT: Boston naming test; DST: Digit span test; P25: Lower quartile; P75: Upper quartile; FAQ: Functional activities questionnaire.
MBI-C total and subdomain scores of non-MBI and MBI groups in MCI
Significant differences (P < 0.05) were observed in all five MBI dimensions between the non-MBI and MBI groups. The median scores for each subdomain were as follows: Decreased motivation (n = 2), emotional dysregulation (n = 2), impulse dyscontrol (n = 4), and social inappropriateness (n = 0). Of these, the highest score was recorded in the impulse dyscontrol subdomain (Table 3).
Table 3.
Mild behavioral impairment-checklist subdomain scores of non-mild behavioral impairment and mild behavioral impairment groups in mild cognitive impairment
|
MCI (n = 105)
|
K-W
|
P value
|
||
|
MBI- (n = 89)
|
MBI+ (n = 16)
|
|||
| Decreased motivation median (P25, P75) | 0 (0, 0) | 2 (0, 3) | 4.561 | < 0.001 |
| Emotional dysregulation median (P25, P75) | 0 (0, 0) | 2 (0, 5) | 5.143 | < 0.001 |
| Impulse dyscontrol median (P25, P75) | 0 (0, 1) | 4 (1.3, 7.5) | 5.233 | < 0.001 |
| Social inappropriateness median (P25, P75) | 0 (0, 0) | 0 (0, 1) | 5.349 | < 0.001 |
| Abnormal perception or thought content median (P25, P75) | 0 (0, 0) | 0 (0, 0) | ||
P values were calculated using Mann Whitney U Test. MBI: Mild behavioral impairment; MCI: Mild cognitive impairment; P25: Lower quartile; P75: Upper quartile.
Factors related to MBI
The total MBI-C score exhibited a negative correlation with diabetes (r = -0.234, P < 0.05) and a significant positive correlation with FAQ (r = 0.402, P < 0.05). However, no discernible correlation was found between the MBI-C total score and factors such as age, years of education, hypertension, and cognitive function (all P > 0.05) (Supplementary Table 3).
Comparison of plasma biomarkers scores between different genders
There were no statistically significant differences in plasma p-tau217, Aβ40, Aβ42, Aβ42/Aβ40 (all P > 0.05), see Supplementary Table 4.
Comparison of plasma biomarkers between non-MBI and MBI groups in MCI
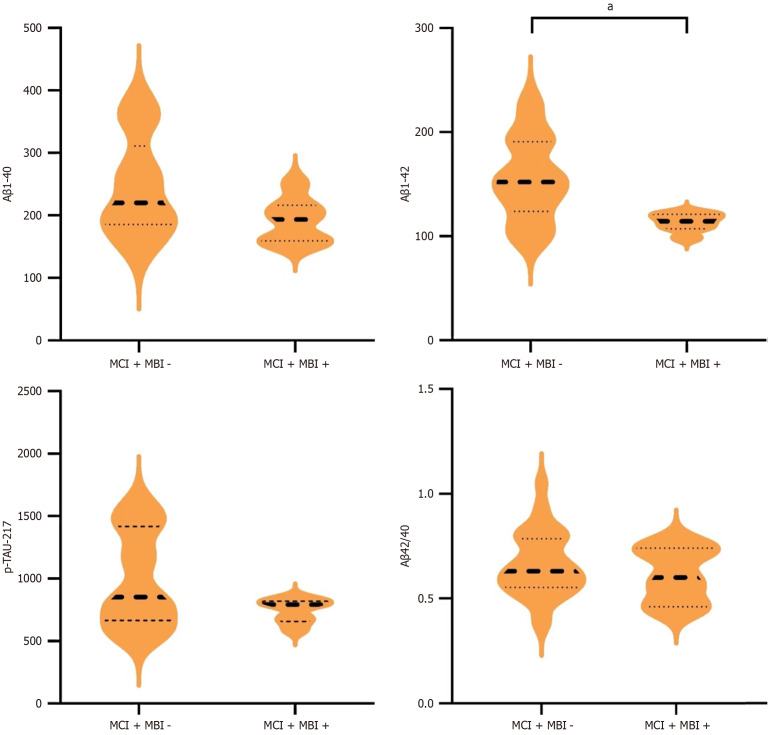
In the MCI group, Aβ42 levels were significantly lower in the MBI + group than in the MBI - group, showing a statistically significant difference (t = 2.40, P = 0.021). However, no statistically significant differences were observed in Aβ40, Aβ42/Aβ40, and p-tau217 between MBI +/- groups (all P > 0.05, Figure 2).
