Abstract
Purpose
Adrenomedullin (AM), a peptide produced by various cells, exerts diverse physiological effects and is regulated by receptor activity-modifying proteins (RAMP2 and 3). Experimental autoimmune uveitis (EAU) is a well-established model for studying human autoimmune uveitis. Hence, we investigated the pathophysiological roles of the AM-RAMP system in uveitis using an optimized EAU mouse model.
Methods
Female wild-type (WT), AM knockout, RAMP2KO, and RAMP3KO mice were immunized with the human interphotoreceptor retinoid-binding protein. The expression of macrophage-related genes and inflammatory cytokines in the retina and spleen was analyzed using real-time polymerase chain reaction. EAU-induced WT mice received human recombinant AM; therapeutic effects were evaluated via clinical and histologic scores, quantification of T-cell and macrophage infiltration in the retina, and the number of splenic regulatory T cells (Tregs) and M2 macrophages using flow cytometry.
Results
Compared with WT mice, EAU-induced AMKO and RAMP2KO mice had significantly increased retinal inflammatory cell infiltration and worsened clinical scores, whereas RAMP3KO mice did not. Proinflammatory cytokine expression was suppressed in the retina of EAU-induced WT mice that received AM. However, anti-inflammatory cytokine expression was upregulated compared with that in the vehicle group. Additionally, there was reduced retinal infiltration of T cells and macrophages, leading to improved clinical and histologic scores. AM administration also suppressed EAU-induced splenomegaly and increased the number of Tregs and M2 macrophages, possibly contributing to resolving inflammation.
Conclusions
AM exerts an anti-inflammatory effect in uveitis by activating Tregs and M2 macrophages through RAMP2. Its administration is a potential adjunctive therapy for uveitis.
Keywords: adrenomedullin, EAU, macrophages, T cells, tissue repair
Uveitis is a sight-threatening ocular inflammatory disorder characterized by retinal vascular leakage, inflammatory lesions, and macular edema,1–4 accounting for 5% to 20% of legal blindness in the United States and Europe and approximately 25% of blindness in developing nations.5,6 Etiologically, it can be classified as infectious and noninfectious, with noninfectious uveitis (NIU) being the most common, which tends to recur once triggered and often has a chronic course.7,8 The etiopathogenesis of NIU remains unclear; however, a combination of polygenic and environmental factors may contribute to the pathogenesis by disrupting the balance between inflammatory and regulatory immune mechanisms.9,10
Notably, experimental autoimmune uveitis (EAU) is an animal model that is widely used because it replicates many clinicopathologic features of human autoimmune uveitis.11 The etiology of EAU closely resembles human uveitis based on an autoimmune nature, in which patients show immunologic responses of circulating lymphocytes to retinal antigens. In the murine model, EAU is triggered by the systemic activation of ocular-specific CD4-positive T cells, which are typically located in or around the photoreceptor segments.12 EAU is mediated by pathologic T cells, particularly T helper 1 (Th1) and Th17 cells, which are derived from naive T cells in response to exposure to proinflammatory cytokines.13 This model facilitates the investigation of the underlying mechanisms of uveitis and the evaluation of potential therapeutics for ocular inflammatory diseases.14–16 However, current treatment options, primarily systemic corticosteroids, are limited by systemic side effects. Additionally, they can lead to ocular complications such as cataracts and glaucoma.17–23 Furthermore, immunosuppressive drugs help to control the disease with lower corticosteroid doses. However, drugs such as cyclosporine may cause side effects that are not associated with steroid use, such as proximal tubular damage.24–27 Additionally, biological agents, including monoclonal antibodies, are a relatively new therapeutic class; however, achieving stable anti-inflammatory effects with these agents may require several months. Moreover, some patients may be refractory to these treatments or encounter adverse drug reactions.28–34 Notably, biologics may increase the risk of opportunistic infections when combined with corticosteroids or immunosuppressive drugs.35
Adrenomedullin (AM) is a potential therapeutic avenue that is underexplored in uveitis. It is a bioactive peptide isolated from human pheochromocytoma.36 It belongs to the calcitonin superfamily and acts through the calcitonin receptor–like receptor (CLR), regulated by three receptor activity-modifying proteins (RAMPs): RAMP1, RAMP2, and RAMP3. CLR-RAMP2 and CLR-RAMP3 complexes have a high affinity for AM.37 Various physiological effects are attributable to AM, including anti-inflammatory, antiapoptotic, and antioxidative stress effects,38–41 which contribute to homeostasis across various organs and tissues,39,42 including the eyes.43
We previously reported that AM ameliorates pathologic angiogenesis and hyperpermeability in the retina in ophthalmic disease models of diabetic retinopathy44 and retinal vein occlusion (RVO).45 Additionally, we reported that AM improves the pathogenesis of neovascular age-related macular degeneration (AMD).46 In the RVO model, AM and RAMP2 knockout (KO) mice had more severe phenotypes, such as retinal hemorrhage and vein dilatation with tortuousness, than the other mice. Furthermore, AM administration reduced inflammation and improved vascular reperfusion in the RVO-induced wild-type (WT) mice.45
In the AMD model, the AM KO and RAMP2 KO mice showed increased laser-induced choroidal neovascularization, fibrosis, and inflammation compared with those in the WT mice. Notably, exogenous AM administration suppressed these pathologic features and downregulated fibrosis-related molecules.46 These findings highlight the potential of the AM-RAMP2 system as a novel therapeutic target for RVO and AMD.
We hypothesized that the AM-RAMP2 system could have therapeutic value in uveitis, considering that the condition involves abnormal autoimmune responses and shares inflammatory and vascular permeability features with RVO and AMD. However, the specific effects of AM on local inflammation in uveitis, including its role in T-cell differentiation and macrophage recruitment, remain unclear. Herein, we optimized experimental conditions to enhance the reproducibility of the EAU model and conducted investigations, including EAU-induced AM, RAMP2, and RAMP3 KO mice and exogenous AM administration in EAU-induced WT mice, to investigate the therapeutic potential of the AM-RAMP2 and AM-RAMP3 systems for autoimmune uveitis.
Methods
Animals
Eight-week-old female C57BL/6J WT mice were obtained from a supplier of laboratory animals (Charles River Laboratories Japan, Kanagawa, Japan). The AM,47 RAMP2,48 and RAMP349 KO mice were previously generated by our group. AM KO mice were generated using C57BL/6J and 129 hybrids, RAMP2 KO mice were generated using C57BL/6J, and RAMP3 KO mice were generated using C57BL/6J and 129 hybrids but crossed back to the C57BL/6J strain after five generations. Therefore, it is not appropriate to simply compare the WT (+/+) in the two KO mouse lines. Each KO mouse must be compared to the WT (+/+) in its respective littermate. To make this distinction clear, we have labeled the WT model “+/+” as opposed to “±” for the heterozygous model. However, as we previously reported that generating homozygous AM (−/−) and RAMP2 (−/−) mice is embryonically lethal because of systemic edema and hemorrhage, which is primarily caused by abnormal vascular development,47 heterozygous AM (±) and RAMP2 (±) mice were used. In these mice, the affected gene expression is reduced to approximately half of that in WT mice.48 The following mice were used in this study: 8- to 10-week-old female AM (±), RAMP2 (±), RAMP3 (−/−), and WT mice, along with C57BL/6J WT mice that received systemic AM administration using osmotic pumps. All mice were bred and maintained under specific pathogen-free conditions, with controlled circadian rhythms, temperature, and humidity. The mice were anesthetized via intraperitoneal injection comprising an anesthetic that included 0.3 mg/kg medetomidine (Nippon Zenyaku Kogyo, Koriyama, Japan), 4.0 mg/kg midazolam (Astellas Pharma, Tokyo, Japan), and 5.0 mg/kg butorphanol (Meiji Seika Pharma, Tokyo, Japan). All experiments adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and our institutional guidelines. Furthermore, all animal handling procedures were approved by the Ethics Committee of Shinshu University School of Medicine (#024040, #024041).
AM Administration in Mice
Human AM (Peptide Institute, Osaka, Japan), dissolved in phosphate-buffered saline (PBS), was infused into subcutaneous tissues using osmotic pumps (Alzet; DURECT, Cupertino, CA, USA) immediately after EAU induction. The delivery rate was set at 29 µg/kg/d, according to the doses in our previous RVO45 and AMD46 models, where no adverse effects from AM treatment were observed, and the mice received AM for 7 or 14 days (Fig. 1A). The PBS-treated mice served as controls.
