ABSTRACT
Objective: To quantify and compare the carotico-oculomotor triangle (COT) area before and after extradural anterior clinoidectomy (AC). Methods: Ten cadaveric heads were dissected bilaterally. Before and after an extradural AC, the following points were measured: (1) the internal carotid artery (ICA) bifurcation to the tip of the anterior clinoid process (ACP) (A) and to the distal dural ring (A′), (2) the ICA bifurcation to the point where the oculomotor nerve becomes obscured by the tentorial fold (B) and to the porus oculomotoris after incision of the tentorial fold (B′), and (3) the tip of the ACP to the point where the oculomotor nerve becomes obscured by the tentorial incisura (C) and from the distal dural ring to the porus oculomotoris (C′). The area of the COT was calculated before and after AC (ΔABC and ΔA′B′C′, respectively). Results: The mean values were as follows: A: 9.15 ± 0.93 mm, A′: 13.45 ± 0.82 mm; B: 7.80 ± 1.24 mm, B′: 9.90 ± 1.21 mm; C: 7.15 ± 0.99 mm, C′: 9.3 ± 1.26 mm; ΔABC: 26.26 ± 6.05 mm2, ΔA′B′C′: 45.06 ± 8.92 mm2. Conclusions: Extradural AC enhances the exposure of the COT almost twofold. This increased exposure can be of significant help during resection of lesions of the parasellar and basilar apex regions.
Keywords: Anterior clinoid, carotico-oculomotor triangle, clinoidectomy, extradural
Removal of the anterior clinoid process (ACP) was initially reported by Hauser and Gass1 and was further refined by Drake2 and Yasargil et al3 as an intradural procedure. Dolenc4 pioneered the extradural technique of complete anterior clinoidectomy (AC) for approaching the cavernous sinus for the direct management of carotid-cavernous fistulas and intracavernous aneurysms. Later, the application of this technique was extended for cavernous sinus tumors, basilar tip aneurysms,5,6,7,8 craniopharyngiomas, peri- and suprasellar meningiomas, and giant pituitary adenomas in selected cases.9,10,11,12
Despite the wide use of AC in clinical settings,2,3,11,13,14,15,16,17,18 few studies have attempted to quantify the spatial advantages of removing the ACP.8,13,19,20 The present cadaveric study was conducted to quantify and compare the area of the carotico-oculomotor triangle (COT) before and after extradural AC.
MATERIALS AND METHODS
Ten cadaveric adult heads (five males and five females) were injected and preserved in a solution of formalin and glutaraldehyde. The same investigator took all measurements before bilaterally and after AC (total, 20 sets).
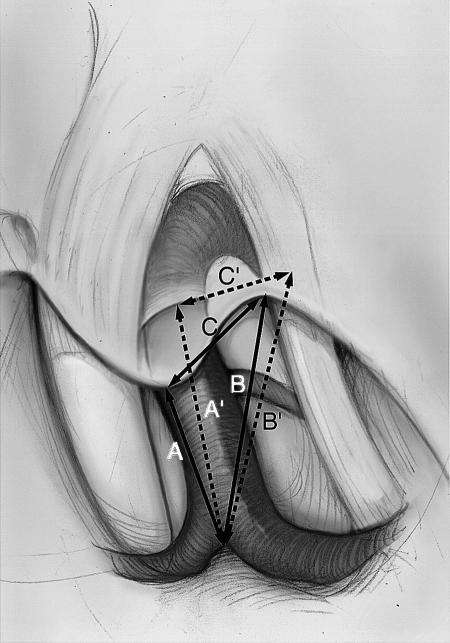
The specimens were fixed in a three-point Mayfield clamp (Ohio Medical Instrument Company Inc., Cincinnati, OH). The head was rotated 30 degrees to the side contralateral to the incision. A standard frontotemporal skin incision was performed followed by a craniotomy. The dura was opened in a semicircular fashion and elevated from the sphenoid ridge. The sylvian fissure was dissected carefully, while retraction of the frontal and temporal lobes was held constant. The optic nerve (CN II), internal carotid artery (ICA), oculomotor nerve (CN III), and the COT were exposed (Figs. 1 and 2). The in situ pre-AC measurements consisted of (1) the distance from the ICA bifurcation to the tip of the ACP (A), (2) the distance from the ICA bifurcation to the point where CN III becomes obscured by the tentorial incisura (B), and (3) the distance from the tip of the ACP to the point where CN III becomes obscured by the tentorial incisura (C) (Fig. 2, solid lines).