Figure 2.
Comparison of plasma biomarkers between mild cognitive impairment groups with or without mild behavioral impairment. The sample size for mild cognitive impairment (MCI) + mild behavioral impairment (MBI) - was 89, and 16 for MCI + MBI +. aP < 0.05. Significance star for independent sample t-test. MCI: Mild cognitive impairment; MBI: Mild behavioral impairment; p-tau217: Hyperphosphorylated tau; Aβ: Amyloid β.
Correlation analysis between MBI-C total score, subdomains, and AD plasma biomarkers
The total MBI score displayed a negative correlation with Aβ42 and p-tau217 (r = -0.385, P = 0.019; r = -0.33, P = 0.041), whereas no correlation was observed with Aβ40 and Aβ42/40. Within the subdomains, it was determined that the score for impulse dyscontrol significantly negatively correlated with Aβ42 (r = -0.401, P = 0.025, Supplementary Table 5).
Factors influencing Aβ42
Aβ42 levels were employed as dependent variables, whereas age, education level, MMSE scores, and MBI-C scores were used as independent variables to formulate a multivariable linear regression equation. The results indicated that the MBI-C score significantly influenced Aβ42 (B = -5.277, t = -2.638, P = 0.0113), and it negatively predicted the Aβ42 level. However, age and cognitive status had no discernible influence on the Aβ42 level (Table 4).
Table 4.
Analysis of multivariable linear regression for factors influencing amyloid β 42
| Variable |
Unstandardized coefficients
|
Standardized coefficients
|
t
|
P value
|
|
|
B
|
SE
|
β
|
|||
| Constant | 288.851 | 93.373 | 3.094 | 0.004 | |
| Age | 0.864 | 0.940 | 0.155 | 0.919 | 0.366 |
| Years of education | -0.641 | 1.807 | -0.077 | -0.355 | 0.725 |
| MMSE total score | -2.984 | 5.140 | -0.177 | -0.581 | 0.566 |
| MBI-C total score | -5.277 | 2.000 | -0.445 | -2.638 | 0.013 |
P values were calculated using multivariable linear regression. MBI-C: Mild behavioral impairment-checklist; MMSE: Mini-mental state examination.
DISCUSSION
In this study, we found a prevalence of 4.4% MBI in HOAs and 15.3% in those with MCI, with a notably higher prevalence in the MCI group. These results align with past research. For instance, Mortby et al[30] conducted a large-scale survey of 1377 community-dwelling older adults and discovered a higher prevalence of MBI in the MCI group when compared to cognitively healthy adults (48.9% vs 27.6%). Similarly, a study in Iran[31] reported similar results, indicating a 50% prevalence of MBI in its sample of 96 MCI patients at memory clinics. The findings from the present study, along with prior research, suggest that behavioral impairment symptoms are highly prevalent at the pre-dementia stage, especially so in MCI cases. It has been previously demonstrated that NPS is a risk factor for MCI and AD dementia[32,33], thereby emphasizing the need to understand the relationship between MBI, MCI, and AD.
In this cross-sectional study, we determined that symptoms of MBI were nearly unrelated to cognitive symptoms. Nonetheless, individuals with MBI symptoms generally exhibited poorer social functioning, aligning with previous research conclusions[34]. We did not discover any relationship between MBI and age or years of education. Additionally, no differences in MBI prevalence and its subdomains were found between genders. Prior research results on this topic remain inconsistent. Some studies have reported that males exhibit decreased motivation and impulse dyscontrol more frequently than females[30]. They further propose that abnormal perception or thought content domains are more prevalent in females[34], while others have found no significant gender differences[32]. A recent large-scale United Kingdom study confirmed gender differences in the correlation between MBI and cognition[35]. Hence, larger-scale studies in China required to clarify these asserted gender differences in MBI prevalence.