Figure 1.
Protocol and optimization of the EAU model by fine-tuning various factors. (A) The timeline of immunization with hIRBP and administration of AM or PBS using an osmotic pump for C57BL/6J mice. Additionally, different sacrifice time points after immunization are shown. (B) Eyes are photographed using a portable camera on day 14 postimmunization. (C) To induce EAU, hIRBP is suspended in PBS and emulsified with an equal volume of CFA. Emulsion quality is satisfactory if the extruded emulsion does not separate and remains as droplets on a water surface for >2 hours. (D) Mice are immunized by injecting 80 µL of the emulsion mixture subcutaneously at approximately 1 cm from the base of the tail. Successful administration results in a white band discoloration at the injection site. (E) Fundus photographs representing a range of disease scores. (F) HE staining of the retinal section represents a range of disease scores. (G, H) Anterior ocular segment appearance of EAU before and after fine-tuning the reagent volume (G); the fundus or even pupil is obscured due to severe hyphema, mature cataract, or corneal opacity. After fine-tuning the reagent volume (H), a translucent appearance with the pupil and fundus is clearly visible. (I) Representative photographs and EAU clinical scores for mice in the no-treatment control (n = 10) and topical steroid treatment (n = 10) groups at 14 days after EAU induction. The clinical scores for EAU determined from the fundus photographs are indicated. Yellow arrowheads, retinal linear exudate; red arrows, retinal vessel engorgement. Bars: mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired t-test).
Topical Endoscopy Fundus Imaging
The mice were anesthetized, as previously described, at 14 days post-EAU induction. The pupils were dilated using topical 0.5% phenylephrine and 0.5% tropicamide (Mydrin-P; Santen, Osaka, Japan). Eye gel (Scopisol; Senju, Osaka, Japan) was introduced into the cornea to facilitate contact with a digital medical scope (VersaCam; NIDEK, Aichi, Japan). The endoscope's position was adjusted by horizontally moving its tip against the gel-covered cornea. Subsequently, focus and illumination were adjusted, and the images of the fundus were obtained (Fig. 1B).50
EAU Induction
EAU was induced in 8- to 10-week-old female mice using a subcutaneous injection of 200 µg of an amine-terminal peptide fragment (residues 1–20, GPTHLFQPSLVLDMAKVLLD) of human interphotoreceptor retinoid-binding protein (hIRBP) (MedChemExpress, Middlesex County, NJ, USA), which was emulsified in complete Freund's adjuvant (CFA) containing 2 mg/mL Mycobacterium tuberculosis H37Ra (Iwai Chemicals, Tokyo, Japan).51–54 Equal volumes of hIRBP and CFA were mixed thoroughly (approximately 2 minutes of continuous vortexing with 3- to 5-minute intervals between sets for 10–15 sets) until homogeneity was achieved. The final emulsion did not separate when dripped onto a water surface (Fig. 1C). This emulsion (80 µL in total) was injected subcutaneously at approximately 1 cm from the base of the tail (Fig. 1D). The fluid entry site showed a slight discoloration after successful administration. Concurrently, 1.2 µg pertussis toxin (PTX) (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was administered intraperitoneally.
Scoring of EAU
Topical endoscopy fundus imaging was performed as described above. The EAU clinical score was graded based on the photographs by retinal specialists in a blinded manner, using a 0 to 5 scale to evaluate disease severity. This was based on previous reports with some modifications (Fig. 1E; Table 1).55–61
Table 1.
The Clinical Score for Experimental Autoimmune Uveitis
| Score | Description |
|---|---|
| Score 0 | No evidence of inflammation |
| Score 1 | Retinal vessel engorgement without exudate |
| Score 2 | Retinal vessel engorgement and/or spotted exudate of fewer than five spots |
| Score 3 | Retinal vessel engorgement and/or spotted exudate of more than five spots |
| Score 4 | Retinal vessel engorgement and/or linear exudates along with vasculitis |
| Score 5 | Exudative retinal detachment or subretinal hemorrhage |
Histologic Examination
The mice were euthanized by cervical dislocation 14 days post-EAU induction for histologic assessment. Eyeballs were removed, fixed in 4% paraformaldehyde, and processed into paraffin-embedded sections (5 µm thick) along the papillary–optic nerve plane. The sections were stained with hematoxylin and eosin (HE) and examined using a microscope (BZX710; Keyence, Osaka, Japan). Next, HE-stained eye sections were magnified 4× and photographed to include the entire eye. The EAU severity was scored for each eye using a 0 to 4 grading, based on previous reports with some modifications (Fig. 1F; Table 2),55–61 and subsequently quantified using a BZ analyzer (Keyence). For CD3 or F4/80 staining, the number of positive cells was counted in a 400× field of view. The data were obtained in a blinded manner.
Table 2.
The Histologic Score for Experimental Autoimmune Uveitis
| Score | Description |
|---|---|
| Score 0 | No retinal destruction and normal retinal architecture |
| Score 1 | One retinal fold without the presence of retinal detachment |
| Score 2 | Two retinal folds or one focal retinal detachment* |
| Score 3 | More than two focal retinal detachments |
| Score 4 | Diffuse retinal detachment |
Two retinal folds are equivalent to one focal retinal detachment.
Quantitative Reverse Transcription Polymerase Chain Reaction Analysis
cDNA was synthesized from total RNA, which was extracted from the retina or spleen. This was followed by quantitative reverse transcription polymerase chain reaction (qRT-PCR) to quantify relative mRNA levels using specific primers. The detailed methods of the cDNA synthesis, primer design, and qRT-PCR conditions are provided in the Supplementary Methods. PCR primers are listed in Table 3.
Table 3.
Primers Used for Real-Time PCR
| Primer Name | Primer Type | Primer Sequence |
|---|---|---|
| Adm | Forward | 5′-GGACACTGCAGGGCCAGAT-3′ |
| (adrenomedullin) | Reverse | 5′-GTAGTTCCCTCTTCCCACGACTTA-3′ |
| Calcrl | Forward | 5′-AGGCGTTTACCTGCACACACT-3′ |
| (CLR) | Reverse | 5′-CAGGAAGCAGAGGAAACCCC-3′ |
| Ramp2 | Forward | 5′-ACTGAGGACAGCCTTGTGTCAAA-3′ |
| (RAMP2) | Reverse | 5′-CCTTGACAGAGTCCATGCAACTC-3′ |
| Ramp3 | Forward | 5′-AAAGCCTTCGCTGACATGATG-3′ |
| (RAMP3) | Reverse | 5′-ATCTCGGTGCAGTTAGTGAAGCT-3′ |
| Il1b | Forward | 5′-CTACAGGCTCCGAGATGAACAAC-3′ |
| (IL-1b) | Reverse | 5′-TCCATTGAGGTGGAGAGCTTTC-3′ |
| Il4 | Forward | 5′-TCATCGGCATTTTGAACGAG-3′ |
| (IL-4) | Reverse | 5′-CGAGCTCACTCTCTGTGGTG-3′ |
| Il6 | Forward | 5′-CCCAATTTCCAATGCTCTCC-3′ |
| (IL-6) | Reverse | 5′-TGAATTGGATGGTCTTGGTCC-3′ |
| Il10 | Forward | 5′-CAGCCGGGAAGACAATAACTG-3′ |
| (IL-10) | Reverse | 5′-CCGCAGCTCTAGGAGCATGT-3′ |
| Il13 | Forward | 5′-CCTCTGACCCTTAAGGAGCTT-3′ |
| (IL-13) | Reverse | 5′-ATGTTGGTCAGGGAATCCAG-3′ |
| Il17a | Forward | 5′-TGGCGCAAAAGTGAGCTCCAGAAG -3′ |
| (IL-17A) | Reverse | 5′-CGGCACTGAGCTTCCCAGATCAC -3′ |
| Ifng | Forward | 5′-TCAAGTGGCATAGATGTGGAAGAA-3′ |
| (IFN-g) | Reverse | 5′-AGAGATAATCTGGCTCTGCAGGAT-3′ |
| Tnf | Forward | 5′-ACGGCATGGATCTCAAAGAC-3′ |
| (TNF-a) | Reverse | 5′-AGATAGCAAATCGGCTGACG-3′ |
| Ccl2 | Forward | 5′-GCAGTTAACGCCCCACTCA-3′ |
| (MCP-1) | Reverse | 5′-CCTACTCATTGGGATCATCTTGCT-3′ |
| Cd3e | Forward | 5′-ACGATGCCGAGAACATTGAATA-3′ |
| (CD3e) | Reverse | 5′-CATGCTTCTGAGGCAGCTCTT-3′ |
| Cd4 | Forward | 5′-AGGAAGTGAACCTGGTGGTG-3′ |
| (CD4) | Reverse | 5′-CTCCTGCTTCAGGGTCAGTC-3′ |
| Cd8a | Forward | 5′-CTTCCAGAACTCCAGCTCCAAA-3′ |
| (CD8) | Reverse | 5′-GTGTCCCTCATGGCAGAAAAC-3′ |
| Foxp3 | Forward | 5′-CTGCATCGTAGCCACCAGTA-3′ |
| (Foxp3) | Reverse | 5′-TGGAAGAACTCTGGGAAGGA-3′ |
| Itga | Forward | 5′-CTGGATAGCCTTTCTTCTGCTG-3′ |
| x (CD11c) | Reverse | 5′-GCACACTGTGTCCGAACTC-3′ |
| Mrc1 | Forward | 5′-TCAGCTATTGGACGCGAGGCA-3′ |
| (CD206) | Reverse | 5′-TCCGGGTTGCAAGTTGCCGT-3′ |
| Adgre1 | Forward | 5′-GATGAATTCCCGTGTTGTTGGT-3′ |
| (F4/80) | Reverse | 5′-ACATCAGTGTTCCAGGAGACACA-3′ |
| Nos2 | Forward | 5′-ATGTGGCTACCACATTGAAGAAGC-3′ |
| (iNOS) | Reverse | 5′-AAGACTGCACCGAAGATATCTTCATG-3′ |
Transcriptome Analysis and Data Processing
The transcriptome analysis was conducted using a mouse Clariom S array (Thermo Fisher Scientific, Waltham, MA, USA). The complete description of this analysis and data processing are provided in Supplementary Methods.
