ABSTRACT
ELANA, excimer laser-assisted nonocclusive anastomosis, is a technique using an excimer laser/catheter system for intracranial bypass surgery of the brain. The technique has been developed over the past 12 years by Tulleken and colleagues at UMC Utrecht in The Netherlands for treatment of primarily untreatable giant aneurysms. We review here the emergence of transplanted conduit bypass as a valuable technique for managing these lesions and the subsequent development of ELANA bypass. The ELANA technique allows the operating surgeon to perform an extracranial-to-intracranial or intracranial-to-intracranial bypass using a transplanted large caliber conduit without occlusion of the recipient artery, thus eliminating intraoperative ischemic insult related to temporary occlusion time. We describe the ELANA technique, illustrate it with intraoperative photos, and review the relevant literature. ELANA is shown to be safe; we discuss its advantages over conventional techniques.
Keywords: Excimer laser-assisted nonocclusive bypass, extracranial-to-intracranial bypass, high-flow bypass
ELANA, excimer laser-assisted nonocclusive anastomosis, is a technique using an excimer laser/catheter system that has been developed over the past 12 years by Tulleken and colleagues in UMC Utrecht. The technique and system have been designed for the purpose of treating primarily giant or untreatable aneurysms of the intracranial circulation. The ELANA technique allows the operating surgeon to perform an extracranial-to-intracranial (EC-IC) or intracranial-to-intracranial (IC-IC) bypass using a transplanted large caliber conduit without occlusion of the recipient artery eliminating intraoperative ischemic insult related to temporary occlusion time.
Giant aneurysms of the cerebral circulation, those over 25 mm in size, maintain a formidable natural history. Whether ruptured or unruptured, symptomatic or not, these aneurysms maintain rupture, rebleed, and death rates from 20 to 70% at 5 years in most series,1,2,3,4,5,6 indicating that treatment should be attempted in most patients (independent of age) who are in good medical and neurological condition. These aneurysms emanate from vital arteries in the brain that subserve significant volumes of tissue making direct vessel sacrifice high risk. Collateral cerebral circulation often is robust enough to allow parent vessel sacrifice as a direct treatment for these lesions. This technique has been well described in the literature for treatment of many large aneurysms7,8,9,10,11 but is now reserved only for those that cannot be treated directly either through surgery or interventional neuroradiological technique. Untreatable aneurysms of the internal carotid artery can be treated with parent vessel sacrifice ∼80% of the time, due to collateral flow to the affected region provided via the Circle of Willis combined with increased flow volume via the contralateral internal carotid.12,13 Patients who fail have been shown to primarily harbor hypoplastic or absent A1 segments or anterior communicating arteries.14 Internal carotid artery sacrifice along with EC-IC bypass using a superficial temporal to middle cerebral artery (STA-MCA) procedure was subsequently developed to treat these individuals.15,16,17,18
EC-IC bypass was originally developed by Yasargil and Yonekawa as a safe means of direct cerebral revascularization.19 The technique involved attaching the superficial temporal artery (STA) to a distal middle cerebral arterial (MCA) branch vessel and was primarily utilized as a cerebral blood flow augmentative procedure in the treatment of patients harboring middle cerebral ischemia. This methodology was called into question in 1985 by the EC-IC Bypass Study which found the procedure to be of no statistical benefit to patients. It was essentially discontinued as a treatment for cerebral ischemia.20 The procedure, however, continued to be used for cerebral blood flow replacement as an adjunct to parent vessel sacrifice in patients with aneurysms and tumors who had poor collateral circulation. The STA conduit and distal middle cerebral recipient were both relatively small arteries and the volume of replacement flow was limited. Significant stroke complications have been noted in patients with apparently intact bypasses8,9,21 due to inadequate flow replacement. Strategies to improve flow replacement volume were developed.10,11,22,23,24,25,26 These techniques involved increasing the size and therefore flow potential of both the donor and recipient vessel. These modifications of the original EC-IC technique also brought additional risks. The conduits were now vessels transplanted either from the leg in the form of the saphenous vein or from the forearm's radial artery. The vein graft can be complicated by kinking and thrombosis due to its patulous nature and requires relatively high flows to remain patent. Radial artery grafts have been shown to develop vasospasm which has been overcome in vivo by overdilation26; however, maximal flow through a radial artery is less than that of the larger saphenous conduit and the risks of arterial harvest to the hand and forearm are not insignificant.27,28,29 The recipient artery for these higher flow grafts also must be of similar large diameter, forcing the surgeon to graft into deeper more proximal arteries in the vascular tree. This created both new technical and physiological challenges which did not exist in the more superficially performed STA-MCA bypass. Grafting into these more proximal arteries required exposure of middle cerebral branch vessels within the Sylvian fissure requiring modest brain retraction combined on occasion with vein sacrifice, slightly increasing operative risk as compared with the superficially performed STA-MCA procedure. Use of intraoperative heparin and preoperative and postoperative aspirin to improve graft patency have increased the risks of postoperative hemorrhage. Embolic stroke risk is increased due to vessel manipulation. Perioperative aneurysm rupture, likely due to alterations in intraluminal blood flow in the aneurysm itself, has been reported.30,31
STA-MCA bypass requires temporary occlusion of superficial, small distal middle cerebral branches with little risk of intraoperative ischemic injury. Higher-flow bypasses, however, require temporary occlusion of the larger M2 or M3 branches, decreasing the safe time of temporary occlusion and increasing the risk of intraoperative ischemia as well as the anxiety of the operating surgeon. Grafting into more proximal arteries such as the parent MCA or ICA, which could provide even greater possible replacement flow, was of even greater technical difficulty due to the depth of the anastomosis and even higher risk of ischemia.
High-flow replacement EC-IC bypass has been performed in specialized centers for many years.3,25,32 The reported risk of performing these bypasses is significant, with perioperative morbidity and mortality ranging ∼7 to 15%.25,32 While safer than the natural history of the lesions themselves, these risks remain high and argue for a careful consideration of alternative options in the face of a giant or untreatable aneurysm. The definition of “untreatable” itself is changing as interventional neuroradiological devices improve and make the aneurysm that was untreatable yesterday quite treatable today. These devices themselves, however, have resulted in new treatment challenges with aneurysms reopening or bleeding following interventional therapy, resulting in intravascular hardware within or in close proximity to the aneurysm fundus, leaving them untreatable by direct surgery. Vessel sacrifice alone following balloon-test occlusion remains a favored method to this day, despite a risk of delayed stroke of up to 10%.13 Vessel sacrifice, particularly in young patients, may have additional long-term risks.33,34 Unfortunately, the reduction of risk of high-flow replacement bypass utilizing the current technique is difficult. Attempts at reduction of perioperative risks have been made by eliminating the use of heparin and utilizing barbiturate cerebral protection. However, the inherent risk of transplanting an autologous vessel to the brain has not been overcome. One factor which has been directly addressed has been temporary occlusion time and it is here that the ELANA technique has been focused.