In addition, we found no significant difference between various risk factors and MBI, aligning with previous findings. However, studies have indicated that demographic factors such as alcohol consumption and smoking are associated with AD biomarkers[36]. A recent study involving Mexican AD patients and controls of older adults with cholesterol levels over the healthy range (< 200 mg/dL) observed no significant difference in plasma cholesterol levels between the AD and control groups. Furthermore, some carriers of apolipoprotein E (APOE) 4 have reported both hypertriglyceridemia and hypercholesterolemia, and APOE4 is associated with low glucose metabolism in the brain of older adults. This suggests that elevated lipid concentrations can lead to systemic hyperlipidemia, possibly impairing the blood-brain barrier. This may promote the bypass of proteins such as Aβ and tau into the bloodstream, and provide a mechanism for increased amyloid plaque formation[37].
This study did not uncover a gender difference in dementia biomarkers, diverging from some previous research. There are a limited number of relevant studies and their findings vary. One 25-year follow-up study included 2284 participants with a median age of 59.2 ± 5.2 years, 57% of whom were female. It reported that plasma Aβ42 and Aβ40 were higher among male subjects than females, and the Aβ42 to Aβ40 ratio was slightly lower, with a smaller increase in Aβ42 and Aβ40 from middle age to later life[36]. Differences in study results may correlate to participant ethnicity, given that most of the studies were conducted in European countries or the United States[37].
While this study did not identify a correlation between MBI and clinical symptoms of cognitive decline, we discovered that patients with MBI + had significantly lower plasma Aβ42 levels, but not Aβ40. Aβ42 is the primary component of senile plaques[38], which has greater neurotoxicity than Aβ40, and plays a noteworthy role in brain amyloid angiopathy[39]. The association between Aβ42 levels and cognitive impairment might be more pronounced than that between Aβ40 and cognitive impairment[40]. Sun et al[41] identified the predictive relationship between baseline MBI and the progression of amyloid pathology in individuals free of dementia. This suggests that the relationship between MBI and cognitive impairment could be linked to alterations in amyloid pathology. Furthermore, a cross-sectional study from the Mayo Clinic indicated that patients with MCI and cerebral Aβ deposition are at a heightened risk of developing NPS[42]. However, the mechanism through which MBI influences amyloid changes remains undefined.
Furthermore, we also discovered a link between MBI and late-stage AD tau-217 pathology. However, some controversy currently exists in this field of study. One study by Lussier et al[12], which conducted 18F Aβ-PET and 18F tau-PET scans on 96 cognitively normal older adults, observed that increased MBI-C scores strongly correlated with Aβ-PET uptake, particularly in early-stage AD brain regions like the neocortex, including the frontal neocortex, and then the striatum. However, there was no observed association between MBI and an increase in tau protein PET uptake. This suggests that MBI is related to the early-stage pathophysiology of AD[2] in cognitively healthy older populations, but not to the pathophysiology characteristic of the late stages.
In contrast to other findings, a Swedish BioFINDER study[43] that included Aβ-positive cognitively normal older adults, found MBI to be related to cortical tau deposition in the entorhinal cortex. Additional findings revealed the pathological changes related to the olfactory system occur early in AD[44,45]. This study proves that Aβ-positive cognitively normal older adults show signs of both early and late-stage AD.
Pathological deposition related to AD follows a specific temporal sequence. In early-stage AD, Aβ-related pathophysiological abnormalities, followed by downstream neuronal biomarker damage, like tau pathology and neurodegenerative change markers[46]. Though late-stage tau protein alterations changes can also contribute to cognitive decline, significant tau protein aggregation is seldom observed in cognitively intact individuals[12].
In our study, we incorporated MCI patients and discovered that higher MBI total scores were linked to lower Aβ42 and tau-217 levels. The contrasting conclusions in these studies could be attributed to differences in inclusion criteria, sample size, blood-biomarker quantification methods, and the type of information obtained (PET vs fluid biomarkers), among other factors.