Flow Cytometry
Single-cell suspensions from spleens were prepared, followed by viability staining and Fc receptor blocking. Subsequently, fluorochrome-conjugated antibodies were used to stain specific cell populations for analysis using fluorescence-activated cell sorting (FACS). Detailed procedures are provided in Supplementary Methods. The regents and fluorochrome-conjugated antibodies used are listed in Table 4. Gating was performed as shown in Supplementary Figs. S1A, S2.
Table 4.
Antibodies Used for Fluorescence-Activated Cell Sorting
| Name of Antibody | RRID | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Host Species, Monoclonal or Polyclonal |
|---|---|---|---|
| F4/80 | AB_2563102 | BioLegend (San Diego, CA, USA), #123137 | Rat, monoclonal |
| CD11b | AB_2629529 | BioLegend (San Diego, CA, USA), #101263 | Rat, monoclonal |
| CX3CR1 | AB_2565707 | BioLegend (San Diego, CA, USA), #149025 | Mouse, monoclonal |
| CD4 | AB_2563054 | BioLegend (San Diego, CA, USA), #100548 | Rat, monoclonal |
| CD11c | AB_2562414 | BioLegend (San Diego, CA, USA), #117339 | American hamster, monoclonal |
| Ly6c | AB_2565852 | BioLegend (San Diego, CA, USA), #128041 | Rat, monoclonal |
| MHCⅡ | AB_313320 | BioLegend (San Diego, CA, USA), #107605 | Rat, monoclonal |
| CD45 | AB_893340 | BioLegend (San Diego, CA, USA), #103131 | Rat, monoclonal |
| CD206 | AB_2562247 | BioLegend (San Diego, CA, USA), #141719 | Rat, monoclonal |
| SiglecF | AB_2750236 | BioLegend (San Diego, CA, USA), #155507 | Rat, monoclonal |
| Ly6G | AB_10643269 | BioLegend (San Diego, CA, USA), #127622 | Rat, monoclonal |
| iNOS | AB_2572641 | eBioscience (Carlsbad, CA, USA), #12592080 | Rat, monoclonal |
| CD3 | AB_394595 | BD Bioscience (Franklin Lakes, NJ, USA), #553061 | American hamster, monoclonal |
| Foxp3 | AB_465936 | eBioscience (Carlsbad, CA, USA), #12577382 | Rat, monoclonal |
| Anti-CD16/CD32 (Mouse Fc Block) (2.4G2) | AB_394656 | BD Bioscience (Franklin Lakes, NJ, USA), #553142 | Rat, monoclonal |
RRID, Research Resource Identifier.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 9.3.1 (GraphPad Software, La Jolla, CA, USA). The values are expressed as mean ± SEM. A t-test and one-way analysis of variance, followed by Dunnett's test, were used to determine significance levels. Statistical significance was set at P < 0.05.
Results
Optimization of the EAU Model by Fine-Tuning Various Factors
EAU is a widely used animal model of human autoimmune uveitis; however, the quantity of reagent and administration methods used to induce inflammation, along with the evaluation approaches for inflammatory findings, are significantly varied across studies. Thus, we investigated the optimal protocol for effective EAU induction in C57BL/6J mice, drawing references from previous reports.62
Initially, EAU was induced using 250 µg hIRBP and emulsified in CFA containing 2.5 mg/mL M. tuberculosis H37Ra. The mixed hIRBP–CFA emulsion (100 µL) was subcutaneously injected, followed by 1.5 µg intraperitoneal PTX. However, this approach approximately led to a 50% mortality rate among the mice, and those that survived had excessive anterior segment inflammation, which precluded fundus examination because of issues such as anterior chamber hemorrhage, posterior iris synechiae, mature cataracts, and corneal opacity (Fig. 1G).
Notably, the EAU incidence and inflammation severity are highly variable across different studies using the C57BL/6 mouse strain. The variability is attributable to inconsistent susceptibility, leading to induction uncertainties.63–66 Various factors, particularly the hIRBP1-20 peptide and PTX doses, can influence EAU incidence.67 In this study, we used a vortex device to mix hIRBP and CFA, which enhanced both speed and ease compared with the conventional method of manually mixing two syringes using a three-way stopcock.62
The injection sites for hIRBP administration also varied across studies, including the back, neck, thighs, flanks, and footpad.68–70 We selected tail injections to avoid subtle differences resulting from skin pinching and injection site variances.
Reagent volumes were fine-tuned, including reducing hIRBP to 200 µg, M. tuberculosis H37Ra in CFA to 2 mg/mL, and PTX to 1.2 µg, each by 20%. Consequently, anterior segment inflammation was no longer observed, allowing for clear visualization of the fundus (Fig. 1H).
However, the model was unsuitable for reflecting clinically observed uveitis when posterior segment inflammation was too severe for treatment, even if the anterior segment was clear and the fundus was visible. Therefore, we confirmed that this model was also responsive to treatment, as posterior segment inflammation was somewhat mitigated by topical steroids (Sanbetason; Santen, Osaka, Japan) (Fig. 1I).
Additionally, the scoring system in our model for assessing the degree of inflammation was based on specific inflammatory findings (rather than vague descriptors), reducing examiner subjectivity (Tables 1 and 2). Experiments were conducted using the EAU model refined under these conditions.
AM (±) Mice Exhibited More Pronounced Inflammatory Findings
We examined heterozygous AM (±) and AM (+/+) mice to clarify the pathophysiologic role of endogenous AM in uveitis. In the absence of EAU induction, neither AM (+/+) nor AM (±) mice showed any inflammatory change in the fundus. After EAU induction, both showed mild inflammation, including vascular engorgement and dilation. Additionally, AM (±) mice developed linear exudative lesions. Consequently, the clinical score in AM (±) mice was significantly higher than that in AM (+/+) mice (Fig. 2A). Furthermore, the number of CD3-positive T cells and F4/80-positive macrophages infiltrating the retina of AM (±) mice was significantly higher than that in the AM (+/+) mice (Figs. 2B, 2C). The expression of inflammatory cytokines (IL-1β and IL-6) in the retina tended to increase in AM (±) mice with and without EAU (Fig. 2D). Furthermore, the increase in spleen weight (along with splenomegaly) in EAU was significantly greater in the AM (±) mice than in the AM (+/+) mice (Fig. 2E and Supplementary Fig. S3). These exacerbated inflammatory findings suggest that AM considerably contributes to alleviating EAU-induced inflammation.