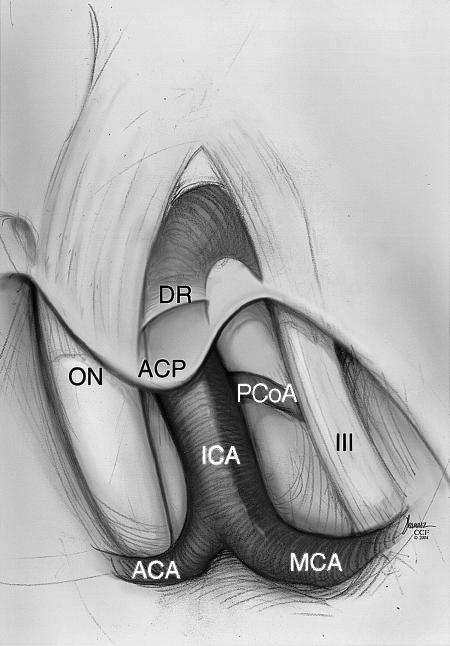
Figure 1.
Illustration of the relevant anatomy surrounding the right carotico-oculomotor triangle. ACP, anterior clinoid process; DR, distal carotid dural ring; ON, optic nerve; ICA, internal carotid artery; MCA, middle cerebral artery; PCoA, posterior communicating artery; III, cranial nerve III; ACA, anterior cerebral artery. Copyright © 2005 by The Cleveland Clinic Foundation, Cleveland, Ohio. Reprinted with permission.
Figure 2.
Illustration of the carotico-oculomotor triangle before (ΔABC) and after (ΔA'B'C') complete extradural anterior clinoidectomy (Δ, triangle). Copyright © 2005 by The Cleveland Clinic Foundation, Cleveland, Ohio. Reprinted with permission.
The ACP was then removed extradurally as follows. The lateral sphenoid ridge was flattened, and the dura was separated from the ACP. A limited posterior orbitotomy, bony decompression of the superior orbital fissure, and unroofing of the optic canal were performed. The ACP was removed by fracturing its last attachment at the optic strut. Finally, the optic sheath was opened distally to the annulus of Zinn.
After the ACP, the second measurements were as follows: (1) the distance from the ICA bifurcation to the distal dural ring of ICA (A′), (2) the distance from the ICA bifurcation to the porus oculomotoris (B′), and (3) the distance from the distal dural ring of the ICA to the porus oculomotoris (C′) (Fig. 2, broken lines).
The measurements were made directly using a microcaliper and recorded in millimeters. The area of the COT was calculated using Heron’s method (i.e., area of a triangle in mm2: S = 2s(s − a)(s − b)(s − c); where S is the area of a triangle; 2s = a + b + c; and a, b, c each represent the length of each side of the triangle, respectively) before and after AC. The mean change in area of the COT was calculated by subtracting the mean values of its area before and after AC (Table 1).
Table 1.