ELANA allows the construction of EC-IC bypass grafts utilizing saphenous vein or radial artery without requiring any temporary occlusion of the recipient. This allows the construction of deep anastomoses of extracranial to intracranial circulation without risk of intraoperative ischemic insult. Grafting to the large proximal arteries improves maximal graft flow and creates a graft which provides a more proximal point of origin of flow, mimicking innate cerebral flow more closely, which may be important.35 Postoperative magnetic resonance (MR) blood flow studies have shown these proximally placed grafts to maintain flows of 199 +/− 72 cc/min.14 Conventional high-flow replacement grafts have not been studied as such, leaving the ELANA graft flow without a good source of comparison.36 The concept of higher flows with more proximally placed recipient arteries has been studied with flows of 40 to 140 cc/min reported in grafts to the M2, M3, and basilar arteries.23,36,37 Mathematical model studies also support this concept and lend additional evidence to the likely benefit of a high-flow proximally placed ELANA bypass.35
ELANA does not directly impact the other sources of risk that are unrelated to temporary occlusion; however, these grafts are performed without systemic heparin given the lack of flow arrest and without barbiturate protection given the lack of cerebral ischemia, eliminating the incremental risks that these factors contribute. Also, somewhat less exposure is required to perform an ELANA bypass due to the fact that room on the recipient artery for temporary clip application is not required, leaving a shorter segment of vessel exposure required to place the bypass graft. This has important ramifications for not only local exposure but for different, less invasive approaches to be considered through which conventional bypass techniques could not be used. Most notable are the demonstrations by Tulleken et al of trans-Sylvian approaches to the P1 segment for EC-P1 or IC-P1 bypass.38,39 Conventional techniques do not permit safe grafting into the P1 or basilar without circulatory arrest and any high-flow direct posterior circulation bypass requires a subtemporal approach. Unlike a trans-Sylvian approach, this requires significant brain retraction.
The ELANA technique has been well described in the literature both in the early laboratory animal phase as well as the more recent clinical results and case reports.14,36,38,39,40,41,42,43,44,45,46,47,48 Patients selected for ELANA have either failed balloon-occlusion tests or demonstrate local anatomy by angiography that makes carotid sacrifice high risk. The technique involves the attachment of an anastomotic ring to a recipient artery of at least 3 mm in diameter either on its own or as part of the distal donor vessel (Fig. 1). The ring and distal donor segment are sewn to the recipient utilizing conventional microneurosurgical technique (Fig. 2). Following the suture attachment step of the donor to the recipient, a laser suction catheter is passed down the lumen of the open donor vessel from proximal to distal and is placed against the sidewall of the recipient from within the donor lumen itself (Figs. 3, 4). The laser suction catheter suction portion is then activated, allowing the catheter to firmly affix the proposed arteriotomy defining the portion of the recipient to be removed. Following 2 minutes of active vacuum pressure the laser portion of the catheter is activated. The laser broaches the recipient arterial wall and separates the arteriotomy flap from the recipient, while the suction portion of the catheter maintains this small flap in contact with the catheter thus preventing its migration into the lumen of the recipient. The catheter is removed from the donor lumen with the small flap attached (Fig. 5). The proximal donor lumen is then sewn end to end to a second portion of donor vessel attached to the extracranial donor artery. completing the bypass with an extra anastomosis step as the two halves of the donor must be sewn end to end (Figs. 6, 7, 8). The ELANA technique therefore is really a subtle modification of the existing conventional EC-IC high-flow bypass. The only real differences involve how the recipient artery is broached. In the conventional technique the artery is temporarily occluded and opened using a microscissors and knife, while in the ELANA technique it is left unoccluded and is entered with laser light. This subtle difference, however, has important ramifications for the safety of the procedure.
Figure 1.
Suturing of platinum ring to distal end of saphenous vein graft.
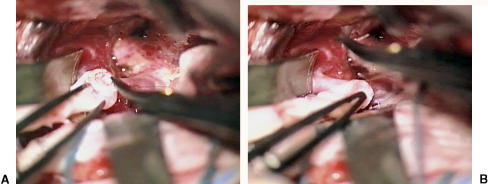
Figure 2.
(A,B) Left frontal trans-Sylvian approach to supraclinoidal segment of left internal carotid artery (frontal retractor in foreground). Suturing of saphenous graft/ring complex to recipient internal carotid artery.
Figure 3.
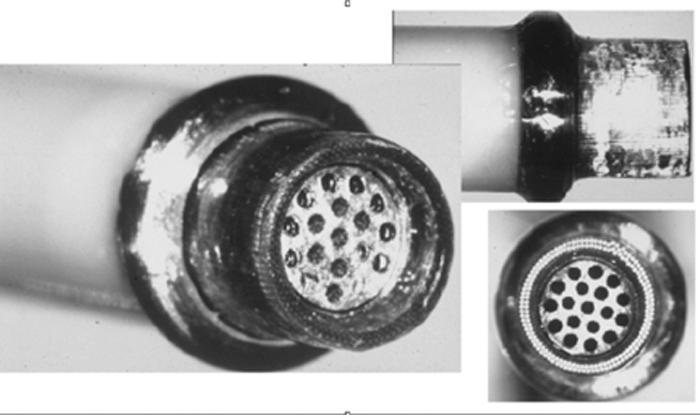
Catheter tip is designed as shown. Note inner suction portion surrounded by outer excimer laser array.
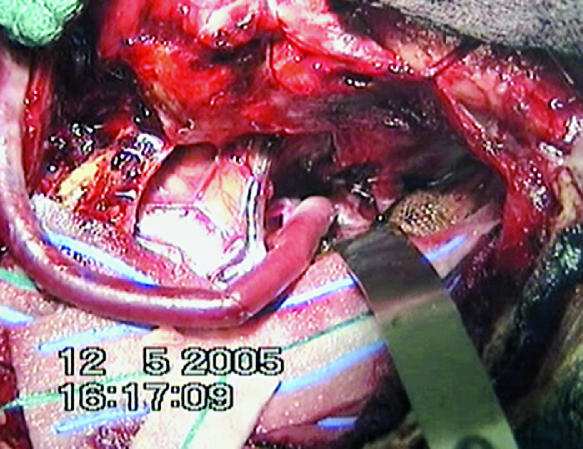
Figure 4.
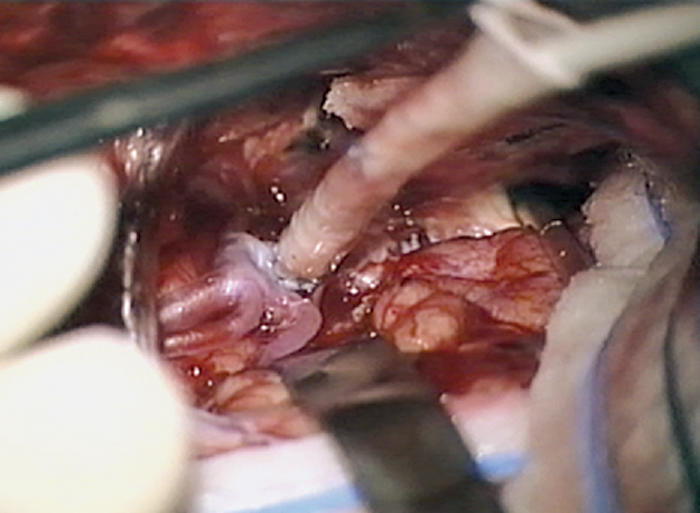
Laser catheter is placed within graft and advanced to wall of recipient with application of suction followed by laser activation.