Figure 2.
AM (±) mice exhibited more pronounced inflammatory findings. (A) Representative fundus photographs and EAU clinical scores for mice in the AM (+/+) (n = 18) and AM (±) (n = 23) groups at 14 days after EAU induction. Naive mice (n = 6) were similarly examined. The clinical score for EAU determined from the fundus photographs is indicated. Yellow arrowheads, retinal linear exudate; red arrows, retinal vessel engorgement. (B, C) Staining for immunohistochemical analysis of CD3-positive T-cell (B) and F4/80-positive macrophage (C) infiltration at 14 days following EAU induction. The number of immune cells per retina in one cross section of the AM (+/+) (n = 6) and AM (±) (n = 5) mice was counted. Red dot circles, CD-3 positive T cells; red circles, F4/80-positive macrophages. (D) Quantitative reverse transcription PCR analysis of inflammation-related genes (IL-1β and IL-6) in retinas on day 7 after immunization (n = 6–24). (E) Body weight, spleen weight, and the spleen weight/body weight ratio (×1/1000) of control (n = 6), AM (+/+) (n = 8), and AM (±) (n = 14) mice after EAU induction. (F) Quantitative reverse transcription PCR analysis of AM and AM-related receptor genes in spleens on day 7 after immunization (n = 6–17). Bars: mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (unpaired t-test or one-way analysis of variance).
Additionally, the RAMP3 gene expression in the spleen showed near-complete depletion following either KO or EAU induction, regardless of the genotype. AM expression was halved in the heterozygous KO mice with or without inflammation, and the RAMP2 expression did not decrease or differ among groups, regardless of the presence of inflammation or genotype (Fig. 2F).
RAMP2 (±) Mice Exhibited More Pronounced Inflammatory Findings
The EAU model in the AM (±) group revealed that RAMP2 significantly contributes to EAU pathogenesis among the RAMP sub-isoforms of AM. To verify this, we examined the importance of endogenous RAMP2 using RAMP2 (±) mice. Notably, both RAMP2 (+/+) and RAMP2 (±) mice had significantly increased clinical scores, with RAMP2 (±) having a significantly higher score than that of the RAMP2 (+/+) mice (Fig. 3A).
Figure 3.
RAMP2(±) mice exhibited more pronounced inflammatory findings. (A) Representative fundus photographs and EAU clinical scores for mice in the RAMP2 (+/+) (n = 12) and RAMP2 (±) (n = 8) groups at 14 days after EAU induction. Naive mice (n = 6) were similarly examined. The clinical score for EAU determined from the fundus photographs is indicated. Yellow arrowheads, retinal linear exudate; red arrows, retinal vessel engorgement. (B, C) Staining for immunohistochemical analysis of CD3-positive T-cell (B) and F4/80-positive macrophage (C) infiltration at 14 days after EAU induction. The number of immune cells per retina in one cross section of the RAMP2 (+/+) (n = 12) and RAMP2 (±) (n = 8) mice was counted. Red dot circles, CD-3 positive T cells; red circles, F4/80-positive macrophages. (D) Quantitative reverse transcription PCR analysis of inflammation-related genes (TNF-α, IFN-γ, monocyte chemoattractant protein [MCP] 1, IL-1β, IL-6, and IL-10) in the retinas on day 7 after immunization (n = 3–8). (E) Body weight, spleen weight, and the spleen weight/body weight ratio (×1/1000) of control (n = 3–5), RAMP2 (+/+) (n = 11), and RAMP2 (±) (n = 7) mice after EAU induction. Bars: mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (unpaired t-test or one-way ANOVA).
Furthermore, significantly more CD3-positive T cells infiltrated the retinas of the RAMP2 (±) mice than that of RAMP2 (+/+) mice (Fig. 3B). Similarly, F4/80-positive macrophages tended to increase more among the RAMP2 (±) mice than in the RAMP2 (+/+) mice (Fig. 3C). In RAMP2 (±) mice with EAU, the expression of inflammatory cytokines in the retina significantly increased, and certain markers tended to be elevated, even in the absence of EAU. Only IL-13 expression tended to be higher in the RAMP2 (+/+) mice (Fig. 3D). Additionally, the increase in spleen weight due to EAU was greater in the RAMP2 (±) mice than in the RAMP2 (+/+) mice, similar to the findings in AM (±) mice (Fig. 3E), suggesting that the AM-RAMP2 system plays a role in the inflammatory response and the splenomegaly caused by EAU.
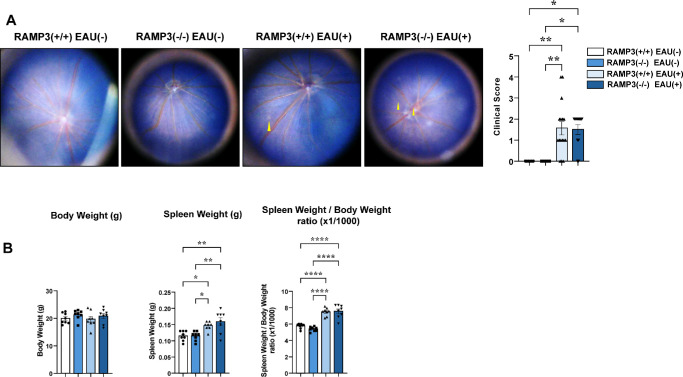
No Significant Difference Was Detected in Inflammatory Findings Between RAMP3 (+/+) and RAMP3 (−/−) Mice
RAMP3 expression in the spleen was significantly reduced in this model, suggesting that it does not significantly contribute to exacerbating EAU pathology. Subsequent experiments with RAMP3 (−/−) mice confirmed this; no significant differences were observed between the groups. After EAU induction, both RAMP3 (+/+) and RAMP3 (−/−) mice showed a significant increase in clinical scores; however, the two groups did not significantly differ (Fig. 4A). The EAU induced a significant increase in spleen weight; however, the increase did not significantly differ between RAMP3 (+/+) and RAMP3 (−/−) mice (Fig. 4B). These results confirm that, compared with the AM-RAMP2 system, the AM-RAMP3 system is less integral to the pathogenesis of EAU. However, the downregulation of RAMP3 gene expression caused by EAU induction may contribute to the lack of differences in outcomes between the two groups.
Figure 4.
No significant difference was detected in inflammatory findings between RAMP3 (+/+) and RAMP3 (−/−) mice. (A) Representative fundus photographs and EAU clinical scores for mice in the RAMP3 (+/+) (n = 16) and RAMP3 (−/−) (n = 11) groups at 14 days after EAU induction. Naive mice (n = 6) were similarly examined. The clinical score for EAU determined from the fundus photographs is indicated. Yellow arrowheads, retinal linear exudate; red arrows, retinal vessel engorgement. (B) Body weight, spleen weight, and the spleen weight/body weight ratio (×1/1000) of control, RAMP3 (+/+), and RAMP3 (−/−) mice after EAU induction (n = 8).
Exogenous AM Administration in C57BL/6J Mice Significantly Improved Various Inflammatory Outcomes
The aforementioned experiments in genetically engineered mice demonstrated that the endogenous AM-RAMP2 system ameliorates uveitis pathogenesis in the EAU model by suppressing T-cell and macrophage infiltration into the retina and reducing proinflammatory cytokine expression in the retina. Accordingly, we administered exogenous AM to C57BL/6J WT mice to explore the potential therapeutic application of AM in uveitis.
The results showed that the EAU induction significantly increased clinical scores; however, inflammatory findings, such as vasodilation and linear exudative spots, were alleviated in the AM-treated group, resulting in lower scores than those in the PBS-treated group (Fig. 5A). The infiltration of CD3-positive T cells and F4/80-positive macrophages was also significantly increased by EAU induction; however, the infiltration was significantly suppressed by AM treatment compared with that in the PBS-treated group (Figs. 5B, 5C).