Measurements before (A-B-C) and after (A′-B′-C′) Extradural Anterior Clinoidectomy*
| Cadaver | A | B | C | ΔA-B-C Area (mm2) | A′ | B′ | C′ | ΔA′-B′-C′ Area (mm2) |
|---|---|---|---|---|---|---|---|---|
| R, right; L, left; SD, standard deviation. | ||||||||
| 1R | 9 | 6 | 7 | 20.98 | 13 | 10 | 9 | 44.90 |
| 1L | 10 | 6 | 6 | 16.58 | 13 | 9 | 8 | 35.50 |
| 2R | 9 | 8 | 6 | 23.53 | 15 | 12 | 10 | 59.81 |
| 2L | 8 | 6 | 6 | 17.89 | 14 | 9 | 9 | 39.60 |
| 3R | 10 | 7 | 6 | 20.66 | 13 | 9 | 8 | 35.50 |
| 3L | 9 | 6 | 5 | 14.14 | 13 | 8 | 7 | 24.25 |
| 4R | 10 | 10 | 8 | 36.66 | 14 | 12 | 9 | 53.51 |
| 4L | 9 | 7 | 7 | 24.13 | 15 | 9 | 10 | 43.61 |
| 5R | 7 | 9 | 8 | 26.83 | 14 | 11 | 9 | 49.48 |
| 5L | 8 | 9 | 7 | 26.83 | 12 | 11 | 10 | 51.52 |
| 6R | 10 | 8 | 7 | 27.81 | 13 | 10 | 8 | 39.98 |
| 6L | 9 | 8 | 8 | 29.77 | 13 | 9 | 10 | 44.90 |
| 7R | 9 | 8 | 7 | 26.83 | 14 | 9 | 12 | 53.51 |
| 7L | 8 | 9 | 8 | 29.77 | 12 | 11 | 10 | 51.52 |
| 8R | 9 | 9 | 8 | 32.25 | 14 | 12 | 9 | 53.51 |
| 8L | 9 | 7 | 9 | 29.02 | 13 | 10 | 12 | 57.00 |
| 9R | 9 | 7 | 7 | 24.13 | 14 | 9 | 8 | 33.67 |
| 9L | 10 | 9 | 7 | 30.59 | 14 | 9 | 9 | 39.60 |
| 10R | 11 | 9 | 8 | 35.50 | 13 | 9 | 10 | 44.90 |
| 10L | 10 | 8 | 8 | 31.23 | 13 | 10 | 9 | 44.90 |
| Mean | 9.15 | 7.80 | 7.15 | 26.26 | 13.45 | 9.90 | 9.30 | 45.06 |
| SD | 0.933 | 1.240 | 0.988 | 6.048 | 0.826 | 1.210 | 1.261 | 8.917 |
All measurements are in millimeters (mm).
RESULTS
The mean values and standard deviation (SD) of each measurement for each cadaveric dissection are shown in Table 1. The combined mean values (± SD) of the right and left sides before and after extradural AC were as follows: (1) A = 9.15 ± 0.93 mm and A′ = 13.45 ± 0.82 mm, (2) B = 7.80 ± 1.24 mm and B′ = 9.90 ± 1.21 mm, and (3) C = 7.15 ± 0.99 mm and C′ = 9.3 ± 1.26 mm, respectively. The area of COT (± SD) before and after extradural AC was as follows: ΔABC = 26.26 ± 6.05 mm2 and ΔA′B′C′ = 45.06 ± 8.92 mm2, respectively.
DISCUSSION
The anatomy of the ACP and surrounding structures has been studied extensively.4,5,12,13,14,16,21 The ACP is located at the medial end of the lesser wing of the sphenoid bone and forms the lateral wall of the intracranial end of the optic canal.13 Its average length is 7.7 mm and its average width is 4 mm.21 A normal ACP is usually composed of a thin shell of cortical and inner trabecular bone21; however, it may contain air cells in communication with the sphenoid sinus in 6.4 to 14.5% of the cases.12,22 The AC creates an anatomical space that does not exist when the clinoid is intact.12,21 The floor of this space, the clinoid space, is occupied by the clinoid segment of the ICA. The optic sheath is located medially, and the superior orbital fissure is located laterally.