When observing MBI subdomains, it was demonstrated that the decrease in plasma Aβ42 was significantly associated with the impulse dyscontrol subdomain. Interestingly, this association was not identified for the decreased motivation or emotional dysregulation subdomains. Impulse dyscontrol includes agitation, aggression, irritability, and abnormal motor behaviors[30]. Gill et al[47] discovered that impulse dyscontrol was linked to gray matter atrophy, particularly in the parahippocampal gyrus cortical thickness. This suggests a strong connection between impulse dyscontrol and typical early AD-related brain structural alterations. Other studies have found associations between AD biomarkers such as CSF Aβ42, tau protein, and agitation and aggression, but not with other subdomains[48]. Moreover, the emotional dysregulation subdomain is significantly associated with decreased plasma Aβ42/Aβ40[49]. Longitudinal follow-up studies have found a relationship between impulse dyscontrol and sudden cognitive decline[50,51]. Therefore, the impulse dyscontrol subdomain of MBI may be of particular importance in predicting cognitive decline and dementia risk. Thus, a comprehensive exploration of the relationship between MBI structural domains and AD biomarkers is needed. Notably, NPS has a high rate of consultation rate in memory clinics[10], and impulse dyscontrol is the most common occurrence in the population, consistent with previous findings[8,52,53]. Consequently, when older adults exhibit impulsive and unregulated behaviors, they can significantly impact families and society. This also enables family members to notice these behaviors and seek professional medical help earlier. Therefore, further exploration of impulse dyscontrol is necessary, as it may be associated with a higher risk of sudden cognitive decline and dementia.
The strength of this study lies in its verification of these manifestations within the context of the Chinese population’s context, revealing robust sensitivity and specificity in diagnosing MBI status using an MBI-C cut-off of 6.5 points. Additionally, it is notable that the patient didn’t take any dementia medication or psychotropic drugs that could potentially interfere with the behavioral assessment. However, this study’s limitations include a small sample size that didn’t allow for any sex-based differences to be detected in MBI patients. Future studies require larger sample sizes for validation of these findings. MBI, a concept only recently proposed, suffers from a dearth of long-term follow-up studies examining it directly. Given that our study is cross-sectional, it lacks evidence to confirm causal relationships. Future, longitudinal studies may help unearth a causal relationship between MBI and cognitive decline. Lastly, our focus on evaluating markers for AD hindered us from assessing the test’s specificity for AD detection when other neurodegenerative diseases could be present.
CONCLUSION
In conclusion, this study further validates the relationship between behavioral impairment and AD biomarkers within the Chinese population. It suggests that plasma Aβ42 levels help identify populations with MBI. Before cognitive decline, significant changes occur in behavioral impairment. This could contribute to establishing the MBI-C scale as a testing tool for use in the preclinical stages of dementia.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of The First Hospital of Hebei Medical University (No. 20210902).
Informed consent statement: Each participant provided signed informed consent.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
STROBE statement: The authors have read the STROBE Statement—a checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-a checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade C
Novelty: Grade B, Grade B, Grade B
Creativity or Innovation: Grade B, Grade B, Grade B
Scientific Significance: Grade B, Grade B, Grade B
P-Reviewer: Liu DF; Lyu WQ S-Editor: Fan M L-Editor: A P-Editor: Zhang L
Contributor Information
Wei Liang, Mental Health Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China; Xi’an Mental Health Center, Xi’an 710061, Shaanxi Province, China.
Lan Wang, Mental Health Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China; Hebei Key Laboratory of Forensic Medicine, Shijiazhuang 050017, Hebei Province, China.
Mei Song, Mental Health Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China.
Hao Geng, Mental Health Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China.
Xin-Yang Jing, Mental Health Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China.
Wei Li, Mental Health Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China.
Ya-Xin Huo, Mental Health Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China.
An-Qi Huang, Mental Health Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China.
Xue-Yi Wang, Mental Health Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China.
Cui-Xia An, Mental Health Center, The First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China. acxsunny@hebmu.edu.cn.
Data sharing statement
No additional data are available.