Figure 5.
Exogenous adrenomedullin administration in C57BL/6J mice significantly improved various inflammatory outcomes. (A) Representative fundus photographs and EAU clinical score for mice in the control (n = 20), PBS-treated (n = 20), and AM-treated (n = 18) groups at 14 days after EAU induction. The clinical score for EAU determined from the fundus photographs is indicated. Yellow arrowheads, retinal linear exudate; red arrows, retinal vessel engorgement. (B, C) Staining for immunohistochemical analysis of CD3-positive T-cell (B) and F4/80-positive macrophage (C) infiltration at 14 days after EAU induction. The number of immune cells per retina in one cross section of the control (n = 10), PBS-treated (n = 9), and AM-treated (n = 8) mice was counted. Red dot circles, CD-3 positive T cells; red circles, F4/80-positive macrophages. (D) Representative histologic findings and EAU histologic scores for mice in the control (n = 15), PBS-treated (n = 15), and AM-treated (n = 13) groups at 14 days after EAU induction. Sections of the eye from control and EAU mice at 14 days after injection with hIRBP, stained with HE. Yellow arrows, retinal folds. Scale bars: 500 µm. (E) Quantitative reverse transcription PCR analysis of inflammation-related genes (TNF-α, IFN-γ, MCP-1, IL-1β, IL-6, and IL-10) in retinas on day 7 after immunization (n = 6–16). (F) Transcriptomic analysis of the EAU model in C57BL/6J mice identified candidate genes associated with the suppressive effects of AM. Volcano plots of the differentially expressed genes in 14-day samples by comparison between AM- and PBS-administered mice are shown (n = 2 in each). Red spots represent the upregulated genes, and blue spots represent the downregulated genes, with the fold change on the x-axis and P value on the y-axis. The gray-colored region represents the insignificantly differentially expressed genes. Gene Ontology (GO) enrichment histogram shows the top six most significantly enriched GO terms. (G) Body weight, spleen weight, and the spleen weight/body weight ratio (×1/1000) of control, PBS-, and AM-treated mice after EAU administration (n = 9–10). Bars: mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (unpaired t-test or one-way ANOVA).
In addition, HE staining revealed normal retinal structure in the absence of EAU. In the PBS-treated group, during EAU, retinal folds and exudative retinal detachment were observed; however, these findings considerably improved, and the histologic score was significantly suppressed in the AM-treated group (Fig. 5D). Furthermore, EAU induction elevated the expression levels of various inflammatory cytokines in the retina, and the AM treatment tended to suppress these compared with those in the PBS-treated group. Conversely, IL-13 and the anti-inflammatory cytokine IL-10 were significantly upregulated by AM treatment (Fig. 5E).
Moreover, transcriptome analysis of the retina 2 weeks post-EAU induction revealed enhanced activation of factors associated with cell adhesion and cytoskeletal remodeling, such as TGF-β and Smad3 (Fig. 5F). Furthermore, TGF-β and its downstream signaling molecule Smad3 suppressed inflammatory responses and prevented excessive inflammation, promoting tissue repair by inhibiting cell proliferation and extracellular matrix formation.71,72 This aligned with the improvement in exudative spots and serous retinal detachment secondary to increased vascular permeability, which led to significantly improved clinical and histological scores in the AM-treated group.
Furthermore, EAU significantly increased spleen weight; however, the AM-treated group showed significantly less weight gain compared with that in the PBS-treated group (Fig. 5G). Further examination of cell populations using splenic FACS revealed that Tregs (expressing Foxp3) and M2 macrophages (expressing CD206) were upregulated in the AM-treated group (Figs. 6A, 6B).
Figure 6.
Further examinations for the mechanism of EAU suppression by the AM-RAMP2 system. (A, B) The absolute numbers and the percentage of the population of T regs (A) and M2 macrophages (B) in spleens of PBS-treated (n = 3) and AM-treated (n = 3) C57BL/6J mice in splenic flow cytometric analysis on day 7 after immunization. (C, D) Quantitative reverse transcription PCR analysis of T-cell-related (C) and macrophage-related genes (D) in retinas on day 7 after immunization (n = 7–18). (E, F) Quantitative reverse transcription PCR analysis of macrophage-related (E) and AM and AM-related receptor genes (F) in spleens on day 7 after immunization (n = 5–11). Bars: mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (unpaired t-test or one-way ANOVA).
The increase in M2 macrophages, which promote inflammatory convergence and tissue repair, in the spleen of the AM-treated group indicates that AM has a systemic anti-inflammatory effect, consistent with the upregulated expression of Foxp3, a Treg marker, in the retina (Fig. 6C), as well as CD206, an M2 macrophage marker, in both the retina and spleen (Figs. 6D, 6E). These are also consistent with the observed improvement in inflammatory findings (scores) in the fundus and suppression of proinflammatory cytokine expression in the retina, along with reduced weight gain in the spleen. The increased expression of cytokines that activate M2 macrophages (IL-4 and, especially, IL-13) in the retina corroborated the anti-inflammatory effect.
Discussion
In humans, elevated AM levels have been observed in eyes with ocular lesions such as proliferative diabetic retinopathy, glaucoma, retinitis pigmentosa, and uveitis.73 Furthermore, CLR and RAMP2 showed similar localization patterns to AM in the retina and stimulation of isolated retinas with AM increased immunoreactivity, suggesting that the AM pathway is also associated with retinal lesions.74 Notably, high glucose-induced human retinal endothelial cell migration and proliferation were previously inhibited by AM administration.75
Additionally, AM and its receptors are expressed in the iris ciliary body, where AM binding relaxes the iris sphincter muscle and reduces intraocular pressure.76,77 Reportedly, genetic mutations in RAMP2 in mice disrupt the AM-RAMP2/CLR and cAMP signaling pathways, leading to primary open-angle glaucoma through retinal ganglion cell death.78
Similarly, in our EAU model, exogenous AM administration significantly improved clinical scores and reduced inflammatory cell infiltration and cytokine expression compared with that in the vehicle group. This suggests that exogenous AM administration ameliorates the pathogenesis of uveitis. Furthermore, EAU induction strongly suppressed RAMP3 expression, whereas RAMP2 expression was unchanged. Exogenous AM administration tended to increase RAMP2 expression, whereas RAMP3 expression remained suppressed (Fig. 6F), suggesting that AM administration ameliorates the pathogenesis of uveitis via RAMP2 but not RAMP3. Notably, AM (±) and RAMP2 (±) mice exhibited worsening clinical scores, higher inflammatory cell infiltration, and elevated inflammatory cytokine expression compared to WT mice. In contrast, compared with the WT mice, the severity of the disease, including clinical scores in the RAMP3 (−/−) mice, did not significantly differ.
In addition, AM protects against experimental autoimmune encephalomyelitis,79 arthritis,80 and inflammatory bowel disease (IBD)81 by downregulating inflammatory cytokine production and Th1 response and inducing Tregs. In addition, AM-stimulated dendritic cells had lower levels of costimulator expression and proinflammatory cytokine release.82
In the EAU model, AM administration decreased the expression of IFN-γ and IL-17, cytokines produced by Th1 and Th17 cells, respectively. However, the expression of IL-13, a cytokine derived from Th2 cells, Foxp3, a Treg marker, and IL-10, an anti-inflammatory cytokine derived from Tregs, was increased. This shift in the balance of immune responses may transition the pathogenesis of uveitis from a proinflammatory to an anti-inflammatory state, thereby improving clinical outcomes.
Furthermore, we found that AM administration significantly enhanced M2 macrophage marker (CD206) expression in the retina and spleen. Splenic FACS analysis also showed an increase in M2 macrophages. Our findings also revealed that the TGF-β–Smad3 system was upregulated in the transcriptome analysis of the retina. Th2 cells secrete cytokines, such as IL-4 and IL-13, which bind to macrophage surface receptors and are transmitted via the STAT6 pathway,83 causing their differentiation into M2 macrophages. This activation is known as “alternative activation.”84 Notably, M2 macrophages secrete anti-inflammatory cytokines and growth factors, such as IL-10 and TGF-β, which promote anti-inflammatory responses, tissue repair, and regeneration and are crucial in regulating chronic inflammation.85 Autoimmune diseases, such as multiple sclerosis86 and type 1 diabetes,87 and chronic inflammatory diseases, such as Crohn disease,88 exhibit reduced M2 macrophage function, causing excessive inflammation and impaired tissue repair functions, aggravating the disease.