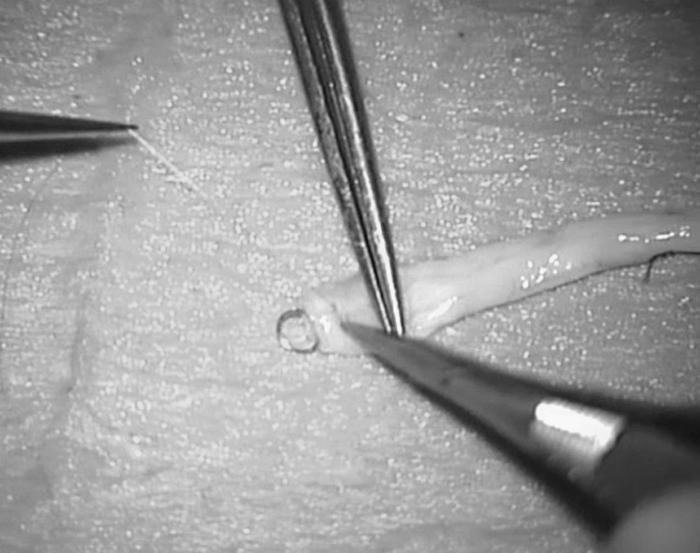
Figure 5.
(A) Arteriotomy flap is left attached to suction portion of laser tip and (B) is removed.
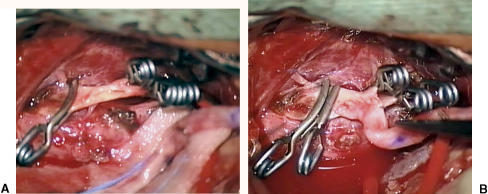
Figure 6.
(A) Left external carotid donor vessel is shown after temporary occlusion with arteriotomy. (B) Proximal anastomosis is made between donor artery and proximal graft using standard microanastomosis technique.
Figure 7.
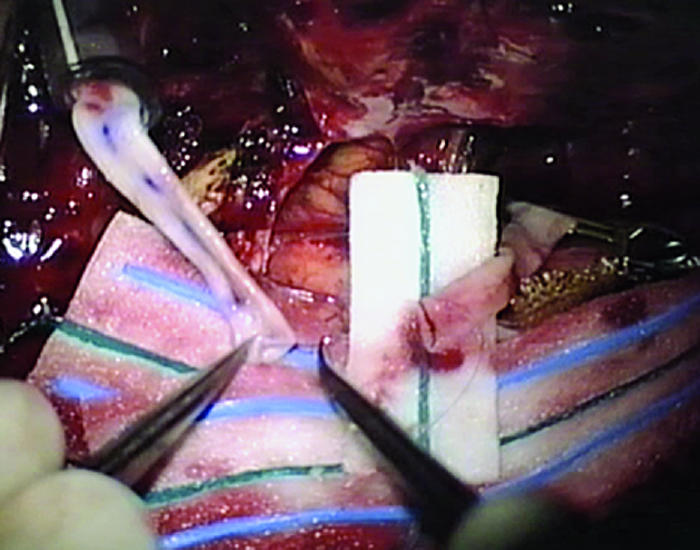
Proximal and distal ends of graft are sewn end to end completing bypass. Note proximal segment on left with Sylvian portion on right.
Figure 8.
Temporary clips are released with bypass connected.
What follows is a review of the literature of the ELANA technique. The literature supports the safety of the laser arteriotomy and both in animal experiments and in human cases appears to be safe and reliable. The treatment of giant and untreatable aneurysms remains high risk. The ELANA technique reduces the risk of bypass only incrementally by eliminating intraoperative temporary occlusion but certainly does not affect the other real risks of these procedures. The addition of ELANA to the treatment armamentarium of the operating surgeon will give the neurosurgeon greater flexibility and treatment options which will become complementary to and not exclusionary to the current conventional EC-IC bypass techniques.
LITERATURE REVIEW
The published literature on the ELANA technique can be divided into three distinct phases which partially overlap in time. All of the work in the literature has been based in UMC Utrecht under the guidance of Prof. C.A.F. Tulleken. The initial laboratory phase of the work overlaps with the initial case reports in humans and the case reports continue to overlap with more recent series of retrospective reviews of clinical results. Additionally, unpublished data from the Utrecht experience has also been reviewed.
CITATION REVIEW
Laboratory Studies
Using the excimer laser, Tulleken and colleagues44 essentially established the basic thinking regarding the excimer laser nonocclusive anastomosis technique. The laboratory experiment was well designed and allowed the evaluation of two laser arteriotomies per rabbit. The nonocclusive technique was first developed in this study and is quite similar to the technique used today. Fifteen rabbits underwent excimer laser-assisted anastomoses of an explanted portion of the left common carotid artery to the right carotid with two anastomoses performed per rabbit. Thirteen rabbits maintained patent grafts in the follow-up period of 4 hours to 2 months following surgery. Scanning electron microscopy of patent grafts showed no evidence of thermal injury to the recipient or donor vessel and showed an extremely sharp cut edge in the recipient with full endothelialization by 6 weeks after surgery. Laboratory observations were also made with regard to the theoretical concern of debris embolization from the laser arteriotomy site. The debris was noted to consist of miniscule particles that freely pass through the microcirculation of the brain, overcoming an early major technical concern. There are no significant data or studies of these particles and they are referred to in the discussion only, unfortunately leaving the reader without the ability to objectify the authors’ conclusions. Following good success in 13 of 15 rabbits, the authors report their experience with one patient with good results and also report of a second patient successfully treated as well.
Tulleken and associates46 published a second laboratory study in 1996 that included an early in vivo human series as well. A new catheter design was introduced for the first time and was nearly identical to that which is currently being used. The design alteration was driven by the observation that the previous catheter caused irregular cuts in the recipient that were not reliable. The authors hypothesized as to the reason for these irregular cuts and conceived of a new type of catheter tip that consisted of a central suction portion with an outer laser array that proved to more reliably excise an arteriotomy flap from the recipient artery. The platinum ring device was also introduced for the first time. The ring helped to insure a completely flush interface between the laser tip and the arteriotomy site, allowing the laser tip to broach the arterial wall along its entire circumference. It also allowed the surgeon to define the arteriotomy on the recipient and acted as a barrier on the vessel itself, providing a “dock” point for the catheter and preventing it from entering deeply into the recipient vessel lumen after the wall has been broached. Last, by applying vacuum pressure through the suction portion of the tip, the authors solved the difficulties not only of getting a flush fit of the catheter against the recipient but also of maintaining an elegant way to prevent the arteriotomy flap from migrating into the lumen of the recipient artery. The authors modified the experimental system and utilized the rabbit abdominal aorta for graft placement. The rabbit aorta more closely mimics that of the human internal carotid and provided a better substrate for the laser tip to be tested.
This paper, titled “The Modified Excimer Laser-Assisted High-Flow Bypass Operation,”46 represents a major advance in the technology and is the true origin of the technique as it is today. It is primarily a technical paper with cartoon figures and very little actual data but adequately describes the “modified” technique and shows some early human results as well. This paper clearly had the greatest impact on the future project and remains the definitive reference for the ELANA project.