References
- 1.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsunoda K, Yamashita T, Osakada Y, Sasaki R, Tadokoro K, Matsumoto N, Nomura E, Morihara R, Nakano Y, Takahashi Y, Hatanaka N, Shang J, Sato K, Takemoto M, Hishikawa N, Ohta Y, Abe K. Early Emergence of Neuropsychiatric Symptoms in Cognitively Normal Subjects and Mild Cognitive Impairment. J Alzheimers Dis. 2020;73:209–215. doi: 10.3233/JAD-190669. [DOI] [PubMed] [Google Scholar]
- 3.Taragano FE, Allegri RF, Heisecke SL, Martelli MI, Feldman ML, Sánchez V, García VA, Tufro G, Castro DM, Leguizamón PP, Guelar V, Ruotolo E, Zegarra C, Dillon C. Risk of Conversion to Dementia in a Mild Behavioral Impairment Group Compared to a Psychiatric Group and to a Mild Cognitive Impairment Group. J Alzheimers Dis. 2018;62:227–238. doi: 10.3233/JAD-170632. [DOI] [PubMed] [Google Scholar]
- 4.Taragano FE, Allegri RF, Krupitzki H, Sarasola DR, Serrano CM, Loñ L, Lyketsos CG. Mild behavioral impairment and risk of dementia: a prospective cohort study of 358 patients. J Clin Psychiatry. 2009;70:584–592. doi: 10.4088/jcp.08m04181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassam F, Chen H, Nosheny RL, McGirr A, Williams T, Ng N, Camacho M, Mackin RS, Weiner MW, Ismail Z. Cognitive profile of people with mild behavioral impairment in Brain Health Registry participants. Int Psychogeriatr. 2023;35:643–652. doi: 10.1017/S1041610221002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creese B, Brooker H, Ismail Z, Wesnes KA, Hampshire A, Khan Z, Megalogeni M, Corbett A, Aarsland D, Ballard C. Mild Behavioral Impairment as a Marker of Cognitive Decline in Cognitively Normal Older Adults. Am J Geriatr Psychiatry. 2019;27:823–834. doi: 10.1016/j.jagp.2019.01.215. [DOI] [PubMed] [Google Scholar]
- 7.Ismail Z, McGirr A, Gill S, Hu S, Forkert ND, Smith EE. Mild Behavioral Impairment and Subjective Cognitive Decline Predict Cognitive and Functional Decline. J Alzheimers Dis. 2021;80:459–469. doi: 10.3233/JAD-201184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouse HJ, Small BJ, Schinka JA, Loewenstein DA, Duara R, Potter H. Mild behavioral impairment as a predictor of cognitive functioning in older adults. Int Psychogeriatr. 2021;33:285–293. doi: 10.1017/S1041610220000678. [DOI] [PubMed] [Google Scholar]
- 9.Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, Agüera-Ortiz L, Sweet R, Miller D, Lyketsos CG ISTAART Neuropsychiatric Symptoms Professional Interest Area. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12:195–202. doi: 10.1016/j.jalz.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail Z, Agüera-Ortiz L, Brodaty H, Cieslak A, Cummings J, Fischer CE, Gauthier S, Geda YE, Herrmann N, Kanji J, Lanctôt KL, Miller DS, Mortby ME, Onyike CU, Rosenberg PB, Smith EE, Smith GS, Sultzer DL, Lyketsos C NPS Professional Interest Area of the International Society of to Advance Alzheimer’s Research and Treatment (NPS-PIA of ISTAART) The Mild Behavioral Impairment Checklist (MBI-C): A Rating Scale for Neuropsychiatric Symptoms in Pre-Dementia Populations. J Alzheimers Dis. 2017;56:929–938. doi: 10.3233/JAD-160979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Li T, Xiong L, Wang X, Ismail Z, Fukuda M, Sun Z, Wang J, Gauthier S, Yu X, Wang H. Reliability and Validity of the Chinese Version of Mild Behavioral Impairment Checklist in Mild Cognitive Impairment and Mild Alzheimer's Disease. J Alzheimers Dis. 2021;81:1141–1149. doi: 10.3233/JAD-210098. [DOI] [PubMed] [Google Scholar]