Furthermore, the EAU, similar to other autoimmune disease models, showed significant pathologic changes due to AM-induced alterations in M2 macrophages. In the AM-treated group, the number of M2 macrophages increased, alongside a rise in inducible nitric oxide synthase (iNOS)–positive cell numbers and gene expression (Supplementary Figs. S1A, S1B). These iNOS-expressing cells, which lacked the macrophage marker F4/80, were likely granulocytes (possibly eosinophils) because of their relative size and complexity. Eosinophils, like M2 macrophages, are activated by IL-4 and IL-13,89,90 indicating that AM administration enhances the production of these cytokines. Furthermore, AM administration upregulates the IL-13 receptor α2 chain.91 Figure 7 summarizes the proposed mechanisms by which AM administration improves disease status in EAU. AM suppressed chronic inflammation, promoted tissue repair, and improved the pathology of uveitis by activating Tregs and M2 macrophages.
Figure 7.

Schematic illustration showing the possible mechanism by which adrenomedullin promotes tissue repair and regeneration following the inflammatory response in EAU mice. In the EAU model, the AM-RAMP2 system is thought to exert anti-inflammatory effects and promote tissue repair by activating Tregs and M2 macrophages, thereby contributing to the improvement of disease pathology. Exogenous administration of AM reduced the expression of IFN-γ and IL-17, which are produced by Th1 and Th17 cells, respectively. In contrast, the expression of IL-13, a cytokine derived from Th2 cells, and IL-10, an anti-inflammatory cytokine derived from Tregs, was increased. Furthermore, M2 macrophages may promote tissue repair by activating the TGF-β–Smad3 signaling pathway.
In the present study, we demonstrated that AM is a promising therapeutic target for preventing and treating autoimmune uveitis. As a bioactive peptide naturally present in the body, AM is believed to be safe and has fewer side effects compared to traditional immunosuppressants, which exert strong effects. In contrast, AM modulates immune function, potentially reducing side effects. Furthermore, the tissue repair effect of AM may bring further benefits in improving pathologic conditions.
However, continuous administration is necessary to achieve therapeutic effects because of the conventionally short half-life of peptides in the blood, which can be inconvenient for patients in clinical settings. In this regard, polyethylene glycol (PEG)–modified AM (PEG-AM) is effective in animal models of IBD without compromising the original efficacy of AM, avoiding degradation by peptidases, and extending blood duration by reducing renal clearance.92–94
To our knowledge, this is the first study to investigate the therapeutic efficacy of AM in uveitis. While AM was administered via osmotic pumps, extended half-life formulations such as PEGylated peptides could allow for less frequent dosing, thereby enhancing convenience for patients through regular outpatient injections, similar to other biologics.
In addition, in this study, we adopted a prophylactic dosing approach by starting AM immediately after EAU induction. This experiment was not a therapeutic administration but rather an initial study to evaluate the potential therapeutic effect of AM. While this approach is common in experimental disease models, future studies should evaluate AM's efficacy in advanced stages of uveitis.
In conclusion, we investigated the pathophysiologic significance of the AM-RAMP2 and AM-RAMP3 systems in EAU and clarified the potential mechanism of EAU suppression by the AM-RAMP2 system. AM may exert anti-inflammatory and tissue repair effects by activating Tregs and M2 macrophages; therefore, it has the potential to become a novel therapeutic option for uveitis.
Supplementary Material
Acknowledgments
The authors thank Kenji Kangawa for providing recombinant AM.
Supported by JST SOHATSU (grant JPMJFR220V), Grants-in-Aid for Scientific Research (KAKENHI), grants from the Santen Scholarship Donation Program and SENSHIN Medical Research Foundation (TS), and research grants from Life Science Foundation of Japan and YOKOYAMA Foundation for Clinical Pharmacology (MT).
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request. Data are available with the permission of Shinshu University School of Medicine.
Disclosure: Y. Matsuda, None; M. Tanaka, None; Y. Zhao, None; S. Kakihara, None; K. Hoshiyama, None; T. Sakurai, None; A. Kamiyoshi, None; Y. Ichikawa-Shindo, None; H. Kawate, None; Y. Zhang, None; Q. Guo, None; P. Li, None; J. Li, None; J. Duan, None; M. Hayashi, None; H. Sanjo, None; T. Murata, None; T. Shindo, None
References
- 1. Chen SC, Sheu SJ. Recent advances in managing and understanding uveitis. F1000Res. 2017; 6: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004; 111(3): 491–500. [DOI] [PubMed] [Google Scholar]
- 3. Rothova A, Suttorp-van Schulten MS, Treffers WF, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996; 80(4): 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsirouki T, Dastiridou A, Symeonidis C, et al.. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. 2018; 26(1): 2–16. [DOI] [PubMed] [Google Scholar]
- 5. de Smet MD, Taylor SRJ, Bodaghi B, et al.. Understanding uveitis: the impact of research on visual outcomes. Prog Retin Eye Res. 2011; 30(6): 452–470. [DOI] [PubMed] [Google Scholar]
- 6. Bodaghi B, Cassoux N, Wechsler B, et al.. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Med (Baltim). 2001; 80(4): 263–270. [DOI] [PubMed] [Google Scholar]
- 7. Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004; 88(9): 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sonoda KH, Hasegawa E, Namba K, et al.. Epidemiology of uveitis in Japan: a 2016 retrospective nationwide survey. Jpn J Ophthalmol. 2021; 65(2): 184–190. [DOI] [PubMed] [Google Scholar]
- 9. Airody A, Heath G, Lightman S, Gale R. Non-infectious uveitis: optimising the therapeutic response. Drugs. 2016; 76(1): 27–39. [DOI] [PubMed] [Google Scholar]
- 10. Wu X, Tao M, Zhu L, Zhang T, Zhang M. Pathogenesis and current therapies for non-infectious uveitis. Clin Exp Med. 2023; 23(4): 1089–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caspi RR, Silver PB, Luger D, et al.. Mouse models of experimental autoimmune uveitis. Ophthalmic Res. 2008; 40(3–4): 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rizzo LV, Silver P, Wiggert B, et al.. Establishment and characterization of a murine CD4+ T cell line and clone that induce experimental autoimmune uveoretinitis in B10.A mice. J Immunol. 1996; 156(4): 1654–1660. [PubMed] [Google Scholar]
- 13. Caspi RR, Roberge FG, McAllister CG, et al.. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986; 136(3): 928–933. [PubMed] [Google Scholar]
- 14. Gasparin F, Takahashi BS, Scolari MR, Gasparin F, Pedral LS, Damico FM. Experimental models of autoimmune inflammatory ocular diseases. Arq Bras Oftalmol. 2012; 75(2): 143–147. [DOI] [PubMed] [Google Scholar]
- 15. Forrester JV, Liversidge J, Dua HS, Towler H, McMenamin PG. Comparison of clinical and experimental uveitis. Curr Eye Res. 1990; 9(suppl): 75–84. [DOI] [PubMed] [Google Scholar]
- 16. Dick AD. Experimental approaches to specific immunotherapies in autoimmune disease: future treatment of endogenous posterior uveitis? Br J Ophthalmol. 1995; 79(1): 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001; 33(4): 289–294. [DOI] [PubMed] [Google Scholar]
- 18. Kapugi M, Cunningham K. Corticosteroids. Orthop Nurs. 2019; 38(5): 336–339. [DOI] [PubMed] [Google Scholar]
- 19. Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013; 23(5): 705–717. [DOI] [PubMed] [Google Scholar]