Tulleken et al47 published an extensive laboratory study with a clinical case review of 40 patients. The first part focuses upon the laboratory experience and consists of the results of 100 acute and chronic experiments performed in rabbits. This portion of the paper is essentially a review of the laboratory experience up to that point, with little new data with the exception that the results using the new catheter are more clearly described. Thirty of the 100 rabbits were operated using the newly designed laser suction catheter. These were all acute experiments and resulted in 28/30 arteriotomies with excellent results. Early graft patency was 100% in these 28 animals at 4 to 5 hours prior to euthanizing the rabbits. This paper, while somewhat redundant, does reflect the authors’ technical progress and is important and highly valuable in reflecting the safety of the new laser catheter in the laboratory.
Wolfs and associates48 and Streefkerk et al49 involve study of the histological features of the excimer laser-assisted anastomosis. The purpose of the first study was to evaluate the endothelialization process of the laser-assisted arteriotomy. Scanning electron microscopy was used to carefully study the appearance of the laser-assisted anastomosis site in rabbits. Grafts were studied in both the acute stage and in survival animals as well. The paper is well illustrated with photographs and cartoons of the anastomotic segments. The endothelialization of the anastomosis and laser edge appears to begin within 1 day of surgery and is complete at 9 days, which is much earlier than the earlier study had suggested. No control group for comparison (i.e., conventional grafts) is performed, leaving the reader unable to conclude how these findings compare with the conventional sutured anastomosis. These questions are addressed in the last laboratory paper, which was recently accepted for publication in the Journal of Neurosurgery. Here, long term re-endothelialization is compared between conventionally sutured anastomoses and excimer laser-assisted grafts in a pig model. Twenty-eight pigs underwent bypass with one excimer laser and one conventional anastomosis. Twenty-four bypasses remained patent during the study period, leaving 48 anastomosis sites for evaluation. Excimer laser sites were placed both proximal and distal on the bypasses to compare outflow and inflow zones. Bypass flows were checked intraoperatively and intraoperative angiograms were checked. Animals were sacrificed and evaluated at 2 weeks, 2 months, 3 months, and 6 months postop. Anastomotic segments were examined using scanning electron microscopy and light microscopy. There did not appear to be any negative ramifications of the ring at the anastomosis site as compared with conventional bypass with complete endothelialization of the rim and ring by 2 weeks. There did appear to be some tissue thickening near the rim of the anastomosis in the excimer group which was not seen on the conventional end and was not fully discussed. The authors discuss the findings of Lidman et al who noted hyperplasia of the recipient wall in end-to-side and end-to-end arteriovenous (a-v) fistulae in a pig model, possibly consistent with medial hyperplasia.50 While this is a possible explanation, it is left somewhat unclear what the authors’ conclusions are regarding the different apparent findings between conventional and excimer-assisted anastomosis, with the exception of the more irregular nature of the conventional graft site. While there was no apparent difference in patency or endothelialization, this must be studied further. The authors point out that the pig carotid is thicker than the human intracranial internal carotid, necessitating a higher number of laser energy pulses to broach the pig vessel. No thermal effects were noted due to these extra pulses on the recipient.
Clinical Case Reports
Eight case reports or series of cases have been published. The first case report is included in the initial laboratory paper using the first-generation laser catheter, with the most recent report published in 2004, which is essentially an autopsy study of the anastomosis.
Tulleken et al44 published the first case reports along with the first laboratory series of 15 rabbit surgeries. The patient harbored bilateral carotid occlusions and had recurrent episodes of hemispheric ischemia with blood flow studies showing marked hypoperfusion. Right hemispheric revascularization augmentation was planned using excimer laser-assisted anastomosis. The conduit was an epigastric artery connected proximally to the STA and the bypass was shown to be patent 5 days postoperatively. No clinical outcome is reported on the patient other than that she was intact postoperatively. An angiogram showed a patent bypass at 5 days after surgery.
In the next case series, the procedure is once again outlined45 and for the first time is mentioned as a tool to revascularize patients with giant aneurysms by creating high-flow bypasses to replace carotid flow prior to deconstuctive carotid occlusion. A short series of nine patients is presented. This reference was written during the ELANA technical improvement phase during the time that the catheter was being modified. The authors report improved success in rabbits with the modified laser tip but do not mention in the article whether the new or old tip was utilized in the nine patients treated. Seven of these patients underwent laser-assisted bypass using epigastric artery while two had saphenous vein grafts. Unlike the clinical paper by Tulleken et al,46 the authors review the clinical results in a bit more detail. Four patients with giant aneurysms were bypassed, one with a carotid cavernous fistula and four with cerebral ischemia. In the nonischemic cases, the clinical outcomes were discussed but patency was not adequately studied as none of these patients underwent postoperative angiograms. Doppler exams, however, were performed in three patients with apparent patent grafts who underwent subsequent carotid sacrifice successfully with aneurysm thrombosis. The fourth aneurysm patient had an occluded graft following occlusion of the aneurysm with a good outcome and so should be considered a treatment success despite a failed bypass. The cavernous carotid fistula patient did poorly and likely had an occluded graft. Most important, however, was the apparent early safety of the procedure with no reported ELANA-related intraoperative complications. This series does add relatively high value to the measure of ELANA safety in general without adding as much information regarding its effectiveness versus conventional bypass. The authors do point out apparently improving patency rates noted in chronic rabbit experiments, suggestive of improvements in the catheter design.
A subsequent study by Tulleken and associates46 combined laboratory data as outlined above with a large group of patients treated with high-flow bypass for various indications using saphenous vein graft. Twenty patients had interposition grafts between the superficial temporal artery and the intracranial internal carotid, while five patients underwent external carotid to intracranial internal carotid bypass. Six patients were bypassed with giant internal carotid aneurysms, two for skull base meningioma and 12 due to hemodynamic insufficiency; the bypass was performed with good technical success. Five additional patients had failed attempts at excimer-assisted bypass. Two of these patients underwent attempts at laser activation with failure to create an arteriotomy, resulting in conversion to standard anastomosis to a cortical middle cerebral branch. Two other patients maintained significantly atherosclerotic intracranial internal carotids and were not felt to be suitable for laser arteriotomy, with conventional bypasses similarly performed. One patient suffered a spontaneous disruption of the anastomosis. No further information regarding the follow-up is given and there is no mention of outcome other than intraoperative measurements to determine patency. The authors conclude that the procedure is safe with “minimal complications” and a “satisfactory patency rate” yet report only a modest amount of substantive data to support their conclusions and offer little substance in regards to clinical outcome. One must infer that this has much to do with this being the first real attempt at documenting outcome and was published during the period of rapid progress in the development of the technique.