- 12.Lussier FZ, Pascoal TA, Chamoun M, Therriault J, Tissot C, Savard M, Kang MS, Mathotaarachchi S, Benedet AL, Parsons M, Qureshi MNI, Thomas ÉM, Shin M, Dion LA, Massarweh G, Soucy JP, Tsai IH, Vitali P, Ismail Z, Rosa-Neto P, Gauthier S. Mild behavioral impairment is associated with β-amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement. 2020;16:192–199. doi: 10.1002/alz.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson M, Stomrud E, Insel PS, Leuzy A, Johansson PM, Smith R, Ismail Z, Janelidze S, Palmqvist S, van Westen D, Mattsson-Carlgren N, Hansson O. Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer's disease. Transl Psychiatry. 2021;11:76. doi: 10.1038/s41398-021-01206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naude JP, Gill S, Hu S, McGirr A, Forkert ND, Monchi O, Stys PK, Smith EE, Ismail Z Alzheimer’s Disease Neuroimaging Initiative. Plasma Neurofilament Light: A Marker of Neurodegeneration in Mild Behavioral Impairment. J Alzheimers Dis. 2020;76:1017–1027. doi: 10.3233/JAD-200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews SJ, Ismail Z, Anstey KJ, Mortby M. Association of Alzheimer's genetic loci with mild behavioral impairment. Am J Med Genet B Neuropsychiatr Genet. 2018;177:727–735. doi: 10.1002/ajmg.b.32684. [DOI] [PubMed] [Google Scholar]
- 16.Creese B, Arathimos R, Brooker H, Aarsland D, Corbett A, Lewis C, Ballard C, Ismail Z. Genetic risk for Alzheimer's disease, cognition, and mild behavioral impairment in healthy older adults. Alzheimers Dement (Amst) 2021;13:e12164. doi: 10.1002/dad2.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu J, Qiang Q, Yan Y, Wen Y, Ren Y, Wei W, Zhang L. Distinct Patterns of Brain Atrophy associated with Mild Behavioral Impairment in Cognitively Normal Elderly Adults. Int J Med Sci. 2021;18:2950–2956. doi: 10.7150/ijms.60810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallo SC, Ismail Z, Pereiro AX, Facal D, Lojo-Seoane C, Campos-Magdaleno M, Juncos-Rabadán O. Assessing mild behavioral impairment with the mild behavioral impairment checklist in people with subjective cognitive decline. Int Psychogeriatr. 2019;31:231–239. doi: 10.1017/S1041610218000698. [DOI] [PubMed] [Google Scholar]
- 19.Philipps V, Amieva H, Andrieu S, Dufouil C, Berr C, Dartigues JF, Jacqmin-Gadda H, Proust-Lima C. Normalized Mini-Mental State Examination for assessing cognitive change in population-based brain aging studies. Neuroepidemiology. 2014;43:15–25. doi: 10.1159/000365637. [DOI] [PubMed] [Google Scholar]
- 20.Lu J, Li D, Li F, Zhou A, Wang F, Zuo X, Jia XF, Song H, Jia J. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol. 2011;24:184–190. doi: 10.1177/0891988711422528. [DOI] [PubMed] [Google Scholar]
- 21.Nyunt MS, Chong MS, Lim WS, Lee TS, Yap P, Ng TP. Reliability and Validity of the Clinical Dementia Rating for Community-Living Elderly Subjects without an Informant. Dement Geriatr Cogn Dis Extra. 2013;3:407–416. doi: 10.1159/000355122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 23.Expert Consensus Group on Prevention and Treatment of Cognitive Impairment in China. [Expert Group on the Consensus of Chinese Experts on the Prevention and Treatment of Cognitive Dysfunction] Zhonghua Laonian Yixue Zazhi. 2006;2:485–487. [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 25.Ueda H, Kitabayashi Y, Narumoto J, Nakamura K, Kita H, Kishikawa Y, Fukui K. Relationship between clock drawing test performance and regional cerebral blood flow in Alzheimer's disease: a single photon emission computed tomography study. Psychiatry Clin Neurosci. 2002;56:25–29. doi: 10.1046/j.1440-1819.2002.00940.x. [DOI] [PubMed] [Google Scholar]
- 26.Cheung RW, Cheung MC, Chan AS. Confrontation naming in Chinese patients with left, right or bilateral brain damage. J Int Neuropsychol Soc. 2004;10:46–53. doi: 10.1017/S1355617704101069. [DOI] [PubMed] [Google Scholar]
- 27.Yang CC, Kao CJ, Cheng TW, Yang CC, Wang WH, Yu RL, Hsu YH, Hua MS. Cross-cultural effect on suboptimal effort detection: an example of the Digit Span subtest of the WAIS-III in Taiwan. Arch Clin Neuropsychol. 2012;27:869–878. doi: 10.1093/arclin/acs081. [DOI] [PubMed] [Google Scholar]