- 20. Patten SB, Neutel CI. Corticosteroid-induced adverse psychiatric effects: incidence, diagnosis and management. Drug Saf. 2000; 22(2): 111–122. [DOI] [PubMed] [Google Scholar]
- 21. Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017; 39(11): 2216–2229. [DOI] [PubMed] [Google Scholar]
- 22. Stacey SK, McEleney M. Topical corticosteroids: choice and application. Am Fam Physician. 2021; 103(6): 337–343. [PubMed] [Google Scholar]
- 23. Tempest-Roe S, Joshi L, Dick AD, Taylor SRJ. Local therapies for inflammatory eye disease in translation: past, present and future. BMC Ophthalmol. 2013; 13(1): 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet. 1993; 24(6): 472–495. [DOI] [PubMed] [Google Scholar]
- 25. Graham RM. Cyclosporine: mechanisms of action and toxicity. Cleve Clin J Med. 1994; 61(4): 308–313. [DOI] [PubMed] [Google Scholar]
- 26. Mihatsch MJ, Kyo M, Morozumi K, Yamaguchi Y, Nickeleit V, Ryffel B. The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol. 1998; 49(6): 356–363. [PubMed] [Google Scholar]
- 27. Robert N, Wong GW, Wright JM. Effect of cyclosporine on blood pressure. Cochrane Database Syst Rev. 2010;(1): CD007893. [DOI] [PubMed] [Google Scholar]
- 28. Borrás-Blasco J, Casterá DE, Cortes X, Abad FJ, Rosique-Robles JD, Mallench LG. Effectiveness of infliximab, adalimumab and golimumab for non-infectious refractory uveitis in adults. Int J Clin Pharmacol Ther. 2015; 53(5): 377–390. [DOI] [PubMed] [Google Scholar]
- 29. Dingerkus VLS, Becker MD, Doycheva D. Biologics in the treatment of uveitis. Klin Monbl Augenheilkd. 2022; 239(5): 686–694. [DOI] [PubMed] [Google Scholar]
- 30. Leclercq M, Desbois AC, Domont F, et al.. Biotherapies in uveitis. J Clin Med. 2020; 9(11): 3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leone GM, Mangano K, Petralia MC, Nicoletti F, Fagone P. Past, present and (foreseeable) future of biological anti-TNF alpha therapy. J Clin Med. 2023; 12(4): 1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu W, Bai D, Kou L. Comparison of infliximab with adalimumab for the treatment of non-infectious uveitis: a systematic review and meta-analysis. BMC Ophthalmol. 2023; 23(1): 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martel JN, Esterberg E, Nagpal A, Acharya NR. Infliximab and adalimumab for uveitis. Ocul Immunol Inflamm. 2012; 20(1): 18–26. [DOI] [PubMed] [Google Scholar]
- 34. Mase O, Qasem M, Beare N. Systematic review of studies comparing infliximab and adalimumab in autoimmune uveitis. BMJ Open Ophthalmol. 2023; 8(1): e001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agrawal H, Doan H, Pham B, et al.. Systemic immunosuppressive therapies for uveitis in developing countries. Indian J Ophthalmol. 2020; 68(9): 1852–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kitamura K, Kangawa K, Kawamoto M, et al.. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993; 192(3): 553–560. [DOI] [PubMed] [Google Scholar]
- 37. Shindo T, Tanaka M, Kamiyoshi A, et al.. Regulation of cardiovascular development and homeostasis by the adrenomedullin-RAMP system. Peptides. 2019; 111: 55–61. [DOI] [PubMed] [Google Scholar]
- 38. Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004; 84(3): 903–934. [DOI] [PubMed] [Google Scholar]
- 39. Kato J, Tsuruda T, Kita T, Kitamura K, Eto T. Adrenomedullin: a protective factor for blood vessels. Arterioscler Thromb Vasc Biol. 2005; 25(12): 2480–2487. [DOI] [PubMed] [Google Scholar]
- 40. Shimosawa T, Ogihara T, Matsui H, Asano T, Ando K, Fujita T. Deficiency of adrenomedullin induces insulin resistance by increasing oxidative stress. Hypertension. 2003; 41(5): 1080–1085. [DOI] [PubMed] [Google Scholar]
- 41. Shindo T, Kurihara H, Maemura K, et al.. Hypotension and resistance to lipopolysaccharide-induced shock in transgenic mice overexpressing adrenomedullin in their vasculature. Circulation. 2000; 101(19): 2309–2316. [DOI] [PubMed] [Google Scholar]
- 42. Shimosawa T, Shibagaki Y, Ishibashi K, et al.. Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation. 2002; 105(1): 106–111. [DOI] [PubMed] [Google Scholar]
- 43. Udono-Fujimori R, Udono T, Totsune K, Tamai M, Shibahara S, Takahashi K. Adrenomedullin in the eye. Regul Pept. 2003; 112(1–3): 95–101. [DOI] [PubMed] [Google Scholar]
- 44. Imai A, Toriyama Y, Iesato Y, et al.. Adrenomedullin suppresses vascular endothelial growth factor-induced vascular hyperpermeability and inflammation in retinopathy. Am J Pathol. 2017; 187(5): 999–1015. [DOI] [PubMed] [Google Scholar]
- 45. Hirabayashi K, Tanaka M, Imai A, et al.. Development of a novel model of central retinal vascular occlusion and the therapeutic potential of the adrenomedullin-receptor activity-modifying protein 2 system. Am J Pathol. 2019; 189(2): 449–466. [DOI] [PubMed] [Google Scholar]
- 46. Tanaka M, Kakihara S, Hirabayashi K, et al.. Adrenomedullin-receptor activity-modifying protein 2 system ameliorates subretinal fibrosis by suppressing epithelial-mesenchymal transition in Age-related macular degeneration. Am J Pathol. 2021; 191(4): 652–668. [DOI] [PubMed] [Google Scholar]
- 47. Shindo T, Kurihara Y, Nishimatsu H, et al.. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001; 104(16): 1964–1971. [DOI] [PubMed] [Google Scholar]
- 48. Ichikawa-Shindo Y, Sakurai T, Kamiyoshi A, et al.. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J Clin Invest. 2008; 118(1): 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamauchi A, Sakurai T, Kamiyoshi A, et al.. Functional differentiation of RAMP2 and RAMP3 in their regulation of vascular system. J Mol Cell Cardiol. 2014; 77: 73–85. [DOI] [PubMed] [Google Scholar]
- 50. Paques M, Guyomard JL, Simonutti M, et al.. Panretinal, high-resolution color photography of the mouse fundus. Invest Ophthalmol Vis Sci. 2007; 48(6): 2769–2774. [DOI] [PubMed] [Google Scholar]
- 51. Satoh M, Namba KI, Kitaichi N, et al.. Invariant natural killer T cells play dual roles in the development of experimental autoimmune uveoretinitis. Exp Eye Res. 2016; 153: 79–89. [DOI] [PubMed] [Google Scholar]
- 52. Sonoda KH, Yoshimura T, Takeda A, Ishibashi T, Hamano S, Yoshida H. WSX-1 plays a significant role for the initiation of experimental autoimmune uveitis. Int Immunol. 2007; 19(1): 93–98. [DOI] [PubMed] [Google Scholar]
- 53. Takeda A, Hasegawa E, Fukuhara T, et al.. EBI3 is pivotal for the initiation of experimental autoimmune uveitis. Exp Eye Res. 2014; 125: 107–113. [DOI] [PubMed] [Google Scholar]
- 54. Takeda A, Yamada H, Hasegawa E, et al.. Crucial role of P2X7 receptor for effector T cell activation in experimental autoimmune uveitis. Jpn J Ophthalmol. 2018; 62(3): 398–406. [DOI] [PubMed] [Google Scholar]
- 55. Copland DA, Wertheim MS, Armitage WJ, Nicholson LB, Raveney BJE, Dick AD. The clinical time-course of experimental autoimmune uveoretinitis using topical endoscopic fundal imaging with histologic and cellular infiltrate correlation. Invest Ophthalmol Vis Sci. 2008; 49(12): 5458–5465. [DOI] [PubMed] [Google Scholar]
- 56. Inoki T, Yamagami S, Sakai R, Isobe M, Tsuru T, Kawashima H. Suppression of experimental autoimmune uveoretinitis by anti-alphabeta TCR monoclonal antibody. Jpn J Ophthalmol. 2002; 46(5): 518–524. [DOI] [PubMed] [Google Scholar]
- 57. Okunuki Y, Mukai R, Nakao T, et al.. Retinal microglia initiate neuroinflammation in ocular autoimmunity. Proc Natl Acad Sci U S A. 2019; 116(20): 9989–9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Osada M, Sakai T, Kuroyanagi K, Kohno H, Tsuneoka H. Treatment of experimental autoimmune uveoretinitis with peroxisome proliferator-activated receptor α agonist fenofibrate. Mol Vis. 2014; 20: 1518–1526. [PMC free article] [PubMed] [Google Scholar]