The largest case series, representing 40 patients, was published by Tulleken et al.47 It is included in the paper along with laboratory results as reviewed above. The authors report that the procedure was well tolerated by the patients with a high patency. Five complications were encountered. One patient with severe atherosclerotic disease suffered graft disconnection with serious neurological injury that is not fully spelled out. Severe atherosclerosis thus became an early contraindication to an ELANA bypass. No patient with a giant aneurysm has had a similar outcome. A second severely atherosclerotic patient had the procedure aborted due to a sclerotic carotid wall soon after this case, reflecting the author's recognition of ELANA's limitations. Only 3 of the remaining 38 patients had arteriotomy flaps that were not recovered, reflecting a significant improvement in the effectiveness of the new catheter and ring concept. The authors went so far as to say that in 2 of these 3 patients, catheter design was the cause of the failure. Most significant is that good follow-up was made on this group with no complications reported and 92% patency at 4 weeks to 4 years postop. Seven patients underwent intraoperative flow measurements with carotid occlusion and flows of 120 to 190 cc/min were reported. This is the first report of the flow obtained in “high-flow” venous conduit bypass, confirming its value for high-flow requirement cases. This clinical case review is the most extensive published to that point and significantly advances our understanding of the pitfalls as well as the strengths of the ELANA system. The results do suffer once again from inconsistency in follow-up and data acquisition, making it somewhat difficult to make broad conclusions concerning long-term effectiveness. This paper does, however, add significant weight to performance data regarding the system and allows the reader to conclude that the ELANA system appears to be safe and low risk in patients found suitable for laser-assisted anastomosis. It carries significant weight in regards to safety because it identifies a high-risk subgroup with data supporting its apparent low risk in a well-defined group of patients. This paper succeeds where the previous paper had failed because it addresses the risks directly and reports them in a clear way. The paper also has much more data on long-term outcome and clearly reflects the improvements in the system and its apparent long-term effectiveness as well.
Tulleken and colleagues38 describe the creation of a novel EC-IC bypass between the external carotid and the posterior cerebral artery through a trans-Sylvian approach. The authors report that this is the only such report in the literature which is borne out on an independent literature search. This case report highlights the uniqueness of the technique in that it is the first to describe an ELANA operation that has no equivalent conventionally. The authors correctly point out the unique technical challenge of grafting into the posterior cerebral artery and delineate the advantages of the ELANA technique. The posterior cerebral artery cannot be temporarily occluded safely and the exposure of enough vessel to allow for safe placement of temporary clips is exceedingly difficult through a trans-Sylvian approach. Short-term outcome is described but no long-term outcome is mentioned. This paper is the first to recognize the ELANA technique as providing novel types of revascularization conduits. The majority of ELANA procedures provide a nonocclusive method of opening vessels which otherwise mimics the conventional technique—replacing a knife with a laser. The authors point out how the ELANA technique allows grafts to be placed where access for temporary occlusion is impossible, leaving certain patients without a diversity of options if only conventional methods are available.
Graamans and Tulleken41 report treatment of one patient with a skull base angiofibroma involving the internal carotid artery requiring its sacrifice for total resection. This report is essentially a focused review of a single case of a bypass done in anticipation of internal carotid sacrifice for treatment of an aggressive nasopharyngeal tumor. The patient outcome is reported as good with near-complete tumor resection. No postoperative studies were shown. Also, the discussion of indications for bypass was not presented. There is no mention of the patient having undergone a balloon-test occlusion, but there is reference to the question of whether carotid sacrifice is safe. This is the third tumor case reported and represents the first full case report of a bypass using the ELANA technique in tumor surgery. Once again the technique is shown to be safe and effective.
Tulleken and associates39 produced a second report describing an application of the ELANA technique which cannot be performed without a nonocclusive system. The construction of a new posterior communicating artery without hypothermic arrest had never been reported and cannot be performed safely using current conventional methods. This is also the first to describe the use of ELANA for an intra-intracranial (IC-IC) bypass using saphenous vein—a truly novel concept. Unlike conventional EC-IC bypass and the standard external carotid-to-internal carotid bypass employed in the majority of ELANA surgeries in which the proximal anastomosis is made using temporary occlusion, this case describes the use of nonocclusive ELANA technique at the proximal and distal ends of the anastomosis. The patient described presented with vertebrobasilar ischemia and had failed a prior lower-flow occipital artery-to-posterior cerebellar artery bypass. He tolerated the surgery well with measurement of intraoperative flows suggestive of a patent bypass. Postoperative angiography confirmed a patent bypass and the patient was described as having been in good condition several months postop despite a hemispheric transient ischemic attack (TIA) suffered during the postop period. The etiology of the left hemispheric TIA was not explained, but its origination from the internal carotid proximal anastomosis cannot be ruled out. This article represents an expansion upon the indications for ELANA and describes the ELANA group's thinking regarding new applications and indications. Undoubtedly ELANA IC-IC bypass is the only option for a very small subgroup of bypass patients. These primarily include patients with posterior circulation ischemia who require higher-flow replacement bypasses and patients with poor extracranial proximal graft sites, such as previously operated or radiated patients. A second group of patients can be treated with an IC-IC bypass as an alternative to a conventional approach. The only real advantage of these bypasses over conventional ones would be their shorter length, more anatomic flow direction, and lack of a cervical incision. Overall this paper is of high value in that it identifies new indications for the ELANA system while once again supporting its safety in a difficult case.
Streefkerk et al43 once again highlight the creation of an IC-IC bypass, albeit with a distal conventional anastomosis on the superior cerebellar artery in a very complex vasculopathic patient with a giant basilar aneurysm. The patient suffered a large postoperative infarct in the left middle cerebral distribution and died; however, his graft appeared to be patent and the infarct not clearly related to the graft procedure. The value of this paper is in the study that followed. Using scanning electron microscopy which had been used in earlier laboratory experiments, the authors studied the ELANA anastomosis postoperatively confirming their prior histologic findings in pigs and rabbits. A small thrombus was visualized beneath the previously described residual rim—a small piece of tissue left at the anastomosis following laser arteriotomy. This rim was noted to fully endothelialize in animals within 9 days and early re-endothelialization is reported in this study which is somewhat difficult to discern. The middle cerebral thrombus appears to be massive, clearly embolic in origin, and, given the findings of multiple lung emboli, likely to have originated from an extracranial source. This is the second reported IC-IC bypass and the second to have an apparent hemispheric ischemic event in a middle cerebral distribution. While the source of embolus is not clearly the proximal graft site, it cannot be entirely ruled out, especially in light of some thrombus noted at the proximal anastomosis in this study. The flows in these IC-IC bypasses were also noted to be somewhat lower than the more common higher-flow EC-IC grafts. It does seem possible that these lower flow grafts could be somewhat more thrombogenic. Nevertheless, this study is important as it is the first to demonstrate a human postmortem ELANA specimen confirming earlier light and scanning electron microscopic findings in small animals.
Neurology Studies
Klijn et al51 were the first to focus exclusively on ischemic patients who harbor symptomatic carotid occlusion and were thought to be at high risk for subsequent stroke. These patients behave very differently than those treated for giant aneurysms and their clinical outcome has little direct relevance to the outcome for aneurysm patients. This paper adds little to our understanding of surgical morbidity and mortality of the ELANA procedure in patients with giant or untreatable aneurysms, but does illustrate the danger in operating on ischemic patients whether utilizing ELANA or conventional technique. The authors do, however, mention that flap retrieval is not an absolute and that bypasses with good flows measured intraoperatively without retrieved flaps are not necessarily at risk. They report unpublished data in animal experiments that suggest that these retained flaps do not obstruct the bypass and don’t migrate distally. They suggest that bypass functionality is what is important as quantified by measuring flow. Actual data from the laboratory are not presented to support this finding. Given the prior electron microscopic and light microscopy studies, the reader is left to wonder how these retained flaps appear under high magnification. Overall, this paper lends little to the analysis of the ELANA system primarily due to its choice of patients but also due to its focus on long-term outcome in a very high-risk group. No real new data can be gained from this article other than the authors’ recommendations concerning retained arteriotomy flaps.