Within the cavernous sinus, the CN III lies between the two layers of the dura forming the lateral wall of the cavernous sinus. It pierces the roof of the cavernous sinus lateral to the posterior clinoid process and courses in the upper part of the lateral wall. It exits the cavernous sinus by passing below the lower margin of the ACP to enter the superior orbital fissure. This nerve provides a good landmark for opening the lateral wall of the cavernous sinus.13 The layer of dura that lines the lower margin of the clinoid and separates it from the CN III and extends medially to form the lateral part of the lower dural ring is called the oculomotor membrane.23 The triangular space between the ICA and CN III, which is called the COT, has been an important surgical corridor for accessing neoplastic lesions of the parasellar, suprasellar, and paracavernous spaces as well as vascular lesions of the basilar trunk and apex. An extradural AC, by enlarging the COT window, enhances access to such locations, enabling more aggressive tumor removal or easier aneurysm clipping.
Scant data are available on the morphometric analysis of extradural AC. We believe that it is important to support clinical experience with cadaveric data. Evans and colleagues19 have demonstrated that an AC increases the exposure of the optic nerve two-fold and increases the width of the opticocarotid triangle three- to four-fold. They did not observe a marked increase of ICA exposure, but opening the distal dural ring enhanced its mobilization. In the present study, extradural removal of the ACP led to an almost two-fold increase in the area of the COT. Youssef and associates8 have also reported a 44% increase in the carotico-oculomotor distance by adding an AC to the standard pterional approach. Additional retraction of the CN II and the ICA, facilitated by an AC during an actual surgery, can dramatically increase the surgical window.
CONCLUSIONS
An extradural AC increases the surgical exposure of the COT by almost two-fold. Enlargement of this window facilitates not only the management of parasellar and paracavernous tumors but also vascular lesions of the basilar artery. This technique improves the working area, early visualization, decompression, and mobilization of the structures that define its borders, and reduces the risk of intraoperative neurovascular injury.
REFERENCES
- Hauser M J, Gass H. Optic nerve pressure by aneurysm relieved by decompression of optic nerve: report of a case. AMA Arch Ophthalmol. 1952;48:627–631. doi: 10.1001/archopht.1952.00920010638011. [DOI] [PubMed] [Google Scholar]
- Drake C G. The surgical treatment of aneurysms of the basilar artery. J Neurosurg. 1968;29:436–446. doi: 10.3171/jns.1968.29.4.0436. [DOI] [PubMed] [Google Scholar]
- Yasargil M G, Gasser J C, Hodosh R M, Rankin T V. Carotid-ophthalmic aneurysms: direct microsurgical approach. Surg Neurol. 1977;8:155–165. [PubMed] [Google Scholar]
- Dolenc V V. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985;62:667–672. doi: 10.3171/jns.1985.62.5.0667. [DOI] [PubMed] [Google Scholar]
- Dolenc V V, Skrap M, Sustersic J, Skrbec M, Morina A. A transcavernous-transsellar approach to the basilar tip aneurysms. Br J Neurosurg. 1987;1:251–259. doi: 10.3109/02688698709035309. [DOI] [PubMed] [Google Scholar]
- Dolenc V. Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983;58:824–831. doi: 10.3171/jns.1983.58.6.0824. [DOI] [PubMed] [Google Scholar]
- Sato S, Sato M, Oizumi T, et al. Removal of anterior clinoid process for basilar tip aneurysm: clinical and cadaveric analysis. Neurol Res. 2001;23:298–303. doi: 10.1179/016164101101198523. [DOI] [PubMed] [Google Scholar]