- 28.González DA, Gonzales MM, Resch ZJ, Sullivan AC, Soble JR. Comprehensive Evaluation of the Functional Activities Questionnaire (FAQ) and Its Reliability and Validity. Assessment. 2022;29:748–763. doi: 10.1177/1073191121991215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallo SC, Ismail Z, Pereiro AX, Facal D, Lojo-Seoane C, Campos-Magdaleno M, Juncos-Rabadán O. Assessing Mild Behavioral Impairment with the Mild Behavioral Impairment-Checklist in People with Mild Cognitive Impairment. J Alzheimers Dis. 2018;66:83–95. doi: 10.3233/JAD-180131. [DOI] [PubMed] [Google Scholar]
- 30.Mortby ME, Ismail Z, Anstey KJ. Prevalence estimates of mild behavioral impairment in a population-based sample of pre-dementia states and cognitively healthy older adults. Int Psychogeriatr. 2018;30:221–232. doi: 10.1017/S1041610217001909. [DOI] [PubMed] [Google Scholar]
- 31.Kianimehr G, Fatehi F, Noroozian M. Prevalence of mild behavioral impairment in patients with mild cognitive impairment. Acta Neurol Belg. 2022;122:1493–1497. doi: 10.1007/s13760-021-01724-z. [DOI] [PubMed] [Google Scholar]
- 32.Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJ, Pankratz VS, Boeve BF, Sochor O, Tangalos EG, Petersen RC, Rocca WA. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171:572–581. doi: 10.1176/appi.ajp.2014.13060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pink A, Stokin GB, Bartley MM, Roberts RO, Sochor O, Machulda MM, Krell-Roesch J, Knopman DS, Acosta JI, Christianson TJ, Pankratz VS, Mielke MM, Petersen RC, Geda YE. Neuropsychiatric symptoms, APOE ε4, and the risk of incident dementia: a population-based study. Neurology. 2015;84:935–943. doi: 10.1212/WNL.0000000000001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuoka T, Ismail Z, Narumoto J. Prevalence of Mild Behavioral Impairment and Risk of Dementia in a Psychiatric Outpatient Clinic. J Alzheimers Dis. 2019;70:505–513. doi: 10.3233/JAD-190278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfova K, Creese B, Aarsland D, Ismail Z, Corbett A, Ballard C, Hampshire A, Cermakova P. Gender/Sex Differences in the Association of Mild Behavioral Impairment with Cognitive Aging. J Alzheimers Dis. 2022;88:345–355. doi: 10.3233/JAD-220040. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan KJ, Blackshear C, Simino J, Tin A, Walker KA, Sharrett AR, Younkin S, Gottesman RF, Mielke MM, Knopman D, Windham BG, Griswold ME, Mosley TH. Association of Midlife Plasma Amyloid-β Levels With Cognitive Impairment in Late Life: The ARIC Neurocognitive Study. Neurology. 2021;97:e1123–e1131. doi: 10.1212/WNL.0000000000012482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castillo-Mendieta T, Arana-Lechuga Y, Campos-Peña V, Sosa AL, Orozco-Suarez S, Pinto-Almazán R, Segura-Uribe J, Javier Rodríguez-Sánchez de Tagle A, Ruiz-Sánchez E, Guerra-Araiza C. Plasma Levels of Amyloid-β Peptides and Tau Protein in Mexican Patients with Alzheimer's Disease. J Alzheimers Dis. 2021;82:S271–S281. doi: 10.3233/JAD-200912. [DOI] [PubMed] [Google Scholar]
- 38.Verbeek MM, Eikelenboom P, de Waal RM. Differences between the pathogenesis of senile plaques and congophilic angiopathy in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:751–761. [PubMed] [Google Scholar]
- 39.Peng X, Xu Z, Mo X, Guo Q, Yin J, Xu M, Peng Z, Sun T, Zhou L, Peng X, Xu S, Yang W, Bao W, Shan Z, Li X, Liu L. Association of plasma β-amyloid 40 and 42 concentration with type 2 diabetes among Chinese adults. Diabetologia. 2020;63:954–963. doi: 10.1007/s00125-020-05102-x. [DOI] [PubMed] [Google Scholar]