Brilstra et al40 reported the first study of the ELANA technique outcome that includes nonsurgeons and those not involved in its early development. The goals of the study were good and well thought out with the data collection much improved over prior studies. Unfortunately, the study was designed in such a way as to point out the high-risk nature of cerebral bypass in general without a clear way to measure risk associated with the ELANA system. Nearly one third of the patients had suffered a subarachnoid hemorrhage and two thirds harbored giant aneurysms. This would seem to suggest that a significant number of these bypasses were done to allow the surgeon to be aggressive with a difficult and high-risk aneurysm by bypassing the parent vessel prior to an attempt at direct treatment. This heterogenous group is evaluated with multivariate analysis with the conclusion that poor condition initially was associated with poor outcome—a well-known finding of all aneurysm series and irrespective of bypass studies. While this article seemed to hold promise regarding conclusions being drawn in reference to ELANA, it ultimately added very little to our understanding of the risks of ELANA and was not designed to address these risks directly.
Clinical Research Studies
Van der Zwan and colleagues36 offered the first of two studies designed to study flow through ELANA saphenous vein bypasses both intraoperatively as well as postoperatively. Twenty-six giant aneurysm patients were studied along with eight ischemic patients. Intraoperative flows were measured using a flow probe. Significant flow increases were measured during recipient artery occlusion when compared with when the parent vessel was left patent. The decision to deconstruct the parent artery was often based upon flow volumes, with higher-flow grafts left with patent parent arteries. Late intraoperative flows were found significantly higher than early flow checks, possibly consistent with short-term graft adaptation. Postoperative flows were measured using MR angiography (MRA). Patients studied with MRA showed consistent increases in flow from those measured intraoperatively, consistent with possible long-term graft adaptation. Conclusions drawn regarding hyperperfusion injury in giant aneurysm and ischemic patients were discussed. Nonocclusive (ELANA) bypass patients should theoretically be less susceptible to this sort of injury, but absolute conclusions regarding this could not be made from these data. This is primarily a study of graft flow and no real attempt was made to assess ELANA safety versus conventional techniques. The measured flows are novel and, given the lack of equivalent types of measurements made for any significant conventional bypass series, represent a significant contribution and are suggestive of the value of the ELANA system. This paper certainly should be weighted highly in its ability to demonstrate the true “high flow” of a high-flow replacement bypass and should be the standard to which future studies of conventional bypass are compared.
Hendrikse et al14 wrote the second of the two studies devoted to flow measurements. This study is a clinical research paper that measures bypass flow as well as preoperative and postoperative individual intracranial vessel blood flow in seven consecutive patients treated with high-flow carotid replacement bypass for giant intracranial aneurysms. The study reports good outcome in all patients with 2/7 having the deconstructive portion of the operation performed intraoperatively and 5/7 having the carotid sacrifice performed postoperatively in the interventional suite. Measured flows were excellent in the group, reflective of the benefits of high-flow bypass using saphenous vein. The uniqueness of the ELANA system is not directly addressed but it is suggested that by safely grafting in a nonocclusive fashion into the proximal vasculature, higher bypass flows are generated. Once again, as no good studies of flows in groups of conventional bypass cases exist, it is difficult to compare the ELANA technique to conventional techniques on the basis of flow. This paper, however, does carry some weight in that it substantiates the flow capacity of the ELANA bypass and provides further data to support its acceptance.
Review Articles
Tulleken et al52 review the state of the ELANA system at the time of its publication. No new data are presented. The concept of the IC-IC bypass is reviewed as well. Specific pitfalls are once again reviewed and pointed out. The paper makes clear that the technique does not prohibit the surgeon from performing a conventional anastomosis should the ELANA technique fail or if the patient is found to maintain vessels not suitable for its application. The authors do relate to the reader several venous complications, not unique to ELANA, that have resulted in morbidity.
Streefkerk and colleagues42 essentially outline the history of the development of ELANA, monitor the clinical progress made to date, discuss laboratory progress, and offer thoughts on the utility of the ELANA system in the future. It is an exhaustive review of not only the ELANA system but the field of revascularization in general. One of the more interesting sections is the portion devoted to complications. Intraoperative complications primarily are related to graft problems such as early thrombosis and to deficits due to temporary vessel occlusion, stroke from occlusion of bypassed vessels, subdural hematomas, intracerebral hemorrhages, and myocardial infarction as outlined by Sundt and associates in 1986.22 Sundt and Sundt additionally published a review of vein graft preparation, classifying graft occlusion into acute (30 days or less), subacute (1 to 2 years) and late (5 years or more).53 Sekhar and coworkers characterized graft problems somewhat differently, preferring to divide them into general, proximal end, tunnel-related, and distal-related problems.24 Utilizing these complication algorithms to evaluate ELANA is helpful in that it allows one to identify which risks are increased, decreased, or not applicable when one compares the ELANA system to conventional technique. ELANA has indirect benefit over conventional bypass in several ways. The need for less exposure could result in less brain retraction and therefore fewer intracerebral hematomas and diminished incidence of postoperative cerebral swelling or edema. Heparin and barbiturate use is obviated, resulting in fewer side effects due to use of these two drugs. Medical risks, risks of embolic stroke from the aneurysm itself or the recipient as well as recipient thrombosis is essentially equal in the two techniques. General, proximal, and tunnel-related graft problems are also theoretically identical, because the problems with vein, radial artery, or other graft conduits would not be improved upon by ELANA. ELANA does in fact require an extra end-to-end anastomosis that can complicate the vein graft patency. This extra anastomosis step, however, may lower the incidence of venous kinking. The ELANA group reports in this review as well as in other articles that the 45-degree angle venous anastomosis at the end-to-end site may assist in preventing venous kinking.
ELANA clearly has direct benefits in two important ways. First, as noted by Sundt et al, stroke due to temporary occlusion of a vessel does occur.22 This risk is reduced to zero by the ELANA technique and is the primary reason to support its acceptance. A less obvious direct benefit is the platinum ring. By placing the ring within the graft's distal end prior to fastening it to the recipient, the vessel is maintained patent and distended around the ring. This allows the surgeon to place sutures to attach graft and recipient with much less chance of sewing to the “back wall.” This benefit should with time result in fewer distal-end graft complications.
CONCLUSIONS
From the data reviewed as outlined above, it appears that the ELANA system is at least as safe as conventional high-flow bypass surgery and in all likelihood has significant advantages over current techniques. Unfortunately, the literature is not comprehensive regarding the true risks of the conventional procedure itself, making it somewhat difficult to compare the ELANA outcomes at one center with the published EC-IC outcomes. ELANA represents a major technological advance in the construction of EC-IC bypasses and may have significant impact on the field of cerebrovascular surgery.
ACKNOWLEDGMENTS
The authors would like to thank Professor C.A.F. Tulleken for providing the vision and inspiration that led to what is now called ELANA. We are grateful to Dr. Albert van der Zwan for his review of the manuscript. We would also like to thank Pablo Pena, M.D. for technical assistance with operative photography.