- Youssef A S, Aziz K MA, Kim E Y, Keller J T, Zuccarello M, Loveren H R van. The carotid-oculomotor window in exposure of upper basilar artery aneurysms: a cadaveric morphometric study. Neurosurgery. 2004;54:1181–1187. discussion 1187–1189. doi: 10.1227/01.neu.0000119757.28390.98. [DOI] [PubMed] [Google Scholar]
- Al-Mefty O. Clinoidal meningiomas. J Neurosurg. 1990;73:840–849. doi: 10.3171/jns.1990.73.6.0840. [DOI] [PubMed] [Google Scholar]
- el-Kalliny M, Loveren H van, Keller J T, Tew J M., Jr Tumors of the lateral wall of the cavernous sinus. J Neurosurg. 1992;77:508–514. doi: 10.3171/jns.1992.77.4.0508. [DOI] [PubMed] [Google Scholar]
- Lee J H, Jeun S S, Evans J, Kosmorsky G. Surgical management of clinoidal meningiomas. Neurosurgery. 2001;48:1012–1019. discussion 1019–1021. doi: 10.1097/00006123-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Yonekawa Y, Ogata N, Imhof H G, et al. Selective extradural anterior clinoidectomy for supra- and parasellar processes. Technical note. J Neurosurg. 1997;87:636–642. doi: 10.3171/jns.1997.87.4.0636. [DOI] [PubMed] [Google Scholar]
- Inoue T, Rhoton A L, Jr, Theele D, Barry M E. Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery. 1990;26:903–932. doi: 10.1097/00006123-199006000-00001. [DOI] [PubMed] [Google Scholar]
- Day A L. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. 1990;72:677–691. doi: 10.3171/jns.1990.72.5.0677. [DOI] [PubMed] [Google Scholar]
- Day J D, Giannotta S L, Fukushima T. Extradural temporopolar approach to lesions of the upper basilar artery and infrachiasmatic region. J Neurosurg. 1994;81:230–235. doi: 10.3171/jns.1994.81.2.0230. [DOI] [PubMed] [Google Scholar]
- Coscarella E, Baskaya M K, Morcos J J. An alternative extradural exposure to the anterior clinoid process: the superior orbital fissure as a surgical corridor. Neurosurgery. 2003;53:162–166. discussion 166–167. doi: 10.1227/01.neu.0000068866.22176.07. [DOI] [PubMed] [Google Scholar]
- Hakuba A, Tanaka K, Suzuki T, Nishimura S. A combined orbitozygomatic infratemporal epidural and subdural approach for lesions involving the entire cavernous sinus. J Neurosurg. 1989;71:699–704. doi: 10.3171/jns.1989.71.5.0699. [DOI] [PubMed] [Google Scholar]
- Sundt T M, Jr, Piepgras D G. Surgical approach to giant intracranial aneurysms. Operative experience with 80 cases. J Neurosurg. 1979;51:731–742. doi: 10.3171/jns.1979.51.6.0731. [DOI] [PubMed] [Google Scholar]
- Evans J J, Hwang Y S, Lee J H. Pre- versus post-anterior clinoidectomy measurements of the optic nerve, internal carotid artery, and opticocarotid triangle: a cadaveric morphometric study. Neurosurgery. 2000;46:1018–1021. discussion 1021–1023. [PubMed] [Google Scholar]
- Day J D, Tsachabitscher M. Microsurgical Dissection of the Cranial Base. New York, NY: Churchill Livingstone; 1996. pp. 39–42.
- Kim J M, Romano A, Sanan A, Loveren H R van, Keller J T. Microsurgical anatomic features and nomenclature of the paraclinoid region. Neurosurgery. 2000;46:670–680. discussion 680–682. doi: 10.1097/00006123-200003000-00029. [DOI] [PubMed] [Google Scholar]
- Huynh-Le P, Natori Y, Sasaki T. Surgical anatomy of the anterior clinoid process. J Clin Neurosci. 2004;11:283–287. doi: 10.1016/j.jocn.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Seoane E, Rhoton A L, Jr, Oliveira E de. Microsurgical anatomy of the dural collar (carotid collar) and rings around the clinoid segment of the internal carotid artery. Neurosurgery. 1998;42:869–884. discussion 884–886. doi: 10.1097/00006123-199804000-00108. [DOI] [PubMed] [Google Scholar]