- 40.Gomis M, Sobrino T, Ois A, Millán M, Rodríguez-Campello A, Pérez de la Ossa N, Rodríguez-González R, Jiménez-Conde J, Cuadrado-Godia E, Roquer J, Dávalos A. Plasma beta-amyloid 1-40 is associated with the diffuse small vessel disease subtype. Stroke. 2009;40:3197–3201. doi: 10.1161/STROKEAHA.109.559641. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Xu W, Chen KL, Shen XN, Tan L, Yu JT Alzheimer’s Disease Neuroimaging Initiative. Mild behavioral impairment correlates of cognitive impairments in older adults without dementia: mediation by amyloid pathology. Transl Psychiatry. 2021;11:577. doi: 10.1038/s41398-021-01675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krell-Roesch J, Vassilaki M, Mielke MM, Kremers WK, Lowe VJ, Vemuri P, Machulda MM, Christianson TJ, Syrjanen JA, Stokin GB, Butler LM, Traber M, Jack CR Jr, Knopman DS, Roberts RO, Petersen RC, Geda YE. Cortical β-amyloid burden, neuropsychiatric symptoms, and cognitive status: the Mayo Clinic Study of Aging. Transl Psychiatry. 2019;9:123. doi: 10.1038/s41398-019-0456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansson M, Smith R, Stomrud E, Johansson P, Janelidze S, van Westen D, Mattsson N, Hansson O. Mild behavioral impairment is predictive of tau deposition in the earliest stages of Alzheimer's disease. Alzheimers Dement. 2020;16 [Google Scholar]
- 44.Park SJ, Lee JE, Lee KS, Kim JS. Comparison of odor identification among amnestic and non-amnestic mild cognitive impairment, subjective cognitive decline, and early Alzheimer's dementia. Neurol Sci. 2018;39:557–564. doi: 10.1007/s10072-018-3261-1. [DOI] [PubMed] [Google Scholar]
- 45.Khurshid K, Crow AJD, Rupert PE, Minniti NL, Carswell MA, Mechanic-Hamilton DJ, Kamath V, Doty RL, Moberg PJ, Roalf DR. A Quantitative Meta-analysis of Olfactory Dysfunction in Epilepsy. Neuropsychol Rev. 2019;29:328–337. doi: 10.1007/s11065-019-09406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wirth M, Oh H, Mormino EC, Markley C, Landau SM, Jagust WJ. The effect of amyloid β on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimers Dement. 2013;9:687–698.e1. doi: 10.1016/j.jalz.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill S, Wang M, Mouches P, Rajashekar D, Sajobi T, MacMaster FP, Smith EE, Forkert ND, Ismail Z Alzheimer's Disease Neuroimaging Initiative. Neural correlates of the impulse dyscontrol domain of mild behavioral impairment. Int J Geriatr Psychiatry. 2021;36:1398–1406. doi: 10.1002/gps.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Showraki A, Murari G, Ismail Z, Barfett JJ, Fornazzari L, Munoz DG, Schweizer TA, Fischer CE. Cerebrospinal Fluid Correlates of Neuropsychiatric Symptoms in Patients with Alzheimer's Disease/Mild Cognitive Impairment: A Systematic Review. J Alzheimers Dis. 2019;71:477–501. doi: 10.3233/JAD-190365. [DOI] [PubMed] [Google Scholar]
- 49.Miao R, Chen HY, Gill S, Naude J, Smith EE, Ismail Z Alzheimer’s Disease Neuroimaging Initiative. Plasma β-Amyloid in Mild Behavioural Impairment - Neuropsychiatric Symptoms on the Alzheimer's Continuum. J Geriatr Psychiatry Neurol. 2022;35:434–441. doi: 10.1177/08919887211016068. [DOI] [PubMed] [Google Scholar]
- 50.Wise EA, Rosenberg PB, Lyketsos CG, Leoutsakos JM. Time course of neuropsychiatric symptoms and cognitive diagnosis in National Alzheimer's Coordinating Centers volunteers. Alzheimers Dement (Amst) 2019;11:333–339. doi: 10.1016/j.dadm.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masters MC, Morris JC, Roe CM. "Noncognitive" symptoms of early Alzheimer disease: a longitudinal analysis. Neurology. 2015;84:617–622. doi: 10.1212/WNL.0000000000001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Creese B, Griffiths A, Brooker H, Corbett A, Aarsland D, Ballard C, Ismail Z. Profile of mild behavioral impairment and factor structure of the Mild Behavioral Impairment Checklist in cognitively normal older adults. Int Psychogeriatr. 2020;32:705–717. doi: 10.1017/S1041610219001200. [DOI] [PubMed] [Google Scholar]
- 53.Fan S, Liang X, Yun T, Pei Z, Hu B, Ismail Z, Yang Z, Xu F. Mild behavioral impairment is related to frailty in non-dementia older adults: a cross-sectional study. BMC Geriatr. 2020;20:510. doi: 10.1186/s12877-020-01903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.