REFERENCES
- Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339:1725–1733. doi: 10.1056/NEJM199812103392401. [No authors listed] [DOI] [PubMed] [Google Scholar]
- Barrow D L, Alleyne C. Natural history of giant intracranial aneurysms and indications for intervention. Clin Neurosurg. 1995;42:214–244. [PubMed] [Google Scholar]
- Lawton M T, Spetzler R F. Surgical strategies for giant intracranial aneurysms. Neurosurg Clin N Am. 1998;9:725–742. [PubMed] [Google Scholar]
- McCormick W F, Acosta-Rua G J. The size of intracranial saccular aneurysms. An autopsy study. J Neurosurg. 1970;33:422–427. doi: 10.3171/jns.1970.33.4.0422. [DOI] [PubMed] [Google Scholar]
- Orz Y, Kobayashi S, Osawa M, Tanaka Y. Aneurysm size: a prognostic factor for rupture. Br J Neurosurg. 1997;11:144–149. doi: 10.1080/02688699746500. [DOI] [PubMed] [Google Scholar]
- Wiebers D O, Whisnant J P, Sundt T M, Jr, O'Fallon W M. The significance of unruptured intracranial saccular aneurysms. J Neurosurg. 1987;66:23–29. doi: 10.3171/jns.1987.66.1.0023. [DOI] [PubMed] [Google Scholar]
- Erba S M, Horton J A, Latchaw R E, et al. Balloon test occlusion of the internal carotid artery with stable xenon/CT cerebral blood flow imaging. AJNR Am J Neuroradiol. 1988;9:533–538. [PMC free article] [PubMed] [Google Scholar]
- Fox A J, Vinuela F, Pelz D M, et al. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg. 1987;66:40–46. doi: 10.3171/jns.1987.66.1.0040. [DOI] [PubMed] [Google Scholar]
- Vazquez Anon V, Aymard A, Gobin Y P, et al. Balloon occlusion of the internal carotid artery in 40 cases of giant intracavernous aneurysm: technical aspects, cerebral monitoring, and results. Neuroradiology. 1992;34:245–251. doi: 10.1007/BF00596347. [DOI] [PubMed] [Google Scholar]
- Sen C, Sekhar L N. Direct vein graft reconstruction of the cavernous, petrous, and upper cervical internal carotid artery: lessons learned from 30 cases. Neurosurgery. 1992;30:732–742. discussion 742–743. [PubMed] [Google Scholar]
- Sekhar L N, Sen C N, Jho H D. Saphenous vein graft bypass of the cavernous internal carotid artery. J Neurosurg. 1990;72:35–41. doi: 10.3171/jns.1990.72.1.0035. [DOI] [PubMed] [Google Scholar]
- Rutgers D R, Blankensteijn J D, Grond J van der. Preoperative MRA flow quantification in CEA patients: flow differences between patients who develop cerebral ischemia and patients who do not develop cerebral ischemia during cross-clamping of the carotid artery. Stroke. 2000;31:3021–3028. doi: 10.1161/01.str.31.12.3021. [DOI] [PubMed] [Google Scholar]
- Linskey M E, Jungreis C A, Yonas H, et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994;15:829–843. [PMC free article] [PubMed] [Google Scholar]
- Hendrikse J, Zwan A van der, Ramos L M, Tulleken C A, Grond J van der. Hemodynamic compensation via an excimer laser-assisted, high-flow bypass before and after therapeutic occlusion of the internal carotid artery. Neurosurgery. 2003;53:858–863. discussion 863–865. doi: 10.1227/01.neu.0000083552.45265.46. [DOI] [PubMed] [Google Scholar]
- Hopkins L N, Grand W. Extracranial-intracranial arterial bypass in the treatment of aneurysms of the carotid and middle cerebral arteries. Neurosurgery. 1979;5(1 Pt 1):21–31. doi: 10.1227/00006123-197907010-00004. [DOI] [PubMed] [Google Scholar]
- Spetzler R F, Roski R A, Schuster H, Takaoka Y. The role of EC-IC in the treatment of giant intracranial aneurysms. Neurol Res. 1980;2:345–359. doi: 10.1080/01616412.1980.11739587. [DOI] [PubMed] [Google Scholar]
- Spetzler R F, Schuster H, Roski R A. Elective extracranial-intracranial arterial bypass in the treatment of inoperable giant aneurysms of the internal carotid artery. J Neurosurg. 1980;53:22–27. doi: 10.3171/jns.1980.53.1.0022. [DOI] [PubMed] [Google Scholar]
- Peerless S J, Ferguson G G, Drake C G. Extracranial-intracranial (EC/IC) bypass in the treatment of giant intracranial aneurysms. Neurosurg Rev. 1982;5:77–81. doi: 10.1007/BF01743477. [DOI] [PubMed] [Google Scholar]
- Yasargil M G, Yonekawa Y. Results of microsurgical extra-intracranial arterial bypass in the treatment of cerebral ischemia. Neurosurgery. 1977;1:22–24. doi: 10.1227/00006123-197707000-00005. [DOI] [PubMed] [Google Scholar]
- Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191–1200. doi: 10.1056/NEJM198511073131904. [No authors listed] [DOI] [PubMed] [Google Scholar]
- Abruzzo T, Joseph G J, Owens D S, Dawson R C, III, Reid J, Barrow D L. Prevention of complications resulting from endovascular carotid sacrifice: a retrospective assessment. Neurosurgery. 2000;46:910–916. discussion 916–917. doi: 10.1097/00006123-200004000-00025. [DOI] [PubMed] [Google Scholar]
- Sundt T M, Jr, Piepgras D G, Marsh W R, Fode N C. Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg. 1986;65:439–450. doi: 10.3171/jns.1986.65.4.0439. [DOI] [PubMed] [Google Scholar]
- Sekhar L, Kalavakonda C. Saphenous vein and radial artery grafts in the management of skull base tumors and aneurysms. Oper Tech Neurosurg. 1999;2:129–141. [Google Scholar]
- Sekhar L N, Bucur S D, Bank W O, Wright D C. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions: evolution of surgical treatment and improved graft results. Neurosurgery. 1999;44:1207–1223. discussion 1223–1224. doi: 10.1097/00006123-199906000-00028. [DOI] [PubMed] [Google Scholar]
- Lawton M T, Hamilton M G, Morcos J J, Spetzler R F. Revascularization and aneurysm surgery: current techniques, indications, and outcome. Neurosurgery. 1996;38:83–92. discussion 94–94. doi: 10.1097/00006123-199601000-00020. [DOI] [PubMed] [Google Scholar]
- Sekhar L N, Duff J M, Kalavakonda C, Olding M. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms: techniques and outcomes for 17 patients. Neurosurgery. 2001;49:646–658. discussion 658–659. doi: 10.1097/00006123-200109000-00023. [DOI] [PubMed] [Google Scholar]
- Siminelakis S, Karfis E, Anagnostopoulos C, Toumpoulis I, Katsaraki A, Drossos G. Harvesting radial artery and neurologic complications. J Card Surg. 2004;19:505–510. doi: 10.1111/j.0886-0440.2004.04090.x. [DOI] [PubMed] [Google Scholar]
- Sudhakar C B, Forman D L, Dewar M L, Shaw R K, Fusi S. Free radial artery grafts: surgical technique and results. Ann Plast Surg. 1998;40:408–411. discussion 412. doi: 10.1097/00000637-199804000-00015. [DOI] [PubMed] [Google Scholar]