- 59. Sakai T, Kohno H, Ishihara T, et al.. Treatment of experimental autoimmune uveoretinitis with poly(lactic acid) nanoparticles encapsulating betamethasone phosphate. Exp Eye Res. 2006; 82(4): 657–663. [DOI] [PubMed] [Google Scholar]
- 60. Shoda H, Yanai R, Yoshimura T, et al.. Dietary omega-3 fatty acids suppress experimental autoimmune uveitis in association with inhibition of Th1 and Th17 cell function. PLoS ONE. 2015; 10(9): e0138241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thurau SR, Chan CC, Nussenblatt RB, et al.. Oral tolerance in a murine model of relapsing experimental autoimmune uveoretinitis (EAU): induction of protective tolerance in primed animals. Clin Exp Immunol. 1997; 109(2): 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang M, Yang Z, Huang J, et al.. Optimization of determinant factors associated with the efficiency of experimental autoimmune uveitis induction in C57BL/6 mice. Ann Transl Med. 2022; 10(23): 1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fang J, Fang D, Silver PB, et al.. The role of TLR2, TRL3, TRL4, and TRL9 signaling in the pathogenesis of autoimmune disease in a retinal autoimmunity model. Invest Ophthalmol Vis Sci. 2010; 51(6): 3092–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Klaska IP, Muckersie E, Martin-Granados C, Christofi M, Forrester JV. Lipopolysaccharide-primed heterotolerant dendritic cells suppress experimental autoimmune uveoretinitis by multiple mechanisms. Immunology. 2017; 150(3): 364–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yadav UCS, Shoeb M, Srivastava SK, Ramana KV. Aldose reductase deficiency protects from autoimmune- and endotoxin-induced uveitis in mice. Invest Ophthalmol Vis Sci. 2011; 52(11): 8076–8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yazid S, Gardner PJ, Carvalho L, et al.. Annexin-A1 restricts Th17 cells and attenuates the severity of autoimmune disease. J Autoimmun. 2015; 58: 1–11. [DOI] [PubMed] [Google Scholar]
- 67. Klimova A, Stangova PS, Svozilkova P, Forrester JV, Klaska I, Heissigerova J. The critical points in induction of experimental autoimmune uveitis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016; 160(1): 140–142. [DOI] [PubMed] [Google Scholar]
- 68. Fan NW, Li J, Mittal SK, et al.. Characterization of clinical and immune responses in an experimental chronic autoimmune uveitis model. Am J Pathol. 2021; 191(3): 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hu Y, Chen G, Huang J, et al.. The calcium channel inhibitor nimodipine shapes the uveitogenic T cells and protects mice from experimental autoimmune uveitis through the p38-MAPK signaling pathway. J Immunol. 2021; 207(12): 2933–2943. [DOI] [PubMed] [Google Scholar]
- 70. Hu Y, Li Z, Chen G, et al.. Hydroxychloroquine alleviates EAU by inhibiting uveitogenic T cells and ameliorating retinal vascular endothelial cells dysfunction. Front Immunol. 2022; 13: 859260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tanaka M, Koyama T, Sakurai T, et al.. The endothelial adrenomedullin-RAMP2 system regulates vascular integrity and suppresses tumour metastasis. Cardiovasc Res. 2016; 111(4): 398–409. [DOI] [PubMed] [Google Scholar]
- 72. Wei Y, Tanaka M, Sakurai T, et al.. Adrenomedullin ameliorates pulmonary fibrosis by regulating TGF-ß-Smads signaling and myofibroblast differentiation. Endocrinology. 2021; 162(8): bqab090. [DOI] [PubMed] [Google Scholar]
- 73. Udono T, Takahashi K, Abe T, Shibahara S, Tamai M. Elevated immunoreactive-adrenomedullin levels in the aqueous humor of patients with uveitis and vitreoretinal disorders. Peptides. 2002; 23(10): 1865–1868. [DOI] [PubMed] [Google Scholar]
- 74. Blom J, Give TJ, Pong WW, Blute TA, Eldred WD. Evidence for a functional adrenomedullin signaling pathway in the mouse retina. Mol Vis. 2012; 18: 1339–1353. [PMC free article] [PubMed] [Google Scholar]
- 75. Chen Z, Liu G, Xiao Y, Lu P. Adrenomedullin22-52 suppresses high-glucose-induced migration, proliferation, and tube formation of human retinal endothelial cells. Mol Vis. 2014; 20: 259–269. [PMC free article] [PubMed] [Google Scholar]
- 76. Yousufzai SY, Ali N, Abdel-Latif AA. Effects of adrenomedullin on cyclic AMP formation and on relaxation in iris sphincter smooth muscle. Invest Ophthalmol Vis Sci. 1999; 40(13): 3245–3253. [PubMed] [Google Scholar]
- 77. Taniguchi T, Kawase K, Gu ZB, et al.. Ocular effects of adrenomedullin. Exp Eye Res. 1999; 69(5): 467–474. [DOI] [PubMed] [Google Scholar]
- 78. Gong B, Zhang H, Huang L, et al.. Mutant RAMP2 causes primary open-angle glaucoma via the CRLR-cAMP axis. Genet Med. 2019; 21(10): 2345–2354. [DOI] [PubMed] [Google Scholar]
- 79. Pedreño M, Morell M, Robledo G, et al.. Adrenomedullin protects from experimental autoimmune encephalomyelitis at multiple levels. Brain Behav Immun. 2014; 37: 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gonzalez-Rey E, Chorny A, O'Valle F, Delgado M. Adrenomedullin protects from experimental arthritis by down-regulating inflammation and Th1 response and inducing regulatory T cells. Am J Pathol. 2007; 170(1): 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martínez-Herrero S, Martínez A. Adrenomedullin: not just another gastrointestinal peptide. Biomolecules. 2022; 12(2): 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rullé S, Kioon MDA, Asensio C, et al.. Adrenomedullin, a neuropeptide with immunoregulatory properties induces semi-mature tolerogenic dendritic cells. Immunology. 2012; 136(2): 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bhattacharjee A, Shukla M, Yakubenko VP, Mulya A, Kundu S, Cathcart MK. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med. 2013; 54: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014; 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016; 44(3): 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boven LA, Van Meurs M, Van Zwam M, et al.. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006; 129(pt 2): 517–526. [DOI] [PubMed] [Google Scholar]
- 87. Grinberg-Bleyer Y, Baeyens A, You S, et al.. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010; 207(9): 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hunter MM, Wang A, Parhar KS, et al.. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010; 138(4): 1395–1405. [DOI] [PubMed] [Google Scholar]
- 89. Robinson D, Humbert M, Buhl R, et al.. Revisiting type 2-high and type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017; 47(2): 161–175. [DOI] [PubMed] [Google Scholar]
- 90. Wechsler ME, Munitz A, Ackerman SJ, et al.. Eosinophils in health and disease: a state-of-the-art review. Mayo Clin Proc. 2021; 96(10): 2694–2707. [DOI] [PubMed] [Google Scholar]
- 91. Joshi BH, Leland P, Calvo A, Green JE, Puri RK. Human adrenomedullin up-regulates interleukin-13 receptor alpha2 chain in prostate cancer in vitro and in vivo: a novel approach to sensitize prostate cancer to anticancer therapy. Cancer Res. 2008; 68(22): 9311–9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Akashi E, Nagata S, Yamasaki M, Kitamura K. Activation of calcitonin gene-related peptide and adrenomedullin receptors by PEGylated adrenomedullin. Biol Pharm Bull. 2020; 43(11): 1799–1803. [DOI] [PubMed] [Google Scholar]
- 93. Nagata S, Yamasaki M, Kitamura K. Anti-inflammatory effects of PEGylated human adrenomedullin in a mouse DSS-induced colitis model. Drug Dev Res. 2017; 78(3–4): 129–134. [DOI] [PubMed] [Google Scholar]
- 94. Nagata S, Yamasaki M, Kitamura K. Polyethylene glycol-conjugated human adrenomedullin as a possible treatment for vascular dementia. Peptides. 2019; 121: 170133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.