- Budillon A M, Nicolini F, Agostinelli A, et al. Complications after radial artery harvesting for coronary artery bypass grafting: our experience. Surgery. 2003;133:283–287. doi: 10.1067/msy.2003.43. [DOI] [PubMed] [Google Scholar]
- Evans J J, Sekhar L N, Rak R, Stimac D. Bypass grafting and revascularization in the management of posterior circulation aneurysms. Neurosurgery. 2004;55:1036–1049. doi: 10.1227/01.neu.0000140822.64362.c6. [DOI] [PubMed] [Google Scholar]
- Anson J A, Stone J L, Crowell R M. Rupture of a giant carotid aneurysm after extracranial-to-intracranial bypass surgery. Neurosurgery. 1991;28:142–147. doi: 10.1097/00006123-199101000-00020. [DOI] [PubMed] [Google Scholar]
- Sekhar L N, Kalavakonda C. Cerebral revascularization for aneurysms and tumors. Neurosurgery. 2002;50:321–331. doi: 10.1097/00006123-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Roski R A, Spetzler R F, Nulsen F E. Late complications of carotid ligation in the treatment of intracranial aneurysms. J Neurosurg. 1981;54:583–587. doi: 10.3171/jns.1981.54.5.0583. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Fujii K, Fukui M. De novo aneurysm formation and aneurysm growth following therapeutic carotid occlusion for intracranial internal carotid artery (ICA) aneurysms. Acta Neurochir (Wien) 1993;120:20–25. doi: 10.1007/BF02001464. [DOI] [PubMed] [Google Scholar]
- Hillen B, Hoogstraten H W, Overbeek J J van, Zwan A van der. Functional anatomy of the circulus arteriosus cerebri (Willisii) Bull Assoc Anat (Nancy) 1991;75:123–126. [PubMed] [Google Scholar]
- Zwan A van der, Tulleken C A, Hillen B. Flow quantification of the non-occlusive excimer laser-assisted EC-IC bypass. Acta Neurochir (Wien) 2001;143:647–654. doi: 10.1007/s007010170042. [DOI] [PubMed] [Google Scholar]
- Charbel F T, Misra M, Clarke M E, Ausman J I. Computer simulation of cerebral blood flow in moyamoya and the results of surgical therapies. Clin Neurol Neurosurg. 1997;99 Suppl 2:S68–S73. doi: 10.1016/s0303-8467(97)00073-5. [DOI] [PubMed] [Google Scholar]
- Tulleken C A, Zwan A van der, Rooij W J van, Ramos L M. High-flow bypass using nonocclusive excimer laser-assisted end-to-side anastomosis of the external carotid artery to the P1 segment of the posterior cerebral artery via the sylvian route. Technical note. J Neurosurg. 1998;88:925–927. doi: 10.3171/jns.1998.88.5.0925. [DOI] [PubMed] [Google Scholar]
- Tulleken C A, Streefkerk H J, Zwan A van der. Construction of a new posterior communicating artery in a patient with poor posterior fossa circulation: technical case report. Neurosurgery. 2002;50:415–419. discussion 419–420. doi: 10.1097/00006123-200202000-00036. [DOI] [PubMed] [Google Scholar]
- Brilstra E H, Rinkel G J, Klijn C J, et al. Excimer laser-assisted bypass in aneurysm treatment: short-term outcomes. J Neurosurg. 2002;97:1029–1035. doi: 10.3171/jns.2002.97.5.1029. [DOI] [PubMed] [Google Scholar]
- Graamans K, Tulleken C A. Laser-assisted bypass of the internal carotid artery prior to treatment of an extensive angiofibroma. Skull Base Surg. 1998;8:205–210. doi: 10.1055/s-2008-1058184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streefkerk H J, Zwan A van der, Verdaasdonk R M, Beck H J, Tulleken C A. Cerebral revascularization. Adv Tech Stand Neurosurg. 2003;28:145–225. doi: 10.1007/978-3-7091-0641-9_3. [DOI] [PubMed] [Google Scholar]
- Streefkerk H J, Wolfs J F, Sorteberg W, Sorteberg A G, Tulleken C A. The ELANA technique: constructing a high flow bypass using a non-occlusive anastomosis on the ICA and a conventional anastomosis on the SCA in the treatment of a fusiform giant basilar trunk aneurysm. Acta Neurochir (Wien) 2004;146:1009–1019. doi: 10.1007/s00701-004-0296-2. [DOI] [PubMed] [Google Scholar]
- Tulleken C A, Verdaasdonk R M, Berendsen W, Mali W P. Use of the excimer laser in high-flow bypass surgery of the brain. J Neurosurgery. 1993;78:477–480. doi: 10.3171/jns.1993.78.3.0477. [DOI] [PubMed] [Google Scholar]
- Tulleken C A, Verdaasdonk R M. First clinical experience with Excimer assisted high flow bypass surgery of the brain. Acta Neurochir (Wien) 1995;134:66–70. doi: 10.1007/BF01428506. [DOI] [PubMed] [Google Scholar]
- Tulleken C A, Verdaasdonk R M, Beck R J, Mali W P. The modified excimer laser-assisted high-flow bypass operation. Surg Neurol. 1996;46:424–429. doi: 10.1016/s0090-3019(96)00096-1. [DOI] [PubMed] [Google Scholar]
- Tulleken C A, Verdaasdonk R M, Mansvelt Beck H J. Nonocclusive excimer laser-assisted end-to-side anastomosis. Ann Thor Surg. 1997;63:S138–S142. doi: 10.1016/s0003-4975(97)00355-x. [DOI] [PubMed] [Google Scholar]
- Wolfs J F, Ham L F van den , Laak M P ter, Zwan A van der, Tulleken C A. Scanning electron microscopic evaluation of nonocclusive excimer laser-assisted anastomosis in rabbits. Acta Neurochir (Wien) 2000;142:1399–1407. doi: 10.1007/s007010070012. [DOI] [PubMed] [Google Scholar]
- Streefkerk H JN, Kleinvald S, Koedam E LGE, et al. The ELANA technique: long-term re-endothelialization of ELANA anastomoses versus conventionally sutured anastomoses in pigs. J Neurosurg. 2005 doi: 10.3171/jns.2005.103.2.0328. in press. [DOI] [PubMed] [Google Scholar]
- Lidman D, Thomsen M B. Patency, blood flow and histologic characteristics in end-to-end and end-to-side arteriovenous fistulas. Comparisons in an experimental model. Acta Chir Scand. 1986;152:103–109. [PubMed] [Google Scholar]
- Klijn C J, Kappelle L J, Zwan A van der, Gijn J van, Tulleken C A. Excimer laser-assisted high-flow extracranial/intracranial bypass in patients with symptomatic carotid artery occlusion at high risk of recurrent cerebral ischemia: safety and long-term outcome. Stroke. 2002;33:2451–2458. doi: 10.1161/01.str.0000030319.78212.51. [DOI] [PubMed] [Google Scholar]
- Tulleken C AF, Zwan A van der, Verdaasdonk R M, et al. High flow excimer laser assisted extra-intracranial and intra-intracranial by pass. Oper Tech Neurosurg. 1999;2:142–148. [Google Scholar]
- Sundt T M, III, Sundt T M., Jr Principles of preparation of vein bypass grafts to maximize patency. J Neurosurg. 1987;66:172–180. doi: 10.3171/jns.1987.66.2.0172. [DOI] [PubMed] [Google Scholar]